Abstract
Heat shock proteins (Hsps) are ubiquitous proteins that are induced following exposure to sublethal heat shock, are highly conserved during evolution, and protect cells from damage through their function as molecular chaperones. Some cancers demonstrate elevated levels of Hsp70, and their expression has been associated with cell proliferation, disease prognosis, and resistance to chemotherapy. In this study, we developed a tetracycline-regulated gene expression system to determine the specific effects of inducible Hsp70 on cell growth and protection against hyperthermia in MCF-7 breast cancer cells. MCF-7 cells expressing high levels of Hsp70 demonstrated a significantly faster doubling time (39 hours) compared with nonoverexpressing control cells (54 hours). The effect of elevated Hsp70 on cell proliferation was characterized further by 5-bromo-2′deoxyuridine labeling, which demonstrated a higher number of second and third division metaphases in cells at 42 and 69 hours, respectively. Estimates based on cell cycle analysis and mean doubling time indicated that Hsp70 may be exerting its growth-stimulating effect on MCF-7 cells primarily by shortening of the G0/G1 and S phases of the cell cycle. In addition to the effects on cell growth, we found that elevated levels of Hsp70 were sufficient to confer a significant level of protection against heat in MCF-7 cells. The results of this study support existing evidence linking Hsp70 expression with cell growth and cytoprotection in human cancer cells.
INTRODUCTION
Heat shock proteins (Hsps) belong to the highly conserved family of stress proteins, some of which are induced by a variety of cellular stresses, environmental factors, and pathological conditions (Lindquist 1986). Several major classes of Hsps (Hsp110, Hsp90, Hsp70, Hsp25) commonly are found in mammalian cells and named in accordance with their molecular weights. The Hsp70 family includes 2 major forms: a constitutively expressed, 73-kDa protein (Hsc70) and a stress-inducible, 72-kDa protein (Hsp70). A major role of Hsps resides in their ability to function as molecular chaperones. Hsp70 binds nascent polypeptide chains; assists protein transport into the mitochondria, endoplasmic reticulum, and nucleus; maintains proper folding of precursor proteins; and protects proteins from stress (Georgopoulos and Welch 1993; Craig et al 1994). Overexpression of Hsp70 is frequently observed in several types of tumors, including breast and cervical cancers (Yano et al 1996; Kim et al 1998; Park et al 1999) and may be involved with cell proliferation, prognosis, and drug resistance.
Accumulating evidence indicates that Hsp70 plays an important role in the control of cell cycling and growth. Under normal conditions, inducible Hsp70 is expressed in proliferating cells during G1/S and S phases of the cell cycle (Kao et al 1985; Milarski and Morimoto 1986; Taira et al 1997). Expression of the hsp70 genes are induced by a number of oncogenes, including c-myc (Kaddurah-Daouk et al 1987; Taira et al 1999), p53 (Tsutsumi-Ishii et al 1995), and adenovirus E1A (Simon et al 1988; Williams et al 1989). In SHOK cells, the overexpression of Hsp72 using a metallothionein IIA promoter causes stimulation of cell growth (Suzuki and Watanabe 1994). Immunohistochemical studies of breast tumors also demonstrate a positive correlation between Hsp70 levels and proliferative activity (Yano et al 1996; Vargas-Roig et al 1997).
When living cells are exposed to nonlethal elevated temperatures, they acquire a transient resistance to a subsequent heat shock. This well-studied phenomenon of thermotolerance is paralleled by the expression of Hsps and includes members of the Hsp70 family (Landry et al 1982; Li and Werb 1982; Subjeck et al 1982; Li et al 1995). Other Hsp members, including Hsp90 and Hsp27, have been implicated in the development of thermotolerance (Chretien and Landry 1988; Bansal et al 1991; Lavoie et al 1993; Heads et al 1995). In studies where the synthesis of Hsps is inhibited, either by the expression of a high copy of heat shock elements (Johnston and Kucey 1988), disruption of the HSF1 gene (McMillan et al 1998), or antisense technology (Wei et al 1995), there is a loss of heat resistance. To date, it is unclear if thermotolerance is primarily due to one particular Hsp or is achieved through cooperation from several members of the Hsp family. In the present studies, we have created a tetracycline-regulated gene expression system in MCF-7 breast cancer cells to examine the specific effect of inducible Hsp70 on cell growth and protection against the cytotoxicity of hyperthermia.
MATERIALS AND METHODS
MCF-7 Tet-off cells, plasmids, and constructs
MCF-7 Tet-off cells (Clontech, Palo Alto, CA, USA) containing the plasmid pUHD15-1neo express the tTA (tet transactivator) regulator protein from the strong immediate early promoter of cytomegalovirus (PCMV). The pTRE response plasmid pUHD10-3 (Clontech) contains the compound promoter PhCMV*-1, which consists of the tetracycline response element (TRE) located upstream of PminCMV. pTK-Hyg (Clontech) encodes the hygromycin resistance gene under the control of a thymidine kinase minimal promoter from herpes simplex virus. The plasmid pLitHsp70-1 (generous gift from Clayton Hunt) contains the human hsp70-1 cDNA. The plasmid pTRE/Hsp70-1 was constructed by ligating a 2.1-kb BamHI/BfaI fragment from pLitHsp70-1 downstream of the tTA-regulated promoter of pUHD10-3.
Cell culture and transfections
Human MCF-7 Tet-off breast tumor cells and transfected derivatives were cultured in Dulbecco modified Eagle medium supplemented with 10% Tet system–approved fetal bovine serum (Clontech), 2 mM l-glutamine, 100 U/mL of penicillin, 100 μg/mL of streptomycin, and 100 μg/mL of G418. Fugene (Boehringer Mannheim, Palo Alto, CA, USA) was used according to the manufacturer's directions for all stable transfections. MCF-7 Tet-off cells were transfected with pTRE/Hsp70-1 and pTK-Hyg (3:1). Stable clones were selected in medium containing 50 μg/mL of hygromycin. Seventeen different clones were isolated, expanded, and screened for Hsp70 and Hsc70 expression by Western analysis. Clones positive for elevated Hsp70 expression were screened for down-regulation of Hsp70 levels by culturing cells in media containing doxycycline (2 μg/mL).
