Abstract
Recent predictive processing accounts of perception and action point towards a key challenge for the nervous system in dynamically optimizing the balance between incoming sensory information and existing expectations regarding the state of the environment. Here, we report differences in the influence of the preceding sensory context on motor function, varying with respect to both clinical and subclinical features of autism spectrum disorder (ASD). Reach-to-grasp movements were recorded subsequent to an inactive period in which illusory ownership of a prosthetic limb was induced. We analysed the sub-components of reach trajectories derived using a minimum-jerk fitting procedure. Non-clinical adults low in autistic features showed disrupted movement execution following the illusion compared to a control condition. By contrast, individuals higher in autistic features (both those with ASD and non-clinical individuals high in autistic traits) showed reduced sensitivity to the presence of the illusion in their reaching movements while still exhibiting the typical perceptual effects of the illusion. Clinical individuals were distinct from non-clinical individuals scoring high in autistic features, however, in the early stages of movement. These results suggest that the influence of high-level representations of the environment differs between individuals, contributing to clinical and subclinical differences in motor performance that manifest in a contextual manner. As high-level representations of context help to explain fluctuations in sensory input over relatively longer time scales, more circumscribed sensitivity to prior or contextual information in autistic sensory processing could contribute more generally to reduced social comprehension, sensory impairments and a stronger desire for predictability and routine.
Keywords: predictive coding, movement, autism, autistic traits, rubber-hand illusion
1. Introduction
An influential idea in cognitive science is that for the brain to successfully represent and interact with its environment, it engages in an unconscious process of inference about the external causes of sensory stimulation. This idea arises in response to the ambiguous relationship between sensory inputs and worldly states, which seems to necessitate that sensory information be integrated with prior and contextual information regarding the likely causes of input. Contemporary predictive processing accounts of cortical function provide a computationally and biologically plausible mechanism through which this process might occur via the implementation of probabilistic generative models [1,2]. In a recurrent hierarchical arrangement, hypotheses regarding the present causes of input are used to generate predictions of sensory activity at subordinate levels. Hypotheses at each level are then updated iteratively to more closely match predictions to incoming data. In this manner, a dynamic representation of the causal structure of the world comes to be encoded across the neocortex, graded from lower to higher levels of spatial and temporal abstraction. Action is situated within this framework as a process of manipulating the sensory input to match predictions (e.g. predictions regarding the parameters of unfolding proprioceptive feedback) [3,4].
When the brain is cast in this light, we gain a nuanced perspective on how systematic differences in perception and action may emerge between individuals. To improve predictions over time, the influence of sensory input on cortical representations must be weighted by how informative the input is expected to be concerning regularities in the world (i.e. weighted in proportion to the expected precision of the input relative to the precision of existing expectations) [5]. This captures the intuitive principle that sensory information should be drawn upon to a greater degree (at the expense of prior or contextual information) in contexts when the present input is expected to be more highly reliable in determining the state of the external world. Thus, a key task for the nervous system is in optimizing the relative influence of (top-down) prior or contextual information on low-level, local processing. This task can be challenging because different contexts require recruitment of different levels of the cortical hierarchy to accurately represent the causal structure of the world. Changes in sensory input could be best accounted for by inferring the presence of either shorter- or longer-term regularities, for example, and could reflect changes in first- or second-order statistics (for discussion, see [6]).
These concepts have been drawn upon very recently to understand autism spectrum disorder (ASD) and non-clinical variance in autistic features [6–13]. ASD is a highly prevalent developmental condition (approx. 1%) characterized in significant part by social, communicative and behavioural atypicalities [14,15]. Other well-established features include sensory hyper- and hypo-sensitivities, a detail-oriented processing style, a strong preference for predictability and routine, cognitive inflexibility and poor motor coordination [16–19]. Social and non-social ASD-like characteristics vary to a significant degree across the general population, in both children and adults (e.g. [20–24]). Pellicano & Burr [8] argue that non-social features of ASD can be understood as a reduced influence of prior experience on sensory processing (see also [25]). Within the predictive processing framework, this idea has been developed in terms of an increased effect of sensory stimulation on cortical representations of the world, such that perception is bound more closely to lower levels of representation—where the weighting of sensory input is tied to estimations of state-dependent uncertainty [6,9–11]. As mentioned, differences between individuals in the modulation of lower-level processing by higher-level representations can arise because there is not an unequivocal answer regarding the appropriate levels of the hierarchy to recruit in a given situation. Work has just begun in unpacking the implications of this type of account for our understanding of sensory, motor and social symptoms in ASD, as well as for individual differences more broadly (e.g. [7,9,10,13,26–28]).
Here, we examine the influence of the preceding sensory context on sensorimotor function with respect to both clinical and subclinical features of ASD. Specifically, we investigate how reach-to-grasp movements unfold following exposure to the rubber-hand illusion (RHI)—a multisensory illusion of ownership for a prosthetic limb [29,30]. Induction of this illusion (via synchronous tactile stimulation of a visible prosthetic limb and the occluded real limb) influences bodily representations for perception and action, reflected, for example, in drift in perceived arm position towards the prosthetic limb [31,32]. Moving the arm subsequent to an inactive period of illusion induction is therefore likely to require integration between prior, context-sensitive expectations regarding limb position and sensory (proprioceptive) feedback received once movement is underway. This provides a novel setting for examining consequences of the dynamic interaction between sensory evidence and higher-order expectations specified by predictive processing. In this type of paradigm, sensitivity to the context of the illusion can be understood in terms of the relative influence of higher-level representations filtering down the cortical hierarchy to modulate predictions at lower levels. We therefore expected that divergence between individuals across illusory and non-illusory conditions would be revealing in terms of the processing imbalances hypothesized by inferential accounts of ASD. In particular, we expect individuals higher in autistic features to be increasingly disinclined to let higher-level representations be informative about low-level sensory input; conversely, they should be more inclined to consider their sensory input informative.
Non-clinical individuals grouped by their level of autistic traits show differences in sensitivity to the presence of the RHI in reaching movements [7] (see also [33]). Specifically, individuals low in autistic features exhibit reduced smoothness of movement following the illusion compared with a control condition, while individuals high in autistic features show uniformly smooth movements across conditions. In this study, we examined whether adults with ASD demonstrate similarly reduced sensitivity in movement to the preceding context of the RHI. Crucially, we compare those with a diagnosis of ASD with both non-clinical individuals high in autistic features and non-clinical individuals low in autistic features. This allows us to assess how the predictive processing account of individual differences we described and developed above coheres with clinical and non-clinical features of movement. Additionally, we decompose reaching trajectories into sub-components (described in §2) to more closely examine whether differences in action following the illusion are consistent with differences in the context-sensitive integration of sensory feedback with prior expectations.
