Abstract
Collective decision-making processes emerge from social feedback networks within a group. Many studies on collective behaviour underestimate the role of individual personality and, as a result, personality is rarely analysed in the context of collective dynamics. Here, we show evidence of sheltering behaviour personality in a gregarious insect (Periplaneta americana), which is characterized by a collective personality at the group level. We also highlight that the individuals within groups exhibited consistent personality traits in their probability of sheltering and total time sheltered during the three trials over one week. Moreover, the group personality, which arises from the synergy between the distribution of behaviour profiles in the group and social amplifications, affected the sheltering dynamics. However, owing to its robustness, personality did not affect the group probability of reaching a consensus. Finally, to prove social interactions, we developed a new statistical method that will be helpful for future research on personality traits and group behaviour. This approach will help to identify the circumstances under which particular group compositions may improve the fitness of individuals in gregarious species.
Keywords: personality, collective dynamics, consensus decision-making, arthropods, Periplaneta americana
1. Introduction
Recently, a significant number of publications have highlighted personality and behavioural syndromes in several taxa [1–8]. This personality, corresponding to a consistent and correlated behaviour across time or situations, concerns a wide variety of traits such as boldness–shyness, exploration–avoidance, activity level, sociability or aggression [9]. Hence, from an evolutionary and ecological perspective, different populations across the animal kingdom show stable interindividual behavioural variations in the absence of demographic or morphological correlations [3,9–16]. The most recent theory assumes that these personality traits play an important role for fitness in context-dependent ways: dominance, reproductive success or competitive abilities [8,10,13,17].
Regarding insects, although personality has been reported [18–20], the majority of studies focus on social insects where interindividual differences are mainly associated with the morphological or reproductive caste [8,21]. The physiological constraints predispose individuals to exhibit specific behaviours (e.g. queens and workers), which are often considered as a colony-level adaptation that contributes to the division of labour efficiency [8,13]. The importance of personality observed within castes is still poorly documented. Some studies focusing on behavioural variability in monomorphic species (e.g. ants [18] and bees [22,23]) show the coexistence of different behavioural types of individuals in the same colony [24]. Unfortunately, in the case of gregarious arthropods, only few studies have been devoted to personality, such as in the pea aphid [19], caterpillars [25] or social spiders [26]. In this type of social context, personality is not easy to quantify [13]. Indeed, the observed behaviours result from the synergy between personality and the large number of interactions between individuals [27,28].
The personality's role in social dynamics remains largely unexplored [29,30]. Hence, the main objective of this study is to integrate personality into the study of aggregation dynamics and the related collective decision-making process in a group of cockroaches (Periplaneta americana). The mechanism of collective decision-making resides in the amplification resulting from inter-attractions between individuals in a context where each individual has little environmental information [31,32]. In typical situations, these well-known mechanisms imply density-dependent processes, quorum and consensus [33,34]. Collective decision-making in cockroaches has been largely studied for its importance in the understanding of the aggregation process [34,35]. Studies on problem-solving have demonstrated the cockroaches’ ability to settle together in one shelter (i.e. reach a consensus) when confronted with a choice between several shelters of similar or different quality. Indeed, the probability of joining a shelter decreases with the crowding under the shelter. The probability of leaving a shelter decreases with the increment of shelter quality and the number of individuals it contains. The relationship between this probability and the number of sheltered individuals is the main component of the amplification processes [36–38].
Previous studies on collective decision-making have mainly been focused on the mechanisms underlying the spatio-temporal dynamics arising from social interactions [32]. Such studies have deliberately neglected interindividual differences. This simplification can lead to erroneous interpretations of the mechanisms governing the interaction networks and the resulting collective phenomena [30,39]. Moreover, as the conflict of interest between individuals (e.g. optimal timing of activities or preferences) influences the collective pattern, individual fitness often depends on the within-group individual personality composition [10,25,33,40,41]. Finally, different mixtures of personalities can lead to different group or colony personalities (e.g. social spiders [26], bees [23] or ants [42]). Therefore, it is crucial to study the mechanisms underlying the emergence of group personalities at the individual level [39].
In this context, we have coupled an experimental and a theoretical approach to understand the interplay between personality and collective dynamics and how different parameters influence the biological system [11,35,43–46]. Consequently, the main objectives of this study are (i) to provide evidence for the existence of personality in P. americana at individual and collective levels, and (ii) to investigate the implications of such phenomena for the aggregation dynamics. Furthermore, (iii) we propose a new method to test social inter-attractions within a group and (iv) we discuss how individuals’ behavioural variability can generate group personality.
2. Methods
(a). Experimental set-up
Experiments were carried out on adult males of P. americana (L.) (Dictyoptera: Blattidae) without external damage, which were issued from strains reared for more than 10 years in the same breeding facilities. The experimental set-up was a circular arena limited by a black polyethylene ring (diameter: 100 cm, height: 20 cm) covered with a paper layer (120 g m−2). To prevent cockroaches from escaping, the inner surface of the ring was covered by an electric fence [43]. The lighting source (four lamp bulbs, Phillips ambiance Pro, 20 W) was placed above the arena and provided homogeneous illumination intensity at ground level (355 ± 5 lux). Two shelters made of transparent Plexiglas discs (diameter: 15 cm) were hung by three nylon threads (diameter: 0.3 mm) and covered by a red coloured filter film (Rosco E-Colour 19:fire), creating low luminosity zones (75 ± 5 lux). Cockroaches are photophobic during the diurnal phase, and thus both shelters are perceived as rest sites [34,36,47]. The centre of each disc was located 23 cm from the edge of the arena and 3 cm above the floor arena (electronic supplementary material, S1). The shelters were large enough to potentially shelter all 16 cockroaches within one shelter [34]. The set-up was surrounded by white curtains to avoid spatial cues.
In order to detect the animals when they were under the shelter, cockroaches were tagged with a radio-frequency identification (RFID) chip (diameter: 7.1 ± 0.2 mm and weight: 107 ± 3 mg; Spacecode). This chip was glued to the thorax with latex (Winsor & Newton). Sheltered individuals were detected by a circular RFID reader located below each shelter (electronic supplementary material, S1).
