Abstract
Metabolism fuels all biological activities, and thus understanding its variation is fundamentally important. Much of this variation is related to body size, which is commonly believed to follow a 3/4-power scaling law. However, during ontogeny, many kinds of animals and plants show marked shifts in metabolic scaling that deviate from 3/4-power scaling predicted by general models. Here, we show that in diverse aquatic invertebrates, ontogenetic shifts in the scaling of routine metabolic rate from near isometry (bR = scaling exponent approx. 1) to negative allometry (bR < 1), or the reverse, are associated with significant changes in body shape (indexed by bL = the scaling exponent of the relationship between body mass and body length). The observed inverse correlations between bR and bL are predicted by metabolic scaling theory that emphasizes resource/waste fluxes across external body surfaces, but contradict theory that emphasizes resource transport through internal networks. Geometric estimates of the scaling of surface area (SA) with body mass (bA) further show that ontogenetic shifts in bR and bA are positively correlated. These results support new metabolic scaling theory based on SA influences that may be applied to ontogenetic shifts in bR shown by many kinds of animals and plants.
Keywords: aquatic invertebrates, body shape, metabolic scaling, ontogeny, resource-transport networks, surface area
1. Introduction
All living activities depend on metabolism for energy and materials. Therefore, understanding variation in metabolic rate is of fundamental importance in biology. Much of this variation is related to body size, but how and why these relationships occur remain vexing questions. General models assume that metabolic rate scales monotonically with body size according to the simple power function
| 1.1 |
where R is metabolic rate, a is the scaling coefficient (antilog of the intercept in a log–log plot), M is body mass and b (henceforth bR) is the scaling exponent (linear slope of a log–log plot that frequently approximates 3/4) [1,2]. However, metabolic scaling often shows marked shifts during ontogeny in animals and plants (bR varying mostly between 2/3 and 1, but also showing values outside this range) [3–7] that are not well understood. These metabolic shifts are important because they appear to be fundamentally linked to other ontogenetic changes in the physiology, growth rate, cell size, body composition, behaviour and ecology of a species [3–7].
Here, we show that ontogenetic changes in body shape and associated surface area (SA)-related resource supply predict frequently observed shifts from near isometric (bR ∼ 1) to negatively allometric (bR < 1) intraspecific metabolic scaling (=type III scaling [4]) in diverse aquatic invertebrates. Crucially, we also show that shape shifting predicts more rarely observed changes in metabolic scaling that occur in the opposite direction (from shallow to steep scaling). Our results demonstrate that the prediction of metabolic scaling from the body-shape-related scaling of SA applies more widely than that recently described by Hirst et al. [8]. We show here that this predictive power applies to marked variation in metabolic scaling seen not only among diverse pelagic (open water) animal taxa [8], but also during the intraspecific ontogeny of both pelagic invertebrates and those that exhibit developmental shifts from pelagic to benthic (bottom-dwelling) lifestyles. A critical assumption of our shape-shifting model is that the supply of resources or loss of wastes scales with external body surface, thus implying that exchange of materials such as respiratory gases is distributed over the body surface, which we evaluate herein. We also discuss potential implications of our findings for ontogenetic shifts in metabolic scaling observed in many other kinds of animals and plants.
2. Theoretical background
Metabolic rate may be controlled by the supply of, or demand for, resources [9–11]. Both should be considered in order to attain a comprehensive understanding of the scaling of metabolic rate with body size [4,12,13]. Here, we test opposing predictions from influential metabolic scaling theories that focus on transport of resources and waste products such as respiratory gases between the external environment and metabolizing cells. This transport may be influenced by two major steps: the exchange of materials across body surfaces and the transport of materials through internal networks. These steps are the foci for two prominent theoretical approaches to understanding and predicting biological scaling: SA theory [4,8,14,15] and resource-transport network (RTN) theory [1,2,16,17], respectively.
Although both SA and internal transport networks may be important in influencing metabolic rate and its scaling with body size, SA theory (including widely applied DEB theory, which incorporates SA theory [8,18,19]) predicts that body-shape changes should have opposite effects on the metabolic scaling exponent bR (see equation (1.1)) than those predicted by existing RTN theory [8]. If an organism shows isomorphic growth (i.e. it grows with equal proportions in all three dimensions, so as to maintain a constant shape), SA theory (based on simple Euclidean geometry) predicts that bR = 2/3, whereas RTN theory typically predicts that bR = 2/3 [2,17] or 3/4 [1,2,16], depending on the physical properties of the transport network [20,21]. These predicted scaling exponents of 2/3 and 3/4 have received the most attention by biologists since the seminal studies of Rubner [14] and Kleiber [22] over 80 years ago. However, if an organism displays nearly two-dimensional growth (e.g. it grows in length and width without any significant change in depth, thus appearing increasingly flat), SA theory predicts that bR ∼ 1 [8,15], whereas RTN models predict that bR ∼ 1/2 [17], 5/8 [2,8] or 2/3 [16], depending on network geometry and dynamics. In addition, if an organism exhibits nearly one-dimensional growth (e.g. it grows in length without any significant change in width or depth, thus showing an increasingly elongated shape), SA theory predicts that bR ∼ 1 [8,15], whereas RTN theory predicts that bR ∼ 0 [17], 1/4 [2,8] or 1/2 [16], again depending on network properties. Therefore, increased elongation or flattening during ontogeny (trends towards one- or two-dimensional growth) should lead to an increase in bR, according to SA theory, whereas RTN theory predicts the opposite—a decrease in bR. Conversely, ontogenetic trends away from increasingly elongated one-dimensional or flattened two-dimensional growth, but towards isomorphic three-dimensional growth, reverse the changes in bR predicted by the two theories: SA theory predicts decreasing bR, and RTN theory increasing bR. Studying the effects of ontogenetic shape shifting on metabolic scaling thus provides an excellent opportunity to test the relative validity of models based on two major competing theories of metabolic scaling, which is much needed for the field to advance [23,24].
3. Testing theory using animals with mixed ontogenetic metabolic scaling
Several kinds of aquatic animals with complex life cycles exhibit ontogenetic shifts in the scaling of aerobic metabolism, typically measured as the rate of oxygen consumption. In these animals, respiration scaling exponents (bR) most often change from near 1 in larvae or young juveniles to less than 1 in older juveniles or adults [3,4,25], but reverse shifts also rarely occur [26,27]. From the literature, we collected respiratory and morphometric data on aquatic invertebrates that have complete or partial pelagic (open water) life histories to test whether and how these ontogenetic shifts in metabolic scaling are related to changes in body shape.