Western analysis
MCF-7 cell clones were cultured in medium with (off) or without (on) doxycycline for 48 hours. Cells were harvested by trypsinization and sonicated in buffer containing fresh protease inhibitors. Aliquots from each sample containing 4 μg of protein were heat denatured, subjected to 1-dimensional 7.5% polyacrylamide gel electrophoresis, and transferred to nitrocellulose. The membrane was blocked with 5% nonfat milk in Tris-buffered saline (TBS) and washed in TBS with 0.1% Tween-20 (TTBS). The membrane was incubated for 1 hour with an anti-Hsp70 mouse monoclonal antibody specific for human-inducible Hsp70 (MA3-009, Affinity Bioreagents, Golden, CO, USA) diluted 1:10 000 in TTBS or a goat-polyclonal antibody specific for constitutive Hsc70 (K-19, Santa Cruz Biotechnology, Inc, Santa Cruz, CA, USA) diluted 1:1000 in TTBS and washed 3 times in TTBS. A 1:10 000 dilution of anti-mouse IgG or anti-goat IgG conjugated to horseradish peroxidase was incubated with the membranes for 1 hour, followed by 4 washes in TTBS. Bands were detected using a commercially available chemiluminescent detection system (ECL, Amersham, NJ, USA).
Immunofluorescence
MCF-7 cells (clones C14 and C20) were grown on glass coverslips and fixed in cold methanol for 10 minutes. Coverslips were blocked with 2% bovine serum albumin (BSA) with 0.1% Triton X-100 for 20 minutes. A mouse monoclonal antibody (SPA-810, StressGen Biotechnologies, Victoria, British Columbia, Canada) specific for inducible Hsp70 was diluted 1:500, placed on coverslips, and incubated at 37°C for 1 hour. Coverslips were washed 3 times in PBS/0.02% BSA/0.01% Triton X-100 for 5 minutes each. Secondary antibody consisted of a fluorescein isothiocyanate–labeled anti-mouse IgG diluted 1:100, incubated at 37°C for 1 hour. Coverslips were washed 3 times as performed earlier and mounted on slides with 1 μg/mL of 4′,6-diamidino-2-phenylindole dihydrochloride. Fluorescence was visualized with a Nikon Microphot-FXA microscope and photographs were taken with a Nikon 35-mm camera.
Cell growth curves
MCF-7 cells were seeded at 105 cells per well in 6-well plates and cultured in media with (off) or without (on) doxycycline. Viable cells were counted at 48, 72, 96, and 169 hours after plating using a hemacytometer. Statistical analysis on cell numbers was performed using an analysis of variance (ANOVA) and Bonferroni multiple comparison test at P < 0.05 (GraphPad Prism, GraphPad Software Inc, San Diego, CA, USA). Cell growth curves from 4 to 6 experiments were plotted and doubling times calculated from the linear component of the exponential growth phase. Statistical analysis of cell doubling times in MCF-7 clones expressing control levels of Hsp70 compared with C14-on cells was performed using a t-test at P < 0.05. The percentage of cell death was determined by staining with trypan blue.
BrdU labeling
MCF-7 cells were plated at a density of 4 × 105 cells in T25 flasks. 5-Bromo-2′-deoxyuridine (BrdU, 10 μM) was added to the cultures 24 hours after plating, and the cells were harvested at 42 and 69 hours after BrdU. Colcemid (0.5 μg/mL) was added to each culture 2 hours before harvesting. Cells were trypsinized and cell pellets resuspended in hypotonic solution (0.075 M KCl) for 10 min. Cells were fixed in 3:1 methanol–acetic acid solution 3 times and placed on slides to dry. Hoescht 33258 reagent was added to the slides, incubated for 21 minutes, and rinsed in water. McIlvaine's buffer (pH 8.0) was added and slides were placed on a slide warmer at 55°C under black lights for 21 minutes. Slides were rinsed in water, dried, and stained with 4% Giemsa in Gurr buffer. Two hundred metaphases per group were scored as first, second, or third divisions using a Leitz microscope. A Fisher's exact test and χ2 analysis were performed on data generated from C14-on and -off cells at 42 (n = 4) and 69 hours (n = 3), respectively.
Flow cytometry
Exponentially growing MCF-7 cells were isolated by trypsinization and centrifugation. Lysis buffer (0.2% NP-40 and 0.5 mg/mL of ribonuclease A in PBS) was added to the cell pellets, and incubation proceeded at 25°C for 30 minutes. Propidium iodide (500 μg/mL) was added to each sample and incubated at 4°C for 15 minutes. DNA content of 10 000–12 000 cells per sample was monitored with a Becton Dickinson FACSCalibur Flow Cytometer and data were analyzed using ModFitLT v2.0 software (Verity Software House Inc, Topsham, ME, USA).
Hyperthermia—cell counts and cytotoxicity
MCF-7 cells were seeded at 105 cells per well in 6-well plates and cultured in media with (off) or without (on) doxycycline. On day 5 of growth, triplicate cultures were exposed to 37°C for 60 minutes (control), 43°C for 30 minutes, or 43°C for 60 minutes. Following exposures, the plates were returned to a 37°C incubator. Twenty-four hours following heat treatments, cells from each culture were trypsinized and counted using a hemacytometer. Statistical analysis was performed using an ANOVA and Bonferroni multiple comparison test at P < 0.05. For cytotoxicity measurements, C14-on and C14-off cells were exposed to 43°C for 60 minutes. Twenty-four hours following heat treatment, the cells were trypsinized and resuspended in media containing 1:25 ethidium bromide (100 μg/mL) and acridine orange (100 μg/mL). Cells were placed on slides and examined using a fluorescence microscope. Two hundred cells from each group were evaluated as dead (orange), live (green with normal nuclear/chromatin structure), or apoptotic (green with condensed chromatin/apoptotic bodies). Statistical analysis was performed using a t-test at P < 0.05.
