Healy et al. [1] contribute an excellent analysis of maximum lifespan records for nearly 1400 bird and mammal species. Consistent with the predictions of the evolutionary theory of ageing, their results suggest that behaviours which reduce extrinsic mortality have allowed the evolution of increased longevity. Notably, the ability to escape predators in multiple dimensions [2] seems to be particularly important; confirming previous studies (e.g. [3]), Healy et al. find that both volant and non-volant arboreal species demonstrate longer lifespans than non-volant terrestrial species. The authors also find that fossoriality is associated with increased longevity and suggest that this is due to the ability of fossorial mammals to escape predators and other potential sources of extrinsic mortality such as inclement weather or other unfavourable conditions. Naked mole-rats are touted as an example of an extremely long-lived fossorial mammal, especially when body size is taken into account. While we do not contest this fact, we suggest that naked mole-rats and some relatives in the family Bathyergidae are exceptional among fossorial mammals because other factors, such as sociality, play large and perhaps primary roles in their increased longevity [4,5]. Namely, species in the bathyergid genera Heterocephalus and Fukomys are eusocial [4–7], a factor that has been shown to be highly correlated with longevity in insects [8]. Here, we analyse a large dataset of fossorial mammals (N = 101 compared with N = 10 in [1]) and test the hypothesis that increased longevity is related to fossoriality and eusocial tendencies.
Many fossorial mammals build elaborate tunnel systems that provide protection from surface predators and climatic extremes [9,10]. Therefore, one hypothesis to explain the exceptional lifespan potential observed in Heterocephalus glaber (naked mole-rats) is fossoriality [1,8,11–13]. Alternatively, social factors may play a primary role in Heterocephalus longevity [4,5]. Keller & Genoud [8] demonstrated convincingly that the evolution of eusociality is associated with significant increases in longevity in ants. Like eusocial insects, Heterocephalus and other eusocial bathyergids (genus Fukomys) are characterized by overlapping generations, reproductive division of labour and cooperative breeding [4,6,7], meeting the original requirements of eusociality [14]. This social system, in which the breeding female (i.e. ‘queen’) and one to several breeding males are both protected and provisioned by non-breeders, ensures that breeders rarely encounter predators and therefore experience low extrinsic mortality [6]. Among mammals, ‘true’ eusociality is unknown outside of Bathyergidae; however, some other mammals, namely within Carnivora and Primates, demonstrate eusocial behavioural tendencies [7,15,16], most notably Suricata suricatta (meerkats) and Helogale parvula (dwarf mongooses), cooperatively breeding herpestids that demonstrate marked reproductive skew [7,15,17]. For example, in Helogale, as in eusocial bathyergids, the breeding pair receives food priority and protection from subordinate members and is rarely tasked with predator confrontation [16].
To determine the role of both fossoriality and sociality on senescence, maximum lifespan records and average, pooled-sex body masses for ground-dwelling mammals (excluding arboreal and aquatic mammals) were compiled using AnAge (http://genomics.senescence.info/species/) [18] and other sources [19–21]. Since fossorial and eusocial-like species are restricted to smaller body sizes, we do not include mammals larger than 60 kg, a boundary that is approached by the largest semi-fossorial and eusocial-like mammals, aardvarks and humans, respectively. Healy et al. test the efficacy of data for species with at least 10 maximum lifespan records versus those with 100+ as recorded on the AnAge website and demonstrate that the datasets produce similar results. In an effort to increase sample size of usable taxa while bearing in mind that data can be biased by sample size [2,18], we too use the sample size and data quality criteria of AnAge. However, species whose records are considered ‘not yet established’ due to low sample size or questionable data quality, or those not listed on AnAge, presumably for the same reason, are targeted and compared with closely related taxa using standardized residuals generated from reduced major axis (RMA) regression on the entire dataset. If these species produce residuals that are substantially lower than those of close relatives, they are removed from the dataset; however, if the residuals are similar or higher than those of species with ‘acceptable’ data quality in AnAge, the longevity records in question are retained in the dataset. The reduced dataset consists of 440 ground-dwelling mammals (electronic supplementary material, appendix 1).
Species were organized into two categories: terrestrial (including occasional burrowers that burrow only to construct nests for offspring or those that use shallow, simple burrows, in addition to terrestrial mammals that do not burrow) (N = 339) and fossorial (including mammals that spend time above ground but shelter in sealed or complex burrows, in addition to subterranean mammals that spend the majority of time underground) (N = 101), following Healy et al. [1] and using multiple sources [9,10,19,22] (electronic supplementary material, appendix 1). For social categories, mammals were divided into ‘eusocial’ (those demonstrating strong eusocial behavioural tendencies) (N = 17) and non-eusocial (solitary and social) (N = 423) species using several sources [7,15,16]. Although the definition of eusociality is debated, authorities are in agreement that cooperative breeding and high reproductive skew are two important requirements [14].
