Abstract
Size-selective harvest of fish and crustacean populations has reduced stock numbers, and led to reduced growth rates and earlier maturation. In contrast to the focus on size-selective effects of harvest, here, we test the hypothesis that fishing may select on life-history traits (here, growth rate) via behaviour, even in the absence of size selection. If true, then traditional size-limits used to protect segments of a population cannot fully protect fast growers, because at any given size, fast-growers will be more vulnerable owing to bolder behaviour. We repeatedly measured individual behaviour and growth of 86 crayfish and found that fast-growing individuals were consistently bold and voracious over time, and were subsequently more likely to be harvested in single- and group-trapping trials. In addition, there was some indication that sex had independent effects on behaviour and trappability, whereby females tended to be less active, shyer, slower-growing and less likely to be harvested, but not all these effects were significant. This study represents, to our knowledge, the first across-individual support for this hypothesis, and suggests that behaviour is an important mechanism for fishing selectivity that could potentially lead to evolution of reduced intrinsic growth rates.
Keywords: personality, catchability, bias, behavioural syndromes, life history, boldness
1. Introduction
Few would argue with the suggestion that humans are a major selective pressure on animals. Of the many pressures that humans impose on animals, the persistent selective harvesting of fish and ‘wildlife’ species is thought to have major impacts, extending beyond numerical effects [1,2]. Animal harvest is a particularly strong selective pressure, because larger individuals are usually intensively and selectively targeted, leading to reductions in body size, growth rate, horn size and age at maturity over successive generations [2–5].
In contrast to the literature on size-selective effects of fishing harvest [6], the role of behaviour in mediating selection on life-history traits in fisheries remains under-studied despite the long-known links between behaviour and catchability [reviewed by 7,8]. However, one recent study highlighted how fishing may select on life-history traits via a behavioural mechanism, even in the absence of any size selection [9]. Selection on growth rate via behaviour can occur, because fast-growing individuals tend to be more active, bold and voracious [10,11], and so, in turn, we should expect fast-growers to be more vulnerable to harvest owing to greater encounter rates with fishing gear, and lower gear avoidance. Indeed, at the group level, faster growth is associated with greater catchability, even in the absence of size selection [9,12]. In addition, several recent studies have shown biases towards more active and bold individuals in the catch [7,9,13–15], but see [16]. Nonetheless, a demonstration of selection on growth rate via a behavioural mechanism has not yet been made at the individual level. That is, no study has, to our knowledge, yet assessed vulnerability to harvest of a group of individual animals with known behavioural tendencies and life-history trajectories from within a single population (see also Discussion).
A behavioural mechanism for selection on life history emphasizes the importance of distinguishing between selection on growth rate via size selection (indirect growth selection) from direct selection on growth rate via behaviour which is independent of size [9]. In many instances, behaviour should represent the immediate mechanism for catchability, because by definition inactive or shy individuals are less likely to approach or contact fishing gear, and more likely to avoid approaching fishing gear. By extension, direct selection on growth can occur if a strong correlation exists between intrinsic growth rates and behaviour. In other words, any selection on life-history traits (and the proximate physiology underpinning it, such as metabolism and hunger) may, in general, be through behaviour. If widespread, this mechanism has important management implications, because the common management practice of imposing size-limits to protect segments of the population [6] may not be sufficient to protect fast-growing and productive individuals. Why? Because fast-growers will be always be more vulnerable at any given size owing to greater levels of activity and boldness. However, fast growers can more quickly grow through vulnerable size ranges in a slot-limit, which may compensate for such effects, at least in part.
In this study, we test this mechanism using a freshwater crayfish as a model. We chose the Australian common yabby, Cherax destructor, because it is harvested both recreationally and commercially. More generally, crustaceans are intensively harvested around the world, and declines in size have been documented in shrimp and lobster [17,18], but, to date, none have studied links between behaviour and fishing harvest. We repeatedly assayed the behaviour and growth of 86 yabbies over five months, while fed ad libitum; bold and voracious individuals were also consistently faster-growing in this population [19]. Here, we used those individuals with known behavioural types and intrinsic growth rates, and assessed their vulnerability to harvest in individual trapping trials, and in group trapping trials that contained a mix of behavioural types—importantly, we used traps that were not size-selective. Because behaviour and growth rate are labile traits, and individual means are estimated with considerable uncertainty, we used multivariate mixed models to quantify correlations between behaviour, growth and catchability, using behavioural and growth data collected for one month prior to trapping trials.