2. Material and methods
(a). Participants
Three participant groups were involved in this experiment. Thirty non-clinical adults were recruited via university and hospital advertisements and separated into two groups based on a median-split of autism-spectrum quotient scores (AQ, an adult inventory measure of social and non-social autistic traits [20]). Thus, we examined a Low AQ group of 15 non-clinical individuals (eight female; mean age: M = 30.20, s.d. = 7.31 years; AQ: M = 8.07, s.d. = 3.96) and a High AQ group of 15 non-clinical individuals (five female; mean age: M = 29.87, s.d. = 8.61 years; AQ: M = 22.13, s.d. = 5.74). A third group of 15 adults with ASD were recruited via advertisements and the Monash Alfred Psychiatry Research Centre volunteer database (four female; mean age: M = 29.27, s.d. = 9.17 years; AQ: M = 28.60, s.d. = 10.47).
Diagnoses were of either autistic disorder (high-functioning) or Asperger's disorder. All diagnoses were according to DSM-IV-TR criteria [34] and established by a qualified clinician external to the study (psychiatrist, paediatrician or clinical psychologist). All participants were right-handed. Further demographic and clinical characteristics are reported in electronic supplementary material, table S1.
(b). Procedure
Participants sat at a desk with their right arm resting in a fixed position. A prosthetic right arm was posed in an anatomically plausible position in front of the participant, while the participant's corresponding limb was hidden from view. The limbs were spaced 20 cm apart in the horizontal plane (as measured from the middle fingers), aligned in the vertical plane and positioned with approximately equivalent hand configuration and orientation. The prosthesis was visually similar to a human limb with respect to physical proportions, skin detail and compression to touch. An experimenter applied stroking concurrently to each limb using a pair of soft brushes (2–2.5 × 0.5 cm tip size). Stimulation was applied to the dorsal surface of the fingers and hand. Each trial consisted of either synchronous or asynchronous stimulation, applied for 3 min at approximately 1–2 Hz. In the synchronous stimulation condition, stroking was applied in temporal synchrony to corresponding locations of each limb. In asynchronous stimulation trials, stroking was both temporally and spatially asynchronous. Asynchronous stimulation constitutes the standard control condition in research employing the RHI and tends not to elicit behavioural and physiological responses characteristic of the RHI (for review, see [30]). Participants were instructed to attend to the stroking of the prosthetic limb during stimulation. Sixteen trials were conducted in total (eight with synchronous stimulation; eight with asynchronous stimulation). Trial order was randomized for each participant.
Each trial included pre- and post-stimulation estimates of limb position and a post-stimulation reach-to-grasp movement. At the end of each trial, participants completed a questionnaire to assess their subjective experience of the illusion. Throughout the experiment, the real and prosthetic limbs were situated in separate compartments of an observation box that spanned the length of the desk. Compartmentalized lighting allowed the prosthetic limb to be visible only in the stimulation phase of each trial, while the participant's limb was occluded throughout the experiment. The reach target was presented only in the reaching phase of each trial. A smock blocked the participant's view of how the real and prosthetic limbs entered the box.
(c). Perceptual measures
(i). Illusion statements
Participants reported on their experience of the stimulation period in each trial using a questionnaire comprising 11 statements (see [7] for full description). Three statements were worded to capture the typical phenomenological qualities of the illusion: (i) ‘It seemed as if I was feeling the touch of the paintbrush in the location where I saw the rubber hand being touched’, (ii) ‘It seemed as though the touch I felt was caused by the paintbrush I could see touching the rubber hand’ and (iii) ‘It felt as if the rubber hand was my hand’. Also included were eight control statements that were not expected to differ systematically between synchronous and asynchronous conditions (e.g. ‘It seemed as if I might have more than one right hand or arm’). Participants rated their agreement with each statement on a 20 cm horizontal visual analogue scale. Average ratings across illusion-related and control items were analysed. Statement order was randomized for each trial.
(ii). Illusion onset latency
Participants pressed a footswitch during the stimulation phase of each trial when they first agreed with the statement, ‘It seemed as though the touch I felt was caused by the paintbrush I could see touching the rubber hand’, or the statement, ‘It seemed as if I was feeling the touch of the paintbrush in the location where I saw the rubber hand being touched’. Participants were not required to press the footswitch if they did not agree with either statement (these trials were treated as missing data; missing data owing to equipment issues described below). The illusion onset latency for each trial was recorded as the duration between when the experimenter first began applying stimulation and when the participant first pressed the footswitch.
(iii). Proprioceptive drift
Directly before and after each stimulation period, participants estimated the position of their right hand. A bar was positioned across the box, above the participant's right hand, in the horizontal plane from the participant's perspective. The experimenter slid a marker across the bar, and the participant verbally indicated when the marker was estimated to be directly above the centre knuckle of their hand. The experimenter recorded the position of the marker to the nearest millimetre. Proprioceptive drift was calculated as the difference between pre- and post-stimulation estimates, with positive values indicating drift towards the prosthetic limb.
(d). Kinematic measures
Participants performed a reach-to-grasp movement in each trial using the hand that had received stimulation. The target of the movement was a 4.5 × 18 cm bright yellow cylinder, located 13 cm forward and 5 cm to the right of the participant's hand. Participants began the movement when a light was switched on to allow vision of the upper approximately 2 cm of the target; vision of their hand and the prosthetic limb was blocked throughout the movement.
Displacement was recorded continuously by an electromagnetic tracking device together with a sensor attached centrally to the dorsal surface of the hand (Ascension Technology Corporation 3DGuidance trakStar with mid-range transmitter; reported resolution of 1.4 mm and 0.5°). Recording was at 60 Hz with a 50 Hz notch filter. The three spatial dimensions of movement were the subject of analyses. All trials were visually screened for recording artefacts. Two participants were excluded from reach analyses owing to extensive recording failures in these sessions; across the remaining 43 participants (688 trials), 11 trials in total were similarly excluded owing to equipment issues. For each trial, movement onset and offset were defined as when velocity first exceeded 20 mm s−1 for 0.05 s when proceeding anterograde and retrograde through the time series, respectively (this follows [7,35,36]). A Savitzky–Golay filter was used to smooth and then differentiate displacement data (frame length = 11–19; polynomial order = 2–3).
Area under the curve of the Euclidean jerk profile was a measure of interest. Jerk is the change in acceleration over time (the third derivative of displacement). Minimization of mean or integrated squared jerk is a theoretical criterion for producing smooth, naturalistic point-to-point trajectories [37]. Previous clinical studies have used jerk measures to quantify movement performance (e.g. [38–40]), and we have previously demonstrated that individuals low in autistic features show increased integrated jerk in reaching movements following the RHI [7].
