Abstract
Being the largest and most visible organ of the body and heavily influenced by environmental factors, skin is ideal to study the long-term effects of aging. Throughout our lifetime, we accumulate damage generated by UV radiation. UV causes inflammation, immune changes, physical changes, impaired wound healing and DNA damage that promotes cellular senescence and carcinogenesis. Melanoma is the deadliest form of skin cancer and among the malignancies of highest increasing incidence over the last several decades. Melanoma incidence is directly related to age, with highest rates in individuals over the age of 55 years, making it a clear age-related disease. In this review, we will focus on UV-induced carcinogenesis and photo aging along with natural protective mechanisms that reduce amount of “realized” solar radiation dose and UV-induced injury. We will focus on the theoretical use of forskolin, a plant-derived pharmacologically active compound to protect the skin against UV injury and prevent aging symptoms by up-regulating melanin production. We will discuss its use as a topically-applied root-derived formulation of the Plectranthus barbatus (Coleus forskolii) plant that grows naturally in Asia and that has long been used in various Aryuvedic teas and therapeutic preparations.
Keywords: forskolin, aging, UV radiation, skin, oxidative stress
1. Introduction
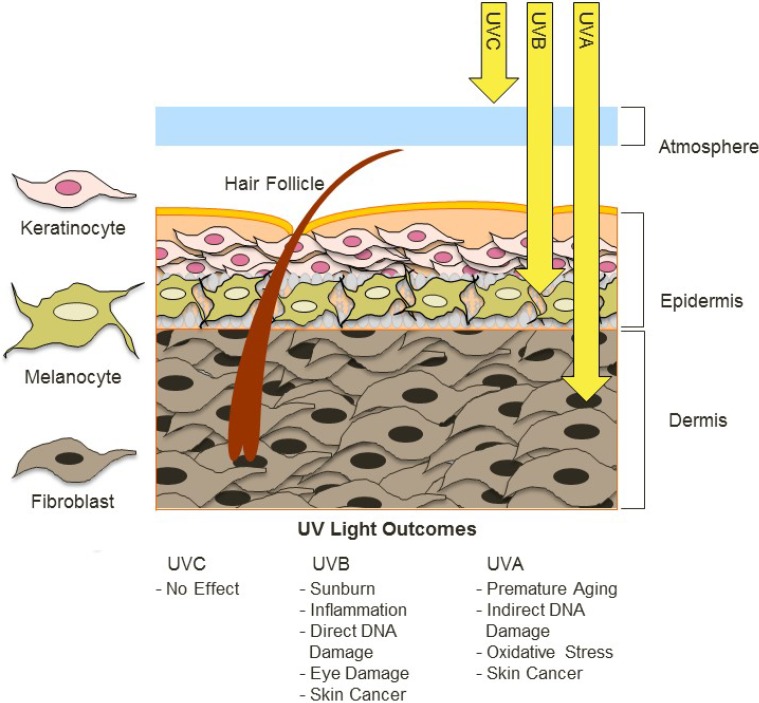
Due to its anatomic location at the external boundary of the body, skin is exposed to a variety of environmental factors such as UV radiation that derives naturally from the sun. Solar UV exposure is a major causative factor for age-related changes such as skin cancer development. UV radiation is composed of UVA, UVB and UVC components based on photon wavelength with UVA having the longest wavelengths (315–400 nm), UVB being mid-range (290–320 nm) and UVC being the shortest wavelengths (100–280 nm). Ambient sunlight is composed primarily of UVA (90%–95%) and UVB (5%–10%) energy, with most solar UVC absorbed by the ozone layer (Figure 1).
Figure 1.
UV radiation in ambient sunlight is composed primarily of UVA and UVB energy. Most UVC is absorbed by the ozone, therefore although it is highly bioactive, terrestrial organisms are not exposed to significant levels of UVC. UVB can cause direct damage to DNA and reach the epidermis. UVA can penetrate the dermis and increases levels of ROS that indirectly induce DNA mutagenesis.
Naturally occurring UV radiation is the environmental mutagen responsible for the largest percentage of environmentally induced skin pathologies, including erythema and inflammation, degenerative aging changes, and cancer [1]. Humans are exposed to UV radiation primarily as a consequence of unprotected exposure to sunlight [2]. UV radiation has many deleterious effects on cells [3,4,5]. UV radiation produces both direct and indirect DNA damage, and each can result in mutagenesis in skin cells. The DNA double helix can absorb energy from shorter-wave UV photons and undergo covalent modification. Neighboring pyrimidines are particularly vulnerable to direct UV damage at the 5–6 double bond position. When UV causes this bond to break, abnormal covalent interactions between adjacent thymines and/or cytosines can result. There are two main DNA lesions that result from UV-induced damage to the 5–6 double bond: (1) cyclobutane dimers formed from two covalent bonds between adjacent pyrimidines to form a ring structure; and (2) pyrimidine 6–4 pyrimidone (6,4)- photoproducts that form upon reaction of the open 5–6 double bond with the exocyclic moiety of an adjacent 3' pyrimidine [6]. Both of these lesions distort the double helix and can lead to mutation, and an individual skin cell may accumulate up to 100,000 such lesions from one day’s worth of sun exposure [7]. UV radiation also damages cellular macromolecules indirectly, through production of oxidative free radicals [8]. Several DNA modifications can result from oxidative injury, including 7,8-dihydro-8-oxoguanine (8-oxoguanine; 8-OH-dG), which promotes mutagenesis (specifically GC-TA transversion mutations [9]. Both direct and indirect DNA changes interfere with transcription and replication, and render skin cells susceptible to mutagenesis. Much of solar UV energy is absorbed by stratospheric ozone, and the gradual depletion of stratospheric ozone over the last several decades has resulted in higher levels of solar UV radiation that strikes the surface of the Earth [10]. Increased ambient UV radiation from global climate change may be an important factor to explain the burgeoning prevalence of melanoma and skin cancer over the last several decades [11,12,13,14].
UVB is a well-characterized mutagen and inducer of skin cancers [15], but recent studies have implicated an increasing role of UVA as a carcinogen [16,17,18] likely through its pro-oxidative effects and possibly through other mechanisms such as telomere shortening [19]. In addition, UVA is less able to induce melanin production compared to UVB, leaving the skin less able to protect itself against further UV insult [15,19,20,21,22]. Increasing attention is being paid to the potential impact of UVA radiation to the body focusing on differential cellular repair and apoptosis depending on anatomic site [23]. The role of UVA in melanoma formation is also suggested by the observation of rising melanoma incidence over the last several decades and sunscreen use in the 1980s when only UVB-blocking sunscreens were used.
2. Factors Contributing to UV Exposure
Geographical variations such as altitude, latitude and urbanization all determine ambient UV strength. Because atmospheric particles such as dust or water droplets can scatter, reflect or otherwise interfere with UV photons, the more atmosphere sunlight has to traverse, the weaker its energy will be at the surface of the Earth. At higher altitudes, with less atmosphere for sunlight to traverse before hitting land, there is higher exposure to UV and a higher risk for melanoma. There is a 2% increase in risk with every 10 m rise in altitude [24] and in addition those living at altitudes over 1,400 m above sea level are at most risk for developing melanoma [25]. In addition to living in high altitudes, occupations routinely operating at high altitudes such as airplane pilots and mountain guides have a higher incidence of melanoma and precancerous lesions [26,27].
UV strength is strongest at the equator because sunlight hits the Earth most directly at the equator. Toward the poles, sunlight hits the earth obliquely and must pass through more atmosphere. Not surprisingly, there is a higher incidence of melanoma in locales closest to the equator, most especially among Caucasians [28] who are most UV-sensitive because of lower cutaneous melanin pigments, but also among lower-risk populations of darker skin tone [29]. In a study of the Norwegian Cancer Registry, decreased latitude by 10° was associated with a 2–2.5 increased risk of melanoma [30]. Another study of 5,700 melanoma cases worldwide found a 1.5-fold increased risk when living at latitudes closer than 20° from the equator [31]. Importantly, though latitude risk for melanoma has been historically strong, recent studies suggest decreased correlation [32], or even an opposite trend. A 2012 study of Northern Europeans, for example, showed an increase in melanoma incidence with increase in latitude beyond 50° north of the equator [33], perhaps due to the dramatic rise in artificial indoor tanning.