2. Methods
(a). Subjects and behavioural assays
We used 86 yabbies that had been housed individually since ‘birth’, and fed ad libitum for nearly five months, before being assessed for trappability. Six berried females were sourced from a single population from a commercial supplier and held until they released their young, at which time 15 individuals from each female were randomly chosen for observations (see Biro et al. [19]). Given these females were trapped, the variation in behaviour and growth rate may be reduced relative to that present in the source population owing to behavioural sampling bias [20,21]. Nonetheless, substantial variation existed in both traits (see Results).
Yabbies were housed individually with an artificial burrow, and were repeatedly and concurrently assayed for ‘boldness', ‘voracity’ and growth rate across an interval of about five months. There was no effect of female identity on behaviour or growth of the young, and there was no detectable individual variation in initial mass at the start of the experiment. However, this does not mean growth rate is not heritable, because any given female may carry offspring fertilized by several fathers. In addition, yabby growth rate is known to be heritable [22]. Animals were held in a constant temperature room, and water was changed every 3 days to ensure water quality. All details of the set-up and husbandry, including detailed descriptions of the behavioural assays, are outlined elsewhere ([18], but see below).
We used the many repeated measures of behaviour and mass taken during one month prior to trapping trials (termed ‘protocol 2’ in Biro et al. [19]) to relate to individual trappability. These data were comprised 13 daytime and five night observations of boldness for each individual (total number of scores taken, n = 1548); voracity was measured eight times for each individual (total scores, n = 688; Biro et al. [19]) and mass was measured three times (no significant differences in individual mass were observed at the start of the experiment). Across the entire dataset, all traits displayed consistency over time, and repeatability values ranged from 0.44 to 0.50 for behavioural traits, and 0.85 to 0.99 for mass changes over time [18]. Consistent individual differences (repeatability) were also confirmed for data collected one month prior to trapping (see Results).
Briefly, boldness was assessed as the tendency to use open areas away from its burrow, whereby we repeatedly scored the position of the individual during a series of 30 point (scan) observations. In each scan, the yabby was given a score reflecting the relative risk of their position: zero for hiding within the refuge, one for fully emerged from the refuge but still in the half of the aquarium with sand on the bottom (nearest the refuge) and two when positioned in the open half of the aquaria where they were least camouflaged; the sum of scores across the 30 scans in a single trial represented a boldness index. Voracity was assessed as the latency to reach bloodworms, introduced in the open area away from its burrow; a shorter latency was inferred to represent greater voracity. Boldness was assessed during day and at night, and voracity only during the day (additional details in Biro et al. [19]).
(b). Individual trapping trials
Each individual was first measured for its trappability in its own home tank. Thirty traps were made for the individual trapping stage of the experiment which was carried out over four nights following the end of all behavioural observations. Each trap was a white PVC tube which was 5 cm long with a diameter of 3 cm. Both ends were covered with mesh, but with an opening at the top on one end which was ca 1 × 2 cm wide for the yabby to enter through. The size of this opening was more than large enough to accommodate the largest individual at that time. The juveniles were not fed the day of trapping. The trap was placed in the open half of their aquaria at 17.00 with the open end facing towards the shelter. Five bloodworms were pippetted into the middle of the trap, and it was checked at 8.00 the following morning when the water temperature was taken. Because temperature varied slightly across the bench in the constant temperature room, we rotated into the same positions the next 30 tanks (and individuals) to be tested to help maintain relatively constant temperature (20–21°C); nonetheless, we tested for temperature effects in our models but found none (see Results).