(b). Experimental procedure and measures
Groups of 16 cockroaches were kept in total darkness for 48 h in Plexiglas boxes (36 × 24 × 14 cm) containing a cardboard shelter, humidified cotton wool and ad libitum food. Afterwards, 16 males were introduced to the centre of the arena. The same group of cockroaches was tested during three consecutive trials (each trial lasted 3 h) over one week (Monday, Wednesday and Friday) with a 45 h gap between trials, where groups were kept in the dark in the same Plexiglas box. This procedure was repeated for 19 different groups.
First, we measured the sheltering time (under both shelters) for each cockroach throughout the experiment. In this article, we refer to it as individual resting time (IRT). The group resting time (GRT) is the mean IRT for each group. The GRT is the measure of the sheltering time for the entire group. Moreover, the faster the cockroaches' settlement under shelters, the greater the GRT; hence, it constitutes a direct result of the sheltering dynamics. Second, for each group, we quantified the time it took for each cockroach to visit a shelter for the first time. Finally, to identify the emergence of a consensus, we compared the distribution of cockroaches aggregated under each shelter at the end of experiments. If this distribution is a symmetrical binomial distribution, it corresponds to a situation without social interaction. A consensus resulting from social interactions is reached when one of the two shelters contains a statistically higher number of sheltered individuals than expected by a binomial law (p-value < 0.05).
(c). Analysis
We used R software (v. 2.14.1) for statistical and modelling analysis. We used analysis of variance (ANOVA) to test differences between the mean GRT of the 19 groups along the three trials. Moreover, the Kruskal–Wallis test was used to test the influence of the week factor over the GRT of different trials. The significance of statistical tests was fixed to α = 0.05.
We included a model in the analysis to test the null hypothesis that aggregation dynamics can be adequately explained under the assumption that all individuals have identical joining and leaving probabilities. For this purpose, we analysed experimental survival curves of all individuals' periods of time (t) spent outside or inside shelters for all trials. These curves were fitted (nonlinear least-squares regression with GraphPad Prism) to an exponential-power law with three parameters (α, β and θ), where x is the fraction of periods of time inside shelters that are not at an end at time t (equation (2.1a); see figure 1 for parameter values). The probabilities of joining (PJ(t), equation (2.1b)) and leaving (PL(t), equation (2.1c)) the shelter for a theoretical mean individual in a social context can be derived from the equation (2.1a). The present equations (2.1b) or (2.1c) provide an improvement of the power law applied in reference [43]. By adding a constant αL (αJ), the probability PL (PJ) reaches a plateau value αL (αJ) for long sheltering times (staying time in the arena). In order to reproduce the observed data, the equations (2.1b) and (2.1c) were subsequently implemented in a numerical model (stochastic simulation) that considers mean individuals (identical probability values). In this simulation, three locations were considered: two shelters and the rest of the arena. There was no direct transition between shelters. At the beginning of a simulation run, the 16 cockroaches were outside the shelters. At each time step (0.1 s), the individual probability of joining a shelter (equation (2.1b)) and leaving (equation (2.1c)) is updated. If individuals change their location, the time t is reset. In simulations, the experimental timescale was preserved (t = 10 800 s). A total of 1000 simulations of 19 groups of 16 individuals were performed.
| 2.1a |
| 2.1b |
| 2.1c |
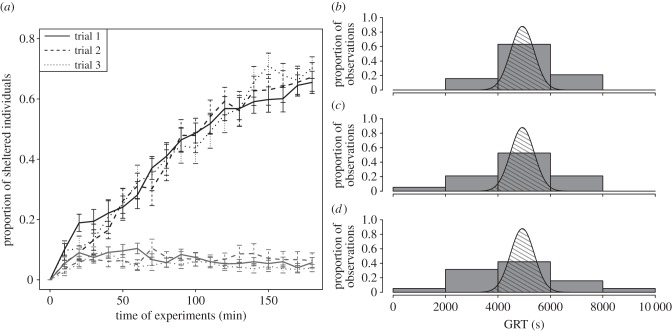
Figure 1.
(a) Evolution of sheltered cockroaches during the experimental time (180 min). The black lines represent the proportion of cockroaches present under the selected shelter (with more sheltered cockroaches at the end of the experiment); grey represents the non-selected shelter. (b) Comparison between the experimental (grey bars) and theoretical (dashed area, calculated with equation (2.1b,c)) group resting time (GRT) distributions for the first trial, (c) the second trial and (d) the third trial. Parametric values of the theoretical model are PJ(t): αJ = 0.00035, βJ = 0.75 and θJ = 6.37. PL(t): αL = 0.00045, βL = 0.76 and θL = 5.61.
(d). Individual resting time test for sociality
In order to highlight the presence of social interactions in sheltering behaviour, we developed a new method called the ‘IRT test for sociality’ based on the IRT of individuals of P. americana. Prior to the analysis, we ensured that the mean GRT had homogeneity of variance and did not differ between trials. If this condition was met, the IRTi1, IRTi2 and IRTi3 of each group member i during trials one to three could be compared. For a two-trial comparison, given a group of N individuals, a score of 1 or 0 is assigned to each individual i based on whether IRTi1 > IRTi2 or IRTi1 < IRTi2, respectively. If experimental group members behave independently (without influence of social attractions), the probability Π of having the IRTi1 > IRTi2 (equation (2.2)) for an individual i is equal to 0.5. The probability P(j,N) to observe j individuals identified by the score 1 among N is given by the symmetric binomial distribution (equation (2.2)). The j value varies between 0 and N.
| 2.2 |
In a social context, the IRT depends on positive feedbacks between individuals; an individual i with a long IRT (short IRT) would stimulate the others to have a long IRT (short IRT), and vice versa. Therefore, for each group, the probability to observe a large proportion of individuals with the same score (0, increasing or 1, decreasing their IRT between two trials) is greater than the probability predicted by the theoretical binomial law (Π = 0.5). In this study, we consider the size of the majority in each group (either score 1 or 0), and the corresponding distribution of 19 groups is compared with the symmetric folded binomial distribution (it arises from a binomial distribution when it is indistinct, whether the majority scores 1 or 0) [48]. When the observed distribution is not different from the theoretical folded binomial distribution, we conclude that the behaviour is not influenced by inter-attraction. By contrast, a skewed distribution demonstrates the contribution of amplifications through social interactions.