Urban-versus-rural lifestyle also seems to be important, with as much as a 50% increased melanoma risk in urban regions [34]. Urbanization may affect cancer risk by bringing together many independent risk factors such as occupational chemical exposure, social pressures regarding skin appearance, easy access to indoor tanning, and higher socio-economic levels lending to increased use of indoor tanning and holiday travel [35]. The increase in urbanization worldwide and the increase in these activities may help explain the rise in melanoma rates for northern Caucasian populations that would otherwise not be exposed to natural risk factors such as latitude [24].
3. Age
UV exposure may account for up to 80% of visible signs of aging in the skin including dry appearance, scalping, wrinkling [15] and impaired pigmentation, and photoaging correlates with cancer risk. A 2012 study of Central Europeans, for example, showed those with early signs of wrinkling on the neck were over four times more susceptible to melanoma than the general population. Freckling on the back also showed over three times the risk [21]. Cutaneous photoaging and melanoma risk both correlate with age and UV exposure. The average age of melanoma diagnosis is about 55 and incidence varies worldwide from five to over 60 cases per 100,000 people per year [12]. Although melanoma is a malignancy mostly diagnosed in the fifth and sixth decade of life, one fifth of cases occur in young adults [36,37]. It is important to note, however, that the UV exposure and accumulation of DNA damage that underlie melanoma formation begin with sun exposure early in youth, which is why sun protection in the pediatric years is so important. There is a significant correlation of melanoma risk with excessive sun exposure before adolescence, perhaps contributed to by structural anatomical differences between the skin of children and adults making it easier for UV to penetrate [38]. Childhood UV exposure also increases the risk of young adult melanoma (melanoma under the age of 30) by over three times, showing how exposure can accelerate the process of carcinogenesis [39]. Furthermore, a new study published in 2014 of over three million people in Sweden showed that accumulation of UV damage begins as early as in the neonate, with melanoma incidence increased in those born in the spring and summer versus those born in the fall or winter [40]. Indeed, some estimates indicate that up to 80% of lifetime UV exposure occurs before the age of 20 because of the outdoor recreational habits of children.
This risk for melanoma among the middle-aged population has risen in the past few decades. An epidemiologic study in Minnesota found an incidence of 60 cases per 100,000 in 2009 compared to just eight per 100,000 in 1970; that is a 24-fold increase in risk for this population. Another unfortunate finding is the steady increase in occurrence in young adults, particularly for young women in the United States (US). Whereas young American women aged 15–39 had a melanoma incidence rate of 6 out of 100,000 cases in 1973, their rate more than doubled to 14 out of 100,000 cases per year in 2006 [41]. Because of ongoing recreational UV trends such as increased use of artificial tanning sources, melanoma rates are expected to continue to rise [37], making this disease an increasing public health threat.
4. Artificial UV and Tanning Beds
Indoor tanning use has dramatically risen in the last thirty years and is predicted to continue rising largely because of societal and commercial incentives for a tanned appearance viewed by many as appealing. In 2013, over 40% of adolescents aged 15 to 18 had tried indoor tanning with about 18% using indoor tanning routinely [42]. A recent large-scale systematic review, published in 2014, reported that over 50% of college-aged students tried indoor tanning, with over 40% using it in the past year. In a 2014 study of college-aged women from 18 to 25 years of age, 25% of current users could be classified as tanning-dependent [43]. Similarly, prevalence among American adults may be as high as 35% [42,44]. Interestingly, tanning behavior has increasingly been compared to classic “substance use” disorders, with some classifying frequent indoor UV patronage as a true addiction [45,46]. Tanning-addictive behaviors have been associated especially with young age, other high-risk behaviors and psychiatric disorders [45,46]. Indoor tanning involves exposure to high doses of UV with the intent to trigger skin pigmentary responses. Tanning beds emit varying blends of UVA and UVB energy, and their use is clearly linked with photoaging, keratinocyte malignancies and melanoma. Generally, most basic tanning beds emit a blend of UVB and UVA radiation with more advanced beds emitting mostly UVA radiation to emulate natural UV radiation. However, with UVA now firmly implicated in melanoma carcinogenesis, such beds may be no safer than those with higher UVB output. Alarmingly, despite strong epidemiologic data correlating younger age of tanning bed use with skin cancers, tanning bed use among minors is poorly regulated and its use increasing. In 2013, the National Conference of State Legislatures of USA approved new laws limiting or banning the use of tanning beds by adolescents, however actual laws that govern indoor tanning among minors vary by state with most states not prohibiting use among minors. Use of tanning salons before the age of 35 years is associated with a 75% increased lifetime melanoma risk [47], therefore the increasing use of tanning beds may be an important factor to explain the increasing incidence of melanoma in recent decades.
5. Sunburns and Melanoma
Overexposure to UV is a key factor in development of skin cancers, and melanoma incidence correlates particularly with intermittent intense UV exposures that cause sunburn. More than five sunburns in a lifetime doubles risk for melanoma and there is increased risk for melanoma as a young adult if there are increased sunburns in childhood [31,39,48]. The melanoma-sunburn link may reflect the contribution of inflammatory mediators to carcinogenesis or perhaps a particular threshold above which the dose of UV must exceed in order to transform melanocytes. Nonetheless, intense blistering sunburns seem to play a role in many cases of melanoma. Unfortunately, over half of all adults in the US suffered from sunburn in 2013 and prevalence of sunburn in the US population today is over 50% in all adults and over 65% in fair-skinned young adults under the age of 30. Furthermore, prevalence of sunburn has not declined despite the variety of lotions, sprays and clothing advertised and available for sun protection [49]. Risk of sunburn is complex and influenced by a variety of factors ranging from geography, cloud cover, climate, societal norms relating to amount of clothing worn, etc. Not surprisingly, incidence of sunburns in children (and indoor tanning use among adolescents) correlates with parental attitudes regarding sun protection. For this reason, educational campaigns targeted at parents’ ideas about UV safety might be particularly useful for prevention and protection against UV-induced skin pathologies [50].
6. The Melanocortin 1 Receptor (MC1R) and the Tanning Response
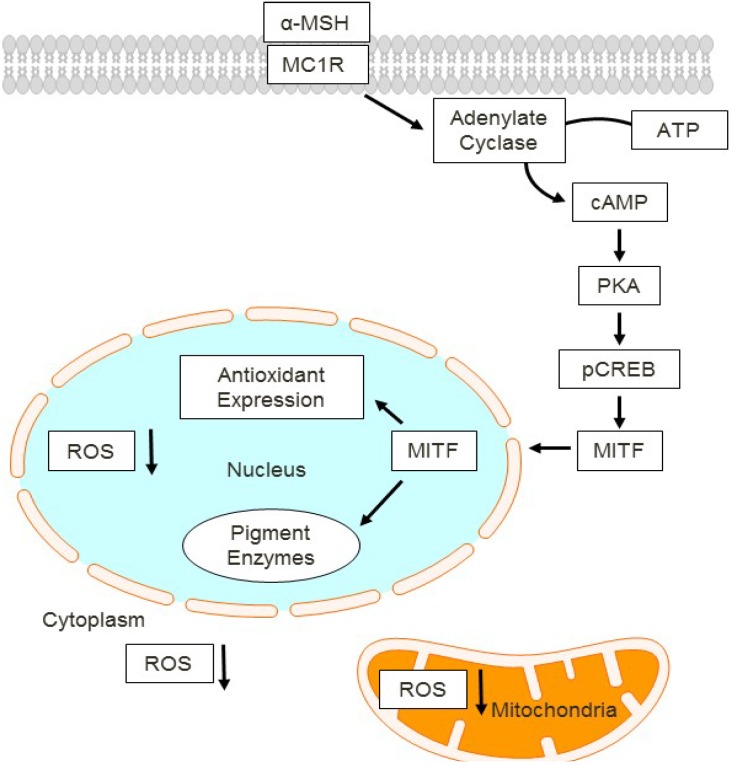
One important aspect of the solar radiation in human’s skin is the adaptive tanning response. After UV exposure, cellular damage response activation induces melanin production by melanocytes and proliferation and melanin deposition in keratinocytes, all of which result in enhanced pigmentation of the skin. This important physiologic pathway is a natural UV-protective response to protect the skin against further UV insult after an initial UV exposure. The ability of the skin to tan depends on the function and activity of the cutaneous melanocortin 1 receptor (MC1R) signaling pathway [51,52,53,54,55] (Figure 2).
Figure 2.
