Abstract
Mammalian cells respond to environmental stress by activating heat shock transcription factors (eg, Hsf1) that regulate increased synthesis of heat shock proteins (Hsps). Hsps prevent the disruption of normal cellular mitosis, meiosis, or differentiation by environmental stressors. To further characterize this stress response, transformed wild-type Hsf1+/+ and mutant Hsf1−/− mouse embryonic fibroblasts (MEFs) were exposed to (1) lethal heat (45°C, 60 minutes), (2) conditioning heat (43°C, 30 minutes), or (3) conditioning followed by lethal heat. Western blot analysis demonstrated that only Hsf1+/+ MEFs expressed inducible Hsp70s and Hsp25 following conditioning or conditioning and lethal heat. Exposure of either Hsf1+/+ or Hsf1−/− MEFs to lethal heat resulted in cell death. However, if conditioning heat was applied 6 hours before lethal heat, more than 85% of Hsf1+/+ MEFs survived, and cells in G2/M transiently increased 3-fold. In contrast, conditioned Hsf1−/− MEFs neither survived lethal heat nor exhibited this G2/M accumulation. Coinfection with adenoviral Hsp70 and Hsp25 constructs did not fully recreate thermotolerance in either Hsf1+/+ or Hsf1−/− MEFs, indicating other Hsf1-mediated gene expression is required for complete thermotolerance. These results demonstrate the necessity of Hsf1-mediated gene expression for thermotolerance and the involvement of cell cycle regulation, particularly the G2/M transition, in this thermotolerant response.
INTRODUCTION
Understanding the regulatory mechanisms involved in cell cycle progression, checkpoint activation, and initiation of apoptosis is essential for elucidating the pathways that orchestrate normal growth and differentiation. Cell cycle checkpoints are essential regulatory events that must be overcome for a cell to progress through the phases of the cell cycle (Elledge 1996). The interactions of environmental factors with mechanisms regulating cell cycle and programmed cell death (apoptosis) have been areas of intensive investigation during the past decade.
Identifying the key components in cell cycle and apoptotic regulatory pathways that are altered following toxicant exposure will help in understanding the mode of action for a wide range of chemicals and their effects on cellular fate. Heat, radiation, hypoxia, heavy metals, and numerous drugs and other chemicals have been demonstrated to alter cell cycle and/or induce apoptosis (Shackelford et al 1999; Tibbles and Woodgett 1999). In response to such environmental stressors, all species studied to date elicit a stress response that includes the expression of highly conserved heat shock proteins (Hsps). Stress-induced expression of a number of mammalian Hsps is mediated by the activation of heat shock transcription factor 1 (Hsf1). Activation of Hsf1 requires the phosphorylation of the inactive cytoplasmic monomer to induce trimerization, translocation to the nucleus, and Hsf1 binding to heat shock elements (HSEs) in the promoter regions of Hsp genes (Cotto et al 1996; Xia and Voellmy 1997; Chu et al 1998; Morano and Thiele 1999). Hsps function as molecular chaperones, assisting in the folding, trafficking, and maintenance of proteins, a property that explains their multifaceted roles in a wide range of cellular pathways, including development, differentiation, cell cycle regulation, apoptosis, and thermotolerance. Thermotolerance occurs when a cell or organism is subjected to a mild, nonlethal dose of heat, which then protects it from subsequent exposure to a lethal dose of heat (Li and Werb 1982; Subjeck et al 1982; Moseley 1997). Numerous studies indicate a requirement for Hsf1-mediated expression of inducible Hsps for thermotolerance (McMillian et al 1998; Nollen et al 1999; Xiang and Rensing 1999). In this study we set out to determine if Hsf1-mediated thermotolerance has any consequences on cell cycle regulation.
To date, a number of studies implicate constitutive and inducible mammalian Hsps in the regulation of the cell cycle. The expression of Hsps following heat shock is associated with cell cycle arrest at various phases of the cell cycle, depending on cell type, the phase of the cell cycle when stressed, and the severity of the stress (Milarski and Morimoto 1986; Milarski et al 1989; Nitta et al 1997). For example, heat-induced expression of Hsp70 correlates with the induction of p21, a cyclin-dependent kinase inhibitor, and subsequent p53-independent G1 cell cycle arrest in human glioblastoma cells (Fuse et al 1996). There is also evidence of direct protein-protein interactions between Hsps and cell cycle regulators, such as the association between Hsc70 and P27Kip1 in rat thyroid cells during the G1/S transition of the cell cycle (Nakamura et al 1999) and Hsp70-2 and CDC2/cyclin B1 during the G2/M transition in mouse spermatocytes (Zhu et al 1997). These data suggest that Hsps might be involved in cell cycle arrest at various phases in both mitotic and meiotic cells. Thus, the sum of the current literature indicates that Hsf1-mediated expression of Hsps is required for thermotolerance and could be associated with heat-induced cell cycle arrest.
These observations led us to the hypothesis that Hsf1-mediated expression of Hsps in thermotolerant cells allows them to accumulate at either the G1/S or G2/M phase of the cell cycle following a lethal heat shock, allowing recovery from the effects of heat stress and eventually resumption of the cell cycle. To test this hypothesis, we used cells from an Hsf1 gene knockout mouse model (McMillian et al 1998; Xiao et al 1999) to analyze effects of heat on Hsp expression, cell death, and cell cycle. Transformed wild-type and gene-knockout Hsf1 (Hsf1+/+ and Hsf1−/−, respectively) mouse embryonic fibroblasts (MEFs) were exposed to 3 treatment protocols: lethal heat stress, conditioning heat, or conditioning heat followed by lethal heat stress. MEFs were then analyzed for cell viability or death and cell cycle alterations linked to Hsf1 genotype and Hsp expression. Results with these cell lines demonstrated that Hsf1-mediated expression of inducible Hsps is necessary for thermotolerance and identified specific effects on the G2/M transition of the cell cycle.
