Abstract
Carbon-fiber-reinforced polyetheretherketone (CFR-PEEK) has been successfully used in orthopedic implants. The aim of this systematic review is to investigate the properties, technical data, and safety of CFR-PEEK biomaterial and to evaluate its potential for new innovation in the design of articulating medical devices. A comprehensive search in PubMed and EMBASE was conducted to identify articles relevant to the outcomes of CFR-PEEK orthopedic implants. The search was also expanded by reviewing the reference sections of selected papers and references and benchmark reports provided by content experts. A total of 23 articles were included in this review. There is limited literature available assessing the performance of CFR-PEEK, specifically as an implant material for arthroplasty systems. Nevertheless, available studies strongly support CFR-PEEK as a promising and suitable material for orthopedic implants because of its biocompatibility, material characteristics, and mechanical durability. Future studies should continue to investigate CFR-PEEK’s potential benefits.
Keywords: carbon-fiber-reinforced polyetheretherketone, implants, CFR-PEEK, review, orthopedics
Background
The ongoing development and improvement of orthopedic implants requires much attention as surgeries performed to treat traumatic injuries and joint diseases, along with their associated complications, are highly prevalent. For example, knee osteoarthritis (OA), a chronic degenerative joint disease, is very common among people over the age of 60 years.1–3 For severe cases of knee OA, total knee replacements are the standard of care, as they relieve the patient’s pain so that they can return to their normal daily activities.4,5 Despite the improvement, the life span of the prosthesis is ultimately finite, as mechanical wear, aseptic loosening, infection, instability, and periprosthetic fractures are reasons for revision of knee arthroplasty.6,7 Consequently, a high percentage of patients are required to undergo a second knee replacement within 10 years of their initial surgery. As a result, new implants made of innovative materials are being introduced and evaluated with the goal of improving implant survival rates and reducing the associated burden of revision surgery.8
Carbon-fiber-reinforced polyetheretherketone (CFR-PEEK) has historically been used in spinal cages, bone fixation screws, and cardiac and neurological leads.9 More recently, CFR-PEEK has been used in orthopedic implants and may be ideal for articulating implants, including knee replacement products. CFR-PEEK is readily accepted by the body and does not break down over time. It has a modulus very similar to bone and an ability to withstand prolonged fatigue strain.9 It can also be manufactured to match the modulus of both cortical and cancellous bone densities.9 Proponents of CFR-PEEK hypothesize that it may help to avoid potential issues such as stress shielding and bone resorption, which are common problems experienced when stainless steel or titanium implants are used in total knee replacements.9 In addition, the x-ray transparency of CFR-PEEK allows the fusion to be readily visualized, and, because it is not metallic, it is compatible with both computed tomography and magnetic resonance imaging technologies. Owing to the chemical stability and resistance of CFR-PEEK, it can be readily sterilized by common methods such as steam and gamma.9
We conducted a systematic review of the literature to summarize the outcomes of studies evaluating CFR-PEEK as an articulating surface in orthopedic implants. The specific objectives of this review are to investigate the properties, technical data, and safety of CFR-PEEK biomaterial and to evaluate its potential for improving the design of orthopedic implants.
Methods
Search strategy
A systematic literature search was performed using MEDLINE and EMBASE from 1950 to February 2014. The search strands were designed to retrieve relevant studies assessing the outcomes of CFR-PEEK orthopedic implants. Keywords used for the searches included CFR-PEEK AND implants, carbon-fiber-reinforced-polyetheretherketone AND implants, CFR-PEEK AND orthopedics OR orthopedics, or carbon-fiber-reinforced-polyetheretherketone AND orthopedics OR orthopedics (Table 1). The search was also expanded by reviewing the reference sections of selected papers to capture all relevant articles. Content experts also provided a list of relevant articles.
Table 1.
EMBASE and MEDLINE search strategy results.
| SEARCH STRATEGY | MEDLINE | EMBASE |
|---|---|---|
| CFR-PEEK AND implants | 12 | 13 |
| Carbon-fiber-reinforced-poly-etheretherketone AND implants | 23 | 0 |
| CFR-PEEK AND orthopedics | 4 | 3 |
| Carbon-fiber-reinforced-poly-etheretherketone AND orthopedics | 9 | 2 |
| Total Number of Articles | 48 | 18 |
Selection criteria
To be included, articles had to be written in English and report information related to the outcomes of CFR-PEEK as an orthopedic implant. Articles were not included if they met any of the following exclusion criteria: (1) were duplicate publications, (2) their full-text versions could not be retrieved, and (3) focused on the chemical composition of the material.
Article selection
The titles identified in the literature searches were screened to determine if the articles should be considered for full-text review. Full-text articles that met the inclusion criteria were selected for data abstraction. Relevant reports provided to us by experts in this field were also incorporated into this review.
Data abstraction
We abstracted data from the full-text articles using a data abstraction table to compare each study, which included the year of publication, location where the study was conducted, materials assessed, tests used, results of the test under study, safety of the materials, and the overall outcome.
Results
Literature search results
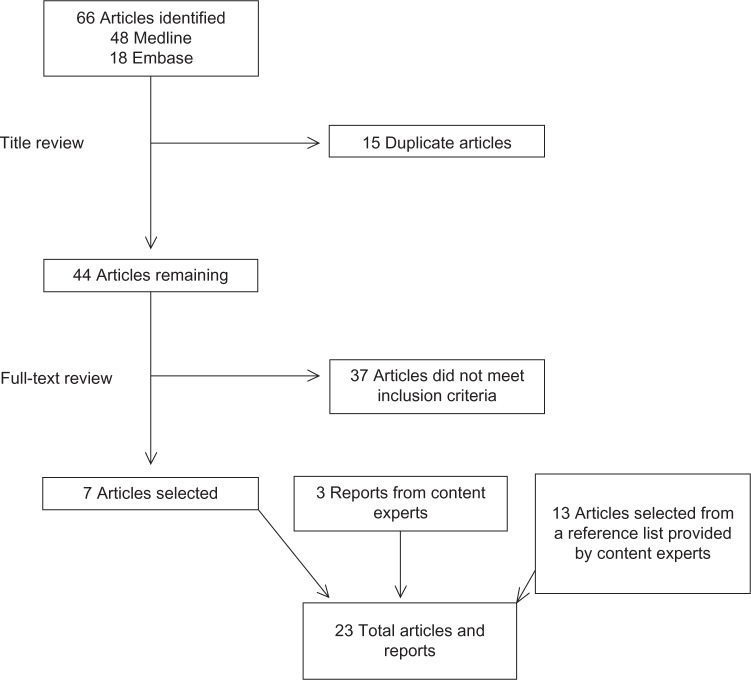
The literature search identified 66 references; 15 of the articles were duplicates. Upon abstract and full-text review of the remaining articles, seven studies met the criteria for inclusion of this review (Fig. 1).10–16 We included three reports that specifically assessed the system performance of CFR-PEEK components in the KineSpring® Knee Implant System and PEEKPower High Tibial Osteotomy Plates provided to us by content experts.17–19 We also identified 13 articles provided by content experts, resulting in a total of 23 included references.20–32
Figure 1.