MC1R signaling cascade in melanocytes. Activated by its agonist alpha-MSH, MC1R promotes cAMP second messenger generation which induces melanocyte differentiation and survival pathways involving PKA, CREB and Mitf. In this way, cAMP induces both melanin production and antioxidants that reduce cellular ROS. cAMP, cyclic adenosine monophosphate. PKA, protein kinase A. pCREB, phosphorylated cAMP response binding element. ROS, reactive oxygen species. MITF, microphthalmia (Mitf) transcription factor.
The MC1R is a Gs-couple protein located in the extracellular membranes of epidermal melanocytes. When bound by agonistic ligands, most notably α-melanocyte stimulating hormone (α-MSH) [51], the MC1R initiates a cascade of UV-protective events mediated by activation of adenylyl cyclase and generation of the second messenger cAMP. MC1R activates adenylate cyclase that converts ATP to cAMP which activates protein kinase A (PKA). PKA phosphorylates the cAMP-responsive binding element (CREB) and induces activation of the microphthalmia (MITF) transcription factor. MITF is a myc-like master transcription factor that, in melanocytes, drives expression of tyrosinase and other pigment biosynthetic enzymes. In this way, epidermal melanocytes produce melanin pigment that gets deposited in the epidermis to physically interfere with penetration of UV photons, thereby protecting skin cells from the damaging effects of sunlight [56]. Importantly, MC1R signaling also influences the ability of melanocytes to recover from UV-induced DNA damage [57,58,59,60,61]. Overall, there is much evidence placing MC1R as a “master regulator” of melanocyte UV physiologic responses.
7. Pigmentation Phenotype Depends on MC1R Signaling
Loss-of-function polymorphisms of MC1R lead to a fair-skinned, sun-sensitive, and cancer-prone phenotype [62,63,64]. The major MC1R polymorphisms among human populations are the so-called “red hair colored” (RHC) genotypes that yield a characteristic UV-sensitive and melanoma-prone phenotype, namely propensity for sun burning rather than tanning, fair skin complexion, freckling and red/blonde hair. The RHC phenotype include R151C, R160W and D294H MC1R genotypes [65,66,67]. In these cases, there is blunted cAMP production in melanocytes, and the skin produces less of the highly UV-protective dark brown/black pigment species known as eumelanin. Instead, there is production of a red/blonde pigment known as pheomelanin that is much less effective at blocking incoming UV energy and may even potentiate UV-induced oxidative injury. Without effective cAMP induction in melanocytes, as is the case in MC1R-defective individuals, the skin cannot accumulate significant amounts of eumelanin and therefore will be prone to UV damage and carcinogenesis.
8. Forskolin Rescues cAMP Deficient Signaling
Forskolin is a naturally derived diterpenoid extracted from the roots of the Plectranthus barbatus (Coleus forskolii) plant that grows naturally in Asia and that has long been used in various Aryuvedic teas and therapeutic preparations. Forskolin, which is a skin-permeable compound, directly activates adenylate cyclase to induce production of cAMP. Our laboratory was among the first to show that topical application of forskolin promoted UV-independent production of eumelanin in an MC1R-defective fair-skinned animal model [53], resulting in robust UV protection by interfering with epidermal penetration of UV photons [68]. Pharmacologic stimulation of cAMP using forskolin may protect the skin in ways other than through melanin induction. For example, cAMP provided enhancement of keratinocyte migration to promote wound healing [69] and it also decreased blister formation [70]. De Vries and co-workers proposed using a topical cAMP approach to regulate beta-adrenergic response in psoriasis patients [71]. Interestingly, cAMP stimulation has also been studied as an activator of hair follicle activity and has been considered as a therapy for age-related hair loss [72,73]. We and others have been interested in the UV-protective consequences of topical cAMP induction to promote melanin protection from UV-mediated DNA damage [68] and to enhance levels and/or activity of key DNA repair and antioxidant enzymes [74]. Forskolin and other cAMP-promoting agents may also protect the skin against UVB-induced apoptosis [75] and by promoting epidermal thickening which also aids in resisting UV damage [76]. In particular, Scott et al. reported that cAMP-mediated accumulation of basal and epidermal keratinocytes resulted in a melanin-independent mechanism of blocking UVA and UVB penetration into the skin [76]. Others reported that forskolin protected against generation of oxidative stress by decreasing levels of nitric oxide [77] and enhancing stimulation of the cytoplasmic antioxidant enzyme copper/zinc superoxide dismutase (Cu/ZnSOD) [78]. Taken together, studies suggest that pharmacologic induction of cAMP in the skin may represent a potential UV-protective strategy for MC1R-defective individuals who are fair-skinned, sun-sensitive and melanoma prone.
9. Oxidative Stress and Aging
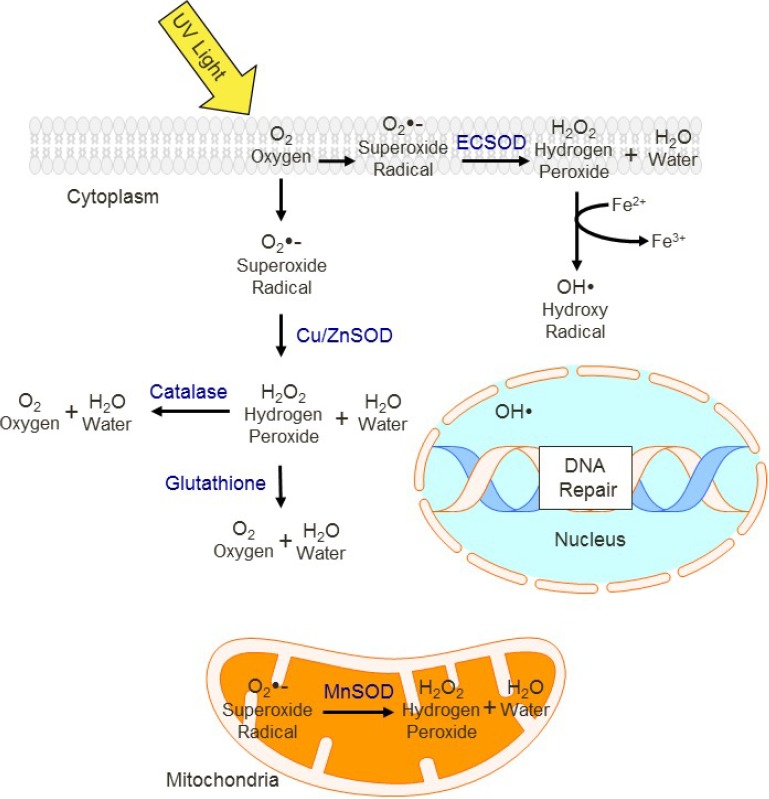
Reactive oxidative species (ROS) are produced by cells during normal metabolic activities such as mitochondrial oxidative phosphorylation, however levels of ROS vary with UV exposure and levels of antioxidant enzymes. Figure 3 shows a simplified scheme of the location of protective antioxidant enzymes in the cell (Figure 3).
Figure 3.
Cellular antioxidant defenses. UV induces a variety of free radical and oxidative molecules, which because of their chemical reactivity alter the molecular structure and damage lipids, proteins and nucleic acids [79]. Antioxidant enzymes mediate the removal of ROS, with different enzymes functioning in specific compartments (e.g., MnSOD localized to mitochondria). If not removed, ROS may react with DNA and other cell signal proteins, impairing their function. ECSOD, Extracellular Superoxide dismutase. Cu/Zn SOD, copper/zinc superoxide dismutase. MnSOD, Manganese Superoxide dismutase.
Without inactivation, ROS damage macromolecules including lipid, proteins and DNA. UV, particularly longer-wavelength UVA, is a well-known inducer of ROS, and UV-induced oxidative stress may be an important contributive factor for melanoma [80,81,82]. ROS can inappropriately activate signaling pathways, interfere with genome maintenance and affect apoptosis. Numerous studies have tested the effects of solar radiation and oxidative stress on the skin [29,83,84,85], and oxidative stress has been linked to age-related loss of skin elasticity [86,87,88], defective cellular signaling [68] and photoaging [89,90]. Because it triggers cellular damage pathways, oxidative stress activates cellular senescence which is thought to directly lead to photoaging [91,92,93,94]. Cellular senescence is associated with a reduced capacity to divide and proliferate, sometimes in conjunction with shortening of telomeres [95,96,97,98]. Yokoo et al. found that exposing cells to a pro-oxidant agent (H2O2) impaired telomerase function which eventually resulted in telomere shortening, decreased proliferation and cellular enlargement [97]. Wrinkling of the skin is one of the most overt signs of photoaging, and UV exposure can induce wrinkling over time [99,100,101,102]. Though the molecular mechanism(s) of wrinkling are likely to be complex, UV exposure may reduce elastic properties of the skin to alter the three-dimensional structure of elastic fibers [103]. Using an animal model, Shin et al. noted an inverse correlation between wrinkling and important antioxidant enzymes that reduce cellular levels of ROS [104]. Thus UV-induced oxidative cutaneous damage may play a major role in photoaging.