MATERIALS AND METHODS
Cell culture
Human papillomaviral E6/E7–transformed Hsf1 MEFs were seeded into T75-cm2 tissue culture flasks and cultivated in Dulbecco modified Eagle medium high glucose (DMEM-H) supplemented with 10% fetal bovine serum (FBS), 1% l-glutamine, 1% penicillin-streptomycin, and 0.001% β-mercaptoethanol (complete medium) in 5% CO2 at 37°C. Thirty-six hours before any experiments, the cells were subcultured to a concentration of 1, 3, or 4 × 105 per 25 cm2 flask depending on the assay.
Heat stress protocols
To induce thermotolerance, 25-cm2 flasks of logarithmically growing cells were conditioned with a mild heat shock by total submersion in a 43°C water bath for 30 minutes and allowed to recover at 37°C for 6 hours (conditioning dose). Lethal heat stress (45°C for 1 hour) was followed by incubation at 37°C for the times indicated. The conditioning and lethal dose (treatment) involved submersion at 43°C for 30 minutes, 37°C for 6 hours, 45°C for 1 hour, and incubation at 37°C for the indicated times.
Protein extraction and Western blot analysis
Single flasks of cells (both adherent and nonadherent) were trypsinized, pelleted by centrifugation (1000 × g for 8 minutes), and washed 3 times in phosphate-buffered saline (PBS). The cell pellet was resuspended in 300 μL of RIPA lysis buffer (150 mM NaCl, 50 mM Tris-HCl [pH 7.5], 1% NP40, 0.1% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 1 mM ethylenediamine-tetraacetic acid (EDTA), 1 mM phenylmethlysulfonyl fluoride (PMSF), 4 μg of leupeptin, 4 μg of apoptinin, and 4 μg of trypsin inhibitors) and placed on ice for 20 minutes. The cell lysate was centrifuged at 10 000 × g for 10 minutes and supernatants were harvested. A total of 10 μL of total cell lysate was added to 5 μL of sample buffer containing 5% β-mercaptoethanol, 15% glycerol, 3% SDS, and 0.1 M Tris (pH 7.4) and subjected to a 7.5% Tris-HCl Ready Gel (Bio-Rad, Hercules, CA, USA) under reducing conditions. Proteins were electrophoretically blotted onto PVDF membranes (Hybond-P, Amersham Pharmacia, Piscataway, NJ, USA). Hsp expression was detected by probing membranes with antibodies specific for the inducible forms of Hsp70 (Hsp70-1 and Hsp70-3), Hsp25, or the constitutive form of Hsc70 (Hsp72, SPA-810; Hsp25, SPA-801; Hsc70, SPA-815; Stressgen Biotechnologies Corp, Victoria, British Columbia, Canada). Horseradish peroxidase–conjugated goat anti-mouse or anti-rabbit immunoglobulin (Roche Molecular Biochemicals, Indianapolis, IN, USA) were used as the detection antibody and the reaction visualized by ECL-Plus (Amersham Pharmacia).
Adenoviral infections
Both the inducible rat hsp70 and hsp25 adenoviral constructs were generated using the strategy previously described (Mestril et al 1996). Briefly, the rat hsp70 or hsp25 was cloned into the multiple cloning site of the adenoviral shuttle plasmid pACCMVpLpASR. This plasmid contains the 5′ end of the adenovirus serotype 5 genome (map units 0–17), where the E1 region has been substituted with the human cytomegalovirus enhancer/promoter followed by the multiple cloning site from pAC19 and the polyadenylation region from simian vacuolating virus No. 40. The resulting plasmid was cotransfected with pJM17, a plasmid that contains the complete adenovirus 5 genome, into the human embryonic kidney cell line 293 using the calcium phosphate transfection method. Infectious viral particles containing the inserted hsp70 were generated by in vivo recombination in the 293 cells and were isolated as single plaques 7 days later.
To produce detectable amounts of Hsp70/Hsp25, Hsf1+/+, and Hsf1−/−, MEFs were plated at 4 × 105 cells per 25-cm2 flask and infected with adenoviral Hsp70, adenoviral Hsp25, or both at 10 viral particles per cell in DMEM-H containing 2% heat-inactivated FBS. The cells were incubated at 37°C for 2 hours with occasional rocking. After the incubation, the 2% medium was replaced with the DMEM-H complete medium and returned to 37°C for 48 hours. Following the 48-hour incubation, the infected cells were challenged with a lethal dose of heat and monitored for adherence throughout a 96-hour period.
Cell cycle analysis
Following the indicated treatments, all cells in a flask (both adherent and nonadherent) were trypsinized, pelleted, and washed twice with PBS. The pellet was hand vortexed and resuspended in 500 μL of lysis buffer (0.2% NP40 in PBS [pH 7.4], 0.5 mg/mL of ribonuclease A) followed by vigorous mechanical vortexing for 30 seconds. The lysates were incubated at room temperature for 30 minutes followed by the addition of 25 μL of 1 mg/mL of propidium iodide (PI) and incubation on ice for 15 minutes. Cell cycle analysis was performed using a FACSCalibur flow cytometer (BD Immunocytometry Systems, San Jose, CA, USA) and modeling of data performed with ModFit LT v2.0 (Verity Software House, Topsham, ME, USA) on at least 10 000 cells.
