Abstract
Treatment of motor symptoms of degenerative cerebellar ataxia remains difficult. Yet there are recent developments that are likely to lead to significant improvements in the future. Most desirable would be a causative treatment of the underlying cerebellar disease. This is currently available only for a very small subset of cerebellar ataxias with known metabolic dysfunction. However, increasing knowledge of the pathophysiology of hereditary ataxia should lead to an increasing number of medically sensible drug trials. In this paper, data from recent drug trials in patients with recessive and dominant cerebellar ataxias will be summarized. There is consensus that up to date, no medication has been proven effective. Aminopyridines and acetazolamide are the only exception, which are beneficial in patients with episodic ataxia type 2. Aminopyridines are also effective in a subset of patients presenting with downbeat nystagmus. As such, all authors agreed that the mainstays of treatment of degenerative cerebellar ataxia are currently physiotherapy, occupational therapy, and speech therapy. For many years, well-controlled rehabilitation studies in patients with cerebellar ataxia were lacking. Data of recently published studies show that coordinative training improves motor function in both adult and juvenile patients with cerebellar degeneration. Given the well-known contribution of the cerebellum to motor learning, possible mechanisms underlying improvement will be outlined. There is consensus that evidence-based guidelines for the physiotherapy of degenerative cerebellar ataxia need to be developed. Future developments in physiotherapeutical interventions will be discussed including application of non-invasive brain stimulation.
Keywords: Cerebellum, Cerebellar ataxia, Motor rehabilitation, Physiotherapy, Drug therapy
Introduction
Treatment of cerebellar ataxia remains a major challenge. In recent years, modest but important progress has been made in the management of patients with cerebellar ataxias. The focus of this consensus paper will be on treatment of patients with slowly progressive cerebellar degeneration.
Results of recent drug trials will be discussed first. Currently, researchers are attempting to identify medications that can provide symptomatic treatment of ataxia, as well as treatment of the cause of the underlying disease. Usage of aminopyridines as a symptomatic treatment will be described in greatest detail. Currently, aminopyridines (and acetazolamide) are the only medication leading to reliable improvements in specific cerebellar symptoms (episodic ataxia type 2, downbeat nystagmus [1, 2]). We also focus on drug trials in Friedreich's ataxia (FRDA). This is a common form of autosomal recessive hereditary ataxia, with a major component being sensory loss. A comparatively large number of drug trials have been performed on FRDA, driven by increasing knowledge about the underlying pathogenesis. Despite promising options, however, as yet there has been no major breakthrough in drug treatment of FRDA. Finally, drug trials will be summarized which have been performed mainly in autosomal dominant ataxias. Success is very limited and findings need to be confirmed in future studies.
Because of the general lack of medication, the mainstays of therapy remain physiotherapy, occupational therapy, and speech therapy. As such, the focus of the second part of this paper will be on physiotherapy and possible ways to enhance motor performance and learning. Studies of patients with cerebellar ataxias are at a very early stage of development when compared to the more advanced work on people with hemiparesis due to stroke ([3–5] for recent reviews). Studies will be presented from both adult and juvenile patients with cerebellar degeneration which for the first time convincingly show that motor training is beneficial in degenerative cerebellar ataxia, despite the known contributions of the cerebellum to motor learning [6, 7]. We think that a better understanding of the underlying pathomechanisms of disordered motor performance and learning offers future opportunity to tailor physiotherapy to the specific needs of patients with cerebellar ataxia. Open questions will be addressed which need to be answered to develop future guidelines for an evidence-based physiotherapy of cerebellar ataxia.
Drug Therapy for Cerebellar Ataxia
Aminopyridines for the Treatment of Cerebellar Disorders (J. Teufel, J. Claaßen, K. Feil, R. Kalla, M. Strupp)
Aminopyridines [3,4-diaminopyridine (3,4-DAP), 4-aminopyridine (4-AP) ]are an effective symptomatic treatment for episodic ataxia type 2(EA2) and a subset of patients with downbeat nystagmus (DBN). They may also benefit gait ataxia. Findings are summarized in Table 1.
Table 1. Trials and case series with aminopyridines in cerebellar disorders.
| Study | Study population | Drug | Daily dose | Trial design | Study period | No. of patients | Outcome |
|---|---|---|---|---|---|---|---|
| Struppetal. [11] | EA 2 | 4-AP | 5 mg rid | Case series | – | 3 | Prevention of attacks |
| Alleviation of ataxic symptoms | |||||||
| Strupp et al. [2] | EA 2 | 4-AP | 5 mg rid | Placebo-controlled | 8–12 weeks | 10 | Reduction of frequency and duration of attacks |
| Improvement in quality of life (VDADL score) | |||||||
| Claassen et al. [14] | EA 2 | 4-AP-SR | 10 mgbid | Observational | 2 weeks | 10 | Decrease of slow phase velocity (DBN) |
| Improvement of visual acuity | |||||||
| NCT01543750 | EA 2 | 4-AP-SR | 10 mg bid | Placebo-controlled | 8 weeks | – | Ongoing |
| EAT-2-TREAT | EA 2 | 4-AP-SR vs. acetazolamide | 10 mg bid | Placebo-controlled | 12 weeks | – | Ongoing |
| Strupp et al. [1] | DBN (different etiology) | 3,4-DAP | 20 mg | Placebo-controlled | Single dose | 17 | Reduction of slow phase velocity |
| Improvement of DBN (oscillopsia, postural stability) | |||||||
| Tsunemi et al. [19] | Pure cerebellar degeneration | 3,4-DAP | 20 mg bid | Controlled | 1 week | 15 | 3,4-DAP effective on DBN and oscillopsia |
| No effect on other ataxic symptoms | |||||||
| Kalla et al. [148] | DBN | 4-AP | 10 mg | Case report | Single dose | 1 | Improvement of DBN, smooth pursuit, VOR gain |
| Kallaetal. [18] | DBN | 4-AP | 10 mg | Controlled | Single dose | 15 | Improvement of slow-phase velocity (DBN) |
| Kalla et al. [20] | DBN | 3,4-DAP | 10 mg 3,4-DAP vs. 10 mg 4-AP | Placebo-controlled | Single dose | 8 | Reduction of slow phase velocity (DBN) |
| 4-AP | 4-AP showed more pronounced effect than equivalent doses of 3,4-DAP | ||||||
| Claassen et al. [22] | DBN | 4-AP | 5 mg qid vs. 10 mg qid | Placebo-controlled | 3 days 20 mg/day vs. 4 days 40 mg/day vs. 4 days placebo | 27 | Reduction of slow phase velocity (DBN), improvement of visual acuity, postural sway, and locomotor parameters |
| Schniepp et al. [26] | CACNA1A mutation | 4-AP | 5 mg rid | Case series | – | 2 | Improvement of gait |
| Reduction of gait variability; improvement of subjective ambulatory scores | |||||||
| Schniepp et al. [27] | DBN, SAOA, CACNA1A mutation, cerebellar stroke | 4-AP | 5 mg rid | Case series | – | 31 | Improvement of gait Increase of mean preferred gait velocity and mean cadence at preferred speed; decrease of the coefficient of variation of stride time |
| Giordano et al. [28] | SCA l,3,6,SAOA, POLG mutation | 4-AP-SR | Observational | 2 weeks | 16 | Modest short-term improvements mainly on gait and speech | |
| Improvements are within the range of placebo effects, long-term trials on prolonged effects are needed | |||||||
| NCT01811706 | SCA 1,2,3,6 | 4-AP-SR | 10 mg bid | Placebo-controlled | 4 weeks | – | Ongoing |
| FACEG | Cerebellar disorders of different etiology | 4-AP-SR | 10 mg bid | Placebo-controlled | 12 weeks | – | Ongoing |
Bid two time a day, tid three times a day, qid four time a day, DBN downbeat nystagmus, EA 2 episodic ataxia type 2, SAOA sporadic adult onset ataxia, SCA spinocerebellar ataxia, 4-AP 4-aminopyridine, SR slow release, 3,4-DAP 3,4-diaminopyridine
Episodic Ataxia Type 2
EA 2 is most often caused by mutations of the CACNA1A gene encoding the alpha-subunit of P/Q-type calcium channel [8]. The clinical manifestations of this channelopathy are recurrent bouts of vertigo, ataxic symptoms, and ocular motor disturbances such as DBN, often overlapping with migraine [9]. The attacks are often provoked by stress, exertion, or alcohol and usually persist for hours or days.
Approximately two thirds of these patients respond to treatment with acetazolamide (250–1,000 mg/day) [9, 10], and acetazolamide has been a first-line treatment of EA 2 for many years. However, there are no placebo-controlled trials about the efficacy of this drug. Furthermore, the side effects of acetazolamide (e.g., kidney stones) often limit its therapeutic use.
In 2004, it was shown in an observational study that the potassium channel blocker 4-AP reduces the number of attacks and was well tolerated [11]. In a randomized, placebo-controlled, double-blind, crossover trial, a significant effect of this agent on the number of attacks and the quality of life was found [2]. These effects of 4-AP on the attacks have been confirmed in several studies with an animal model of EA 2, the tottering mouse [12, 13], which also allows to evaluate the underlying mode of action (see below). More recently, it was also shown in an observational study that the sustained release form of 4-AP [Ampyra™ (Acorda, USA) or Fampyra™ (Biogen Idec, Europe)] is also effective and well tolerated [14]. The use of 4-AP in a dosage of 5 to 10 mg three times a day is recommended for the treatment of EA 2. There are two ongoing placebo-controlled trials with the sustained release form of 4-AP (University of California, NCT01543750) and with the sustained form of 4-AP vs. acetazolamide (University of Munich, EAT-2-TREAT).
Downbeat Nystagmus
DBN is the most frequent type of acquired nystagmus. Affected patients predominantly suffer from postural imbalance and cerebellar gait ataxia, as well as oscillopsia with reduced visual acuity due to a corrective downward saccade following a spontaneous upward drift [15, 16]. In the majority, it is caused by a bilaterally impaired function of the cerebellar floccular lobe [17] due to neurodegenerative disorders.
In a randomized controlled crossover trial, the non-selective potassium channel blocker 3,4-DAP effectively reduced DBN [1]. Furthermore, 4-AP also alleviates the symptoms of DBN, particularly in patients with cerebellar atrophy [18]. Although 3,4-DAP improved DBN in patients with spinocerebellar ataxia type 6 (SCA6), there was no improvement of postural control or other ataxic symptoms after 1 week of 3,4-DAP (20 mg two times a day) [19]. The comparison of equal doses of 3,4-DAP with 4-AP results in a more distinct improvement of DBN following the administration of 4-AP [20]. Since the latter is lipid-soluble and crosses the blood–brain barrier more easily [20, 21], 4-AP is preferred. Recently, a randomized double-blind crossover trial of 4-AP in DBN showed a reduction in slow phase velocity of DBN by half and an improvement of visual acuity at a dosage of 5 mg 4-AP four times a day [22]. Further on, 4-AP at a dosage of 10 mg four times a day reduced postural sway and improved motor performance assessed by the timed “get-up-and-go test.” However, there was no subjective improvement, which may be due to the short half-life of the drug. This shortage may be overcome by the sustained-release form of 4-AP. Therefore, further trials on the new formulation are needed.
Based on the current studies, the use of 4-AP in a dosage of 5 mg two to four times per day is recommended for the treatment of DBN. The sustained-release form Fampyra™ is also efficient. Chlorzoxazone, a non-selective activator of small conductance calcium-activated potassium channels (SK channels), could be a potentially new therapeutic agent for the symptomatic treatment. In an observational proof-of-concept pilot study [23], slow phase velocity of DBN, visual acuity, and postural sway showed significant effects. Other drugs have been shown to lack efficacy in DBN (e.g., baclofen) [24].
Cerebellar Gait Disorders
Beyond the use of aminopyridines in the treatment of fatigue and gait disturbance, a beneficial effect on upper limb tremor has been reported in multiple sclerosis [25]. In a retrospective case series, patients with cerebellar ataxic gait due to different etiologies (multisystem atrophy, sporadic adult-onset ataxia, cerebellar stroke, CACNA1A mutations, DBN syndrome) also benefitted from aminopyridines [26, 27]. In a short-term trial with the sustained release form of 4-AP with 16 patients (SCA1, 3, 6, SAOA, POLG mutation), there were modest short-term improvements [28]. However, these observations need to be confirmed in placebo-controlled trials and more homogeneous patient populations. Currently, there are two ongoing placebo-controlled trials of the sustained release form of 4-AP for cerebellar gait disorders, which will hopefully shed further light upon the impact of this drug for patients with cerebellar disorders [University of Florida (NCT01811706), University of Munich (FACEG)].
Mode of Action of Aminopyridines
Aminopyridines are blockers of so-called Kv1 voltage-activated potassium channels. Thereby, they increase the excitability of neurons. The common mechanism behind the therapeutic influence of aminopyridines lies within the influence on cerebellar Purkinje cells (PCs). In vitro studies show that 4-AP increases the excitability of PCs of the guinea pig cerebellum [29] and the precision of pacemaking of PCs in a mouse model (tottering mouse) of EA 2 [12]. In this model, 4-AP and 3,4-DAP both reduced the frequency of attacks probably by raising the threshold for triggering of attacks [13]. Furthermore, aminopyridines normalize the firing rate and the motor behavior in ataxin-1 mutant mice in vivo [30]. Strikingly, mice treated early demonstrated better motor function, which may be mediated by a neuroprotective effect due to an enhanced electrical activity of PCs [30]. Therefore, long-term treatment starting early in the course of neurodegenerative ataxias may help to improve the outcome.
Drug Therapy of Autosomal Recessive Ataxias (L. Schöls)
Although already rare as a group, autosomal recessive ataxias fall into many subtypes of genetically distinct diseases with different pathomechanisms. Some biomarkers can help to decipher the underlying genotype. Alphafetoprotein is increased in ataxia telangiectasia (AT) and in ataxia with ocular apraxia type 2 [31]. In ataxia with ocular apraxia type 1 (AOA1), cholesterol is increased and albumin decreased especially with more advanced disease. Vitamin E is a reliable marker to identify ataxia with primary vitamin E deficiency and abetalipoproteinemia. Coenzyme Q10 is lacking by definition in ataxia with Q10 deficiency but is difficult to screen since muscle specimen are required and analyses are established in only few labs. Coenzyme Q10 may also be low in AOA1 [32]. Lactate is increased in plasma and cerebrospinal fluid in ataxias due to mtDNA mutations like Kearns–Sayre syndrome but also in the ataxic variant of leukoencephalopathy with brain stem and spinal cord involvement and brain lactate elevation [33]. Decreased ceruloplasmin and increased cholestanol, phytanic acid, and very long chain fatty acid help to filter for ataxia due to metabolic diseases like Wilson disease, cerebrotendinous xanthomatosis, Refsum disease, and adrenoleukodystrophy/adrenomyeloneuropathy, respectively. These causes of ataxia should not be missed since treatment options are available but are not covered by this review.
