Summary
Sirtuins (SIRTs) are critical enzymes that govern genome regulation, metabolism, and aging. Despite conserved deacetylase domains, mitochondrial SIRT4 and SIRT5 have little to no deacetylase activity, and a robust catalytic activity for SIRT4 has been elusive. Here, we establish SIRT4 as a cellular lipoamidase that regulates the pyruvate dehydrogenase complex (PDH). Importantly, SIRT4 catalytic efficiency for lipoyl- and biotinyl-lysine modifications is superior to its deacetylation activity. PDH, which converts pyruvate to acetyl-CoA, has been known to be primarily regulated by phosphorylation of its E1 component. We determine that SIRT4 enzymatically hydrolyzes the lipoamide cofactors from the E2 component dihydrolipoyllysine acetyltransferase (DLAT), diminishing PDH activity. We demonstrate SIRT4-mediated regulation of DLAT lipoyl levels and PDH activity in cells and in vivo, in mouse liver. Furthermore, metabolic flux switching via glutamine stimulation induces SIRT4 lipoamidase activity to inhibit PDH, highlighting SIRT4 as a guardian of cellular metabolism.
Introduction
Sirtuins (SIRTs) are a family of seven mammalian nicotinamide adenine dinucleotide (NAD+)-dependent enzymes that regulate diverse biological processes, including genome regulation, stress response, metabolic homeostasis and aging (Guarente, 2000; Imai et al., 2000). SIRTs display widespread subcellular distributions, as SIRT1, SIRT6, and SIRT7 are nuclear, SIRT2 is predominantly cytoplasmic, and SIRTs3-5 are mitochondrial (Haigis et al., 2006; Michishita et al., 2005). As all SIRTs have a conserved deacetylase domain, these enzymes are generally known as lysine deacetylases, acting in opposition to acetyltransferases to remove acetyl-modifications from lysine residues (Imai et al., 2000). However, SIRTs exhibit varying catalytic efficiencies to this modification. SIRTs1-3 display robust deacetylase activity, in contrast to SIRTs4-5 that show little to no activity (Haigis et al., 2006; Michishita et al., 2005; Schuetz et al., 2007). Emerging evidence has revealed that several SIRTs can hydrolyze alternative lysine modifications more efficiently than acetyl. Specifically, SIRT5 preferentially desuccinylates and demalonylates protein substrates (Du et al., 2011; Peng et al., 2011), while SIRT6 can hydrolyze long-chain fatty acyl lysine modifications (Jiang et al., 2013). These studies have highlighted the functionally dynamic nature of this family of proteins, able to perform different enzymatic reactions, and regulate a wide range of cellular processes.
Mitochondrial SIRTs3-5 regulate ATP production, apoptosis, and cell signalling (Verdin et al., 2010) through distinct enzymatic functions. SIRT3 is considered to be the major deacetylase of the mitochondria, as SIRT3-deficient mice exhibit significant protein hyper-acetylation (Lombard et al., 2007). The desuccinylase activity of SIRT5 was shown to target proteins involved in fatty acid β-oxidation and ketone body synthesis pathways, with SIRT5-deficient mice exhibiting an accumulation of acylcarnitines and a decrease in β-hydroxybutyrate production (Rardin et al., 2013). More recently, SIRT5 was reported to regulate lysine glutarylation levels, thereby modulating the activity of carbamoyl phosphase synthase 1, a critical enzyme in the urea cycle (Tan et al., 2014). In contrast to SIRT3 and SIRT5, SIRT4 enzymatic functions have generally remained elusive (Newman et al., 2012). SIRT4 has been reported to regulate glutamine metabolism (Csibi et al., 2013; Jeong et al., 2013), and fatty acid oxidation via PPAR-α activity (Laurent et al., 2013a). To date, the enzymatic activity of SIRT4 is largely based on its ability to ADP-ribosylate glutamate dehydrogenase (GLUD1), which regulates amino acid-dependent insulin secretion (Haigis et al., 2006). The deacylase activities of SIRT4 have remained less well-characterized. Initial studies reported limited deacetylation activity (Lin et al., 2012; Michishita et al., 2005), yet SIRT4 was recently reported to control lipid catabolism through deacetylation of malonyl-CoA decarboxylase (MCD) (Laurent et al., 2013b). Additionally, acetylated SIRT4 substrate candidates have been identified in vitro via peptide microarrays (Rauh et al., 2013) and by screening the activity of recombinant SIRTs against various acyl-histone peptides (Feldman et al., 2013). Unfortunately, these efforts may have been hampered by difficulty in maintaining soluble and active recombinant SIRT4. Therefore, reconciliation of in vitro enzymatic activities with in vivo biological substrates and downstream physiological functions remains a challenge.
Here, we characterized SIRT4 protein interactions within mitochondria, identifying its association with proteins containing lipoyl and biotinyl modifications. In agreement with this, we demonstrate that SIRT4 removes lipoyl- and biotinyl-lysine modifications more efficiently than acetylations. We discover a physical and functional interaction between SIRT4 and the components of the pyruvate dehydrogenase complex (PDH). PDH is a mitochondrial complex comprised of three catalytic subunits (E1, pyruvate decarboxylase; E2, dihydrolipoyllysine acetyltransferase (DLAT); E3, dihydrolipoyl dehydrogenase), a structural subunit (PDH-binding component X, PDHX) and two regulatory subunits (PDH kinase and PDH phosphatase) (Zhou et al., 2001). The complex catalyzes the decarboxylation of pyruvate to generate acetyl CoA, and links glycolysis to the TCA cycle. Its activity is known to be regulated by phosphorylation of the E1 subunit, phosphorylation that can be also impacted by E1 acetylation (Fan et al., 2014; Jing et al., 2013; Linn et al., 1969; Wieland and Jagow-Westermann, 1969). Here, we show that SIRT4 provides a previously unrecognized, phosphorylation-independent, mechanism of PDH regulation. SIRT4 hydrolyzes lipoamide cofactors from the DLAT E2 component of the PDH complex, thereby inhibiting PDH activity. Finally, as glutamine stimulation in rat liver is also known to inhibit the PDH (Haussinger et al., 1982), we investigated whether SIRT4 may play a role in this process. Indeed, we show that glutamine stimulation induces endogenous SIRT4 lipoamidase activity, triggering a reduction in both DLAT lipoyl levels and PDH activity. As the PDH controls pyruvate decarboxylation, fueling multiple downstream pathways, our findings highlight SIRT4 as a critical regulator of cellular metabolism.
Results
SIRT4 interacts with the three mitochondrial dehydrogenase complexes
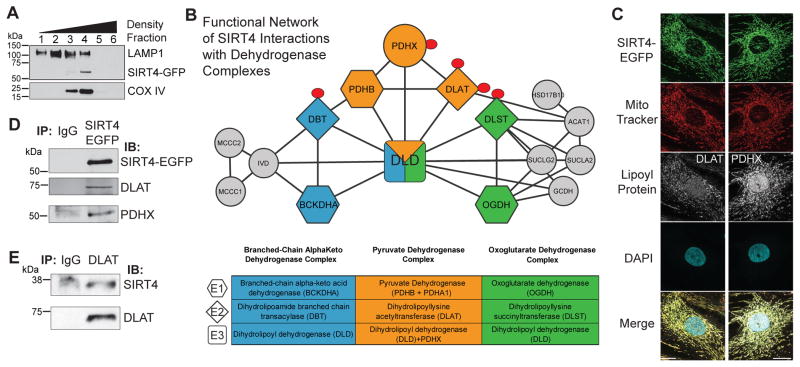
To investigate potential cellular substrates of SIRT4, we used proteomics to define its mitochondrial protein interactions. We constructed MRC5 fibroblasts stably expressing SIRT4-EGFP. Using density-based organelle fractionation (co-isolation with mitochondrial COX IV, Fig. 1A) and direct fluorescence microscopy (co-localization with MitoTracker, Fig. 1C and Fig. S1A), we confirmed its mitochondrial localization. Mitochondria were isolated and the interactions of SIRT4-EGFP were characterized by immunoaffinity purification-mass spectrometry (IP-MS) (Joshi et al., 2013). Interaction specificity was computationally assessed using SAINT (Choi et al., 2011), and 106 significant SIRT4 candidate interactions were identified (Table S1), including the known interactions and substrates, GLUD1, IDE and MLYCD (Ahuja et al., 2007; Haigis et al., 2006; Laurent et al., 2013b). We hypothesized that as yet unrecognized substrates were also identified, and interrogated SIRT4 interactions using bioinformatics to extract enriched metabolic pathways and assemble functional protein networks. Notably, pyruvate metabolism, the TCA cycle, branched-chain amino acid catabolism, and biotin metabolism were significantly enriched pathways (Fig. S1). Interaction of SIRT4 with biotin-dependent carboxylases has been reported (Wirth et al., 2013), validating the reliability of our dataset. Interestingly, we found that SIRT4 associated with all three of the multimeric mammalian dehydrogenase complexes—pyruvate dehydrogenase (PDH), oxoglutarate dehydrogenase (OGDH), and branched-chain alpha-keto acid dehydrogenase (BCKDH) (Fig. 1B). These complexes occupy discrete positions within the cellular metabolic landscape, regulating TCA cycle activity and amino acid metabolism (Fig. S1C). Given its relative prominence within SIRT4 interactions, we focused on PDH. The PDH complex is known to be regulated by reversible phosphorylation of its E1 component (Linn et al., 1969; Wieland and Jagow-Westermann, 1969), with acetylation of E1 also impacting its phosphorylation levels (Fan et al., 2014; Jing et al., 2013). We confirmed that SIRT4-EGFP co-localized (Fig. 1C) and immuno-isolated (Fig. 1D) with DLAT and PDH component X (PDHX), the E2 and E3 subunits of PDH, respectively (Fig. 1B). Furthermore, in wild-type (WT) human fibroblast cells we confirmed that DLAT interacts with endogenous SIRT4 by reciprocal IP (Fig. 1E).