Literature search results.
Study characteristics
The 23 articles and reports selected for this review were published between the years 1990 and 2013. Approximately 17% of the articles and reports included in this review were published in 2013. The selected studies were conducted in North America (39.1%), the United Kingdom (30.4%), Germany (8.7%), Japan (8.7%), France (4.3%), Italy (4.3%), and Israel (4.3%), demonstrating broad international support for CFR-PEEK as an orthopedic material (Table 2). Each study assessed the outcome and performance of various CFR-PEEK composite implant materials such as pins, plates, tibial nails, cups, stems, and spinal cages.
Table 2.
Study characteristics.
| CHARACTERISTIC | N (%) |
|---|---|
| Location | |
| North America | 9 (39.1) |
| United Kingdom | 7 (30.4) |
| Germany | 2 (8.7) |
| Japan | 2 (8.7) |
| France | 1 (4.3) |
| Italy | 1 (4.3) |
| Israel | 1 (4.3) |
| Year of Publication | |
| 1990 | 1 (4.3) |
| 1991 | 0 (0.0) |
| 1992 | 1 (4.3) |
| 1993 | 1 (4.3) |
| 1994 | 1 (4.3) |
| 1995 | 0 (0.0) |
| 1996 | 0 (0.0) |
| 1997 | 0 (0.0) |
| 1998 | 0 (0.0) |
| 1999 | 1 (4.3) |
| 2000 | 0 (0.0) |
| 2001 | 0 (0.0) |
| 2002 | 0 (0.0) |
| 2003 | 0 (0.0) |
| 2004 | 0 (0.0) |
| 2005 | 0 (0.0) |
| 2006 | 0 (0.0) |
| 2007 | 2 (8.7) |
| 2008 | 3 (13.0) |
| 2009 | 2 (8.7) |
| 2010 | 2 (12.5) |
| 2011 | 1 (4.3) |
| 2012 | 4 (17.4) |
| 2013 | 4 (17.4) |
| Number of Studies Included | |
| Peer-review literature | 20 (87.0%) |
| Science-based reports | 3 (13.0%) |
The studies included in this review used several different tests to assess the use of CFR-PEEK material as an implant material based on its properties, technical data, and safety. The safety of the implant material was compared to acceptance criteria and wear rates similar to currently used implant materials. The studies included in this review concluded that CFR-PEEK material performs well in orthopedic implants.10–15 The comprehensive series of tests used to assess the material included bending tests13,19; static torsion tests13; bending fatigue tests13; pin-on-plate tests10,11; animal models14; in vitro wear stimulation16; friction wear tests12,15; durability tests17,18; absorber strength, extension, and compression limits; and durability and corrosion tests.17,18 These tests have been utilized to demonstrate the durability and biocompatibility of the CFR-PEEK material in relation to currently used materials. These tests simulate the everyday stresses that the implants encounter within a patient as well as the safety profile that the material demonstrates.
Summary of outcomes
Out of the 23 studies included in this review, 20 were clinical and/or biomechanical studies and 3 were scientific reports. Of the 20 studies, 16 reported positive outcomes in favor of the use of CFR-PEEK as an implant material. Three studies reported neutral outcomes, stating that CFR-PEEK-based devices were comparable to the current standard and offered no clear advantages. Lastly, one study found that using the CFR-PEEK material in joint replacement therapy was suitable for hip arthroplasty, but not for knee arthroplasty. Biomechanical studies are summarized in Table 3A, and clinical studies are summarized in Table 3B.
Table 3.
(A) Biomechanical studies and (B) clinical studies that examined CFR-PEEK as an implant material.