Unfortunately, actual measurements of body SA during ontogeny are rare [8]. Therefore, as a first-order approximation, we quantified differences in shape-related growth between life-history phases by using Euclidean geometry for smooth surfaces to deduce SA–mass scaling exponents (bA, or the slope of log10 body SA versus log10 body mass). To achieve this, we first used readily available body length and mass data to obtain mass–length scaling exponents (bL), estimated as the slopes of least-squares regressions (LSRs) of log10 body mass versus log10 body length (along the longest axis). Values of bL were calculated separately for larvae, juveniles and adults [28]. Logarithmic transformation was used to permit easy detection of proportional changes [29]. If growth is occurring proportionally in three dimensions without any change in mass density, bL should be 3, whereas if growth involves pure elongation in only one dimension (along the longest axis) or pure flattening because of size increases in only the two longest dimensions, bL should be 1 or 2, respectively [8]. For intermediate patterns of body-shape change, involving disproportionate growth in one or two of the longest dimensions, 1 ≤ bL ≤ 3. Values of bL may even be more than 3, if growth in the shortest dimensions (width and/or depth) is proportionately greater than that for length along the longest axis that is used to calculate bL (i.e. the animal is becoming relatively thicker and/or broader) [8].
Ranges of bA values were inferred from bL values that are less than or equal to 3 by using formulae for the extreme possibilities of different degrees of elongation (one-dimensional growth) and flattening (two-dimensional growth) [8]. The formula for different degrees of elongation is
| 3.1 |
whereas the formula for different degrees of flattening is
| 3.2 |
Equation (3.1) applies for 1 ≤ bL ≤ 3, and equation (3.2) applies for 2 ≤ bL ≤ 3, as represented in figure 1a,b by the left- and right-hand purple curves for bL ≤ 3, respectively. Ranges of potential bA values were also inferred from bL values that are more than 3 by using formulae quantifying disproportionate thickening in one or two of the shortest dimensions. The formula for thickening of only the shortest dimension is the same as equation (3.2), whereas the formula for thickening of only the second shortest dimensions is bA = 1 − (1/bL) (supplementary information 1g in [8]), as represented in figure 1a,b by the bottom and top purple curves for bL > 3, respectively. When data on the scaling of body width with body length were available, it was possible to predict a single value of bA rather than a range (see the electronic supplementary material).
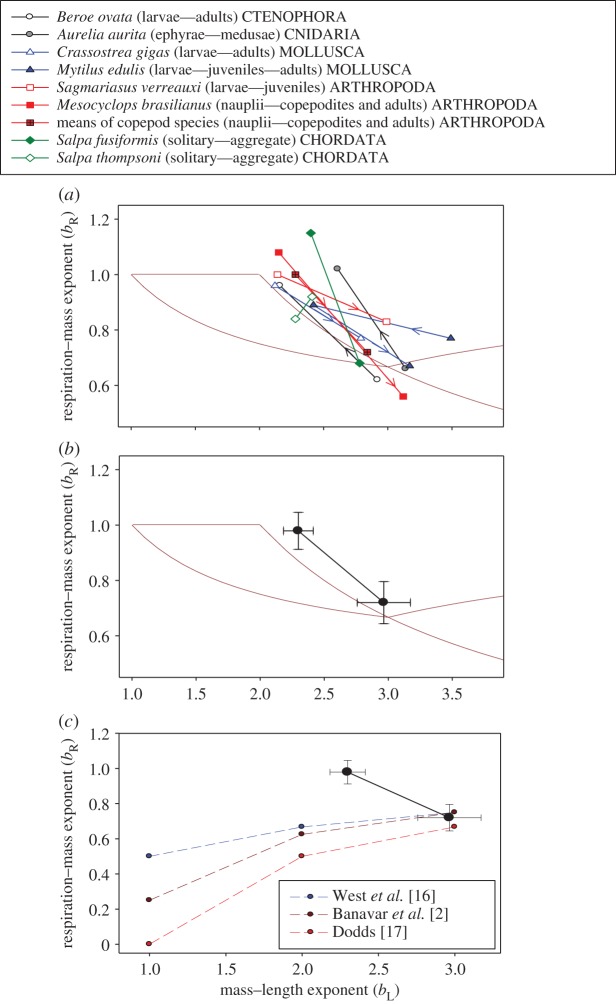
Figure 1.
Ontogenetic shifts of respiration–mass exponents (bR) in relation to mass–length exponents (bL) of several species of aquatic invertebrates in five different phyla (a) and for all species averaged together (b,c), where bR is the scaling exponent for log10 RMR in relation to log10 body mass, and bL is the scaling exponent for log10 body mass in relation to log10 total body length (data from tables 1 and 2). Multiple b values for the life-history stage of a species or group of species (copepods) were averaged. Arrows indicate the direction of ontogenetic change. In (b,c), the upper left and lower right points were calculated by averaging all of the paired bR and bL values that occurred in ontogenetic stages with the higher versus lower bR, respectively. The 95% confidence limits are shown for each mean value of bR and bL.. Also shown in (b) are the bounded range of values of bA (log10 body SA in relation to log10 body mass) in relation to bL (depicted as light purple lines) calculated using equations (3.1) and (3.2) based on Euclidean geometry (also see [8]); and in (c) the predicted effects of body-shape changes (bL) on bR (depicted as dashed coloured lines) according to the RTN models of West et al. [16], Banavar et al. [2] and Dodds [17] (also see [8]). Note that in seven of eight species sampled, ontogenetic shifts in bL are accompanied by inverse shifts in bR, as predicted by SA scaling theory (b), but in contradiction to RTN scaling theory (c). (Online version in colour.)
The empirical bL values and inferred bA values were then compared to scaling exponents (bR) for regressions of log10 routine metabolic (oxygen consumption) rate (RMR) in relation to log10 body mass of different ontogenetic stages both within and among several species of aquatic invertebrates.
Both bL and bR values were based on LSR, the method used for all of the literature scaling analyses included in our study. An alternative, often used method, reduced major axis (RMA) analysis, gave similar b values (bRMA = bLSR/r) to those from LSR, because reported correlation coefficients (r) were always high (more than or equal to 0.8). The r values for bL averaged 0.97 ± 0.01 (±1 s.e., n = 15), and those for bR averaged 0.96 ± 0.01 (n = 19) (calculated from r2 values in the electronic supplementary material for table 1). As a result, bL and bR values based on RMA analyses averaged only approximately 3–4% higher than those based on LSR.
Table 1.