Fig. 1. SIRT4 interacts with the pyruvate dehydrogenase complex.
(A) Density gradient-based cellular fractionation of MRC5 cells isolates SIRT4-EGFP with mitochondrial marker COX IV. (B) Functional network of SIRT4 interactions reveals association with dehydrogenase complexes. The E2 components in each complex (diamonds) contain lipoamide modifications (red circle). (C) SIRT4-EGFP (green) co-localizes with DLAT and PDHX (white) within mitochondria (MitoTracker Red). (D) Immunoaffinity purification of SIRT4-EGFP co-isolates DLAT and PDHX. (E) Reciprocal immunoaffinity purification of DLAT co-isolates endogenous SIRT4 in wild-type fibroblasts. See also Figure S1.
SIRT4 more efficiently catalyzes removal of lipoyl- and biotinyl- than acetyl-lysine modifications
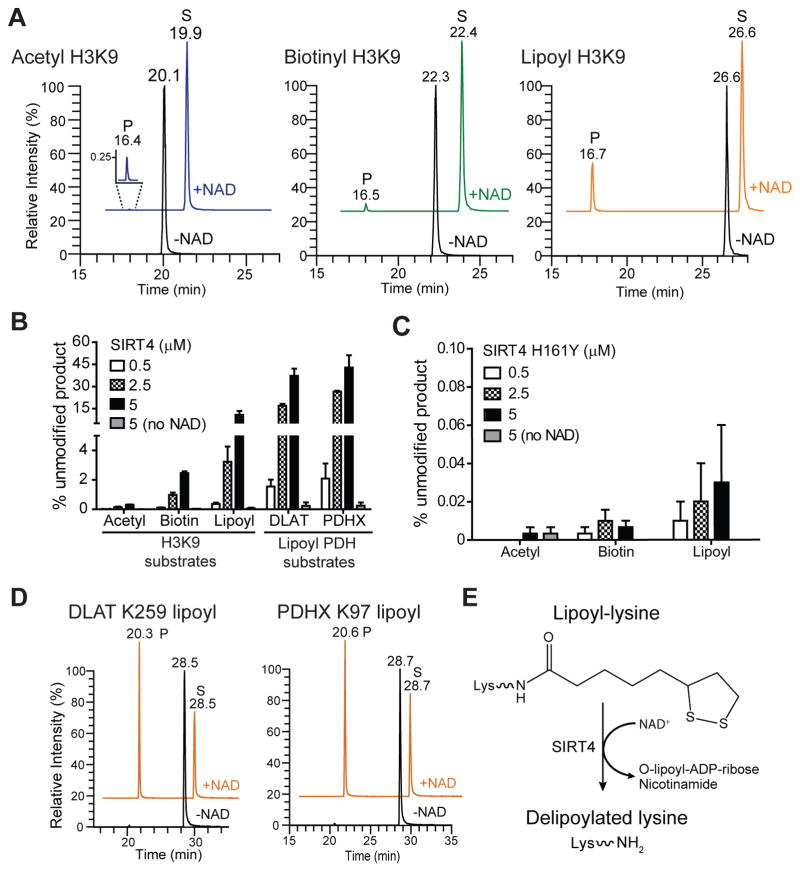
Given our confirmation of SIRT4 interaction with the PDH components, we pursued their functional relationship. The lipoamide cofactors bound to E2 transferase enzymes (Fig. 1B, “L”) are required for PDH activity (Rahmatullah et al., 1990), forming the intermediate S-acetyldihydrolipoyl-lysine in the production of acetyl-CoA. DLAT also has a structural role, constituting the PDH catalytic core. As other mitochondrial SIRTs can hydrolyze various lysine modifications (Du et al., 2011; Jiang et al., 2013), and as DLAT was prominent in our SIRT4 isolation, we speculated that E2 dehydrogenase components may be biological substrates of SIRT4, and that SIRT4 may directly hydrolyze the lipoamide cofactor. To test this we screened the in vitro activity of recombinant SIRT4 against differentially-modified synthetic peptides (Table 1). Initially, SIRT4 was incubated with histone H3 Lys9 (H3K9) peptides modified with acetyl-, biotinyl- or lipoyl-lysine, in the presence or absence of NAD+. Following the reaction, the generated unmodified peptides and remaining unreacted substrates were quantified by LC-MS (Fig. 2A–D and Fig. S2). SIRT4 only exhibited enzymatic activity in the presence of NAD+, shown by the generation of a product peak (P) at ~16.5 min (Fig. 2A), corresponding to the unmodified H3K9 (Fig. S2A). To compare the relative preference of SIRT4 for the different acyl-lysine peptides, we used extracted ion chromatograms to quantify the percentage of unmodified peptide generated for each substrate (Fig. 2B). SIRT4 showed the highest potency for removing the lipoyl modification (Fig 2B). The relative amount of unmodified product generated after reaction with SIRT4 was 11% (lipoyl), 3% (biotinyl), and 0.3% (acetyl) (Fig. 2B, H3K9 substrates). To show that the enzymatic activity of SIRT4 was required for hydrolysis, we purified a recombinant SIRT4 containing a mutation to the critical residue H161. This histidine, conserved among all SIRTs, is critical for NAD+ and substrate binding (Frye, 1999; Smith and Denu, 2006). In contrast to wild-type SIRT4, deacylation assays using the catalytically inactive SIRT4 H161Y resulted in no significant activity against any of these acyl-modified substrates (Fig. 2C).
Table 1.
Determination of in vitro SIRT4 kinetics with acyl-modified peptide substrates
| Peptide substrate | Sequence | kcat (s−1) | Km (μM) | kcat/Km (s−1M−1) |
|---|---|---|---|---|
| H3 K9 Acetyl | KQTARKSTGGWW | ND* | ND* (>2500) | 0.083 ± 0.004 |
| H3 K9 Biotinyl | 0.0005 ± 0.0001 | 719 ± 79 | 0.74 ± 0.05 | |
| H3 K9 Lipoyl | 0.0019 ± 0.0002 | 814 ± 163 | 2.30 ± 0.30 | |
| DLAT K259 Acetyl | EIETDKATIGW | ND* | ND (>2500)* | 0.20 ± 0.01 |
| DLAT K259 Lipoyl | 0.0018 ± 0.0001 | 239 ± 51 | 7.65 ± 1.31 | |
| MCD K471 Acetyl | SYLGSKNIKASEW | ND* | ND* (>2500) | 0.0064 ± 0.0006 |
Synthetic peptide sequences are shown containing the modified lysine residues (underlined) as indicated by peptide substrate. When appropriate, kcat, Km, and kcat/Km values were determined by modeling of kinetic data using the Briggs-Haldane approach (see Figs. 2D–E and Materials and Methods).
ND, kcat and Km could not be determined because v0 vs. [S] was linear. kcat/Km was calculated by linear regression of v0/[SIRT4] vs. [S]. SIRT4 enzyme concentration = 5 μM.
Fig. 2. SIRT4 hydrolyzes lipoyl-, biotin-, and acetyl-lysine modifications in vitro.
