Abstract
Early tetrapods faced an auditory challenge from the impedance mismatch between air and tissue in the transition from aquatic to terrestrial lifestyles during the Early Carboniferous (350 Ma). Consequently, tetrapods may have been deaf to airborne sounds for up to 100 Myr until tympanic middle ears evolved during the Triassic. The middle ear morphology of recent urodeles is similar to that of early ‘lepospondyl’ microsaur tetrapods, and experimental studies on their hearing capabilities are therefore useful to understand the evolutionary and functional drivers behind the shift from aquatic to aerial hearing in early tetrapods. Here, we combine imaging techniques with neurophysiological measurements to resolve how the change from aquatic larvae to terrestrial adult affects the ear morphology and sensory capabilities of salamanders. We show that air-induced pressure detection enhances underwater hearing sensitivity of salamanders at frequencies above 120 Hz, and that both terrestrial adults and fully aquatic juvenile salamanders can detect airborne sound. Collectively, these findings suggest that early atympanic tetrapods may have been pre-equipped to aerial hearing and are able to hear airborne sound better than fish on land. When selected for, this rudimentary hearing could have led to the evolution of tympanic middle ears.
Keywords: urodeles, hearing, vibration detection, early tetrapods, evolution of aerial hearing
1. Introduction
Sound detection and auditory perception are used to navigate, communicate, find food and avoid predators [1]. Hearing hence provides vital information about the surrounding environment to a large range of both aquatic and terrestrial animals. Auditory systems of vertebrates have been shaped by evolution to cope with the physical properties of the two very different media of air and water. Although the functional unit of hearing in all vertebrates is the hair cell, sensitive to displacement [2,3], various transduction elements have evolved to enable detection of the particle motion and pressure components of sound.
Because the impedance of animal tissue is close to the impedance of water, sound waves can travel almost unhindered to the hair cells of the inner ear maculae in aquatic vertebrates. In fish auditory systems, otolithic organs enable hair cell deflection by differential inertial movements of otolith and hair cells [4,5], and so the adequate stimulus for these sensory organs is particle motion in the form of acceleration [6]. To evolve pressure-sensitive ears, pressure waves need to be converted to detectable particle motion [7,8]. In many aquatic vertebrates, this is accomplished by gas-filled structures such as swim bladders [7,9] or bullae [10], or secondarily by middle ear cavities [11]. The enclosed air in such cavities increases the available particle motion when ensonified by a pressure wave providing up to two orders of magnitude more acceleration than particle motion in the surrounding water [12].
In contrast to aquatic vertebrates, tetrapods in air have the problem that the impedance of tissue is much higher than the impedance of air, and thus most of the sound energy is reflected on the air–tissue boundary. Hence, early tetrapods faced an impedance problem as they moved from aquatic to terrestrial lifestyles during the Early Carboniferous. The solution to this problem in recent tetrapods is the evolution of the tympanic middle ear, which converts sound pressure in air to particle motion in the fluid of the inner ear. According to the recent palaeontological record, however, the tympanic middle ear did not appear until the Early Triassic, where tympanic middle ears evolved independently in all the tetrapod lineages [13]. Presumably, terrestrial vertebrates were therefore not adapted to detect aerial sound pressure for up to 100 Myr, raising the question of whether early tetrapods were functionally deaf.
The impedance mismatch is also faced by some extant amphibious vertebrates. In most anurans, it is overcome by development of a tympanic middle ear during or after metamorphosis [14], resulting in improved pressure sensitivity of the adults in addition to the particle-motion-sensitive ear found in the aquatic juveniles [15]. However, not all amphibians develop a tympanic middle ear during metamorphosis. For example, none of the urodeles have tympanic middle ears [16]; instead, the columella articulates distally with the squamosal or palatoquadrate of these animals. Additionally, the urodele middle ear also contains the operculum, which is connected to the scapula of the shoulder girdle through the opercularis muscle and has been proposed to aid the transmission of substrate vibrations into the inner ear via the forelegs [17], play a role in airborne hearing by bone conduction [18] or function as a protective mechanism against loud sound exposures [19]. The morphology of the urodele auditory system resembles that of early ‘lepospondyl’ microsaur tetrapods in the shape of the columella and the lack of a tympanic middle ear [13,20]. Equally important, it can be regarded as a potential model for the intermediate evolutionary developmental stage between the aquatic adapted auditory systems of tetrapod ancestors (exemplified by the auditory system in recent lungfish) and the auditory systems of recent tympanic tetrapods adapted to aerial hearing seen in most anurans (figure 1). Experimental studies on the hearing capabilities in recent urodeles are therefore instructive for uncovering the evolutionary and functional drivers behind the shift from aquatic to aerial hearing in early tetrapods.
Figure 1.
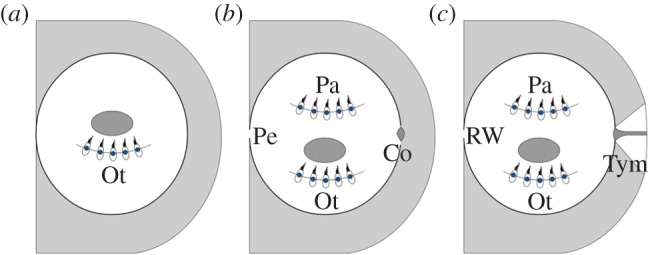
Illustration of the auditory systems in (a) lungfish, (b) urodeles and (c) recent tympanic anurans. The lungfish ear is characterized by a closed otic capsule containing otolithic organs only resembling those of the tetrapodomorph tetrapod ancestors. The otic capsule of urodeles is opened by the oval window and perilymphatic duct, and papillae are found in the inner ear in addition to otolithic organs. In most anurans, the inner ear is further connected to the surrounding air by the tympanic middle ear. Ot, otolithic organ; Pe, perilymphatic duct; Pa, papillae organs; Co, columella; RW, round window; Tym, tympanic middle ear.
However, only a few experimental studies have investigated hearing and vibration detection of urodeles, and changes across metamorphosis have never (to our knowledge) been studied. Overall, the morphology of the urodele middle ear implies good sensitivity to substrate vibrations, but poor sensitivity to aerial sound, suggesting that urodeles may be no more adapted to aerial hearing than are fish. In agreement with the morphological expectations, urodeles have previously been shown to be very sensitive to substrate vibrations [19,21–25], but surprisingly, earlier studies also suggest that urodeles are able to detect airborne sound [19]. Urodeles would seem unable to use pressure-to-particle motion transduction by air volumes in their lungs to enable underwater pressure detection as there is no mechanical connection between the lungs and the inner ears. Yet an earlier study indicates that urodeles may detect underwater sound pressure using an air volume in the mouth cavity for pressure-to-particle motion transduction [26].