Cells have a network of antioxidants and antioxidant enzymes that function to inactivate ROS and limit free radical injury. Because they house the enzymes that mediate the electron transport chain, mitochondria are the main intracellular source of endogenous levels of ROS. Manganese superoxide dismutase (MnSOD), a mitochondrial protein, is a major regulator of ROS in the mitochondria. Glutathione is the most widely expressed antioxidant in the cell, and its levels and oxidation state are regulated by feedback signaling dependent on total ROS level. Increases in ROS lead to use and depletion of glutathione and trigger recruitment of antioxidant enzymes such as catalase and superoxide dismutases (SODs).
Many synthetic and natural products have been reported to enhance levels of antioxidant enzymes, which make them therapeutic candidates to mitigate UV-mediated damage and to prevent the health consequences of UV exposure. Some products include alpha-tocopherol, selenium, phloretin, ferulic acid, flavangenol, lipoic acid, and uric acid [57,105,106,107,108,109,110,111] as well as a variety of flavonoids derived from plants including pomegranate and strawberry [112,113]. Lipid-soluble carotinoids such as lycopene and beta-carotene have been reported to scavenge superoxide radicals [114] and to promote vitamin A activity [115]. However, large doses of UV may inactivate carotenoids in the skin and promote degradation of dermal collagen and elastin [114,116]. Vitamin C is another anti-oxidant compound that has been studied as a UV photoprotective agent [108], particularly in combination with other compounds such as ferulic acid and phloretin [108].
There is emerging evidence implicating MC1R and cAMP signaling in regulating antioxidant proteins. Using keratinocytes transfected with MC1R, Henri et al. reported lower cellular levels of ROS after pharmacologic activation MC1R/cAMP pathway and higher levels of ROS when PKA was pharmacologically inhibited [117]. In other work using human melanocytes, Song and colleagues found that αMSH-induced MC1R signaling increased levels of catalase after UV exposure. Catalase is an antioxidant enzyme that converts excess of hydrogen peroxide molecules to water and molecular oxygen [118]. Finally, Kaderaro and coworkers reported that cAMP stimulation reduced levels of hydrogen peroxide, an important ROS, in human melanocytes after UV exposure [74]. Therefore, there is great interest in exploiting the MC1R UV-protective signaling pathway as a protective mechanism against UV-mediated oxidative injury.
10. Conclusions
UV exposure is one of the most important environmental health hazards, clearly causative for age-related skin changes such as wrinkling, pigmentary changes, thinning and carcinogenesis. Because of complex societal factors, UV exposure may actually be increasing through increased occupational and recreational activities including indoor tanning. As we learn more about innate signaling mechanisms that regulate natural antioxidant defense pathways in the skin such as the MC1R hormonal axis, new approaches are being designed to exploit these signaling pathways to delay or even prevent free-radical induced symptoms of aging. Use of natural extracts such as forskolin derived from the roots of the Plectranthus barbatus (Coleus forskolii) plant may enhance protection against UV-induce molecular damage to the skin. cAMP-induced melanin deposition and antioxidant induction may prove to be an important therapeutic opportunity to reduce UV-mediated pathologies.
Acknowledgments
This work was supported by grants: RO1CA131075 and supplemental RO1CA131075-02S1 from the National Cancer Institute. NIH: UL1TR000117, TL1 TR000115, KL2 TR000116 National Center for Advancing Translational Sciences University of Kentucky. The authors will like to thank Heather Russell-Simmons and the Markey Cancer Center Research Communication Office for assistance with editing and manuscript preparation.
Author Contributions
AA-O, BY and JD each wrote parts of the manuscript, edited the document and approved of the final version.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Elwood J.M., Jopson J. Melanoma and sun exposure: An overview of published studies. Int. J. Cancer. 1997;73:198–203. doi: 10.1002/(SICI)1097-0215(19971009)73:2<198::AID-IJC6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 2.Godar D.E. UV doses worldwide. Photochem. Photobiol. 2005;81:736–749. doi: 10.1562/2004-09-07-IR-308R.1. [DOI] [PubMed] [Google Scholar]
- 3.Wei Q., Lee J.E., Gershenwald J.E., Ross M.I., Mansfield P.F., Strom S.S., Wang L.E., Guo Z., Qiao Y., Amos C.I., et al. Repair of UV light-induced DNA damage and risk of cutaneous malignant melanoma. J. Natl. Cancer Instit. 2003;95:308–315. doi: 10.1093/jnci/95.4.308. [DOI] [PubMed] [Google Scholar]
- 4.Cleaver J.E., Crowley E. UV damage, DNA repair and skin carcinogenesis. Front. Biosci. 2002;7:d1024–d1043. doi: 10.2741/cleaver. [DOI] [PubMed] [Google Scholar]
- 5.Krutmann J., Morita A., Chung J.H. Sun exposure: What molecular photodermatology tells us about its good and bad sides. J. Investig. Dermatol. 2012;132:976–984. doi: 10.1038/jid.2011.394. [DOI] [PubMed] [Google Scholar]
- 6.Sarasin A. The molecular pathways of ultraviolet-induced carcinogenesis. Mutat. Res. 1999;428:5–10. doi: 10.1016/S1383-5742(99)00025-3. [DOI] [PubMed] [Google Scholar]
- 7.Hoeijmakers J.H. DNA damage, aging, and cancer. N. Engl. J. Med. 2009;361:1475–1485. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- 8.Meyskens F.L., Jr., Farmer P., Fruehauf J.P. Redox regulation in human melanocytes and melanoma. Pigment Cell Res. 2001;14:148–154. doi: 10.1034/j.1600-0749.2001.140303.x. [DOI] [PubMed] [Google Scholar]
- 9.Schulz I., Mahler H.C., Boiteux S., Epe B. Oxidative DNA base damage induced by singlet oxygen and photosensitization: Recognition by repair endonucleases and mutagenicity. Mutat. Res. 2000;461:145–156. doi: 10.1016/S0921-8777(00)00049-5. [DOI] [PubMed] [Google Scholar]
- 10.Norval M., Lucas R.M., Cullen A.P., de Gruijl F.R., Longstreth J., Takizawa Y., van der Leun J.C. The human health effects of ozone depletion and interactions with climate change. Photochem. Photobiol. Sci. 2011;10:199–225. doi: 10.1039/c0pp90044c. [DOI] [PubMed] [Google Scholar]
- 11.Rigel D.S., Russak J., Friedman R. The evolution of melanoma diagnosis: 25 years beyond the ABCDs. CA Cancer J. Clin. 2010;60:301–316. doi: 10.3322/caac.20074. [DOI] [PubMed] [Google Scholar]
- 12.Garbe C., Leiter U. Melanoma epidemiology and trends. Clin. Dermatol. 2009;27:3–9. doi: 10.1016/j.clindermatol.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong B.K., Kricker A. The epidemiology of UV induced skin cancer. J. Photochem. Photobiol. B Biol. 2001;63:8–18. doi: 10.1016/s1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- 14.Diffey B.L. The future incidence of cutaneous melanoma within the UK. Br. J. Dermatol. 2004;151:868–872. doi: 10.1111/j.1365-2133.2004.06216.x. [DOI] [PubMed] [Google Scholar]