(A) Recombinant SIRT4 (5 μM) was incubated with various acyl-modified H3K9 peptides (10 μM) with or without NAD (1 mM), and product and residual substrate peptides detected by LC-MS after reaction. Representative extracted ion chromatograms show unreacted acyl-modified H3K9 substrates (S), and unmodified H3K9 products (P, ~16.5 min). +NAD chromatograms are offset for clarity. (B) The percentage of product (unmodified peptide) formed as a function of increasing concentration of wild-type SIRT4 (±NAD) (mean ± S.E.M.; n=3). (C) Same as (B), except product formation from H3K9 substrates after reaction with increasing concentration of catalytically inactive SIRT4 H161Y. (D) Same as (A), except SIRT4 was incubated with putative biological DLAT and PDHX lipoyl lysine peptides (10 μM). Representative extracted ion chromatograms of unreacted DLAT and PDHX lipoyl peptide substrates (S) and unmodified products (P). (E) Scheme depicting the NAD+-dependent delipoylation of lipoyl-lysine mediated by SIRT4 lipoamidase activity. See also Figure S2.
SIRT4 has superior lipoamidase activity for lipoyl-modified PDH peptides
To characterize the putative biological substrates of SIRT4, we tested whether SIRT4 removed lipoamide from DLAT and PDHX peptides (Fig. 2B, D, Fig. S2B–C). SIRT4 showed greater activity towards these substrates than for H3K9, as the proportion of unmodified peptide generated in the presence of NAD+ increased to 33% for DLAT and 42% for PDHX (Fig. 2B, Lipoyl PDH substrates, and Fig. 2D).
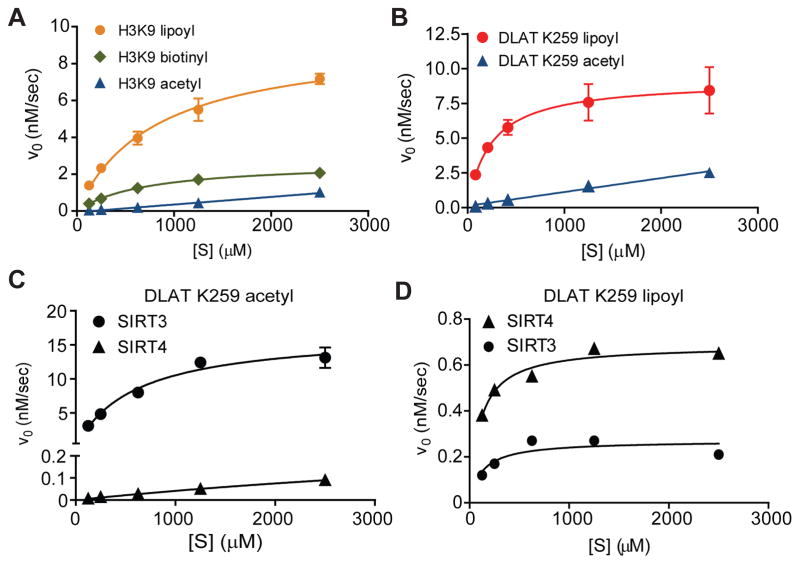
Having established SIRT4 enzymatic activity, we next performed steady-state enzyme kinetic assays. This allowed direct comparison of SIRT4’s catalytic efficiency for the various acyl-modified peptide substrates (Table 1). Compared to H3K9 acetyl, SIRT4 removed lipoyl and biotinyl H3K9 modifications 28-fold and 9-fold more efficiently, respectively (Fig. 3A, Table 1). The DLAT lipoyl peptide displayed a 3.3-fold increase in efficiency compared to H3K9 lipoyl, owing mainly to a decreased Km (Fig. 3A–B, Table 1). As a deacetylase, SIRT4 showed slightly greater efficiency towards DLAT acetyl compared to H3K9 acetyl; however, this efficiency was still 38–fold lower than DLAT lipoyl (Fig. 3B, Fig S3A). We also compared SIRT4’s ability to deacetylate the known biological substrate peptide from MCD (Laurent et al., 2013b) and found that SIRT4 was ~1270-fold more efficient at hydrolyzing DLAT lipoyl (Table 1, Fig. S3A). Altogether, our results demonstrate that SIRT4 has a higher NAD+-dependent lipoamidase than deacetylase activity, directly hydrolyzing the lipoyl-lysine to generate unmodified lysine (Fig. 2E).
Fig. 3. Steady-state kinetics reveals SIRT4 has the highest catalytic efficiency for lipoyl-modified substrates among mitochondrial SIRTs.
(A–B) SIRT4 (5 μM) initial velocity (v0) versus substrate concentration [S] for (A) H3K9- and (B) DLAT-acyl peptides (mean ± S.E.M.; n=3). [SIRT4] = 5 μM. v0 vs. [S] were linear for acetyl substrates and were re-plotted to estimate kcat/Km (see Fig S3A). (C–D) Comparison of SIRT3 (0.5 μM) and SIRT4 (0.5 μM) initial velocity vs. [S] for DLAT K259 (C) acetyl and (D) lipoyl peptide (mean ± S.E.M.; n=3). SIRT4 v0 vs. [S] was linear for DLAT acetyl and was re-plotted to estimate kcat/Km (see Fig S3B). If no error bars are displayed, errors were smaller than the data point size.See also Figure S3.
SIRT4 is the most efficient lipoamidase in vitro among mitochondrial sirtuins
To evaluate the lipoamidase activity for the three known mitochondrial sirtuins (SIRT3-5), we used steady-state enzyme kinetics to compare their ability to hydrolyze lipoyl or acetyl lysine modifications (Fig. 3C–D). For SIRT5, low but detectable activity was measured for DLAT acetyl (Fig. S3B), while no activity was detected for DLAT lipoyl reactions (Fig. S3C). As predicted, SIRT3, a robust mitochondrial deacetylase, showed significant enzymatic activity towards DLAT acetyl, while SIRT4 had minimal activity (~800-fold lower) (Fig 3C, Fig S3C). In contrast, although SIRT3 displayed some enzymatic activity towards DLAT lipoyl (Fig. 3D), its efficiency was 13-fold lower when compared to DLAT acetyl (Fig. 3D vs 3C). Thus, SIRT4 has the highest catalytic efficiency for lipoamide modifications compared to the other mitochondrial SIRTs.
SIRT4 lipoamidase activity diminishes cellular PDH lipoamide levels and inhibits its activity
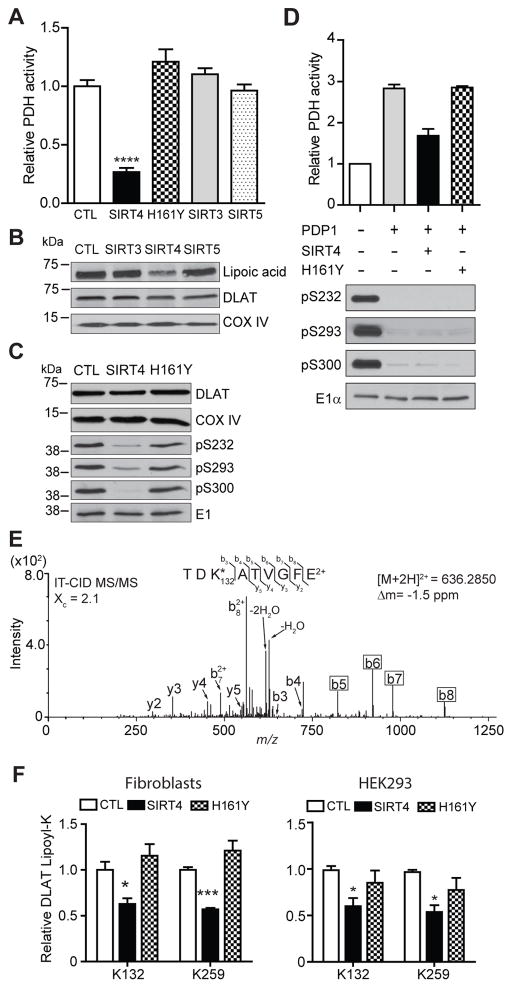
Given that the lipoamide cofactor is essential for PDH function (Perham, 1991), we examined the impact of elevated SIRT levels on the endogenous cellular activity of PDH by over-expression (OE) of each mitochondrial SIRT in cultured human fibroblasts. Strikingly, PDH activity was only diminished in fibroblasts stably expressing SIRT4 compared to cells over-expressing GFP (CTL), SIRT3, or SIRT5 (Fig. 4A, S4A–B). While SIRT3 displayed marginal in vitro enzymatic activity for the DLAT lipoyl peptide (Fig. 3D), OE of either SIRT3 or SIRT5 did not alter cellular PDH activity (Fig. 4A, Fig. S4B), reinforcing the cellular specificity of SIRT4. We further confirmed the direct involvement of SIRT4 activity by showing that PDH activity was not reduced by OE of SIRT4 H161Y (Fig. 4A, S4A). Concomitant with the SIRT4-mediated reduction in PDH activity, we observed reduced lipoylation of endogenous DLAT in SIRT4 OE cells, while total DLAT levels remained constant (Fig. 4B). DLAT lipoyl levels were not altered in SIRT3 or SIRT5 OE cells. To further characterize the correlation between SIRT4 OE and the decrease in PDH activity and lipoyl levels, we measured phosphorylation of PDH-E1rα. Interestingly, we observed reduced phosphorylation at all three sites of E1α in SIRT4 OE cells, while total E1α levels remained constant (Fig. 4C). Expression of H161Y SIRT4 did not change phosphorylation levels at any of the E1 sites (Fig. 4C), indicating that SIRT4 enzymatic activity is required.