(c). Group trapping trials
Crayfish were sorted into six groups, with 15 individuals in each group. Each group had a mixture of behavioural types, and were chosen semi-randomly to avoid any group-related differences in growth or behavioural scores. There were no systematic differences in average predicted behavioural scores or growth rates among groups (ANOVA, all p > 0.7), but we nonetheless tested for a group effect in analyses. Each yabby was numbered using waterproof paper glued to their carapace. Three large tanks (2 m long, 1 m wide) were set up with sand covering the bottom. Each had a heater set to 21°C and an air stone at both ends to circulate and aerate the water (15 cm deep). Three groups of 15 individuals each were placed into the tanks with 22 tubes each for shelter. The tubes were translucent, so that the individual's number could still be viewed while they refuged within. Overhead fluorescent lights were set to be on from 4.30 to 16.30 with two small red lamps on during night.
Crayfish were left to acclimate for two nights, and then trapping was run on the third and fourth nights. Two Plexiglass shrimp/snail traps with a cube of bloodworms inside were placed into each tank each night at 17.30, for a total of two nights of trapping. These traps are entirely enclosed with entry only possible by pushing through flaps which close behind. This trap can catch the smallest, as well as the largest of crayfish we had in our sample (dimensions: 140 × 70 × 70 mm; see http://fischer.en.alibaba.com/product/1780206930-213205875/China_manufacturer_aquarium_crab_shrimp_and_snail_trap.html). One trap was placed by each air stone to spread the scent. The following day at 8.00, the traps were removed, and any trapped individuals placed back into their individual home tanks. The traps were set again that night as before. Trapping was then repeated for the remaining three groups of 15.
(d). Statistical analyses
We tested for across-individual correlations between behaviour, growth rate and harvest (captured, not captured) using multivariate mixed models which assumed normal errors for the behaviours and growth rate, and binomial errors on capture probability (Proc Glimmix, SAS Institute, Cary, NC). We used the individual repeated measures data collected in the final month prior to trapping (see above). Multivariate mixed models allow one to rigorously estimate correlations between traits in a set of individuals that take account of the repeated measures and the effect of variability in the data when calculating parameter estimates and measures of their uncertainty [23]. Individual identity was specified as a random (intercept) effect in the model, sex was specified as a trait-specific fixed effect and we fitted a separate residual variance parameter for each trait (binomial trapping data residual variance set to zero). All variables (except harvest) were standardized to mean zero and variance of one (z-transformed) in order to help with model convergence. We tested for the significance of trait variances (which estimate the variation in individual predicted mean values, i.e. the random intercept effect), and covariances (across-individual correlations between traits), using the ‘covtest’ option. We used the ‘gcorr’ option to output the across-individual correlations between traits, which are calculated as the covariance divided by the square root of the product of the two trait variances [23].
3. Results
(a). Individual trapping trials
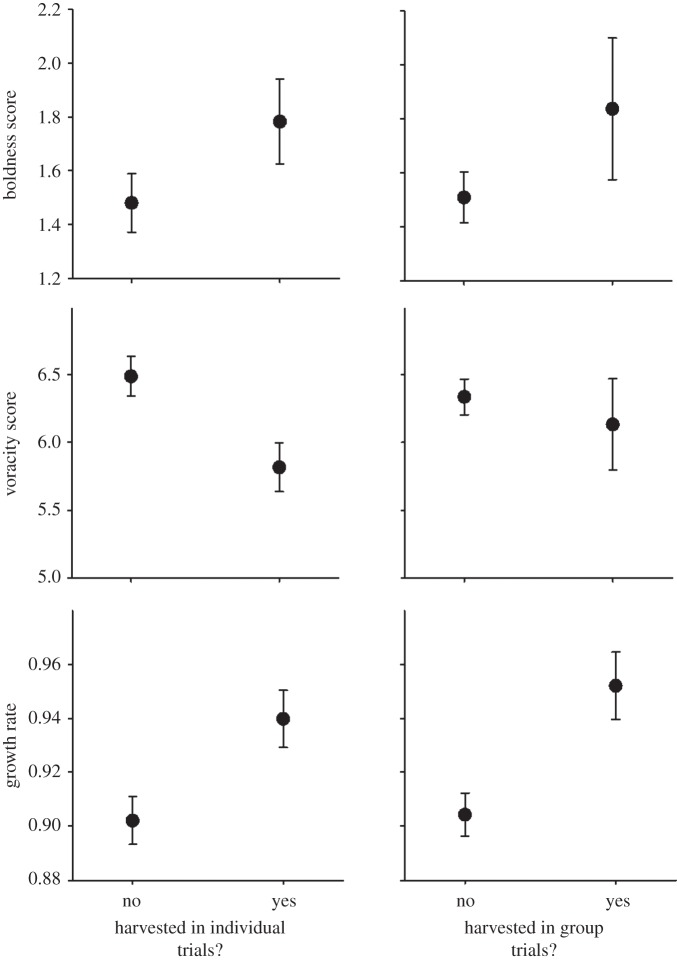