Friedreich's Ataxia
FRDA is the most frequent autosomal recessive ataxia in the Western world with a prevalence of about 1:50,000 [34]. It is caused by lack of frataxin, a mitochondrial protein involved in iron–sulfur cluster synthesis essential for respiratory chain complexes and iron homeostasis. Abnormal respiratory chain function and increased levels of mitochondrial iron are thought to cause increased reactive oxygen species (ROS) production and oxidative stress [35]. Therapeutic approaches address these points by aiming to (a) increase frataxin levels on the transcriptional level by HDAC inhibitors or the protein level by recombinant human erythropoietin (rhuEPO); (b) reduce ROS by antioxidants like coenzyme Q10, its homolog idebenone and vitamin E; (c) lower mitochondrial iron stores with deferiprone; and (d) improve energy metabolism by l-carnitine supplementation.
Idebenone
Idebenone is the most frequently used drug in FRDA (Table 2). It is generally well tolerated even at high doses up to 45 mg/kg body weight [36]. Several open-label studies suggested favorable effects on cardiac hypertrophy associated with FRDA and neurological dysfunction. Early, placebo-controlled pilot trials found a dose-dependent response on neurological function assessed by the International Cooperative Ataxia Rating Scale (ICARS) [37] especially in young and still ambulatory patients [36] and a minor decrease in cardiac mass after 1 year [38] but no effect on primary endpoints like oxidative stress markers and respiratory chain function [36, 39]. Unfortunately, two large phase III trials failed to replicate these effects [40; press release on the MICONOS trial by Santhera Pharmaceuticals]. However, the open-label extension study indicated a trend for improvement in neurological function over an 18-month period in pediatric patients [41]. More details of idebenone trials in FRDA are presented in Table 2. Further controlled studies in subgroups of Friedreich's ataxia like patients with severe hypertrophic cardiomyopathy and early stages of the disease are warranted to clarify potential beneficial effects. For further critical evaluation of antioxidative therapy in FRDA, see the recent Cochrane Review [42].
Table 2. Idebenone trials in Friedreich's ataxia.
| Daily dose | No. of patients | Age (years) | Study period (months) | Trial design: controlled/blinded | Effect on LV mass | Effect on ataxia | Comment/study |
|---|---|---|---|---|---|---|---|
| 5 mg/kg | 3 | 11–21 | 4–9 | −/− | + | − | Cardiac improvement but only patients with cardiac hypertrophy; Rustin et al. [149] |
| 360 mg | 9 | 19–54 | 1.5 | +/+ | − | − | No effect on energy metabolism in 31P-MRS; Schöls et al. [39] |
| 5 mg/kg | 38 | 4–22 | 6 | −/− | + | − | Shortening fraction improved in 5 of 6 pats; Hausse et al. [150], Rustin et al. [151] |
| 5 mg/kg | 9 | 11–19 | 12 | −/− | − | + | Oculomotor disorder improved; Artuch et al. [152] |
| 5 mg/kg | 29 | 20–31 | 12 | +/+ | + | − | Exclusion of patients with normal IVS; Mariotti et al. [38] |
| 5 mg/kg | 8 | 8–27 | 12 | −/− | + | − | Buyse et al. [153] |
| 5 mg/kg | 104 | 13–74 | 6–84 | +/− | + | − | No comparison to untreated group; Ribaï et al. [154] |
| 4–50 mg/kg | 48 | 9–17 | 6 | +/+ | (+) | + | Dose-dependent improvement in ICARS; Di Prospero et al. [36] |
| 5–20 mg/kg | 24 | 8–46 | 36–60 | −/− | − | (+) | Stabilized neurological function in pediatric patients; Pineda et al. [155] |
| 450–900 mg 1,350–2,250 mg |
70 | 8–18 | 6 | +/+ | − | − | IONIA trial phase III; Lynch etal. [156], Lagedrost et al. [40] |
| 180–360 mg | 232 | 8–70 | 12 | +/+ | − | − | MICONOS trial |
| 450–900 mg | Phase III | ||||||
| 1,350–2,250 mg | Santhera communication | ||||||
| 450–900 mg 1,350–2,250 mg |
68 | 8–18 | 12 | −/− | − | (+) | IONIA extension study; Meier et al. [41] |
Effect on left ventricular mass and ataxia
− no effect, (+) temporary effect or effect in subgroup, + significant effect, MRS magnetic resonance spectroscopy, IVS interventricular septum, LV left ventricular
Coenzyme Q10+Vitamin E
A pilot trial of a combined treatment with coenzyme Q10 (400 mg/day) and vitamin E (2,100 IU/day) for 6 months showed positive effects on energy metabolism assessed by 31P-MRS in cardiac and calf muscle without consistent benefits in neurological and echocardiographic evaluations. Follow-up after 47 months indicated effects on fractional shortening in echocardiography and ataxia in comparison to historical natural history data but no clear dose dependence (Table 3)[43–45]. Placebo-controlled trials are warranted to prove the beneficial effect of the combined supplementation of coenzyme Q10 and vitamin E in Friedreich's ataxia.
Table 3. Drug trials in patients with recessive ataxias (for idebenone trials in Friedreich's ataxia, see Table 2).
| Ataxia | Drug | Study design | No. of patients | Outcome | Study |
|---|---|---|---|---|---|
| Friedreich's ataxia | Coenzyme Q10 400 mg/die+ vitamin E 2,100 IU/die 6 months+47 months |
Open label | 10 | Stable neurological condition after 6 months. Echocardiography: increased fractional shortening after 47 months. 3IP-MR spectroscopy: improvement of ATP production in cardiac and calf muscle throughout 47 months | Lodi et al. [43] and Hart et al. [44] |
| Friedreich's ataxia | Conzyme Q10 600 mg/die+ vitamin E 2,100 IU/die vs. Q10 30 mg/die+Vit E 24 IU/die 2 years |
Randomized, double-blind, comparative high-vs. low-dose study | 50 | Progression of ataxia in both groups | Cooper et al. [45] |
| Friedreich's ataxia | rhuEPO 5,000 U thrice a week s.c. 8 weeks |
Open label | 12 | Increase in frataxin levels in lymphocytes. Reduction of oxidative stress markers | Boesch et al. [49] |
| Friedreich's ataxia | rhuEPO 2,000 U thrice a week s.c. 6 months |
Open label | 8 | Improvement in SARA by 5.2 points. Increase in frataxin levels in lymphocytes by 24 %. Reduction of oxidative stress markers Side effects: need of phlebotomy in 4/8 patients due to increase in hematocrit |
Boesch et al. [52] |
| Friedreich's ataxia | rhuEPO Increasing doses from 20,000 U every 3 weeks to 40,000 U every 2 weeks s.c. 6 months |
Randomized placebo-controlled, double-blind | 16 | No effect on SARA, frataxin levels, and hematologic parameters | Mariotti et al. [53] |
| Ataxia with vitamin E deficiency | Vitamin E 800 mg/die 12 months |
Open label | 24 | Reduction of 10 points in ICARS. Improvement mainly of action tremor | Gabsi et al. [54] |
| Ataxia with vitamin E deficiency | Vitamin E 300-2,400 mg/die 2-11 years |
Retrospective, open label | 16 | Stable neurological condition in most patients | Mariotti et al. [55] |
| Ataxia with coenzyme Q10 deficiency | Coenzyme Q10 600-3,000 mg/die 1 month-4 years |
Retrospective, open label | 6 | Reduction of 26 points in ICARS | Musumeci et al. [59] |
| Ataxia telangiectasia | Betamethasone 0.01 mg/kg/die+0.03 mg/kg/die 20 days each |
Open label | 6 patients who previously responded to betamethasone 0.1 mg/kg/die | Reduction in SARA by 2.7 points with 0.01 and by 5.5 points with 0.03 mg/kg/die | Broccoletti et al. [63] |
| Ataxia telangiectasia | Betamethasone 0.1 mg/kg/die 30 days |
Double-blind, randomized, Placebo-controlled crossover | 13 | Improvement of 17 points in ICARS with betamethasone vs. 4.5 points with placebo | Zannolli et al. [64] |
EPO erythropoietin
EPI A0001
EPI A0001 is an α-tocopheryl quinone studied recently for its ability to improve glucose metabolism in Friedreich's ataxia. Whereas this primary endpoint failed, some improvement was observed in neurological function [46]. Further studies have to be awaited.
l-Carnitine
Respiratory chain defects are supposed to cause secondary carnitine deficiency. l-Carnitine supplementation (3 g/day) resulted in some improvement in muscle energy metabolism when assessed by phosphorus spectroscopy (31P-MRS) [47]. Controlled trials are needed to substantiate clinical relevance of this effect.
Deferiprone
This iron chelator is supposed to promote redistribution of iron overload in Friedreich's ataxia. Especially with higher doses (60 mg/kg), adverse events including agranulocytosis, iron-deficient anemia, and neurological deterioration occurred and forced termination of the high-dose arm in yet unpublished placebo controlled trial. In an open study, combined therapy with idebenone (20 mg/kg) and deferiprone (20 mg/kg) led to some improvement in kinetic functions but worsening of gait and posture scores over 11 months. Heart hypertrophy parameters and iron deposits in dentate nucleus improved. Side effects included the risk of neutropenia and progressive reduction of plasma iron parameters [48]. Future studies with well-tolerated dosages of deferiprone should address the long-term effect especially on cardiomyopathy in controlled trials.
Erythropoietin
An open-label pilot trial with 12 Friedreich's ataxia patients found rhuEPO to increase frataxin levels in lymphocytes after 8 weeks treatment with 5,000 U rhuEPO three times weekly and a reduction of oxidative stress markers urinary 8-hydroxydeoxyguanosine and serum peroxides [49]. In another open-label “proof-of-concept” study, the same group investigated safety and efficacy treatment with 2,000 IU rhuEPO thrice a week over 6 months and found significantly increased frataxin levels and improvement in ataxia rating scales (FARS and SARA) [50, 51] while oxidative stress markers decreased. Increases in hematocrit requiring phlebotomies occurred in four of eight patients [52]. A 6-month, randomized placebo-controlled, double-blind, dose–response trial comparing 20,000 IU every 3 weeks, 40,000 IU every 3 weeks, and 40,000 IU every 2 weeks in 16 adult patient with Friedreich's ataxia did not result in any significant hematological, clinical, or biochemical effects (Table 3) [53]. Further trials are needed to clarify the effect of rhuEPO and its derivates on frataxin levels in Friedreich's ataxia.
Ataxia with Isolated Vitamin E Deficiency
The pathomechanism of primary vitamin E deficiency suggests a supplementary approach in ataxia with isolated vitamin E deficiency (AVED). However, due to the rarity of the disease, randomized placebo-controlled trials are missing. In an open-label study of 24 AVED patients, supplementation with vitamin E (800 mg daily) during a 1-year period resulted in a normalization of serum vitamin E levels and a moderate improvement in an ataxia rating scale [54]. Mariotti et al. [55] reported their experience with vitamin E supplementation in 16 patients treated with 1,000–2,400 mg daily over 2–11 years and found a stabilization of the neurological conditions in most of the patients. However, development of spasticity and retinitis pigmentosa was noted in a few patients during therapy (Table 3). Placebo-controlled long-term trials are missing to prove efficacy of vitamin E substitution in AVED but may be difficult due to the broad availability and good tolerability of vitamin E supplementation and its suggestive efficacy.
Abetalipoproteinemia
As cerebellar degeneration in abetalipoproteinemia is thought to be secondary to vitamin E deficiency in parallel to AVED, high-dose vitamin E supplementation is recommended [56]. However, disturbance in lipid metabolism requires a much more complex diet including reduced long-chain fatty acids and higher amounts of medium-chain triglycerides and essential fatty acids of the n-6 and n-3 type [57]. Therapy has not been proven in controlled trials, and composition as well as dosages of supplementary therapy need further evaluation.
Ataxia with Coenzyme Q10 Deficiency
Q10 deficiency is caused by mutations in several genes involved in the biosynthesis of coenzyme Q10 [58]. Clinical manifestation can be variable including a spectrum of cerebellar ataxia, isolated myopathy, encephalomyopathy, nephropathy, and severe multisystemic disease. Supplementation of coenzyme Q10 (300–600 mg per day) can stabilize the disease or even improve symptoms partially (Table 3) [59, 60]. However, response to Q10 supplementation may be only incomplete [61]. Larger series, longer follow-up periods, and genetic differentiation of ataxia with Q10 deficiency are needed.
Ataxia Telangiectasia
Ataxia telangiectasia is caused by lack of ataxia telangiectasia mutated kinase and consecutive deficient DNA repair pathways and DNA damage. Betamethasone possibly improves ataxia symptoms in AT. Treatment with betamethasone (0.1 mg/kg per 24 h) let to substantial improvement of ataxia after 2 weeks in a single case. However, switching to methylprednisolone (2 mg/kg per 24 h) was not effective and had to be stopped after 4 weeks because of adverse effects like increased appetite and body weight and moon face [62]. To prevent these side effects, a low-dose trial with two 20-day cycles of oral betamethasone at 0.01 and 0.03 mg/kg/day was performed. Ataxia scores (SARA) improved in all patients at the dosage of 0.03 mg/kg/day [63]. In a double-blind, randomized, placebo-controlled crossover trial, oral betamethasone (0.1 mg/kg/day for 2×10 days with 10 days intermediate tapering) improved ataxia assessed by ICARS by 17 points (about 30 %) compared to placebo (improvement of 4.5 points) (Table 3) [64]. However, minimal dosage, long-term effectiveness, and safety need to be established.
Drug Therapy of Autosomal Dominant Ataxias (W. Nachbauer, S. Boesch)
SCAs, as referred for autosomal dominant ataxias in the genetic nomenclature, are a rare cause of cerebellar ataxia defined by the mode of inheritance and a progressive cerebellar syndrome that is often associated with other neurological signs such as pyramidal or extra-pyramidal signs, ophthalmoplegia, or cognitive impairment leading to a substantial range of SCA phenotypes [65]. The role of symptomatic treatment for extra-cerebellar features in SCAs will not be addressed in this review. The spinocerebellar ataxias are clinically, genetically, and neuropathologically heterogeneous. So far more than 30 different SCA mutations have been described, harboring heterogeneous types of mutations. Among one to three per 100,000 Europeans with SCA mutations, polyglutamine (polyQ) expansion SCAs account for about 45 %, conventional mutations account for 10 %, and up to 50 % of SCAs are currently of unknown genetic background [65].
Many efforts to delineate natural history of SCA mutations in large multi-national data bases, to validate rating instruments for ataxias, and to develop inventories for additional non-ataxia signs were undertaken in the last decade [51, 66, 67]. Even more, the issue of the onset of disease and thereby the accurate time point for a future therapeutic intervention is addressed in an ongoing study [68]. A major drawback, however, in these efforts is the lack of valid bio-or surrogate markers for SCAs or even SCA subgroups (e.g., polyQ expansion SCAs).