Ontogenetic scaling exponents from LSRs of log10 body mass in relation to log10 body length (bL) and log10 RMR in relation to log10 body mass (bR) for larvae, juveniles and/or adults of the Atlantic ctenophore Beroe ovata, moon jellyfish A. aurita, Pacific oyster C. gigas, common mussel M. edulis and spiny lobster S. verreauxi; for nauplii, copepodites and adults of the copepod M. brasilianus; and for solitary and aggregate life cycle stages of the salps (pelagic tunicates) Salpa fusiformis and S. thompsoni. Values of bL and bR were taken or calculated from published data in the sources listed in the electronic supplementary material, where additional data and methodological information can also be found. Values of bA for log10 body SA in relation to log10 body mass were estimated from bL values and scaling exponents of body width versus length (electronic supplementary material). When data for scaling of width versus length were not available, ranges of potential bA values are given (based on equations (3.1) and (3.2)). Note the parallel changes in bA and bR values (shown in italic), regardless of whether decreases or increases in bR were observed during ontogeny (except possibly for the transition from veliger larvae to juveniles in M. edulis; also see text). Statistically significant ontogenetic shifts in bL and bR values occur in all species, except for between solitary and aggregrate life stages of S. thompsoni.
| species | stage | bL | bA | bR |
|---|---|---|---|---|
| Beroe ovata | juveniles | 2.92 | 0.67 (0.67–0.68) | 0.62 |
| adults | 2.47 | 0.80 (0.70–0.81) | 0.99 | |
| 2.23 | 0.87 (0.72–0.90) | 1.04 | ||
| 1.78 | 1.09 (0.78–1.00) | 0.86 | ||
| Aurelia aurita | ephyra larvae | 3.14 | 0.64 (0.64–0.66) | 0.63 |
| 0.35 | ||||
| 1.01 | ||||
| medusae | 2.50 | 0.80 (0.70–0.80) | 0.93 | |
| 2.72 | 0.74 (0.68–0.74) | 1.11 | ||
| 1.01 | ||||
| Crassostrea gigas | veliger larvae | 2.12 | 0.95 (0.74–0.94) | 0.96 |
| adults | 2.79 | 0.68–0.72 | 0.77 | |
| Mytilus edulis | veliger larvae | 3.49 | 0.57 (0.57–0.71) | 0.90 |
| 0.90 | ||||
| 0.70 | ||||
| 0.59 | ||||
| juveniles | 2.42 | 0.71–0.83 | 0.89 | |
| adults | 3.17 | 0.63–0.68 | 0.66 | |
| 0.68 | ||||
| Sagmariasus verreauxi | phyllosoma larvae | 2.14 | 0.91 (0.73–0.93) | 1.00 |
| juveniles | 2.99 | 0.67 | 0.83 | |
| Mesocyclops brasilianus | nauplii | 2.15 | 0.73–0.93 | 1.08 |
| copepodites and adults | 3.12 | 0.64–0.68 | 0.56 | |
| Salpa fusiformis | solitary zooids | 2.40 | 0.71–0.83 | 1.15 |
| aggregate zooids | 2.78 | 0.68–0.72 | 0.68 | |
| Salpa thompsoni | solitary zooids | 2.28 | 0.72–0.88 | 0.84 |
| aggregate zooids | 2.41 | 0.71–0.83 | 0.92 |
4. Results
We compared conspecific bL, bR and inferred bA values for different ontogenetic phases of a ctenophore, a scyphozoan, two bivalves, two crustaceans and two thaliaceans (table 1), as well as mean heterospecific values for the nauplii, copepodites and adults of several copepod crustaceans (table 2).
Table 2.
Mean ontogenetic scaling exponents from LSRs of log10 body mass in relation to log10 body length (bL) and log10 RMR in relation to log10 body mass (bR) for nauplii, copepodites and adults of several copepod species (bL and bR values were taken from published sources given in the electronic supplementary material, whereas the ranges of potential bA values for log10 body SA in relation to log10 body mass were estimated from bL values using equations (3.1) and (3.2) in the text). Mean scaling exponents were calculated by averaging mean conspecific values among species. The 95% CI and number of species sampled (n) are given for each scaling exponent. Note the parallel changes in bA and bR values (shown in italic).
| stage | bL ± 95% CI (n) | bA | bR ± 95% CI (n) |
|---|---|---|---|
| nauplii | 2.28 ± 0.27a (17) | 0.72–0.88 | 1.00 ± 0.16b (4) |
| copepodites and adults | 2.84 ± 0.20a (20) | 0.68–0.70 | 0.72 ± 0.22b (7) |
aBased on dry mass, carbon mass or nitrogen mass.
bBased on dry body mass.
For seven of the eight species sampled, ontogenetic shifts in the metabolic scaling exponent (bR) are accompanied by inverse shifts in the scaling exponent of body mass in relation to length (bL) (table 1 and figure 1a). The only exception is the pelagic tunicate Salpa thompsoni, which showed no significant difference in bR or bL between the solitary (oozoid) and aggregate (blastozoid) life-cycle stages (table 1 and figure 1a). Remarkably, even this exception supports a link between bR and bL, because both life stages of S. thompsoni have relatively high bR values associated with relatively low bL values, as compared with the exponents exhibited by the other species sampled (figure 1a). Furthermore, inverse shifts in bR and bL occurred in the other seven species sampled regardless of whether bR showed an increase or decrease during ontogeny (table 1 and figure 1a). Inverse relationships between bR and bL, also seen among all of the species averaged together (figure 1b,c), follow SA theory (figure 1b), but contradict all existing RTN models (figure 1c). Although ontogenetic stages with a mean bL ∼ 3 (2.97 ± 0.21 95% confidence interval (CI)) exhibited a mean bR (0.72 ± 0.08) not significantly different from 2/3 or 3/4, as predicted by both SA and RTN theory, stages with a mean bL ∼ 2.3 (2.30 ± 0.12) exhibited a mean bR (0.98 ± 0.07) not significantly different from 1 and significantly greater than 2/3 and 3/4, as predicted by SA theory, but in contradiction to all RTN models, which predict bR values less than 2/3 or 3/4 (figure 1b,c).
As the scaling exponent for body SA (bA), as inferred from Euclidean geometry, is inversely related to bL (figure 1a; equations (3.1) and (3.2)), it follows mathematically that ontogenetic shifts in bR and bA should be positively correlated, as observed in seven of the eight species sampled (table 1 and figure 2a). As predicted by SA theory, bR and bA are also positively correlated for all species averaged together (figure 2b), and when these exponents were compared pairwise among each of the ontogenetic phases of each species (figure 2c). The slope for the latter correlation (1.14) is not significantly different from 1, as expected if bR varied in direct proportion to bA (figure 2c).