Fig. 4. Elevated SIRT4 expression decreases the activity and lipoylation of the pyruvate dehydrogenase complex in cultured cells.
(A) PDH activity in fibroblasts expressing mitochondrial SIRT proteins vs. GFP-expressing cells (CTL) (mean ± S.E.M.; n=3 SIRTs 3–5; n=5 GFP; ****p<0.0001) measured by a PDH immunocapture colorimetric assay, (B) Western blot analysis of endogenous, full length lipoylated DLAT in cells overexpressing mitochondrial SIRTs. DLAT and COX IV – loading controls. (C) Western blot analysis of regulatory PDH-E1α phosphorylation (pS232, pS293, pS300) upon overexpression of SIRT4, catalytically inactive mutant H161Y, or GFP (CTL). E1 is loading control. (D) Relative PDH activity of untreated (control) or “activated” (+pyruvate dehyrogenase phosphatase, PDP1) purified porcine PDH complex incubated with wild-type or H161Y SIRT4. Western blot analysis of PDH-E1α phosphorylation sites; E1 – loading control. (E) Representative MS/MS spectra of K132 lipoyl peptide detected from endogenous DLAT immunopurified from mitochondria of fibroblasts and digested with endoproteinase GluC. K*, reduced and di-carbamidomethylated lipoyl-lysine (Δm = 304 amu vs. unmodified lysine). (F) SRM quantification of endogenous DLAT lipoyl K132 and K259 in fibroblasts (left) and HEK293 cells (right) (mean ± S.E.M; n=3, *p=0.03, ***p=0.0003). See also Figure S4.
To confirm that the inhibition of PDH activity in cells reflects a direct effect of SIRT4 on the complex, we immuno-captured and measured the activity of purified porcine PDH in vitro (Fig. 4D, S4C). Purified PDH was treated with pyruvate dehydrogenase phosphatase catalytic subunit 1 (PDP1), which decreased the phosphorylation of all three inhibitory PDH-E1α sites and, as expected, increased PDH activity (Fig 4D, S4C). Then, “activated” PDH was treated with either recombinant WT or inactive H161Y SIRT4. Only active SIRT4 was able to attenuate PDH activity, which was not due to increased phosphorylation of E1 (Fig. 4D). Overall, these in vitro data with purified PDH support our observations of reduced activity of PDH in SIRT4 OE cells (Fig 4A–C), and together suggest that reduction in PDH activity occurs in a phosphorylation-independent manner, by SIRT4 directly hydrolyzing lipoylated DLAT.
DLAT contains two lipoyl-lysine residues, K132 and K259. Since western blot analysis only assessed overall lipoyl protein content, we designed an assay using LC-MS/MS selected reaction monitoring (SRM) (Sherrod et al., 2012; Tsai et al., 2012) to measure the effect of SIRT4 on specific lipoylated lysines of endogenous cellular DLAT. Towards this goal, we affinity-purified endogenous DLAT from fibroblast mitochondria and then performed protein digestion using the endoproteinase GluC. Using non-targeted LC-MS/MS the two predicted lipoyl-lysine peptides of endogenous DLAT, containing K132 and K259 residues, were identified (Fig 4E, Fig S4D). We confirmed these results using a synthetic K259 lipoyl peptide, which showed a similar LC retention time and fragmentation pattern as the endogenous K259 peptide (Fig S4E). Additionally, fragmentation of these lipoyl-lysine peptides generated b-ions suitable for relative quantification by targeted MS/MS (Fig 4E, boxes). Using this SRM assay, we measured the effect of SIRT4 OE on the relative levels of endogenous DLAT K132 lipoyl and K259 lipoyl in mitochondria (Fig. 4F, S4F). Stable expression of active SIRT4 in fibroblasts reduced levels of DLAT lipoyl at both lysine residues (Fig. 4F, left), consistent with our western blotting results (Fig. 4B). In contrast, expression of SIRT4 H161Y did not reduce DLAT lipoyl levels. We also analyzed relative lipoyl levels on endogenous DLAT in HEK293 cells transiently transfected with either mCherry (CTL), SIRT4, or SIRT4 H161Y. Only expression of active SIRT4 diminished levels of DLAT lipoyl (Fig. 4F, right), suggesting that SIRT4 reduction of DLAT lipoyl levels were not unique to fibroblasts or an artifact from cell line generation. Altogether, our results demonstrate that the E2 component of the PDH is a biological substrate of SIRT4 lipoamidase activity, and that the SIRT4-mediated reduction in PDH lipoyl levels leads to an inhibition of its function.
Glutamine-stimulation induces endogenous SIRT4 lipoamidase activity and inhibits PDH activity
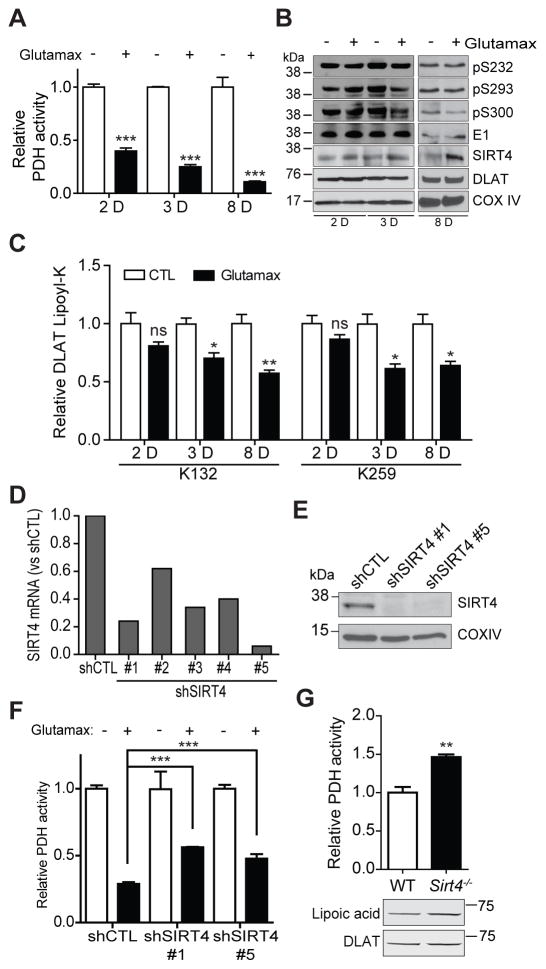
Glutamine stimulation in rat liver is known to cause increased flux through OGDH and decreased flux through PDH, leading to PDH inhibition (Haussinger et al., 1982). Therefore, we investigated whether SIRT4 may play a role in this process. Stimulation of WT fibroblasts with the glutamine supplement glutamax (4 mM) caused a significant time-dependent decrease in PDH activity (Fig. 5A, Fig. S5A–B). Importantly, this reduction in activity was not due to increased levels of inhibitory PDH-E1 phosphorylation relative to unstimulated cells at the same time points (Fig. 5B). While steady-state levels of DLAT were unchanged due to glutamax stimulation (Fig. 5B), a decrease in DLAT lipoyl levels was observed within 72 hr (Fig. 5C). In agreement with these observations, we detected elevated expression of endogenous SIRT4 in cells stimulated with glutamax (Fig. 5B). To validate the dependence of PDH inhibition on SIRT4 activity, we measured PDH activity in SIRT4 OE cells stimulated with glutamax. Following 40 hr culture in glutamax, over-expression of active WT SIRT4 triggered pronounced PDH inhibition, in contrast to the H161Y catalytic mutant (Fig. S5C). To test the specific involvement of endogenous SIRT4, we generated fibroblasts with knock-down SIRT4 expression using shRNA (Table S2). Effective SIRT4 knock-down was confirmed at the mRNA level (shSIRT4 #1 and #5 achieving >75% knock-down) (Fig. 5D) and at the protein level (Fig. 5E). Importantly, SIRT4 knock-down using two different shRNA constructs led to a partial rescue of the glutamax-mediated inhibition of PDH activity (Fig. 5F, Fig. S5D, F). Finally, to confirm a role for PDH regulation via SIRT4 in vivo, PDH activity was measured in mitochondria purified from the liver of SIRT4 knock-out (KO) mice. Indeed, we observed elevated PDH activity (Fig. 5G, Fig. S5E) and DLAT lipoyl levels (Fig. 5G) in SIRT4 KO mice relative to control mice. Altogether, these data demonstrate that endogenous SIRT4 is involved in inhibiting PDH activity and DLAT lipoyl levels in the mitochondria of cells and in vivo in mouse liver.