- 15.Grant W.B. The effect of solar UVB doses and vitamin D production, skin cancer action spectra, and smoking in explaining links between skin cancers and solid tumours. Eur. J. Cancer. 2008;44:12–15. doi: 10.1016/j.ejca.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Paunel A.N., Dejam A., Thelen S., Kirsch M., Horstjann M., Gharini P., Murtz M., Kelm M., de Groot H., Kolb-Bachofen V., et al. Enzyme-independent nitric oxide formation during UVA challenge of human skin: Characterization, molecular sources, and mechanisms. Free Radic. Biol. Med. 2005;38:606–615. doi: 10.1016/j.freeradbiomed.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 17.Venditti E., Bruge F., Astolfi P., Kochevar I., Damiani E. Nitroxides and a nitroxide-based UV filter have the potential to photoprotect UVA-irradiated human skin fibroblasts against oxidative damage. J. Dermatol. Sci. 2011;63:55–61. doi: 10.1016/j.jdermsci.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Akhalaya M.Y., Maksimov G.V., Rubin A.B., Lademann J., Darvin M.E. Molecular action mechanisms of solar infrared radiation and heat on human skin. Ageing Res. Rev. 2014 doi: 10.1016/j.arr.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Yin B., Jiang X. Telomere shortening in cultured human dermal fibroblasts is associated with acute photodamage induced by UVA irradiation. Postepy Dermatol. I Alergol. 2013;30:13–18. doi: 10.5114/pdia.2013.33374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atillasoy E.S., Seykora J.T., Soballe P.W., Elenitsas R., Nesbit M., Elder D.E., Montone K.T., Sauter E., Herlyn M. UVB induces atypical melanocytic lesions and melanoma in human skin. Am. J. Pathol. 1998;152:1179–1186. [PMC free article] [PubMed] [Google Scholar]
- 21.Wendt J., Schanab O., Binder M., Pehamberger H., Okamoto I. Site-dependent actinic skin damage as risk factor for melanoma in a central European population. Pigm. Cell Melanoma Res. 2012;25:234–242. doi: 10.1111/j.1755-148X.2011.00946.x. [DOI] [PubMed] [Google Scholar]
- 22.Flament F., Bazin R., Laquieze S., Rubert V., Simonpietri E., Piot B. Effect of the sun on visible clinical signs of aging in Caucasian skin. Clin. Cosmet. Investig. Dermatol. 2013;6:221–232. doi: 10.2147/CCID.S44686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breger J., Baeva L., Agrawal A., Shindell E., Godar D.E. UVB-induced inflammatory cytokine release, DNA damage and apoptosis of human oral compared with skin tissue equivalents. Photochem. Photobiol. 2013;89:665–670. doi: 10.1111/php.12030. [DOI] [PubMed] [Google Scholar]
- 24.Haluza D., Simic S., Moshammer H. Temporal and spatial melanoma trends in Austria: An ecological study. Int. J. Environ. Res. Public Health. 2014;11:734–748. doi: 10.3390/ijerph110100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aceituno-Madera P., Buendia-Eisman A., Olmo F.J., Jimenez-Moleon J.J., Serrano-Ortega S. Melanoma, altitude, and UV-B radiation. Actas Dermo-Sifiliograficas. 2011;102:199–205. doi: 10.1016/j.ad.2010.08.003. (In Spanish) [DOI] [PubMed] [Google Scholar]
- 26.Hammar N., Linnersjo A., Alfredsson L., Dammstrom B.G., Johansson M., Eliasch H. Cancer incidence in airline and military pilots in Sweden 1961–1996. Aviat. Space Environ. Med. 2002;73:2–7. [PubMed] [Google Scholar]
- 27.Lichte V., Dennenmoser B., Dietz K., Hafner H.M., Schlagenhauff B., Garbe C., Fischer J., Moehrle M. Professional risk for skin cancer development in male mountain guides–A cross-sectional study. J. Eur. Acad. Dermatol. Venereol. 2010;24:797–804. doi: 10.1111/j.1468-3083.2009.03528.x. [DOI] [PubMed] [Google Scholar]
- 28.Eide M.J., Weinstock M.A. Association of UV index, latitude, and melanoma incidence in nonwhite populations–US Surveillance, Epidemiology, and End Results (SEER) Program, 1992 to 2001. Arch. Dermatol. 2005;141:477–481. doi: 10.1001/archderm.141.4.477. [DOI] [PubMed] [Google Scholar]
- 29.Hu S., Ma F., Collado-Mesa F., Kirsner R.S. UV radiation, latitude, and melanoma in US Hispanics and blacks. Arch. Dermatol. 2004;140:819–824. doi: 10.1001/archderm.140.7.819. [DOI] [PubMed] [Google Scholar]
- 30.Cicarma E., Juzeniene A., Porojnicu A.C., Bruland O.S., Moan J. Latitude gradient for melanoma incidence by anatomic site and gender in Norway 1966–2007. J. Photochem. Photobiol. B Biol. 2010;101:174–178. doi: 10.1016/j.jphotobiol.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Chang Y.M., Barrett J.H., Bishop D.T., Armstrong B.K., Bataille V., Bergman W., Berwick M., Bracci P.M., Elwood J.M., Ernstoff M.S., et al. Sun exposure and melanoma risk at different latitudes: A pooled analysis of 5700 cases and 7216 controls. Int. J. Epidemiol. 2009;38:814–830. doi: 10.1093/ije/dyp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qureshi A.A., Laden F., Colditz G.A., Hunter D.J. Geographic variation and risk of skin cancer in US women. Differences between melanoma, squamous cell carcinoma, and basal cell carcinoma. Arch. Intern. Med. 2008;168:501–507. doi: 10.1001/archinte.168.5.501. [DOI] [PubMed] [Google Scholar]
- 33.Armstrong B.K. Epidemiology of malignant melanoma: Intermittent or total accumulated exposure to the sun? J. Dermatol. Surg. Oncol. 1988;14:835–849. doi: 10.1111/j.1524-4725.1988.tb03588.x. [DOI] [PubMed] [Google Scholar]
- 34.Sharp L., Donnelly D., Hegarty A., Carsin A.E., Deady S., McCluskey N., Gavin A., Comber H. Risk of several cancers is higher in urban areas after adjusting for socioeconomic status. results from a two-country population-based study of 18 common cancers. J. Urban Health. 2014 doi: 10.1007/s11524-013-9846-3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brouse C.H., Hillyer G.C., Basch C.E., Neugut A.I. Geography, facilities, and promotional strategies used to encourage indoor tanning in New York City. J. Commun. Health. 2011;36:635–639. doi: 10.1007/s10900-010-9354-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garbe C., Orfanos C.E. Epidemiology of malignant melanoma in central Europe: Risk factors and prognostic predictors. Results of the Central Malignant Melanoma Registry of the German Dermatological Society. Pigm. Cell Res. 1992;2:285–294. doi: 10.1016/B978-0-444-89547-9.50033-3. [DOI] [PubMed] [Google Scholar]
- 37.Bishop J.N., Bataille V., Gavin A., Lens M., Marsden J., Mathews T., Wheelhouse C. The prevention, diagnosis, referral and management of melanoma of the skin: Concise guidelines. Clin. Med. 2007;7:283–290. doi: 10.7861/clinmedicine.7-3-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volkmer B., Greinert R. UV and children’s skin. Progr. Biophys. Mol. Biol. 2011;107:386–388. doi: 10.1016/j.pbiomolbio.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Cust A.E., Jenkins M.A., Goumas C., Armstrong B.K., Schmid H., Aitken J.F., Giles G.G., Kefford R.F., Hopper J.L., Mann G.J. Early-life sun exposure and risk of melanoma before age 40 years. Cancer Causes Control. 2011;22:885–897. doi: 10.1007/s10552-011-9762-3. [DOI] [PubMed] [Google Scholar]