Currently, drugs against cerebellar ataxia irrespective of the underlying mutation aim (a) to exert regulatory effects on the firing rate of cerebellar neurons, (b) to modulate cerebellar function addressing neuronal nicotinic acetylcholine receptors, (c) to alter neuronal firing rates via carbonic anhydrase inhibition, or (d) to ameliorate glutamate mediated neurotoxicity. Disease-modifying therapies that aim to decrease the synthesis of abnormal proteins or to increase their clearance acting on a DNA or RNA level are still not available. Intensive ongoing clinical and neurogenetic research together with applied molecular approaches, however, yields scientific advances that are prone to be translated into developing effective treatments for the spinocerebellar ataxias in the near future. Currently, however, no effective medication for SCAs is available.
Drugs with Proposed Effect on Cerebellar Symptoms
Randomized controlled studies on cerebellar symptoms in SCAs are sparse. Clinical studies are often performed in genetically heterogeneous or genetically non-defined degenerative cerebellar syndromes. Large cohort studies are lacking. Open-label studies and case reports are available in some SCA mutations. Outcome measures are mostly not comparable since different assessments and/or ratings of ataxia were applied in the respective studies. Studies are summarized in Table 4.
Table 4. Drug trials in patients with mainly dominant ataxias.
| Study | Ataxia | Daily dose | Study design | Study period | No. of patients | Outcome |
|---|---|---|---|---|---|---|
| Ristori et al. [69] | Cerebellar ataxias of different etiologies | 100 mg riluzole | Randomised, double-blind, placebo-controlled | 8 weeks | 40 | Significant drop in ICARS score (at least 5 points) especially static function, kinetic functions, dysarthria |
| Zesiewicz et al. [70] | SCA3 | 1 mg varenicline twice daily | Randomized, double-blind, placebo-controlled, (crossover) | 4 weeks titration, 4 weeks 1 mg twice daily | 20 | Significant improvement of SARA scores for gait, stance, rapid alternating movements, timed walking test, no crossover phase because of drop-outs |
| Strupp et al. [73] | Cerebellar ataxia of different etiology | 5 g/day acetyl-dl-leucine | Open label | 1 week | 13 (5 SAO A, 1 SCA2, 1 SCA1,2ADCA, 4AOA) | SARA improvement (16.1 vs. 12.8); improvement in SCAFI and EuroQo1-5d-3l |
| Velazquez-Perez et al. [76] | SCA2 | 50 mg ZnSO(4) daily or placebo | Randomized, double-blind, placebo-controlled | 6 months | 36 | Significant increase Zn levels in the CSF, non-significant decrease in SARA subscores for gait, posture, stance and rapid alternating movements, reduction of lipid's oxidative damage, reduction of saccadic latency in the zinc-treated group |
| Assadietal. [157] | FRDA, CA, OPCA | 30 mg buspirone HC1 twice daily | Double-blind, placebo controlled, crossover | 3 months | 20 (10 SCA, 6 CA, 4 FA) | No evidence of an effect of buspirone HC1 on ICARS |
| Schulte et al. [158] | SCA3 | Combination of trimethoprim 160 mg+sulfamethoxazole 800 mg, twice daily for 2 weeks, followed by a combination of trimethoprim 80 mg+sulfamethoxazole, 400 mg, twice daily for 5.5 months | Randomised, double-blind, placebo-controlled, crossove (2 weeks washout) | 6 months r | 22 | No significant improvement in ataxia ranking scale, self-assessment score, static posturography, and results of motor performance testing after 2 weeks and 6 months. No significant effects on the visual system using the achromatic Vision Contrast Test System and the Farnsworth-Munsell 100-hue test for color discrimination |
| Yabe et al. [74] | SCA6 | 250 or 500 mg acetazolamide daily | Open-label pilot study | 22 months | 9 | Significant improvement in the ataxia rating scale (ICARS) and body sway analysis by stabilometry until week 48, non-significant effect after 88 weeks |
| Botez et al. [75] | FRDA, OPCA | 200 mg amantadine vs. placebo | Randomized, double-blind, placebo-controlled | 3–4 months | 30 | Efficacy evaluation via visual and auditory reaction time and movement time with right and left hand according to Hamsher and Benton real method. Measurement of homovanillinic acid levels in the CSF |
FRDA Friedreich's ataxia, CA cerebellar atrophy, OPCA olivopontocerebellar atrophy, SCA spinocerebellar ataxia (SCA1 spinocerebellar ataxia type 1, SCA2 spinocerebellar ataxia type 2 etc.), CSF cerebrospinal fluid, POLG mutation in the polymerase gamma gene, SAOA sporadic ataxia of unknown origin, ADCA autosomal dominant cerebellar ataxia, AOA ataxia with ocular apraxia, SCAFI SCA functional index (consisting of the PATA test, 8 m walking time, nine-hole PEG test), EQ-VAS visual analog scale of the EQ-5D
Riluzole
Riluzole opens small-conductance potassium channels exerting an important regulatory effect on the firing rate of neurons in deep cerebellar nuclei. Riluzole is suggested to reduce neuronal hyperexcitability. In a randomized, double-blind, placebo-controlled pilot trial, 40 patients presenting with cerebellar ataxias of different etiologies were assigned to riluzole (100 mg/day) or placebo in an 8-week trial. Primary outcome measure was the proportion of patients with a decrease of at least five points in the ICARS score after 4 and 8 weeks. The number of patients with a five-point ICARS drop was significantly higher in the riluzole group than in the placebo group after 4 and 8 weeks. The mean change in the riluzole group ICARS after treatment revealed a decrease in the total score and major sub-scores for static function, for kinetic function, and for dysarthria [69]. Experience of many ataxia clinics is less promising. Findings on a symptomatic effect of riluzole in cerebellar dysfunction need to be confirmed in further (second) placebo-controlled clinical trials.
Varenicline
Varenicline is a partial agonist at α4β2 neuronal nicotinic acetylcholine receptors used for smoking cessation. After several case reports, the efficacy of varenicline in SCA3 was assessed in a randomized controlled trial over 8 weeks comparing 1 mg varenicline twice daily to placebo. Twenty patients (mean age 51±11 years, mean disease duration 14±10 years) entered into the study. Primary outcome values were changes in the SARA after 8 weeks, a timed 25-ft walk test and nine-hole peg test. Varenicline significantly improved SARA subscores for gait, stance, and rapid alternating movements as well as the timed walking test in phase I of the study (4 weeks). However, because of a dropout rate of overall 40 %, the pre-planned crossover design was not performed [70]. Therefore, and because of considerable side effects of varenicline [71], further studies will have to clarify the usefulness of this drug in cerebellar ataxias [72].
Acetyl-dl-leucine
Acetyl-dl-leucine appears to modulate the activity of central vestibular neurons by normalizing the membrane potential of depolarized or hyperpolarized neurons. Acetyl-dl-leucine (Tanganil™) has been widely used in France for symptomatic treatment of vertigo and dizziness. Recently, 13 patients with cerebellar ataxia of different etiologies (SCA1/2, ADCA, AOA, SAOA) were treated with acetyl-dl-leucine (5 g/day) for 1 week in an open label case series [73]. Mean SARA score decreased by about four points. The SCAFI (consisting of 8 m walking, nine-hole peg test, PATA rate) and ratings of quality of life showed improvement. No side effects were reported. Given the good risk–benefit profile of acetyl-dl-leucine, larger placebo-controlled trials using acetyl-dl-leucine are warranted.
Acetazolamide
Acetazolamide, a carbonic anhydrase inhibitor, showed significant improvement of ataxia in six SCA6 patients (mean age 57±9 years, mean disease duration 9±2 years) administered for up to 88 weeks in an open label pilot study. SCA6 patients received either 250 mg or 500 mg acetazolamide daily. Ataxia was measured using an ataxia rating scale [37] and body stabilometry [74]. Significant improvement in ataxia was observed until week 48 and became weaker thereafter. Until to date, these findings have not been replicated in a controlled trial design.
Amantadine
Amantadine, a NMDA antagonist with anti-parkinsonian properties and proposed amelioration of glutamate mediated neurotoxicity in cerebellar granular cells, has been used in genetically heterogeneous degenerative ataxias. A group of 30 patients with olivopontocerebellar atrophy were randomly assigned to placebo or amantadine hydrochloride 200 mg daily for 3 to 4 months. Efficacy was evaluated by simple visual and auditory reaction time and movement time for both right and left hands. Patients with olivopontocerebellar atrophy receiving amantadine showed significant improvement on seven out of eight variables studied by analysis of covariance [75]. Olivopontocerebellar atrophy refers to several disease entities and does not apply to current nomenclature in cerebellar ataxias. Future studies will have to replicate aforementioned findings in well-defined and/or genetically assigned cerebellar ataxias.
Zinc Sulfate Supplementation
In a randomized, double-blind, placebo-controlled trial during 6 months, 36 Cuban SCA2 patients with reduced zinc concentrations in serum and cerebrospinal fluid were randomly assigned to 50 mg ZnSO(4) daily or placebo, together with neurorehabilitation therapy. A significant increase of the Zn levels in the CSF; mild decrease in SARA subscores for gait, posture, stance and alternating hand movements; and a reduction of saccadic latency were observed. The treatment was safe and well tolerated. Zn supplementation was efficacious and safe in Cuban SCA2 patients [76]. SCA2 patients should be checked for lack of zinc concentration, and Zn supplementation may be started.
Ongoing Interventional Clinical Trials in SCAs
Lithium Carbonate
Lithium carbonate used as a mood stabilizer in very low dose over a long time period has been reported not to be harmful in one SCA2 patient with a benign course of disease [77]. Lithium carbonate being proposed as a candidate substance in SCAs [78] has been tested/is currently tested in several trials: SCA1 (completed, not published), SCA3 (active, non-recruiting), and SCA2 (active, non-recruiting). The conduct of these trials is hampered by the aggravation of ataxia symptoms and poor tolerability of lithium (personal communication).
Intravenous Immunoglobulin
Intravenous immunoglobulin effects are currently tested in spinocerebellar ataxias (University of South Florida; NCT 01350440).
Future Treatment Options in SCAs
Cell-based in vivo targeted approaches aiming to reduce the expression of the mutant protein may have implications for novel treatment approaches in polyQ expansion SCAs [79–81]. Still, several issues such as the mode of vector delivery or selective targeting of the mutant protein to reinstall protein homeostasis in polyQ expansion SCAs in the human brain remain to be refined before this therapeutic approach will be applicable.
Stem Cell Therapy
Clinical trials using umbilical stem cell therapy in SCA1 (recruiting) and adipose-derived mesenchymal stem cells for the treatment of cerebellar ataxia (recruiting) are currently under way.
Motor Rehabilitation in Degenerative Cerebellar Disease
Physiotherapeutical Interventions in Adult Patients with Spinocerebellar Degeneration (I. Miyai, W. Ilg)
In general, functional recovery of motor capabilities heavily depends on the cause and site of the cerebellar lesion. Within the spectrum, degenerative cerebellar diseases are especially hard to treat due to their progressive nature and effects on virtually all parts of the cerebellum. In contrast, ataxia following stroke, neurosurgery, trauma, or multiple sclerosis affects generally only some regions of the cerebellum, but leaves other regions intact which might be recruited to compensate for the defective parts.
Rehabilitation of cerebellar-induced motor impairments is additionally complicated by the functional role of the cerebellum in motor learning [82]. Impairments of cerebellar patients in (short-term) error-dependent motor learning have been shown for various motor tasks [83–87]. Therefore, poor recovery or low benefit of physiotherapeutic training may be a consequence of damaging structures critically involved in re-learning of motor skills [88–90]. Until recently, relatively few clinical studies have evaluated physiotherapeutic interventions for patients with cerebellar ataxia. Using increasingly demanding balance and gait tasks, improvements were reached for increased postural stability in clinical measures and less dependency on walking aids in everyday life [91, 92]. Locomotion training on treadmills with [93, 94] or without [95] body-weight support has been proposed in particular for patients with more severe ataxia, which are not able to walk freely. Many of these studies were single cases or based on a very small number of patients with different types of cerebellar disease and severity of ataxia. This heterogeneity in patient populations makes it very difficult to compare and evaluate intervention methods in existing studies.
Recently, two clinical studies on larger cohorts have shown more systematically that motor rehabilitation can be beneficial to individuals with degenerative cerebellar disease [6, 7]. Details of both studies can be found in Table 5. The benefits of intensive whole-body coordinative training on balance and mobility function in degenerative cerebellar disease have been demonstrated in an intra-individual case–control design [6, 96]. Sixteen patients suffering from progressive ataxia due to cerebellar degeneration (n =10) or degeneration of afferent pathways (n = 6) have been tested. Results indicated significant reduction of ataxia symptoms measured by the clinical ataxia scale SARA improvements in motor performance after 4 weeks intervention. Quantitative movement analysis revealed specific improvements in dynamic balance in posture and gait as well as in intra-limb coordination. Patients with cerebellar ataxia profit more substantially from the intervention in comparison with patients with sensory ataxia. This discrepancy is most likely caused by a loss of afferent information in these patients, which removes necessary sensory inputs for adequate cerebellar processing. Retention of effects has been shown to depend crucially on continuous training. Assessments after 12 months show that for patients executing continuous motor training, benefits were meaningful for their everyday life and persisted after 1 year, despite of a gradual decline of motor performance and gradual increase of ataxia symptoms due to progression of underlying neurodegeneration [96].
Table 5. Details of the first two clinical studies of motor rehabilitation in larger cohorts in degenerative cerebellar disease [6, 7].
| Ilg et al. [6, 96] | Miyai et al. [7] | |
|---|---|---|
| Number of patients | 16 | 42 |
| Type of disease | SCA6(2), SCA2(1), ADCA(1), IDCA(6), FRDA(3), SANDO(2), SN(1) | SCA6(20), ADCA(6), IDCA(16) |
| Age±SD (range) | 61.4±11.2 (44–79) | 62.5±8.0 (40–82) |
| Gender | 8 males, 8 females | 22 males, 20 females |
| Duration of disease | 12.9±7.8 (3–25 years) | 9.8±6.2(7 months–0 years) |
| Baseline SARA | 15.8±4.3 (11–24) | 11.3±3.8 (5–21.5) |
| Control | Intra-individual controls for short-term effect | Crossover for short-term effect |
| Intervention | 1 h×3 per week× 4 weeks | 2 h × 5 +1 h × 2 per week × 4 weeks |
| Post-training | Home training protocols | No |
| Outcome measures | SARA, gait speed, balance, BBS, GAS, movement analysis | SARA, FIM, gait speed, cadence, FAC, falls |
| Assessment point | 4 weeks pre, baseline, post 0, 8 weeks | Baseline, post 0, 4, 12, 24 weeks |
| Main results | SARA and gait improved 8 weeks post-rehabilitation only in patients with cerebellar ataxia not afferent ataxia | SARA and gait improved 12 weeks but not 24 weeks |
SCA spinocerebellar ataxia, FRDA Friedreich's ataxia, IDCA idiopathic cerebellar ataxia, ADCA autosomal dominant cerebellar ataxia, SANDO sensory ataxic neuropathy with dysarthria and ophthalmoparesis caused by mutations in the polymerase gamma gene, SN sensory neuropathy, SARA Scale for the Assessment and Rating of Ataxia, BBS Berg Balance score [159], GAS Goal Attainment Scaling [160], FIM Functional Independence Measure [97], FAC Functional Ambulation Categories
Further evidence for the efficiency of motor rehabilitation in degenerative cerebellar disease is given by another study combining physiotherapy with occupational therapy [7]. In 42 patients with pure cerebellar degeneration, a 12-h intervention per week for 4 weeks revealed improvements of ataxia severity, gait speed, fall frequency, and activities of daily living measured by a Functional Independence Measure [97]. Improvements were more prominent in trunk ataxia than in limb ataxia, and patients with mild ataxia severity experienced a more sustained improvement in ataxic symptoms and gait speed [7]. Although functional status tended to decline to the baseline level within 24 weeks, gains were maintained in more than half of the participants.