RESULTS
Construction of Hsp70-1–expressing vector and isolation of MCF-7 clones containing human hsp70-1 gene
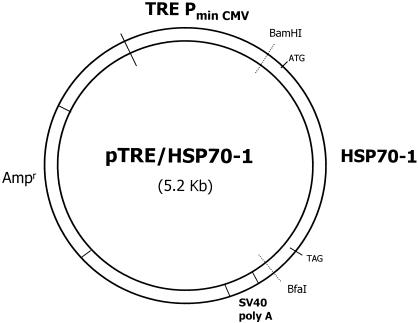
The Hsp70-1 tetracycline-controlled vector pTRE/Hsp70-1 (Fig 1) contained both a 2.1-kb fragment from pLitHsp70-1 and a 3.1-kb fragment of pUHD10-3 (Clontech). The human hsp70-1 gene fragment was ligated downstream of the tTA-regulated promoter, which contains a TRE upstream of the minimal immediate early promoter of cytomegalovirus (PminCMV). The 2.1-kb fragment of the human hsp70-1 gene contained initiation and termination codons. The simian vacuolating virus No. 40 poly A sequence was included in the pUHD10-3.
Fig. 1.
Structure of pTRE/Hsp70-1 plasmid. The vector contains a 2.1-kb fragment of BamHI/BfaI from pLitHsp70-1 and 3.1 kb of pUHD10-3 (Clontech) containing the compound promoter consisting of the TRE located upstream of the early minimal promoter from cytomegalovirus PminCMV
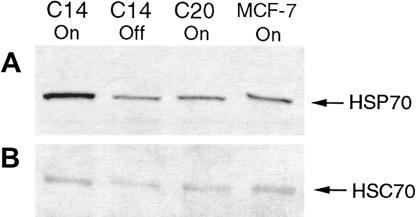
Following cotransfection with pTRE/Hsp70-1 and pTK-Hyg into MCF-7 Tet-off cells, several hygromycin-resistant clones were isolated and expanded. Figure 2 shows results obtained from Western analysis demonstrating inducible Hsp70 levels in parental MCF-7 Tet-off cells and 2 hygromycin-resistant clones grown with (off) or without (on) the presence of 2 μg/mL of doxycycline. MCF-7 Tet-off cells expressed detectable amounts of inducible Hsp70 under normal conditions (Fig 2A). The MCF-7 clone designated C20 was an example of a negative clone that maintained hygromycin resistance but did not incorporate the TRE/Hsp70-1 construct. Even under inducible conditions, C20 cells demonstrated no increase in Hsp70 levels over control. The MCF-7 clone C14 demonstrated integration of both hygromycin and TRE/Hsp70-1 constructs. Under induced conditions, C14 cells demonstrated an approximate 5-fold up-regulation of inducible Hsp70 levels. Induced levels of Hsp70 in C14 cells were down-regulated to control levels with the addition of doxycycline. Constitutive Hsc70 levels were similar in all of the clones grown under either induced or repressed conditions (Fig 2B).
Fig. 2.
Hsp70 and Hsc70 levels in MCF-7 clones. Western analysis was performed with a monoclonal antibody specific for Hsp70 (A) or Hsc70 (B). C14, a positive MCF-7 clone; C20, a negative MCF-7 clone; MCF-7, MCF-7 Tet-off, parental cell line. Cells were cultured with (off) or without (on) doxycycline
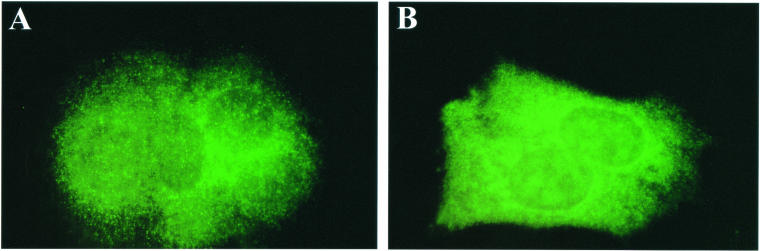
Immunofluorescence analysis further demonstrated induction of Hsp70 in clone C14 cells compared with the negative clone C20. The intracellular localization of the Hsp70 signal in C20 cells was observed to be more intense in the cytoplasm than in the nucleus (Fig 3A). In C14-on cells, the intensity of signal throughout the cells was consistently greater than in C20 cells, and the same relative intensities of cytoplasmic and nuclear staining were maintained (Fig 3B). Overall, these data further validated the elevated expression of inducible Hsp70 and demonstrated no significant alterations in intracellular distribution between the Hsp70 overexpressing cells and controls.
Fig. 3.
Immunofluorescence of inducible Hsp70 in MCF-7 cells. Hsp70 localization was analyzed by immunofluorescence with a monoclonal antibody to Hsp70 and a fluorescein isothiocyanate–labeled secondary antibody. (A) Negative MCF-7 clone C20; (B) Hsp70 overexpressing MCF-7 clone C14 (magnification, 100×)
Enhanced cell growth in Hsp70 overexpressing cells
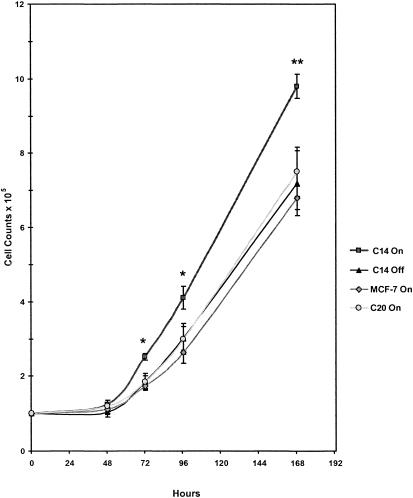
Figure 4 depicts cell growth data from MCF-7 Tet-off (parental), C20, and C14 cells grown under on and off conditions. In general, the MCF-7 cells demonstrated a 2-day lag period before entering the exponential growth phase. All MCF-7 cells expressing control levels of Hsp70 (MCF-7 Tet-off, C20, and C14-off) exhibited similar growth curves, whereas C14-on cells displayed significantly enhanced growth. The number of viable C14-on cells was significantly higher than any of the MCF-7 cells expressing control levels of Hsp70 at 3 (P < 0.05), 4 (P < 0.05), and 7 (P < 0.01) days after plating. Trypan blue staining of C14-on and C14-off cells demonstrated no significant differences in the percentage of cell death at 2, 3, and 4 days after plating, which could account for the differences in cell number and growth rate (Fig 5).