Multivariate analyses, using the binomial trapping data and all the individual assays of behaviour and growth during the month preceding trapping revealed that there were consistent individual differences in daytime boldness, night-time boldness, voracity and growth (each variance parameter p < 0.0001). As expected, all four traits were significantly correlated with one another across individuals, whereby bold individuals (day or night) tended to be voracious (shorter latency to feed) and fast growing (each covariance p < 0.0001; table 1). Probability of harvest was not significantly correlated with daytime boldness (r = 0.11, p = 0.45), but was higher for individuals that tended to be bolder at night (r = 0.32, p = 0.063), and was higher for more voracious and faster-growing individuals (r = 0.37 and 0.34, both p < 0.03; table 1 and figure 1). As expected, there was a significant effect of sex that differed among the traits (trait × sex interaction: F5,335 = 2.70, p < 0.025); females had lower day and night-time activity (both coefficients, p < 0.015) and lower growth rates (coefficient, p < 0.01). Females may also have had higher latencies (shyer) and lower trapping probability, but these were not significant (both coefficients, p > 0.16).
Table 1.
Correlation matrix obtained from multivariate mixed models that examine across-individual correlations between traits (also known as the G-correlation matrix). (Individual identity is specified as a random intercept effect which characterizes individual differences in trait values over time and accounts for multiple repeated measures per individual (except harvest, which is measured once per individual).)
| trait | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|
| (a) individual trapping | ||||||
| boldness-day | 1 | 1 | ||||
| boldness-night | 2 | 0.846 | 1 | |||
| voracity | 3 | 0.676 | 0.756 | 1 | ||
| growth | 4 | 0.808 | 0.958 | 0.670 | 1 | |
| harvest | 5 | 0.083 | 0.217 | 0.275 | 0.295 | 1 |
| (b) group trapping | ||||||
| boldness-day | 1 | 1 | ||||
| boldness-night | 2 | 0.846 | 1 | |||
| voracity | 3 | 0.676 | 0.756 | 1 | ||
| growth | 4 | 0.808 | 0.958 | 0.670 | 1 | |
| harvest | 5 | 0.190 | 0.246 | 0.018 | 0.334 | 1 |
Figure 1.
Raw data for individual mean values for boldness (averaged across day versus night contexts), voracity (here, latency to feed) and growth rate, in relation to whether it was harvested or not, both in individual trapping trials and in groups containing a mixture of behavioural types (‘group’ trials). (Shown are the means (±s.e.) of 86 individuals in total, calculated on a log scale (see Methods for units and details).)
(b). Group trapping trials
Multivariate analyses using the binomial group trapping data were congruent with the individual trapping data. All trait variances and covariances among behavioural and growth measures were significant and near-identical to the preceding analysis (all p < 0.0001). Probability of harvest was higher for individuals with higher daytime boldness (r = 0.19, p < 0.025), higher night-time boldness (r = 0.25, p < 0.005) and faster-growth rate (r = 0.33, p < 0.001; table 1 and figure 1). However, probability of harvest was not related to voracity (r = 0.02, p > 0.8; table 1 and figure 1). Again, there was a significant effect of sex that differed among the traits (trait × sex interaction: F5,335 = 2.58, p < 0.03); females had lower day and night-time activity (both coefficients, p < 0.02) and lower growth rates (coefficient, p < 0.01). Females may also have had higher latencies (shyer) and lower trapping probability, but these were not significant (both coefficients, p > 0.13).