Fig. 5. Endogenous SIRT4 inhibits PDH in cultured fibroblasts and in vivo, in mouse liver.
(A) PDH activity time course in wild type fibroblasts stimulated with glutamax (4mM) for 2, 3, and 8 days, versus unstimulated cells (mean ± S.E.M.; n=4 2D and 3D, p<0.0001; n=3 8D, p=0.0007). (B) Western blot analysis of regulatory PDH-E1α phosphorylation sites and total E1 (loading control), and endogenous SIRT4, DLAT, and COX IV (loading control) levels, following glutamax stimulation. (C) SRM quantification of DLAT lipoyl levels (K132 and K259) in cells stimulated with glutamax versus unstimulated (mean ± S.E.M.; n=3) for 2D (ns), 3D (*p=0.015), and 8D (**p=0.007, *p=0.018). (D) Relative SIRT4 mRNA expression measured by qRT-PCR in fibroblast stably expressing either non-targeting control shRNA (shCTL) or one of five different constructs targeting SIRT4 (shSIRT4 #1–5). (E) Western blot analysis of SIRT4 and COX IV (loading control) from mitochondria purified from fibroblasts expressing shRNA constructs shCTL, shSIRT4 #1, and shSIRT4 #5. (F) PDH activity in fibroblasts with knock-down levels of endogenous SIRT4 (shSIRT4 #1 or #5, mean ± S.E.M; n=4) treated with glutamax (4 mM for 8D), versus control shCTL cells (mean ± S.E.M; n=7, ***p<0.0001). (G) PDH activity, lipoyl levels of endogenous DLAT (lipoic acid), and total DLAT levels (DLAT) from mouse liver mitochondria of Sirt4−/− mice (mean ± S.E.M, n = 3, **p<0.039) versus wild-type control (n = 4). See also Figure S5.
Discussion
Until now, a mammalian cellular lipoamidase has not been characterized. However, our study discovered that SIRT4 can function with this enzymatic capacity in the mitochondria, and that PDH is a biological substrate. We find that, compared to its catalytic efficiency for deacetylation, SIRT4 exhibits far superior enzymatic activity for lipoyl- and biotinyl-lysine modifications. Interestingly, there is precedence for a serum lipoamidase having enzymatic activity for both lipoyl- and biotinyl-lysine modifications (Nilsson and Kagedal, 1993). Importantly, patients with severe serum biotinidase deficiency were observed to exhibit lipoamidase deficiency (Nilsson and Ronge, 1992). Given the serum enzyme was unable to hydrolyze lipoamide from bovine heart PDH (Oizumi and Hayakawa, 1989), it is tempting to speculate that SIRT4 is the mitochondria-specific member of the mammalian class of enzymes that possess both lipoamidase and biotinidase activity, and therefore may be unique among these enzymes to liberate lipoate from PDH.
SIRT4 is among the least abundant proteins in the human proteome (PaxDb, www.paxdb.org), and there is only a limited number of human proteins known to be lipoylated. These proteins play critical roles in cellular metabolism and include DLAT, DLST, DBT, PDHX and GCSH—the first four of which were identified as specific interacting partners of SIRT4 in our IP-MS study. We determined in vitro, in cells, and in an animal mouse model that SIRT4 regulates overall PDH activity by hydrolyzing the lipoamide cofactors from DLAT. Importantly, we showed that a catalytically active SIRT4 is required for this arm of PDH regulation. Overall, PDH controls pyruvate decarboxylation to generate acetyl-CoA, and DLAT specifically performs the transacetylation reaction, transferring the acetyl-group to Coenzyme A. Our finding that SIRT4 regulates DLAT lipoylation suggests PDH is inhibited via diminished transacetylation. Interestingly, the accessory PDH E3-binding subunit (PDHX) also contains a lipoyl modification. Indeed, we detected lipoylated protein(s) at ~ 50KDa by western blotting (Fig. S4D). This signal may represent individual DLST, DBT, PDHX, or a mixture of these proteins (with similar masses at 49–54KDa), or other SIRT4 substrates. Although this band was reduced in cells upon SIRT4 overexpression (Fig. S4D), there was no clear difference in mouse liver from SIRT4 KO when compared to WT mice (Fig S5G). In contrast, DLAT lipoyl levels (~70K band) were reduced in cells overexpressing SIRT4 (Fig. 4B) and enhanced in SIRT4 KO vs wild-type mice (Fig S5G). This highlights the significance of lipoylated DLAT under SIRT4 null conditions. Additionally, rather than performing a catalytic function, PDHX is known to play a structural role, such as in anchoring the E3 to the E2 (DLAT) subunit (Brautigam et al., 2006; Harris et al., 1997). Thus, should SIRT4, in addition to its impact on DLAT, modulate PDH activity through delipoylation of PDHX, this may involve a structural impairment of PDH. In addition, given our identification of SIRT4 interactions with biotin-dependent decarboxylases and demonstration that SIRT4 also has activity for biotinyl lysine, we predict that SIRT4 also regulates these decarboxylases and other biotin-dependent enzymes and metabolic pathways.
Comparison of steady-state enzyme kinetics is important for comparing the catalytic efficiency of an enzyme for particular substrates. However, this has been notoriously difficult for SIRT4 given issues associated with maintaining recombinant protein solubility (Du et al., 2011). Enzyme kinetics have not been reported for SIRT4 substrates prior to this study, and although some in vitro activity of SIRT4 towards reduced lipoamide H3K9 peptide has been reported recently (Feldman et al., 2013), the activity was not reproducible, and at very low levels (presumably due to stability issues). We optimized the expression, purification, and storage of SIRT4, which has allowed us to perform steady-state kinetics assays and discover that SIRT4 has the predominant lipoamidase activity among the mitochondrial sirtuins. Furthermore, SIRT4 catalytic efficiency for lipoylated DLAT is far-superior (1270-fold) to its previously reported substrate, acetylated MCD, making DLAT the best characterized substrate to date. Importantly, the observed SIRT4 catalytic efficiency and binding constant, Km, for DLAT-lipoyl is consistent with the biological conditions within PDH. Specifically, each of the DLAT lipoyl domains is concentrated within PDH at > 1 mM (Roche et al., 1993), supporting a cellular role for SIRT4 lipoamidase activity in regulating PDH activity. Indeed, if SIRT4 is actually embedded within the PDH, this finding may help explain the previously reported inability to detect SIRT4 within the mitochondrial matrix by either proteomic profiling (Rhee et al., 2013) or immunofluorescence (www.proteinatlas.org).
Compared to the other mitochondrial SIRTs, our study shows that the cellular lipoamidase activity on DLAT-lipoyl and PDH activity is unique to SIRT4. Elevated expression levels of SIRT3 and SIRT5 in cells did not affect DLAT-lipoyl levels or PDH activity, despite SIRT3 showing some in vitro enzymatic activity to DLAT-lipoyl in kinetic assays. This highlights that the complex architecture and the local cellular environment facilitate protein interactions that may help to define substrate specificity. SIRT4 substrate specificity may also be determined by the size of the active site and amino acids that line the catalytic pocket. For example, structural analysis of the SIRT5 active site defined its preference for lysine substrates bearing negatively charged carboxylates (Du et al., 2011). However, in contrast to all previously characterized SIRT substrates, lipoamide has a sulfur-containing, dithiolane ring. Assuming the SIRT4 active site is near physiological pH, lipoamide will have a neutral charge and may not require extensive charge stabilization. Overall, our results show SIRT4 lipoamidase activity for non-reduced lipoamide in vitro (Fig 2); however, the oxidative susceptibility of lipoamide raises the possibility of other in vivo substrates. Ultimately, given the current lack of a SIRT4 crystal structure, future studies will be required to delineate the full range of SIRT4 lipoamide substrate specificity under different cellular states.