Fig. 4.
Cell growth curves of MCF-7 clones. MCF-7 cell clones (C14, C20, and parental MCF-7 Tet-off) were cultured with (off) or without (on) doxycycline. The average number of viable cells (±SEM) are plotted vs time. Significant differences in cell numbers at P < 0.05 (*) and P < 0.01 (**) are indicated. n = 6 replicates for C14 cells, n = 5 for C20 cells, and n = 4 for MCF-7 Tet-off cells
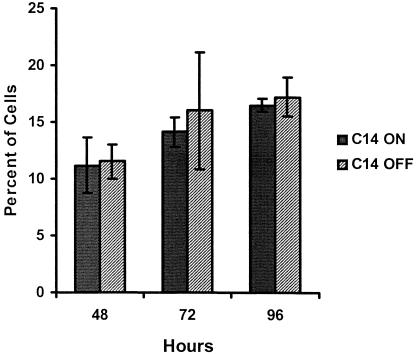
Fig. 5.
Cell death of MCF-7 clones. MCF-7 clone C14 cells were cultured with (off) or without (on) doxycycline. Cell death was determined by trypan blue staining. Data are reported as the percentage of dead cells (±SEM) at 48, 72, and 96 hours after plating (n = 3 replicates)
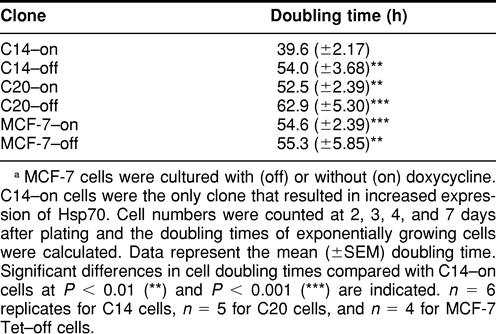
Cell doubling times were calculated using data from the exponential growth phase (Table 1). The average doubling time for MCF-7 cells expressing control levels of Hsp70 was 54 hours, whereas cells expressing elevated levels of Hsp70 exhibited a doubling time of 39 hours. The doubling time of C14-on cells was significantly different from C14-off, C20-on, C20-off, MCF-7-on, and MCF-7-off cells at P < 0.01. There were no significant differences in the doubling times between cells expressing control levels of Hsp70 grown under either induced or repressed conditions.
TABLE 1.
Calculated cell doubling times of MCF-7 clonesa
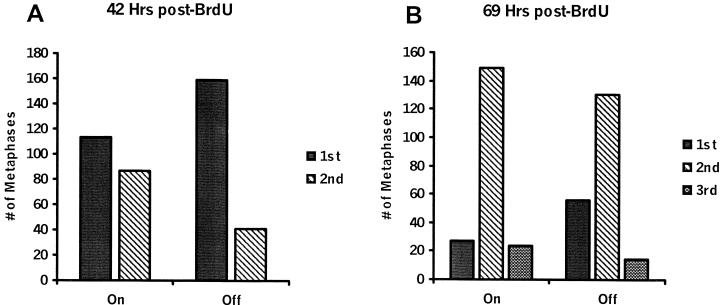
BrdU incorporation and differential staining were used to further confirm differences in growth of MCF-7 cells expressing control vs elevated levels of Hsp70. Figure 6A demonstrates an increase in the average number of second division metaphases at 42 hours after BrdU in C14-on cells compared with C14-off cells. Likewise, at 69 hours after BrdU, C14-on cells also showed an elevation in average number of second and third division metaphases compared with the number observed for C14-off cells (Fig 6B). Statistical analysis indicated a significant difference in the distribution of first, second, and third division metaphases in C14-on cells compared with C14-off cells at 42 hours (P < 0.0001) and 69 hours (P < 0.001).
Fig. 6.
Metaphase analysis of MCF-7 cells. Clone C14 cells cultured with (off) or without (on) doxycycline were harvested for metaphase analysis 42 (A) and 69 (B) hours after BrdU. Two hundred metaphases were counted per group or experiment. Data represent the average number of metaphases observed from 4 experiments (42 hours) or 3 experiments (69 hours). Significant differences in the distribution of first, second, and third division metaphases were observed in C14-on cells compared with C14-off cells at 42 hours (P < 0.0001) and 69 hours (P < 0.001)
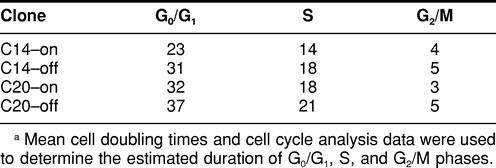
Flow cytometry was used to determine the cell cycle distributions of exponentially growing MCF-7 cells. As seen in Table 2, there were no significant differences in the percentage of cells in G0/G1, S, or G2/M phases of the cycle between C14 or C20 cells grown under either on or off conditions. Flow cytometry can be used to estimate the length of G1, S, and G2 phases when certain conditions are met: the mean generation time for a cell population is known, the fraction of proliferative cells is high, the frequency of cell loss is low, and cells are reasonably homogeneous with respect to their cell cycle kinetics (Merrill 1998). The calculated cell doubling times and cell cycle analysis data were used to provide an estimated duration of G0/G1, S, and G2/M cell cycle phases in MCF-7 cells. Data presented in Table 3 indicate that cells expressing elevated levels of Hsp70 (C14-on) have a shorter duration of G0/G1 (23 vs 33.3 hours), S (14 vs 19 hours), and G2/M (4 vs 4.3 hours) phases compared with cells expressing control levels of Hsp70 (C14-off, C20-on, C20-off). Thus, although all phases appear shortened, these effects were greatest at G0/G1 and S phases.
TABLE 2.
Percentage of G0/G1, S, and G2/M cellsa
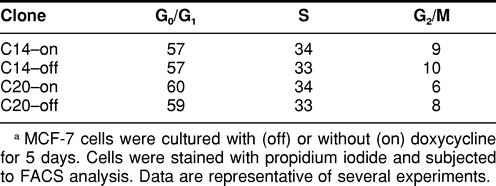
TABLE 3.