Figure 2.
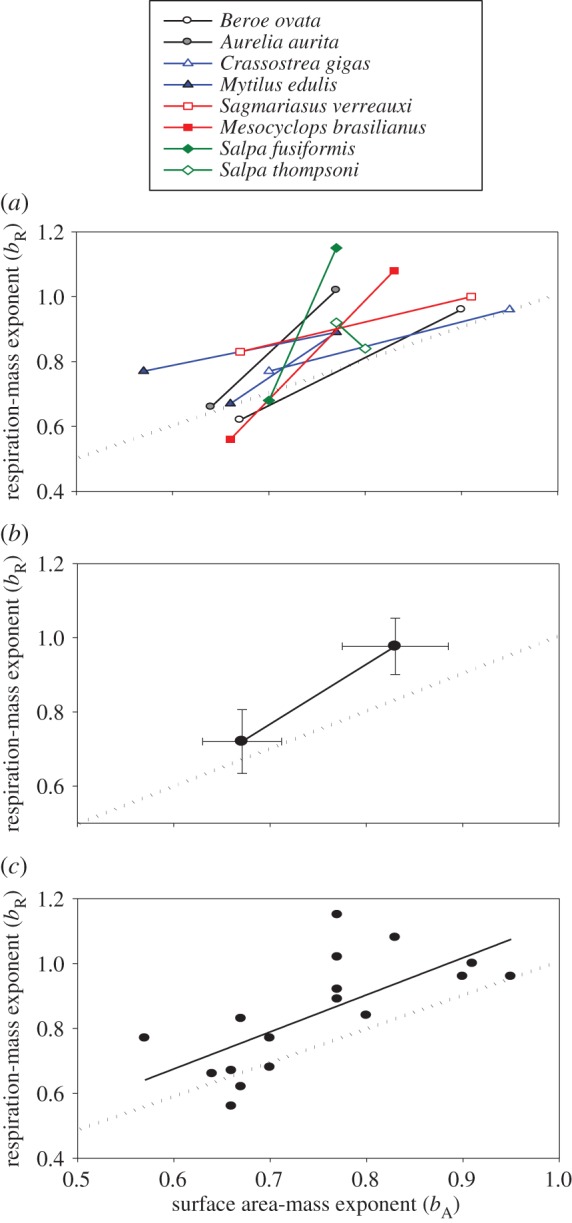
Ontogenetic shifts of respiration–mass exponents (bR) in relation to SA–mass exponents (bA) of several species of aquatic invertebrates (a) and for all species averaged together (b), where bR is the scaling exponent for log10 RMR in relation to log10 body mass, and bA is the scaling exponent for log10 surface area in relation to log10 total body length (data from table 1). Values of bA were calculated using bL values and additional data in the electronic supplementary material. Multiple bR values for the life-history stage of a species were averaged, whereas the midpoint was used when only a range of bA values was available. In (b), the lower left and upper right points were calculated by averaging all of the paired bR and bA values that occurred in ontogenetic stages with the lower versus higher bR, respectively. The 95% confidence limits are shown for each mean value of bR and bA. As predicted by SA scaling theory (also see [8]), in seven of eight species sampled, ontogenetic shifts in bA are accompanied by positively correlated shifts in bR. Also shown in (c) is a significant positive correlation between bR and bA for each ontogenetic stage of each species. The equation for the LSR line is: bR = 1.14 ± 0.64 (95% CI) (bA) − 0.0092 (r2 = 0.454; p = 0.0018, n = 17). In (a–c), the dotted diagonal lines represent bR = bA. (Online version in colour.)
Similar ontogenetic shifts are seen when bR, bL and bA values are compared between nauplii and copepodites/adults averaged among several species of copepods (table 2 and figure 1a). The heterospecific, inversely related shifts in bR and bL almost exactly parallel those observed for the single copepod species Mesocyclops brasilianus (figure 1a).
The spiny lobster Sagmariasus verreauxi nicely exemplifies how ontogenetic changes in metabolic rate and body shape correlate. The phyllosoma larvae are very thin and flat, and show nearly two-dimensional growth, until they metamorphose into adult-looking benthic juveniles that are much thicker and show three-dimensional growth. This marked shift in growth pattern and body form is represented by an abrupt ontogenetic shift in bL values, from 2.142 ± 0.260 (95% CI) in the phyllosoma larvae to 2.991 ± 0.037 in the juveniles (figure 3a and table 1). The phyllosoma bL value is not significantly different from 2, whereas the juvenile bL value is not significantly different from 3. As a result, the scaling exponents predicted for SA (bA) from Euclidean geometry (see the electronic supplementary material) are 0.91 for the phyllosomas and 0.67 for the juveniles (table 1). Like bA, the bR values for RMR are also higher in the phyllosomas (1.002 ± 0.081) than in the juveniles (0.829 ± 0.157) (figure 3b and table 1). Comparisons of the 95% CI [23,32] reveal that the bL and bR values are both significantly different between the two life-history stages. The phyllosoma bR value is not significantly different from 1, but is significantly greater than 1/2, 5/8, 2/3 and 3/4. By contrast, the juvenile bR value is significantly less than 1, not significantly different from 3/4 and significantly greater than 1/2, 5/8, and just barely 2/3.
Figure 3.