Here, we combine imaging techniques with neurophysiological measurements in both water and air in an attempt to resolve how the change from aquatic larva to terrestrial adult through metamorphosis affects the morphology of the ears and the sensory capabilities of urodeles. To allow for a controlled and thus uniform metamorphosis, the axolotl (Ambystoma mexicanum) was chosen as our experimental animal. This neotenic salamander is a convenient choice for comparative studies across metamorphosis as this can easily be induced by thyroid hormone treatment and thereby controlled in an experimental study. Additionally, we investigate the vibration detection and aerial hearing of adult specimens of the closely related tiger salamanders (Ambystoma tigrinum) to compare hearing abilities of aquatic and terrestrial salamanders. Our results show that both juvenile and adult salamanders are able to detect airborne sound. Furthermore, pressure detection is found to enhance underwater hearing sensitivity of salamanders at frequencies above 120 Hz. In combination, these findings suggest that early atympanic tetrapods may have been pre-equipped to aerial hearing and able to hear airborne sound better than fish on land.
2. Material and methods
The study was conducted using 20 axolotls (mass: 68.5 ± 20.3 g and total length: 21.5 ± 2.8 cm, mean ± s.d.) and six adult tiger salamanders (mass: 38.0 ± 4.5 g and total length: 20.7 ± 1.5 cm). Both axolotls and tiger salamanders were obtained commercially and kept in a 12 L : 12 D cycle at room temperature (approx. 20°C). The salamanders were anaesthetized before measurements by submergence in a 0.25‰ Benzocaine (Sigma-Aldrich, St Louis, MO, USA) water solution until they failed to execute the righting reflex. Benzocaine was further added to the water, resulting in a 0.05‰ solution to uphold the anaesthesia during underwater measurements. The juvenile axolotls were kept moist during measurements in air by wrapping them in wet paper towels and dripping them with the Benzocaine solution several times. Animals recovered from anaesthesia in about 20 min when returned to benzocaine-free water after measurements. The animals were sacrificed at the end of the sensitivity experiments by submergence in a high-concentration Benzocaine solution and fixated in buffered 4% formaldehyde solution (VWR, Leuven, Belgium) for later computed tomography (CT) scanning. The fixation did not have any significant effect on the preparations.
(a). Metamorphosis
The vibration and hearing sensitivity was determined in the 20 juvenile axolotls. Next, metamorphosis was induced in 12 axolotls by addition of thyroxin hormone (T4; Sigma-Aldrich) to the water of their aquarium [27]. The remaining eight juveniles were used as controls kept under the same conditions in identical aquaria to enable comparison with animals that also got older, but did not metamorphose. Thirty days after reaching stage 4 of the metamorphosis [28], the adult axolotls, along with the eight controls, were then re-tested to investigate changes across metamorphosis.
(b). Experimental set-up and calibration
The neurophysiological experiments in air were conducted in a combined acceleration and sound pressure set-up [29]. The salamanders had their head resting on a shaker platform 80 cm below a loudspeaker to determine acceleration and sound pressure threshold, respectively. The shaker (Brüel & Kjær Vibration Exciter, Type 4809, Nærum, Denmark) was calibrated using a Brüel & Kjær Accelerometer (Type 4381) calibrated using a Brüel & Kjær Calibration Exciter (Type 4294) with an output of 10 ms−2 at 159.15 Hz. The speaker (8 inch V8 installation speaker, Tannoy Ltd, Coatbridge, UK) was calibrated using a ½ inch free field microphone (Type 40AF, GRAS, Holte, Denmark), calibrated with a Brüel & Kjær Acoustical Calibrator (Type 4231, Brüel & Kjær) with an output of 94 dBRMS re 20 µPa at 1000 Hz.
The underwater experiments were conducted in a standing wave tube set-up [30] where an underwater loudspeaker was placed in the bottom of a 2 m long and 30 cm diameter water-filled steel tube with 1 cm thick walls. Sound stimulation created standing waves in the tube and hence the underwater hearing sensitivity could be investigated under different particle motion-to-pressure conditions by changing the measuring depth in the tube. Both pressure and particle motion of the sound field in the tube was calibrated using two hydrophones (Reson TC 4013), with a flat frequency response in the frequency range used. The hydrophones were calibrated using a Brüel & Kjær hydrophone calibrator (Type 4223) with an output of 165.7 dBRMS re. 1 µPa at 250 Hz. Particle motion was calculated using the instantaneous pressure difference measured between the two hydrophones spaced 2 cm apart [31]. Both pressure and magnitude of particle motion was measured ±5 cm in x- and y-axes, and found not to vary between these axes. Salamanders were suspended in a sling of nylon mesh on a PVC frame during water measurements. The sling did not distort the sound field, but air volumes in lung and mouth cavities of the animals altered the effective sound intensity in the tube significantly. The particle motion-to-pressure ratios were, however, not affected and so calibrations could be corrected for this effect. To do so, the intensity change resulting from introduction of air-filled balloons in the depths used in the electrophysiological experiments was measured (electronic supplementary material, figures S1 and S2) and combined with the median lung volumes of anaesthetized and handled animals found in the CT scans (table 1).
Table 1.
Median air volumes (variance in parentheses) in lungs and mouth before anaesthesia and after handling in anaesthesia for eight juvenile axolotls and 11 adult axolotls.
| awake |
anaesthetized and post handling |
|||||
|---|---|---|---|---|---|---|
| lungs (ml) | mouth (ml) | total (ml) | lungs (ml) | mouth (ml) | total (ml) | |
| juvenile | 2.71 (2.22) | 0 | 2.71 (2.22) | 1.77 (1.06) | 0 (0.01) | 1.80 (1.10) |
| adult | 2.19 (0.48) | 0 | 2.19 (0.51) | 0.16 (0.32) | 0.06 (0.01) | 0.29 (0.41) |
The experimental equipment in both air and water was calibrated and controlled by routines written in Matlab 2007b (The MathWorks, Natick, MA, USA) and RPvdsEx v. 72 (TDT, Alachua, FL, USA). Data collection and analyses were made using Matlab and RPvdsEx v. 72 (TDT). Both sound and vibrational background noise levels were measured and quantified in octave levels to provide a conservative measure of the potential masking noise [29].
(c). Recording of evoked potentials and threshold determination
Evoked potentials were recorded from the brainstem and VIIIth cranial nerve both in air and water by inserting three stainless steel needle electrodes subcutaneously [32]. Two measuring electrodes were inserted on top of the head of the salamanders (one medial dorsal to the brainstem and one mediolateral dorsal to the inner ear and VIIIth cranial nerve). The reference electrode was inserted on the back of the salamanders well away from the VIIIth cranial nerve and brainstem. The neural responses to stimulation were recorded as the voltage difference between the two measuring electrodes relative to the reference electrode.