(e). Personality assessment
To assess the consistency of behavioural traits between trials, we used the W Kendall coefficient for concordance assessment, which is based on a comparison of group ranks for a given variable along the trial period [49,50]. Hence, the stability of two variables was tested: the GRT and the proportion of sheltered individuals at the end of the experiments. W coefficients range from 0 to 1 (the bigger the value, the bigger the agreement of ranks between trials). To determine whether the ranks are significantly conserved between trials or are randomly acquired, the observed W coefficients were compared with the ‘Kendall groups random distribution’. The ‘Kendall groups random distribution’ is computed by randomly ranking the 19 groups for three repeated trials (N = 1000). Similarly, we ranked individuals within groups by their IRT and the first visit order to shelters to assess their stability within the group, hence the individual personality. The within-group W coefficients of the IRT and the first visit order to shelters were compared with the ‘Kendall individual random distribution’, which is the theoretical W coefficient distribution for 1000 groups of 16 cockroaches that were randomly ranked for three repeated trials. These results were compared with the most used method in personality assessment: the repeatability test.
3. Results
(a). Modelling approach
As demonstrated in previous studies, the red shelters were well perceived as resting sites. Indeed, the proportion of sheltered individuals increased over the experimental time until the majority of individuals were sheltered (figure 1a). Concerning our model, the large observed distribution of GRT for the 19 tested groups could not be obtained by assuming a homogeneity of group member behaviours (figure 1b–d). Indeed, the Kolmogorov–Smirnov test (K–S test) showed that the observed distributions for trials 2 and 3 were different from the theoretical distribution obtained with equation (2.1b,c) (figure 1c,d; K–S test: trial 2: D = 0.34, p = 0.03; trial 3: D = 0.35, p = 0.02; all N = 19). Although the curve of trial 1 was not significantly different from the theoretical curve (K–S test: D = 0.29, N = 19, p = 0.07; figure 1b), the variance from the experimental trial 1 is greater than the variance of the theoretical curve (Bartlett test: 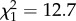 , p < 0.001). Otherwise, three experimental distributions of GRT were not significantly different (K–S test: trials 1–2: D = 0.32, p = 0.31; trials 2–3: D = 0.21, p = 0.81 trials 1–3: D = 0.26, p = 0.53; all N = 19) and had homogeneity of variance (Bartlett test:
, p < 0.001). Otherwise, three experimental distributions of GRT were not significantly different (K–S test: trials 1–2: D = 0.32, p = 0.31; trials 2–3: D = 0.21, p = 0.81 trials 1–3: D = 0.26, p = 0.53; all N = 19) and had homogeneity of variance (Bartlett test: 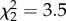 , p = 0.17). These results highlight that our model, which considers all individuals with the same behaviour, is not accurate enough to reproduce the experimental intergroup variability.
, p = 0.17). These results highlight that our model, which considers all individuals with the same behaviour, is not accurate enough to reproduce the experimental intergroup variability.
(b). Group personality
The between-group differences in the proportion of individuals settled under shelters (t = 180 min) were maintained throughout the week (figure 2a). The GRT was not correlated to the week of experiments (Kruskal–Wallis test for trial 1: 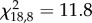 , p = 0.2; trial 2:
, p = 0.2; trial 2: 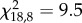 , p = 0.3 and trial 3:
, p = 0.3 and trial 3: 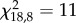 , p = 0.2). The groups showed a high repeatability of their proportion of sheltered cockroaches (N = 19; W = 0.60). Indeed, the obtained W coefficient was significantly different from the ‘Kendall groups random distribution’ (Z-test: Z = 2.58, p = 0.01; see §2 for more details).
, p = 0.2). The groups showed a high repeatability of their proportion of sheltered cockroaches (N = 19; W = 0.60). Indeed, the obtained W coefficient was significantly different from the ‘Kendall groups random distribution’ (Z-test: Z = 2.58, p = 0.01; see §2 for more details).
Figure 2.
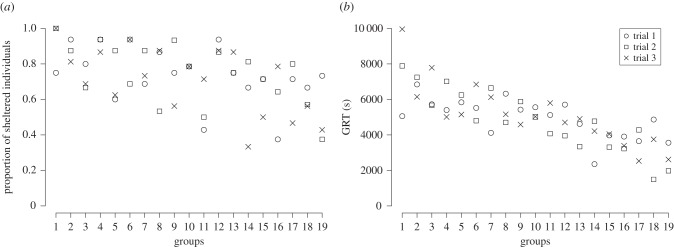
(a) Mean number of sheltered individuals across the three trials at t = 180 min. (b) Mean group resting time (GRT) of three trials for the 19 tested groups. Groups are shown in decreasing mean GRT order in both cases.
The ANOVA results showed a high variability of GRT between groups (ANOVA for repeated measures: F18,36 = 101, p < 0.001). This variability contrasts with the low intragroup GRT variability (figure 2b). The groups expressed a high repeatability of GRT during the trial period. Indeed, the intergroup consistency of GRT along the trial period (N = 19; W = 0.72) was higher than the expected consistency by the ‘Kendall groups random distribution’ (Z-test: Z = 4.2, p < 0.001).
These two sheltering measures (the proportion of sheltered individuals and GRT) were highly repeatable and statistically positively correlated (Kendall tau correlation test: R = 0.47, N = 57, p < 0.001). Hence, groups spending an extended period under shelters (long GRT) were also the groups with a high number of sheltered individuals; the inverse relationship can also be observed.
Finally, in agreement with previous studies, groups were also able to reach a consensus (see §2) in 86% of the cases (N = 57; electronic supplementary material, S2). The distribution of the 19 groups, which reached a consensus once, twice or thrice, was not different from that expected by a binomial law with a probability (Π parameter from equation (2.2)) of 0.86 to reach a consensus (probability given by the percentage of trials during which groups reached it, 86%; K–S test: D = 0.003, N = 19, p = 1; electronic supplementary material, S3). The binomial law assumes the same probability of reaching a consensus for all the groups. If personality were involved in consensus decision-making, we should observe a different distribution (owing to some groups reaching the consensus more often or less often than expected).