Estimated duration (in hours) of G0/G1, S, and G2/M phasesa
Effect of Hsp70 on cytotoxicity of heat shock
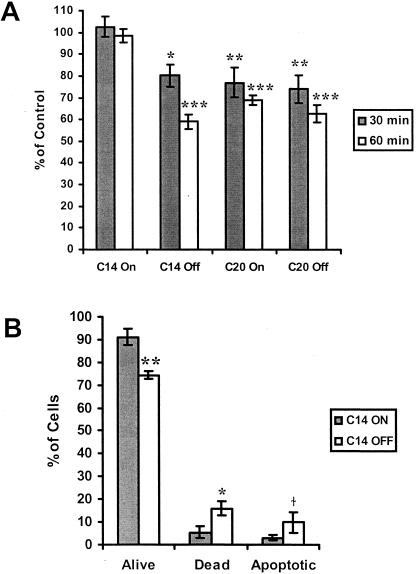
Figure 7A depicts the effect of Hsp70 levels on cell growth and viability following heat shock treatments of 43°C for 30 or 60 minutes. MCF-7 clones expressing control levels of Hsp70 (C20-on, C20-off, C14-off) demonstrated a significant time-dependent decrease in cell number following heat shock compared with MCF-7 cells that expressed elevated Hsp70 (C14-on). Twenty-four hours following a heat shock of 43° for 30 minutes, the number of C14-on cells was significantly higher than that of C14-off (P < 0.05), C20-on (P < 0.01), or C20-off (P < 0.01) cells. After 60 minutes of heat shock, the number of C14-on cells was significantly (P < 0.001) higher than that of MCF-7 cells expressing control levels of Hsp70. Additional experiments using acridine orange and ethidium bromide staining further demonstrated the protective effect of Hsp70 following heat shock. As depicted in Figure 7B, C14-off cells showed decreased cell viability and increased cell death and apoptosis compared with C14-on cells. The effects on cell viability and cell death between C14-on and C14-off cells were statistically significant at P < 0.01 and P < 0.05, respectively. The effect of Hsp70 on apoptosis was not significant (P = 0.051).
Fig. 7.
Effect of heat shock on MCF-7 cells. (A) MCF-7 cell clones (C14 and C20) were cultured with (off) or without (on) doxycycline and exposed to heat shock of 43°C for either 30 or 60 minutes. Cells were returned to 37°C and harvested 24 hours later for cell counts. Data are reported as percentage of control (±SEM). Significant differences in cell numbers at P < 0.05 (*), P < 0.01 (**), and P < 0.001 (***) compared with C14-on cells are indicated. n = 6 replicates for C14 cells; n = 3 for C20 cells. (B) MCF-7 clone C14 cells were cultured with (off) or without (on) doxycycline and exposed to heat shock of 43°C for 60 minutes (n = 3 replicates). Cells were returned to 37°C for 24 hours and stained with ethidium bromide and acridine orange. Percentage of viable, dead, and apoptotic cells are reported (±SEM). Significant differences between C14-on and C14-off cells at P < 0.05 (*), P < 0.01 (**), and P = 0.051 (†) are indicated
DISCUSSION
In this study, we report a role for Hsp70 in enhancing cell proliferation and providing thermoprotection in MCF-7 breast cancer cells. To our knowledge, an investigation into the specific role(s) for Hsp70 has not previously been addressed using a tetracycline-regulated system in MCF-7 cells. In relation to this need, a stably transfected tetracycline-regulated MCF-7 cell line was created that expresses an approximately 5-fold increased amount of inducible Hsp70 when cells are cultured without doxycycline compared with the control level of Hsp70 when cultured with doxycycline. Hsc70 levels were unaltered in the MCF-7 cells regardless of Hsp70 levels. The addition of doxycycline did not affect any of the growth or cytotoxicity end points examined in our studies as indicated by comparing results between C20-on and C20-off.
Tetracycline-regulated expression systems provide several advantages over conventional gene regulatory systems. By testing the effects on genotypically identical cells, the Tet system avoids the problems of variability observed by using different cell lines or clones of cells. In addition, because tetracyclines have fairly low cytotoxicity and limited biological effects on mammalian cells, this system also avoids the problem of nonspecific activation of endogenous genes or certain physiological effects commonly seen with other inducers (Yarranton 1992). Therefore, the use of a tetracycline-regulated expression system significantly strengthens the interpretation that observed experimental effects are specifically due to differences in expression of the singular protein of interest.
Using the tetracycline-regulated system, we show that MCF-7 cells that express elevated levels of Hsp70 exhibit an increase in cell growth and thus a significantly shorter cell doubling time. Our data indicate that the apparently faster cell cycling is not influenced by differences in the rate of cell death or loss between Hsp70-on and -off cells during normal cell culture. The positive effect of elevated Hsp70 on cell proliferation is further supported by BrdU labeling, which demonstrates a higher number of second and third division metaphases in cells at 42 and 69 hours, respectively. Flow cytometry of MCF-7 cells with elevated Hsp70 indicates no significant alterations in cell cycle distribution. Based on cell cycle and doubling time data, Hsp70 appears to be exerting its effect on MCF-7 cells primarily by shortening of the G0/G1 and S phases of the cell cycle.
The significant effects of Hsp70 on cell growth and proliferation could be a general cellular effect or may relate specifically to the MCF-7 cell type. Levels of Hsc70 are significantly higher in proliferating compared with nonproliferating rat glioma cells and are particularly prevalent during the S phase of the cell cycle (Helmbrecht and Rensing 1999). Overexpression of human Hsp72 in the SHOK cell line also stimulates cell growth and the number of S phase cells (Suzuki and Watanabe 1994). Further data demonstrating a role of Hsp70 in proliferation come from hsp70 antisense administration, which interrupts cell proliferation and progress through G1 and S phase in human cancer cells (Wei et al 1995). It is interesting to note that several studies on human breast tumors have shown a positive correlation between Hsp70 immunoreactivity and proliferating cell nuclear antigen (PCNA) labeling (proliferation) index (Yano et al 1996; Vargas-Roig et al 1997). A recent study using proteomic and mass spectrometry analysis found that MCF-7 breast cells stimulated to proliferate by FGF-2 demonstrate up-regulation of Hsp70, Hsp90, and PCNA (Vercoutter-Edouart et al 2001). Our study adds new data to the accumulating evidence for a specific role of inducible Hsp70 in breast cancer cell growth and proliferation.