Detection of cell death
Cells were analyzed for their capacity to bind recombinant human annexin V directly conjugated to fluorescein isothiocyanate (FITC) after the indicated time following heat exposure. Cells were incubated in trypsin/EDTA and washed twice in 1× PBS and once in DMEM-H plus serum. Cell pellet was washed once in 1× binding buffer (Clontech Laboratories, Palo Alto, CA, USA) and resuspended in 200 μL of ×1 binding buffer and 5 μL of annexin V–FITC and PI to a final concentration of 1 μg/mL and incubated for 15 minutes at room temperature per manufacturer's suggestion (Clontech). Samples were analyzed on a FACSCalibur flow cytometer by dual color cytofluorometry.
RESULTS
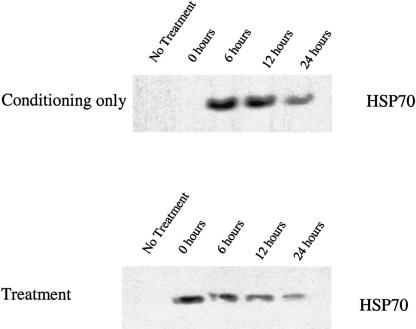
Levels of inducible and constitutive Hsp expression in Hsf1+/+ and Hsf1−/− MEFs are compared in Figure 1. Untreated (cycling) Hsf1+/+ and Hsf1−/− MEFs did not express detectable levels of inducible Hsp70 (Fig 1, top) or Hsp25 (Fig 1, middle). Also, 6 hours following lethal heat treatment, Hsf1+/+ and Hsf1−/− MEFs did not express detectable levels of inducible Hsp70 or Hsp25 (Fig 1). However, 6 hours after a conditioning dose of heat or conditioning and lethal treatment, Hsf1+/+ MEFs expressed significant levels of inducible Hsp70 and Hsp25, whereas Hsf1−/− MEFs did not, indicating that expression of inducible Hsp70 and Hsp25 is dependent on Hsf1 expression. In contrast, the level of constitutive Hsc70 expression did not vary significantly between the 2 cell lines under any condition (Fig 1, bottom), reaffirming that the expression of constitutive Hsc70 is independent of Hsf1. To determine the kinetics of Hsp70 expression in Hsf1+/+ MEFs and validate the timing of the conditioning and lethal heat shock experiments, cell lysates were prepared at various time points following either a conditioning dose of heat or conditioning and lethal treatment (Fig 2). Hsf1+/+ MEFs did not express detectable levels of Hsp70 immediately following the conditioning heat dose (0 hours). The expression of Hsp70 peaks around 6 hours and begins to decrease 24 hours after the conditioning dose of heat (Fig 2, top). The kinetics for Hsf1+/+ MEFs receiving a conditioning and lethal heat shock demonstrates that Hsp70 expression is near its peak level at the time of lethal heat shock (Fig 2, bottom), such that significant levels of Hsp70, and presumably other Hsps, are present when these cells are subjected to lethal heat.
Fig. 1.
Characterization of inducible Hsp expression in Hsf1+/+ and Hsf1−/− MEFs. Western analysis of whole cell extracts from cycling Hsf1+/+ and Hsf1−/− MEFs either untreated or exposed to lethal heat (45°C for 60 minutes), conditioning heat (43°C for 30 minutes), or conditioning and lethal heat (treatment). Total cellular protein was extracted from untreated cycling cells and the heat-exposed cells after a 6-hour recovery at 37°C. Proteins were separated by SDS–polyacrylamide gel electrophoresis, transferred to nitrocellulose, and probed with antibodies specific for inducible Hsp70s (upper panel), inducible Hsp25 (middle panel), or constitutive Hsc70 (lower panel)
Fig. 2.
Kinetics of inducible Hsp70 expression in Hsf1+/+ MEFs. Western analysis of whole cell extracts from cycling Hsf1+/+ MEFs untreated, exposed to conditioning heat (43°C for 30 minutes, upper panel), or exposed to conditioning and lethal heat (treatment, bottom panel). Total cellular protein was extracted from equivalent numbers of cells after 0, 6, 12, or 24 hours of recovery at 37°C. Samples were separated by SDS–polyacrylamide gel electrophoresis, transferred to filters, and probed with an antibody specific for inducible Hsp70s
We subsequently investigated heat-induced apoptosis and necrosis in Hsf1+/+ and Hsf1−/− MEFs exposed to conditioning, lethal, and conditioning and lethal doses of heat. Cells were harvested at 24-hour intervals, stained with fluorescein-conjugated annexin-V and PI, and quantitatively assessed for cellular viability by flow cytometry (Fig 3). The percentage of viable (annexin-negative, PI-negative), early apoptotic (annexin-positive, PI-negative), late apoptotic/necrotic (annexin and PI-positive), and necrotic (annexin-negative, PI-positive) cells were compared. These data demonstrate that both Hsf1+/+ and Hsf1−/− MEFs exposed to lethal heat became significantly more annexin and PI positive, or less viable, during the experiment when compared with untreated (control) cells (Fig 3 A,B). However, Hsf1+/+ MEFs that received a conditioning dose of heat before the lethal dose were protected from the lethal heat dose (Fig 3A). The Hsf1−/− MEFs were not thermotolerant following conditioning and lethal heat treatment and therefore died (Fig 3B).
Fig. 3.