(c). Individual personality
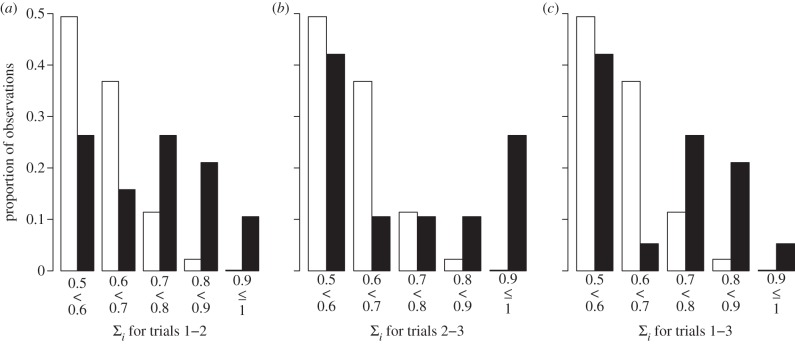
According to the ANOVA, there was no increase or decrease in the mean GRT along the trial period (trial 1: 5014 s ± 1185.5, trial 2: 5016.7 s ± 1477.1, trial 3: 4856.7 s ± 1863.2; mean ± s.d.; ANOVA for repeated measures: F2,36 = 0.27, p = 0.61). Despite showing that GRT displayed a high repeatability between trials, we could not exclude any fluctuations of IRT of group members between trials (figure 2b). The IRTs were normally distributed within groups (Shapiro test: p > 0.05 for 50 over 57 groups). The ‘IRT test for sociality’ confirms the existence of a social influence inside the group resulting from the crossed inter-attractions between group members (figure 3). With this method, we show that the experimental distributions of values were significantly greater than the theoretical folded binomial distribution (equation (2.2)) attempted for the same size groups (K–S test: figure 3a: trial 1–2: D = 0.49, p < 0.001; figure 3b: trial 2–3: D = 0.38, p = 0.01; figure 3c: trial 1–3: D = 0.38, p < 0.01; all N = 19). Moreover, individuals had an IRT for each trial that was not random, which was typically similar during the week. The distribution of W coefficients for the experimental groups was significantly greater than the ‘Kendall individual random distribution’ (figure 4a; K–S test: D = 0.78, N = 19, p < 0.001). In addition to their resting time, group members showed consistency in their shelter discovering behaviour. Indeed, most group members visited shelters for the first time in an order that was not random over three trials (figure 4b; K–S test: D = 0.43, N = 19, p < 0.01). It is noteworthy that the previous W coefficient distributions (IRT and first visit order) were positively correlated (Pearson: R = 0.45, N = 19, p = 0.053) but not significant. Nevertheless, the W coefficients for IRT were highly correlated with the respective results obtained by the repeatability test (Pearson correlation test: R2 = 0.9, p < 0.001; see the electronic supplementary material, S4).
Figure 3.
Comparison between experimental values for the ‘IRT test for sociality’ (black bars) with the binomial distribution (white bars). Results for the transition between (a) trial 1 to trial 2, (b) trial 2 to trial 3 and (c) trial 1 to trial 3.
Figure 4.
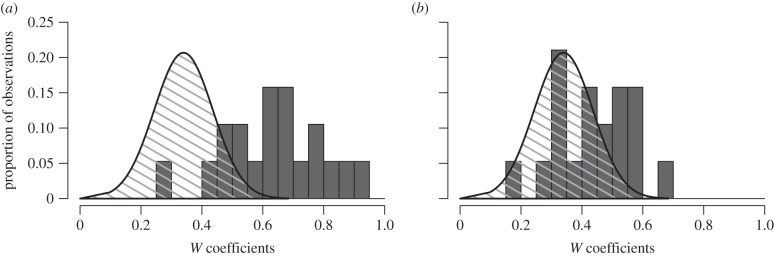
Comparison of W coefficient distribution between experimental values (grey bars) and the theoretical ‘IRT Kendall random distribution’ (dashed area) for (a) individual resting time (IRT) and (b) the first visit order.
(d). Individual personality and group resting time
The distribution of personalities within a group and their consistency over time could influence the sheltering dynamics. In this context, we showed that groups composed by individuals characterized by a low repeatability of sheltering behaviours (i.e. the IRT and the first visit order) might display extreme collective dynamics leading to low or high GRT (figure 5a,b). By contrast, stable groups where individuals maintained their IRT through the trials showed intermediate values of GRT, which is an indicator of the sheltering dynamic (see §2). Indeed, the mean GRT of three trials and the W coefficient for the IRT of each group showed a parabolic correlation with a peak of W values for intermediate GRT (figure 5a; R2 = 0.33, p = 0.01). Moreover, a similar parabolic correlation was found between the consistency in the order of shelter visits and the dynamics (figure 5b; R2 = 0.47, p < 0.01).
Figure 5.
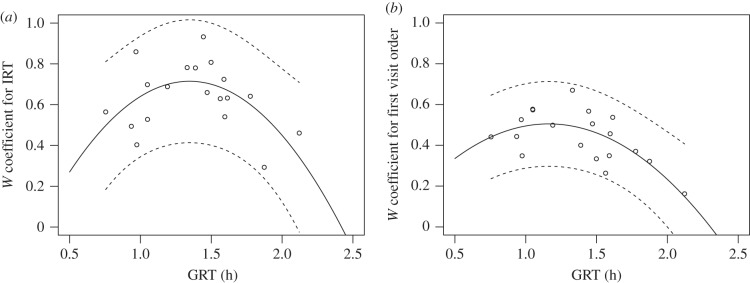
Relationship between the mean group resting time (GRT) of each group with the W coefficients observed for (a) individual resting time (IRT) and (b) the first visit order. The mean GRT shown is the mean GRT of the three trials. Solid lines represent the parabolic equations fitted to data points and the dashed lines represent the 95% confidence interval.