Our discovery that SIRT4 inhibits the PDH via direct hydrolysis of the lipoamide cofactor builds on the knowledge accumulated during several decades to provide a new perspective for understanding PDH regulation. PDH activity is understood to be principally inhibited by kinase-dependent phosphorylation of the E1 subunit, whereby phosphorylation is modulated downstream of E1 acetylation (Fan et al., 2014; Jing et al., 2013; Linn et al., 1969; Wieland and Jagow-Westermann, 1969). Our finding that SIRT4 lipoamidase activity can directly impair the function of the complex underscores that PDH regulation is a highly complex process that involves several mechanisms. For instance, we observed that cells with elevated levels of SIRT4 actually have decreased E1 phosphorylation, in conjunction with reduced PDH activity and lipoyl levels. Reduced phosphorylation would normally be expected to activate PDH. Nevertheless, this result is not entirely unexpected and may be partly explained if one considers that PDH kinases are known to be activated via binding to the lipoyl domain (Radke et al., 1993). Therefore, if SIRT4 reduces DLAT lipoamide levels it also triggers an indirect reduction in kinase binding sites, thereby limiting kinase function and causing reduced phosphorylation. Thus, the complex regulation of PDH likely involves the temporal activation of several mechanisms by different stimuli and cellular responses.
Glutamine stimulation in rat liver was shown to inhibit PDH activity by increasing flux through OGDH, accompanied by decreased flux through PDH (Haussinger et al., 1982). Building on this knowledge, we demonstrated that endogenous SIRT4 lipoamidase activity could be induced via glutamine stimulation. Interestingly, after 48hr of glutamine stimulation the PDH activity decreased by 50%, while the DLAT lipoyl levels decreased to a lesser extent. At subsequent time points, substantial decreases in both PDH activity and DLAT lipoylation were observed. These results suggest that PDH inhibitory mechanisms are temporally regulated. For instance, it is possible that a lipoyl-independent mechanism impacts PDH activity early after glutamine stimulation (up to 48 hr), which is followed by a lipoyl-dependent mechanism that relies on increased SIRT4 levels and lipoamidase activity (72h-8 days). The underlying molecular mechanisms for this temporal regulation are not entirely understood, but may reflect time requirements for increased transcription or translation of SIRT4. Nonetheless, knock-down of SIRT4 in cells led to a partial rescue of the glutamine-mediated inhibition of PDH activity. It remains to be determined whether the lack of a full rescue is due to the function of residual SIRT4 in these knocked-down cells, or if another, yet to be identified, PDH inhibition mechanism is simultaneously active. Finally, PDH activity and DLAT lipoyl levels in SIRT4 KO mouse liver were elevated compared to control animals, further supporting the function of SIRT4 in regulating PDH activity in vivo.
SIRT4 was initially characterized as an ADP-ribosyltransferase that regulates glutamate dehydrogenase and insulin secretion (Haigis et al., 2006). Studies in insulin producing cells with knock-down SIRT4 expression showed elevated insulin secretion in response to glucose (Ahuja et al., 2007). Our discovery that SIRT4 inhibits PDH activity has interesting implication for further understanding this phenotype, as insulin secretion is initiated by the rapid utilization of glucose for glycolysis and the consequent entry into the TCA cycle for oxidative phosphorylation (Newgard and McGarry, 1995). Thus, SIRT4 knock-down would help to accelerate this process. SIRT4 has also been reported to regulate fatty acid oxidation via expression of catabolic genes (Laurent et al., 2013a; Nasrin et al., 2010), and by deacetylation of MCD, which inhibits conversion of malonyl-CoA to acetyl-CoA (Laurent et al., 2013b). Our finding that SIRT4 is a lipoamidase is consistent with a function in this metabolic process, and highlights PDH as another molecular target that also negatively regulates acetyl-CoA production. SIRT4 has also been reported to be involved in cancer progression. Lung tumors spontaneously develop in SIRT4 KO mice, while expression of SIRT4 represses tumor development in vivo (Csibi et al., 2013; Jeong et al., 2013). Reduced levels of SIRT4 have also been detected in human bladder, breast, colon, gastric, ovarian carcinoma, relative to normal tissues (Csibi et al., 2013). The aforementioned observations suggest that SIRT4 acts a as a tumor suppressor (Zhu et al., 2014), primarily by inhibiting carcinogenesis through repression of glutamine anaplerosis (Jeong et al., 2013). Thus, it is tempting to speculate that this regulation may also involve SIRT4 lipoamidase activity towards OGDH, which contains DLST-lipoyl and feeds 2-oxoglutarate into the TCA cycle.
Taken together, our results identify SIRT4 as a cellular lipoamidase, which regulates the activity of the PDH complex via enzymatic hydrolysis of the lipoamide cofactor. As PDH controls pyruvate decarboxylation, fueling multiple downstream pathways, our findings highlight SIRT4 as a critical regulator of cellular metabolism. We anticipate that these findings will trigger numerous future studies aimed at further characterizing the roles of SIRT4’s lipoamidase activity in mitochondrial function in diverse health and disease states.
Experimental Procedures
Cell culture and generation of stable cell lines
Human MRC5 fibroblasts and embryonic kidney HEK293 cells were cultured in DMEM containing 10% (v/v) Benchmark fetal bovine serum and 1% penicillin-streptomycin solution. Stable cell lines that express SIRT4-EGFP, SIRT4-EGFP H161Y, or GFP control, as well as stable cell lines with knockdown expression of SIRT4 (using SIRT4-targeting shRNA) or control shRNA were generated as described in Supplementary Materials and Methods. SIRT4 expression levels were measured by western blotting, and knockdown efficiency was measured by qRT-PCR and western blotting.
Confocal microscopy
For live microscopy, fibroblasts stably expressing SIRT4-EGFP were imaged on a Leica SP5 confocal microscope using the 63X oil immersion objective. For co-localization studies, SIRT4-EGFP stably expressing fibroblasts were fixed and imaged on a Leica SP5 confocal microscope using the 63X glycerol immersion objective, as described in the Supplementary Materials and Methods.
Mitochondrial isolation and Immunoaffinity Purification of SIRT4-GFP
Purified mitochondria fractions were isolated from fibroblasts (25 × 106) by removing nuclei using centrifugation, and further resolving the resulting crude organelle pellet using a discontinuous OptiPrep™ gradient, as detailed in Supplementary Materials and Methods. SIRT4-EGFP and control EGFP were immunoaffinity purified from mitochondrial fractions using anti-GFP antibodies and M270 Epoxy Dynabeads, as described in (Cristea et al., 2005) and in Supplementary Materials and Methods.
Proteomic analysis of SIRT4 and interacting protein partners
SIRT4 immunoisolates were analyzed by mass spectrometry-based proteomics using an in-gel digestion approach followed by nanoliquid chromatography (Dionex Ultimate3000) coupled directly to an LTQ-Orbitrap Velos (ThermoFisher Scientific) mass spectrometer operated in data-dependent acquisition mode, as described in Supplementary Materials and Methods. The specificity of candidate protein interactions was assessed using the SAINT algorithm, as in (Choi et al., 2011) and in Supplementary Materials and Methods. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (Vizcaino et al., 2014) via the PRIDE partner repository with the dataset identifier PXD001447 and DOI 10.6019/PXD001447.
Western Immunoblotting
Proteins were transferred to nitrocellulose membranes, and protein and posttranslational modification levels were detected and visualized as described in the Supplementary Materials and Methods.
Recombinant mitochondrial SIRT proteins
N-terminally truncated human SIRT4 (33–314) was cloned, co-expressed with GroEL and GroES in BL21(DE3) E. coli, and purified as described in Supplementary Materials and Methods. Recombinant SIRT3 and SIRT5 were purchased from Sigma.
Peptide synthesis and LC-MS-based in vitro peptide deacylation assays
Synthetic peptides were designed (Supplementary Table 2), synthesized by GenScript, and validated by infusion into an LTQ Orbitrap XL mass spectrometer (ThermoFisher Scientific). The ability of SIRT4 to hydrolyze various acyl-lysine modifications was measured using LC-MS, as described in Supplementary Materials and Methods.
