Abstract
The question of how animals process stimulus mixtures remains controversial as opposing views propose that mixtures are processed analytically, as the sum of their elements, or holistically, as unique entities different from their elements. Overshadowing is a widespread phenomenon that can help decide between these alternatives. In overshadowing, an individual trained with a binary mixture learns one element better at the expense of the other. Although element salience (learning success) has been suggested as a main explanation for overshadowing, the mechanisms underlying this phenomenon remain unclear. We studied olfactory overshadowing in honeybees to uncover the mechanisms underlying olfactory-mixture processing. We provide, to our knowledge, the most comprehensive dataset on overshadowing to date based on 90 experimental groups involving more than 2700 bees trained either with six odourants or with their resulting 15 binary mixtures. We found that bees process olfactory mixtures analytically and that salience alone cannot predict overshadowing. After normalizing learning success, we found that an unexpected feature, the generalization profile of an odourant, was determinant for overshadowing. Odourants that induced less generalization enhanced their distinctiveness and became dominant in the mixture. Our study thus uncovers features that determine odourant dominance within olfactory mixtures and allows the referring of this phenomenon to differences in neural activity both at the receptor and the central level in the insect nervous system.
Keywords: olfaction, olfactory learning, mixture processing, overshadowing, odourant dominance, honeybees
1. Introduction
Overshadowing [1–4] is a phenomenon that has stirred up the attention of disciplines as diverse as experimental psychology, neuroscience, ecology and animal behaviour. In overshadowing, an animal trained with a mixture composed of two stimuli learns one stimulus better (the overshadowing stimulus) at the expense of the other (the overshadowed stimulus), and its response to the overshadowed component is lower than that obtained when this component is trained alone. This phenomenon can be found from invertebrates to humans, in a variety of sensory modalities and behavioural contexts (spiny lobsters [5], fruit flies [6], honeybees [7–11], fishes [12], toads [13], pigeons [14,15], nutcrackers [16], rats [17–19], rabbits [20], dogs [2], monkeys [21] and humans [22–25]).
Traditionally, overshadowing has been attributed to stimulus salience, which is accessible through the success with which an individual learns about a stimulus [22,26]. Salient stimuli, which have better learning rates when trained alone, would also be learned better within a mixture, in detriment of less salient ones. This salience can be related to the physical properties of the stimuli or to the animals' perceptual characteristics. For instance, it may refer to the physical intensity of stimulation or can result from the animal's sensitivity. The search for specific features that define stimulus strength and allow prediction of overshadowing is a fundamental task for understanding mixture processing and learning.
Here we studied overshadowing in the olfactory modality in the honeybee Apis mellifera. We focused on olfaction because odourants play an essential role in the life of a bee [27,28] and because olfactory learning and processing can be studied in bees using the olfactory conditioning of the proboscis extension response (PER) [29,30]. A prior study using this protocol showed that the learning efficiency of single odourants does not account for odourant dominance in complex-mixture perception by bees. As a consequence, the features determining this dominance remained unclear [31]. We thus aimed at identifying the mechanisms underlying olfactory overshadowing to understand what makes a dominant odourant and how bees process and learn olfactory mixtures.
2. Material and methods
Naive, harnessed bees were trained to respond with PER to an odourant (the conditioned stimulus, CS) paired with sucrose solution (the unconditioned stimulus, US) delivered to the antennae and proboscis [29,30]. We trained bees with six single odourants (referred to as ‘elements’ in this paper, table 1) that varied systematically in their chain length (8 or 9 carbons) and functional group (alcohols, aldehydes and ketones) (see the electronic supplementary material, figure S1a,b), two parameters that define a putative olfactory space in honeybees [32], or with the 15 possible binary mixtures made of these odour components (see the electronic supplementary material, figure S1c,d). For every group trained with a mixture AB (overshadow, OVS group), two control groups were trained with the respective components A or B (Ctrl A and Ctrl B groups). Ten minutes after conditioning, all three groups were tested with A, B and AB in a random order. As the amount of experience with a mixture may strongly influence overshadowing [33], different groups of bees were subjected to one or to three conditioning trials with each binary mixture or component. Overall, our dataset encompasses the performance of 90 experimental groups involving more than 2700 bees trained either to a mixture or a single component, and then tested with the mixture and both components. More details about the materials and procedures used, including statistics, are available in the electronic supplementary material, Methods section.
Table 1.
Characteristics of the six odourants used. (The odourants are listed according to their functional group (aldehydes, secondary ketones and secondary alcohols). Purity (gas chromatograph measurements, GC (commercial description of the product)), vapour pressure values (VP), dilution quantities in mineral oil and pheromone action of the odourants are given. +, repels bees at the hive entrance, releases stinging, encourages foraging activity; −, no action.)