Movement sub-components were also examined to probe for features that may underlie differences in overall execution. This analysis drew on a fitting method recently developed by Friedman and colleagues to decompose recorded trajectories into constituent sub-movements [41,42]. This procedure finds the minimum number of (potentially overlapping) sub-movements that sum together to reproduce the observed velocity profile. Individual sub-movements are assumed to minimize jerk (i.e. show a Gaussian velocity profile) and fit certain temporal and spatial constraints. The fitting procedure described in [42] was run for 1 : 10 sub-movements with an error threshold of 0.03. Spatial bounds were set for x (−200 to 300) and y (−100 to 200) dimensions based on the physical proportions of the task environment. This procedure returned two to three sub-movements for 84% of trials, with a single trial returning no valid solution after not converging. The average reconstruction error was 0.0166 (s.d. = 0.0026), similar to [42]. We examined parameters of the first two velocity sub-movements as more than 99% of trials contained at least two sub-movements. These parameters were peak velocity, time to peak velocity, full width at half maximum (a measure of duration) and onset time.
3. Results
(a). Perceptual measures
The typical perceptual effects of the RHI were observed across groups. A 2 × 2 × 3 mixed ANOVA was performed for self-reported ratings of the illusion, with statement type (control versus illusion-related), stimulation type (synchronous versus asynchronous) and group (low AQ versus high AQ versus clinical) as factors. Importantly, a significant interaction was observed between statement type and stimulation type (F1,42 = 113.09, p < 0.0001). Post hoc t-tests indicated that illusion items were rated higher following synchronous stimulation (M = 12.52, s.d. = 5.55) compared with asynchronous stimulation (M = 3.99, s.d. = 3.79), t44 = 11.09, p < 0.0001, Hedges' gav = 1.81 (figure 1). Similarly, illusion items were rated higher than control items for synchronous stimulation, t44 = 9.03, p < 0.0001, Hedges' gav = 1.32 (illusion ratings: M = 12.52, s.d. = 5.55; control ratings: M = 6.74, s.d. = 3.16), but not for asynchronous stimulation, for which a lesser difference in the opposite direction was observed, t44 = −4.59, p < 0.0001, Hedges' gav = 0.37 (illusion ratings: M = 3.99, s.d. = 3.79; control ratings: M = 5.25, s.d. = 3.00). Together, these results indicate that the phenomenological features of the illusion typically reported in the literature tended to be experienced following synchronous stimulation but not asynchronous stimulation, as expected. A significant interaction effect was also observed between statement type, stimulation type and group (F2,42 = 4.33, p = 0.02). Post hoc tests indicated that all three groups demonstrated the same pattern of effects as reported above for the whole sample, however (these post hoc tests and further main effects are reported in the electronic supplementary material).
Figure 1.
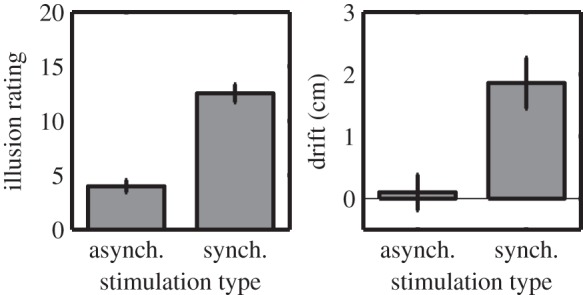
Perceptual measures of the RHI. Mean illusion ratings and drift in perceived arm position are shown across RHI conditions. synch., synchronous; asynch., asynchronous. Error bars indicate s.e.
Groups similarly did not differ in the average time required to induce the illusion in the synchronous stimulation condition. A Kruskal–Wallis test with group as the factor was performed on illusion onset latency during synchronous stimulation. (A non-parametric test was used because the distribution of this data was positively skewed.) There was no significant difference across the three participant groups, χ2 (2, n = 33) = 0.14, p = 0.93. Mean onset latency for the full sample was 51.25 s (s.d. = 42.29; median = 48.65; median absolute difference = 36.60).
Synchronous stimulation induced drift in perceived arm position towards the prosthetic limb, and, consistent with other perceptual measures, this effect did not differ in relation to autistic characteristics. A 2 × 3 mixed ANOVA was performed for proprioceptive drift measurements, with stimulation type (synchronous versus asynchronous) and group (low AQ versus high AQ versus clinical) as factors. Drift in perceived arm position towards the prosthetic limb was significantly greater for synchronous (M = 1.86, s.d. = 2.74) than asynchronous stimulation (M = 0.10, s.d. = 1.91), F1,42 = 43.73, p < 0.0001, 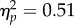 ,
, 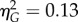 (figure 1). No other main or interaction effects were significant (p > 0.05).
(figure 1). No other main or interaction effects were significant (p > 0.05).
(b). Kinematic measures
(i). Integrated jerk
While the perceptual effects of the RHI were intact across groups, autistic characteristics were found to modulate reaching movements performed subsequent to the experience of the illusion. A 2 × 3 mixed ANOVA was performed for the integrated jerk index of movement performance, with stimulation type (synchronous versus asynchronous) and group (low AQ versus high AQ versus clinical) as factors. A significant interaction was observed between stimulation type and group, F2,40 = 5.26, p = 0.009, 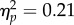 (figure 2). In replication of our previous study in non-clinical individuals [7], the low AQ group showed increased integrated jerk in movements performed subsequent to synchronous stimulation (M = 15.77, s.d. = 3.58) compared with asynchronous stimulation (M = 14.30, s.d. = 3.06), t13 = 3.55, p = 0.004, Hedges' gav = 0.43. By contrast, the high AQ group showed no difference between synchronous (M = 14.14, s.d. = 4.10) and asynchronous (M = 14.06, s.d. = 3.79) stimulation conditions, t13 = 0.23, p = 0.83, Hedges' gav = 0.02. Moreover, in this study, we were able to extend this analysis to individuals with ASD, who similarly showed no change in integrated jerk across synchronous (M = 18.18, s.d. = 5.90) and asynchronous (M = 18.56, s.d. = 6.05) conditions, t14 = −0.79, p = 0.44, Hedges' gav = 0.06. Additionally, there was a main effect of group, F2,40 = 3.59, p = 0.037,
(figure 2). In replication of our previous study in non-clinical individuals [7], the low AQ group showed increased integrated jerk in movements performed subsequent to synchronous stimulation (M = 15.77, s.d. = 3.58) compared with asynchronous stimulation (M = 14.30, s.d. = 3.06), t13 = 3.55, p = 0.004, Hedges' gav = 0.43. By contrast, the high AQ group showed no difference between synchronous (M = 14.14, s.d. = 4.10) and asynchronous (M = 14.06, s.d. = 3.79) stimulation conditions, t13 = 0.23, p = 0.83, Hedges' gav = 0.02. Moreover, in this study, we were able to extend this analysis to individuals with ASD, who similarly showed no change in integrated jerk across synchronous (M = 18.18, s.d. = 5.90) and asynchronous (M = 18.56, s.d. = 6.05) conditions, t14 = −0.79, p = 0.44, Hedges' gav = 0.06. Additionally, there was a main effect of group, F2,40 = 3.59, p = 0.037, 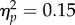 . Pairwise comparisons with Bonferroni adjustment indicated that the clinical group (M = 18.37, s.d. = 5.90) exhibited significantly greater integrated jerk in their reaching movements (across conditions) than the high AQ group (M = 14.10, s.d. = 3.89; p = 0.015). The low AQ group did not differ significantly from either other group (p > 0.05). No other main or interaction effects were significant (p > 0.05).