| (A) FIRST AUTHOR (YEAR) | LOCATION | MATERIALS ASSESSED | TESTS USED | RESULTS OF THE TEST (TECHNICAL DATA) | SAFETY (CONCLUSION) | OUTCOME |
|---|---|---|---|---|---|---|
| Brown, SA (1990) | North America | CFR-PEEK PAN thermoplastic for fracture fixation devices, compared to polysulphone and polybutylene terephthalate. | Flexion tests Fracture toughness testing |
CFR-PEEK material showed no degradation in mechanical properties after contouring and saline soaking. | The CFR-PEEK PAN composite was easiest to contour and had the best mechanical properties. Preliminary biocompatibility test results have been encouraging and the CFR-PEEK PAN composite is an excellent candidate for further study for such applications. |
(+) |
| Jockish, KA (1992) | North America | CFR-PEEK fracture fixation plate, compared to UHMWPE and PEEK. | Muscle implant testing In vivo functional compatibility testing |
Normal muscle tissue with no signs of infection or an adverse tissue response. Very few inflammatory cells present in the tissue surrounding any of the implant materials. PEEK and CFR-PEEK implants showed similar tissue response to UHMWPE. No difference in callus response and soft tissue healing. |
Animal subjects remained very active and the plates did not fail mechanically. CFR-PEEK particles were well tolerated by the subjects. CFR-PEEK plates were mechanically capable of supporting a fracture, even under adverse conditions. |
(+) |
| Scholes, SC (2007) | United Kingdom | (Pin material + Plate material) 1. CFR-PEEK-OPTIMA® PAN +BioLox Delta 2. CFR-PEEK-OPTIMA® pitch + BioLox Delta 3. CFR-PEEK-OPTIMA® PAN + BioLox Forte 4. CFR-PEEK-OPTIMA® pitch + BioLox Forte |
Multidirectional pin-on-plate machine | The BioLox Delta plates seem to wear slightly less than the BioLox Forte plates when artic ulating against the same CFR-PEEK-OPTIMA® pin material. The difference is statistically significant for both the PAN and pitch pins (P < 0.05). No statistically significant difference in the total wear for each material combination (ANOVA P > 0.20). The CFR-PEEK-OPTIMA® pitch pins seem to perform the best when articulating against BioLox Forte. |
CFR-PEEK-OPTIMA® articulating against ceramic has been found to give low wear factors compared to metal-on-metal samples. This material combination may perform well in joint applications. |
(+) |
| Latif, AMH (2008) | United Kingdom | CFR-PEEK acetabular cup | Finite Element Analysis Wear studies using hip joint wear simulator Biocompatibility tests Mechanical tests Stability tests |
Results show that CFR-PEEK cups are comparable to the current standard. Wear study demonstrated that volume of wear particles was an order of magnitude less with CFR-PEEK than UHMWPE. |
The design will provide the benefits previously seen with the current standard, with improve long-term component wear and stability. | (+) |
| Scholes, SC (2008) | United Kingdom | CFR-PEEK acetabular cup, compared to UHMWPE | Wear testing Friction testing |
Wear rates for the CFR-PEEK joints were considerably lower than UHMWPE joints. | The results show that this novel joint couple may potentially be an alternative solution for the reduction of osteolysis. | (+) |
| Scholes, SC (2009) | United Kingdom | (Pin material + Plate material) 1. CFR-PEEK + LC CoCrMo 2. CFR-PEEK PAN+ LC CoCrMo 3. CFR-PEEK PAN+ HC CoCrMo 4. CFR-PEEK pitch + HC CoCrMo |
Pin-on-plate wear tests | Volumetric wear: PEEK LC CoCrMo-high wear rates with no running-in wear phase CFR-PEEK pins against the LC CoCrMo plates- showed no running-in wear period CFR-PEEK-PAN against HC CoCrMo- the running-in wear period was in the first 1.4 million cycles CFR-PEEK Pitch against HC CoCrMo had two wear phases: higher initial wear phase (1 million cycles) followed by a lower steady-state wear phase. |
CFR-PEEK against CoCrMo (HC/LC) provided low wear rates. Study concluded with confidence that this material combination will perform well in orthopedic applications. |
(+) |
| Scholes, SC (2009) | United Kingdom | Oxford® Partial Knee [CoCrMo tibial component and femoral component between a mobile pitch-based CFR-PEEK OPTIMA® meniscal bearing] | Wear test: Durham six-station knee wear simulator Friction Test: Durham friction stimulator II Lubrication Test |
Wear tests: Loaded soak control gave very similar wear results to the unloaded soak control for this material combination. The medial joints gave higher wear than the lateral joints using both the loaded and the unloaded soak controls. Friction tests: The medial components gave slightly lower friction than the lateral components performed using carboxymethyl cellulose (CMC) or bovine serum (BS) as lubricant. A slight decrease in friction factors was found when BS was used as the lubricant for the medial and lateral bearings compared to CMC |
CFR PEEK unicondylar knee joints performed well in these wear tests (lower volumetric wear than conventional metal-on-ultra-high-molecular-weight polyethylene prostheses) The friction tests showed that the joints were working within boundary-mixed lubrication. The low wear produced by these joints was due to the material combination and not the lubrication regime. |
(+) |
| Brockett, CL (2012) | United Kingdom | 36 mm diameter Biolox Delta ceramic and cobalt chrome heads paired with CFR-PEEK Pitch cups. Control: 36 mm diameter cross-linked UHMWPE cups |
Friction wear test using a single station pendulum friction stimulator. Wear test method using a 10-station Prosim hip wear simulator. |
The friction for both the CFR-PEEK bearings was significantly higher than the friction for the cross-linked UHMWPE. The mean volumetric wear rate was 0.3 mm3/Mc indicating that the ceramic-on-CFR-PEEK bearing to be a very low wearing option |
CoCFR-PEEK bearings are a promising alternative bearing option for total hip replacement. | (+) |
| Dickinson, AS (2012) | United Kingdom | Composite hemi-pelvis implanted with acetabular cups made of either: -CoCr -UHMWPE -MOTIS CFR-PEEK |
Surface strain measurement using Digital Image Correlation | CoCr and CFR-PEEK cups generated increased tensile and compressive cortex strains superior to the acetabular rim, whereas UHMWPE cup generated a global reduction in cortex strain. The CFR-PEEK cup produced the closest bone strain pattern to the intact case, increasing the average principal strain magnitude in the gage region by 12%. |
The MOTIS CFR-PEEK composite acetabular cup produced the closest bone strain to the intact pelvis in the main load path compared to CoCr metal and UHMWPE. CFR-PEEK cups may produce a lower extent of internal cancellous bone stress shielding and induce less adverse bone adaptation, offering greater longevity. |
(+) |
| Nakahara, I (2013) | Japan | CFR-PEEK composite (Cup + Stem) 1. Cement + Cement 2. Cement + Cementless 3. Cementless + Cement 4. Cementless + Cementless |
Ovine model-unilateral hip replacement was performed using either a cementless or cemented prosthesis. | All animals recovered from surgery uneventfully. 11/15 animals were used to examine fixation of the cups and stems. Both cementless and cemented CFR-PEEK stems work well for fixation. Cup fixation may be difficult for both cemented and cementless types. |
Reported stable in vivo fixation of CFR/PEEK prostheses in both cemented and cementless cases even under load-bearing condition. Results encourage the development of CFR/PEEK hip prostheses. |
(+) |
| Steinberg, EL (2012) | Israel | Piccolo composite CFR-PEEK items: 1. 10 mm tibial nail 2. Dynamic compression plate 3. Proximal humeral plate 4. Distal volar radial plate |