4. Discussion
We hypothesized that if fishing directly selects on growth rate via consistent individual differences in behaviour, we should observe that harvested individuals are generally bold, voracious and fast-growing. The weight of evidence here suggests this hypothesis is supported—we observed consistent individual differences in daytime boldness, night-time boldness, voracity and growth rate across 86 individuals during the month preceding our trapping trials. In both multivariate analyses, one for individual trapping and one for group trapping, all of the behavioural and growth traits were strongly and positively correlated with one another and, most importantly, faster-growing individuals and those that were bold at night were always more likely to be harvested. However, daytime boldness was not related to individual trappability, and voracity (measured during the day) was not related to trappability in a group setting. Given that traps were set overnight as is typically done in fisheries, and crayfish are largely nocturnal, it is perhaps not surprising that night-time behavioural measures were consistently related to the probability of trapping whereas daytime measures were not.
Overall, this study indicates for the first time to the best of our knowledge that consistent across-individual differences in behaviour are a probable mechanism explaining why fishing harvest can select against fast-growing individuals, even when using gear that is not size selective. Our study distinguishes itself from previous similar work which showed that across-group differences in behaviour (wild and domesticated genotypes) in fishes resulted in differential harvest when gear was not size selective [9]. Our study also distinguishes itself from a selection experiment which demonstrated changes over time in life-history and behavioural traits owing to fishing [24], but did not control for size selection. Here, we build upon this previous work, and find support for the hypothesis regarding the direct role of behaviour in catchability, by conducting a similar experiment but at the individual level (as opposed to group level), using a large number of individuals (albeit from few females) that were repeatedly tested to establish consistent behavioural differences. It is crucial to perform such a test, because selection acts at the individual level, and the hypothesis centres on individuals that consistently differ in behaviour within a given population, and its link to growth rate.
The strongest correlations we observed were between intrinsic growth rate and trappability, rather than were behavioural traits and trappability. We might expect that if behaviour is the immediate determinant of whether individuals are captured or not, then correlations should be highest between behaviour and catchability (all else being equal). However, behaviour is inherently a more labile trait than is growth rate, and repeatability of behavioural traits reached a maximum of 0.50 in comparison with growth rate which reached a maximum of 0.99 at the end of the experiment (see Methods; [25]). Thus, we can a priori expect that behaviour–catchability correlations will be lower [25]. Nonetheless, behaviour must be the immediate cause of catchability in this study because it must choose to move and choose to enter the trap, even if other more proximate factors like hunger are motivating behaviour in the first instance. Given that behavioural variation cannot affect differences in foraging success and impact upon growth under ad libitum food conditions, it would seem that intrinsic differences in growth rates (life-history strategy) are what affect differences in behaviour which, in turn, affect catchability.
Given that our observations are based on fishing gear that was not size selective, an important implication of our results is that size-limits may not by themselves sufficiently protect fast-growing individuals. They are unlikely to fully protect fast-growers, because bold/fast-growing phenotypes will always be harvested at a higher rate relative to other individuals at any given size. So while we might try to protect larger individuals in a fishery using size-limits, which may help in the short term, we will nonetheless continue to differentially remove the fast-growing individuals among the smaller size classes. This would also apply to minimum size-limits as well, which give fishes a chance to attain maturity, but, thereafter, fast-growing individuals would be harvested at higher rates. However, the extent to which this is true will depend upon other factors, particularly on how quickly fast-growers move through vulnerable size ranges in a slot-limit which will be compensatory. Protecting fast-growers is important as these individuals reach larger sizes more quickly, tend to be more fecund and probably contribute more to overall population productivity than slow growers.
Given the significant correlations between behaviour, voracity, growth rate and catchability, we might speculate that the directional selection we observed on growth rate could extend beyond numerical effects, and lead to evolution of decreased growth rates over time, at least in the absence of compensatory processes [8]. Indeed, a realistic recent study on fishes showed that fishing harvest can lead to changes over time [24], but that study did not exclude size-selection as a factor. The very high repeatability of growth rate observed (reaching 0.99) suggests a heritable component to intrinsic growth rate in our yabbies. What remains unknown is whether size-independent selection owing to behaviour can lead to evolutionary change. This is now the focus of our current experiments.