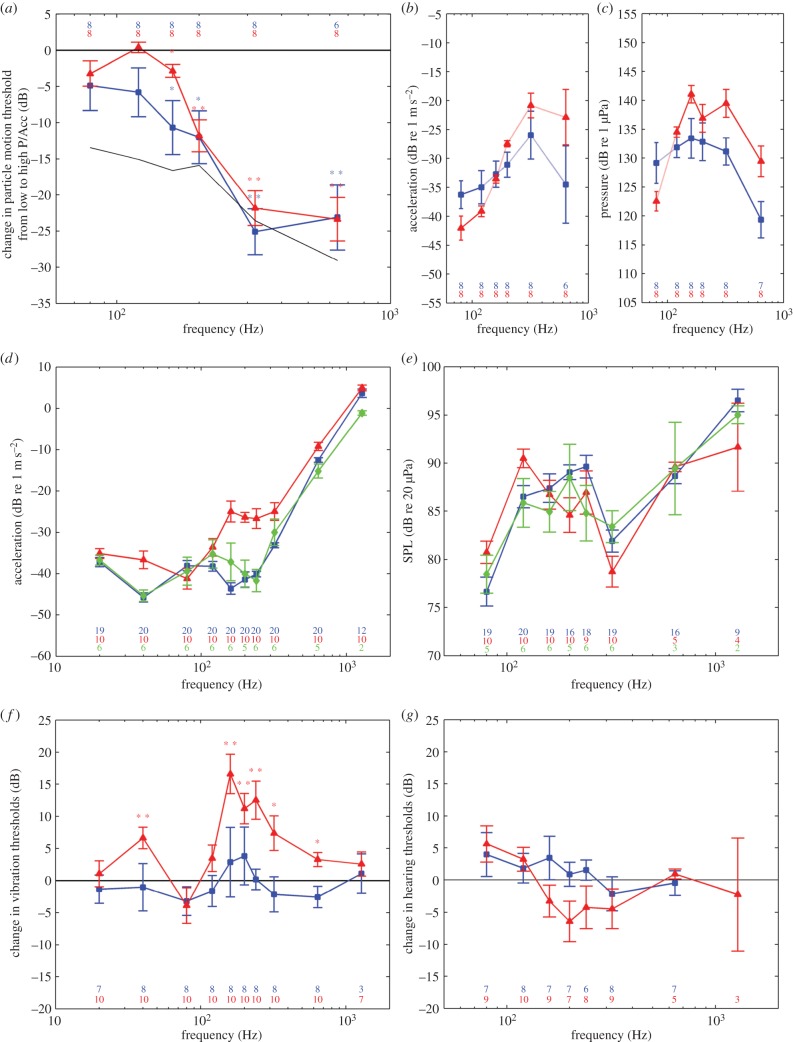
Both vibrational and sound stimulus consisted of 390 ms pure tones in both air and water, gated with a frequency-dependent Tukey window to avoid transients and provide a ramped rise and fall of the tone of 10 cycles. Each trial consisted of 20 tone bursts interspaced by periods of no stimulation with same length as the gated pure tones. In water, pure tones of 80, 120, 160, 200, 320 and 640 Hz were used (figure 3a–c), as the sound field contained a range in pressure-to-particle motion ratio of 15 dB or more at these frequencies (figure 3a). In air, pure tones of 20–1280 Hz were used to determine vibration sensitivity (figure 3d). The speaker could not be calibrated adequately below 80 Hz, however, and therefore sound pressure experiments in air were conducted at frequencies from 80 to 1280 Hz (figure 3e).
Figure 3.
Hearing and vibration sensitivity of juvenile and adult axolotls, and adult tiger salamanders in (a–c) water and (d–g) air. (a) Relative change in particle motion thresholds from high-particle-motion to high-pressure depth for juvenile (blue squares) and adult axolotls (red triangles) along with the change in particle motion-to-pressure ratio (black line). Asterisks indicate statistical significance (paired t-test, *p < 0.05; **p < 0.01). (b) Average particle motion audiograms of juvenile (blue squares) and adult axolotls (red triangles) from the high-particle-motion depth. (c) Average pressure audiogram of juvenile (blue squares) and adult axolotls (red triangles) from the high-pressure depth. (d) Vibration sensitivity of juvenile axolotls (blue squares), adult axolotls (red triangles) and tiger salamanders (green diamonds) in response to vertical shaker vibrations. (e) Sound pressure sensitivity of juvenile axolotls (blue squares), adult axolotls (red triangles) and tiger salamanders (green diamonds). (f) Average change in vibration thresholds of axolotls across the metamorphosis (red triangles) along with juvenile controls (blue squares). Asterisks indicate statistical significance (paired t-test, *p < 0.05; **p < 0.01). (g) Average change in sound pressure thresholds of axolotls across the metamorphosis (red triangles) along with juvenile controls (blue squares). Asterisks indicate statistical significance (paired t-test, *p < 0.05; **p < 0.01). Bars are in all plots ±s.e.m. n-values are indicated in each plot.
Pure tone stimulation resulted in characteristic pure tone signal in the evoked potential at double the stimulation frequency (electronic supplementary material, figure S3), which could be recognized in the fast Fourier transform (FFT) of the combined neural response. The FFT peak size at the second harmonic increased sigmoidally with stimulation, whereas no increase in peak size was found for periods of no stimulation or in a dead animal used as control (electronic supplementary material, figure S3b). Thresholds were determined as the intensity at the zero crossing when making a linear regression on the steep part of the sigmoid curve [33] (electronic supplementary material, figure S3c). Only data points more than 3 s.d. above the electrical noise were included in the regression.
(d). Computed tomography and micro computed tomography
Internal air volume in the lungs and pharynx of awake and anaesthetized salamanders was measured by CT. This was performed using a Siemens Somatom Definition (Siemens Medical Solutions, Germany) with the following parameters: 472 × 472 mm2 field-of-view, 512 × 512 matrix; 0.6 mm slice thickness; 100 kVp tube voltage; 260 mAs tube current, resulting in an acquisition time of 20 s. Eight juvenile axolotls and 11 adult axolotls were placed in separate plastic containers on the scanner bed and CT was performed in the sequence: awake undisturbed, anaesthetized undisturbed, anaesthetized and handled, to evaluate any effect on anaesthesia and handling on internal air volumes.
Micro computed tomography (µCT) imaging was performed using two different systems to obtain high-resolution ex vivo information of cephalic and thoracic anatomy of the salamanders under study: a Scanco Medical XtremeCT system and a Scanco Medical µCT-35 system (Brüttisellen, Switzerland). A staining protocol was applied to some of the specimens to reveal soft tissue structures [34]. After formaldehyde fixation, samples were washed for three weeks in phosphate buffer to remove any residual formaldehyde, thereafter immersed for four weeks in diluted Lugol's solution (0.33% I2 and 0.67% KI). Imaging parameters of the XtremeCT system were: 63.63 × 28.54 mm2 field of view; 776 × 348 matrix; 0.082 mm slice thickness; 59.4 kVp tube voltage; 119 µAs tube current, resulting in an acquisition time of 35 min. Higher-resolution data were acquired using the µCT-35 system with parameters: 21.04 × 23.56 mm2 field-of-view; 1403 × 1571 matrix; 0.015 mm slice thickness; 55 kVp tube voltage; 116 µA tube current, resulting in an acquisition time of 10 h.