- 40.Crump C., Sundquist K., Sieh W., Winkleby M.A., Sundquist J. Season of birth and other perinatal risk factors for melanoma. Int. J. Epidemiol. 2014 doi: 10.1093/ije/dyt277. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Purdue M.P., Freeman L.E., Anderson W.F., Tucker M.A. Recent trends in incidence of cutaneous melanoma among US Caucasian young adults. J. Investig. Dermatol. 2008;128:2905–2908. doi: 10.1038/jid.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wehner M.R., Chren M.M., Nameth D., Choudhry A., Gaskins M., Nead K.T., Boscardin W.J., Linos E. International prevalence of indoor tanning: A systematic review and meta-analysis. JAMA Dermatol. 2014 doi: 10.1001/jamadermatol.2013.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heckman C.J., Cohen-Filipic J., Darlow S., Kloss J.D., Manne S.L., Munshi T. Psychiatric and addictive symptoms of young adult female indoor tanners. Am. J. Health Promot. 2014;28:168–174. doi: 10.4278/ajhp.120912-QUAN-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dore J.F., Chignol M.C. Tanning salons and skin cancer. Photochem. Photobiol. Sci. 2012;11:30–37. doi: 10.1039/c1pp05186e. [DOI] [PubMed] [Google Scholar]
- 45.Petit A., Karila L., Chalmin F., Lejoyeux M. Phenomenology and psychopathology of excessive indoor tanning. Int. J. Dermatol. 2014 doi: 10.1111/ijd.12336. [DOI] [PubMed] [Google Scholar]
- 46.Petit A., Lejoyeux M., Reynaud M., Karila L. Excessive indoor tanning as a behavioral addiction: A literature review. Curr. Pharmaceut. Des. 2013 doi: 10.2174/13816128113199990615. in press. [DOI] [PubMed] [Google Scholar]
- 47.Lazovich D., Vogel R.I., Berwick M., Weinstock M.A., Anderson K.E., Warshaw E.M. Indoor tanning and risk of melanoma: A case-control study in a highly exposed population. Cancer Epidemiol. Biomark. Prev. 2010;19:1557–1568. doi: 10.1158/1055-9965.EPI-09-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pfahlberg A., Kolmel K.F., Gefeller O., Febim Study G. Timing of excessive ultraviolet radiation and melanoma: Epidemiology does not support the existence of a critical period of high susceptibility to solar ultraviolet radiation-induced melanoma. Br. J. Dermatol. 2001;144:471–475. doi: 10.1046/j.1365-2133.2001.04070.x. [DOI] [PubMed] [Google Scholar]
- 49.Centers for Disease C. Prevention, sunburn and sun protective behaviors among adults aged 18–29 years–United States, 2000–2010. MMWR. Morbid. Mortal. Wkl. Rep. 2012;61:317–322. [PubMed] [Google Scholar]
- 50.Behrens C.L., Thorgaard C., Philip A., Bentzen J. Sunburn in children and adolescents: Associations with parents’ behaviour and attitudes. Scand. J. Public Health. 2013;41:302–310. doi: 10.1177/1403494813476158. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki I., Cone R.D., Im S., Nordlund J., Abdel-Malek Z.A. Binding of melanotropic hormones to the melanocortin receptor MC1R on human melanocytes stimulates proliferation and melanogenesis. Endocrinology. 1996;137:1627–1633. doi: 10.1210/endo.137.5.8612494. [DOI] [PubMed] [Google Scholar]
- 52.Spry M.L., Vanover J.C., Scott T., Abona-Ama O., Wakamatsu K., Ito S., D’Orazio J.A. Prolonged treatment of fair-skinned mice with topical forskolin causes persistent tanning and UV protection. Pigm. Cell Melanoma Res. 2009;22:219–229. doi: 10.1111/j.1755-148X.2008.00536.x. [DOI] [PubMed] [Google Scholar]
- 53.D’Orazio J.A., Nobuhisa T., Cui R., Arya M., Spry M., Wakamatsu K., Igras V., Kunisada T., Granter S.R., Nishimura E.K., et al. Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning. Nature. 2006;443:340–344. doi: 10.1038/nature05098. [DOI] [PubMed] [Google Scholar]
- 54.Cui R., Widlund H.R., Feige E., Lin J.Y., Wilensky D.L., Igras V.E., D’Orazio J., Fung C.Y., Schanbacher C.F., Granter S.R., et al. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128:853–864. doi: 10.1016/j.cell.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 55.Amaro-Ortiz A., Vanover J.C., Scott T.L., D’Orazio J.A. Pharmacologic induction of epidermal melanin and protection against sunburn in a humanized mouse model. J. Vis. Exp. 2013 doi: 10.3791/50670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tolleson W.H. Human melanocyte biology, toxicology, and pathology. J. Environ. Sci. Health Part C Environ. Carcinogen. Ecotoxicol. Rev. 2005;23:105–161. doi: 10.1080/10590500500234970. [DOI] [PubMed] [Google Scholar]
- 57.Scott M.C., Wakamatsu K., Ito S., Kadekaro A.L., Kobayashi N., Groden J., Kavanagh R., Takakuwa T., Virador V., Hearing V.J., et al. Human melanocortin 1 receptor variants, receptor function and melanocyte response to UV radiation. J. Cell Sci. 2002;115:2349–2355. doi: 10.1242/jcs.115.11.2349. [DOI] [PubMed] [Google Scholar]
- 58.Kadekaro A.L., Kanto H., Kavanagh R., Abdel-Malek Z. Significance of the melanocortin 1 receptor in regulating human melanocyte pigmentation, proliferation, and survival. Ann. N. Y. Acad. Sci. 2003;994:359–365. doi: 10.1111/j.1749-6632.2003.tb03200.x. [DOI] [PubMed] [Google Scholar]
- 59.Rouzaud F., Kadekaro A.L., Abdel-Malek Z.A., Hearing V.J. MC1R and the response of melanocytes to ultraviolet radiation. Mutat. Res. 2005;571:133–152. doi: 10.1016/j.mrfmmm.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 60.Bohm M., Wolff I., Scholzen T.E., Robinson S.J., Healy E., Luger T.A., Schwarz T., Schwarz A. alpha-Melanocyte-stimulating hormone protects from ultraviolet radiation-induced apoptosis and DNA damage. J. Biol. Chem. 2005;280:5795–5802. doi: 10.1074/jbc.M406334200. [DOI] [PubMed] [Google Scholar]
- 61.Hauser J.E., Kadekaro A.L., Kavanagh R.J., Wakamatsu K., Terzieva S., Schwemberger S., Babcock G., Rao M.B., Ito S., Abdel-Malek Z.A. Melanin content and MC1R function independently affect UVR-induced DNA damage in cultured human melanocytes. Pigm. Cell Res. 2006;19:303–314. doi: 10.1111/j.1600-0749.2006.00315.x. [DOI] [PubMed] [Google Scholar]
- 62.Abdel-Malek Z., Suzuki I., Tada A., Im S., Akcali C. The melanocortin-1 receptor and human pigmentation. Ann. N. Y. Acad. Sci. 1999;885:117–133. doi: 10.1111/j.1749-6632.1999.tb08669.x. [DOI] [PubMed] [Google Scholar]
- 63.Abdel-Malek Z., Scott M.C., Suzuki I., Tada A., Im S., Lamoreux L., Ito S., Barsh G., Hearing V.J. The melanocortin-1 receptor is a key regulator of human cutaneous pigmentation. Pigm. Cell Res. 2000;13:156–162. doi: 10.1034/j.1600-0749.13.s8.28.x. [DOI] [PubMed] [Google Scholar]
- 64.Abdel-Malek Z.A., Knittel J., Kadekaro A.L., Swope V.B., Starner R. The melanocortin 1 receptor and the UV response of human melanocytes–A shift in paradigm. Photochem. Photobiol. 2008;84:501–508. doi: 10.1111/j.1751-1097.2008.00294.x. [DOI] [PubMed] [Google Scholar]
- 65.Bastiaens M.T., ter Huurne J.A., Kielich C., Gruis N.A., Westendorp R.G., Vermeer B.J., Bavinck J.N., Leiden Skin Cancer Study T. Melanocortin-1 receptor gene variants determine the risk of nonmelanoma skin cancer independently of fair skin and red hair. Am. J. Hum. Genet. 2001;68:884–894. doi: 10.1086/319500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gruis N.A., van Doorn R. Melanocortin 1 receptor function: Shifting gears from determining skin and nevus phenotype to fetal growth. J. Investig. Dermatol. 2012;132:1953–1955. doi: 10.1038/jid.2012.216. [DOI] [PubMed] [Google Scholar]
- 67.Quint K.D., van der Rhee J.I., Gruis N.A., Ter Huurne J.A., Wolterbeek R., van der Stoep N., Bergman W., Kukutsch N.A. Melanocortin 1 receptor (MC1R) variants in high melanoma risk patients are associated with specific dermoscopic ABCD features. Acta Dermato-Venereol. 2012;92:587–592. doi: 10.2340/00015555-1457. [DOI] [PubMed] [Google Scholar]