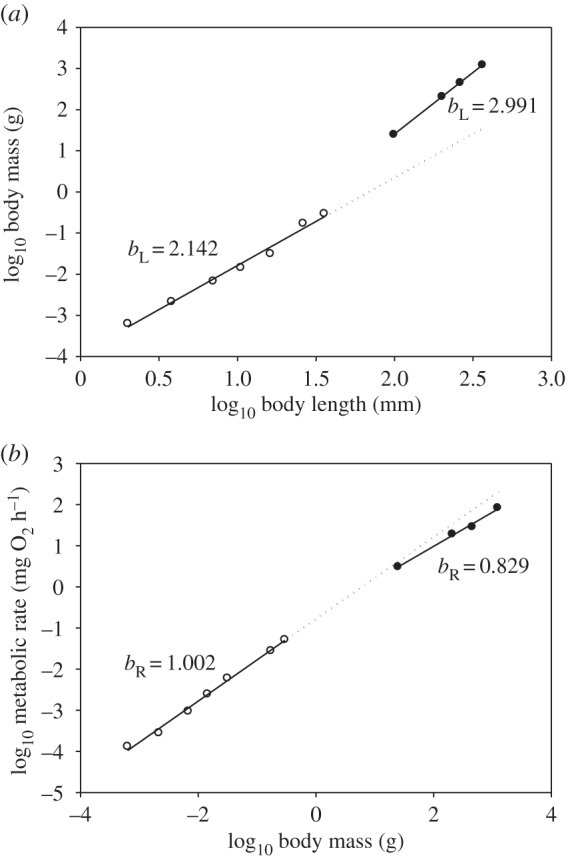
Scaling of log10 wet body mass in relation to log10 total body length (a) and log10 RMR in relation to log10 wet body mass (b) in phyllosoma larvae (open points) and juveniles (filled points) of the spiny lobster S. verreauxi maintained at 21–23°C. Each point in (a,b) is based on 4–11 replicate measurements, respectively [30,31]. The phyllosoma points represent instars 1, 3, 6, 9, 12, 15 and 17. The standard errors for each RMR value are all less than 20% of the mean. The LSR equations for the scaling lines and their coefficients of determination (r2) and significance levels (p) for phyllosoma larvae and juveniles are: (a) Y = −3.931 + 2.142 (X), r2 = 0.988, p = 0.00001; Y = −4.574 + 2.991 (X), r2 = 1.000, p < 0.00001; and (b) Y = −0.773 + 1.002 (X), r2 = 0.995, p < 0.00001; Y = −0.673 + 0.829 (X), r2 = 0.993, p = 0.00353, respectively. The number by each line is the scaling slope (exponent). Linear extrapolations of the empirical scaling lines for the phyllosoma larvae are shown as dotted lines to highlight the ontogenetic scaling shifts for both body shape (a) and metabolic rate (b).
5. Discussion
The parallel changes in the scaling exponents for RMR and inferred SA observed in seven of the eight species sampled supports the importance of SA changes in the observed ontogenetic shifts in metabolic scaling, but contradicts predictions of all current models emphasizing internal RTNs. SA theory predicts positive correlations between the scaling exponents for SA (bA) and RMR (bR), as observed, whereas current RTN theory incorrectly predicts negative correlations between bA and bR. Although RTN theory has been claimed to be universally applicable to all of life [1,16], or at least to macroscopic multicellular eukaryotes [33], RTN theory may not apply to animals without circulatory systems, or with open or incompletely closed circulatory systems [2,4,21], which are far more common taxonomically than those with completely closed circulatory systems (including only vertebrates, cephalopod molluscs, and some annelid and nemertean worms [34,35]). In fact, two of the animal species included in our study have no or very rudimentary circulatory systems (a ctenophore and scyphozoan), whereas the other six have open or incompletely closed circulatory systems (the bivalve, crustacean and thaliacean species) [35]. Nevertheless, Hirst et al. [8] have shown that the similarly steep ontogenetic scaling of both metabolic rate and SA with body mass in squids (cephalopods), which have closed circulatory systems, is also consistent with SA theory, but not with RTN theory. This additional deviation from RTN theory may be because squids use both their skin and gills for gas exchange, and also distribute resource-laden blood by means of multiple hearts found throughout the body [35], rather than from a single centralized heart or distribution centre, as assumed by the theory [1,2,16,17]. If current RTN theory does not apply to squids for these reasons, then annelid worms that use multiple hearts to pump blood [34] and nemertean worms that have no heart at all [35] may also be exceptions. Therefore, current RTN theory appears to apply to only a small subset of animals: perhaps only vertebrates with single central hearts in a completely closed vascular system. Even the application of RTN theory to vertebrates may be of limited use, because currently it cannot explain large variation in the metabolic scaling exponent observed in various vertebrate classes, which appears to be related to physiological state, ecological lifestyle or environmental conditions [12,36–39]. Perhaps next-generation resource exchange and supply theory may resolve these problems (see §6).
In any case, the positive associations between bA and bR observed in this study are remarkable because they occur in animals with very different body designs, including five different phyla, and furthermore they occur regardless of whether bA increases or decreases during ontogeny. Therefore, it is unlikely that these correlations are merely side effects of bA and bR being independently related to other unmeasured factors associated with developmental maturation or ontogenetic age. Rather, it is more likely that shape shifting directly affects metabolic scaling via changes in SA available for resource uptake and waste excretion.
This hypothetical mechanism for how shape shifting may affect metabolic scaling seems especially applicable to the thin-skinned pelagic animals (larval or adult) that we have studied here and in a related paper [8]. Phyllosoma, veliger and other pelagic larvae of many marine animals that show biphasic or triphasic metabolic scaling appear to be ‘skin-breathers’ (i.e. they can absorb oxygen and expel dissolved waste products such as CO2 through their thin, permeable integuments) [40,41]. Some can even absorb nutrients through their body surfaces and metabolize them in their tissues [40,42]. Remarkably, the body-mass scaling slope for metabolism in larvae of the Pacific oyster Crassostrea gigas is not significantly different from that for uptake of the amino acid alanine [40]. However, it is not known how much cutaneous absorption of nutrients contributes to the overall energy budget of these larvae and other pelagic animals under natural conditions [43]. Our findings point towards the importance of further research on integumentary material exchange by aquatic animals, as a way to better understand variation in their metabolic scaling.
Although parallel shifts in bA and bR values have usually been seen in this study, possible exceptions invite further scrutiny. For example, although bA and bR decrease in tandem in the common mussel Mytilus edulis as juveniles mature into adults, more research is needed to determine whether shifts in SA and metabolic scaling also match as veliger larvae develop into juveniles. This is because, although the inferred bA shift from 0.57 to 0.71–0.83 parallels the shift of bR from 0.77 (based on an average of four values) to 0.89, the individual bR estimates for veligers are highly variable, ranging from 0.59 to 0.90 (table 1). Some of this variation appears to be related to temperature, because as temperature increases from 6°C to 18°C, bR decreases from 0.90 to 0.59 (see the electronic supplementary material), as predicted by the metabolic-level boundaries hypothesis [12]. Sampling error may also be important because the body-size range of growing veligers is small (less than 2 orders of magnitude), thus potentially increasing variation in bR estimates [36,44,45] relative to those of juveniles and adults with larger body-size ranges (more than 3 and 4 orders of magnitude, respectively; see the electronic supplementary material, table S1). A similar explanation may apply to the variable bR estimates (0.35 and 1.01) for two ephyra samples of the scyphozoan Aurelia aurita that have body-size ranges less than 1.5 orders of magnitude and that deviate markedly from the inferred bA value of 0.64 (table 1). By contrast, the bR estimate (0.63) for a third sample of ephyra larvae, with a larger body-size range (more than 2 orders of magnitude), is very close to the estimated bA value. Moreover, the bR value (0.65) for ephyra larvae of A. aurita, estimated from the metabolic data of several studies taken together, is also not significantly different from the inferred bA value of 0.64 [46]. It is also possible that our simple Euclidean estimates of SA scaling (bA) do not adequately represent the ontogenetic SA changes occurring in a mussel veliger or an ephyra larva. A veliger's velum (foot), which has an extensive, highly permeable surface [40], can extend far beyond the measured shell length used in calculating bL and bA [47]. In addition, the ephyra larva shows complex changes in body shape as it grows, thus making it difficult to accurately estimate the scaling of its SA using our method based on Euclidean geometry (see footnote 7 in the electronic supplementary material, table S1).