The XtremeCT system was used on the anterior of three stained and three unstained juvenile axolotls, three stained and three unstained adult axolotls, and two unstained tiger salamanders. In addition to these specimens, a single tiger salamander was stained, and of these specimens one stained and one unstained specimen from each phenotype were selected for high-resolution µCT. Images of interrelated samples were registered and three-dimensional models generated using Amira v. 5.6 as described by Ruthensteiner & Heß [35].
3. Results
(a). Morphology
Metamorphosis caused distinct and easily recognizable changes in the outer morphology of the axolotls. The animals showed an average weight reduction (±s.d.) of 38 ± 8%, and tail keel and gills shrunk, and were completely lost in the adult stage. X-ray CT data showed that the columella is free in both the juvenile and adult axolotl, but fused to the otic capsule in the tiger salamander (figure 2; also see interactive three-dimensional models in electronic supplementary material, figures S4–S6). The opercularis muscle connecting scapula and operculum was easily recognized in iodine-stained specimens of tiger salamanders and adult axolotls, but could not be found in the juvenile axolotls. The cartilaginous operculum could not be recognized in the CT scans in any specimens (figure 2), but a large aperture was found in the otic capsule posterior to the columella (i.e. at the location of the operculum) in both juvenile and adult salamanders. Dissections of inner and middle ears showed that all three phenotypes had a movable operculum, which in the adult axolotls and tiger salamanders clearly responded to movement of the scapula. Moreover, the columella was movable in both juvenile and adult axolotls, but rigid in tiger salamanders, confirming the findings from the CT data.
Figure 2.
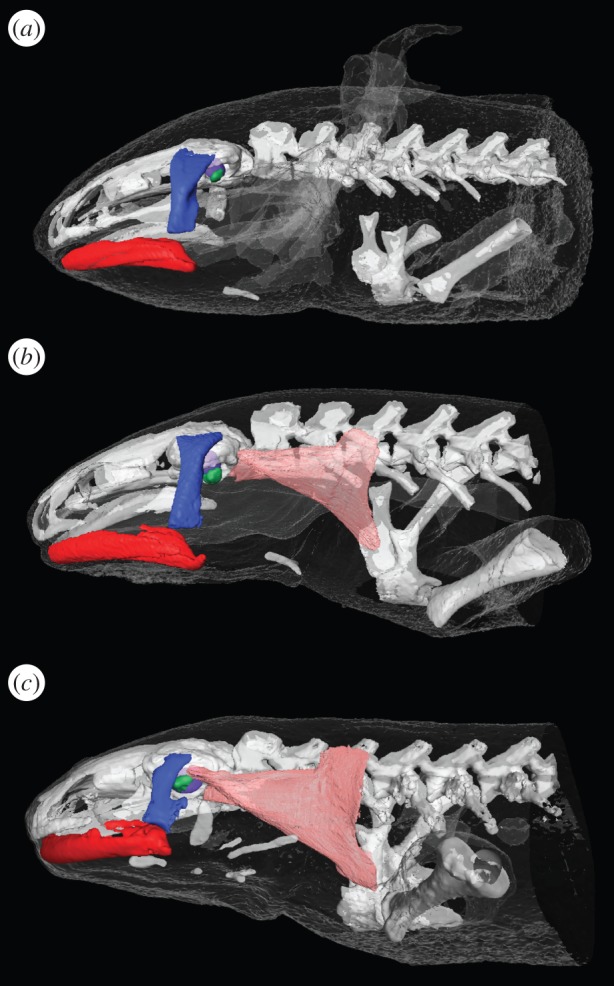
Middle ear anatomy. (a) Juvenile axolotl. (b) Adult axolotl. (c) Adult tiger salamander. Red, mandible; dark blue, quadrate; green, columella auris; purple, saccule; pink, opercularis and levator scapulae muscles. See also interactive models in electronic supplementary material, figures S4–S6.
CT was also used to determine air volumes in lung and mouth cavities of the axolotls (table 1). In awake animals, air volumes were only found in the lung cavities, whereas no air was observed in the mouth cavities. Anaesthesia and handling reduced lung air volumes in both groups, but mostly in the adult axolotls (table 1; paired t-test: juveniles: t = −4.179, p = 0.004; adults t = −6.34, p < 0.001). In addition to air volumes in the lungs, small air volumes were occasionally found in the mouth cavity of anaesthetized and handled animals.
(b). Adequate stimuli under water
No significant change was found in particle motion thresholds for 80 and 120 Hz from high particle motion to high-pressure conditions for either juvenile or adult axolotls (figure 3a). At frequencies above 120 Hz, however, particle motion sensitivity was significantly increased in both these phenotypes under high-pressure conditions relative to sensitivity found under high-particle-motion conditions (figure 3a; paired t-test: juveniles: 160 Hz: t = −2.868, p = 0.024; 200 Hz: t = −3.299, p = 0.013; 320 Hz: t = −7.906, p < 0.001; 640 Hz: t = −5.099, p = 0.004; adults: 160 Hz: t = −3.096, p = 0.017; 200 Hz: t = −5.362, p = 0.001; 320 Hz: t = −9.089, p < 0.001; 640 Hz: t = −7.761, p < 0.001). Particle motion thresholds determined under high-particle-motion conditions and pressure thresholds determined under high-pressure conditions are plotted in figure 3b and 3c, respectively. All thresholds found were at least 15 dB above the octave noise level of the frequencies tested. As the critical bands in amphibians are smaller than one octave [36,37], the octave noise level generates an upper bound estimate of the masking by ambient noise.
(c). Vibration and sound pressure sensitivity in air
Vibration sensitivity curves (vibrograms) of both axolotls and tiger salamanders had a W-shape with two distinct peaks (figure 3d). Juvenile axolotls had best frequencies of 40 and 160 Hz with mean thresholds (±s.e.m.) of −46 ± 1.1 and −44 ± 1.4 dB re 1 m s−2, respectively, whereas adult axolotls were less sensitive and had higher best frequencies of 80 and 240 Hz with mean thresholds of −41 ± 2.4 and −27 ± 2.4 dB re 1 m s−2. Adult tiger salamanders had a lower best frequency of 40 Hz with a mean threshold of −45 ± 1.4 dB re 1 m s−2 and a high best frequency of 240 Hz with a mean threshold of −42 ± 2.7 dB re 1 m s−2. Individual thresholds were at least 16 dB above the octave noise level of the frequencies tested.
Sound pressure sensitivity curves were also W-shaped (figure 3e). All groups had best frequencies of 80 and 320 Hz. Juvenile axolotls had mean thresholds of 77 ± 1.5 and 82 ± 1.1 dB re 20 µPa, adult axolotls 81 ± 1.2 and 79 ± 1.6 dB re 20 µPa, and tiger salamanders had mean thresholds of 78 ± 2 and 83 ± 1.6 dB re 20 µPa, respectively. All thresholds were at least 29.5 dB above the octave noise level of the frequencies tested. Further, sound-induced shaker vibrations were below vibration thresholds at all frequencies tested for all three phenotypes.