. Pairwise comparisons with Bonferroni adjustment indicated that the clinical group (M = 18.37, s.d. = 5.90) exhibited significantly greater integrated jerk in their reaching movements (across conditions) than the high AQ group (M = 14.10, s.d. = 3.89; p = 0.015). The low AQ group did not differ significantly from either other group (p > 0.05). No other main or interaction effects were significant (p > 0.05).
Figure 2.
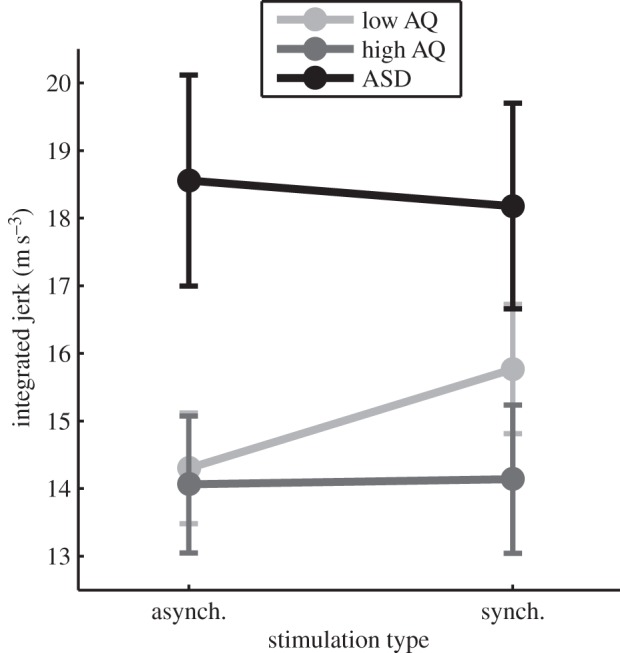
Mean integrated jerk of reaching movements performed subsequent to the RHI. synch., synchronous; asynch., asynchronous. Error bars indicate s.e.
(ii). Sub-movement analysis
Modelling the constituent sub-components of the reach-to-grasp movements performed subsequent to the illusion shed light on the features of movement that likely contributed to the differences in performance noted in the previous section. Fitted sub-components for a single trial are illustrated in figure 3a. Parameters of the first and second sub-movements were each analysed in a 2 × 3 mixed ANOVA, with stimulation type (synchronous versus asynchronous) and group (low AQ versus high AQ versus clinical) as the factors.
Figure 3.
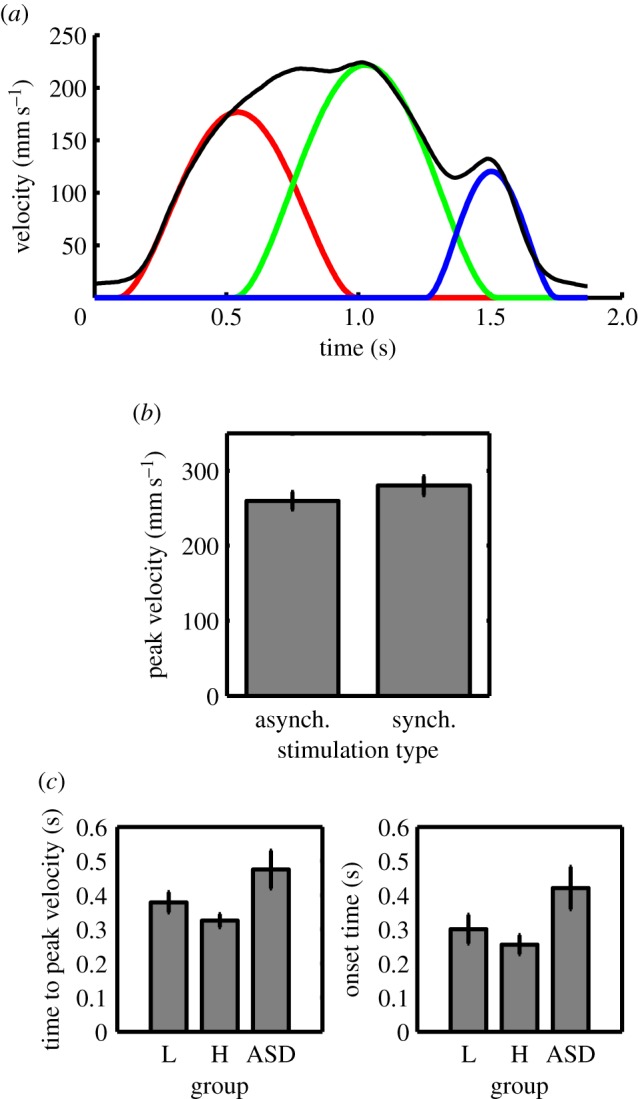
Sub-component analysis of reach-to-grasp movements. (a) Modelled sub-components of a single reach-to-grasp movement. Black, recorded data; red, first sub-movement; green, second sub-movement; blue, third sub-movement. (b) Mean peak velocity of the second sub-movement for the low AQ group across RHI conditions. (c) Mean time to peak velocity (first sub-movement) and onset time (second sub-movement) across participant groups. L = low AQ; H = high AQ. Error bars indicate s.e. (Online version in colour.)
Peak velocity of the first sub-movement did not differ across groups or RHI conditions, nor was there an interaction effect (p > 0.05). However, for the second sub-movement, there was a significant interaction between stimulation type and group, F2,40 = 4.88, p = 0.013. There was no main effect of either factor (p > 0.05). Post hoc t-tests indicated that the low AQ group showed greater peak velocity in the second sub-movement following synchronous stimulation (M = 280.06, s.d. = 46.87) compared with asynchronous stimulation (M = 259.82, s.d. = 43.09), t13 = 3.527, p = 0.004, Hedges' gav = 0.44 (figure 3b). By contrast, the high AQ and clinical groups showed no difference in peak velocity between the synchronous (high AQ: M = 260.46, s.d. = 44.50; clinical: M = 288.96, s.d. = 112.95) and asynchronous (high AQ: M = 273.35, s.d. = 45.50; clinical: M = 301.48, s.d. = 121.01) stimulation conditions (high AQ: t13 = −1.39, p = 0.19, Hedges' gav = 0.28; clinical: t14 = −1.28, p = 0.22, Hedges' gav = 0.10).