In this study, the G1 and S phases of the cycle appear to be considerably shortened by Hsp70 overexpression. Some members of the Hsp70 family are known to express in a cell-cycle dependent manner. In mouse cells, Hsp70 demonstrates 2 peaks of expression, one in G1 and the other during S phase. A G1-specific enhancer element has been identified and is thought to be responsible for cell cycle-dependent expression of hsp70 (Taira et al 1997). Several cell lines have demonstrated S phase–dependent increases in Hsp70 or Hsc70 message and protein (Milarski and Morimoto 1986; Zeise et al 1998; Helmbrecht and Rensing 1999), and translocation of Hsc70 into the nucleus during S phase has also been detected (Zeise et al 1998). Our immunofluorescence studies demonstrated that elevated levels of Hsp70 were primarily located within the cytoplasm of MCF-7 cells. Therefore, the overall effect on enhanced cell growth could not be attributed to a significant alteration in the intracellular distribution of Hsp70 produced from the transgene construct. To date, the exact role(s) Hsp70s play in the cell cycle and/or the mechanisms responsible for their effects on cell growth are not clear.
Hsp70s may influence cell proliferation through their association with a number of proteins, such as c-myc (Henriksson et al 1992), pRb (Inoue et al 1995), and p53 (Hainaut and Milner 1992; Selkirk et al 1996). A recent study found that Hsc73 forms complexes with p27Kip1, an inhibitor of cyclin-dependent kinase. This interaction appears to target degradation of p27Kip1 during the G1/S transition phase of the cell cycle (Nakamura et al 1999). Several members of the Hsp family, including Hsp70, also have the capacity to bind to nuclear receptors, such as estrogen receptor alpha (Smith and Toft 1993). Hsps have been linked to the intracellular transport of steroid receptors (De Franco et al 1997; Sai Padma et al 2000) and can influence receptor-DNA interactions (Landel et al 1997). Recent work by Hurd et al (2000) found that transient transfection of Hsp70 produced a 2- to 3-fold increase in estrogen-stimulated estrogen response element–luciferase reporter activity in MCF-7 cells. Hsps may likewise contribute to enhanced cell growth through interactions with microtubules and/or centrosomes. Hsp70s have long been identified as microtubule-associated proteins (Weller 1988; Sanchez et al 1994) and are also localized to the centrosome during cell division (Rattner 1991; Brown et al 1994). In human breast samples, Hsp70 was found complexed to microtubules and clearly associated with the mitotic spindles (Vargas-Roig et al 1997). Given the wide range of proteins that Hsps chaperone, it is reasonable that Hsp70 overexpression could have significant effects on cell division through interactions with one or more cellular proteins and/or structures.
In addition to the effects on cell proliferation, the present study also investigated the ability of inducible Hsp70 to provide thermotolerance. Elevated levels of inducible Hsp70 produced by clone C14 were sufficient to confer a significant level of protection against the cytotoxic effects of heat in MCF-7 cells. This demonstrated that the Hsp70 protein produced by the transgene construct was adequate, in both quantity and quality, to provide cytoprotection. However, in addition to its value as a validation of the cell line, the results of these experiments also identify a specific role for Hsp70 in the phenomenon of thermotolerance. Although thermotolerance has been generally linked to the expression of several Hsps (Landry et al 1982; Subjeck et al 1982), it has not been entirely clear which or how many members of the Hsp family are required to produce this effect. Other studies have linked thermotolerance to the expression of Hsp27 (Chretien and Landry 1988), Hsp40 (Kaneko et al 1997), Hsp90 (Bansal et al 1991), Hsp110 (Oh et al 1997), Hsc70, and Hsp70 (Li and Werb 1982; Li et al 1995). Overexpression of Hsp70 in hamster fibroblasts using the Tet system demonstrated that Hsp70 significantly contributed to heat tolerance, even though it was not able to protect cells to the same degree as thermotolerant cells (Nollen et al 1999). Another study using the Tet system in a human T-cell line found that elevated Hsp70 was sufficient to confer increased viability and reduced levels of apoptosis following heat stress (Mosser et al 1997). Our study similarly demonstrates that in human MCF-7 cells, a protective effect against cell death and apoptosis following heat stress can be obtained by elevated levels of inducible Hsp70.
In conclusion, results of this study demonstrate a significant effect of inducible Hsp70 on cell growth and protection against heat stress in MCF-7 breast cancer cells. This cell system provides an important tool to selectively study the effects of Hsp70 without the potentially confounding effects from induction of other members of the Hsp family. Further studies will be required to fully elucidate the specific molecular mechanisms responsible for the effects of inducible Hsp70 on cell growth and cytoprotection. Nevertheless, the described MCF-7 cell system has potential application for studies into the clinical significance of Hsp70s, particularly in regard to the development of cancer and their utility in diagnosis, treatment, and prevention.