Open Questions and Future Research Directions
These findings raise many intriguing questions and stimulate further studies in the rehabilitation of degenerative cerebellar disorders [98]. Among these are long-term learning studies as well as imaging analyses in order to clarify whether the degenerating cerebellum is still able to adapt motor coordination, or whether the learning deficit is compensated by other brain structures (see next sections). In the following, we will highlight several questions directly important for the rehabilitation management in degenerative cerebellar disease.
First, an important goal is to find effective strategies for long-term interventions in order to minimize decline due to progression of underlying neurodegeneration. The above-described studies provided evidence that intensive motor training can be efficient in degenerative cerebellar disease with an intensity of at least 3 h per week for 4 weeks. It is not clear whether greater dose of intervention results in further improvement. For retention, 1-h of homework training per day showed at least a partially persistence of effects [96], whereas improvements declined to the baseline level at 6 months after the intervention if no specific follow-up training was provided [7]. Thus, for patients with degenerative diseases, continuous training seems crucial for stabilizing improvements. Possible interventions for long-term meaningful gains include re-boost intensive rehabilitation, home rehabilitation, and combination of both interventions. The effect of these interventions can be tested by comparing outcome with natural history of the diseases. The natural disease progression of degenerative cerebellar ataxias is 0.35–2.5 points per year on the SARA scale depending on genotypes [66].
New follow-up data (Miyai, unpublished) over 3 years in four cases revealed that gains in SARA after repeated boost rehabilitation were comparable with those obtained after the initial intervention (Fig. 1). In terms of activity of daily living (ADL), the effect of re-boost rehabilitation on Functional Independence Measure [97] was also meaningful (Miyai, unpublished). However, low-intensity home therapy up to 80 min per week failed to maintain their gains in ataxia and ADL.
Fig. 1.
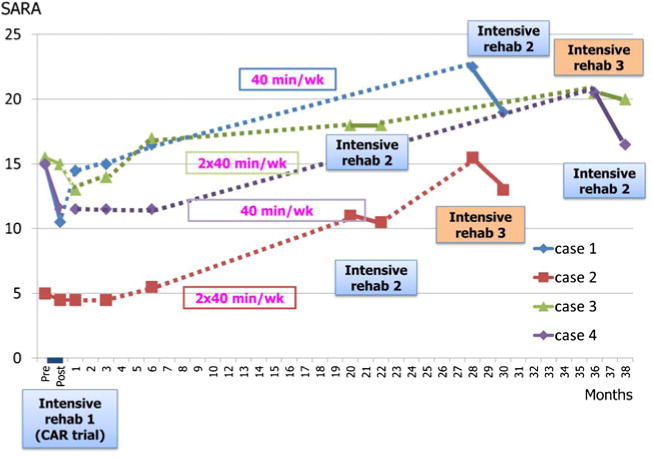
Effect of re-boost rehabilitation on cerebellar ataxia as assessed by SARA scores in patients with cerebellar degenerative diseases. All four patients underwent 4-week intensive rehabilitation (CAR trial; Miyai, unpublished). Cases 2 and 3 had re-boost intensive rehabilitation twice, and cases 1 and 4 had re-boost rehabilitation once. First intervention results in 2.5 point improvement of SARA on average, and after average of 26 weeks, SARA worsened by 7.6 points and the re-boost therapy resulted in 2.0 point improvement. This suggests that the effect of re-boost rehabilitation on SARA was comparable with the first intensive rehabilitation. However, low-intensity home therapy up to 80 min per week failed to maintain the gains. Solid lines represent intervention periods of intensive rehabilitation. Dotted lines represent follow-up periods with home-based rehabilitation of 40 to 80 min per week
Most of the existing studies including the above described were focused on ambulatory patients, who are able to walk with or without walking aid. Importantly, further studies are needed to examine whether patients with more severe impairments would also benefit from physiotherapeutical training (adjusted to their impairments, e.g., for arm movements) or whether the capacity to improve motor performance relies on a specific level of residual cerebellar integrity. The aim is to find adequate predictors for the potential benefits an individual patient can expect from a specific motoric training and thus the possibility to provide efficient training programs for different stages of cerebellar impairment. Indeed, one of such predictors for the rehabilitation outcome could be the capability for short-term motor adaptation. In Hatakenaka et al. [99], it was shown that ataxic patients with infratentorial stroke that impaired short-term motor adaptation correlated with reduced rehabilitation gains.
Such individual training strategies also include the question which training program is most beneficial for a specific level of ataxia severity. In general, a combination of restorative and compensatory techniques may be utilized; the relative emphasis depends on the severity of cerebellar ataxia and its pattern of progression [89, 100, 101]. Such compensatory techniques can include (a) replacing rapid multi-joint movements with slower movements with sequential single joints movements [100], (b) the rehearsal of eye movements for goal-directed stepping [102], and (c) the training of secure fall strategies instead of training to avoid falls. In even more severe cases, in which free standing and walking is not possible anymore, treadmill training [93–95] with potential weight support may be helpful to increase walking capabilities (with the use of mobility aids) and to preserve general fitness as far as possible. The use of mobility aids is dependent on disease stage and individual preferences. Furthermore, in severe cases of upper limb ataxia, when basic activities of daily living like eating are impaired, the use of orthotics—in order to stiffen mechanically several degrees of freedom—can help to improve functional performance [101, 103].
In summary, further research will have to focus on the development of individual rehabilitation strategies potentially including the combination of motor training with other rehabilitation techniques like non-invasive stimulation and bio-feedback approaches (see next sections).
Motor Rehabilitation Training in Children and Adolescents with Spinocerebellar Degeneration (M. Synofzik, W. Ilg)
As described in the last section, studies on the effectiveness of physiotherapy and coordinative training in degenerative ataxia are rare already in adult populations. This paucity is even more pronounced in children with degenerative cerebellar ataxia. Only very few and only small case studies exist: In a child with a vascular cerebellar lesion, some beneficial effects were reported by using locomotor training with body-weight support on a treadmill [93]. A single case study of a multi-component rehabilitation intervention in a juvenile FA patient revealed mixed results [104]. In a study with a child suffering from AVED, results of a postural biofeedback therapy were confounded by simultaneous starting of treatment with vitamin E [105].
Comparable to the motor rehabilitation of adults, the goal is to come up with a rehabilitation program which enables the children to train their motor performance concerning dynamic balance and multi-joint coordination intensively and continuously over a long period of time. The described physiotherapy program for adults, however, has several drawbacks for children, including their lack of motivation for intensive and continuous physiotherapy. Thus, we used recently developed whole-body controlled video game technology for an intensive coordinative training [106]. Ten children with progressive spinocerebellar ataxia trained 8 weeks with three Microsoft Xbox Kinect® video games, first for 2 weeks under instruction and supervision by a physiotherapist, then for 6 weeks at home. The strategy of the videogame-based training aimed to train motor capacities known to be dysfunctional in ataxia, namely goal-directed limb movements, dynamic balance, and whole-body coordination (Fig. 2). Moreover, children had to train a cognitively demanding interaction in a virtual environment, forcing them to rapidly react to novel situations and to constantly recalibrate predictions about upcoming events.
Fig. 2.
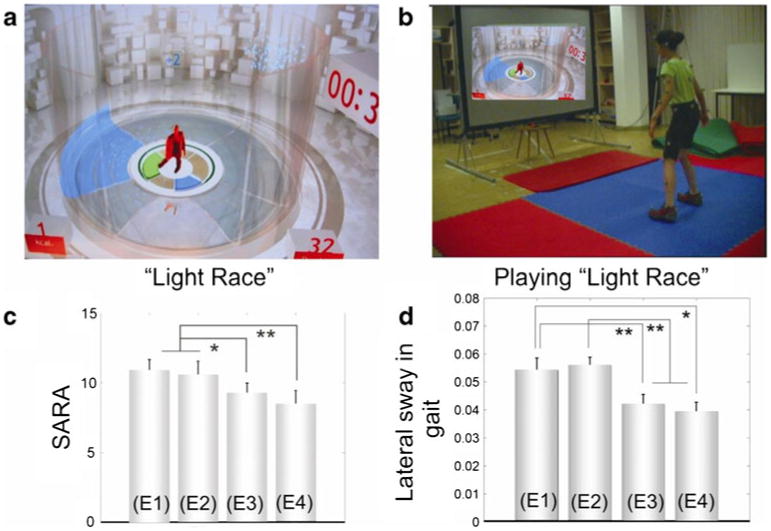
a Screenshots from the game “Light Race” used in the exercises. b Snapshot from the “Light Race” game. Patient C1 performs dynamic stepping movements in order to control the avatar to step onto the highlighted areas on the floor (figures reproduced with permission from Microsoft Xbox Kinect® (a, b)). c, d Group comparisons of the clinical ataxia scores (SARA) and lateral sway in gait at examinations E1–E4. Patients were examined four times: 2 weeks before intervention (E1), immediately before the first training session (E2), after the 2-week lab-training period (E3), and after the 6-week home-training phase (E4)[106]. Stars denote significance: *p <0.05; **p <0.01
For evaluating intervention outcome, patients were examined four times: 2 weeks before intervention (E1), immediately before the first training session (E2), after the 2-week lab-training period (E3), and after the 6-week home-training phase (E4). Rater-blinded assessment revealed a reduction in ataxia symptoms by ∼2 points on average in the SARA ataxia score [51] after 8 weeks. The improvement in ataxia (SARA scale) during home training was thereby dependent on the intensity of home training: The more intensive the training periods at home, the higher the reduction in SARA gait and posture. Importantly, children were highly motivated throughout the whole demanding training period, and they experienced feelings of success about their own movements. The clinical improvement was paralleled by improvements in biomechanical measures of gait (lateral sway, step length variability) and goal-directed leg placement (see Fig. 2).
In summary, this study provides proof-of-principle evidence that, despite progressive cerebellar degeneration, children are able to improve motor performance by intensive coordination training. Moreover, it suggests that directed training of whole-body controlled video games might present a highly motivational, cost-efficient, and home-based rehabilitation strategy to train dynamic balance and interaction with dynamic environments for these children. However, our results provide still rather preliminary evidence. Larger cohort studies, studies including also more severely affected children, assessment of long-term benefits, finding of predictors for training success, and studies comparing different training interventions and different ataxia types have to be performed in the future.
How Neuroimaging Studies Can Help to Understand and Improve Cerebellar Rehabilitation (R. G. Burciu, D. Timmann)
The cortical organization in the human brain is not fixed, but there are ongoing modifications based on experience [107]. With the advent of imaging tools, reorganization of the brain and recovery from brain disease can be studied in a non-invasive way. Imaging correlates of motor recovery in patients with cerebral stroke are well documented [108, 109]. However, little is known about the potential for neural plasticity of patients with cerebellar dysfunction and even less about the ability to regain function through rehabilitative exercises. To date, only a few studies described changes in the organization of the human brain following cerebellar dysfunction. A first study using positron emission tomography (PET) found that patients with cerebellar degeneration performing a self-paced sequential finger opposition task with the right hand had more increase in regional cerebral blood flow in the motor cortex, supplementary motor area, caudal cingulate motor area, and putamen than healthy controls [110]. Moreover, an additional increase of movement-related cortical potential (NS1) was also found in the same degenerative patients [111]. These PET and EEG results suggest an increased motor and attentional effort by which cerebellar patients attempted to compensate for their disease-related deficit. This compensation process appeared to rely heavily on the basal ganglia motor loop rather than the cerebrocerebellar loop. In patients with cerebellar infarction, Kinomoto and colleagues [112] found a different pattern. Using functional magnetic resonance imaging, self-paced grasping of the hand ipsilateral to the lesioned side was associated with a broader activation of the sensorimotor cortex ipsilateral to the hand movement but also a greater recruitment of the cerebellar hemisphere contralateral to the hand movement. Results suggested that the contralesional cerebrocerebellar loop plays an important role in recovery of motor function of patients with cerebellar stroke, unlike the basal ganglia loop which prevailed in degenerative patients. A functional near-infrared spectroscopy (fNIRS) study revealed an increase of hemoglobin oxygenation (oxyHb) in the sensorimotor cortex, supplementary motor area, and prefrontal cortex in patients with cerebellar stroke during treadmill walking [113]. The pattern of increase during the acceleration phase of the gait period did not differ between groups. However, while controls had a reduction of oxyHb during the steady phase, patients manifested the opposite, sustaining the activation of the previously mentioned areas. A more recent fNIRS study of Hatakenaka and colleagues [99] showed similar patterns of the hemoglobin oxygenation during a pursuit rotor task in patients with ataxia following cerebellar stroke. In cerebellar patients, cortical mapping based on oxyHb signal changes revealed sustained activation of the prefrontal regions compared with controls, whereas in controls, a shift of activation from the pre-SMA toward SMA was observed.
The former results are based on cross-sectional assessments. For a better understanding of training-related reorganization of the brain in cerebellar patients, information derived from longitudinal studies is needed. An increasing number of studies show that physical therapy can enhance motor performance in patients with cerebellar degeneration [6, 7, 96]. In an attempt to depict the brain changes that contribute to improvement of motor function, Burciu et al. [114] performed a voxel-based morphometry (VBM) study in patients with cerebellar degeneration. A 2-week postural training resulted in a significant improvement of balance in degenerative patients. Comparing gray matter volumes before and after training revealed an increase primarily within non-affected neocortical regions of the cerebrocerebellar loop, more specifically the premotor cortex. Gray matter changes were observed within the cerebellum as well but were less pronounced. Thus, training may at least to some extent improve function of remaining cerebellar circuitry. Likewise, an animal model of alcohol toxic cerebellar degeneration has shown that training-related improvement of motor performance is accompanied by synaptogenesis of remaining Purkinje cells and increased size of astrocytes [115]. Cerebellar contributions are likely more pronounced in patients with focal disorders leaving parts of the cerebellum intact (e.g., following stroke). In particular, it likely plays a role whether or not the deep cerebellar nuclei are lesioned [116, 117].