There was a significant main effect of group for time to peak velocity of the first sub-movement, F2,40 = 3.85, p = 0.03, 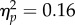 (figure 3c). Pairwise comparisons with Bonferroni adjustment indicated that the clinical group (M = 0.48, s.d. = 0.21) took longer to reach peak velocity of the first sub-movement than the high AQ group (M = 0.33, s.d. = 0.07; p = 0.009). The low AQ group did not differ significantly from either other group (p > 0.05). The other main and interaction effects for this variable were non-significant (p > 0.05). Time to peak velocity of the second sub-movement showed no main or interaction effects (p > 0.05).
(figure 3c). Pairwise comparisons with Bonferroni adjustment indicated that the clinical group (M = 0.48, s.d. = 0.21) took longer to reach peak velocity of the first sub-movement than the high AQ group (M = 0.33, s.d. = 0.07; p = 0.009). The low AQ group did not differ significantly from either other group (p > 0.05). The other main and interaction effects for this variable were non-significant (p > 0.05). Time to peak velocity of the second sub-movement showed no main or interaction effects (p > 0.05).
A significant main effect of group also existed for the onset time of the second sub-movement, F2,40 = 3.39, p = 0.044, 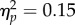 (figure 3c). Similar to the difference in time to peak velocity of the first sub-movement, pairwise comparisons with Bonferroni adjustment indicated that the onset time of the second sub-movement was later for the clinical group (M = 0.42, s.d. = 0.24) than for the high AQ group (M = 0.25, s.d. = 0.10; p = 0.016). The low AQ group did not differ significantly from either other group (p > 0.05), and the further main and interaction effects for this variable were non-significant (p > 0.05). Further analysis of the reach data is reported in the electronic supplementary material.
(figure 3c). Similar to the difference in time to peak velocity of the first sub-movement, pairwise comparisons with Bonferroni adjustment indicated that the onset time of the second sub-movement was later for the clinical group (M = 0.42, s.d. = 0.24) than for the high AQ group (M = 0.25, s.d. = 0.10; p = 0.016). The low AQ group did not differ significantly from either other group (p > 0.05), and the further main and interaction effects for this variable were non-significant (p > 0.05). Further analysis of the reach data is reported in the electronic supplementary material.
4. Discussion
This study was designed to examine sensitivity to the preceding sensory context in motor function with respect to clinical and subclinical autistic features. In non-clinical individuals, those higher in autistic characteristics were less sensitive to the presence of the RHI in their reaching movements than those lower in autistic characteristics. Adults with a diagnosis of ASD similarly exhibited little difference in kinematic parameters across illusory and control conditions. Sensitivity to the illusion manifested in the low AQ group as increased integrated jerk (in replication of our previous study of subclinical autistic characteristics; [7]) and increased peak velocity in the second sub-component of movement. The latter feature is likely to contribute to or underlie increased integrated jerk, as integrated jerk is responsive to changes in the shape of the velocity profile. All groups reported the typical subjective effects of the illusion and exhibited the same degree of drift in perceived arm position towards the prosthetic limb. Thus, the observed differences in reaching movements are explained better in terms of an association between autistic features and sensitivity to prior (contextual) information rather than a general resistance to the illusion in the high AQ and clinical groups.
This pattern of results supports and extends to movement the recent notion that the autism spectrum is characterized by reduced top-down modulation of sensory processing [6–10]. In a predictive processing view of the RHI paradigm, initial expectations for arm position influenced by the illusion are updated iteratively with sensory (proprioceptive) feedback received once movement is underway. In the low AQ group, movement in illusory and control conditions began in the same manner (as indicated by the lack of difference between conditions in the parameters of the first sub-movement) but differed in peak velocity of the second sub-movement. This is consistent with the hypothesis that movement performance in this group is modulated by conflict between sensory feedback and illusory expectations for arm position: as movement unfolds, participants in the low AQ group accumulate sensory evidence for the true position of the arm and in that light make in-flight corrections to the movement. Following on from this, we can understand the reduced sensitivity to the illusion in the high AQ and clinical groups as reflecting a greater weighting of sensory feedback in determining arm position during movement, such that prior representations of the environment are relatively circumscribed in their influence.
Individuals with ASD were also distinct from non-clinical individuals high in autistic traits, the former showing greater integrated jerk in movements across conditions. The sub-movement analysis was again revealing about the features of movement that may contribute to this performance difference; specifically, the clinical group (compared to the high AQ group) showed a later time to peak velocity of the first sub-component of movement and a later onset time of the second sub-component of movement. This sluggishness in the early stage of movement coheres with previous research investigating abnormalities in movement initiation and preparation in ASD (e.g. [43,44]). Thus, while non-clinical individuals higher in autistic traits resemble clinical individuals in their lack of motor sensitivity to the RHI, these groups are distinct in early features of movement that manifest across contexts. A regression analysis indicated no relationship across the sample between AQ score and movement performance across conditions (reported in the electronic supplementary material), similarly suggesting that these features of movement are specific to clinical individuals. This highlights how examining both clinical individuals and non-clinical variation in autistic traits in the same experiment can provide a more complete characterization of how the clinical condition presents with respect to variation that exists across the general population. Furthermore, this pattern of results points to the possibility that the processing differences that contribute to reduced sensitivity to the RHI in the high AQ and ASD groups occurs to such an extent in the latter that difficulties in motor performance are experienced across contexts.
Motor incoordination (e.g. clumsiness) occurs commonly in ASD across a range of different motor behaviours, including reaching movements and gait [18,19]. The results of this study furnish an account of the motor symptoms of ASD in terms of the relative weighting of prior or contextual information against sensory feedback. A fundamental assumption of the Bayesian approach to perception is that sensory information is noisy and ambiguous, such that drawing upon prior and contextual information is necessary to determine the state of the external world. Thus, the increased weighting of sensory information in perceptual inference that is suggested to occur in ASD leads directly to an account of motor incoordination in terms of how the brain estimates the state of the body during movement. Namely, relying too highly on the incoming sensory information at the expense of prior information should typically lead to a less accurate sense of body position, which may contribute to clinical symptoms of motor incoordination (and the reduced smoothness of movement observed for the clinical group in this study). Moreover, while the context of the illusion misleads performance in the RHI, sensitivity to higher-order contextual information may more commonly be of benefit to accurate motor performance (e.g. when performing movement without visual feedback, or when contextual factors like weight on the arm modulate the relationship between actions and their sensory consequences).