(d). Change across metamorphosis
Vibration thresholds were increased significantly across metamorphosis at 40, 160, 200, 240, 320 and 640 Hz (figure 3f; paired t-test: 40 Hz: t = 3.952, p = 0.003; 160 Hz: t = 5.385, p < 0.001; 200 Hz: t = 4.732, p = 0.001; 240 Hz: t = 4.186, p = 0.002; 320 Hz: t = 2.738, p = 0.023; 640 Hz: t = 3.011, p = 0.015), whereas no significant difference was found in the control group. Neither the sound pressure thresholds of the metamorphosed axolotls nor of the control group changed significantly across metamorphosis, although thresholds of 160–240 Hz were lower in the adult than in the juvenile stage (figure 3g).
4. Discussion
Here, we used evoked potential measurements to determine the underwater hearing of both juvenile and adult axolotls to test the null hypothesis that urodeles are unable to detect sound pressure under water. We show that particle motion is the adequate stimulus at frequencies up to 120 Hz. Sound pressure is, however, the adequate stimulus at higher frequencies, and we therefore reject the null hypothesis. Evoked potential measurements were also used to determine aerial hearing and vibration sensitivity to elucidate how the auditory abilities of urodeles are affected by the change from an aquatic to a terrestrial lifestyle. Specifically, we wanted to test the null hypothesis that terrestrial adult urodeles, having no special adaptations to aerial hearing, are no better in detecting airborne sound than fully aquatic vertebrates such as fish. It is demonstrated that urodeles indeed are more sensitive to aerial sound than fully aquatic vertebrates, and thus we find that urodeles are better than fish on land when it comes to hearing.
(a). Underwater sound detection
We investigated the underwater hearing capabilities of the axolotl in a standing wave tube system where the particle motion-to-pressure ratio of the sound field changes with depth. Sound detection can therefore be investigated under both high-particle-motion conditions and high-pressure conditions, allowing us to establish the adequate stimulus. Similar hearing sensitivity (in terms of particle motion) under both conditions suggests detection of particle motion, whereas increased sensitivity from high-particle-motion to high-pressure conditions demonstrates pressure detection. No difference was found in particle motion thresholds at frequencies of 80 and 120 Hz for either juvenile or adult axolotls (figure 3a). At frequencies above 120 Hz, however, thresholds determined under high-pressure conditions were significantly lower than thresholds determined under high-particle-motion conditions (figure 3a). Our results therefore show that the adequate stimulus is particle motion at low frequencies (figure 3b), but that both juvenile and adult axolotls are able to detect sound pressure in water at frequencies above 120 Hz (figure 3c). Consistent with our results, Hetherington & Lombard [26] showed that both juvenile (whole body) and adult tiger salamanders (head preparations) responded to the pressure component of an underwater sound field using pressure-to-particle motion transduction by air volumes trapped in the mouth cavity. Here, acquired CT data showed no air volumes in the mouth cavity of awake axolotls, and only small volumes in the mouth cavity of some anaesthetized and handled animals (table 1). Therefore, axolotls do not seem to rely on an air volume in the mouth cavity for pressure-to-particle motion transduction. By contrast, the lungs of both juvenile and adult awake axolotls contained 2–3 ml air, which, compared with a model of fish swim-bladder vibrations [12], corresponds to a resonance frequency of 4–500 Hz. This finding matches the frequency range where axolotls can detect the sound pressure (figure 3a), suggesting that axolotls sense the underwater sound pressure by detecting the pressure-induced particle motion caused by the air volumes in their lungs, as seen in lungfish [30].
(b). Detection of substrate vibrations
While sound pressure is the adequate stimulus of ears adapted to aerial hearing, good vibration sensitivity may enable atympanic, terrestrial vertebrates to use substrate vibrations as a source of information regarding potential prey, predators and conspecifics, and for communication, as shown for many animals [38].
We found that both axolotls and tiger salamanders are very sensitive to vertical substrate vibrations (figure 3d), consistent with both sensitivity and frequency ranges found in earlier studies [19,21–25]. The high sensitivity is underlined by the fact that thresholds determined by evoked potentials may be 10–30 dB above thresholds determined by single cell recordings or behavioural studies in a variety of animals [39–41]. Moreover, the low-frequency vibration sensitivity found here is comparable with the vibration sensitivity of lungfish [30,42] in terms of best frequency and sensitivity, but whereas the lungfish vibrogram is U-shaped, the salamander vibrograms were W-shaped, having an additional peak at higher frequencies. In frogs, both the saccule and the amphibian papilla are involved in detection of substrate vibrations: the saccule being most sensitive at frequencies below 100 Hz, and the amphibian papilla to frequencies between 60 and 600 Hz [43–45]. Coupling of vibrations to the ventral surface cause endolymphatic displacements through operculum vibrations at 50–400 Hz in urodeles [46], and the amphibian papilla is therefore probably also responsible for detection of high-frequency substrate vibrations in these animals. In line with this suggestion, we found that only the high-frequency sensitivities (figure 3f) changed across metamorphosis when accounting for an increase in best frequencies (figure 3d), indicating that the two peaks observed in the vibrogram originate from two different end organs.
If assuming similar sensitivity of papilla hair cells in the juvenile and the adult axolotls, the reduction in vibration sensitivity may originate from changes in the middle ear morphology across metamorphosis. Dissections and CT data from juvenile axolotls and adult tiger salamanders (figure 2) support the hypothesis that the columella is the functional element in the middle ear of aquatic juvenile salamanders, whereas the operculum after fusion of the columella to the otic capsule, and development of the opercularis muscle, is the functional element in the middle ear of the terrestrial adult tiger salamander [16,17]. The adult axolotls, however, apparently have both a movable columella and a functional opercularis system (figure 2; electronic supplementary material, figure S5). Comparing the vibration sensitivity with the morphology, our results suggest that the columella system and the opercularis system on their own may be equally efficient ways of coupling substrate vibrations to the amphibian papilla of the inner ear: juvenile axolotls and adult tiger salamanders had comparable sensitivity at high frequencies (figure 3d). The reduction in high-frequency vibration sensitivity found across metamorphosis in the axolotl (figure 3d,f), however, suggests that having both systems is less efficient. Thus, input through one system could be short circuited by output through the other. Our results therefore seem to oppose the proposed functions of the opercularis system in aiding the transmission of substrate vibrations to the inner ear [17], at least in the axolotl. Collectively, the results suggest that the increased frequency range and vibration sensitivity at high frequencies of urodeles compared with those of animals with otolith auditory systems only, such as fish, are enabled by the additional structures of the urodele ear: the oval window containing movable inertial elements (columella and/or operculum) and the possession of the amphibian papilla (figure 1).