Four-point bending, static torsion of the nail and bending fatigue; debris generated at the connection between the CF-PEEK plate and titanium alloy screws | 4-point bending stress of the tibial nail and dynamic and distal radius plates met acceptance criteria (similar to other commercially used nails). Torsion stiffness test results for CF were similar to commercial nails. All CF-PEEK devices underwent one million fatigue cycles without failure Debris filters indicated that the CF-PEEK had a lower debris weight than the titanium control. |
CFR-PEEK OPTIMA passed the tests meeting the acceptable criteria and behaving similar to other commercial nails and plates | (+) |
| Maharaj, G (1994) | North America | CFR-PEEK in the femoral component of a total hip arthroplasty | Wear debris characterization Mechanical testing |
Amount of debris generated is relatively small compared to UHMWPE. Zirconia heads remained well-attached to CFR-PEEK trunnions after 10 million cycles. |
Indicates a stable, well-fixed interface. | (+/−) |
| Bruner, HJ (2010) | North America - | Titanium 6 x 45-mm pedicle screws (EXPEDIUM, DePuy Spine) couple with 5.5-mm rods made of either titanium, PEEK, or CFR-PEEK. | Biomechanical loading: -Pure moment loading Combined loading |
No statistically significant differences between constructs. | CFR-PEEK rods have similar stiffness to PEEK and titanium alloy constructs in single-cycle, in vitro, biomechanical loading in the human lumbar spine. | (+/−) |
| Grupp, TM (2010) | Germany | Univariation F fixed bearing unicom-partmental knee design compared to experimental gliding surfaces made of CFR-PEEK pitch and CFR-PEEK PAN. | In vitro wear stimulation: Univariation F medial unicompartmental knee replacement with UHMWPE/CoCr29Mo6 compared to the experimental gliding surfaces made of CFR-PEEK Pitch and CFR-PEEK-PAN. | CFR-PEEK Pitch instead of polyethylene led to a significant reduction of cumulative wear but not substantially different from the wear rate of the clinical reference. The CFR-PEEK-PAN group showed no significant difference in the cumulative wear and wear rate. There was a wide scattering in wear behavior. There were huge variances in individual wear rates. |
CFR-PEEK-PAN is unsuitable as a bearing material for fixed bearing knee articulations with low congruency. CFR-PEEK Pitch cannot be recommended as it remains doubtful whether it reduces wear compared to polyethylene. |
(+/−) |
| Wang, A (1999) | North America | CFR-PEEK as bearing surfaces for total joint replacements (hip and knee) | Wear tests | Knee: CFR-PEEK composites exhibited significant higher wear rates than UHMWPE. Hip: CFR-PEEK composites showed a reduction in the wear rate compared to UHMWPE. |
CFR-PEEK composites offer a far superior wear resistance over UHMWPE in the hip joint, but it not recommended for total knee replacements. | Hip (+) Knee (−) |
| (B) FIRST AUTHOR (YEAR) | LOCATION | MATERIALS ASSESSED | TESTS USED | RESULTS OF THE TEST (TECHNICAL DATA) | SAFETY (CONCLUSION) | OUTCOME |
| Brantigan, JW (1993) | North America | CFR-PEEK implant cage | Clinical and radiographic assessment | Successful radiographic fusion in all cases. Clinically, 20/26 patients categorized as either “excellent” or “good”. |
The clinical and fusion results represent the justification for a larger formal study of the safety and efficacy of the device. | (+) |
| Rousseau, MA (2007) | France | CFR-PEEK interbody cage at the lumbar spine | Physical examination using the classification of Brantigan and Steffee Radiologic evaluation |
Clinical outcomes were excellent or good in 49 of 57 patients. Fusion was definite in 56 of 57 cases. No revision surgery. At follow up, local lordosis decreased in 13 cases. |
Lumbar circumferential arthrodesis using CFR-PEEK cages provided good clinical results and fusion rate, but lordosis correction was not maintained at follow-up. | (+) |
| Nakahara, I (2012) | Japan | CFR-PEEK hip stem | Radiographic and histologic assessment | CFR-PEEK stems remained intact without any evidence of delamination, microfracture, or particulate migration in appearance No evidence of any wear debris or any inflammatory reaction in the surrounding soft tissue. Bone on growth fixation achieved in 2 of 4 cases of CFR-PEEK stems and in all 5 cases of titanium stems. CFR-PEEK cases showed minimal stress shielding while 3 of 5 titanium cases demonstrated typical osteopenia associated with stiff metal stems. Bone resorption and osteopenia observed with titanium stems was not found with CFR-PEEK design. |
The lower stiffness of the CFR-PEEK has a positive effect on suppression of the bone resorption due to stress shielding. | (+) |
| Pace, N (2008) | Italy | CFR-PEEK acetabular cup | Clinical assessment Harris Hip Score (HHS) Radiographic examination |
Mean preoperative HHS improved from 52 to 90 points. 1 of 28 patients had a revision due to a septic loosening. Radiographs revealed that everyone was stable. |
The presented findings show the short-term efficacy of the implant, but longer follow-ups and a larger number of patients are needed. These results should be considered encouraging. |
(+) |
| Heary, RF (2011) | North America | CFR-PEEK cage (DePuy Spine) | Clinical examination (Odom criteria) and plain radiographs. | 39 (97.5%) of patients had solid fusions at a mean follow-up of 43 months. No deterioration of neurological function compared with preoperative status. |
CFR-PEEK cages are effective for achieving thoracolumbar fusion. Excellent clinical and radiographic results were achieved. |
(+) |
Joint arthroplasty
One study investigated the wear of CFR-PEEK pins articulating against both BIOLOX delta and BIOLOX forte plates using a multidirectional pin-on-plate machine.10 The results of the study indicated that the materials articulating against ceramic demonstrated low wear factors and may perform well in articulating joint replacement applications.10
Similarly, a study conducted by Scholes and Unsworth used multidirectional pin-on-plate wear tests on various combinations of nonreinforced PEEK and CFR-PEEK against CoCrMo (low and high carbon) to assess the potential of this material combination for use in orthopedic implants.11 This study concluded that carbon-reinforced PEEK performed better than standard PEEK alone.11
Another study performed tribological tests (friction, lubrication, and wear) on CFR-PEEK mobile unicondylar knee prostheses.12 The Durham six-station knee wear simulator and the Durham friction simulator II were used to assess the friction, lubrication, and wear properties of this material combination. The results of the wear simulation test indicated that CFR-PEEK knee joints gave lower wear rates than metal-on-ultra-high-molecular-weight polyethylene prostheses tested under similar conditions.12 The friction test results indicated that these joints operated in the boundary-mixed lubrication (carboxymethyl cellulose (CMC) and bovine serum (BS)). Therefore, the low wear produced by these joints was suggested to be attributable to the favorable material combination, and not the lubrication.12
Nakahara et al conducted one of the few studies to investigate in vivo implant fixation of cementless and cemented CFR-PEEK hip prostheses up to 52 weeks after implantation at a radiographic and histological level.14 A unilateral total hip replacement was performed in skeletally mature sheep using either cemented or cementless CFR-PEEK cups and stems. There was no significant difference in stability whether the CFR-PEEK prostheses were cemented or cementless. The CFR-PEEK implant performed at an acceptable level. Their positive results encouraged the further development of CFR-PEEK in hip prostheses.14