Available imaging data show that patients with cerebellar degeneration increasingly rely on extracerebellar areas during motor performance and training. It may depend on the task whether corticobasal ganglia loop is recruited, or parts of the corticocerebellar loop unaffected by the disease. For example, in the PET study by Wessel et al. [110], an internally paced movement was used, and basal ganglia loop was engaged. In contrast, in the VBM study by Burciu et al. [114], movements were externally paced (using markers on a screen), and the premotor cortex was of particular importance. On the other hand, additional use of prefrontal areas may reflect increased reliance on strategic learning. Finally, in training situations where additional explicit motor knowledge becomes available, cerebellar patients may make excessive use of brain regions involved in explicit, rule-based learning (that is basal ganglia, prefrontal cortex, and hippocampus) [118].
To conclude with, present findings stimulate further studies including larger sample sizes, longer training periods, precisely defined training concepts as well as newer imaging analyses in order to clarify how patients with cerebellar dysfunction are still able to learn motor tasks and to what extent other brain structures compensate for the learning deficit inherent to the disease. Pattern and extent of training-related brain changes likely depend on the localization and degree of the underlying cerebellar disease.
Non-invasive Cerebellar Stimulation: Could It Become a Therapeutic Intervention? (P. Celnik)
Since early 1990, the use of non–invasive brain stimulation has grown exponentially. Scientists have applied this technique to understand the physiology and alter how the CNS works. Despite early studies showing that it was possible to stimulate non-invasively the cerebellum, only recently the number of reports using this approach to understand its role in health and disease has increased.
Here, we will refer to the use of two non–invasive cerebellar stimulation techniques, transcranial magnetic stimulation (TMS), and transcranial direct current stimulation (tDCS). In TMS, a rapid electrical current is delivered through a coil generating a magnetic field. If the field is strong enough, it can reach neural tissue inducing a rapidly changing electrical field that can depolarize neurons (for review, see [119]). In tDCS, a small steady current is passed between two large electrodes applied over the scalp. This current, under the right circumstances, has a neuromodulatory effect eliciting increases or decreases of neuronal excitability underneath the electrodes [120].
In recent years, different investigations have applied these techniques to test the role of the cerebellum in cognitive, motor, and sensory behaviors. Although a great number of studies focused on testing the behavioral, imaging, and/or physiological consequences of disrupting cerebellar activity or assessing patients with cerebellar disorders, only few have described how cerebellar physiology in healthy humans changes in association to behavior. In two recent studies, we have shown how the cerebellar motor cortex connectivity changes in association to motor learning. Normally, the cerebellum exerts an inhibitory tone over the primary motor cortex (M1), a phenomenon known as cerebellar brain inhibition (CBI). This can be detected using TMS paired pulse paradigms. Here, a conditioning TMS pulse is applied over the cerebellum and is followed by a test pulse delivered over M1 few milliseconds after. When TMS pulses are combined in this manner, it is possible to observe a reduction in motor evoked potential amplitudes, relative to a single pulse over M1. This effect has been interpreted as an activation of Purkinje cells by the first pulse, which in turn inhibit the dentate and ultimately M1 via a disynaptic excitatory connection through the thalamus [121, 122]. Using this previously described technique, we have found that when healthy young adults learn a motor task, CBI is reduced during the early phase of learning [123] in a proportionally manner to the amount of learning [124]. These studies are interesting because they are the first ones to start unveiling the cerebellar physiological mechanisms, in this case connectivity to M1, underlying motor learning in healthy individuals.
Understanding how cerebellar physiology changes during behavior is important, but to be able to use this information to develop interventions, we also need to learn whether we can modulate cerebellar physiological processes. One study applied cerebellar anodal and cathodal tDCS in young healthy individuals and showed that CBI (cerebellar–M1 connectivity) can be modulated in a polarity-specific manner, without affecting M1 and brainstem excitability [125]. Other studies also showed that it is possible to modulate cerebellar function by applying inhibitory or excitatory forms of repetitive TMS over the cerebellum and assessing the consequent changes in CBI [126] or other distant interconnected brain regions (M1 excitability) [127–129].
Altogether, this information becomes crucial to design interventions geared to change behavior. Indeed, recent studies showed that cerebellar stimulation might have a role as a therapeutic intervention. We have shown that cerebellar direct current stimulation can affect motor learning processes in healthy individuals. For instance, anodal tDCS (the excitatory form of this stimulation) can speed up how healthy people learn reaching and locomotor adaptation tasks [130, 131]. Importantly, unpublished preliminary data [124] suggest that anodal tDCS has similar beneficial effects when stroke patients train a locomotor task geared to improve their walking symmetry.
Other investigations have shown that cerebellar stimulation can be beneficial in other domains beyond motor function. For instance, a recent study showed that applying the inhibitory form of tDCS (cathodal) healthy subjects improved in a word generation task that tests working memory. This beneficial effect was more prominent when the task was more difficult and interpreted as a dis–inhibition of prefrontal areas as a consequence of cerebellar depression by cathodal tDCS [132]. Another study showed that cerebellar tDCS could enhance emotional expression recognition of facial sadness and anger [133].
Finally, other series of studies have applied cerebellar stimulation to decrease disease symptoms. For instance, a sham-controlled study in 74 spinocerebellar degeneration patients delivered multiple single pulses of TMS over the entire cerebellum and described a significant reduction of truncal ataxia [134]. Another investigation showed that applying theta burst stimulation, a repetitive inhibitory form of TMS, in Parkinson's disease patients with l–Dopa–induced dyskinesias, resulted in a significant reduction of symptoms [135]. Similarly, a recent study in patients with essential tremor showed that another inhibitory form of TMS (1 Hz) applied bilaterally over the cerebellum five consecutive days reduced tremors amplitude and severity, an effect that lasted at least for 3 weeks [136]. This line of work, supported by other investigations applying deep thalamic stimulation in patients with essential tremor [137], suggests that stimulating the cerebellum non-invasively may be a way to access cerebellar thalamic cortical circuits to address abnormalities involving these structures.
In sum, studies so far indicate a very interesting role for cerebellar non–invasive stimulation. These techniques have been useful to improve our understanding of cerebellar physiology in humans. More importantly, recent investigations show that cerebellar stimulation can affect behavior and diminish different symptoms in patients with neurological conditions. Although research so far has focused for the most part on patients with neurological conditions that affect non–cerebellar structures, future investigations will determine the role of these techniques in patients who suffer cerebellar damage. Thus, whether applied to enhance a partially damaged cerebellum or to improve cerebellar function to compensate lesions in other non-cerebellar structures, non–invasive cerebellar stimulation has clear potential to become a beneficial therapeutic intervention in the field of neurological rehabilitation.
Consensus and Future Directions
Up to today, there is very little to offer to patients with cerebellar ataxia in terms of medication. One exception for some cerebellar symptoms is aminopyridines. Aminopyridines are recommended as an alternative to acetazolamide in patients with episodic ataxia type 2 and should be tested in patients presenting with downbeat nystagmus. They are beneficial in a subset of patients with downbeat nystagmus [1, 2]. Whether aminopyridines benefit gait ataxia remains to be seen. In all other situations, there is no medication which is of reliable and reproducible help in patients with cerebellar ataxia. Some drugs appear to be promising, particularly acetyl-dl-leucine and chlorzoxazone, but effects need to be verified in large controlled trials [23, 73]. There is consensus that, as yet, treatment is largely limited to physiotherapy, speech therapy, and occupational therapy. This may change in the future, given the increasing knowledge about the pathomechanisms in heredoataxias and advances in genetic therapies.
Training has been recommended for patients with cerebellar disease for many years. Patients, medical doctors, and therapists generally report that training improves function and that they deteriorate when physiotherapy has to be interrupted [138]. Only in very recent years, however, controlled studies have been performed in larger patient populations, both in adults and in children with degenerative disease, indeed showing that motor performance gets better with the help of continuous and intensive motor training [6, 7, 106]. There is agreement that this is an important step forward given the known contributions of the cerebellum to motor learning. However, many open questions remain.
As yet, physiotherapeutical interventions have been studied with a focus on stance and gait ataxia in ambulatory patients [6, 7]. Future studies need to show that training of upper extremities improves function as well and to what extent patients are able to improve who are wheelchair dependent. Another open question is to decide the best moment to introduce which kind of auxiliary device. Furthermore, there is lack of larger well-controlled studies showing that speech therapy and occupational therapy are indeed beneficial [139].
Because disease is ongoing in cerebellar degeneration, there is consensus that life-long treatment is likely required. A limited number of sessions do not make a difference [140]. Studies in cerebral stroke show that massed training is advantageous compared to physiotherapy once or twice a week [141]. The same principle likely applies to cerebellar ataxia, although this needs to be shown. Patients with cerebellar degeneration may gain the most with massed training once or twice per year for a couple of weeks, with continuous self-training under physiotherapeutical supervision in the intervals (see Fig. 1). Furthermore, recent studies in animal models of cerebellar ataxia suggest that intensive motor training slows down the process of degeneration [142, 143]. Long-term rehabilitation studies in humans are needed to determine whether these promising results also hold for cerebellar patients.
At present, little is known which mechanisms of motor learning underly the observed motor improvements and in which learning conditions cerebellar patients perform best. Taylor and coworkers [144] observed that explicit cognitive strategies are helpful in patients with cerebellar degeneration in implicit learning. Whereas in healthy subjects, explicit strategies are helpful early during learning, but impede later stages of intrinsic learning, this detrimental effect was not observed in patients. Thus, instructions by a physical therapist appear to be of particular importance in training of cerebellar patients. Initial findings of Criscimagna-Hemminger et al. [145] suggested that learning to small changes should be emphasized in motor training. However, their observation that patients with cerebellar degeneration have problems to adapt to abrupt perturbations, but are able to adapt to gradually induced perturbations, could not be replicated in two subsequent studies [146, 147].
In summary, there is consensus that evidence-based guidelines for the physiotherapy of degenerative cerebellar ataxia need to be developed. Future studies are needed to design more specific training programs based on the increasing knowledge of the pathophysiology of disordered motor learning in cerebellar patients. These studies should include neuromodulatory approaches. Transcranial direct current stimulation of the cerebellum or other motor areas during training may be the first to test given the most recent results that anodal stimulation of the cerebellum improves motor learning.
Footnotes
Conflict of Interest: None of the authors stated any conflicts of interest.
Contributor Information
W. Ilg, Department of Cognitive Neurology, Hertie Institute for Clinical Brain Research and Centre for Integrative Neuroscience, Tübingen, Germany
A. J. Bastian, Department of Neuroscience, Kennedy Krieger Institute, Johns Hopkins University, Baltimore, MD, USA
S. Boesch, Department of Neurology, Medical University Innsbruck, Innsbruck, Austria
R. G. Burciu, Department of Neurology, University Clinic Essen, University Duisburg—Essen, Hufelandstrasse 55, 45138 Essen, Germany
P. Celnik, Department of Neuroscience, Kennedy Krieger Institute, Johns Hopkins University, Baltimore, MD, USA
J. Claaßen, Department of Neurology, University Clinic Essen, University Duisburg—Essen, Hufelandstrasse 55, 45138 Essen, Germany; Department of Neurology and German Center for Vertigo and Balance Disorders, University Hospital Munich, Munich, Germany
K. Feil, Department of Neurology and German Center for Vertigo and Balance Disorders, University Hospital Munich, Munich, Germany
R. Kalla, Department of Neurology and German Center for Vertigo and Balance Disorders, University Hospital Munich, Munich, Germany
I. Miyai, Neurorehabilitation Research Institute, Morinomiya Hospital, Osaka, Japan
W. Nachbauer, Department of Neurology, Medical University Innsbruck, Innsbruck, Austria
L. Schöls, Department of Neurodegeneration, Hertie Institute for Clinical Brain Research and Centre of Neurology; Tübingen, GermanyGerman Research Center for Neurodegenerative Diseases (DZNE), Tübingen, Germany
M. Strupp, Department of Neurology and German Center for Vertigo and Balance Disorders, University Hospital Munich, Munich, Germany
M. Synofzik, Department of Neurodegeneration, Hertie Institute for Clinical Brain Research and Centre of Neurology; Tübingen, GermanyGerman Research Center for Neurodegenerative Diseases (DZNE), Tübingen, Germany
J. Teufel, Department of Neurology and German Center for Vertigo and Balance Disorders, University Hospital Munich, Munich, Germany
D. Timmann, Email: dagmar.timmann-braun@uni-duisburg-essen.de, Department of Neurology, University Clinic Essen, University Duisburg—Essen, Hufelandstrasse 55, 45138 Essen, Germany.