Another major pattern evident in our results is that although bR is significantly correlated with bA, it is usually greater than that predicted by bA alone (see tables 1 and 2, and figures 1a,b and 2). Two major factors may help account for these upward deviations of bR. First, metabolically costly growth throughout ontogeny may elevate bR values, as has been observed in other animals and plants [3–7,11,23,48]. These growth effects prompt the question: what are the relative influences of resource supply versus metabolic demand by growth and other biological processes on ontogenetic metabolic scaling [4,9–13]? For example, does the steep scaling of SA (and presumably resource uptake) of many kinds of pelagic animals (including larvae) documented here and by Hirst et al. [8] permit or even drive the steep scaling of metabolism, or is steep SA scaling a secondary adjustment to steep metabolic scaling that is driven by high resource demand (e.g. mass-specific growth rates that often continue at a high rate throughout ontogeny in pelagic animals [4,49,50])? Attempting to answer this critical question brings into focus the importance of understanding the various factors influencing all of the steps of energy flow through an organism, and their integration, as a way to improve our knowledge of how and why metabolic rate varies with body size (see also [4]).
A second major factor that may help to explain apparent upward deviations in bR from that predicted by our SA model (figure 1a) is that we may have underestimated SA changes during ontogeny in our study species. Our geometric estimates of bA based on bL do not allow the detection of increases in SA related to increased convolutions (or fractal dimensionality) of the body surface, or to the development of special respiratory and nutrient-absorption structures (e.g. the velum in veligers, and the gills in other larval or juvenile forms). In addition, half of our inferred bA values (see table 1) may have been underestimated because they were based on the midpoint of a potential range of values, whereas in most cases the upper limit of this range, which involves shape flattening, is probably closer to the actual bA value than the lower limit, which involves shape elongation (also see §3 and [8]). This claim is supported by two observations. First, when data on both bL and the scaling of body width versus length were available (electronic supplementary material), the inferred bA value was almost always closer (in 9 of 10 cases) to the predicted upper limit involving mainly two-dimensional growth (flattening) than the lower limit involving mainly one-dimensional growth (elongation) (see table 1). Second, the mean empirical relationship between bL and bR (and by association bA; see figure 2a–c) for all of our study species more closely parallels the slope of the upper predicted boundary involving flattening than that of the lower boundary involving elongation (figure 1b).
Other data consistent with effects of ontogenetic shifts in body shape and SA scaling on metabolic scaling include significant correlations between bL and bR values among diverse ‘skin-breathing’ pelagic animals, for which species-specific values also show a closer match to the upper boundary curve for predicted bA than to the lower boundary curve [8], and parallel steep scaling of SA and metabolic rate in echinoid larvae [51] and epipelagic squid [52]. Steep (often near-isometric) scaling of metabolic rate in larval fishes may not only be related to their sustained rapid growth rates [4], but also to their ability to absorb oxygen (and possibly nutrients, though rarely observed) through their entire body surface [53,54]. In addition, shifts from isometric to negatively allometric metabolic scaling seen in plants may be related, at least in part, to decreases in their relative ‘leafiness’ (i.e. reductions in the relative contribution of high SA leaves to total biomass) as they grow [6].
6. Conclusion
Ontogenetic shifts in metabolic scaling may often be linked to developmental changes in body shape and SA scaling. Our SA model predicts that this should happen in any organism that relies heavily on body surfaces for resource uptake, such as many kinds of thin-skinned pelagic animals and leafy plants. Ontogenetic shifts in metabolic scaling in other organisms with impermeable integuments may be related to shifts in the SA scaling of specialized respiratory structures (such as occur for gills in developing fish [53] and for tracheae during moulting in insect larvae [13]) or to other metabolically demanding developmental changes unrelated to body shape (e.g. shifts from ectothermy to endothermy in developing mammals [4]). Nevertheless, evidence reported here and elsewhere (including near-2/3-power interspecific scaling of basal and cold-induced metabolic rates in birds and small mammals with closed circulatory systems [38]) suggests that the effect of external SA on metabolic scaling may often outweigh or modify the influence of RTNs [4,8,12,48,51,52], contrary to currently influential general models [1,2]. New resource-transport theory is needed that can accommodate organisms that distribute resources from external surfaces rather than from (or in addition to) internal hubs or ‘pumps’ (e.g. heart and stomach; see also [8]). More generally, our work and a recent review [48] suggest that a comprehensive explanation of the wide diversity of metabolic scaling relationships that have been observed must consider multiple mechanisms, including those related to SA, all of which have variable effects depending upon taxon, environment and physiological state.
Supplementary Material
Acknowledgements
We thank Andrew Kerkhoff for useful discussions, Mark Jensen for access to his raw data on metabolic rates in the spiny lobster S. verreauxi, and Peter Girguis and two anonymous reviewers for helpful comments.
Funding statement
D.S.G. was supported by a sabbatical leave granted by Juniata College, A.G.H. was supported by the Natural Environment Research Council and Department for Environment, Food and Rural Affairs (grant no. NE/L003279/1, Marine Ecosystems Research Programme), and D.A. was supported by a Leverhulme Trust Fellowship.