Brockett et al investigated the wear performance of a ceramic-on-CFR-PEEK total hip replacement through a simulator study.15 The CFR-PEEK bearings were reported to exhibit step-like wear behavior throughout the study. This step-like wear behavior was attributable to periods of higher and lower wear rates (loss of carbon fiber material and loss of PEEK matrix material). Despite this pattern, overall wear of the bearings was low compared to previously documented experiments. There were significantly higher levels of friction in the CFR-PEEK bearings when compared to conventional methods, but no evidence existed to suggest it is a clinically detrimental factor.15
Grupp et al tested different materials of bearings in a unicompartmental knee arthroplasty (UKA) system.16 Specifically, standard polyethylene was compared to multiple CFR-PEEK designs. One variation of the CFR-PEEK bearing showed that there was no significant difference in wear rates compared to the polyethylene bearing, while another variation of the product had significantly less wear than the polyethylene version. However, it was noted that these results should be interpreted with caution because of the high standard deviation of the CFR-PEEK measurements.16
A 2012 study by Dickinson et al assessed surface strain measurement in the pelvis using acetabular cups made of cobalt chromium, polyethylene, or CFR-PEEK. CFR-PEEK produced the closest bone strain to the intact hip in the main load path compared to the cobalt chromium and polyethylene devices. The authors concluded that CFR-PEEK could promote less adverse bone adaption than stiffer press-fitted implants in current use.20
Latif et al conducted wear studies on a CFR-PEEK acetabular cup.21 The investigators determined that this device was comparable to the current standard. They found that there was a lower volume of wear particles when using the CFR-PEEK cup, which could improve long-term component wear and stability.21 Maharaj et al came to a similar conclusion when using the CFR-PEEK material in the femoral component of a hip arthroplasty system.22
In 2012, Nakahara et al studied the results of a CFR-PEEK hip stem in ovine models compared to titanium after a 12-month implantation period. The cases that had the CFR-PEEK design inserted showed minimal stress shielding, and did not exhibit the same bone resorption and osteopenia observed with the titanium stems.23
In another study using CFR-PEEK material for an acetabular cup, Pace et al followed 30 patients for, an average of, 36 months (range: 30–48 months).24 They discovered that the mean Harris hip score (HHS) improved to 90 points, from an average of 52 points preoperatively. Only one revision surgery was performed, because of septic loosening, at 28 months, and the radiographic examinations showed that everyone was stable.24
The CFR-PEEK acetabular cup device was examined again by Scholes et al to assess wear rates using a hip simulator.25 They found that, compared to standard polyethylene, the CFR-PEEK cup resulted in lower wear rates, which may reduce revisions because of osteolysis.25
Wang et al assessed the wear behavior of CFR-PEEK as bearing surfaces for both total hip and total knee replacements.26 Though CFR-PEEK is more dense and offers greater tensile strength compared to polyethylene, the study provided mixed results, as the authors concluded that the material appears to be suitable for the hip joint but not the knee joint after comparing wear rates.26
Fracture fixation
Steinberg et al compared CFR-PEEK tibial nail, dynamic compression plate, proximal humeral plate, and distal radius volar plate biomechanically to commercially available devices for wear/debris. The biomechanics were assessed by four-point bending, static torsion of the nail, and bending fatigue.13 The wear/debris was assessed based on the amount of the debris generated at the connection between the CFR-PEEK plate and titanium alloy screws. The CFR-PEEK tibial nail met all acceptance criteria, thereby behaving similarly to other commercially available nails and plates under similar circumstances.13 The CFR-PEEK nail performed better than titanium alternatives as it generated a significantly lower debris weight.13
In 1990, Brown et al compared CFR-PEEK to CFR-polysulfone and CFR-polybutylene terephthalate for use in fracture fixation. CFR-PEEK showed no degradation in mechanical properties (flexural strength, strain to failure, fracture toughness) compared to the other two materials.27
Bruner et al prepared four fresh human cadavers to conduct pure moment and combine loading studies examining rods made with PEEK, CFR-PEEK, or titanium alloy that are used for posterior lumbar fusion.28 The results were inconclusive as there was no evidence of statistical significance.28
Jockisch et al evaluated CFR-PEEK as a potential material for use as a fracture fixation plate.29 The first phase of the study observed the short-term biocompatibility of CFR-PEEK in rabbit muscle implant testing, which was similar to the polyethylene material. The second phase required that the plates be implanted as internal fixation devices for transverse midshaft femoral osteotomies in beagles. The authors stated that the CFR-PEEK plates were effective in promoting fracture healing.29
Spinal cage
Brantigan and Steffee used a CFR-PEEK implant cage for posterior lumbar interbody fusion in 26 patients.30 At two years follow-up, radiographic fusion was successful. In terms of the clinical results, 20 of the 26 patients (76.9%) were deemed either excellent or good. Two patients were considered to have poor clinical results, but the reasons for failure were unrelated to the material. Also, minimal complications were seen.30
In a 40-patient retrospective study, Heary et al analyzed the fusion rates, clinical outcomes, and percentage of vertebral body coverage for CFR-PEEK cages used in the reconstruction of the thoracolumbar spine.31 They found that successful fusion had occurred in 39 patients and concluded that carbon fiber cages were effective for achieving thoracolumbar fusion with excellent clinical and radiographic results.31
A CFR-PEEK cage design was used in an investigation conducted by Rousseau et al for treatment of degenerative lumbar spine disorders.32 The study included 57 patients, with an average follow-up period of 5.7 years. Clinical outcomes were categorized as either excellent or good in 49 cases, and definite fusion was evident in 56 cases. No patients required a revision surgery. In all, 47 patients displayed no change in lordosis after surgery, while 10 of them had an increase in lordosis. At follow-up, local lordosis decreased in 13 cases. The authors stated that using CFR-PEEK cages provided good clinical results and fusion rate, but that lordosis correction was not maintained at follow-up.32
Scientific reports
The three reports included in this review support the use of CFR-PEEK as an implant device material (Table 4). The first report collected benchmark data for the PEEK Element Absorber (KineSpring System). The PEEK Element Absorber for use in the KineSpring System was evaluated for strength under displacement-controlled static loading conditions to failure.18 The absorber strength, extension limit, and compression limit results passed the acceptance criteria as demonstrated by testing static loading to failure.17
Table 4.