The concepts that we draw upon to elucidate differences in sensitivity to the RHI across the groups may also be useful in understanding the observed differences in movement initiation in ASD. In their application of predictive processing to action, Friston and co-workers [45] have emphasized the role of sensory attenuation in movement initiation. In brief, their notion is that action comes about when predictions regarding the flow of proprioceptive input are fulfilled by peripheral responses that engage the muscles to change the bottom-up signal [3,4]. This contrasts with the more passive process of updating predictions to match input suggested to occur in the perceptual system. To begin movement, sensory evidence for the hypothesis concerning the current (true) arm position must be down-weighted such that alternative hypotheses regarding arm position are favoured. As noted, the mechanism thought to underlie a reduced influence of prior or higher-level expectations in autistic perception is a tendency to weight the sensory input highly relative to top-down predictions (i.e. increased gain on prediction errors) [7,9,10]. An implication of increased sensory weighting may be that the attenuation of sensory evidence that is suggested to facilitate movement initiation is compromised, contributing to differences in the early stages of movement.
Is increased sensory weighting in ASD contextually driven or a chronic feature of sensory processing? In the predictive processing framework, the relative weighting of sensory information against prior expectations is adjusted top-down in a state-dependent manner in response to changes in the expected precision of sensory signals (i.e. the estimated uncertainty of the environment). This is a mechanism that has the potential to add further nuance regarding how subtle differences in sensory processing may manifest in a complex manner across contexts, which may be crucial in accounting for the complex pattern of sensory differences in ASD and the heterogeneity in symptoms reported between and within individuals with ASD. The results of this study point to both context-independent differences in ASD, exhibited in movement performance across conditions, and context-dependent differences in ASD, exhibited in differing responses to the illusion in movement. While there is evidence that proprioceptive estimates in non-illusory conditions are no more accurate or precise in ASD than in controls [46], this study differs in incorporating an uncertain context in which conflict between cues for arm position derived from the illusion and proprioceptive input during illusion induction may induce an expectation for low precision in the sensory input. That is, sensorimotor input can normally be interpreted unequivocally under long-held, very stable expectations about body-image, body-schema and bodily self-awareness, but the RHI challenges these expectations and throws doubt on the sensory input. Those higher in the autism spectrum may therefore weight sensory input more strongly than others in particular contexts owing to a reduced response to cues that suggest that sensory input should be distrusted.
Importantly, we can begin to see how differences in the depth of the cortical hierarchy through which updating occurs could underlie clinical and subclinical autistic features. Specifically, we can situate non-clinical individuals lower in autistic traits as tending to appeal more to higher-level causes to explain sensory input when compared with those higher in autistic features, while clinical individuals have a tendency to predominantly recruit levels that are lower again. It is clear that differences in this regard could be adaptive (as in non-clinical variation in autistic features) or not (as in the clinically defined condition), given the equivocal challenge of determining where in the causal hierarchy to account for changes in sensory input from within the skull. We can speculate that differences in the recruitment of higher levels in the hierarchy may be reflected in neurobiological features found in ASD, such as reduced long-range connectivity (e.g. [47]) and greater intra-individual variability in evoked cortical responses [48]. As representations of context help to explain fluctuations in sensory input over relatively longer time scales, more circumscribed context sensitivity in autistic sensory processing could contribute more generally to reduced social comprehension, sensory impairments and a stronger desire for predictability and routine.
There is evidence that the perceptual experience of the RHI is facilitated by prior expectations regarding bodily representation (e.g. [31,49]). That the typical perceptual effects of the RHI were exhibited by the ASD group thus suggests that individuals with ASD are able to learn informative priors but differ instead in the relative weighting of priors against conflicting sensory signals (see [9,12,13] for discussion). This is consistent with the mixed evidence on visual illusions in ASD, which tends to suggest that prior information influences visual perception in ASD but to a lesser extent than controls [50]. The differences in motor behaviour following the illusion despite the similar perceptual experience across groups points to how subtle atypicalities in the integration of prior expectations with sensory information may manifest differently across tasks depending on factors such as the nature of the priors involved (e.g. expectations regarding bodily representation developed over long time scales versus shorter-term contextual information regarding body position during movement). Similarly, the predictive processing account emphasizes the continual and dynamic integration of incoming sensory signals with existing expectations regarding the causes of sensory input. The reaching task used in the present experiment is likely to be more sensitive to this process when compared to perceptual measures of the illusion, owing to the immediate demand of integrating sensory feedback with existing estimates of arm position as movement unfolds. Thus, it may similarly be important for future research in this area to consider the temporal nature of perceptual inference and include measures that are sensitive to this process.
Reduced sensitivity to the RHI in movement fits broadly with established theories of autistic perception that suggest reduced sensitivity to more global or contextual information (weak central coherence, WCC; [17]) or enhanced lower-level perceptual functioning (EPF; [51]). Specifically, WCC may predict a reduced sensitivity to the illusion in general owing to reduced global integration of sensory information, while EPF may predict better movement performance in the context of the RHI owing to enhanced discriminability of proprioceptive input (though previous research has not supported better proprioceptive discrimination in ASD [46]). The predictive processing account has an advantage in this regard in accounting for why the RHI is experienced similarly in the ASD group but modulates subsequent movement differently in low AQ controls (as discussed earlier), and further, why movement performance is generally poor in the ASD group in addition to this group showing insensitivity to the context of the RHI. Inferential accounts of autistic perception formalize the distinction between bottom-up and top-down sensory processes [8] within a computational framework and situate clinical symptoms and related non-clinical variation within a general model of brain function. Drawing on the biological and computational depth of inferential models may be important for elucidating the mechanisms underlying autistic symptoms; for example, the concept of WCC can be cast in predictive processing terms, leading to corresponding hypotheses regarding the cortical circuitry and neurotransmitter systems involved [9,10]. Further research may also be able to distinguish predictive processing accounts from other theories by examining the role of sensory uncertainty in modulating differences in perceptual or sensorimotor outcomes between ASD and controls.
In this study, we find that both individuals with ASD and non-clinical individuals high in autistic traits show reduced sensitivity to a multisensory illusion of limb ownership in subsequent reaching movements despite experiencing the perceptual effects of this illusion. In addition, clinical and non-clinical individuals high in autistic traits are distinguishable in the integrated jerk and earlier phases of movement across conditions. These sensorimotor differences can be understood in terms of the relative weighting of sensory feedback against existing expectations for body position as movement unfolds; these results thus extend recent predictive processing accounts of ASD to sensorimotor function and to differences across the non-clinical population.
Supplementary Material
Acknowledgements
The authors wish to thank Uta Frith for helpful comments on this manuscript, and Owen Hammond and colleagues at the Monash Instrumentation Facility for creating the mirror box apparatus used in this experiment.