Our results suggest that intrinsically fast-growing individuals are hungrier on average, and therefore spend more time outside of their burrow in search of food, because in nature, greater boldness would increase feeding rates. Indeed, the fastest-growers rarely resided in their burrows, whereas some others hardly ever left their burrow, did not approach the food item in voracity trials, and hardly grew at all relative to others (see figures in Biro et al. [19]). In the light of this, we might speculate that not only are slow-growers not vulnerable to fishing harvest in the short term, but they may also rarely if ever approach traps, and might therefore effectively represent an ‘insurance policy’ that could prevent local extinction.
A caveat to be noted regarding the extent to which our findings are general is that this was a laboratory study, where traps and crayfish were necessarily in close proximity, and that our trials involved yabbies of a small size (several grams in mass) that would not normally be retained by a fishery (but fishers targeting animals for bait would). At the same time, an advantage of the laboratory setting that is not easily duplicated in the field is the ability to have every individual assayed for its behavioural and life-history type, obtained through multiple repeated assays of mass and behaviour. Given the relatively low repeatability of behaviour in this study (0.4–0.5), it would indeed be difficult to obtain reliable estimates of an individuals' behavioural type without several repeated measures in a laboratory setting, and low repeatability and low sample size would together contribute to lower power to detect correlations across individuals between any two traits of interest [25]. Finally, an unavoidable consequence of the correlation between behaviour and growth rate in this experiment is that we could not control for the effect of size on trappability. Consequently, it is possible that size per se may play a role in catchability that interacts with (or is in addition to) the effects of behaviour, and this would represent an interesting topic for future research.
Acknowledgements
Thanks to Bart Adriaenssens for advice, and for his assistance with the group trapping trials. Thanks also to two reviewers for their helpful and constructive comments.
Authorship contribution
P.A.B. conceived the project, wrote the manuscript, and performed all analyses. P.S. collected and compiled all data and took care of animals.
Funding statement
This work was supported by an ARC Discovery grant and an ARC Future Fellowship to P.A.B.
References
- 1.Allendorf FW, England P, Luikart G, Ritchie P, Ryman N. 2008. Genetic effects of harvest on wild animal populations. Trends Ecol. Evol. 23, 327–337. ( 10.1016/j.tree.2008.02.008) [DOI] [PubMed] [Google Scholar]
- 2.Jørgensen C, et al. 2007. Managing evolving fish stocks. Science 318, 1247–1248. ( 10.1126/science.1148089) [DOI] [PubMed] [Google Scholar]
- 3.Law R. 2000. Fishing, selection, and phenotypic evolution. ICES J. Mar. Sci. 57, 659–668. ( 10.1006/jmsc.2000.0731) [DOI] [Google Scholar]
- 4.Coltman DW, O'Donoghue P, Jorgenson JT, Hogg JT, Strobeck C, Festa-Bianchet M. 2003. Undesirable evolutionary consequences of trophy hunting. Nature 426, 655–658. ( 10.1038/nature02177) [DOI] [PubMed] [Google Scholar]
- 5.Sharpe DMT, Wandera SB, Chapman LJ. 2012. Life history change in response to fishing and an introduced predator in the East African cyprinid Rastrineobola argentea. Evol. Appl. 5, 677–693. ( 10.1111/j.1752-4571.2012.00245.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou S, et al. 2010. Ecosystem-based fisheries management requires a change to the selective fishing philosophy. Proc. Natl Acad. Sci. USA 107, 9485–9489. ( 10.1073/pnas.0912771107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uusi-Heikkilä S, Wolter C, Klefoth T, Arlinghaus R. 2008. A behavioral perspective on fishing-induced evolution. Trends Ecol. Evol. 23, 419–421. ( 10.1016/j.tree.2008.04.006) [DOI] [PubMed] [Google Scholar]