- 68.Prunier C., Masson-Genteuil G., Ugolin N., Sarrazy F., Sauvaigo S. Aging and photo-aging DNA repair phenotype of skin cells-evidence toward an effect of chronic sun-exposure. Mutat. Res. 2012;736:48–55. doi: 10.1016/j.mrfmmm.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 69.Pullar C.E., Isseroff R.R. Cyclic AMP mediates keratinocyte directional migration in an electric field. J. Cell Sci. 2005;118:2023–2034. doi: 10.1242/jcs.02330. [DOI] [PubMed] [Google Scholar]
- 70.Spindler V., Vielmuth F., Schmidt E., Rubenstein D.S., Waschke J. Protective endogenous cyclic adenosine 5'-monophosphate signaling triggered by pemphigus autoantibodies. J. Immunol. 2010;185:6831–6838. doi: 10.4049/jimmunol.1002675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Vries G.W., Amdahl L.D., Lowe N., Wheeler L.A. Effect of forskolin on beta-adrenergic hyporesponsiveness in skin. Skin Pharmacol. 1988;1:106–114. doi: 10.1159/000210756. [DOI] [PubMed] [Google Scholar]
- 72.Harmon C.S., Nevins T.D. Evidence that activation of protein kinase A inhibits human hair follicle growth and hair fibre production in organ culture and DNA synthesis in human and mouse hair follicle organ culture. Br. J. Dermatol. 1997;136:853–858. doi: 10.1111/j.1365-2133.1997.tb03924.x. [DOI] [PubMed] [Google Scholar]
- 73.Michelet J.F., Gautier B., Gaillard O., Bernard B.A., Benech F. Human hair follicle pigmentary unit as a direct target for modulators of melanogenesis, as studied by [14C]-2-thiouracil incorporation. Exp. Dermatol. 2009;18:414–416. doi: 10.1111/j.1600-0625.2008.00804.x. [DOI] [PubMed] [Google Scholar]
- 74.Kadekaro A.L., Chen J., Yang J., Chen S., Jameson J., Swope V.B., Cheng T., Kadakia M., Abdel-Malek Z. Alpha-melanocyte-stimulating hormone suppresses oxidative stress through a p53-mediated signaling pathway in human melanocytes. Mol. Cancer Res. 2012;10:778–786. doi: 10.1158/1541-7786.MCR-11-0436. [DOI] [PubMed] [Google Scholar]
- 75.Passeron T., Namiki T., Passeron H.J., le Pape E., Hearing V.J. Forskolin protects keratinocytes from UVB-induced apoptosis and increases DNA repair independent of its effects on melanogenesis. J. Investig. Dermatol. 2009;129:162–166. doi: 10.1038/jid.2008.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scott T.L., Christian P.A., Kesler M.V., Donohue K.M., Shelton B., Wakamatsu K., Ito S., D'Orazio J. Pigment-independent cAMP-mediated epidermal thickening protects against cutaneous UV injury by keratinocyte proliferation. Exp. Dermatol. 2012;21:771–777. doi: 10.1111/exd.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Al-Ayadhi L.Y., Korish A.A., Al-Tuwaijri A.S. The effect of vitamin E, L-arginine, N-nitro L-arginine methyl ester and forskolin on endocrine and metabolic changes of rats exposed to acute cold stress. Saudi Med. J. 2006;27:17–22. [PubMed] [Google Scholar]
- 78.Mishima K., Baba A., Matsuo M., Itoh Y., Oishi R. Protective effect of cyclic AMP against cisplatin-induced nephrotoxicity. Free Radic. Biol. Med. 2006;40:1564–1577. doi: 10.1016/j.freeradbiomed.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 79.Zastrow L., Groth N., Klein F., Kockott D., Lademann J., Renneberg R., Ferrero L. The missing link--light-induced (280–1600 nm) free radical formation in human skin. Skin Pharmacol. Physiol. 2009;22:31–44. doi: 10.1159/000188083. [DOI] [PubMed] [Google Scholar]
- 80.Afanas’ev I.B. Signaling by reactive oxygen and nitrogen species in skin diseases. Curr. Drug MeTable. 2010;11:409–414. doi: 10.2174/138920010791526060. [DOI] [PubMed] [Google Scholar]
- 81.Bossi O., Gartsbein M., Leitges M., Kuroki T., Grossman S., Tennenbaum T. UV irradiation increases ROS production via PKCdelta signaling in primary murine fibroblasts. J. Cell. Biochem. 2008;105:194–207. doi: 10.1002/jcb.21817. [DOI] [PubMed] [Google Scholar]
- 82.Choi Y.J., Uehara Y., Park J.Y., Chung K.W., Ha Y.M., Kim J.M., Song Y.M., Chun P., Park J.W., Moon H.R., et al. Suppression of melanogenesis by a newly synthesized compound, MHY966 via the nitric oxide/protein kinase G signaling pathway in murine skin. J. Dermatol. Sci. 2012;68:164–171. doi: 10.1016/j.jdermsci.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 83.Gabe Y., Osanai O., Takema Y. The relationship between skin aging and steady state ultraweak photon emission as an indicator of skin oxidative stress in vivo. Skin Res. Technol. 2013 doi: 10.1111/srt.12121. [DOI] [PubMed] [Google Scholar]
- 84.Lan C.C., Wu C.S., Yu H.S. Solar-simulated radiation and heat treatment induced metalloproteinase-1 expression in cultured dermal fibroblasts via distinct pathways: Implications on reduction of sun-associated aging. J. Dermatol. Sci. 2013;72:290–295. doi: 10.1016/j.jdermsci.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 85.Poljsak B., Dahmane R. Free radicals and extrinsic skin aging. Dermatol. Res. Pract. 2012;2012:135206:1–135206:4. doi: 10.1155/2012/135206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Langton A.K., Sherratt M.J., Griffiths C.E., Watson R.E. A new wrinkle on old skin: The role of elastic fibres in skin ageing. Int. J. Cosmet. Sci. 2010 doi: 10.1111/j.1468-2494.2010.00574. [DOI] [PubMed] [Google Scholar]
- 87.Naylor E.C., Watson R.E., Sherratt M.J. Molecular aspects of skin ageing. Maturitas. 2011;69:249–256. doi: 10.1016/j.maturitas.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 88.Mahmood T., Akhtar N., Khan B.A., Shoaib Khan H.M., Saeed T. Changes in skin mechanicalproperties after long-term application of cream containing green tea extract. Aging Clin. Exp. Res. 2011;23:333–336. doi: 10.1007/BF03325232. [DOI] [PubMed] [Google Scholar]
- 89.Lee C.W., Park N.H., Kim J.W., Um B.H., Shpatov A.V., Shults E.E., Sorokina I.V., Popov S.A. Study of skin anti-ageing and anti-inflammatory effects of dihydroquercetin, natural triterpenoids, and their synthetic derivatives. Bioorg. Khim. 2012;38:374–381. doi: 10.1134/s1068162012030028. [DOI] [PubMed] [Google Scholar]
- 90.Stohs S.J. The role of free radicals in toxicity and disease. J. Basic Clin. Physiol. Pharmacol. 1995;6:205–228. doi: 10.1515/JBCPP.1995.6.3-4.205. [DOI] [PubMed] [Google Scholar]
- 91.Liu L., Xie H., Chen X., Shi W., Xiao X., Lei D., Li J. Differential response of normal human epidermal keratinocytes and HaCaT cells to hydrogen peroxide-induced oxidative stress. Clin. Exp. Dermatol. 2012;37:772–780. doi: 10.1111/j.1365-2230.2011.04315.x. [DOI] [PubMed] [Google Scholar]
- 92.Yun J.S., Pahk J.W., Lee J.S., Shin W.C., Lee S.Y., Hong E.K. Inonotus obliquus protects against oxidative stress-induced apoptosis and premature senescence. Mol. Cells. 2011;31:423–429. doi: 10.1007/s10059-011-0256-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Velarde M.C., Flynn J.M., Day N.U., Melov S., Campisi J. Mitochondrial oxidative stress caused by Sod2 deficiency promotes cellular senescence and aging phenotypes in the skin. Aging. 2012;4:3–12. doi: 10.18632/aging.100423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sakura M., Chiba Y., Kamiya E., Furukawa A., Kawamura N., Niwa M., Takeuchi M., Hosokawa M. Spontaneous occurrence of photoageing-like phenotypes in the dorsal skin of old SAMP1 mice, an oxidative stress model. Exp. Dermatol. 2013;22:62–64. doi: 10.1111/exd.12059. [DOI] [PubMed] [Google Scholar]