Conditioned Hsf1+/+ MEFs resist heat-induced cell death. Data from FACS analysis of cell viability or death comparing Hsf1+/+ (A) and Hsf1−/− (B) MEFs. Untreated cycling cells are compared with cells exposed to lethal heat or conditioning and lethal heat treatment (Tx), followed by incubation at 37°C for 24, 48, or 72 hours. Cell viability and cell death were determined with annexin V–FITC and PI (ApoAlert kit, Clontech) staining and analyzed by flow cytometry (FACSCalibur, Becton Dickinson). Viable cells were unstained; early apoptotic cells stained only with annexin V; late apoptotic/necrotic cells were doubly positive; and necrotic cells were PI positive. Data were subjected to a 1-way analysis of variance and Tukey-Kramer multiple comparison test. Results are the means of 3 independent experiments; error bars indicate SD. The letters a and b indicate significant differences relative to corresponding untreated cells (P < 0.001)
To determine if thermotolerance affected cell proliferation, we performed growth analyses on Hsf1+/+ and Hsf1−/− MEFs following exposure to lethal and conditioning and lethal doses of heat. Cells were counted 24, 48, 72, and 96 hours after plating and heat exposure. The rate of proliferation of untreated Hsf1+/+ and Hsf1−/− cell lines was equivalent (Fig 4A) and all cells remained adherent. However, once either cell line was exposed to a lethal dose of heat, proliferation ceased and the cells lost the ability to adhere to plastic (Fig 4B). Following conditioning and lethal treatment, Hsf1+/+ MEFs stopped proliferating until 72 hours after lethal heat (Fig 4C), suggesting that thermotolerance required these cells to arrest in the cell cycle.
Fig. 4.
Growth analyses of Hsf1+/+ and Hsf1−/− MEFs. Hsf1+/+ and Hsf1−/− MEFs were untreated (A), subjected to lethal heat (B), exposed to conditioning and lethal heat (C), or coinfected with adenoviruses expressing inducible Hsp70 and Hsp25 and subsequently challenged with lethal heat (D). Adherent and nonadherent cells were counted at various times after plating or heat exposure. Data were subjected to a 1-way analysis of variance and Tukey-Kramer multiple comparison test. Results are the means of 3 independent experiments; error bars indicate SD. Asterisks indicate significant differences (P < 0.001) between WT and KO cells at corresponding times after conditioning and lethal heat (C) or between adenoviral infected/lethal heat challenged cells and untreated cells (D). Western blot analysis of whole cell extracts from Hsf1+/+ and Hsf1−/− MEFs coinfected with adenovirus vectors demonstrate Hsp70 and Hsp25 expression before lethal heat exposure (E)
Since the development of thermotolerance closely correlates with Hsf1 genotype and expression of inducible Hsps, Hsf1+/+ and Hsf1−/− MEFs were infected with adenoviral constructs containing either inducible Hsp72 or Hsp25 or coinfected with both constructs to determine if expression of these Hsps could replicate thermotolerance. To attain detectable levels of Hsp70 and Hsp25 expression similar to heat-conditioned cells, the initial number of cells infected had to be increased to 4 × 105 per flask (see “Materials and Methods” section). Partial protection was observed in the Hsf1+/+ MEFs infected with both inducible Hsp70 and Hsp25 adenoviral constructs (Fig 4D). Hsf1−/− MEFs were not protected following coinfection (Fig 4D), although equivalent levels of Hsp70/25 were expressed in each cell line before lethal heat exposure (Fig 4E). Neither Hsf1+/+ nor Hsf1−/− cell lines were protected by singular infection and expression of either Hsp70 or Hsp25 protein alone, and adenoviral infection without heat shock did not disrupt cell cycle (data not shown).
Because of the effects of heat and Hsf1 genotype on cell proliferation, a more rigorous examination of cell cycle distribution was conducted. The same experimental conditions used for the proliferation analysis were implemented for cell cycle analysis. Nuclear DNA content and cell viability of Hsf1+/+ and Hsf1−/− MEFs were determined 24 hours after heat treatment (Fig 5). The DNA content or viability of Hsf1+/+ and Hsf1−/− cells was not affected following exposure to conditioning heat (data not shown). The DNA content and cell cycle of both cell types were also unaffected by lethal heat; however, cell viability decreased in both cell types based on increased nuclear debris (Fig 5 C,D). Following conditioning and lethal heat, Hsf1+/+ MEFs accumulated in the G2/M phase of the cell cycle, with only a slight increase in debris (Fig 5E). In contrast, the Hsf1−/− cells did not accumulate in any phase of the cell cycle, and debris (dead cells) increased significantly (Fig 5F).
Fig. 5.