4. Discussion
(a). Individual personality
We show evidence of personality in aggregation behavioural traits in the American cockroach: P. americana. Within a group, individuals consistently differed in their rate of joining the shelters and their resting time over the week. Such robustness of personalities within a group was partly unexpected, for three main reasons. First, behaviours that are more sensitive to the environment, and therefore more plastic, tend to be less repeatable than behaviours under morphological or physiological constraints [3]. In our experiments, the behavioural traits were largely related to the different responses to the environment. The insects are introduced in a ‘stressing environment’ and must quickly find a safe place (i.e. the shelter). Moreover, cockroaches explore shelters by walking randomly; therefore, the time needed for an individual to encounter a shelter and the time spent under it (IRT) are highly variable. Second, we should expect a homogenization of behavioural profiles owing to the strong genetic relatedness between tested individuals (see §2). Third, as age and the associated life history and experience generate interindividual behavioural variability [21], only adult males (± four months) were used in our experiments. Moreover, knowing that activity and mobility rates are affected by food deprivation [47], all individuals were fed ad libitum for 48 h before the experiments.
Despite these effects on personalities, we highlight the existence of personality coupled with social amplification between individuals. This social amplification was observed by using the ‘IRT test for sociality’. This result is in agreement with previous studies on social amplification [34,37,51]. We suggest generalizing the use of the ‘IRT test for sociality’, in association with tests based on the binomial distribution, for testing social inter-attractions within groups or populations when a single variable between days is explored.
Previous studies have demonstrated that the sheltering behaviour of gregarious insects is characterized by a set of parameters governing the probability of joining (PJ) and leaving (PL) a shelter [35,43]. If PJ is quite constant during the week for the same individual, it strongly differs between individuals. Indeed, the experimental distribution of the W coefficients indicates that individuals kept a stable rank order for the first visit to shelters, which is directly linked to the PJ. At the current stage, it is not clear whether the between-individual differences in their IRT are solely owing to PJ or also to differences in the PL. Previous results show that PL is clearly modulated by social interactions; however, this has not been found for PJ [37].
Two hypotheses can be formulated to account for the influence of the interindividual variability on PJ and the social interactions during the aggregation process. First, unlike species where individuals are often identified as ‘leaders’ and ‘followers’ [46], cockroach groups are characterized by a self-organized process of aggregation. However, this self-organization does not exclude that the influence or attractiveness between individuals can differ by population. For example, as cuticular hydrocarbons act as aggregation pheromones [52], ‘leader’ individuals producing more cuticular hydrocarbons will act as key individuals [53] and contribute unintentionally more than others to the nucleation of aggregates and consequently to the collective decision. Indeed, it will influence ‘followers’ with lower attractiveness to stay longer under the ‘leader’ shelter. A second hypothesis assumes that all individuals exercise the same attraction (e.g. same production of cuticular hydrocarbons), but the response to conspecifics varies from one individual to another owing to differences in the threshold response to hydrocarbon concentration [54]. According to previous studies [20,22], threshold response variability can lead to interindividual differences [21].
(b). Group personality
We have shown stable between-group differences (GRT and proportion of individuals sheltered) in the exploitation pattern of the environment. This stable between-group variability originates from a double effect. First, it arises from the individual personality, which is generated by a non-negligible variation of individual preferences associated with low levels of flexibility [13]. Indeed, small random sampling from a population produces groups that differ both between themselves and from the initial population in their personality distributions. Second, a social group is not the mere sum of individual behaviours. Social amplification is at work and stands in opposite effect to our system: it favours similar sheltering behaviour within a group, but can contribute to increase differentiation between groups [37]. Moreover, the positive correlation between the GRT and the final proportion of sheltered individuals is important to explain how both behavioural traits are related to the aggregation's dynamics. Owing to the amplification process, the presence of individuals with high PJ in a group would influence the behaviour of other group members, leading to a large number of sheltered individuals. Although the GRT is the mean IRT within a group, the GRT is not an approximation of the mean personality traits. It constitutes a measure of the sheltering time and therefore is a direct result of the sheltering dynamics.
The sheltering dynamics are sensitive to the composition of the group. The groups present different sheltering dynamics according to the distribution of personalities within the groups, each individual differing by their PJ and possibly PL (figure 5a,b). Hence, the heterogeneity of personalities within such groups (i.e. scattered values of PJ) will lead to high repeatability (high W coefficients) of behavioural traits. Groups with heterogeneity of personalities display an intermediate sheltering speed as shown by the intermediate GRT. Instead, the homogeneity of personalities (i.e. slight differences in PJ) would lead to low values of the W coefficients, indicating that there is little agreement between measurements of an individual's rank. Groups comprising homogeneous individuals are more likely to perform extremely fast or slowly in aggregation decision-making processes. The large number of experiments with either fast or slow dynamics supports the hypothesis that personality distribution within the population must present a high variance, with a high frequency of extreme behaviours.
The study of animal personalities generally seeks to understand how and why individuals maintain personalities, as well as their ecological and evolutionary implications [33,55]. We have shown that the probability of reaching consensus in a patchy environment does not vary between groups and is independent of the distributions of personalities within groups. Indeed, consensus is robust and contrasts with the intergroup variability observed in sheltering dynamics (i.e. GRT and proportion of sheltered individuals). From an evolutionary perspective, consensus decision-making should be strongly integrated into the biology of the species and related to predation risk and stress reduction. As reaching a consensus is crucial to maintaining group cohesion and maximizing individual and group benefits [56], most groups would reach a consensus whatever their distribution of personalities. However, fitness depends not only on the probability of reaching a consensus, but also on the speed and accuracy of reaching a consensus [57]. In our experiments, which were carried out with shelters of similar quality, the accuracy of the choice is irrelevant. In this context, groups with higher fitness would be those with all individuals sheltered within the same shelter [32,58–60] and those that had spent the maximum time under shelters. In other words, the faster the aggregation dynamics, the lower the predation and desiccation risk and therefore the greater the fitness. Nevertheless, in a natural environment, not all shelters may have the same quality, and accuracy becomes as relevant as speed. In such cases, the distribution of personalities within a group could contribute to solving the conflict between accuracy and speed [61]. For example, groups composed by solely long-GRT individuals, owing to their high probabilities of joining the worst shelter, risk being unable to make the correct choice. Therefore, groups characterized by a large distribution of personalities could be the best trade-off between speed and accuracy. They would benefit from a better fitness by minimizing the time outside the shelter while maximizing the probability of selecting the better one. In cockroaches, as observed in other insects, a limited plasticity can be the least costly and evolutionarily most beneficial strategy [13,62]. In the future, experiments with different quality shelters should reveal the optimal proportion of short-IRT/long-IRT individuals to reach the most efficient consensus decision-making.