References
- 1.Strupp M, Schuler O, Krafczyk S, Jahn K, Schautzer F, Buttner U, et al. Treatment of downbeat nystagmus with 3,4-diaminopyridine: a placebo-controlled study. Neurology. 2003;61:165–70. doi: 10.1212/01.wnl.0000078893.41040.56. [DOI] [PubMed] [Google Scholar]
- 2.Strupp M, Kalla R, Claassen J, Adrion C, Mansmann U, Klopstock T, et al. A randomized trial of 4-aminopyridine in EA2 and related familial episodic ataxias. Neurology. 2011;77:269–75. doi: 10.1212/WNL.0b013e318225ab07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kandel M, Beis JM, Le Chapelain L, Guesdon H, Paysant J. Non-invasive cerebral stimulation for the upper limb rehabilitation after stroke: a review. Ann Phys Rehabil Med. 2012;55:657–80. doi: 10.1016/j.rehab.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Bowden MG, Woodbury ML, Duncan PW. Promoting neuroplasticity and recovery after stroke: future directions for rehabilitation clinical trials. Curr Opin Neurol. 2013;26:37–42. doi: 10.1097/WCO.0b013e32835c5ba0. [DOI] [PubMed] [Google Scholar]
- 5.Walker MF, Sunnerhagen KS, Fisher RJ. Evidence-based community stroke rehabilitation. Stroke. 2013;44:293–7. doi: 10.1161/STROKEAHA.111.639914. [DOI] [PubMed] [Google Scholar]
- 6.Ilg W, Synofzik M, Brotz D, Burkard S, Giese MA, Schols L. Intensive coordinative training improves motor performance in degenerative cerebellar disease. Neurology. 2009;73:1823–30. doi: 10.1212/WNL.0b013e3181c33adf. [DOI] [PubMed] [Google Scholar]
- 7.Miyai I, Ito M, Hattori N, Mihara M, Hatakenaka M, Yagura H, et al. Cerebellar ataxia rehabilitation trial in degenerative cerebellar diseases. Neurorehabil Neural Repair. 2012;26:515–22. doi: 10.1177/1545968311425918. [DOI] [PubMed] [Google Scholar]
- 8.Ophoff RA, Terwindt GM, Vergouwe MN, van Eijk R, Oefner PJ, Hoffman SM, et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell. 1996;87:543–52. doi: 10.1016/s0092-8674(00)81373-2. [DOI] [PubMed] [Google Scholar]
- 9.Jen J, Kim GW, Baloh RW. Clinical spectrum of episodic ataxia type 2. Neurology. 2004;62:17–22. doi: 10.1212/01.wnl.0000101675.61074.50. [DOI] [PubMed] [Google Scholar]
- 10.Griggs RC, Moxley RT, 3rd, Lafrance RA, McQuillen J. Hereditary paroxysmal ataxia: response to acetazolamide. Neurology. 1978;28:1259–64. doi: 10.1212/wnl.28.12.1259. [DOI] [PubMed] [Google Scholar]
- 11.Strupp M, Kalla R, Dichgans M, Freilinger T, Glasauer S, Brandt T. Treatment of episodic ataxia type 2 with the potassium channel blocker 4-aminopyridine. Neurology. 2004;62:1623–5. doi: 10.1212/01.wnl.0000125691.74109.53. [DOI] [PubMed] [Google Scholar]
- 12.Alvina K, Khodakhah K. The therapeutic mode of action of 4-aminopyridine in cerebellar ataxia. J Neurosci. 2010;30:7258–68. doi: 10.1523/JNEUROSCI.3582-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weisz CJ, Raike RS, Soria-Jasso LE, Hess EJ. Potassium channel blockers inhibit the triggers of attacks in the calcium channel mouse mutant tottering. J Neurosci. 2005;25:4141–5. doi: 10.1523/JNEUROSCI.0098-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Claassen J, Teufel J, Kalla R, Spiegel R, Strupp M. Effects of dalfampridine on attacks in patients with episodic ataxia type 2: an observational study. J Neurol. 2013;260:668–9. doi: 10.1007/s00415-012-6764-3. [DOI] [PubMed] [Google Scholar]
- 15.Hufner K, Stephan T, Kalla R, Deutschlander A, Wagner J, Holtmannspotter M, et al. Structural and functional MRIs disclose cerebellar pathologies in idiopathic downbeat nystagmus. Neurology. 2007;69:1128–35. doi: 10.1212/01.wnl.0000276953.00969.48. [DOI] [PubMed] [Google Scholar]
- 16.Wagner JN, Glaser M, Brandt T, Strupp M. Downbeat nystagmus: aetiology and comorbidity in 117 patients. J Neurol Neurosurg Psychiatry. 2008;79:672–7. doi: 10.1136/jnnp.2007.126284. [DOI] [PubMed] [Google Scholar]
- 17.Kalla R, Deutschlander A, Hufner K, Stephan T, Jahn K, Glasauer S, et al. Detection of floccular hypometabolism in downbeat nystagmus by fMRI. Neurology. 2006;66:281–3. doi: 10.1212/01.wnl.0000194242.28018.d9. [DOI] [PubMed] [Google Scholar]
- 18.Kalla R, Glasauer S, Buttner U, Brandt T, Strupp M. 4-Aminopyridine restores vertical and horizontal neural integrator function in downbeat nystagmus. Brain. 2007;130:2441–51. doi: 10.1093/brain/awm172. [DOI] [PubMed] [Google Scholar]
- 19.Tsunemi T, Ishikawa K, Tsukui K, Sumi T, Kitamura K, Mizusawa H. The effect of 3,4-diaminopyridine on the patients with hereditary pure cerebellar ataxia. J Neurol Sci. 2010;292:81–4. doi: 10.1016/j.jns.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Kalla R, Spiegel R, Claassen J, Bardins S, Hahn A, Schneider E, et al. Comparison of 10-mg doses of 4-aminopyridine and 3,4-diaminopyridine for the treatment of downbeat nystagmus. J Neuroophthalmol. 2011;31:320–5. doi: 10.1097/WNO.0b013e3182258086. [DOI] [PubMed] [Google Scholar]
- 21.Judge SI, Bever CT., Jr Potassium channel blockers in multiple sclerosis: neuronal Kv channels and effects of symptomatic treatment. Pharmcol Ther. 2006;111:224–59. doi: 10.1016/j.pharmthera.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Claassen J, Spiegel R, Kalla R, Faldon M, Kennard C, Danchaivijitr C, et al. A randomised double-blind, cross-over trial of 4-aminopyridine for downbeat nystagmus—effects on slowphase eye velocity, postural stability, locomotion and symptoms. J Neurol Neurosurg Psychiatry. 2013 doi: 10.1136/jnnp-2012-304736. [DOI] [PubMed] [Google Scholar]
- 23.Feil K, Claaβen J, Bardins S, Teufel J, Krafczyk S, Schneider E, et al. Effect of chlorzoxazone in patients with downbeat nystagmus: a pilot trial. Neurology. 2013;81:1152–8. doi: 10.1212/WNL.0b013e3182a55f6d. [DOI] [PubMed] [Google Scholar]
- 24.Averbuch-Heller L, Tusa RJ, Fuhry L, Rottach KG, Ganser GL, Heide W, et al. A double-blind controlled study of gabapentin and baclofen as treatment for acquired nystagmus. Ann Neurol. 1997;41:818–25. doi: 10.1002/ana.410410620. [DOI] [PubMed] [Google Scholar]
- 25.Schniepp R, Jakl V, Wuehr M, Havla J, Kumpfel T, Dieterich M, et al. Treatment with 4-aminopyridine improves upper limb tremor of a patient with multiple sclerosis: a video case report. Mult Scler. 2013;19:506–8. doi: 10.1177/1352458512461394. [DOI] [PubMed] [Google Scholar]
- 26.Schniepp R, Wuehr M, Ackl N, Danek A, Brandt T, Strupp M, et al. 4-Aminopyridine improves gait variability in cerebellar ataxia due to CACNA 1A mutation. J Neurol. 2011;258:1708–11. doi: 10.1007/s00415-011-5987-z. [DOI] [PubMed] [Google Scholar]
- 27.Schniepp R, Wuehr M, Neuhaeusser M, Benecke AK, Adrion C, Brandt T, et al. 4-Aminopyridine and cerebellar gait: a retrospective case series. J Neurol. 2012;259:2491–3. doi: 10.1007/s00415-012-6595-2. [DOI] [PubMed] [Google Scholar]
- 28.Giordano I, Bogdanow M, Jacobi H, Jahn K, Minnerop M, Schoels L, et al. Experience in a short-term trial with 4-aminopyridine in cerebellar ataxia. J Neurol. 2013;260:2175–6. doi: 10.1007/s00415-013-7029-5. [DOI] [PubMed] [Google Scholar]
- 29.Etzion Y, Grossman Y. Highly 4-aminopyridine sensitive delayed rectifier current modulates the excitability of guinea pig cerebellar Purkinje cells. Exp Brain Res. 2001;139:419–25. doi: 10.1007/s002210100788. [DOI] [PubMed] [Google Scholar]
- 30.Hourez R, Servais L, Orduz D, Gall D, Millard I, de Kerchove d'Exaerde A, et al. Aminopyridines correct early dysfunction and delay neurodegeneration in a mouse model of spinocerebellar ataxia type 1. J Neurosci. 2011;31:11795–807. doi: 10.1523/JNEUROSCI.0905-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anheim M, Monga B, Fleury M, Charles P, Barbot C, Salih M, et al. Ataxia with oculomotor apraxia type 2: clinical, biological and genotype/phenotype correlation study of a cohort of 90 patients. Brain. 2009;132:2688–98. doi: 10.1093/brain/awp211. [DOI] [PubMed] [Google Scholar]
- 32.Le Ber I, Dubourg O, Benoist JF, Jardel C, Mochel F, Koenig M, et al. Muscle coenzyme Q10 deficiencies in ataxia with oculomotor apraxia 1. Neurology. 2007;68:295–7. doi: 10.1212/01.wnl.0000252366.10731.43. [DOI] [PubMed] [Google Scholar]
- 33.Synofzik M, Schicks J, Lindig T, Biskup S, Schmidt T, Hansel J, et al. Acetazolamide-responsive exercise-induced episodic ataxia associated with a novel homozygous DARS2 mutation. J Med Genet. 2011;48:713–5. doi: 10.1136/jmg.2011.090282. [DOI] [PubMed] [Google Scholar]
- 34.Epplen C, Epplen JT, Frank G, Miterski B, Santos EJ, Schöls L. Differential stability of the (GAA)n tract in the Friedreich ataxia (STM7) gene. Hum Genet. 1997;99:834–6. doi: 10.1007/s004390050458. [DOI] [PubMed] [Google Scholar]
- 35.Stemmler TL, Lesuisse E, Pain D, Dancis A. Frataxin and mitochondrial FeS cluster biogenesis. J Biol Chem. 2010;285:26737–43. doi: 10.1074/jbc.R110.118679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Prospero NA, Baker A, Jeffries N, Fischbeck KH. Neurological effects of high-dose idebenone in patients with Friedreich's ataxia: a randomised, placebo-controlled trial. Lancet Neurol. 2007;6:878–86. doi: 10.1016/S1474-4422(07)70220-X. [DOI] [PubMed] [Google Scholar]
- 37.Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, et al. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci. 1997;145:205–11. doi: 10.1016/s0022-510x(96)00231-6. [DOI] [PubMed] [Google Scholar]
- 38.Mariotti C, Solari A, Torta D, Marano L, Fiorentini C, Di Donato S. Idebenone treatment in Friedreich patients: one-year-long randomized placebo-controlled trial. Neurology. 2003;60:1676–9. doi: 10.1212/01.wnl.0000055872.50364.fc. [DOI] [PubMed] [Google Scholar]
- 39.Schöls L, Vorgerd M, Schillings M, Skipka G, Zange J. Idebenone in patients with Friedreich ataxia. Neurosci Lett. 2001;306:169–72. doi: 10.1016/s0304-3940(01)01892-4. [DOI] [PubMed] [Google Scholar]
- 40.Lagedrost SJ, Sutton MS, Cohen MS, Satou GM, Kaufman BD, Perlman SL, et al. Idebenone in Friedreich ataxia cardiomyopathy-results from a 6-month phase III study (IONIA) Am Heart J. 2011;161:639–45. doi: 10.1016/j.ahj.2010.10.038. [DOI] [PubMed] [Google Scholar]
- 41.Meier T, Perlman SL, Rummey C, Coppard NJ, Lynch DR. Assessment of neurological efficacy of idebenone in pediatric patients with Friedreich's ataxia: data from a 6-month controlled study followed by a 12-month open-label extension study. J Neurol. 2012;259:284–91. doi: 10.1007/s00415-011-6174-y. [DOI] [PubMed] [Google Scholar]
- 42.Kearney M, Orrell RW, Fahey M, Pandolfo M. Antioxidants and other pharmacological treatments for Friedreich ataxia. Cochrane Database Syst Rev. 2012;4:CD007791. doi: 10.1002/14651858.CD007791.pub3. Review. [DOI] [PubMed] [Google Scholar]
- 43.Lodi R, Hart PE, Rajagopalan B, Taylor DJ, Crilley JG, Bradley JL, et al. Antioxidant treatment improves in vivo cardiac and skeletal muscle bioenergetics in patients with Friedreich's ataxia. Ann Neurol. 2001;49:590–6. [PubMed] [Google Scholar]
- 44.Hart PE, Lodi R, Rajagopalan B, Bradley JL, Crilley JG, Turner C, et al. Antioxidant treatment of patients with Friedreich ataxia: four-year follow-up. Arch Neurol. 2005;62:621–6. doi: 10.1001/archneur.62.4.621. [DOI] [PubMed] [Google Scholar]
- 45.Cooper JM, Korlipara LV, Hart PE, Bradley JL, Schapira AH. Coenzyme Q10 and vitamin E deficiency in Friedreich's ataxia: predictor of efficacy of vitamin E and coenzyme Q10 therapy. Eur J Neurol. 2008;15:1371–9. doi: 10.1111/j.1468-1331.2008.02318.x. [DOI] [PubMed] [Google Scholar]
- 46.Lynch DR, Willi SM, Wilson RB, Cotticelli MG, Brigatti KW, Deutsch EC, et al. A0001 in Friedreich ataxia: biochemical characterization and effects in a clinical trial. Mov Disord. 2012;27:1026–33. doi: 10.1002/mds.25058. [DOI] [PubMed] [Google Scholar]
- 47.Schöls L, Zange J, Abele M, Schillings M, Skipka G, Kuntz-Hehner S, et al. l-Carnitine and creatine in Friedreich's ataxia. A randomized, placebo-controlled crossover trial. J Neural Transm. 2005;112:789–96. doi: 10.1007/s00702-004-0216-x. [DOI] [PubMed] [Google Scholar]
- 48.Velasco-Sánchez D, Aracil A, Montero R, Mas A, Jiménez L, O'Callaghan M, et al. Combined therapy with idebenone and deferiprone in patients with Friedreich's ataxia. Cerebellum. 2011;10:1–8. doi: 10.1007/s12311-010-0212-7. [DOI] [PubMed] [Google Scholar]
- 49.Boesch S, Sturm B, Hering S, Goldenberg H, Poewe W, Scheiber-Mojdehkar B. Friedreich's ataxia: clinical pilot trial with recombinant human erythropoietin. Ann Neurol. 2007;62:521–4. doi: 10.1002/ana.21177. [DOI] [PubMed] [Google Scholar]
- 50.Subramony SH, May W, Lynch D, Gomez C, Fischbeck K, Hallett M, et al. Cooperative Ataxia Group. Measuring Friedreich ataxia: interrater reliability of a neurologic rating scale. Neurology. 2005;64:1261–2. doi: 10.1212/01.WNL.0000156802.15466.79. [DOI] [PubMed] [Google Scholar]
- 51.Schmitz-Hubsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006;66:1717–20. doi: 10.1212/01.wnl.0000219042.60538.92. [DOI] [PubMed] [Google Scholar]
- 52.Boesch S, Sturm B, Hering S, Scheiber-Mojdehkar B, Steinkellner H, Goldenberg H, et al. Neurological effects of recombinant human erythropoietin in Friedreich's ataxia: a clinical pilot trial. Mov Disord. 2008;23:1940–4. doi: 10.1002/mds.22294. [DOI] [PubMed] [Google Scholar]