References
- 1.West GB, Brown JH, Enquist BJ. 1997. A general model for the origin of allometric scaling laws in biology. Science 276, 122–126. ( 10.1126/science.276.5309.122) [DOI] [PubMed] [Google Scholar]
- 2.Banavar JR, Moses ME, Brown JH, Damuth J, Rinaldo A, Sibly RM, Maritan A. 2010. A general basis for quarter-power scaling in animals. Proc. Natl Acad. Sci. USA 107, 15 816–15 820. ( 10.1073/pnas.1009974107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riisgård HU. 1998. No foundation of a ‘3/4 power scaling law’ for respiration in biology. Ecol. Lett. 1, 71–73. ( 10.1046/j.1461-0248.1998.00020.x) [DOI] [Google Scholar]
- 4.Glazier DS. 2005. Beyond the ‘3/4-power law’: variation in the intra- and interspecific scaling of metabolic rate in animals. Biol. Rev. 80, 611–662. ( 10.1017/S1464793105006834) [DOI] [PubMed] [Google Scholar]
- 5.Czarnołeski M, Kozłowski J, Dumiot G, Bonnet J-C, Mallard J, DuPont-Nivet M. 2008. Scaling of metabolism in Helix aspersa snails: changes through ontogeny and response to selection for increased size. J. Exp. Biol. 211, 391–399. ( 10.1242/jeb.013169) [DOI] [PubMed] [Google Scholar]
- 6.Peng Y, Niklas KJ, Reich PB, Sun S. 2010. Ontogenetic shift in the scaling of dark respiration with whole-plant mass in seven shrub species. Funct. Ecol. 24 502–512. ( 10.1111/j.1365-2435.2009.01667.x) [DOI] [Google Scholar]
- 7.Kutschera U, Niklas KJ. 2011. Ontogenetic changes in the scaling of cellular respiration with respect to size among sunflower seedlings. Plant Signal. Behav. 6, 72–76. ( 10.4161/psb.6.1.14001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirst AG, Glazier DS, Atkinson D. 2014. Body shape shifting during growth permits tests that distinguish between competing geometric theories of metabolic scaling. Ecol. Lett. 17, 1274–1281. ( 10.1111/ele.12334) [DOI] [PubMed] [Google Scholar]
- 9.Darveau C-A, Suarez RK, Andrews RD, Hochachka PW. 2002. Allometric cascade as a unifying principle of body mass effects on metabolism . Nature 417, 166–170. ( 10.1038/417166a) [DOI] [PubMed] [Google Scholar]
- 10.Hofmeyr JHS, Rohwer JM. 2011. Supply–demand analysis: a framework for exploring the regulatory design of metabolism. Methods Enzymol. 500, 533–554. ( 10.1016/B978-0-12-385118-5.00025-6) [DOI] [PubMed] [Google Scholar]
- 11.Glazier DS. In press. Is metabolic rate a universal ‘pacemaker’ for biological processes? Biol. Rev. ( 10.1111/brv.12115) [DOI] [PubMed] [Google Scholar]
- 12.Glazier DS. 2010. A unifying explanation for diverse metabolic scaling in animals and plants. Biol. Rev. 85, 111–138. ( 10.1111/j.1469-185X.2009.00095.x) [DOI] [PubMed] [Google Scholar]
- 13.Callier V, Nijhout HF. 2012. Supply-side constraints are insufficient to explain the ontogenetic scaling of metabolic rate in the tobacco hornworm, Manduca sexta. PLoS ONE 7, e45455 ( 10.1371/journal.pone.0045455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubner M. 1883. Über den Einfluss der Körpergrösse auf Stoff- und Kraftwechsel. Zeit. Biol. 19, 535–562. [Google Scholar]
- 15.Okie JG. 2013. General models for the spectra of surface area scaling strategies of cells and organisms: fractality, geometric dissimilitude, and internalization. Am. Nat. 181, 421–439. ( 10.1086/669150) [DOI] [PubMed] [Google Scholar]
- 16.West GB, Brown JH, Enquist BJ. 1999. The fourth dimension of life: fractal geometry and allometric scaling of organisms. Science 284, 1677–1679. ( 10.1126/science.284.5420.1677) [DOI] [PubMed] [Google Scholar]
- 17.Dodds PS. 2010. Optimal form of branching supply and collection networks. Phys. Rev. Lett. 104, 048702 ( 10.1103/PhysRevLett.104.048702) [DOI] [PubMed] [Google Scholar]
- 18.Kooijman SALM. 2010. Dynamic energy budget theory for metabolic organization. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 19.Maino JL, Kearney MR, Nisbet RM, Kooijman SALM. 2014. Reconciling theories of metabolic scaling. J. Anim. Ecol. 83, 20–29. ( 10.1111/1365-2656.12085) [DOI] [PubMed] [Google Scholar]
- 20.Savage VM, Deeds EJ, Fontana W. 2008. Sizing up allometric scaling theory. PLoS Comp. Biol. 4, e1000171 ( 10.1371/journal.pcbi.1000171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price CA, et al. 2012. Testing the metabolic theory of ecology. Ecol. Lett. 15, 1465–1474. ( 10.1111/j.1461-0248.2012.01860.x) [DOI] [PubMed] [Google Scholar]
- 22.Kleiber M. 1932. Body size and metabolism. Hilgardia 6, 315–353. ( 10.3733/hilg.v06n11p315) [DOI] [Google Scholar]
- 23.Glazier DS, Butler EM, Lombardi SA, Deptola TJ, Reese AJ, Satterthwaite EV. 2011. Ecological effects on metabolic scaling: amphipod responses to fish predators in freshwater springs. Ecol. Monogr. 81, 599–618. ( 10.1890/11-0264.1) [DOI] [Google Scholar]
- 24.Kearney MR, White CR. 2012. Testing metabolic theories. Am. Nat. 180, 546–565. ( 10.1086/667860) [DOI] [PubMed] [Google Scholar]
- 25.Zeuthen E. 1953. Oxygen uptake as related to body size in organisms. Q. Rev. Biol. 28, 1–12. (http://www.jstor.org/stable/2810299) [DOI] [PubMed] [Google Scholar]
- 26.Kinoshita J, Hiromi J, Kadota S. 1997. Do respiratory metabolic rates of the scyphomedusa Aurelia aurita scale isometrically throughout ontogeny in a sexual generation? Hydrobiologia 347, 51–55. ( 10.1023/A:1002942806113) [DOI] [Google Scholar]
- 27.Svetlichny LS, Abolmasova GI, Hubareva ES, Finenko GA, Bat L, Kideys AE. 2004. Respiration rates of Beroe ovata in the Black Sea. Mar. Biol. 145, 585–593. ( 10.1007/s00227-004-1336-4) [DOI] [Google Scholar]
- 28.Hirst AG. 2012. Intra-specific scaling of mass to length in pelagic animals: ontogenetic shape change and its implications. Limnol. Oceanogr. 57, 1579–1590. ( 10.4319/lo.2012.57.5.1579) [DOI] [Google Scholar]