Cell cycle phase distribution of cycling and heat-exposed Hsf1+/+ and Hsf1−/− MEFs. Unsynchronized cycling cells were untreated (A and B) or subjected to lethal heat (C and D) or conditioning and lethal heat (E and F). Cells were harvested 24 hours after heat, lysed, and stained with PI. Samples were analyzed for DNA content by flow cytometry (FACSCalibur, Becton Dickinson) and cell cycle profiles calculated for a minimum of 10 000 cells. The asterisk (E) indicates the increased G2/M peak in Hsf1+/+ MEFs following conditioning and lethal treatment
Results from a minimum of 5 independent experiments analyzing cell cycle phase distribution are presented in Table 1. As mentioned herein, Hsf1+/+ and Hsf1−/− cells exposed to lethal heat do not accumulate in any phase of the cell cycle, but cell debris significantly increased, indicative of cellular damage and death. Hsf1+/+ cells subjected to conditioning and lethal heat accumulated in the G2/M phase 24 hours after lethal heat. Forty-eight hours after conditioning and lethal exposure, this accumulation at G2/M decreased, with no significant increase in cellular debris. Forty-eight hours after lethal or conditioning and lethal heat, Hsf1−/− cells did not accumulate in any phase of the cell cycle and cell debris had significantly increased. Results from the proliferation assay and from the 48-hour cell cycle analysis indicate that the G2/M arrest in the Hsf1+/+ was transient. To confirm this, we extended the cell cycle assay to 96 hours. Conditioning and lethal-treated Hsf1+/+ cells possess the highest percentage of G2/M arrested cells 24 hours after treatment (Fig 6B) and return to an untreated DNA profile by 64 hours (compare Fig 6 D,A). These data demonstrate that the G2/M arrest observed in the conditioning and lethal–treated Hsf1+/+ cells is transient.
TABLE 1.
Cumulative cell cycle phase distribution of heat-exposed WT and KO Hsf1 MEFsa

Fig. 6.
The G2/M arrest in Hsf1+/+ MEFs is transient. Unsynchronized cycling cells were untreated (A) or subjected to conditioning and lethal heat treatment. Cells were harvested 24 (B), 48 (C) or 64 hours (D) after heat, lysed, and stained with PI. Samples were analyzed for DNA content by flow cytometry (FACSCalibur, Becton Dickinson) and cell cycle profiles were calculated for a minimum of 10 000 cells. The G2/M accumulation present 24 hours after treatment (B) was absent by 64 hours after treatment (D)
DISCUSSION
Hsps provide essential biological functions in the cell. Two of these functions include molecular chaperoning of other proteins and protection from various stressors (reviewed in Feige et al 1996; Kabakov and Gabai 1997; Gabai et al 1998). The chaperone function of Hsps assists protein folding, transport, and degradation and is characteristic of both constitutively and inducibly expressed Hsp family members. Inducible hsp genes are regulated at the transcriptional level by Hsf1, which is a sequence-specific transcription factor that interacts with HSEs in the hsp gene promoters (Morimoto 1993; Wu 1995). Inducible Hsps are synthesized in response to stresses that cause protein damage and denaturation and are involved in prevention and/or repair of stress-induced protein damage. There is also evidence to suggest that inducible Hsps are synthesized and prevent cell death after stresses that do not cause detectable protein damage (Jaattela 1993; Kampinga 1993; Bellman et al 1996; Samali and Cotter 1996; Kim et al 1997). Therefore, the quantity of inducible Hsps in a cell may be one of the pivotal conditions determining cellular resistance to numerous stress factors and is largely dependent on stress-regulated activation of Hsf1.
In the present study, we have demonstrated that the ability to elicit an inducible heat shock response enhances cell survival and prevents cell death. Using transformed Hsf1+/+ and Hsf1−/− MEF cell lines, we confirmed results from an earlier study with Hsf1+/+ and Hsf1−/− primary cultures of MEFs (McMillian et al 1998) demonstrating that Hsf1 is required for expression of stress-inducible Hsps but not required for the expression of constitutive Hsps. The present data also demonstrate that thermotolerance and subsequent cellular proliferation are dependent on Hsf1 expression. Hsf1 expression and activation direct the expression of inducible Hsp70 and Hsp25. However, our results with the Hsp70 and Hsp25 adenoviral constructs indicate that coexpression of Hsp70 and Hsp25 only partially protects Hsf1+/+ cells. Increased numbers of cells were coinfected with adenoviral Hsp70 and Hsp25 to achieve equivalent levels of Hsp70/25 protein expression as were detected in conditioned Hsf1+/+ cells. It is possible that the apparent partial protection in Hsf1+/+ cells was the result of the following: (1) all cells were coinfected, but expressed insufficient amounts of Hsp70 and Hsp25 protein to afford full protection; (2) only a fraction of cells were coinfected with adenovirus and expressed Hsp70 and Hsp25; or (3) all cells were coinfected and expressed sufficient levels of Hsp70 and Hsp25, but thermotolerance requires additional Hsf1-mediated protein expression for complete thermotolerance.
Like the earlier study with primary cell lines (McMillian et al 1998), the present study also demonstrates that Hsf1 is essential for inducible Hsp-dependent protection from heat-induced cell death. We used flow cytometry to try and characterize this cell death as either necrotic or apoptotic. Numerous studies have demonstrated that the expression of inducible Hsps protect cells from apoptosis-inducing agents (Simon et al 1995; Karlseder et al 1996; Kim et al 1997; Buzzard et al 1998; Kwak et al 1998; Creagh and Cotter 1999), and there appear to be multiple steps at which inducible Hsps can inhibit apoptosis. For example, inducible Hsp70 was able to inhibit apoptosis at points both upstream and downstream of SAPK/JNK activation (Mosser et al 1997). Hsps also regulate the expression and/or homo/heterodimer formation within the Bcl-2 protein family (Strasser and Anderson 1995; Polla et al 1996; Takayana et al 1997). This potential to influence the ratios of Bcl-2 homodiners and heterodimers could determine whether the cell survives or undergoes apoptosis. Another mechanism by which Hsp70s may directly block apoptosis is by inhibiting cytochrome c release from mitochondria or directly binding to cytosolic cytochrome c to prevent activation of the caspases (Greene et al 1995; Creagh and Cotter 1999). Recent studies report Hsp70 prevents cell death by interfering with the ability of cytochrome c and Apaf-1 to recruit procaspase-9 (Beere et al 2000; Saleh et al 2000). Thus, inducible Hsps might be regulating apoptosis through a variety of mechanisms in heat-stressed Hsf1+/+ MEFs.