(c). Aerial hearing
Auditory systems of terrestrial animals are challenged by the large impedance mismatch between animal tissue and air, and therefore most of the sound energy is reflected when impinging on a terrestrial animal. Adult urodeles are atympanic and their auditory system therefore seems no more adapted to aerial hearing than fully aquatic vertebrates such as fish. Nevertheless, we confirm earlier indications of aerial hearing in adult urodeles [19] by showing that the adult axolotls and tiger salamanders are able to detect aerial sound with W-shaped audiograms and best sensitivity of approximately 80 dB re 20 µPa at 80 and 320 Hz (figure 3e). Surprisingly, no significant improvement in hearing sensitivities was found across the metamorphosis and so also the completely aquatic juvenile axolotls are able to detect aerial sound pressure with comparable sensitivity and frequency range to the adult salamanders (figure 3e). Again, evoked potential thresholds may be elevated 10–30 dB relative to actual thresholds and urodeles may therefore be able to detect sound pressures of approximately 50 dB re 20 µPa at best hearing frequencies. This is comparable with the sound detection of atympanic frogs [47] (accounting for the difference in methodology) and atympanic reptiles [29], but is still relatively insensitive compared with the most sensitive tympanic anuran species [48]. The morphological and functional change in the urodele middle ear from the columella to the opercularis system [16,17] (figure 2) occurring during metamorphosis only had a minor effect on the aerial hearing of the axolotl (figure 3g). This is consistent with the fact that no morphological adaption to detection of aerial sound pressure develops in the middle ear across metamorphosis. The lack of increase in sensitivity found across metamorphosis is therefore likely to be rather a consequence of the relative good pressure sensitivity found in juveniles than of poor pressure sensitivity found in adult salamanders. In comparison, lungfish are also able to detect aerial sound [30], but only at low frequencies (less than 200 Hz) and at higher intensities than found here for salamanders. The possession of the oval window with movable inertial elements and the otoconia-free sensory epithelia in the inner ear therefore seem to enable urodeles to improve the sensitivity and frequency range when hearing in air despite being atympanic. The lack of middle ear adaptions for detection of aerial sound pressure, however, implies that urodeles are unable to detect sound pressure per se. Rather, urodeles may be hypothesized to detect sound-induced vibrations (figure 3d). When testing that notion, we found that sound-induced shaker vibrations were below vibration thresholds, and therefore unable to explain the sound pressure detection of the salamanders. Sound-induced head vibrations have previously been shown to be sufficient to explain sound detection in atympanic reptiles [29]. Use of the transfer function, from aerial sound pressure to head vibrations, determined for pythons with similar head size to the salamanders investigated here, suggests that urodeles detect aerial sound by detection of sound-induced head vibrations. Thus, the equivalent vibration thresholds calculated from salamander sound pressure thresholds (figure 3e) and python transfer functions [29] correspond to the vibration thresholds of the salamanders (figure 3d).
(d). Evolutionary perspectives
In many recent tetrapods, exemplified by most anurans, the impedance mismatch between air and animal tissue is overcome by the tympanic middle ear, which relays pressure-induced vibration of the tympanum via the middle ear ossicle to the endolymph of the inner ear (figure 1). There, fluid oscillations between the oval and the round window lead to hair cell deflection in papillae and otoconia end organs by which the animal hears. By contrast, the auditory system of lungfish, the closest living relative of tetrapods [49], is regarded as primitive for tetrapods resembling the auditory system of the tetrapod ancestors [50]. The ears of lungfish can be characterized as unspecialized fish ears with a closed otic capsule and otolith end organs only [51] (figure 1), and so lungfish are completely unadapted to aerial hearing. The palaeontological record suggests that the otic capsule was open already in the early tetrapods [13,50] and that the columella resided in the oval window of these vertebrates [13,52]. The tympanic middle ear, however, apparently did not evolve until the Early Triassic [13,50] some 100 Myr after the water-to-land transition, and consequently, the columella was not connected to the outer surface in early tetrapods. Moreover, the round window, enabling pressure relief in tympanic tetrapods, may not have evolved until the Late Permian or Early Triassic [50]. Though speculative, early tetrapods may, however, have possessed a perilymphatic duct between the inner ear and the cranial cavity, which in urodeles functions as a pressure relief window [46]. The palaeontological record does not provide any specific information about the appearance of papilla organs, but comparative morphological studies of modern tetrapods [53,54] suggest that the amphibian basilar papilla is homologous with that in the amniotes [55] and thus is a primitive character in tetrapods.
The morphology of the urodele auditory system resembles that of early ‘lepospondyl’ microsaur tetrapods [13,20], but it can be further regarded as an intermediate evolutionary stage between the primitive system of early tetrapods, as shown by lungfish and the tympanic ear of most anurans (figure 1). The auditory system of recent urodeles is therefore a relevant model for the auditory systems of early tetrapods before the evolution of the tympanic middle ear [13,20,52]. Caution should, of course, be taken when assuming that hearing of recent urodeles is representative of early tetrapods living some 300–350 Myr ago, but the morphological similarities between the urodele ear and the ears in early microsaur tetrapods lend support to the assumption of comparable auditory abilities.
We show that not only the terrestrial adult tiger salamanders, but also semi-terrestrial adult axolotls, and even completely aquatic juvenile axolotls, are able to detect airborne sound (figure 3e) despite their atympanic middle ears. Our results hence suggest that the urodele auditory system, with an oval window containing free inertial elements, together with a papilla organ and the perilymphatic duct in the inner ear, enable them to have increased frequency range and sensitivity in air compared with fully aquatic vertebrates, such as fish. It follows from this suggestion that early tetrapods also may have been able to detect aerial sound before the appearance of the tympanic middle ear. This limited sensitivity may have provided the rudimentary hearing that, when selected for, led to gradual evolution of low-mass skin areas and bony structures that eventually formed the tympanic middle ear. Further, we show that urodeles are able to detect high-frequency sound pressure underwater (figure 3a). This suggests that possession of air-filled structures enables pressure detection, as the lungs of urodeles are not mechanically connected to the inner ears. Hence, detection of underwater sound pressure may have appeared as a passive consequence of air breathing already in the aquatic ancestors of tetrapods. As the pressure-to-particle motion transduction of such gas-filled structures has the largest effect at frequencies above the resonance frequencies of the otolithic end organs, possession of such structures may have driven the evolution of high-frequency tuned hair cells. Accompanied by development of a lightweight, free inertial element in the oval window, this could have driven the evolution of free hair cell organs without an otolithic mass, a precursor of the basilar papilla, sensitive to high frequencies and responsible for aerial hearing in extant tympanic tetrapods. The evolutionary basis for pressure hearing could therefore have been formed already in water before the water-to-land transition. In concert, our results therefore imply a gradual change from particle motion detection in water to the pressure hearing on land, where high-frequency tuning in the aquatic air-breathing tetrapod ancestors and subsequent detection of sound-induced head vibrations in early tetrapods drove the evolution of aerial hearing, leading to the tympanic auditory systems of most modern tetrapods.
Supplementary Material
Acknowledgements
We thank J. S. Jensen for technical support with the standing wave tube and J. S. Thomsen for support and help with µCT scanning.
Ethics statement
The experiments were licensed by the Danish Animal Experimentation Board nos. 2012-15-2934-00233 and 2012-15-2934-00353.
Data accessibility
Individual AEP thresholds: Dryad doi:10.5061/dryad.mg5ng.