In conclusion, the individuals of P. americana showed behavioural stability over one week. This stability was observed for groups and for the individuals composing them. These significant differences observed among groups in terms of collective decision-making and sheltering behaviour are owing to interindividual differences. Individuals have idiosyncrasy in their probability of joining shelters, but a deeper investigation is needed to determine the other behavioural traits affecting the aggregation (e.g. probability of leaving). Moreover, experiments with different group sizes and isolated individuals will be able to highlight the feedback loop between sociality and personality. Longer studies would determine whether these traits are involved in a behavioural syndrome across situations and time. Therefore, the origins of variability in individual sheltering behaviour remain unclear. It is important to shed some light on the role of genetic determinism. For example, similar to the role of the ‘foraging gene’ observed in other insect species [21], we could potentially find the genetic origins of personality implied in the cockroach aggregation dynamics. However, we cannot exclude the epigenetic factors, as experience influences adult behaviour [63], and nutritional intake during larval stages affects the adult size, activity and mobility rates [47]. Further, there are a large variety of behavioural syndromes, as observed in other animals [9], which may be explored in the case of insects. Future studies should address the identification of the evolutionary forces that contribute to the conservation of divergent traits within gregarious organisms [64,65]. Finally, owing to the multiple feedbacks participating in such social systems, theoretical approaches are needed to understand the relationship between individuals and collective behaviour [44]. However, self-organized models that only take into account the means of individuals cannot accurately predict the observed collective behaviour (i.e. aggregation). Therefore, the global understanding of collective dynamics requires that the personality of individuals and its synergies with social interactions be taken into account.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Rob Flynn, Jessica Williams, Alexandra Latham and the team of Jesús Scrofa for their corrections and suggestions. We acknowledge four anonymous referees for their valuable comments.
Funding statement
This study was supported by a PhD grant from Fonds pour la Formation à la Recherche dans l'Industrie et dans l'Agriculture (FRIA).
References
- 1.Sih A, Bell A, Johnson JC. 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378. ( 10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 2.Biro PA, Stamps JA. 2008. Are animal personality traits linked to life-history productivity? Trends Ecol. Evol. 23, 361–368. ( 10.1016/j.tree.2008.04.003) [DOI] [PubMed] [Google Scholar]
- 3.Bell AM, Hankison SJ, Laskowski KL. 2009. The repeatability of behaviour: a meta-analysis. Anim. Behav. 77, 771–783. ( 10.1016/j.anbehav.2008.12.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trillmich F, Hudson R. 2011. The emergence of personality in animals: the need for a developmental approach. Dev. Psychobiol. 53, 505–509. ( 10.1002/dev.20573) [DOI] [PubMed] [Google Scholar]
- 5.Dingemanse NJ, Dochtermann NA. 2013. Quantifying individual variation in behaviour: mixed-effect modelling approaches. J. Anim. Ecol. 82, 39–54. ( 10.1111/1365-2656.12013) [DOI] [PubMed] [Google Scholar]
- 6.Dingemanse NJ, Wolf M. 2013. Between-individual differences in behavioural plasticity within populations: causes and consequences. Anim. Behav. 85, 1031–1039. ( 10.1016/j.anbehav.2012.12.032) [DOI] [Google Scholar]
- 7.Han CS, Brooks RC. 2013. Evolution of individual variation in behaviour and behavioural plasticity under scramble competition. Anim. Behav. 86, 435–442. ( 10.1016/j.anbehav.2013.05.039) [DOI] [Google Scholar]
- 8.Jandt JM, Bengston S, Pinter-Wollman N, Pruitt JN, Raine NE, Dornhaus A, Sih A. 2014. Behavioural syndromes and social insects: personality at multiple levels. Biol. Rev. Camb. Philos. Soc. 89, 48–67. ( 10.1111/brv.12042) [DOI] [PubMed] [Google Scholar]
- 9.Réale D, Reader SM, Sol D, Mcdougall PT, Dingemanse NJ. 2007. Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318. ( 10.1111/j.1469-185X.2007.00010.x) [DOI] [PubMed] [Google Scholar]
- 10.Smith BR, Blumstein DT. 2008. Fitness consequences of personality: a meta-analysis. Behav. Ecol. 19, 448–455. ( 10.1093/beheco/arm144) [DOI] [Google Scholar]
- 11.King AJ, Sueur C. 2011. Where next? Group coordination and collective decision making by primates. Int. J. Primatol. 32, 1245–1267. ( 10.1007/s10764-011-9526-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carter A, Goldizen A, Heinsohn R. 2012. Personality and plasticity: temporal behavioural reaction norms in a lizard, the Namibian rock agama. Anim. Behav. 84, 471–477. ( 10.1016/j.anbehav.2012.06.001) [DOI] [Google Scholar]
- 13.Dall SRX, Bell AM, Bolnick DI, Ratnieks FLW. 2012. An evolutionary ecology of individual differences. Ecol. Lett. 15, 1189–1198. ( 10.1111/j.1461-0248.2012.01846.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Briffa M. 2013. The influence of personality on a group-level process: shy hermit crabs make longer vacancy chains. Ethology 119, 1014–1023. ( 10.1111/eth.12148) [DOI] [Google Scholar]