A recent study by Nakai et al (2000) found that transgenic mice constitutively expressing an active form of human Hsf1 had apoptotic spermatocytes and experienced male infertility. These data appear counterintuitive to our findings, suggesting that active Hsf1 results in apoptosis, whereas we conclude Hsf1 expression is essential for protection from heat-induced cell death. Considering that activation of Hsf1 is tightly regulated within cells, the apoptosis seen by Nakai et al may be due to inappropriate constitutive Hsf1 activation in these transgenic mice. Constitutively active Hsf1 could result in expression of normally stress-inducible genes at sensitive stages of spermatogenic differentiation, resulting in apoptosis. Unfortunately, Nakai et al are not able to link the germ cell apoptosis to expression of any specific Hsps. Further studies with meiotic germ cells and mitotic MEFs are likely to reveal significant differences in how Hsps function in these diverse cell types.
Varying expression levels of Hsps corresponding to particular phases of the cell cycle have been reported (Milarski and Morimoto 1986; Milarski et al 1989; Zhu et al 1997; Nakamura et al 1999), suggesting Hsps might modulate cell cycle. Milarski and Morimoto and Milarski et al demonstrated that the synthesis, intracellular distribution, and protein-protein associations of stress-inducible human Hsp70 are tightly controlled during the cell cycle. Overexpression of Hsp70 also controlled the duration of the G2 arrest during doxorubicin treatment in murine fibrosarcoma WEHI-S cells (Karlseder et al 1996). Zhu et al (1997) characterized interactions between Hsp70-2 and CDC2 and determined that Hsp70-2 was required for formation of CDC2/cyclin B1, CDC2 kinase activity, and progression through the G2/M meiotic transition. The experimental results we have presented herein demonstrate that Hsf1 protects cells from heat by directing transient expression of Hsps and a G2/M cell cycle arrest. This cell cycle arrest was seen only when a conditioning dose of heat was administered 6 hours before a lethal heat shock, causing inducible Hsps to be present during lethal heat exposure. Cell cycle arrest did not occur following conditioning heat exposure only. This demonstrates that along with the expression of inducible Hsps other cellular events associated with the lethal heat stress must take place for G2/M arrest to occur. Also, the cell cycle arrest is transient, since G2/M-arrested Hsf1+/+ fibroblasts resumed cycling within 64 hours. In the absence of Hsf1, there was no inducible Hsp expression or G2/M arrest and the cells died. Since most Hsf1−/− cells are not viable and are nonadherent following treatment, it is highly likely that the lack of a G2/M block is due to an inability to reenter the cell cycle. Constitutive expression of Hsf1 in the aforementioned transgenic mice may have a similar effect, causing meiotic arrest at G2/M in spermatocytes (Nakai et al 2000). However, in the testis of Hsf1 transgenics, G2/M-arrested spermatocytes do not recover and become apoptotic, similar to what has been reported in hsp70-2 gene knockout mice (Dix et al 1996, 1997). One possible explanation of these results is that meiotic spermatocytes in the testis are less tolerant of a G2/M arrest, whereas mitotic fibroblasts growing in vitro abide the delay and take advantage of the opportunity to prepare for reinitiation of the cell cycle.
Hyperthermia likely damages many proteins in the cell, requiring Hsf1/Hsp protection from the resulting stress. Our findings indicate that the transient G2/M arrest mediated by Hsf1 appears to act as a “cellular brake” in thermotolerant Hsf1+/+ cells, allowing time for cellular recovery and repair. This G2/M delay may be due to inducible Hsps binding essential cell cycle protein complexes (ie, cyclins/cyclin-dependent kinases) and affecting a cell cycle arrest. Alternately, denatured proteins may deplete inducible Hsps from necessary cell cycle protein complexes. Once the environmental stress and its effects are no longer a threat, inducible Hsps may also play a role in the resumption of the cell cycle. It is also possible that the observed G2/M arrest is a consequence of surviving the lethal heat shock and not responsible for it. Further studies with the Hsf1+/+ and Hsf1−/− cells will include investigations into the protein-protein interactions of Hsps during a stress response that affect the cell cycle and recovery process. Further identification of genes whose expression is dependent on or affected by Hsf1 will also be useful for defining cell cycle and cell death mechanisms affected by the stress response.