Acknowledgments
The authors would like to thank Dr Alex Merrick for his insightful comments and suggestions during the development of this cell line. This work was supported by a National Research Council Senior Associate Award. This article has been reviewed by the US Environmental Protection Agency and approved for publication. Approval does not signify that the contents necessarily reflect the views or policies of the agency nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
REFERENCES
- Bansal GS, Norton PM, Latchman DS. The 90-kDa heat shock protein protects mammalian cells from thermal stress but not from viral infection. Exp Cell Res. 1991;195:303–306. doi: 10.1016/0014-4827(91)90377-7. [DOI] [PubMed] [Google Scholar]
- Brown CR, Doxsey SJ, White E, Welch WJ. Both viral (adenovirus E1B) and cellular (hsp 70, p53) components interact with centrosomes. J Cell Physiol. 1994;160:47–60. doi: 10.1002/jcp.1041600107. [DOI] [PubMed] [Google Scholar]
- Chretien P, Landry J. Enhanced constitutive expression of the 27-kDa heat shock proteins in heat-resistant variants from Chinese hamster cells. J Cell Physiol. 1988;137:157–166. doi: 10.1002/jcp.1041370119. [DOI] [PubMed] [Google Scholar]
- Craig EA, Weissman JS, Horwich AL. Heat shock proteins and molecular chaperones: mediators of protein conformation and turnover in the cell. Cell. 1994;78:365–372. doi: 10.1016/0092-8674(94)90416-2. [DOI] [PubMed] [Google Scholar]
- De Franco DB, Ramakrishnan C, Tang Y. Molecular chaperones and subcellular trafficking of steroid receptors. J Steroid Biochem Mol Biol. 1997;65:51–58. doi: 10.1016/s0960-0760(97)00177-5. [DOI] [PubMed] [Google Scholar]
- Georgopoulous C, Welch WJ. Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol. 1993;9:601–634. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- Hainaut P, Milner J. Interaction of heat-shock protein 70 with p53 translated in vitro: evidence for interaction with dimeric p53 and for a role in the regulation of p53 conformation. EMBO J. 1992;11:3513–3520. doi: 10.1002/j.1460-2075.1992.tb05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heads RJ, Yellon DM, Latchman DS. Differential cytoprotection against heat stress or hypoxia following expression of specific stress protein genes in myogenic cells. J Mol Cell Cardiol. 1995;27:1669–1678. doi: 10.1016/s0022-2828(95)90722-x. [DOI] [PubMed] [Google Scholar]
- Helmbrecht K, Rensing L. Different constitutive heat shock protein 70 expression during proliferation and differentiation of rat C6 glioma cells. Neurochem Res. 1999;24:1293–1299. doi: 10.1023/a:1020933308947. [DOI] [PubMed] [Google Scholar]
- Henriksson M, Classon M, Axelson H, Klein G, Thyberg J. Nuclear colocalization of c-myc protein and hsp70 in cells transfected with human wild-type and mutant c-myc genes. Exp Cell Res. 1992;203:383–394. doi: 10.1016/0014-4827(92)90012-w. [DOI] [PubMed] [Google Scholar]
- Hurd AM, Schiff R, Parra I, Friedrichs WE, Osborne CK, Morimoto RI, Hopp T, Fuqua SAW. Heat shock protein 70 can modulate estrogen receptor activity in breast cancer cells. Proc Am Assoc Cancer Res. 2000;41:73. [Google Scholar]
- Inoue A, Torigoe T, and Sogahata K. et al. 1995 70-kDa heat shock cognate protein interacts directly with the N-terminal region of the retinoblastoma gene product pRb: identification of a novel region of pRb-mediating protein interaction. J Biol Chem. 270(2257):1–6. [DOI] [PubMed] [Google Scholar]
- Johnston RN, Kucey BL. Competitive inhibition of hsp70 gene expression causes thermosensitivity. Science. 1988;242:1551–1554. doi: 10.1126/science.3201244. [DOI] [PubMed] [Google Scholar]
- Kaddurah-Daouk R, Greene JM, Baldwin AS Jr,, Kingston RE. Activation and repression of mammalian gene expression by the c-myc protein. Genes Dev. 1987;1:347–357. doi: 10.1101/gad.1.4.347. [DOI] [PubMed] [Google Scholar]
- Kaneko R, Hayashi Y, Tohnai I, Ueda M, Ohtsuka K. Hsp40, a possible indicator for thermotolerance of murine tumour in vivo. Int J Hyperthermia. 1997;13:507–516. doi: 10.3109/02656739709023549. [DOI] [PubMed] [Google Scholar]
- Kao HT, Capasso O, Heintz N, Nevins JR. Cell cycle control of the human Hsp70 gene: implications for the role of a cellular E1A-like function. Mol Cell Biol. 1985;5:628–633. doi: 10.1128/mcb.5.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KK, Jang TJ, Kim JR. Hsp70 and ER expression in cervical intraepithelial neoplasia and cervical cancer. J Korean Med Sci. 1998;13:383–388. doi: 10.3346/jkms.1998.13.4.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landel CC, Potthoff SJ, Nardulli AM, Kushner PJ, Greene GL. Estrogen receptor accessory proteins augment receptor-DNA interaction and DNA bending. J Steroid Biochem Mol Biol. 1997;63:59–73. doi: 10.1016/s0960-0760(97)00073-3. [DOI] [PubMed] [Google Scholar]
- Landry J, Bernier D, Chretien P, Nicole LM, Tanguay RM, Marceau N. Synthesis and degradation of heat shock proteins during development and decay of thermotolerance. Cancer Res. 1982;42:2457–2461. [PubMed] [Google Scholar]
- Lavoie JN, Gingras-Breton G, Tanguay RM, Landry J. Induction of Chinese hamster Hsp27 gene expression in mouse cells confers resistance to heat shock: Hsp27 stabilization of the microfilament organization. J Biol Chem. 1993;268:3420–3429. [PubMed] [Google Scholar]
- Li GC, Mivechi NF, Weitzel G. Heat shock proteins, thermotolerance, and their relevance to clinical hyperthermia. Int J Hyperthermia. 1995;11:459–488. doi: 10.3109/02656739509022483. [DOI] [PubMed] [Google Scholar]
- Li GC, Werb Z. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc Natl Acad Sci U S A. 1982;79:3218–3222. doi: 10.1073/pnas.79.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- McMillan DR, Xiao X, Shao L, Graves K, Benjamin IJ. Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J Biol Chem. 1998;273:7523–7528. doi: 10.1074/jbc.273.13.7523. [DOI] [PubMed] [Google Scholar]