Ethics statement
Approval for this research was granted by the Monash University Human Research Ethics Committee and the Alfred Hospital Ethics Committee. All participants provided informed consent.
Data accessibility
Data are available at http://profiles.arts.monash.edu.au/colin-palmer/publications/.
Funding statement
This work was funded by an Australian Research Council Discovery grant (DP1311336). J.H. is supported by an Australian Research Council Future Fellowship (FT100100322). P.G.E. is supported by a NHMRC Career Development Fellowship (GNT1052073). B.P. is in part supported by a Monash Inter-Disciplinary Research grant, 2013.
References
- 1.Friston K. 2005. A theory of cortical responses. Phil. Trans. R. Soc. B 360, 815–836. ( 10.1098/rstb.2005.1622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friston K. 2009. The free-energy principle: a rough guide to the brain? Trends Cogn. Sci. 13, 293–301. ( 10.1016/j.tics.2009.04.005) [DOI] [PubMed] [Google Scholar]
- 3.Adams RA, Shipp S, Friston KJ. 2013. Predictions not commands: active inference in the motor system. Brain Struct. Funct. 218, 611–643. ( 10.1007/s00429-012-0475-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shipp S, Adams RA, Friston KJ. 2013. Reflections on agranular architecture: predictive coding in the motor cortex. Trends Neurosci. 36, 706–716. ( 10.1016/j.tins.2013.09.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldman H, Friston KJ. 2010. Attention, uncertainty, and free-energy. Front. Hum. Neurosci. 4, 215 ( 10.3389/fnhum.2010.00215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hohwy J. 2013. The predictive mind. Oxford, UK: Oxford University Press. [Google Scholar]
- 7.Palmer CJ, Paton B, Hohwy J, Enticott PG. 2013. Movement under uncertainty: the effects of the rubber-hand illusion vary along the nonclinical autism spectrum. Neuropsychologia 51, 1942–1951. ( 10.1016/j.neuropsychologia.2013.06.020) [DOI] [PubMed] [Google Scholar]
- 8.Pellicano E, Burr D. 2012. When the world becomes ‘too real’: a Bayesian explanation of autistic perception. Trends Cogn. Sci. 16, 504–510. ( 10.1016/j.tics.2012.08.009) [DOI] [PubMed] [Google Scholar]
- 9.Lawson RP, Rees G, Friston KJ. 2014. An aberrant precision account of autism. Front. Hum. Neurosci. 8, 302 ( 10.3389/fnhum.2014.00302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van de Cruys S, Evers K, Van der Hallen R, Van Eylen L, Boets B, de-Wit L, Wagemans J. 2014. Precise minds in uncertain worlds: predictive coding in autism. Psychol. Rev. 121, 649–675 ( 10.1037/a0037665) [DOI] [PubMed] [Google Scholar]
- 11.van Boxtel JJ, Lu H. 2013. A predictive coding perspective on autism spectrum disorders. Front. Psychol. 4, 19 ( 10.3389/fpsyg.2013.00019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brock J. 2012. Alternative Bayesian accounts of autistic perception: comment on Pellicano and Burr. Trends Cogn. Sci. 16, 573–574; Author reply 574–575 ( 10.1016/j.tics.2012.10.005) [DOI] [PubMed] [Google Scholar]
- 13.Skewes JC, Jegindo EM, Gebauer L. In press. Perceptual inference and autistic traits. Autism. ( 10.1177/1362361313519872) [DOI] [PubMed] [Google Scholar]
- 14.Lai MC, Lombardo MV, Baron-Cohen S. 2014. Autism. Lancet 383, 896–910. ( 10.1016/S0140-6736(13)61539-1) [DOI] [PubMed] [Google Scholar]
- 15.American Psychiatric Association. 2013. Diagnostic and statistical manual of mental disorders: DSM-5, 5th edn, xliv, 947pp Arlington, VA: American Psychiatric Association. [Google Scholar]
- 16.Simmons DR, Robertson AE, McKay LS, Toal E, McAleer P, Pollick FE. 2009. Vision in autism spectrum disorders. Vis. Res. 49, 2705–2739. ( 10.1016/j.visres.2009.08.005) [DOI] [PubMed] [Google Scholar]
- 17.Happé F, Frith U. 2006. The weak coherence account: detail-focused cognitive style in autism spectrum disorders. J. Autism Dev. Disord. 36, 5–25. ( 10.1007/s10803-005-0039-0) [DOI] [PubMed] [Google Scholar]
- 18.Fournier KA, Hass CJ, Naik SK, Lodha N, Cauraugh JH. 2010. Motor coordination in autism spectrum disorders: a synthesis and meta-analysis. J. Autism Dev. Disord. 40, 1227–1240. ( 10.1007/s10803-010-0981-3) [DOI] [PubMed] [Google Scholar]
- 19.Gowen E, Hamilton A. 2013. Motor abilities in autism: a review using a computational context. J. Autism Dev. Disord. 43, 323–344. ( 10.1007/s10803-012-1574-0) [DOI] [PubMed] [Google Scholar]
- 20.Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. 2001. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J. Autism Dev. Disord. 31, 5–17. ( 10.1023/A:1005653411471) [DOI] [PubMed] [Google Scholar]
- 21.Hurst RM, Mitchell JT, Kimbrel NA, Kwapil TK, Nelson-Gray RO. 2007. Examination of the reliability and factor structure of the Autism Spectrum Quotient (AQ) in a non-clinical sample. Pers. Individual Differ. 43, 1938–1949. ( 10.1016/j.paid.2007.06.012) [DOI] [Google Scholar]
- 22.Constantino JN, Todd RD. 2003. Autistic traits in the general population: a twin study. Arch. Gen. Psychiatry 60, 524–530. ( 10.1001/archpsyc.60.5.524) [DOI] [PubMed] [Google Scholar]
- 23.Posserud MB, Lundervold AJ, Gillberg C. 2006. Autistic features in a total population of 7–9-year-old children assessed by the ASSQ (Autism Spectrum Screening Questionnaire). J. Child Psychol. Psychiatry 47, 167–175. ( 10.1111/j.1469-7610.2005.01462.x) [DOI] [PubMed] [Google Scholar]
- 24.Palmer CJ, Paton B, Enticott PG, Hohwy J. In press. ‘Subtypes’ in the presentation of autistic traits in the general adult population. J. Autism Dev. Dis. ( 10.1007/s10803-014-2289-1) [DOI] [PubMed] [Google Scholar]
- 25.Mitchell P, Ropar D. 2004. Visuo-spatial abilities in autism: a review. Infant Child Dev. 13, 185–198. ( 10.1002/icd.348) [DOI] [Google Scholar]