Reports that evaluated CFR-PEEK as an implant material.
| FIRST AUTHOR (YEAR) | LOCATION | MATERIALS ASSESSED | TESTS USED | RESULTS OF THE TEST (TECHNICAL DATA) | SAFETY (CONCLUSION) | OUTCOME |
|---|---|---|---|---|---|---|
| Lowe, D (2013) | North America | 1. Femoral Socket 2. Tibial Socket 3. Tapered Sleeves 4. PEEK Absorber Piston 5. P EEK Absorber Arbor |
PEEK Absorber module evaluated under displacement controlled static loading conditions to failure | Absorber Strength: PASS: All strengths were almost an order of magnitude more than 60 lbf. Absorber extension limit: PASS: The amount of lengthening before dissociation >27 mm. Absorber compression limit: PASS: The amount of compressive travel is ≥3 mm by design |
All applicable acceptance criteria have been met as demonstrated by testing static loading to failure. | (+) |
| Lowe, D (2013) | North America | 1. PEEK Absorber Piston 2. P EEK Absorber Arbor |
In vitro fatigue durability of the absorber portion of the KineSpring PEEK System was assessed under displacement controlled, uniaxial dynamic loading conditions | Durability: PASS: No evidence of fracture, permanent deformation, or loss in absorber functionality was identified. Corrosion Resistance: PASS: No evidence of corrosion was identified on any components. |
All applicable acceptance criteria have been met, as demonstrated by testing to 11,391,551 cycles with a target maximum compression of 4.5 mm | (+) |
| Arthrex Medizinische Instrumente GmbH (2013) | Germany | 1. PEEKPower HTO Plate 2. T itanium plates (TomoFix Plate) |
Preclinical and dynamic compression bending tests | The titanium plates demonstrated an early functional failure by fracturing of the plate at the superior screw hole close to the resection line while the PEEK-carbon composite plate specimens failed due to distal screw back outs. The PEEK-carbon composite plates resisted higher dynamic loadings and a higher static flexural rigidity. The titanium plates evidenced more elastic construct behavior with increased deformations. |
The results of this study specified the need for an appropriate ratio between rigidity and load bearing over a defined number of cycles for a long-term functioning implant | (+) |
A similar report was conducted on the KineSpring PEEK System to assess the absorber durability and corrosion resistance.19 The acceptance criterion that was used to assess the durability of the KineSpring PEEK System was that the system’s functionality must be maintained for ≥10 million loading cycles. No evidence of fracture, permanent deformation, or loss in absorber functionality was identified. The acceptance criteria for corrosion resistance were that the system materials must demonstrate corrosion resistance in simulated in vivo environments. There was no evidence of corrosion identified on any of the components. Therefore, acceptance criteria were met for the durability and corrosion resistance of the Kine-Spring PEEK System.18
A PEEK-carbon composite PEEKPower High Tibial Osteotomy Plate was compared to a titanium plate using a sawbone tibia model under static and dynamic loadings. The study evaluated the biomechanical behavior of these two plate systems through a comparable worst case compression bending test design.20 The static bending tests revealed distinct elastic–plastic deformation behavior between both plate constructs. The titanium plates demonstrated an early functional failure by fracturing of the plate at the proximal screw hole close to the osteotomy line, while the PEEK-carbon composite plate specimens failed because of distal screw back outs.20 The PEEK-carbon composite plates resisted higher dynamic loadings and had a higher static flexural rigidity. Although a balance needs to be maintained between rigidity and load bearing, it appears that the PEEKPower Plate has some significant advantages over a titanium comparison.19
Discussion
The objective of this review study was to evaluate properties, technical data, and safety of CFR-PEEK as a potential orthopedic implant material. The strengths of this review include the systematic and thorough literature search and comprehensive data abstraction. In addition, the research studies included have been conducted in various countries, which supports that a high level of generalizability can be attributed to the findings.
There have been several studies that have assessed the performance of variations of CFR-PEEK in a variety of different orthopedic implants using a range of different testing techniques. Many of these articles reported favorable findings regarding the properties, technical outcomes, and safety of CFR-PEEK in orthopedic implants.10–15,17–19
One study reported that one variation of CFR-PEEK was an unsuitable alternative implant material, but the study was limited to low-congruency knee designs. However, this study also reported that the same experiment, with a high congruency design, such as a ball and socket, resulted in significantly less wear than standard polyethylene, and16 consequently, was given a neutral rating for broadly supporting CFR-PEEK as a material. We also noted that there were mixed results when examining the use of CFR-PEEK in knee replacement devices. The study by Wang et al indicated that CFR-PEEK composites should not be used in the development of bearing surfaces for total knee arthoplasty, while other reports, such as those using the KineSpring System, supported their use.17–19 The contrast between these reports was that the investigation performed by Wang et al was conducted over a decade prior to these other studies, and the design of the knee replacement systems was vastly different, as Wang et al evaluated a device that was only composed of a CFR-PEEK-based tibial component.
Composites of CFR-PEEK can be used in a number of different applications.33 This is no exception when concerning orthopedic implants. As an articulating surface, variations of CFR-PEEK performed admirably as in a pin-on-plate wear tests,10,11 as a nail,13 as a bearing,15,16 as a plate,19 or as complete unit of multiple components.12,14,17,18 The use of CFR-PEEK also showed benefits regardless of whether it was interacting with a ceramic material,10,15 metal alloys,11,13,16 or polymers12 in cemented or cementless conditions.14 The multitude of applications in several experimental contexts speaks to the versatility of CFR-PEEK as an articulating surface in orthopedic implants.