- Merrill GF. Cell synchronization. Methods Cell Biol. 1998;57:229–249. [PubMed] [Google Scholar]
- Milarski KL, Morimoto RI. Expression of human hsp70 during the synthetic phase of the cell cycle. Proc Natl Acad Sci U S A. 1986;83:9517–9521. doi: 10.1073/pnas.83.24.9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DD, Caron AW, Bourget L, Denis-Larose C, Massie B. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol Cell Biol. 1997;17:5317–5327. doi: 10.1128/mcb.17.9.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Tatuno I, Noguchi Y, Kitagawa M, Kohn LD, Saito Y, Hirai A. 73-kDa heat shock cognate protein interacts directly with P27Kip1, a cyclin-dependent kinase inhibitor, during G1/S transition. Biochem Biophys Res Commun. 1999;257:340–343. doi: 10.1006/bbrc.1999.0442. [DOI] [PubMed] [Google Scholar]
- Nollen EA, Brunsting JF, Roelofsen H, Weber LA, Kampinga HH. In vivo chaperone activity of heat shock protein 70 and thermotolerance. Mol Cell Biol. 1999;19:2069–2079. doi: 10.1128/mcb.19.3.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh HJ, Chen X, Subjeck JR. Hsp110 protects heat-denatured proteins and confers cellular thermoresistance. J Biol Chem. 1997;272:31636–31640. doi: 10.1074/jbc.272.50.31636. [DOI] [PubMed] [Google Scholar]
- Park CS, Joo IS, Song SY, Kim DS, Bae DS, Lee JH. An immunohistochemical analysis of heat shock protein 70, p53, and estrogen receptor status in carcinoma of the uterine cervix. Gynecol Oncol. 1999;74:53–60. doi: 10.1006/gyno.1999.5429. [DOI] [PubMed] [Google Scholar]
- Rattner JB. hsp70 is localized to the centrosome of dividing HeLa cells. Exp Cell Res. 1991;195:110–113. doi: 10.1016/0014-4827(91)90505-o. [DOI] [PubMed] [Google Scholar]
- Sai Padma A, Renil M, Varman Thampan R. Protein-protein interactions that precede the nuclear entry of goat uterine estrogen receptor under cell-free conditions. J Cell Biochem. 2000;78:650–665. [PubMed] [Google Scholar]
- Sanchez C, Padilla R, Paciucci R, Zabala JC, Avila J. Binding of heat-shock protein 70 (hsp70) to tubulin. Arch Biochem Biophys. 1994;310:428–432. doi: 10.1006/abbi.1994.1188. [DOI] [PubMed] [Google Scholar]
- Selkirk JK, He C, Patterson RM, Merrick BA. Tumor suppressor p53 gene forms multiple isoforms: evidence for single locus origin and cytoplasmic complex formation with heat shock proteins. Electrophoresis. 1996;17:1764–1771. doi: 10.1002/elps.1150171114. [DOI] [PubMed] [Google Scholar]
- Simon MC, Fisch TM, Benecke BJ, Nevins JR, Heintz N. Definition of multiple, functionally distinct TATA elements, one of which is a target in the hsp70 promoter for E1A regulation. Cell. 1988;52:723–729. doi: 10.1016/0092-8674(88)90410-2. [DOI] [PubMed] [Google Scholar]
- Smith DF, Toft DO. Steroid receptors and their associated proteins. Mol Endocrinol. 1993;7:4–11. doi: 10.1210/mend.7.1.8446107. [DOI] [PubMed] [Google Scholar]
- Subjeck JR, Sciandra JJ, Johnson RJ. Heat shock proteins and thermotolerance: a comparison of induction kinetics. Br J Radiol. 1982;55:579–584. doi: 10.1259/0007-1285-55-656-579. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Watanabe M. Modulation of cell growth and mutation induction by introduction of the expression vector of human hsp70 gene. Exp Cell Res. 1994;215:75–81. doi: 10.1006/excr.1994.1317. [DOI] [PubMed] [Google Scholar]
- Taira T, Narita T, Iguchi-Ariga SM, Ariga H. A novel G1-specific enhancer identified in the human heat shock protein 70 gene. Nucleic Acids Res. 1997;25:1975–1983. doi: 10.1093/nar/25.10.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira T, Sawai M, Ikeda M, Tamai K, Iguchi-Ariga SM, Ariga H. Cell cycle-dependent switch of up-and down-regulation of human hsp70 gene expression by interaction between c-Myc and CBF/NF-Y. J Biol Chem. 1999;274:24270–24279. doi: 10.1074/jbc.274.34.24270. [DOI] [PubMed] [Google Scholar]
- Tsutsumi-Ishii Y, Tadokoro K, Hanaoka F, Tsuchida N. Response of heat shock element within the human Hsp70 promoter to mutated p53 genes. Cell Growth Differ. 1995;6:1–8. [PubMed] [Google Scholar]
- Vargas-Roig LM, Fanelli MA, Lopez LA, Gago FE, Tello O, Aznar JC, Ciocca DR. Heat shock proteins and cell proliferation in human breast cancer biopsy samples. Cancer Detect Prev. 1997;21:441–451. [PubMed] [Google Scholar]
- Vercoutter-Edouart AS, Czeszak X, Crepin M, Lemoine J, Boilly B, Le Bourhis X, Peyrat JP, Hondermarck H. Proteomic detection of changes in protein synthesis induced by fibroblast growth factor-2 in MCF-7 human breast cancer cells. Exp Cell Res. 2001;262:59–68. doi: 10.1006/excr.2000.5066. [DOI] [PubMed] [Google Scholar]
- Wei YQ, Zhao X, Kariya Y, Teshigawara K, Uchida A. Inhibition of proliferation and induction of apoptosis by abrogation of heat-shock protein (Hsp) 70 expression in tumor cells. Cancer Immunol Immunother. 1995;40:73–78. doi: 10.1007/BF01520287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller NK. A 70 kDa microtubule-associated protein in NIL8 cells comigrates with the 70 kDa heat shock protein. Biol Cell. 1988;63:307–317. [PubMed] [Google Scholar]
- Williams GT, McClanahan TK, Morimoto RI. E1a transactivation of the human Hsp70 promoter is mediated through the basal transcriptional complex. Mol Cell Biol. 1989;9:2574–2587. doi: 10.1128/mcb.9.6.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M, Naito Z, Tanaka S, Asano G. Expression and roles of heat shock proteins in human breast cancer. Jpn J Cancer Res. 1996;87:908–915. doi: 10.1111/j.1349-7006.1996.tb02119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarranton GT. Inducible vectors for expression in mammalian cells. Curr Opin Biotechnol. 1992;3:506–511. doi: 10.1016/0958-1669(92)90078-w. [DOI] [PubMed] [Google Scholar]
- Zeise E, Kuhl N, Kunz J, Rensing L. Nuclear translocation of stress protein Hsc70 during S phase in rat C6 glioma cells. Cell Stress Chaperones. 1998;3:94–99. doi: 10.1379/1466-1268(1998)003<0094:ntosph>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]