Funding statement
The study was funded by the Oticon Foundation (grant no. 09-3856 to C.B.C.), and the Danish Natural Science Research Council (P.T.M. and J.C-D.).
References
- 1.Webster DB, Fay RR, Popper AN. 1992. The evolutionary biology of hearing. New York, NY: Springer. [Google Scholar]
- 2.Harris GG, Frishkop LS, Flock Å. 1970. Receptor potentials from hair cells of the lateral line. Science 167, 76–79. ( 10.1126/science.167.3914.76) [DOI] [PubMed] [Google Scholar]
- 3.Sand O, Ozawa S, Hagiwara S. 1975. Electrical and mechanical stimulation of hair cells in mudpuppy. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 102, 13–26. [Google Scholar]
- 4.Popper AN, Fay RR. 2011. Rethinking sound detection by fishes. Hear. Res. 273, 25–36. ( 10.1016/j.heares.2009.12.023) [DOI] [PubMed] [Google Scholar]
- 5.Chapman CJ, Sand O. 1974. Field studies of hearing in 2 species of flatfish Pleuronectes platessa (L) and Limanda limanda (L) (family Pleuronectidae). Comp. Biochem. Physiol. 47, 371–385. ( 10.1016/0300-9629(74)90082-6) [DOI] [PubMed] [Google Scholar]
- 6.Kalmijn AJ. 1989. Functional evolution of lateral line and inner ear sensory systems. In The mechanosensory lateral line (eds Coombs S, Görner P, Münz H.), pp. 187–215. New York, NY: Springer. [Google Scholar]
- 7.Fay RR, Popper AN. 1974. Acoustic stimulation of the ear of the goldfish (Carassius auratus). J. Exp. Biol. 61, 243–260. [DOI] [PubMed] [Google Scholar]
- 8.Sand O, Karlsen HE. 2000. Detection of infrasound and linear acceleration in fishes. Phil. Trans. R. Soc. Lond. B 355, 1295–1298. ( 10.1098/rstb.2000.0687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman CJ, Hawkins AD. 1973. A field study of hearing in the cod, Gadus morhua L. J. Comp. Physiol. 85, 147–167. ( 10.1007/bf00696473) [DOI] [Google Scholar]
- 10.Wilson M, Montie EW, Mann KA, Mann DA. 2009. Ultrasound detection in the Gulf menhaden requires gas-filled bullae and an intact lateral line. J. Exp. Biol. 212, 3422–3427. ( 10.1242/jeb.033340) [DOI] [PubMed] [Google Scholar]
- 11.Christensen-Dalsgaard J, Brandt C, Wilis KL, Christensen CB, Ketten D, Edds-Walton P, Fay RR, Madsen PT, Carr CE. 2012. Specialization for underwater hearing by the tympanic middle ear of the turtle, Trachemys scripta elegans. Proc. R. Soc. B 279, 2816–2924. ( 10.1098/rspb.2012.0290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander RM. 1966. Physical aspects of swimbladder function. Biol. Rev. Camb. Phil. Soc. 41, 141–176. ( 10.1111/j.1469-185X.1966.tb01542.x) [DOI] [PubMed] [Google Scholar]
- 13.Clack JA. 1997. The evolution of tetrapod ears and the fossil record. Brain Behav. Evol. 50, 198–212. ( 10.1159/000113334) [DOI] [PubMed] [Google Scholar]
- 14.Hetherington TE. 1987. Timing of development of the middle ear of Anura (Amphibia). Zoomorphology 106, 289–300. ( 10.1007/bf00312003) [DOI] [Google Scholar]
- 15.Simmons A, Flores V. 2012. Particle motion is broadly represented in the vestibular medulla of the bullfrog across larval development. J. Comp. Physiol. A 198, 253–266. ( 10.1007/s00359-011-0705-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monath T. 1965. The opercular apparatus of salamanders. J. Morphol. 116, 149–170. ( 10.1002/jmor.1051160202) [DOI] [Google Scholar]
- 17.Kingsbury BD, Reed HD. 1909. The columella auris in amphibia. Second contribution. J. Morphol. 20, U549–U550. ( 10.1002/jmor.1050200403) [DOI] [Google Scholar]
- 18.Jaslow AP, Hetherington TE, Lombard RE. 1988. Structure and function of the amphibian middle ear. In The evolution of the amphibian auditory system (eds Fritzsch B, Ryan MJ, Wilczynski W, Hetherington TE, Walkowiak W.), pp. 69–91. New York, NY: John Wiley & Sons. [Google Scholar]
- 19.Wever EG. 1985. The amphibian ear. Princeton, NJ: Princeton University Press. [Google Scholar]
- 20.Clack JA, Allin E. 2004. The evolution of single-and multiple-ossicle ears in fishes and tetrapods. In Evolution of the vertebrate auditory system, pp. 128–163. New York, NY: Springer. [Google Scholar]
- 21.Wever EG. 1978. Sound transmission in the salamander ear. Proc. Natl Acad. Sci. USA 75, 529–530. ( 10.1073/pnas.75.1.529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross RJ, Smith JJB. 1982. Responses of the salamander inner-ear to vibrations of the middle-ear. Can. J. Zool. 60, 220–226. ( 10.1139/z82-030) [DOI] [Google Scholar]
- 23.Ross RJ, Smith JJB. 1980. Detection of substrate vibrations by salamanders—frequency sensitivity of the ear. Comp. Biochem. Physiol. A Physiol. 65, 167–172. ( 10.1016/0300-9629(80)90218-2) [DOI] [Google Scholar]
- 24.Ross RJ, Smith JJB. 1979. Detection of substrate vibrations by salamanders—8th cranial nerve activity. Can. J. Zool. 57, 368–374. ( 10.1139/z79-043) [DOI] [Google Scholar]
- 25.Ross RJ, Smith JJB. 1978. Detection of substrate vibrations by salamanders: inner-ear sense organ activity. Can. J. Zool. 56, 1156–1162. ( 10.1139/z78-159) [DOI] [Google Scholar]
- 26.Hetherington TE, Lombard RE. 1983. Mechanisms of underwater hearing in larval and adult tiger salamanders Ambystoma tigrinum. Comp. Biochem. Physiol. A Comp. Physiol. 74, 555–559. ( 10.1016/0300-9629(83)90547-9) [DOI] [PubMed] [Google Scholar]
- 27.Page RB, Monaghan JR, Samuels AK, Smith JJ, Beachy CK, Voss SR. 2007. Microarray analysis identifies keratin loci as sensitive biomarkers for thyroid hormone disruption in the salamander Ambystoma mexicanum. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 145, 15–27. ( 10.1016/j.cbpc.2006.06.003) [DOI] [PubMed] [Google Scholar]
- 28.Cano-Martinez A, Vargas-González A, Asai M. 1994. Metamorphic stages in Ambystoma mexicanum. Axolotl Newsl. 23, 64–71. [Google Scholar]
- 29.Christensen CB, Christensen-Dalsgaard J, Brandt C, Madsen PT. 2012. Hearing with an atympanic ear: good vibration and poor sound-pressure detection in the royal python, Python regius. J. Exp. Biol. 215, 331–342. ( 10.1242/jeb.062539) [DOI] [PubMed] [Google Scholar]