Acknowledgments
The information in this document has been funded in part by the US Environmental Protection Agency. It has been subjected to review by the National Health and Environmental Effects Research Laboratory and approved for publication. Approval does not signify that the contents reflect the views of the agency nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
REFERENCES
- Beere HM, Wolf BB, and Cain K. et al. 2000 Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2:469–475. [DOI] [PubMed] [Google Scholar]
- Bellman K, Jaattela M, Wissing D, Burkart V, Kolb H. Heat shock protein hsp70 overexpression confers resistance against nitric oxide. FEBS Lett. 1996;391:185–188. doi: 10.1016/0014-5793(96)00730-2. [DOI] [PubMed] [Google Scholar]
- Buzzard KA, Giaccia AJ, Killender M, Anderson RL. Heat shock protein 72 modulates pathways of stress-induced apoptosis. J Biol Chem. 1998;273:17147–17153. doi: 10.1074/jbc.273.27.17147. [DOI] [PubMed] [Google Scholar]
- Chu B, Zhong R, Soncin F, Steveson MA, Calderwood SK. Transcriptional activity of heat shock factor 1 at 37°C is repressed through phosphorylation on two distinct serine residues by glycogen synthase kinase 3α and protein kinases Cα and Cζ. J Biol Chem. 1998;273:18640–18646. doi: 10.1074/jbc.273.29.18640. [DOI] [PubMed] [Google Scholar]
- Cotto JJ, Kline M, Morimoto RI. Activation of heat shock factor 1 DNA binding precedes stress-induced serine phosphorylation: evidence for a multistep pathway of regulation. J Biol Chem. 1996;271:3355–3358. doi: 10.1074/jbc.271.7.3355. [DOI] [PubMed] [Google Scholar]
- Creagh EM, Cotter TG. Selective protection by hsp70 against cytotoxicdrug-, but not Fas-induced T-cell apoptosis. Immunology. 1999;97:36–44. doi: 10.1046/j.1365-2567.1999.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix DJ, Allen JW, Collins BW, Mori C, Nakamura N, Poorman-Allen P, Goulding EH, Eddy EM. Targeted gene disruption of Hsp70–2 results in failed meiosis, germ cell apoptosis, and male infertility. Proc Natl Acad Sci U S A. 1996;93:3264–3268. doi: 10.1073/pnas.93.8.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix DJ, Allen JW, and Collins BW. et al. 1997 Hsp70–2 is required for desynapsis of synaptonemal complexes during meiotic prophase in juvenile and adult mouse spermatocytes. Development. 124:4595–603. [DOI] [PubMed] [Google Scholar]
- Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- Feige U, Morimoto R, Yahara I, and Polla B. eds. 1996 Stress-Inducible Cellular Responses. Birkhauser, Basel. [PubMed] [Google Scholar]
- Fuse T, Yamada K, Asai K, Kato T, Nakanishi M. Heat shock-mediated cell cycle arrest is accompanied by induction of p21 CKI. Biochem Biophys Res Commun. 1996;225:759–763. doi: 10.1006/bbrc.1996.1247. [DOI] [PubMed] [Google Scholar]
- Gabai VL, Meriin AB, Yaglom JA, Volloch VZ, Sherman MY. Role of Hsp70 in regulation of stress-kinase JNK: implications in apoptosis and aging. FEBS Lett. 1998;438:1–4. doi: 10.1016/s0014-5793(98)01242-3. [DOI] [PubMed] [Google Scholar]
- Greene LE, Zinner R, Naficy S, Eisenberg E. Effect of nucleotide on the binding of peptides to 70-kDa heat shock protein. J Biol Chem. 1995;270:2967–2973. doi: 10.1074/jbc.270.7.2967. [DOI] [PubMed] [Google Scholar]
- Jaattela M. Overexpression of major heat shock protein hsp70 inhibits tumor necrosis factor-induced activation of phospholipase A2. J Immunol. 1993;151:4286–4294. [PubMed] [Google Scholar]
- Kabakov A, Gabai V 1997 Heat Shock Proteins and Cytoprotection: ATP-Deprived Mammalian Cells. Landes, Austin, TX. [Google Scholar]
- Kampinga HH. Thermotolerance in mammalian cells: protein denaturation and aggregation, and stress proteins. J Cell Sci. 1993;104:11–17. doi: 10.1242/jcs.104.1.11. [DOI] [PubMed] [Google Scholar]
- Karlseder J, Wissing D, Holzer G, Orel L, Sliutz G, Aueer H, Jaattela M, Simon MM. Hsp70 overexpression mediates the escape of a doxorubicin-induced G2 cell cycle arrest. Biochem Biophys Res Comm. 1996;220:153–159. doi: 10.1006/bbrc.1996.0373. [DOI] [PubMed] [Google Scholar]
- Kim Y-M, de Vera ME, Watkins SC, Billiar TR. Nitric oxide protects cultured rat hepatocytes from tumor necrosis factor-α-induced apoptosis by inducing heat shock protein 70 expression. J Biol Chem. 1997;272:1402–1411. doi: 10.1074/jbc.272.2.1402. [DOI] [PubMed] [Google Scholar]
- Kwak H-J, Jun C-D, and Pae H-O. et al. 1998 The role of inducible 70-kDa heat shock protein in cell cycle control, differentiation, and apoptotic cell death of the human myeloid leukemic HL-60 cells. Cell Immunol. 187:1–12. [DOI] [PubMed] [Google Scholar]
- Li GC, Werb Z. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc Natl Acad Sci U S A. 1982;79:3218–3222. doi: 10.1073/pnas.79.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillian DR, Xiao X, Shao L, Graves K, Benjamin IJ. Targeted disruption of heat shock transcription factor 1 abolishes thermotolerance and protection against heat-inducible apoptosis. J Biol Chem. 1998;273:7523–7528. doi: 10.1074/jbc.273.13.7523. [DOI] [PubMed] [Google Scholar]
- Mestril R, Giordano FJ, Conde AG, Dillmann WH. Adenovirus-mediated gene transfer of a heat shock protein (hso70i) protects against simulated ischemia. J Mol Cell Cardiol. 1996;28:2351–2358. doi: 10.1006/jmcc.1996.0228. [DOI] [PubMed] [Google Scholar]