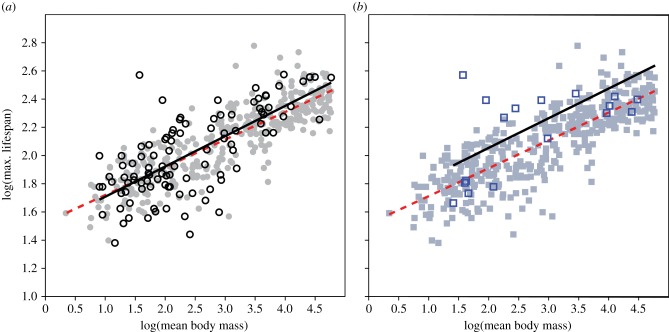
ANOVA demonstrates that body mass explains 31.4% of the variation in maximum lifespan, sociality 3.3% and habitat less than 0.01%. A generalized linear model shows that, as with body mass, the inclusion of sociality significantly improves the model (p < 0.001), whereas habitat does not (p = 0.802). Using RMA regression and analysis of covariance (ANCOVA) with a Bonferroni post-hoc test for multiple comparisons (electronic supplementary material, appendix 2), we find that terrestrial and fossorial slopes are not significantly different (p = 0.807), whereas an elevated ‘eusocial’ regression line over the non-eusocial line reaches borderline significance (p = 0.064) (figure 1). Analyses of phylogenetically independent contrasts (PIC) take relatedness among species into account and thus correct for phylogenetic structure of the dataset [23]. Results of analyses of PIC generate significant results for both habitat and sociality (p < 0.05), suggesting that both factors play significant roles in increases in longevity (electronic supplementary material, appendix 2). Therefore, we find support for Healy et al.'s [1] results on fossoriality with a 10-fold larger sample size, and we additionally identify eusocial-like behaviour as another factor that is associated with increased longevity.
Figure 1.
log10–log10 maximum lifespan and mean body mass in ground-dwelling mammals. Data points and ordinary least squares regression lines are shown for fossorial (open circles; (a) solid-black line) and terrestrial (closed circles; (a) dashed-red line) and ‘eusocial’ (open squares; (b) solid-black line) and non-eusocial (closed squares; (b) dashed-red line) mammals. The human data point is not shown but is included in some analyses; its inclusion or exclusion, however, does not significantly affect the results.
Indeed, high LQs (longevity quotients) are observed in subterranean mammals (e.g. Spalax ehrenbergi, the blind mole-rat, LQ = 1.7, and Spalacopus cyanus, the cururo, LQ = 1.4) and mammals that demonstrate eusocial tendencies (e.g. Helogale = 1.8, and Suricata = 1.6, compared with other herpestids, mean LQ = 1.0) (electronic supplementary material, appendix 1). The family Bathyergidae includes eusocial and non-eusocial species, all of which are entirely fossorial [4–7]. While maximum lifespan records are not yet well-established for the solitary (Georychus capensis) and social (genus Cryptomys) species, the eusocial species produce some of the highest LQs in the dataset (Heterocephalus = 4.9; Fukomys anselli = 2.7; Fukomys damarensis = 1.7). We suggest that these extreme longevities evolved in the context of eusociality and protection in the relative safety of a complex and heavily defended burrow system. By contrast, while pocket gophers live on average slightly less than expected (average LQ = 0.94), afrotherian and placental moles are characteristically short-lived (average LQ = 0.38 and 0.62, respectively).
Although not strictly eusocial, another highly social mammal that demonstrates overlapping generations, reproductive division of labour, and a unique kind of cooperative breeding and reproductive skew, Homo sapiens [24,25], has adopted additional cultural and defensive strategies to reduce extrinsic mortality [26] and likewise demonstrates a high LQ (3.5). We commend Healy et al. on their excellent work, probably the best and most sophisticated study of its kind to date. Our preliminary data suggest that other factors such as sociality have influenced the evolution of longevity in birds and particularly mammals, a hypothesis that requires subsequent testing, ideally using nuanced phylogenetic approaches such as those developed by Healy et al. [1].