- 30.Christensen CB, Christensen-Dalsgaard J, Madsen PT. In press Hearing of the African lungfish (Protopterus annectens) suggests underwater pressure detection and rudimentary aerial hearing in early tetrapods. J. Exp. Biol. [DOI] [PubMed]
- 31.Christensen-Dalsgaard J, Breithaupt T, Elepfandt A. 1990. Underwater hearing in the clawed frog, Xenopus laevis. Naturwissenschaften 77, 135–137. ( 10.1007/bf01134478) [DOI] [PubMed] [Google Scholar]
- 32.Schrode KM, Buerkle NP, Brittan-Powell EF, Bee MA. 2014. Auditory brainstem responses in Cope's gray treefrog (Hyla chrysoscelis): effects of frequency, level, sex and size. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 200, 221–238. ( 10.1007/s00359-014-0880-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mooney TA, Hanlon RT, Christensen-Dalsgaard J, Madsen PT, Ketten DR. 2010. Sound detection by the longfin squid (Loligo pealeii) studied with auditory evoked potentials: sensitivity to low-frequency particle motion and not pressure. J. Exp. Biol. 213, 3748–3759. ( 10.1242/jeb.048348) [DOI] [PubMed] [Google Scholar]
- 34.Metscher BD. 2009. MicroCT for comparative morphology: simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiol. 9, 11 ( 10.1186/1472-6793-9-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruthensteiner B, Heß M. 2008. Embedding 3D models of biological specimens in PDF publications. Microsc. Res. Tech. 71, 778–786. ( 10.1002/jemt.20618) [DOI] [PubMed] [Google Scholar]
- 36.Ehret G, Capranica RR. 1980. Masking patterns and filter characteristics of auditory-nerve fibers in the green treefrog (Hyla cinerea). J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 141, 1–12. ( 10.1007/BF00611872) [DOI] [Google Scholar]
- 37.Ehret G, Gerhardt HC. 1980. Auditory masking and effects of noise on responses of the green treefrog (Hyla cinerea) to synthetic mating calls. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 141, 13–18. ( 10.1007/BF00611873) [DOI] [Google Scholar]
- 38.Hill PM. 2009. How do animals use substrate-borne vibrations as an information source? Naturwissenschaften 96, 1355–1371. ( 10.1007/s00114-009-0588-8) [DOI] [PubMed] [Google Scholar]
- 39.Brittan-Powell EF, Christensen-Dalsgaard J, Tang Y, Carr C, Dooling RJ. 2010. The auditory brainstem response in two lizard species. J. Acoust. Soc. Am. 128, 787–794. ( 10.1121/1.3458813) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gorga MP, Kaminski JR, Beauchaine KA, Jesteadt W. 1988. Auditory brainstem responses to tone bursts in normally hearing subjects. J. Speech Lang. Hear. Res. 31, 87–97. ( 10.1044/jshr.3101.87) [DOI] [PubMed] [Google Scholar]
- 41.Köppl C, Gleich O. 2007. Evoked cochlear potentials in the barn owl. J. Comp. Physiol. A 193, 601–612. ( 10.1007/s00359-007-0215-0) [DOI] [PubMed] [Google Scholar]
- 42.Christensen-Dalsgaard J, Brandt C, Wilson M, Wahlberg M, Madsen PT. 2011. Hearing in the African lungfish (Protopterus annectens): pre-adaptation to pressure hearing in tetrapods? Biol. Lett. 7, 139–141. ( 10.1098/rsbl.2010.0636) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Christensen-Dalsgaard J, Narins PM. 1993. Sound and vibration sensitivity of VIIIth nerve fibers in the frogs Leptodactylus albilabris and Rana pipiens pipiens. J. Comp. Physiol. A 172, 653–662. ( 10.1007/bf00195391) [DOI] [PubMed] [Google Scholar]
- 44.Yu X, Lewis E, Feld D. 1991. Seismic and auditory tuning curves from bullfrog saccular and amphibian papular axons. J. Comp. Physiol. A 169, 241–248. ( 10.1007/bf00215871) [DOI] [PubMed] [Google Scholar]
- 45.Jørgensen MB, Christensen-Dalsgaard J. 1991. Peripheral origins and functional characteristics of vibration-sensitive VIIIth nerve fibers in the frog Rana temporaria. J. Comp. Physiol. A 169, 341–347. ( 10.1007/bf00206998) [DOI] [Google Scholar]
- 46.Smith JJB. 1968. Hearing in the terrestrial urodeles—a vibration-sensitive mechanism in the ear. J. Exp. Biol. 48, 191–205. [DOI] [PubMed] [Google Scholar]
- 47.Walkowiak W. 1980. Sensitivity, range and temperature dependence of hearing in the grass frog and fire-bellied toad. Behav. Process. 5, 363–372. ( 10.1016/0376-6357(80)90019-4) [DOI] [PubMed] [Google Scholar]
- 48.Fay RR. 1988. Hearing in vertebrates: a psychophysics databook. Winnetka, IL: Hill-Fay Associates. [Google Scholar]
- 49.Liang D, Shen XX, Zhang P. 2013. One thousand two hundred ninety nuclear genes from a genome-wide survey support lungfishes as the sister group of tetrapods. Mol. Biol. Evol. 33, 1803–1807. ( 10.1093/molbev/mst072) [DOI] [PubMed] [Google Scholar]
- 50.Clack JA. 2011. Gaining ground: the origin and evolution of tetrapods, second edition Bloomington, IN: Indiana University Press. [Google Scholar]
- 51.Platt C, Jørgensen JM, Popper AN. 2004. The inner ear of the lungfish Protopterus. J. Comp. Neurol. 471, 277–288. ( 10.1002/cne.20038) [DOI] [PubMed] [Google Scholar]
- 52.Clack JA, Ahlberg PE, Finney SM, Dominguez Alonso P, Robinson J, Ketcham RA. 2003. A uniquely specialized ear in a very early tetrapod. Nature 425, 65–69. ( 10.1038/nature01904) [DOI] [PubMed] [Google Scholar]
- 53.White J, Baird I. 1982. Comparative morphological features of the caecilian inner ear with comments on the evolution of amphibian auditory structures. Scanning Electron Microsc. 3, 1301–1312. [PubMed] [Google Scholar]
- 54.Baird IL. 1974. Some aspects of the comparative anatomy and evolution of the inner ear in submammalian vertebrates. Brain Behav. Evol. 10, 11–36. ( 10.1159/000124300) [DOI] [PubMed] [Google Scholar]
- 55.Smotherman M, Narins P. 2004. Evolution of the amphibian ear. In Evolution of the vertebrate auditory system (eds Manley GA, Fay RR, Popper AN.), pp. 164–199. New York, NY: Springer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual AEP thresholds: Dryad doi:10.5061/dryad.mg5ng.