HPLC-based SIRT kinetic assays
Kinetic assays for mitochondrial sirtuins and determination of kinetic parameters were performed using HPLC-UV detection as described by others (Du et al., 2011; Jiang et al., 2013) and in the Supplementary Materials and Methods. All analyses were performed in a minimum of three biological replicates.
Relative quantification of lipoyl-lysine by targeted mass spectrometry
The relative abundance of lipoyl-lysine-containing DLAT peptides were measured in mitochondria lysates using a selected reaction monitoring (SRM/PRM) full-scan tandem mass spectrometry assay, as described in the Supplementary Materials and Methods.
PDH activity assay
The activity of the PDH was measured using the Pyruvate Dehydrogenase Enzyme Activity Microplate Assay Kit (Abcam) according to manufacturer’s instructions, as detailed in Supplementary Materials and Methods. All measurements were performed in at least three biological replicates.
Animal studies
Experiments in mice were conducted in compliance with Institutional Animal Care and Use Committee (IACUC) of Princeton University. SIRT4 knock-out (Jackson Laboratory, Stock number 012756), and control (WT) (Jackson Laboratory, Stock number 002448) adult female mice (N=4) were euthanized, livers excised, and mitochondria isolated as previously described (Rardin et al., 2009) with modifications detailed in Supplementary Materials and Methods.
Supplementary Material
Highlights.
SIRT4 is a lipoamidase that functions in cells and mouse liver mitochondria
Lipoamidase activity of SIRT4 is superior to its deacetylase activity
SIRT4 inhibits PDH activity via enzymatic hydrolysis of the lipoamide cofactor
Endogenous SIRT4 lipoamidase activity can be induced by glutamine stimulation
Acknowledgments
We thank the PRIDE team for assistance in submitting the mass spectrometry data and interactions to the ProteomeXchange and Intact repositories. The authors are supported by grants from the NIDA (DP1DA026192), NIAID (R21AI102187), and NICHD (R21HD073044) to I.M.C., and NIAID (AI78063) and NCI (CA82396) to T.S. R.A.M. is funded by a NHMRC of Australia Early CJ Martin Fellowship #APP1037043, T.M.G. by an NJCCR postdoctoral fellowship, and H.G.B. by an NSF graduate fellowship.
Footnotes
Author Contributions
The study was conceived by R.A.M., A.O., T.S., and I.M.C. Experiments performed by R.A.M, T.M.G, H.G.B., and E.A.R. were done in the I.M.C. lab, by A.O. in the T.S. lab, and by R.C. in the Y.K. lab. Studies for identifying SIRT4 substrates were done by R.A.M. Recombinant SIRT4 proteins were purified by A.O. R.A.M. performed MS-based in vitro peptide deacylation assays. R.A.M., A.O., and T.M.G performed kinetics assays, and R.A.M. and H.G.B. generated the cell lines. H.G.B. performed microscopy and qRT-PCR experiments. T.M.G. performed the SRM-based quantification of lipoylated DLAT, and E.A.R. assessed DLAT lipoyl by western blotting. R.A.M carried out glutamax experiments and PDH activity assays, and E.A.R measured SIRT4 mRNA levels. R.C. and Y.K. acquired the mice; R.C. isolated the mice liver and R.A.M, T.M.G. and H.G.B. performed all assays in liver tissue. R.A.M., T.M.G., and I.M.C. prepared the figures and all authors wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahuja N, Schwer B, Carobbio S, Waltregny D, North BJ, Castronovo V, Maechler P, Verdin E. Regulation of insulin secretion by SIRT4, a mitochondrial ADP-ribosyltransferase. J Biol Chem. 2007;282:33583–33592. doi: 10.1074/jbc.M705488200. [DOI] [PubMed] [Google Scholar]
- Brautigam CA, Wynn RM, Chuang JL, Machius M, Tomchick DR, Chuang DT. Structural insight into interactions between dihydrolipoamide dehydrogenase (E3) and E3 binding protein of human pyruvate dehydrogenase complex. Structure. 2006;14:611–621. doi: 10.1016/j.str.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Larsen B, Lin ZY, Breitkreutz A, Mellacheruvu D, Fermin D, Qin ZS, Tyers M, Gingras AC, Nesvizhskii AI. SAINT: probabilistic scoring of affinity purification-mass spectrometry data. Nat Methods. 2011;8:70–73. doi: 10.1038/nmeth.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristea IM, Williams R, Chait BT, Rout MP. Fluorescent proteins as proteomic probes. Mol Cell Proteomics. 2005;4:1933–1941. doi: 10.1074/mcp.M500227-MCP200. [DOI] [PubMed] [Google Scholar]
- Csibi A, Fendt SM, Li C, Poulogiannis G, Choo AY, Chapski DJ, Jeong SM, Dempsey JM, Parkhitko A, Morrison T, et al. The mTORC1 pathway stimulates glutamine metabolism and cell proliferation by repressing SIRT4. Cell. 2013;153:840–854. doi: 10.1016/j.cell.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Zhou Y, Su X, Yu JJ, Khan S, Jiang H, Kim J, Woo J, Kim JH, Choi BH, et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science. 2011;334:806–809. doi: 10.1126/science.1207861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Shan C, Kang HB, Elf S, Xie J, Tucker M, Gu TL, Aguiar M, Lonning S, Chen H, et al. Tyr phosphorylation of PDP1 toggles recruitment between ACAT1 and SIRT3 to regulate the pyruvate dehydrogenase complex. Mol Cell. 2014;53:534–548. doi: 10.1016/j.molcel.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Baeza J, Denu JM. Activation of the protein deacetylase SIRT6 by long-chain fatty acids and widespread deacylation by mammalian sirtuins. J Biol Chem. 2013;288:31350–31356. doi: 10.1074/jbc.C113.511261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RA. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem Biophys Res Commun. 1999;260:273–279. doi: 10.1006/bbrc.1999.0897. [DOI] [PubMed] [Google Scholar]
- Guarente L. Sir2 links chromatin silencing, metabolism, and aging. Genes Dev. 2000;14:1021–1026. [PubMed] [Google Scholar]
- Haigis MC, Mostoslavsky R, Haigis KM, Fahie K, Christodoulou DC, Murphy AJ, Valenzuela DM, Yancopoulos GD, Karow M, Blander G, et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126:941–954. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Harris RA, Bowker-Kinley MM, Wu P, Jeng J, Popov KM. Dihydrolipoamide dehydrogenase-binding protein of the human pyruvate dehydrogenase complex. DNA-derived amino acid sequence, expression, and reconstitution of the pyruvate dehydrogenase complex. J Biol Chem. 1997;272:19746–19751. doi: 10.1074/jbc.272.32.19746. [DOI] [PubMed] [Google Scholar]
- Haussinger D, Gerok W, Sies H. Inhibition of pyruvate dehydrogenase during the metabolism of glutamine and proline in hemoglobin-free perfused rat liver. Eur J Biochem. 1982;126:69–76. doi: 10.1111/j.1432-1033.1982.tb06747.x. [DOI] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- Jeong SM, Xiao C, Finley LW, Lahusen T, Souza AL, Pierce K, Li YH, Wang X, Laurent G, German NJ, et al. SIRT4 has tumor-suppressive activity and regulates the cellular metabolic response to DNA damage by inhibiting mitochondrial glutamine metabolism. Cancer Cell. 2013;23:450–463. doi: 10.1016/j.ccr.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Khan S, Wang Y, Charron G, He B, Sebastian C, Du J, Kim R, Ge E, Mostoslavsky R, et al. SIRT6 regulates TNF-alpha secretion through hydrolysis of long-chain fatty acyl lysine. Nature. 2013;496:110–113. doi: 10.1038/nature12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing E, O’Neill BT, Rardin MJ, Kleinridders A, Ilkeyeva OR, Ussar S, Bain JR, Lee KY, Verdin EM, Newgard CB, et al. Sirt3 regulates metabolic flexibility of skeletal muscle through reversible enzymatic deacetylation. Diabetes. 2013;62:3404–3417. doi: 10.2337/db12-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi P, Greco TM, Guise AJ, Luo Y, Yu F, Nesvizhskii AI, Cristea IM. The functional interactome landscape of the human histone deacetylase family. Mol Syst Biol. 2013:9. doi: 10.1038/msb.2013.