- 53.Mariotti C, Fancellu R, Caldarazzo S, Nanetti L, Di Bella D, Plumari M, et al. Erythropoietin in Friedreich ataxia: no effect on frataxin in a randomized controlled trial. Mov Disord. 2012;27:446–9. doi: 10.1002/mds.24066. [DOI] [PubMed] [Google Scholar]
- 54.Gabsi S, Gouider-Khouja N, Belal S, Fki M, Kefi M, Turki I, et al. Effect of vitamin E supplementation in patients with ataxia with vitamin E deficiency. Eur J Neurol. 2001;8:477–81. doi: 10.1046/j.1468-1331.2001.00273.x. [DOI] [PubMed] [Google Scholar]
- 55.Mariotti C, Gellera C, Rimoldi M, Mineri R, Uziel G, Zorzi G, et al. Ataxia with isolated vitamin E deficiency: neurological phenotype, clinical follow-up and novel mutations in TTPA gene in Italian families. Neurol Sci. 2004;25:130–7. doi: 10.1007/s10072-004-0246-z. [DOI] [PubMed] [Google Scholar]
- 56.Hentati F, El-Euch G, Bouhlal Y, Amouri R. Ataxia with vitamin E deficiency and abetalipoproteinemia. Handb Clin Neurol. 2012;103:295–305. doi: 10.1016/B978-0-444-51892-7.00018-8. [DOI] [PubMed] [Google Scholar]
- 57.Kohlschütter A. Abetalipoproteinemia. In: Klockgether T, editor. Handbook of ataxia disorders. New York: Marcel Dekker; 2000. pp. 206–17. [Google Scholar]
- 58.Emmanuele V, López LC, Berardo A, Naini A, Tadesse S, Wen B, et al. Heterogeneity of coenzyme Q10 deficiency: patient study and literature review. Arch Neurol. 2012;69:978–83. doi: 10.1001/archneurol.2012.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Musumeci O, Naini A, Slonim AE, Skavin N, Hadjigeorgiou GL, Krawiecki N, et al. Familial cerebellar ataxia with muscle coenzyme Q10 deficiency. Neurology. 2001;56:849–55. doi: 10.1212/wnl.56.7.849. [DOI] [PubMed] [Google Scholar]
- 60.Artuch R, Brea-Calvo G, Briones P, Aracil A, Galvan M, Espinos C, et al. Cerebellar ataxia with coenzyme Q(10) deficiency: diagnosis and follow-up after coenzyme Q(10) supplementation. J Neurol Sci. 2006;246:153–8. doi: 10.1016/j.jns.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 61.Auré K, Benoist JF, Ogier de Baulny H, Romero NB, Rigal O, Lombès A. Progression despite replacement of a myopathic form of coenzyme Q10 defect. Neurology. 2004;63:727–9. doi: 10.1212/01.wnl.0000134607.76780.b2. [DOI] [PubMed] [Google Scholar]
- 62.Buoni S, Zannolli R, Sorrentino L, Fois A. Betamethasone and improvement of neurological symptoms in ataxia-telangiectasia. Arch Neurol. 2006;63:1479–82. doi: 10.1001/archneur.63.10.1479. [DOI] [PubMed] [Google Scholar]
- 63.Broccoletti T, Del Giudice E, Cirillo E, Vigliano I, Giardino G, Ginocchio VM, et al. Efficacy of very-low-dose betamethasone on neurological symptoms in ataxia-telangiectasia. Eur J Neurol. 2011;18:564–70. doi: 10.1111/j.1468-1331.2010.03203.x. [DOI] [PubMed] [Google Scholar]
- 64.Zannolli R, Buoni S, Betti G, Salvucci S, Plebani A, Soresina A, et al. A randomized trial of oral betamethasone to reduce ataxia symptoms in ataxia telangiectasia. Mov Disord. 2012;27:1312–6. doi: 10.1002/mds.25126. [DOI] [PubMed] [Google Scholar]
- 65.Durr A. Autosomal dominant cerebellar ataxias: polyglutamine expansions and beyond. Lancet Neurol. 2010;9:885–94. doi: 10.1016/S1474-4422(10)70183-6. [DOI] [PubMed] [Google Scholar]
- 66.Jacobi H, Bauer P, Giunti P, Labrum R, Sweeney MG, Charles P, et al. The natural history of spinocerebellar ataxia type 1, 2, 3, and 6: a 2-year follow-up study. Neurology. 2011;77:1035–41. doi: 10.1212/WNL.0b013e31822e7ca0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmitz-Hubsch T, Coudert M, Bauer P, Giunti P, Globas C, Baliko L, et al. Spinocerebellar ataxia types 1, 2, 3, and 6: disease severity and nonataxia symptoms. Neurology. 2008;71:982–9. doi: 10.1212/01.wnl.0000325057.33666.72. [DOI] [PubMed] [Google Scholar]
- 68.Jacobi H, Reetz K, du Montcel ST, Bauer P, Mariotti C, Nanetti L, et al. Biological and clinical characteristics of individuals at risk for spinocerebellar ataxia types 1, 2, 3, and 6 in the longitudinal RISCA study: analysis of baseline data. Lancet Neurol. 2013;12:650–8. doi: 10.1016/S1474-4422(13)70104-2. [DOI] [PubMed] [Google Scholar]
- 69.Ristori G, Romano S, Visconti A, Cannoni S, Spadaro M, Frontali M, et al. Riluzole in cerebellar ataxia: a randomized, double-blind, placebo-controlled pilot trial. Neurology. 2010;74:839–45. doi: 10.1212/WNL.0b013e3181d31e23. [DOI] [PubMed] [Google Scholar]
- 70.Zesiewicz TA, Greenstein PE, Sullivan KL, Wecker L, Miller A, Jahan I, et al. A randomized trial of varenicline (Chantix) for the treatment of spinocerebellar ataxia type 3. Neurology. 2012;78:545–50. doi: 10.1212/WNL.0b013e318247cc7a. [DOI] [PubMed] [Google Scholar]
- 71.Connolly BS, Prashanth LK, Shah BB, Marras C, Lang AE. Comment on a randomized trial of varenicline (Chantix) for the treatment of spinocerebellar ataxia type 3. Neurology. 2012;79:2218. doi: 10.1212/WNL.0b013e318278a059. [DOI] [PubMed] [Google Scholar]
- 72.Filla A, Sacca F, De Michele G. Comment on a randomized trial of varenicline (Chantix) for the treatment of spinocerebellar ataxia type 3. Neurology. 2012;78:1538. doi: 10.1212/WNL.0b013e318257ea5d. [DOI] [PubMed] [Google Scholar]
- 73.Strupp M, Teufel J, Habs M, Feuerecker R, Muth C, van de Warrenburg BP, et al. Effects of acetyl-dl-leucine in patients with cerebellar ataxia: a case series. J Neurol. 2013;260:2556–61. doi: 10.1007/s00415-013-7016-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yabe I, Sasaki H, Yamashita I, Takei A, Tashiro K. Clinical trial of acetazolamide in SCA6, with assessment using the Ataxia Rating Scale and body stabilometry. Acta Neurol Scand. 2001;104:44–7. doi: 10.1034/j.1600-0404.2001.00299.x. [DOI] [PubMed] [Google Scholar]
- 75.Botez MI, Botez-Marquard T, Elie R, Pedraza OL, Goyette K, Lalonde R. Amantadine hydrochloride treatment in heredodegenerative ataxias: a double blind study. J Neurol Neurosurg Psychiatry. 1996;61:259–64. doi: 10.1136/jnnp.61.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Velázquez-Pérez L, Rodríguez-Chanfrau J, García-Rodríguez JC, Sánchez-Cruz G, Aguilera-Rodríguez R, Rodríguez-Labrada R, et al. Oral zinc sulphate supplementation for six months in SCA2 patients: a randomized, double-blind, placebo-controlled trial. Neurochem Res. 2011;36:1793–800. doi: 10.1007/s11064-011-0496-0. [DOI] [PubMed] [Google Scholar]
- 77.Hering S, Achmuller C, Kohler A, Poewe W, Schneider R, Boesch SM. Phenotype variability in spinocerebellar ataxia type 2: a longitudinal family survey and a case featuring an unusual benign course of disease. Mov Disord. 2009;24:774–7. doi: 10.1002/mds.22465. [DOI] [PubMed] [Google Scholar]
- 78.Watase K, Gatchel JR, Sun Y, Emamian E, Atkinson R, Richman R, et al. Lithium therapy improves neurological function and hippocampal dendritic arborization in a spinocerebellar ataxia type 1 mouse model. PLoS Med. 2007;4:e182. doi: 10.1371/journal.pmed.0040182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xia H, Mao Q, Eliason SL, Harper SQ, Martins IH, Orr HT, et al. RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat Med. 2004;10:816–20. doi: 10.1038/nm1076. [DOI] [PubMed] [Google Scholar]
- 80.Alves S, Nascimento-Ferreira I, Auregan G, Hassig R, Dufour N, Brouillet E, et al. Allele-specific RNA silencing of mutant ataxin-3 mediates neuroprotection in a rat model of Machado–Joseph disease. PLoS One. 2008;3:e3341. doi: 10.1371/journal.pone.0003341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tsou WL, Soong BW, Paulson HL, Rodriguez-Lebron E. Splice isoform-specific suppression of the Cav2.1 variant underlying spinocerebellar ataxia type 6. Neurobiol Dis. 2011;43:533–42. doi: 10.1016/j.nbd.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ilg W, Timmann D. Gait ataxia—specific cerebellar influences and their rehabilitation. Mov Disord. 2013;28:1566–75. doi: 10.1002/mds.25558. [DOI] [PubMed] [Google Scholar]
- 83.Deuschl G, Toro C, Zeffiro T, Massaquoi S, Hallett M. Adaptation motor learning of arm movements in patients with cerebellar disease. J Neurol Neurosurg Psychiatry. 1996;60:515–9. doi: 10.1136/jnnp.60.5.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. I. Focal olivocerebellar lesions impair adaptation. Brain. 1996;119:1183–98. doi: 10.1093/brain/119.4.1183. [DOI] [PubMed] [Google Scholar]
- 85.Maschke M, Gomez CM, Ebner TJ, Konczak J. Hereditary cerebellar ataxia progressively impairs force adaptation during goal-directed arm movements. J Neurophysiol. 2004;91:230–8. doi: 10.1152/jn.00557.2003. [DOI] [PubMed] [Google Scholar]
- 86.Smith MA, Shadmehr R. Intact ability to learn internal models of arm dynamics in Huntington's disease but not cerebellar degeneration. J Neurophysiol. 2005;93:2809–21. doi: 10.1152/jn.00943.2004. [DOI] [PubMed] [Google Scholar]
- 87.Synofzik M, Lindner A, Thier P. The cerebellum updates predictions about the visual consequences of one's behavior. Curr Biol. 2008;18:814–8. doi: 10.1016/j.cub.2008.04.071. [DOI] [PubMed] [Google Scholar]
- 88.Bastian AJ. Learning to predict the future: the cerebellum adapts feedforward movement control. Curr Opin Neurobiol. 2006;16:645–9. doi: 10.1016/j.conb.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 89.Marsden J, Harris C. Cerebellar ataxia: pathophysiology and rehabilitation. Clin Rehabil. 2011;25:195–216. doi: 10.1177/0269215510382495. [DOI] [PubMed] [Google Scholar]
- 90.Thach WT, Bastian AJ. Role of the cerebellum in the control and adaptation of gait in health and disease. Prog Brain Res. 2004;143:353–66. doi: 10.1016/s0079-6123(03)43034-3. [DOI] [PubMed] [Google Scholar]
- 91.Balliet R, Harbst KB, Kim D, Stewart RV. Retraining of functional gait through the reduction of upper extremity weight-bearing in chronic cerebellar ataxia. Int Rehabil Med. 1987;8:148–53. doi: 10.3109/03790798709166204. [DOI] [PubMed] [Google Scholar]
- 92.Gill-Body KM, Popat RA, Parker SW, Krebs DE. Rehabilitation of balance in two patients with cerebellar dysfunction. Phys Ther. 1997;77:534–52. doi: 10.1093/ptj/77.5.534. [DOI] [PubMed] [Google Scholar]
- 93.Cernak K, Stevens V, Price R, Shumway-Cook A. Locomotor training using body-weight support on a treadmill in conjunction with ongoing physical therapy in a child with severe cerebellar ataxia. Phys Ther. 2008;88:88–97. doi: 10.2522/ptj.20070134. [DOI] [PubMed] [Google Scholar]
- 94.Freund JE, Stetts DM. Use of trunk stabilization and locomotor training in an adult with cerebellar ataxia: a single system design. Physiother Theory Pract. 2010;26:447–58. doi: 10.3109/09593980903532234. [DOI] [PubMed] [Google Scholar]
- 95.Vaz DV, Schettino Rde C, Rolla de Castro TR, Teixeira VR, Cavalcanti Furtado SR, de Mello Figueiredo E. Treadmill training for ataxic patients: a single-subject experimental design. Clin Rehabil. 2008;22:234–41. doi: 10.1177/0269215507081578. [DOI] [PubMed] [Google Scholar]
- 96.Ilg W, Brötz D, Burkard S, Giese MA, Schöls L, Synofzik M. Long-term effects of coordinative training in degenerative cerebellar disease. Mov Disord. 2010;25:2239–46. doi: 10.1002/mds.23222. [DOI] [PubMed] [Google Scholar]
- 97.Keith RA, Granger CV, Hamilton BB, Sherwin FS. The functional independence measure: a new tool for rehabilitation. Adv Clin Rehabil. 1987;1:6–18. [PubMed] [Google Scholar]
- 98.Morton SM, Bastian AJ. Can rehabilitation help ataxia? Neurology. 2009;73:1818–9. doi: 10.1212/WNL.0b013e3181c33b21. [DOI] [PubMed] [Google Scholar]
- 99.Hatakenaka M, Miyai I, Mihara M, Yagura H, Hattori N. Impaired motor learning by a pursuit rotor test reduces functional outcomes during rehabilitation of poststroke ataxia. Neurorehabil Neural Repair. 2012;26:293–300. doi: 10.1177/1545968311412053. [DOI] [PubMed] [Google Scholar]
- 100.Bastian AJ. Mechanisms of ataxia. Phys Ther. 1997;77:672–5. doi: 10.1093/ptj/77.6.672. [DOI] [PubMed] [Google Scholar]
- 101.Cassidy E, Kilbridge C, Holland A. Management of the ataxias: towards best clinical practice—physiotherapy supplement. London: Ataxia UK; 2009. [Google Scholar]
- 102.Crowdy KA, Kaur-Mann D, Cooper HL, Mansfield AG, Offord JL, Marple-Horvat DE. Rehearsal by eye movement improves visuomotor performance in cerebellar patients. Exp Brain Res. 2002;146:244–7. doi: 10.1007/s00221-002-1171-0. [DOI] [PubMed] [Google Scholar]
- 103.Gillen G. Improving mobility and community access in an adult with ataxia. Am J Occup Ther. 2002;56:462–6. doi: 10.5014/ajot.56.4.462. [DOI] [PubMed] [Google Scholar]
- 104.Harris-Love MO, Siegel KL, Paul SM, Benson K. Rehabilitation management of Friedreich ataxia: lower extremity force-control variability and gait performance. Neurorehabil Neural Repair. 2004;18:117–24. doi: 10.1177/0888439004267241. [DOI] [PubMed] [Google Scholar]
- 105.Battisti C, Toffola ED, Verri AP, Serra E, Dotti MT, Formichi P, et al. Clinical and stabilometric monitoring in a case of cerebellar atrophy with vitamin E deficiency. Brain Dev. 1998;20:253–7. doi: 10.1016/s0387-7604(98)00026-6. [DOI] [PubMed] [Google Scholar]
- 106.Ilg W, Schatton C, Schicks J, Giese MA, Schoels L, Synofzik M. Video game-based coordinative training improves ataxia in children with degenerative ataxia. Neurology. 2012;79:2056–60. doi: 10.1212/WNL.0b013e3182749e67. [DOI] [PubMed] [Google Scholar]
- 107.Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–86. doi: 10.1146/annurev.neuro.21.1.149. Review. [DOI] [PubMed] [Google Scholar]