- 15.Brown C, Irving E. 2013. Individual personality traits influence group exploration in a feral guppy population. Behav. Ecol. 25, 95–101. ( 10.1093/beheco/art090) [DOI] [Google Scholar]
- 16.Kappeler PM, Barrett L, Blumstein DT, Clutton-Brock TH. 2013. Constraints and flexibility in mammalian social behaviour: introduction and synthesis. Phil. Trans. R. Soc. B 368, 20120337 ( 10.1098/rstb.2012.0337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trompf L, Brown C. 2014. Personality affects learning and trade-offs between private and social information in guppies, Poecilia reticulata. Anim. Behav. 88, 99–106. ( 10.1016/j.anbehav.2013.11.022) [DOI] [Google Scholar]
- 18.Chapman BB, Thain H, Coughlin J, Hughes WOH. 2011. Behavioural syndromes at multiple scales in Myrmica ants. Anim. Behav. 82, 391–397. ( 10.1016/j.anbehav.2011.05.019) [DOI] [Google Scholar]
- 19.Schuett W, Dall SRX, Baeumer J, Kloesener MH, Nakagawa S, Beinlich F, Eggers T. 2011. Personality variation in a clonal insect: the pea aphid, Acyrthosiphon pisum. Dev. Psychobiol. 53, 631–640. ( 10.1002/dev.20538) [DOI] [PubMed] [Google Scholar]
- 20.Pinter-Wollman N. 2012. Personality in social insects: how does worker personality determine colony personality? Curr. Zool. 58, 580–588. [Google Scholar]
- 21.Jeanson R, Weidenmüller A. 2014. Interindividual variability in social insects—proximate causes and ultimate consequences. Biol. Rev. 89, 671–687. ( 10.1111/brv.12074) [DOI] [PubMed] [Google Scholar]
- 22.Weidenmüller A. 2004. The control of nest climate in bumblebee (Bombus terrestris) colonies: interindividual variability and self reinforcement in fanning response. Behav. Ecol. 15, 120–128. ( 10.1093/beheco/arg101) [DOI] [Google Scholar]
- 23.Wray MK, Seeley TD. 2011. Consistent personality differences in house-hunting behavior but not decision speed in swarms of honey bees (Apis mellifera). Behav. Ecol. Sociobiol. 65, 2061–2070. ( 10.1007/s00265-011-1215-1) [DOI] [Google Scholar]
- 24.Sih A, Del Giudice M. 2012. Linking behavioural syndromes and cognition: a behavioural ecology perspective. Phil. Trans. R. Soc. B 367, 2762–2772. ( 10.1098/rstb.2012.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dussutour A, Nicolis SC, Despland E, Simpson SJ. 2008. Individual differences influence collective behaviour in social caterpillars. Anim. Behav. 76, 5–16. ( 10.1016/j.anbehav.2007.12.009) [DOI] [Google Scholar]
- 26.Pruitt JN, Riechert SE. 2011. Within-group behavioral variation promotes biased task performance and the emergence of a defensive caste in a social spider. Behav. Ecol. Sociobiol. 65, 1055–1060. ( 10.1007/s00265-010-1112-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dewitt TJ, Scheiner SM. 2004. Phenotypic plasticity: functional and conceptual approaches, pp. 1–9. Oxford, UK: Oxford University Press. [Google Scholar]
- 28.Jablonka E, Lamb MJ. 2007. Précis of evolution in four dimensions. Behav. Brain Sci. 30, 353–392. ( 10.1017/S0140525X07002221) [DOI] [PubMed] [Google Scholar]
- 29.Michelena P, Jeanson R, Deneubourg J-L, Sibbald AM. 2010. Personality and collective decision-making in foraging herbivores. Proc. R. Soc. B 277, 1093–1099. ( 10.1098/rspb.2009.1926) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Webster MM, Ward AJW. 2011. Personality and social context. Biol. Rev. Camb. Philos. Soc. 86, 759–773. ( 10.1111/j.1469-185X.2010.00169.x) [DOI] [PubMed] [Google Scholar]
- 31.Parrish JK, Edelstein-Keshet L. 1999. Complexity, pattern, and evolutionary trade-offs in animal aggregation. Science 284, 99–101. ( 10.1126/science.284.5411.99) [DOI] [PubMed] [Google Scholar]
- 32.Sumpter DJT. 2010. Collective animal behavior. Princeton, NJ: Princeton University Press. [Google Scholar]
- 33.Conradt L, Roper TJ. 2005. Consensus decision making in animals. Trends Ecol. Evol. 20, 449–456. ( 10.1016/j.tree.2005.05.008) [DOI] [PubMed] [Google Scholar]
- 34.Sempo G, Canonge S, Detrain C, Deneubourg J-L. 2009. Complex dynamics based on a quorum decision-making process by cockroaches in a patchy environment. Ethology 115, 1150–1161. ( 10.1111/j.1439-0310.2009.01699.x) [DOI] [Google Scholar]
- 35.Jeanson R, Rivault C, Deneubourg J-L, Blanco S, Fournier R, Jost C, Theraulaz G. 2005. Self-organized aggregation in cockroaches. Anim. Behav. 69, 169–180. ( 10.1016/j.anbehav.2004.02.009) [DOI] [Google Scholar]
- 36.Halloy J, et al. 2007. Social integration of robots into groups of cockroaches to control self-organized choices. Science 318, 1155–1158. ( 10.1126/science.1144259) [DOI] [PubMed] [Google Scholar]
- 37.Canonge S, Deneubourg J-L, Sempo G. 2011. Group living enhances individual resources discrimination: the use of public information by cockroaches to assess shelter quality. PLoS ONE 6, e19748 ( 10.1371/journal.pone.0019748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sempo G, Canonge S, Deneubourg J-L. 2013. From aggregation to dispersion: how habitat fragmentation prevents the emergence of consensual decision making in a group. PLoS ONE 8, e78951 ( 10.1371/journal.pone.0078951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petit O, Bon R. 2010. Decision-making processes: the case of collective movements. Behav. Process. 84, 635–647. ( 10.1016/j.beproc.2010.04.009) [DOI] [PubMed] [Google Scholar]