Supplementary Material
Supplementary Material
References
- 1.Healy K, et al. 2014. Ecology and mode-of-life explain lifespan variation in birds and mammals. Proc. R. Soc. B 281, 20140298 ( 10.1098/rspb.2014.0298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Møller AP. 2010. Up, up, and away: relative importance of horizontal and vertical escape from predators for survival and senescence. J. Evol. Biol. 23, 1689–1698. ( 10.1111/j.1420-9101.2010.02034.x) [DOI] [PubMed] [Google Scholar]
- 3.Shattuck MR, Williams SA. 2010. Arboreality has allowed for the evolution of increased longevity in mammals. Proc. Natl Acad. Sci. USA 107, 4635–4639. ( 10.1073/pnas.0911439107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett NC, Faulkes CG. 2000. African mole-rats: ecology and eusociality. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 5.Dammann P, Burda H. 2007. Senescence patterns in African mole-rats (Bathyergidae, Rodentia). In Subterranean rodents: news from underground (eds Begall S, Burda H, Schleich CE.), pp. 251–263. Berlin, Germany: Springer. [Google Scholar]
- 6.Lacey EA, Sherman PW. 1991. Social organization of naked mole-rat colonies: evidence for a division of labor. In The biology of the naked mole-rat (eds Sherman PW, Jarvis JUM, Alexander RD.), pp. 275–336. Princeton, NJ: Princeton University Press. [Google Scholar]
- 7.Solomon NG, French JA. 1997. Cooperative breeding in mammals. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 8.Keller L, Genoud M. 1997. Extraordinary lifespans in ants: a test of evolutionary theories of aging. Nature 389, 958–960. ( 10.1038/40130) [DOI] [Google Scholar]
- 9.Nevo E. 1999. Mosaic evolution of subterranean mammals: regression, progression and global convergence. Oxford, UK: Oxford University Press. [Google Scholar]
- 10.Reichman OJ, Smith SC. 1990. Burrows and burrowing behavior by mammals. In Current mammalogy (ed. Genoways HH.), pp. 197–244. New York, NY: Plenum Press. [Google Scholar]
- 11.de Magalhaes JP, Costa J, Church GM. 2007. An analysis of the relationship between metabolism, developmental schedules, and longevity using phylogenetic independent contrasts. J. Gerontol. 62A, 149–160. ( 10.1093/gerona/62.2.149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez VI, et al. 2009. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proc. Natl Acad. Sci. USA 106, 3059–3064. ( 10.1073/pnas.0809620106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorbunova V, Seluanov A, Zhang Z, Gladyshev VN, Vijg J. 2014. Comparative genetics of longevity and cancer: insights from long-lived rodents. Nat. Rev. Genet. 15, 531–540. ( 10.1038/nrg3728) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costa JT, Fitzgerald TD. 1996. Developments in social terminology: semantic battles in a conceptual war. Trends Ecol. Evol. 11, 285–289. ( 10.1016/0169-5347(96)10035-5) [DOI] [PubMed] [Google Scholar]
- 15.Clutton-Brock T. 2009. Structure and function in mammalian societies. Phil. Trans. R. Soc. B 364, 3229–3242. ( 10.1098/rstb.2009.0120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hager R, Jones CB. 2009. Reproductive skew in vertebrates: proximate and ultimate causes. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 17.Rasa OAE. 1987. The dwarf mongoose: a study of behavior and social structure in relation to ecology in a small, social carnivore. Adv. Stud. Behav. 17, 121–163. ( 10.1016/S0065-3454(08)60178-3) [DOI] [Google Scholar]
- 18.de Magalhaes JP, Costa J. 2009. A database of vertebrate longevity records and their relation to other life-history traits. J. Evol. Biol. 22, 1770–1774. ( 10.1111/j.1420-9101.2009.01783.x) [DOI] [PubMed] [Google Scholar]
- 19.Nowak RM. 1999. Walker‘s mammals of the world, 6th edn Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- 20.Carey JR, Judge DS. 2000. Longevity records: life spans of mammals, birds, amphibians, reptiles, and fish. Odense, Denmark: Odense University Press. [Google Scholar]
- 21.Jones KE, et al. 2009. PanTHERIA: a species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology 90, 2648 ( 10.1890/08-1494.1) [DOI] [Google Scholar]
- 22.Hutchins M, Kleiman DG, Geist V, McDade MC. 2003. Grzimek‘s animal life encyclopedia, 2nd edn Farmington Hills, MI: Gale Group. [Google Scholar]
- 23.Felsenstein J. 1985. Phylogenies and the comparative method. Am. Nat. 125, 1–15. ( 10.1086/284325) [DOI] [Google Scholar]
- 24.Hawkes K. 2003. Grandmothers and the evolution of human longevity. Am. J. Hum. Biol. 15, 380–400. ( 10.1002/ajhb.10156) [DOI] [PubMed] [Google Scholar]
- 25.Foster KR, Ratnieks FLW. 2005. A new eusocial vertebrate? Trends Ecol. Evol. 20, 263–364. ( 10.1016/j.tree.2005.03.001) [DOI] [PubMed] [Google Scholar]
- 26.Burkart JM, Hrdy SB, van Schaik CP. 2009. Cooperative breeding and human cognitive evolution. Evol. Anthropol. 18, 175–186. ( 10.1002/evan.20222) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.