- 108.Stinear CM, Ward NS. How useful is imaging in predicting outcomes in stroke rehabilitation? Int J Stroke. 2013;8:33–7. doi: 10.1111/j.1747-4949.2012.00970.x. [DOI] [PubMed] [Google Scholar]
- 109.Grefkes C, Fink GR. Reorganization of cerebral networks after stroke: new insights from neuroimaging with connectivity approaches. Brain. 2011;134:1264–76. doi: 10.1093/brain/awr033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wessel K, Zeffiro T, Lou JS, Toro C, Hallett M. Regional cerebral blood flow during a self-paced sequential finger opposition task in patients with cerebellar degeneration. Brain. 1995;118:379–93. doi: 10.1093/brain/118.2.379. [DOI] [PubMed] [Google Scholar]
- 111.Wessel K, Nitschke MF. Cerebellar somatotopic representation and cerebro-cerebellar interconnections in ataxic patients. Prog Brain Res. 1997;114:577–88. doi: 10.1016/s0079-6123(08)63388-9. [DOI] [PubMed] [Google Scholar]
- 112.Kinomoto K, Takayama Y, Watanabe T, Kawasaki T, Onishi K, Yagi H, et al. The mechanisms of recovery from cerebellar infarction: an fMRI study. Neuroreport. 2003;14:1671–5. doi: 10.1097/00001756-200309150-00003. [DOI] [PubMed] [Google Scholar]
- 113.Mihara M, Miyai I, Hatakenaka M, Kubota K, Sakoda S. Sustained prefrontal activation during ataxic gait: a compensatory mechanism for ataxic stroke? Neuroimage. 2007;37:1338–45. doi: 10.1016/j.neuroimage.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 114.Burciu RG, Fritsche N, Granert O, Schmitz L, Spönemann N, Konczak J, et al. Brain changes associated with postural training in patients with cerebellar degeneration: a voxel-based morphometry study. J Neurosci. 2013;33:4594–604. doi: 10.1523/JNEUROSCI.3381-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Klintsova AY, Scamra C, Hoffman M, Napper RM, Goodlett CR, Greenough WT. Therapeutic effects of complex motor training on motor performance deficits induced by neonatal binge-like alcohol exposure in rats: II. A quantitative stereological study of synaptic plasticity in female rat cerebellum. Brain Res. 2002;937:83–93. doi: 10.1016/s0006-8993(02)02492-7. [DOI] [PubMed] [Google Scholar]
- 116.Schoch B, Dimitrova A, Gizewski ER, Timmann D. Functional localization in the human cerebellum based on voxelwise statistical analysis: a study of 90 patients. Neuroimage. 2006;30:36–51. doi: 10.1016/j.neuroimage.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 117.Konczak J, Schoch B, Dimitrova A, Gizewski E, Timmann D. Functional recovery of children and adolescents after cerebellar tumour resection. Brain. 2005;128:1428–41. doi: 10.1093/brain/awh385. [DOI] [PubMed] [Google Scholar]
- 118.Fernández-Seara MA, Aznárez-Sanado M, Mengual E, Loayza FR, Pastor MA. Continuous performance of a novel motor sequence leads to highly correlated striatal and hippocampal perfusion increases. Neuroimage. 2009;47:1797–808. doi: 10.1016/j.neuroimage.2009.05.061. [DOI] [PubMed] [Google Scholar]
- 119.Siebner HR, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res. 2003;148:1–16. doi: 10.1007/s00221-002-1234-2. [DOI] [PubMed] [Google Scholar]
- 120.Nitsche MA, Paulus W. Transcranial direct current stimulation—update 2011. Restor Neurol Neurosci. 2011;29:463–92. doi: 10.3233/RNN-2011-0618. [DOI] [PubMed] [Google Scholar]
- 121.Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation over the cerebellum in humans. Ann Neurol. 1995;37:703–13. doi: 10.1002/ana.410370603. [DOI] [PubMed] [Google Scholar]
- 122.Daskalakis ZJ, Paradiso GO, Christensen BK, Fitzgerald PB, Gunraj C, Chen R. Exploring the connectivity between the cerebellum and motor cortex in humans. J Physiol. 2004;557:689–700. doi: 10.1113/jphysiol.2003.059808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schlerf JE, Galea JM, Bastian AJ, Celnik PA. Dynamic modulation of cerebellar excitability for abrupt, but not gradual, visuomotor adaptation. J Neurosci. 2012;32:11610–7. doi: 10.1523/JNEUROSCI.1609-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jayaram G, Celnik P, Bastian A. Program No 276 21/MM14 2012 Neuroscience Meeting Planner. New Orleans: Society for Neuroscience; 2012. Cerebellar stimulation prolongs symmetric walking post stroke. Online. [Google Scholar]
- 125.Galea JM, Jayaram G, Ajagbe L, Celnik P. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J Neurosci. 2009;29:9115–22. doi: 10.1523/JNEUROSCI.2184-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Popa T, Russo M, Meunier S. Long-lasting inhibition of cerebellar output. Brain Stimul. 2010;3:161–9. doi: 10.1016/j.brs.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 127.Oliveri M, Koch G, Torriero S, Caltagirone C. Increased facilitation of the primary motor cortex following 1 Hz repetitive transcranial magnetic stimulation of the contralateral cerebellum in normal humans. Neurosci Lett. 2005;376:188–93. doi: 10.1016/j.neulet.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 128.Koch G, Oliveri M, Torriero S, Salerno S, Lo Gerfo E, Caltagirone C. Repetitive TMS of cerebellum interferes with millisecond time processing. Exp Brain Res. 2007;179:291–9. doi: 10.1007/s00221-006-0791-1. [DOI] [PubMed] [Google Scholar]
- 129.Fierro B, Giglia G, Palermo A, Pecoraro C, Scalia S, Brighina F. Modulatory effects of 1 Hz rTMS over the cerebellum on motor cortex excitability. Exp Brain Res. 2007;176:440–7. doi: 10.1007/s00221-006-0628-y. [DOI] [PubMed] [Google Scholar]
- 130.Galea JM, Vazquez A, Pasricha N, de Xivry JJ, Celnik P. Dissociating the roles of the cerebellum and motor cortex during adaptive learning: the motor cortex retains what the cerebellum learns. Cereb Cortex. 2011;21(8):1761–70. doi: 10.1093/cercor/bhq246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jayaram G, Tang B, Pallegadda R, Vasudevan EV, Celnik P, Bastian A. Modulating locomotor adaptation with cerebellar stimulation. J Neurophysiol. 2012;107:2950–7. doi: 10.1152/jn.00645.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pope PA, Miall RC. Task-specific facilitation of cognition by cathodal transcranial direct current stimulation of the cerebellum. Brain Stimul. 2012;5:84–94. doi: 10.1016/j.brs.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ferrucci R, Giannicola G, Rosa M, Fumagalli M, Boggio PS, Hallett M, et al. Cerebellum and processing of negative facial emotions: cerebellar transcranial DC stimulation specifically enhances the emotional recognition of facial anger and sadness. Cogn Emot. 2012;26:786–99. doi: 10.1080/02699931.2011.619520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shiga Y, Tsuda T, Itoyama Y, Shimizu H, Miyazawa KI, Jin K, et al. Transcranial magnetic stimulation alleviates truncal ataxia in spinocerebellar degeneration. J Neurol Neurosurg Psychiatry. 2002;72:124–6. doi: 10.1136/jnnp.72.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Koch G. rTMS effects on levodopa induced dyskinesias in Parkinson's disease patients: searching for effective cortical targets. Restor Neurol Neurosci. 2010;28:561–8. doi: 10.3233/RNN-2010-0556. [DOI] [PubMed] [Google Scholar]
- 136.Popa T, Russo M, Vidailhet M, Roze E, Lehéricy S, Bonnet C, et al. Cerebellar rTMS stimulation may induce prolonged clinical benefits in essential tremor, and subjacent changes in functional connectivity: an open label trial. Brain Stimul. 2012;6:175–9. doi: 10.1016/j.brs.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 137.Flora ED, Perera CL, Cameron AL, Maddern GJ. Deep brain stimulation for essential tremor: a systematic review. Mov Disord. 2010;25:1550–9. doi: 10.1002/mds.23195. [DOI] [PubMed] [Google Scholar]
- 138.Fonteyn EM, Keus SH, Verstappen CC, van de Warrenburg BP. Physiotherapy in degenerative cerebellar ataxias: utilisation, patient satisfaction, and professional expertise. Cerebellum. 2013;12:841–7. doi: 10.1007/s12311-013-0495-6. [DOI] [PubMed] [Google Scholar]
- 139.Fonteyn EM, Keus SH, Verstappen CC, Schöls L, de Groot IJ, van de Warrenburg BP. The effectiveness of allied health care in patients with ataxia: a systematic review. J Neurol. 2013 doi: 10.1007/s00415-013-6910-6. [DOI] [PubMed] [Google Scholar]
- 140.Daker-White G, Greenfield J, Ealing J. “Six sessions is a drop in the ocean”: an exploratory study of neurological physiotherapy in idiopathic and inherited ataxias. Physiotherapy. 2013 doi: 10.1016/j.physio.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 141.Vearrier LA, Langan J, Shumway-Cook A, Woollacott M. An intensive massed practice approach to retraining balance post-stroke. Gait Posture. 2005;22:154–63. doi: 10.1016/j.gaitpost.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 142.Fryer JD, Yu P, Kang H, Mandel-Brehm C, Carter AN, Crespo-Barreto J, et al. Exercise and genetic rescue of SCA1 via the transcriptional repressor Capicua. Science. 2011;334:690–3. doi: 10.1126/science.1212673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Safdar A, Bourgeois JM, Ogborn DI, Little JP, Hettinga BP, Akhtar M, et al. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proc Natl Acad Sci U S A. 2011;108:4135–40. doi: 10.1073/pnas.1019581108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Taylor JA, Klemfuss NM, Ivry RB. An explicit strategy prevails when the cerebellum fails to compute movement errors. Cerebellum. 2010;9:580–6. doi: 10.1007/s12311-010-0201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Criscimagna-Hemminger SE, Bastian AJ, Shadmehr R. Size of error affects cerebellar contributions to motor learning. J Neurophysiol. 2010;103:2275–84. doi: 10.1152/jn.00822.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gibo TL, Criscimagna-Hemminger SE, Okamura AM, Bastian AJ. Cerebellar motor learning: are environment dynamics more important than error size? J Neurophysiol. 2013;110:322–33. doi: 10.1152/jn.00745.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schlerf JE, Xu J, Klemfuss NM, Griffiths TL, Ivry RB. Individuals with cerebellar degeneration show similar adaptation deficits with large and small visuomotor errors. J Neurophysiol. 2013;109:1164–73. doi: 10.1152/jn.00654.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kalla R, Glasauer S, Schautzer F, Lehnen N, Büttner U, Strupp M, et al. 4-Aminopyridine improves downbeat nystagmus, smooth pursuit, and VOR gain. Neurology. 2004;62:1228–9. doi: 10.1212/01.wnl.0000118287.68294.e5. [DOI] [PubMed] [Google Scholar]
- 149.Rustin P, von Kleist-Retzow JC, Chantrel-Groussard K, Sidi D, Munnich A, Rötig A. Effect of idebenone on cardiomyopathy in Friedreich's ataxia: a preliminary study. Lancet. 1999;354:477–9. doi: 10.1016/S0140-6736(99)01341-0. [DOI] [PubMed] [Google Scholar]
- 150.Hausse AO, Aggoun Y, Bonnet D, Sidi D, Munnich A, Rötig A, et al. Idebenone and reduced cardiac hypertrophy in Friedreich's ataxia. Heart. 2002;87:346–9. doi: 10.1136/heart.87.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rustin P, Rötig A, Munnich A, Sidi D. Heart hypertrophy and function are improved by idebenone in Friedreich's ataxia. Free Radic Res. 2002;36:467–9. doi: 10.1080/10715760290021333. [DOI] [PubMed] [Google Scholar]
- 152.Artuch R, Aracil A, Mas A, Colomé C, Rissech M, Monrós E, et al. Friedreich's ataxia: idebenone treatment in early stage patients. Neuropediatrics. 2002;33:190–3. doi: 10.1055/s-2002-34494. [DOI] [PubMed] [Google Scholar]
- 153.Buyse G, Mertens L, Di Salvo G, Matthijs I, Weidemann F, Eyskens B, et al. Idebenone treatment in Friedreich's ataxia: neurological, cardiac, and biochemical monitoring. Neurology. 2003;60:1679–81. doi: 10.1212/01.wnl.0000068549.52812.0f. [DOI] [PubMed] [Google Scholar]
- 154.Ribaï P, Pousset F, Tanguy ML, Rivaud-Pechoux S, Le Ber I, Gasparini F, et al. Neurological, cardiological, and oculomotor progression in 104 patients with Friedreich ataxia during long-term follow-up. Arch Neurol. 2007;64:558–64. doi: 10.1001/archneur.64.4.558. [DOI] [PubMed] [Google Scholar]
- 155.Pineda M, Arpa J, Montero R, Aracil A, Domínguez F, Galván M, et al. Idebenone treatment in paediatric and adult patients with Friedreich ataxia: long-term follow-up. Eur J Paediatr Neurol. 2008;12:470–5. doi: 10.1016/j.ejpn.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 156.Lynch DR, Perlman SL, Meier T. A phase 3, double-blind, placebo-controlled trial of idebenone in Friedreich ataxia. Arch Neurol. 2010;67:941–7. doi: 10.1001/archneurol.2010.168. [DOI] [PubMed] [Google Scholar]
- 157.Assadi M, Campellone JV, Janson CG, Veloski JJ, Schwartzman RJ, Leone P. Treatment of spinocerebellar ataxia with buspirone. J Neurol Sci. 2007;260:143–6. doi: 10.1016/j.jns.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 158.Schulte T, Mattern R, Berger K, Szymanski S, Klotz P, Kraus PH, et al. Double-blind crossover trial of trimethoprim-sulfamethoxazole in spinocerebellar ataxia type 3/Machado–Joseph disease. Arch Neurol. 2001;58:1451–7. doi: 10.1001/archneur.58.9.1451. [DOI] [PubMed] [Google Scholar]
- 159.Berg K, Wood-Dauphinee S, Williams J, Gayton D. Measuring balance in the elderly: preliminary development of an instrument. Physiother Can. 1989;41:304–11. [Google Scholar]
- 160.Kiresuk TJ, Smith A, Cardillo JEE. Goal attainment scaling: applications, theory and measurement. Hillsdale: Lawrence Erlbaum Associates; 1994. [Google Scholar]