The increased durability of CFR-PEEK,33 combined with less-invasive techniques, such as the KineSpring,17,18 may provide an opportunity to benefit a growing number of patients who would like to minimize the number of reoperations they require.34,35
The increased durability of the device suggests that the occurrence of adverse events from device failure will likely be decreased. The successful use of CFR-PEEK implants in an ovine model also lends support to the safety of these devices in regular medical use.14 Nevertheless, continued research should investigate the safety of orthopedic devices manufactured with CFR-PEEK components. Specifically, increased levels of friction may have clinically relevant consequences that have yet to be understood.15 A review of the discussion by Brockett et al also identified additional articles that support the safety of CFR-PEEK-based implants, suggesting that the biological response to particles from CFR-PEEK materials is not worse than other materials regularly used in medical devices.15,36,37
The current review has some limitations. First, despite the general consensus derived from included articles that CFR-PEEK materials are beneficial for use in orthopedic implants, there were only 24 articles and reports included in this review. The small number of included articles is further compounded by the increased heterogeneity among the studies. Although initial results are promising, confirmatory findings would increase confidence in utilizing this material more regularly.
Conclusion
The use of CFR-PEEK materials in orthopedic implants is strongly supported by the current systematic review. Components of an orthopedic implant made from a CFR-PEEK composite have shown significant benefits under a number of tests, specifically with regard to durability. Continued research should be conducted to support the safe implementation of CFR-PEEK materials.
Footnotes
Author Contributions
Conceived and designed the study: CSL, MB. Collected the data: CSL. Analyzed the data: CSL, CV. Wrote the first draft of the manuscript: CSL. Contributed to the writing of the manuscript: CSL, CV, SS, MB. Agreed with manuscript results and conclusions: CSL, CV, SS, MB. Jointly developed the structure and arguments for the paper: CSL, CV, SS, MB. Made critical revisions and approved the final version: CSL, CV, SS, MB. All authors reviewed and approved the final manuscript.
ACADEMIC EDITOR: Chuanju Liu, Editor in Chief
FUNDING: This study was funded by Moximed Inc. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: CSL, SS and MB are employees of Global Research Solutions Inc. and McMaster University. CV is an employee of Global Research Solutions Inc. SS and MB are employees of Canada Medical Education. MB declares consultancy payments from Smith & Nephew, Stryker, Amgen, Zimmer, Moximed, and Bioventus, and grant support from Smith & Nephew, DePuy, Eli Lilly, and Bioventus.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
REFERENCES
- 1.Valderrabano V, Steiger C. Treatment and prevention of osteoarthritis through exercise and sports. J Aging Res. 2010;2011:374653. doi: 10.4061/2011/374653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chodosh J, Morton SC, Mojica W, et al. Meta-analysis: chronic disease self-management programs for older adults. Ann Intern Med. 2005;143(6):427–38. doi: 10.7326/0003-4819-143-6-200509200-00007. [DOI] [PubMed] [Google Scholar]
- 3.Bosomworth NJ. Exercise and knee osteoarthritis: benefit or hazard? Can Fam Physician. 2009;55(9):871–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Escobar A, Quintana JM, Bilbao A, Arostegui I, Lafuente I, Vidaurreta I. Responsiveness and clinically important differences for the WOMAC and SF-36 after total knee replacement. Osteoarthritis Cartilage. 2007;15(3):273–80. doi: 10.1016/j.joca.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Brander VA. Predicting total knee replacement pain: a prospective, observation study. Clin Orthop Relat Res. 2003;416:27–36. doi: 10.1097/01.blo.0000092983.12414.e9. [DOI] [PubMed] [Google Scholar]
- 6.Tay KS, Lo NN, Yeo SJ, Chia S, Tay DKJ, Chin PL. Revision total knee arthroplasty: causes and outcomes. Ann Acad Med Singapore. 2013;42(4):178–83. [PubMed] [Google Scholar]
- 7.Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Why are total knee arthroplastics failing today? Clin Orthop Relat Res. 2002;404:7–13. doi: 10.1097/00003086-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Kurtz SM, Devine JN. PEEK biomaterials in trauma, orthopedics, and spinal implants. Biomaterials. 2007;28(32):4845–69. doi: 10.1016/j.biomaterials.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kostecki K. PEEK usage climbs for devices. Med Des. 2011. [Accessed July 2, 2014]. Available from: http://medicaldesign.com/materials/peek-usage-climbs-devices.
- 10.Scholes SC, Unsworth A. The wear properties of CFR-PEEK-OPTIMA articulating against ceramic assessed on a multidirectional pin-on-pin machine. Proc Inst Mech Eng H. 2007;221(3):281–9. doi: 10.1243/09544119JEIM224. [DOI] [PubMed] [Google Scholar]
- 11.Scholes SC, Unsworth A. Wear studies on the likely performance of CFR-PEEK/CoCrMo for use as artificial joint bearing materials. J Mater Sci Mater Med. 2009;20(1):163–70. doi: 10.1007/s10856-008-3558-3. [DOI] [PubMed] [Google Scholar]
- 12.Scholes SC, Unsworth A. Pitch-based carbon-fibre-reinforced poly(ether-ether-ketone) OPTIMA® assessed as a bearing material in a mobile bearing unicondylar knee joint. Proc Inst Mech Eng H. 2009;223(1):13–25. doi: 10.1243/09544119JEIM471. [DOI] [PubMed] [Google Scholar]