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent G, de Boer VC, Finley LW, Sweeney M, Lu H, Schug TT, Cen Y, Jeong SM, Li X, Sauve AA, et al. SIRT4 represses peroxisome proliferator-activated receptor alpha activity to suppress hepatic fat oxidation. Mol Cell Biol. 2013a;33:4552–4561. doi: 10.1128/MCB.00087-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent G, German NJ, Saha AK, de Boer VC, Davies M, Koves TR, Dephoure N, Fischer F, Boanca G, Vaitheesvaran B, et al. SIRT4 coordinates the balance between lipid synthesis and catabolism by repressing malonyl CoA decarboxylase. Mol Cell. 2013b;50:686–698. doi: 10.1016/j.molcel.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Su X, He B. Protein lysine acylation and cysteine succination by intermediates of energy metabolism. ACS Chem Biol. 2012;7:947–960. doi: 10.1021/cb3001793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn TC, Pettit FH, Reed LJ. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1969;62:234–241. doi: 10.1073/pnas.62.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27:8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. 2005;16:4623–4635. doi: 10.1091/mbc.E05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrin N, Wu X, Fortier E, Feng Y, Bare OC, Chen S, Ren X, Wu Z, Streeper RS, Bordone L. SIRT4 regulates fatty acid oxidation and mitochondrial gene expression in liver and muscle cells. J Biol Chem. 2010;285:31995–32002. doi: 10.1074/jbc.M110.124164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgard CB, McGarry JD. Metabolic coupling factors in pancreatic beta-cell signal transduction. Annu Rev Biochem. 1995;64:689–719. doi: 10.1146/annurev.bi.64.070195.003353. [DOI] [PubMed] [Google Scholar]
- Newman JC, He W, Verdin E. Mitochondrial protein acylation and intermediary metabolism: regulation by sirtuins and implications for metabolic disease. J Biol Chem. 2012;287:42436–42443. doi: 10.1074/jbc.R112.404863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson L, Kagedal B. Co-purification of human serum lipoamidase and biotinidase: evidence that the two enzyme activities are due to the same enzyme protein. Biochem J. 1993;291(Pt 2):545–551. doi: 10.1042/bj2910545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson L, Ronge E. Lipoamidase and biotinidase deficiency: evidence that lipoamidase and biotinidase are the same enzyme in human serum. Eur J Clin Chem Clin Biochem. 1992;30:119–126. [PubMed] [Google Scholar]
- Oizumi J, Hayakawa K. Liberation of lipoate by human serum lipoamidase from bovine heart pyruvate dehydrogenase. Biochem Biophys Res Commun. 1989;162:658–663. doi: 10.1016/0006-291x(89)92361-9. [DOI] [PubMed] [Google Scholar]
- Peng C, Lu Z, Xie Z, Cheng Z, Chen Y, Tan M, Luo H, Zhang Y, He W, Yang K, et al. The first identification of lysine malonylation substrates and its regulatory enzyme. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M1111.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perham RN. Domains, motifs, and linkers in 2-oxo acid dehydrogenase multienzyme complexes: a paradigm in the design of a multifunctional protein. Biochemistry (Mosc) 1991;30:8501–8512. doi: 10.1021/bi00099a001. [DOI] [PubMed] [Google Scholar]
- Radke GA, Ono K, Ravindran S, Roche TE. Critical role of a lipoyl cofactor of the dihydrolipoyl acetyltransferase in the binding and enhanced function of the pyruvate dehydrogenase kinase. Biochem Biophys Res Commun. 1993;190:982–991. doi: 10.1006/bbrc.1993.1146. [DOI] [PubMed] [Google Scholar]
- Rahmatullah M, Radke GA, Andrews PC, Roche TE. Changes in the core of the mammalian-pyruvate dehydrogenase complex upon selective removal of the lipoyl domain from the transacetylase component but not from the protein X component. J Biol Chem. 1990;265:14512–14517. [PubMed] [Google Scholar]
- Rardin MJ, He W, Nishida Y, Newman JC, Carrico C, Danielson SR, Guo A, Gut P, Sahu AK, Li B, et al. SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab. 2013;18:920–933. doi: 10.1016/j.cmet.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rardin MJ, Wiley SE, Naviaux RK, Murphy AN, Dixon JE. Monitoring phosphorylation of the pyruvate dehydrogenase complex. Anal Biochem. 2009;389:157–164. doi: 10.1016/j.ab.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh D, Fischer F, Gertz M, Lakshminarasimhan M, Bergbrede T, Aladini F, Kambach C, Becker CF, Zerweck J, Schutkowski M, et al. An acetylome peptide microarray reveals specificities and deacetylation substrates for all human sirtuin isoforms. Nat Commun. 2013;4 doi: 10.1038/ncomms3327. [DOI] [PubMed] [Google Scholar]
- Rhee HW, Zou P, Udeshi ND, Martell JD, Mootha VK, Carr SA, Ting AY. Proteomic mapping of mitochondria in living cells via spatially restricted enzymatic tagging. Science. 2013;339:1328–1331. doi: 10.1126/science.1230593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche TE, Powers-Greenwood SL, Shi WF, Zhang WB, Ren SZ, Roche ED, Cox DJ, Sorensen CM. Sizing of bovine heart and kidney pyruvate dehydrogenase complex and dihydrolipoyl transacetylase core by quasielastic light scattering. Biochemistry (Mosc) 1993;32:5629–5637. doi: 10.1021/bi00072a019. [DOI] [PubMed] [Google Scholar]
- Schuetz A, Min JR, Antoshenko T, Wang CL, Allali-Hassani A, Dong AP, Loppnau P, Vedadi M, Bochkarev A, Sternglanz R, et al. Structural basis of inhibition of the human NAD(+)-dependent deacetylase SIRT5 by suramin. Structure. 2007;15:377–389. doi: 10.1016/j.str.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Sherrod SD, Myers MV, Li M, Myers JS, Carpenter KL, Maclean B, Maccoss MJ, Liebler DC, Ham AJ. Label-free quantitation of protein modifications by pseudo selected reaction monitoring with internal reference peptides. J Proteome Res. 2012;11:3467–3479. doi: 10.1021/pr201240a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BC, Denu JM. Sir2 protein deacetylases: evidence for chemical intermediates and functions of a conserved histidine. Biochemistry (Mosc) 2006;45:272–282. doi: 10.1021/bi052014t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M, Peng C, Anderson KA, Chhoy P, Xie Z, Dai L, Park J, Chen Y, Huang H, Zhang Y, et al. Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metab. 2014;19:605–617. doi: 10.1016/j.cmet.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai YC, Greco TM, Boonmee A, Miteva Y, Cristea IM. Functional proteomics establishes the interaction of SIRT7 with chromatin remodeling complexes and expands its role in regulation of RNA polymerase I transcription. Mol Cell Proteomics. 2012;11:60–76. doi: 10.1074/mcp.A111.015156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdin E, Hirschey MD, Finley LW, Haigis MC. Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem Sci. 2010;35:669–675. doi: 10.1016/j.tibs.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizcaino JA, Deutsch EW, Wang R, Csordas A, Reisinger F, Rios D, Dianes JA, Sun Z, Farrah T, Bandeira N, et al. ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat Biotechnol. 2014;32:223–226. doi: 10.1038/nbt.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland O, Jagow-Westermann B. ATP-dependent inactivation of heart muscle pyruvate dehydrogenase and reactivation by Mg(++) FEBS Lett. 1969;3:271–274. doi: 10.1016/0014-5793(69)80156-0. [DOI] [PubMed] [Google Scholar]
- Wirth M, Karaca S, Wenzel D, Ho L, Tishkoff D, Lombard DB, Verdin E, Urlaub H, Jedrusik-Bode M, Fischle W. Mitochondrial SIRT4-type proteins in Caenorhabditis elegans and mammals interact with pyruvate carboxylase and other acetylated biotin-dependent carboxylases. Mitochondrion. 2013;13:705–720. doi: 10.1016/j.mito.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZH, McCarthy DB, O’Connor CM, Reed LJ, Stoops JK. The remarkable structural and functional organization of the eukaryotic pyruvate dehydrogenase complexes. Proc Natl Acad Sci U S A. 2001;98:14802–14807. doi: 10.1073/pnas.011597698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Yan Y, Principe DR, Zou X, Vassilopoulos A, Gius D. SIRT3 and SIRT4 are mitochondrial tumor suppressor proteins that connect mitochondrial metabolism and carcinogenesis. Cancer & metabolism. 2014;2:15. doi: 10.1186/2049-3002-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.