- 40.Bon R, Deneubourg J-L, Gerard J-F, Michelena P. 2005. Sexual segregation in ungulates: from individual mechanisms to collective patterns. In Sexual segregation in vertebrates (eds Ruckstuhl K, Neuhaus P.), pp. 180–199 Cambridge, UK: Cambridge University Press. [Google Scholar]
- 41.Sih A, Cote J, Evans M, Fogarty S, Pruitt J. 2012. Ecological implications of behavioural syndromes. Ecol. Lett. 15, 278–289. ( 10.1111/j.1461-0248.2011.01731.x) [DOI] [PubMed] [Google Scholar]
- 42.Scharf I, Modlmeier AP, Fries S, Tirard C, Foitzik S. 2012. Characterizing the collective personality of ant societies: aggressive colonies do not abandon their home. PLoS ONE 7, e33314 ( 10.1371/journal.pone.0033314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Canonge S, Sempo G, Jeanson R, Detrain C, Deneubourg JL. 2009. Self-amplification as a source of interindividual variability: shelter selection in cockroaches. J. Insect Physiol. 55, 976–982. ( 10.1016/j.jinsphys.2009.06.011) [DOI] [PubMed] [Google Scholar]
- 44.Dingemanse NJ, Wolf M. 2010. Recent models for adaptive personality differences: a review. Phil. Tran. R. Soc. B 365, 3947–3958. ( 10.1098/rstb.2010.0221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller N, Garnier S, Hartnett AT, Couzin ID. 2013. Both information and social cohesion determine collective decisions in animal groups. Proc. Natl Acad. Sci. USA 110, 5263–5268. ( 10.1073/pnas.1217513110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaffer Z, Sasaki T, Pratt SC. 2013. Linear recruitment leads to allocation and flexibility in collective foraging by ants. Anim. Behav. 86, 967–975. ( 10.1016/j.anbehav.2013.08.014) [DOI] [Google Scholar]
- 47.Bell WJ, Adiyodi KG. 1982. The American cockroach. London, UK: Chapman and Hall. [Google Scholar]
- 48.Urbakh VY. 1967. Statistical testing of differences in causal behaviour of two morphologically indistinguishable objects. Biometrics 23, 137–143. ( 10.2307/2528286) [DOI] [PubMed] [Google Scholar]
- 49.Kendall MG. 1938. A new measure of rank correlation. Biometrika 30, 81–93. ( 10.1093/biomet/30.1-2.81) [DOI] [Google Scholar]
- 50.Zar JH. 1999. Biostatistical analysis, 4th edn Englewood Cliffs, NJ: Prentice Hall. [Google Scholar]
- 51.Jeanson R, Deneubourg J-L. 2007. Conspecific attraction and shelter selection in gregarious insects. Am. Nat. 170, 47–58. ( 10.1086/518570) [DOI] [PubMed] [Google Scholar]
- 52.Saïd I, Costagliola G, Leoncini I, Rivault C. 2005. Cuticular hydrocarbon profiles and aggregation in four Periplaneta species (Insecta: Dictyoptera). J. Insect Physiol. 51, 995–1003. ( 10.1016/j.jinsphys.2005.04.017) [DOI] [PubMed] [Google Scholar]
- 53.Robson SK, Traniello JFA. 2000. Key individuals and the organisation of labour in ants. In Information processing in social insects (eds Detrain C, Pasteels JF, Deneubourg JL.), pp. 239–259. Basel, Switzerland: Birkhäuser. [Google Scholar]
- 54.Bonabeau E, Theraulaz G, Deneubourg J-L, Aron S, Camazine S. 1997. Self-organization in social insects. Trends Evol. Ecol. 12, 188–193. ( 10.1016/S0169-5347(97)01048-3) [DOI] [PubMed] [Google Scholar]
- 55.Beckmann C, Biro PA. 2013. On the validity of a single (boldness) assay in personality research. Ethology 119, 937–947. ( 10.1111/eth.12137) [DOI] [Google Scholar]
- 56.Krause J, Ruxton GD. 2002. Living in groups. Oxford, UK: Oxford University Press. [Google Scholar]
- 57.Chittka L, Skorupski P, Raine NE. 2009. Speed–accuracy tradeoffs in animal decision making. Trends Ecol. Evol. 24, 400–407. ( 10.1016/j.tree.2009.02.010) [DOI] [PubMed] [Google Scholar]
- 58.Yoder JA, Grojean C. 1997. Group influence on water conservation in the giant Madagascar hissing-cockroach, Gromphadorhina portentosa (Dictyoptera: Blaberidae). Physiol. Entomol. 22, 79–82. ( 10.1111/j.1365-3032.1997.tb01143.x) [DOI] [Google Scholar]
- 59.Dambach M, Goehlen B. 1999. Aggregation density and longevity correlate with humidity in first-instar nymphs of the cockroach (Blattella germanica L., Dictyoptera). J. Insect Physiol. 45, 423–429. ( 10.1016/S0022-1910(98)00141-3) [DOI] [PubMed] [Google Scholar]
- 60.Amé J-M, Halloy J, Rivault C, Detrain C, Deneubourg JL. 2006. Collegial decision making based on social amplification leads to optimal group formation. Proc. Natl Acad. Sci. USA 103, 5835–5840. ( 10.1073/pnas.0507877103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sumpter DJT, Pratt SC. 2009. Quorum responses and consensus decision making. Phil. Trans. R. Soc. B 364, 743–753. ( 10.1098/rstb.2008.0204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nicolis SC, Detrain C, Demolin D, Deneubourg JL. 2003. Optimality of collective choices: a stochastic approach. Bull. Math. Biol. 65, 795–808. ( 10.1016/S0092-8240(03)00040-5) [DOI] [PubMed] [Google Scholar]
- 63.Watanabe H, Kobayashi Y, Sakura M, Matsumoto Y, Mizunami M. 2003. Classical olfactory conditioning in the cockroach Periplaneta americana. Zool. Sci. 20, 1447–1454. ( 10.2108/zsj.20.1447) [DOI] [PubMed] [Google Scholar]
- 64.Bell AM, Aubin-Horth N. 2010. What can whole genome expression data tell us about the ecology and evolution of personality? Phil. Trans. R. Soc. B 365, 4001–4012. ( 10.1098/rstb.2010.0185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kralj-Fiser S, Schuett W. 2014. Studying personality variation in invertebrates: why bother? Anim. Behav. 91, 41–52. ( 10.1016/j.anbehav.2014.02.016) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.