- 13.Steinberg EL, Rath E, Shlaifer A, Chechik O, Maman E, Salai M. Carbon fiber reinforced PEEK Optima-A composite material biomechanical properties and wear/debris characteristics of CF-PEEK composites for orthopedic trauma implants. J Mech Behav Biomed Mater. 2013;17:221–8. doi: 10.1016/j.jmbbm.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Nakahara I, Takao M, Bandoh S, Bertollo N, Walsk WR, Sugano N. In vivo implant fixation of carbon fiber-reinforced PEEK hip prostheses in an ovine model. J Orthop Res. 2013;31(3):485–92. doi: 10.1002/jor.22251. [DOI] [PubMed] [Google Scholar]
- 15.Brockett CL, John G, Williams S, Jin Z, Isacc GH, Fisher J. Wear of ceramic-on-carbon fiber-reinforced poly-ether ether ketone hip replacements. J Biomed Mater Res B Appl Biomater. 2012;100(6):1459–65. doi: 10.1002/jbm.b.32664. [DOI] [PubMed] [Google Scholar]
- 16.Grupp TM, Utzschneider S, Schröder C, et al. Biotribology of alternative bearing materials for unicompartmental knee arthroplasty. Acta Biomater. 2010;6(9):3601–10. doi: 10.1016/j.actbio.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 17.MoxiMed . Compressive Strength & Travel, PEEK Absorber [Benchmark Report-Product Design] Jan 21, 2013. Released. [Google Scholar]
- 18.MoxiMed . Absorber Durability, KineSpring PEEK System [Benchmark Report-Product Design] Jan 2, 2013. Released. [Google Scholar]
- 19.Anthrex Medizinische Instrumente GmbH . PEEKPower High Tibial Osteotomy Plate. 2013. (White Literature). [Google Scholar]
- 20.Dickinson AS, Taylor AC, Browne M. The influence of acetabular cup material on pelvis cortex surface strains, measured using digital image correlation. J Biomech. 2012;45(4):719–23. doi: 10.1016/j.jbiomech.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 21.Latif AM, Mehats A, Elcocks M, Rushton N, Field RE, Jones E. Pre-clinical studies to validate the MITCH PCR Cup: a flexible and anatomically shaped acetabular component with novel bearing characteristics. J Mater Sci Mater Med. 2008;19(4):1729–36. doi: 10.1007/s10856-007-3256-6. [DOI] [PubMed] [Google Scholar]
- 22.Maharaj G, Bleser S, Albert K, Lambert R, Jani S, Jamison R. Characterization of wear in composite material orthopedic implants. Part I: the composite trunnion/ceramic head interface. Biomed Mater Eng. 1994;4(3):193–8. [PubMed] [Google Scholar]
- 23.Nakahara I, Takao M, Bandoh S, Bertollo N, Walsh WR, Sugano N. Novel surface modifications of carbon fiber-reinforced polyetheretherketone hip stem in an ovine model. Artif Organs. 2012;36(1):62–70. doi: 10.1111/j.1525-1594.2011.01275.x. [DOI] [PubMed] [Google Scholar]
- 24.Pace N, Marinelli M, Spurio S. Technical and histologic analysis of a retrieved carbon fiber-reinforced poly-ether-ether-ketone composite alumina-bearing liner 28 months after implantation. J Arthroplasty. 2008;23(1):151–5. doi: 10.1016/j.arth.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 25.Scholes SC, Inman IA, Unsworth A, Jones E. Tribological assessment of a flexible carbon-fibre-reinforced poly(ether-ether-ketone) acetabular cup articulating against an alumina femoral head. Proc Inst Mech Eng H. 2008;222(3):273–83. doi: 10.1243/09544119JEIM334. [DOI] [PubMed] [Google Scholar]
- 26.Wang A, Lin R, Stark C, Dumbleton JH. Suitability and limitations of carbon fiber reinforced PEEK composites as bearing surfaces for total joint replacements. Wear. 1999;225–9:724–7. [Google Scholar]
- 27.Brown SA, Hastings RS, Mason JJ, Moet A. Characterization of short-fibre reinforced thermoplastics for fracture fixation devices. Biomaterials. 1990;11(8):541–7. doi: 10.1016/0142-9612(90)90075-2. [DOI] [PubMed] [Google Scholar]
- 28.Bruner HJ, Guan Y, Yoganandan N, Pintar FA, Maiman DJ, Slivka MA. Biomechanics of polyaryletherketone rod composites and titanium rods for posterior lumbosacral instrumentation. Presented at the 2010 Joint Spine Section Meeting. Laboratory investigation. J Neurosurg Spine. 2010;13(6):766–72. doi: 10.3171/2010.5.SPINE09948. [DOI] [PubMed] [Google Scholar]
- 29.Jockisch KA, Brown SA, Bauer TW, Merritt K. Biological response to chopped-carbon-fiber-reinforced peek. J Biomed Mater Res. 1992;26(2):133–46. doi: 10.1002/jbm.820260202. [DOI] [PubMed] [Google Scholar]
- 30.Brantigan JW, Steffee AD. A carbon fiber implant to aid interbody lumbar fusion. Two-year clinical results in the first 26 patients. Spine (Phila Pa 1976) 1993;18(14):2106–7. doi: 10.1097/00007632-199310001-00030. [DOI] [PubMed] [Google Scholar]
- 31.Heary RF, Kheterpal A, Mammis A, Kumar S. Stackable carbon fiber cages for thoracolumbar interbody fusion after corpectomy: long-term outcome analysis. Neurosurgery. 2011;68(3):810–8. doi: 10.1227/NEU.0b013e3182077a9f. discussion 818–9. [DOI] [PubMed] [Google Scholar]
- 32.Rousseau MA, Lazennec JY, Saillant G. Circumferential arthrodesis using PEEK cages at the lumbar spine. J Spinal Disord Tech. 2007;20(4):278–81. doi: 10.1097/01.bsd.0000211284.14143.63. [DOI] [PubMed] [Google Scholar]
- 33.Green S. Compounds and composite materials. In: Kurtz SM, editor. PEEK Biomaterials Handbook. Waltham, MA: William Andrew; 2012. pp. 23–48. [Google Scholar]
- 34.Green S. Invivo Biostability Study on a Polyaryletheretherketone Biomaterial. [Accessed July 2, 2014]. Available from: http://cayennemedical.com/wordpress/wp-content/uploads/PEEK_Optima_Bio-stability_Data_Sheet1.pdf.
- 35.Kinbrum A. The PEEK of large joint performance? Orthop Des Technol. 2009. [Accessed July 2, 2014]. Available from: http://www.odtmag.com/articles/2009/03/the-peek-of-large-joint-performance.
- 36.Howling GI, Sakoda H, Antonarulrajah A, et al. Biological response to wear debris generated in carbon based composites as potential bearing surfaces for artificial hip joints. J Biomed Mater Res B Appl Biomater. 2003;67(2):758–64. doi: 10.1002/jbm.b.10068. [DOI] [PubMed] [Google Scholar]
- 37.Utzschneider S, Becker F, Grupp TM, et al. Inflammatory response against different carbon fiber-reinforced PEEK wear particles compared with UHMWPE in vivo. Acta Biomater. 2010;6(11):4296–304. doi: 10.1016/j.actbio.2010.06.002. [DOI] [PubMed] [Google Scholar]