- 8.Enberg K, et al. 2012. Fishing-induced evolution of growth: concepts, mechanisms and the empirical evidence. Mar. Ecol. 33, 1–25. ( 10.1111/j.1439-0485.2011.00460.x) [DOI] [Google Scholar]
- 9.Biro PA, Post JR. 2008. Rapid depletion of genotypes with fast growth and bold personality traits from harvested fish populations. Proc. Natl Acad. Sci. USA 105, 2919–2922. ( 10.1073/pnas.0708159105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stamps JA. 2007. Growth-mortality tradeoffs and ‘personality’ traits in animals. Ecol. Lett. 10, 355–363. ( 10.1111/j.1461-0248.2007.01034.x) [DOI] [PubMed] [Google Scholar]
- 11.Biro PA, Stamps JA. 2008. Are animal personality traits linked to life-history productivity? Trends Ecol. Evol. 23, 361–368. ( 10.1016/j.tree.2008.04.003) [DOI] [PubMed] [Google Scholar]
- 12.Biro P. 2013. Are most samples of animals systematically biased? Consistent individual trait differences bias samples despite random sampling. Oecologia 171, 339–345. ( 10.1007/s00442-012-2426-5) [DOI] [PubMed] [Google Scholar]
- 13.Cooke SJ, Suski CD, Ostrand KG, Wahl DH, Philipp DP. 2007. Physiological and behavioural consequences of long-term artificial selection for vulnerability to recreational angling in a teleost fish. Physiol. Biochem. Zool. 80, 480–490. ( 10.1086/520618) [DOI] [PubMed] [Google Scholar]
- 14.Wilson DS, Coleman K, Clark AB, Biederman L. 1993. Shy–bold continuum in pumpkinseed sunfish (Lepomis gibbosus): an ecological study of a psychological trait. J. Comp. Psychol. 107, 250–260. ( 10.1037/0735-7036.107.3.250) [DOI] [Google Scholar]
- 15.Côté IM, et al. 2014. What doesn't kill you makes you wary? Effect of repeated culling on the behaviour of an invasive predator. PLoS ONE 9, e94248 ( 10.1371/journal.pone.0094248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson ADM, Binder TR, McGrath KP, Cooke SJ, Godin J-GJ, Kraft C. 2011. Capture technique and fish personality: angling targets timid bluegill sunfish, Lepomis macrochirus. Can. J. Fish. Aquat. Sci. 68, 749–757. ( 10.1139/f2011-019) [DOI] [Google Scholar]
- 17.Melville-Smith R, De Lestang S. 2006. Spatial and temporal variation in the size at maturity of the western rock lobster Panulirus cygnus George. Mar. Biol. 150, 183–195. ( 10.1007/s00227-006-0349-6) [DOI] [Google Scholar]
- 18.Charnov E. 1981. Sex reversal in Pandalus borealis: effect of a shrimp fishery? Mar. Biol. Lett. 2, 53–57. [Google Scholar]
- 19.Biro PA, Adriaenssens B, Sampson P. 2014. Individual and sex-specific differences in intrinsic growth rate covary with consistent individual differences in behaviour. J. Anim. Ecol. 83, 1186–1195. ( 10.1111/1365-2656.12210) [DOI] [PubMed] [Google Scholar]
- 20.Carter AJ, Heinsohn R, Goldizen AW, Biro PA. 2012. Boldness, trappability and sampling bias in wild lizards. Anim. Behav. 83, 1051–1058. ( 10.1016/j.anbehav.2012.01.033) [DOI] [Google Scholar]
- 21.Biro PA, Abrahams MV, Post JR. 2007. Direct manipulation of behaviour reveals a mechanism for variation in growth and mortality among prey populations. Anim. Behav. 73, 891–896. ( 10.1016/j.anbehav.2006.10.019) [DOI] [Google Scholar]
- 22.Jerry DR, Purvis IW, Piper LR, Dennis CA. 2005. Selection for faster growth in the freshwater crayfish Cherax destructor. Aquaculture 247, 169–176. ( 10.1016/j.aquaculture.2005.02.010) [DOI] [Google Scholar]
- 23.Littell RC, et al. 2006. SAS for mixed models, p. 828, 2nd edn Cary, NC: SAS Press. [Google Scholar]
- 24.Sutter DAH, Suski CD, Philipp DP, Klefoth T, Wahl DH, Kersten P, Cooke SJ, Arlinghaus R. 2012. Recreational fishing selectively captures individuals with the highest fitness potential. Proc. Natl Acad. Sci. USA 109, 20 960–20 965. ( 10.1073/pnas.1212536109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adolph S, Hardin J. 2007. Estimating phenotypic correlations: correcting for bias due to intraindividual variability. Funct. Ecol. 21, 178–184. ( 10.1111/j.1365-2435.2006.01209.x) [DOI] [Google Scholar]