- Milarski KL, Morimoto RI. Expression of human Hsp70 during the synthetic phase of the cell cycle. Proc Natl Acad Sci U S A. 1986;83:9517–9521. doi: 10.1073/pnas.83.24.9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milarski KL, Welch WJ, Morimoto RI. Cell cycle-dependent association of Hsp70 with specific cellular proteins. J Cell Biol. 1989;108:413–423. doi: 10.1083/jcb.108.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI. Cells in stress: transcriptional activation of heat shock genes. Science. 1993;259:1409–1410. doi: 10.1126/science.8451637. [DOI] [PubMed] [Google Scholar]
- Moseley PL. Heat shock proteins and heat adaptation of the whole organism. J Appl Physiol. 1997;83:1413–1417. doi: 10.1152/jappl.1997.83.5.1413. [DOI] [PubMed] [Google Scholar]
- Mosser DD, Caron AW, Bourget L, Denis-Larose C, Massie B. Role of the heat shock protein hsp70 in protection against stress-induced apoptosis. Mol Cell Biol. 1997;17:5317–5327. doi: 10.1128/mcb.17.9.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai A, Suzuki M, Tanabe M. Arrest of spermatogenesis in mice expressing an active heat shock transcription factor 1. EMBO J. 2000;19:1545–1554. doi: 10.1093/emboj/19.7.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Tatuno I, Noguchi Y, Kitagawa M, Kohn LD, Saito Y, Hirai A. 73-kDa heat shock cognate protein interacts directly with P27Kip1, a cyclin-dependent kinase inhibitor, during G1/S transition. Biochem Biophys Res Commun. 1999;257:340–343. doi: 10.1006/bbrc.1999.0442. [DOI] [PubMed] [Google Scholar]
- Nitta M, Okamura H, Aizawa S, Yamaizumi M. Heat shock induces transient p53-dependent cell cycle arrest at G1/S. Oncogene. 1997;15:561–568. doi: 10.1038/sj.onc.1201210. [DOI] [PubMed] [Google Scholar]
- Nollen EA, Brunsting JF, Roelofsen H, Weber LA, Kampinga HH. In vivo chaperone activity of heat shock protein 70 and thermotolerance. Mol Cell Biol. 1999;19:2069–2079. doi: 10.1128/mcb.19.3.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polla BS, Kantengwa S, Francois D, Salvioli S, Franceschi C, Marsac C, Cossarizza A. Mitochondria are selective targets for the protective effects of heat shock against oxidative injury. Proc Natl Acad Sci U S A. 1996;93:6458–6463. doi: 10.1073/pnas.93.13.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh A, Srinivasula SM, Balkir L, Robbins PD, Alnemri ES. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat Cell Biol. 2000;2:476–483. doi: 10.1038/35019510. [DOI] [PubMed] [Google Scholar]
- Samali A, Cotter TG. Heat shock proteins increase resistance to apoptosis. Exp Cell Res. 1996;223:163–170. doi: 10.1006/excr.1996.0070. [DOI] [PubMed] [Google Scholar]
- Shackelford RE, Kaufmann WK, and Paules RS 1999 Cell cycle control, checkpoint mechanisms, and genotoxic stress. Environ Health Perspect. 107(Suppl 1). 5–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MM, Reikerstorfer A, Schwarz A, Krone C, Luger TA, Jaattela M, Schwarz T. Heat shock protein 70 overexpression affects the response to ultraviolet light in murine fibroblasts: evidence for increased cell viability and suppression of cytokine release. J Clin Invest. 1995;95:926–933. doi: 10.1172/JCI117800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A, Anderson RL. Bcl-2 and thermotolerance cooperate in cell survival. Cell Growth Differ. 1995;6:799–805. [PubMed] [Google Scholar]
- Subjeck JR, Sciandra JJ, Johnson RJ. Heat shock proteins and thermotolerance: a comparison of induction kinetics. Br J Radiol. 1982;55:579–584. doi: 10.1259/0007-1285-55-656-579. [DOI] [PubMed] [Google Scholar]
- Takayana S, Bimston DN, Matsuzawa S, Freeman BC, Aime-Sempe C, Xie Z, Morimoto RI, Reed JC. BAG-1 modulates the chaperone activity of Hsp70/Hsc70. EMBO J. 1997;16:4887–4896. doi: 10.1093/emboj/16.16.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbles LA, Woodgett JR. The stress-activated protein kinase pathways. Cell Mol Life Sci. 1999;55:1230–1254. doi: 10.1007/s000180050369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. Heat shock transcription factors: structure and regulation. Annu Rev Cell Dev Biol. 1995;11:441–469. doi: 10.1146/annurev.cb.11.110195.002301. [DOI] [PubMed] [Google Scholar]
- Xia W, Voellmy R. Hyperphosphorylation of heat shock transcription factor 1 is correlated with transcriptional competence and slow dissociation of active factor trimers. J Biol Chem. 1997;272:4094–4102. doi: 10.1074/jbc.272.7.4094. [DOI] [PubMed] [Google Scholar]
- Xiang W, Rensing L. Changes in cell morphology and actin organization during heat shock in Dictyostelium discoideum: does Hsp70 play a role in acquired themotolerance? FEMS Microbiol Lett. 1999;178:95–107. doi: 10.1111/j.1574-6968.1999.tb13764.x. [DOI] [PubMed] [Google Scholar]
- Xiao X, Zuo X, Davis AA, McMillan DR, Curry BB, Richardson JA, Benjamin IJ. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J. 1999;18:5943–5952. doi: 10.1093/emboj/18.21.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Dix DJ, Eddy EM. Hsp70–2 is required for CDC2 kinase activity in meiosis I of mouse spermatocytes. Development. 1997;124:3007–3014. doi: 10.1242/dev.124.15.3007. [DOI] [PubMed] [Google Scholar]