- 29.Glazier DS. 2013. Log-transformation is useful for examining proportional relationships in allometric scaling. J. Theor. Biol. 334, 200–203. ( 10.1016/j.jtbi.2013.06.017) [DOI] [PubMed] [Google Scholar]
- 30.Jensen MA, Fitzgibbon QP, Carter CG, Adams LR. 2013. Effect of body mass and activity on the metabolic rate and ammonia-N excretion of the spiny lobster Sagmariasus verreauxi during ontogeny. Comp. Biochem. Physiol. A 166, 191–198. ( 10.1016/j.cbpa.2013.06.003) [DOI] [PubMed] [Google Scholar]
- 31.Jensen MA, Fitzgibbon QP, Carter CG, Adams LR. 2013. The effect of stocking density on growth, metabolism and ammonia-N excretion during larval ontogeny of the spiny lobster Sagmariasus verreauxi. Aquaculture 376–379, 45–53. ( 10.1016/j.aquaculture.2012.10.033) [DOI] [Google Scholar]
- 32.Cumming G. 2008. Inference by eye: reading the overlap of independent confidence intervals. Stat. Med. 28, 205–220. ( 10.1002/sim.3471) [DOI] [PubMed] [Google Scholar]
- 33.DeLong JP, Okie JG, Moses ME, Sibly RM, Brown JH. 2010. Shifts in metabolic scaling, production and efficiency across major evolutionary transitions of life. Proc. Natl Acad. Sci. USA 107, 12 941–12 945. ( 10.1073/pnas.1007783107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reiber CL, McGaw IJ. 2009. A review of the ‘open’ and ‘closed’ circulatory systems: new terminology for complex invertebrate circulatory systems in light of current findings. Int. J. Zool. 2009, 301284 ( 10.1155/2009/301284) [DOI] [Google Scholar]
- 35.McMahon BR, Wilkens JL, Smith PJS. 2011. Invertebrate circulatory systems. Compr. Physiol. 2011, 931–1008. ( 10.1002/cphy.cp130213) [DOI] [Google Scholar]
- 36.Bokma F. 2004. Evidence against universal metabolic allometry. Funct. Ecol. 18, 184–187. ( 10.1111/j.0269-8463.2004.00817.x) [DOI] [Google Scholar]
- 37.White CR, Phillips NF, Seymour RS. 2006. The scaling and temperature dependence of vertebrate metabolism. Biol. Lett. 2, 125–127. ( 10.1098/rsbl.2005.0378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glazier DS. 2008. Effects of metabolic level on the body-size scaling of metabolic rate in birds and mammals. Proc. R. Soc. B 275, 1405–1410. ( 10.1098/rspb.2008.0118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Killen SS, Atkinson D, Glazier DS. 2010. The intraspecific scaling of metabolic rate with body mass in fishes depends on lifestyle and temperature. Ecol. Lett. 13, 184–193. ( 10.1111/j.1461-0248.2009.01415.x) [DOI] [PubMed] [Google Scholar]
- 40.Manahan DT. 1990. Adaptations by invertebrate larvae for nutrient acquisition from seawater. Am. Zool. 30, 147–160. ( 10.1093/icb/30.1.147) [DOI] [Google Scholar]
- 41.Haond C, Charmantier G, Flik G, Bonga SE. 2001. Identification of respiratory and ion-transporting epithelia in the phyllosoma larvae of the slipper lobster Scyllarus arctus. Cell Tissue Res. 305, 445–455. ( 10.1007/s004410100405) [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez Souza JC, Strüssmann CA, Takashima F, Satoh H, Sekine S, Shima Y, Matsuda H. 2010. Oral and integumental uptake of free exogenous glycine by the Japanese spiny lobster Panulirus japonicas phyllosoma larvae. J. Exp. Biol. 213, 1859–1867. ( 10.1242/jeb.040030) [DOI] [PubMed] [Google Scholar]
- 43.Gomme J. 2001. Transport of exogenous organic substances by invertebrate integuments: the field revisited. J. Exp. Zool. 289, 254–265. () [DOI] [PubMed] [Google Scholar]
- 44.White CR, Seymour RS. 2005. Sample size and mass range effects on the allometric exponent of basal metabolic rate. Comp. Biochem. Physiol. A 142, 74–78. ( 10.1016/j.cbpa.2005.07.013) [DOI] [PubMed] [Google Scholar]
- 45.Moses ME, Hou C, Woodruff WH, West GB, Nekola JC, Zuo W, Brown JH. 2008. Revisiting a model of ontogenetic growth: estimating model parameters from theory and data. Am. Nat. 171, 632–645. (http://www.jstor.org/stable/10.1086/587073) [DOI] [PubMed] [Google Scholar]
- 46.Gambill M, Peck MA. 2014. Respiration rates of the polyps of four jellyfish species: potential thermal triggers and limits. J. Exp. Mar. Biol. Ecol. 459, 17–22. ( 10.1016/j.jembe.2014.05.005) [DOI] [Google Scholar]
- 47.Hamburger K, Møhlenberg F, Randløv A, Riisgård HU. 1983. Size, oxygen consumption and growth in the mussel Mytilus edulis. Mar. Biol. 75, 303–306. ( 10.1007/BF00406016) [DOI] [Google Scholar]
- 48.Glazier DS. 2014. Metabolic scaling in complex living systems. Systems 2, 451–540. ( 10.3390/systems2040451) [DOI] [Google Scholar]
- 49.Glazier DS. 2006. The 3/4-power law is not universal: evolution of isometric, ontogenetic metabolic scaling in pelagic animals. Bioscience 56, 325–332. ( 10.1641/0006-3568(2006)56[325:TPLINU]2.0.CO;2) [DOI] [Google Scholar]
- 50.Hirst AG, Forster J. 2013. When growth models are not universal: evidence from marine invertebrates. Proc. R. Soc. B 280, 20131546 ( 10.1098/rspb.2013.1546) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McEdward LR. 1984. Morphometric and metabolic analysis of the growth and form of an echinopluteus. J. Exp. Mar. Biol. Ecol. 82, 259–287. ( 10.1016/0022-0981(84)90109-6) [DOI] [Google Scholar]
- 52.Seibel BA. 2007. On the depth and scale of metabolic rate variation: scaling of oxygen consumption rates and enzymatic activity in the Class Cephalopoda (Mollusca). J. Exp. Biol. 210, 1–11. ( 10.1242/jeb.02588) [DOI] [PubMed] [Google Scholar]
- 53.Post JR, Lee JA. 1996. Metabolic ontogeny of teleost fishes. Can. J. Fish. Aquat. Sci. 53, 910–923. ( 10.1139/f95-278) [DOI] [Google Scholar]
- 54.Glover CN, Bucking C, Wood CM. 2013. The skin of fish as a transport epithelium: a review. J. Comp. Physiol. B 183, 877–891. ( 10.1007/s00360-013-0761-4) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.