- 95.Makrantonaki E., Zouboulis C.C., William J. Cunliffe Scientific Awards. Characteristics and pathomechanisms of endogenously aged skin. Dermatology. 2007;214:352–360. doi: 10.1159/000100890. [DOI] [PubMed] [Google Scholar]
- 96.Davis T., Wyllie F.S., Rokicki M.J., Bagley M.C., Kipling D. The role of cellular senescence in Werner syndrome: Toward therapeutic intervention in human premature aging. Ann. N. Y. Acad. Sci. 2007;1100:455–469. doi: 10.1196/annals.1395.051. [DOI] [PubMed] [Google Scholar]
- 97.Yokoo S., Furumoto K., Hiyama E., Miwa N. Slow-down of age-dependent telomere shortening is executed in human skin keratinocytes by hormesis-like-effects of trace hydrogen peroxide or by anti-oxidative effects of pro-vitamin C in common concurrently with reduction of intracellular oxidative stress. J. Cell. Biochem. 2004;93:588–597. doi: 10.1002/jcb.20208. [DOI] [PubMed] [Google Scholar]
- 98.Kashino G., Kodama S., Nakayama Y., Suzuki K., Fukase K., Goto M., Watanabe M. Relief of oxidative stress by ascorbic acid delays cellular senescence of normal human and Werner syndrome fibroblast cells. Free Radic. Biol. Med. 2003;35:438–443. doi: 10.1016/S0891-5849(03)00326-5. [DOI] [PubMed] [Google Scholar]
- 99.Kong S.Z., Shi X.G., Feng X.X., Li W.J., Liu W.H., Chen Z.W., Xie J.H., Lai X.P., Zhang S.X., Zhang X.J., et al. Inhibitory effect of hydroxysafflor yellow a on mouse skin photoaging induced by ultraviolet irradiation. Rejuven. Res. 2013;16:404–143. doi: 10.1089/rej.2013.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Felippi C.C., Oliveira D., Stroher A., Carvalho A.R., van Etten E.A., Bruschi M., Raffin R.P. Safety and efficacy of antioxidants-loaded nanoparticles for an anti-aging application. J. Biomed. Nanotechnol. 2012;8:316–321. doi: 10.1166/jbn.2012.1379. [DOI] [PubMed] [Google Scholar]
- 101.Cornacchione S., Sadick N.S., Neveu M., Talbourdet S., Lazou K., Viron C., Renimel I., de Queral D., Kurfurst R., Schnebert S., et al. In vivo skin antioxidant effect of a new combination based on a specific Vitis vinifera shoot extract and a biotechnological extract. J. Drugs Dermatol. 2007;6:s8–s13. [PubMed] [Google Scholar]
- 102.Raschke T., Koop U., Dusing H.J., Filbry A., Sauermann K., Jaspers S., Wenck H., Wittern K.P. Topical activity of ascorbic acid: From in vitro optimization to in vivo efficacy. Skin Pharmacol. Physiol. 2004;17:200–206. doi: 10.1159/000078824. [DOI] [PubMed] [Google Scholar]
- 103.Imokawa G. Mechanism of UVB-induced wrinkling of the skin: Paracrine cytokine linkage between keratinocytes and fibroblasts leading to the stimulation of elastase. J. Investig. Dermatol. Symp. Proc. 2009;14:36–43. doi: 10.1038/jidsymp.2009.11. [DOI] [PubMed] [Google Scholar]
- 104.Shin M.H., Seo J.E., Kim Y.K., Kim K.H., Chung J.H. Chronic heat treatment causes skin wrinkle formation and oxidative damage in hairless mice. Mechan. Ageing Dev. 2012;133:92–98. doi: 10.1016/j.mad.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 105.Gonzalez S., Pathak M.A., Cuevas J., Villarrubia V.G., Fitzpatrick T.B. Topical or oral administration with an extract of Polypodium leucotomos prevents acute sunburn and psoralen-induced phototoxic reactions as well as depletion of Langerhans cells in human skin. Photodermatol. Photoimmunol. Photomed. 1997;13:50–60. doi: 10.1111/j.1600-0781.1997.tb00108.x. [DOI] [PubMed] [Google Scholar]
- 106.Kalka K., Mukhtar H., Turowski-Wanke A., Merk H. Biomelanin antioxidants in cosmetics: Assessment based on inhibition of lipid peroxidation. Skin Pharmacol. Appl. Skin Physiol. 2000;13:143–149. doi: 10.1159/000029919. [DOI] [PubMed] [Google Scholar]
- 107.Cesarini J.P., Michel L., Maurette J.M., Adhoute H., Bejot M. Immediate effects of UV radiation on the skin: Modification by an antioxidant complex containing carotenoids. Photodermatol. Photoimmunol. Photomed. 2003;19:182–189. doi: 10.1034/j.1600-0781.2003.00044.x. [DOI] [PubMed] [Google Scholar]
- 108.Oresajo C., Stephens T., Hino P.D., Law R.M., Yatskayer M., Foltis P., Pillai S., Pinnell S.R. Protective effects of a topical antioxidant mixture containing vitamin C, ferulic acid, and phloretin against ultraviolet-induced photodamage in human skin. J. Cosmet. Dermatol. 2008;7:290–297. doi: 10.1111/j.1473-2165.2008.00408.x. [DOI] [PubMed] [Google Scholar]
- 109.Kimura Y., Sumiyoshi M. French maritime pine bark (Pinus maritima Lam.) extract (Flavangenol) prevents chronic UVB radiation-induced skin damage and carcinogenesis in melanin-possessing hairless mice. Photochem. Photobiol. 2010;86:955–963. doi: 10.1111/j.1751-1097.2010.00751.x. [DOI] [PubMed] [Google Scholar]
- 110.Veratti E., Rossi T., Giudice S., Benassi L., Bertazzoni G., Morini D., Azzoni P., Bruni E., Giannetti A., Magnoni C. 18beta-glycyrrhetinic acid and glabridin prevent oxidative DNA fragmentation in UVB-irradiated human keratinocyte cultures. Anticancer Res. 2011;31:2209–2215. [PubMed] [Google Scholar]
- 111.Valacchi G., Pecorelli A., Mencarelli M., Maioli E., Davis P.A. Beta-carotene prevents ozone-induced proinflammatory markers in murine skin. Toxicol. Ind. Health. 2009;25:241–247. doi: 10.1177/0748233709103030. [DOI] [PubMed] [Google Scholar]
- 112.Afaq F., Zaid M.A., Khan N., Dreher M., Mukhtar H. Protective effect of pomegranate-derivedproducts on UVB-mediated damage in human reconstituted skin. Exp. Dermatol. 2009;18:553–561. doi: 10.1111/j.1600-0625.2008.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Giampieri F., Alvarez-Suarez J.M., Tulipani S., Gonzales-Paramas A.M., Santos-Buelga C., Bompadre S., Quiles J.L., Mezzetti B., Battino M. Photoprotective potential of strawberry (Fragaria x ananassa) extract against UV-A irradiation damage on human fibroblasts. J. Agric. Food Chem. 2012;60:2322–2327. doi: 10.1021/jf205065x. [DOI] [PubMed] [Google Scholar]
- 114.Lademann J., Schanzer S., Meinke M., Sterry W., Darvin M.E. Interaction between carotenoids and free radicals in human skin. Skin Pharmacol. Physiol. 2011;24:238–244. doi: 10.1159/000326074. [DOI] [PubMed] [Google Scholar]
- 115.Haugen L., Bjornson T. Beta Carotene: Dietary Sources, Cancer and Cognition. Nova Biomedical Books; New York, NY, USA: 2009. p. 358. [Google Scholar]
- 116.Darvin M.E., Richter H., Ahlberg S., Haag S.F., Meinke M.C., le Quintrec D., Doucet O., Lademann J. Influence of sun exposure on the cutaneous collagen/elastin fibers and carotenoids: Negative effects can be reduced by application of sunscreen. J. Biophoton. 2014 doi: 10.1002/jbio.201300171. [DOI] [PubMed] [Google Scholar]
- 117.Henri P., Beaumel S., Guezennec A., Poumes C., Stoebner P.E., Stasia M.J., Guesnet J., Martinez J., Meunier L. MC1R expression in HaCaT keratinocytes inhibits UVA-induced ROS production via NADPH oxidase- and cAMP-dependent mechanisms. J. Cell. Physiol. 2012;227:2578–2585. doi: 10.1002/jcp.22996. [DOI] [PubMed] [Google Scholar]
- 118.Song X., Mosby N., Yang J., Xu A., Abdel-Malek Z., Kadekaro A.L. Alpha-MSH activates immediate defense responses to UV-induced oxidative stress in human melanocytes. Pigm. Cell Melanoma Res. 2009;22:809–818. doi: 10.1111/j.1755-148X.2009.00615.x. [DOI] [PubMed] [Google Scholar]