- 26.Gómez C, et al. 2014. Reduced predictable information in brain signals in autism spectrum disorder. Front. Neuroinform. 8, 9 ( 10.3389/fninf.2014.00009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Boxtel JJ, Lu H. 2013. Impaired global, and compensatory local, biological motion processing in people with high levels of autistic traits. Front. Psychol. 4, 209 ( 10.3389/fpsyg.2013.00209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hohwy J, Palmer CJ. 2014. Social cognition as causal inference: implications for common knowledge and autism. In Social ontology and social cognition (eds Gallotti M, Michael J.), pp. 167–189. New York, NY: Springer. [Google Scholar]
- 29.Botvinick M, Cohen J. 1998. Rubber hands ‘feel’ touch that eyes see. Nature 391, 756 ( 10.1038/35784) [DOI] [PubMed] [Google Scholar]
- 30.Ehrsson HH. 2012. The concept of body ownership and its relation to multi-sensory integration. In The new handbook of multisensory processing (ed. Stein BE.), pp. 775–792. Cambridge, MA: MIT Press. [Google Scholar]
- 31.Tsakiris M, Haggard P. 2005. The rubber hand illusion revisited: visuotactile integration and self-attribution. J. Exp. Psychol. Hum. Percept. Perform. 31, 80–91. ( 10.1037/0096-1523.31.1.80) [DOI] [PubMed] [Google Scholar]
- 32.Zopf R, Truong S, Finkbeiner M, Friedman J, Williams MA. 2011. Viewing and feeling touch modulates hand position for reaching. Neuropsychologia 49, 1287–1293. ( 10.1016/j.neuropsychologia.2011.02.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paton B, Hohwy J, Enticott PG. 2012. The rubber hand illusion reveals proprioceptive and sensorimotor differences in autism spectrum disorders. J. Autism Dev. Disord. 42, 1870–1883. ( 10.1007/s10803-011-1430-7) [DOI] [PubMed] [Google Scholar]
- 34.American Psychiatric Association. 2000. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4th edn, xxxvii, 943 pp Washington, DC: American Psychiatric Association. [Google Scholar]
- 35.Kammers MP, de Vignemont F, Verhagen L, Dijkerman HC. 2009. The rubber hand illusion in action. Neuropsychologia 47, 204–211. ( 10.1016/j.neuropsychologia.2008.07.028) [DOI] [PubMed] [Google Scholar]
- 36.Kammers MP, Verhagen L, Dijkerman HC, Hogendoorn H, De Vignemont F, Schutter DJ. 2009. Is this hand for real? Attenuation of the rubber hand illusion by transcranial magnetic stimulation over the inferior parietal lobule. J. Cogn. Neurosci. 21, 1311–1320. ( 10.1162/jocn.2009.21095) [DOI] [PubMed] [Google Scholar]
- 37.Hogan N, Flash T. 1987. Moving gracefully: quantitative theories of motor coordination. Trends Neurosci. 10, 170–174. ( 10.1016/0166-2236(87)90043-9) [DOI] [Google Scholar]
- 38.Teulings HL, Contreras-Vidal JL, Stelmach GE, Adler CH. 1997. Parkinsonism reduces coordination of fingers, wrist, and arm in fine motor control. Exp. Neurol. 146, 159–170. ( 10.1006/exnr.1997.6507) [DOI] [PubMed] [Google Scholar]
- 39.Romero DH, Van Gemmert AW, Adler CH, Bekkering H, Stelmach GE. 2003. Altered aiming movements in Parkinson's disease patients and elderly adults as a function of delays in movement onset. Exp. Brain Res 151, 249–261. ( 10.1007/s00221-003-1452-2) [DOI] [PubMed] [Google Scholar]
- 40.Nobile M, Perego P, Piccinini L, Mani E, Rossi A, Bellina M, Molteni M. 2011. Further evidence of complex motor dysfunction in drug naive children with autism using automatic motion analysis of gait. Autism 15, 263–283. ( 10.1177/1362361309356929) [DOI] [PubMed] [Google Scholar]
- 41.Friedman J. 2012. How to decompose 2D trajectory data into submovements using matlab. See http://noisyaccumulation.blogspot.com.au/. [Google Scholar]
- 42.Friedman J, Brown S, Finkbeiner M. 2013. Linking cognitive and reaching trajectories via intermittent movement control. J. Math. Psychol. 57, 140–151. ( 10.1016/j.jmp.2013.06.005) [DOI] [Google Scholar]
- 43.Enticott PG, Bradshaw JL, Iansek R, Tonge BJ, Rinehart NJ. 2009. Electrophysiological signs of supplementary-motor-area deficits in high-functioning autism but not Asperger syndrome: an examination of internally cued movement-related potentials. Dev. Med. Child Neurol. 51, 787–791. ( 10.1111/j.1469-8749.2009.03270.x) [DOI] [PubMed] [Google Scholar]
- 44.Rinehart NJ, Tonge BJ, Bradshaw JL, Iansek R, Enticott PG, Johnson KA. 2006. Movement-related potentials in high-functioning autism and Asperger's disorder. Dev. Med. Child Neurol. 48, 272–277. ( 10.1017/S0012162206000594) [DOI] [PubMed] [Google Scholar]
- 45.Brown H, Adams RA, Parees I, Edwards M, Friston K. 2013. Active inference, sensory attenuation and illusions. Cogn. Process. 14, 411–427. ( 10.1007/s10339-013-0571-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fuentes CT, Mostofsky SH, Bastian AJ. 2011. No proprioceptive deficits in autism despite movement-related sensory and execution impairments. J. Autism Dev. Disord. 41, 1352–1361. ( 10.1007/s10803-010-1161-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Just MA, Cherkassky VL, Keller TA, Minshew NJ. 2004. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain 127, 1811–1821. ( 10.1093/brain/awh199) [DOI] [PubMed] [Google Scholar]
- 48.Dinstein I, Heeger DJ, Lorenzi L, Minshew NJ, Malach R, Behrmann M. 2012. Unreliable evoked responses in autism. Neuron 75, 981–991. ( 10.1016/j.neuron.2012.07.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hohwy J, Paton B. 2010. Explaining away the body: experiences of supernaturally caused touch and touch on non-hand objects within the rubber hand illusion. PLoS ONE 5, e9416 ( 10.1371/journal.pone.0009416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitchell P, Mottron L, Soulières I, Ropar D. 2010. Susceptibility to the Shepard illusion in participants with autism: reduced top-down influences within perception? Autism Res. 3, 113–119. ( 10.1002/aur.130) [DOI] [PubMed] [Google Scholar]
- 51.Mottron L, Dawson M, Soulières I, Hubert B, Burack J. 2006. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. J. Autism Dev. Disord. 36, 27–43. ( 10.1007/s10803-005-0040-7) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available at http://profiles.arts.monash.edu.au/colin-palmer/publications/.