| odourant | purity (%) | vapour pressure (mm Hg; 25°C) | dilution (in 1 ml) odourant/mineral oil (µl) | pheromone action |
|---|---|---|---|---|
| octanal | 100 | 1.18 | 8.5/991.5 | − |
| nonanal | 95.0 | 0.37 | 27.0/973.0 | − |
| 2-octanone | 97.0 | 1.35 | 7.4/992.6 | − |
| 2-nonanone | 99.0 | 0.62 | 16.0/984.0 | − |
| 2-octanol | 97.8 | 0.24 | 41.7/958.3 | − |
| 2-nonanol | 99.0 | 0.07 | 147.9/852.1 | + |
3. Results
(a). Bees did not weight all odourant components similarly upon mixture learning
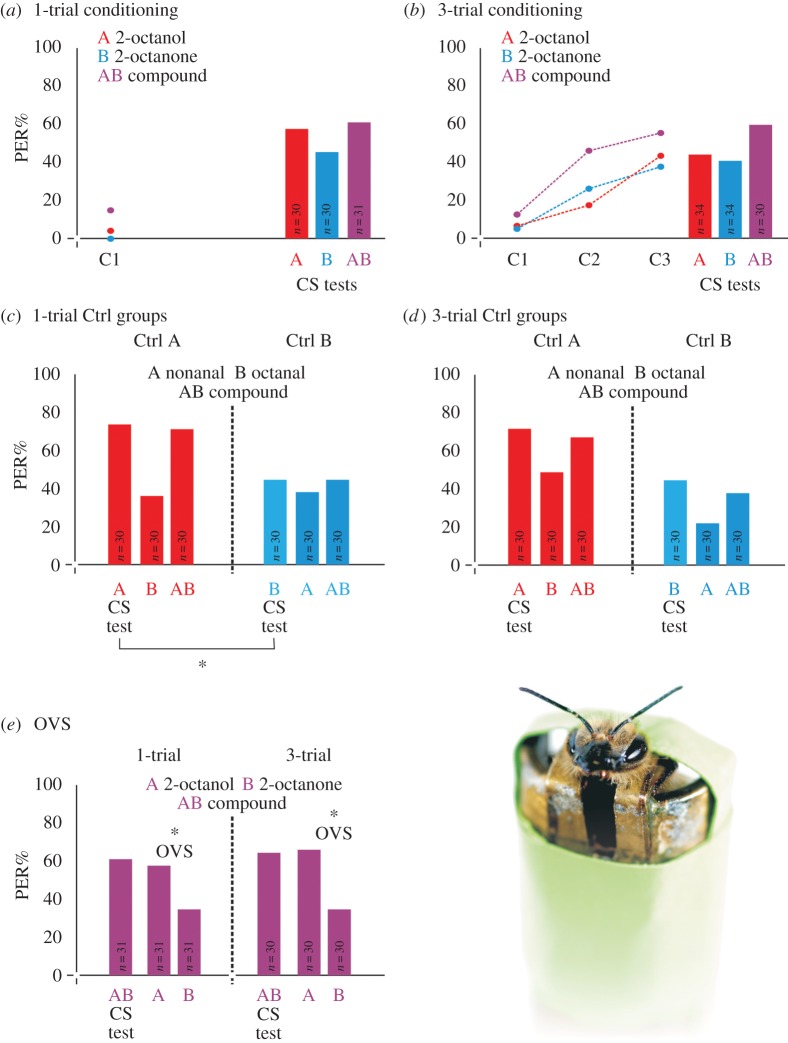
Under both training regimes (1- or 3-trial conditioning), bees learned to respond to all single odourants and mixtures used as the CS (see the electronic supplementary material, figure S1). Figure 1a,b shows an example of how bees learned their corresponding CS and how they responded to it in the subsequent test (‘CS test’) after one or three conditioning trials. The response values (% of PER) to A, B and AB of the 90 conditioned groups are shown in the electronic supplementary material, table S1.
Figure 1.
Conditioning and testing of individual odourants and binary mixtures. (a) 1-trial conditioning. The graph shows the proboscis extension responses (PER%) to the odourant conditioned in the single conditioning trial (C1) and in the tests (CS test); one of the 15 odour combinations included in the study is shown as example (A: 2-octanol in red, B: 2-octanone in blue and their binary mixture AB in magenta). Sample sizes (n) of each conditioned group are indicated within the test bars. (b) 3-trial conditioning. Same as in (a) but for the conditioning with three trials C1, C2 and C3 (intertrial interval of 10 min). (c) Test responses of the control groups Ctrl A (red bars) and Ctrl B (blue bars) after 1-trial conditioning. In this example, another odour combination included in the study is shown as example (A, nonanal; B, octanal; and their binary mixture AB). Only test responses are shown. The CS test responses differed between Ctrl groups; responses to A of the Ctrl A group (red bars) were higher than those to B of the Ctrl B group, thus revealing a higher salience of A compared with B. Generalization responses of the Ctrl A group to the unconditioned odourant B were low, but the generalization to the unconditioned mixture AB was as high as the test response to the CS A itself. In the Ctrl B group (blue bars), generalization to the unconditioned odourant A and to the mixture AB were comparable to the response to the CS B. (d) Test responses of the control groups Ctrl A (red bars) and Ctrl B (blue bars) after 3-trial conditioning. Same as in (c) but after three conditioning trials. In this case, generalization responses of the Ctrl A group showed the same pattern as those in the 1-trial experiment. Generalization responses of the Ctrl B group to the unconditioned odourant A decreased. (e) An example of overshadowing after 1-trial (left) and 3-trial (right) conditioning. Overshadowing is illustrated by the combination of A, 2-octanol, and B, 2-octanone. Only the test responses of the overshadowing group (OVS) trained to the AB mixture are depicted. The generalization test responses to the single components A and B reveal a higher response rate for element A than for B, promoting thereby a significant overshadowing effect both after 1-trial and 3-trial conditioning.
The performance of bees trained with the single odourants A or B (groups Ctrl A or Ctrl B, respectively) confirmed that some odourants were learned better than others as they elicited a higher level of responses in the tests (see e.g. figure 1c). Henceforth, within a pair of odourants we attributed the label ‘A’ to the more salient odourant and ‘B’ to the less salient one. Note that a given odourant could be A in a particular mixture and B in another one. Salience values (average conditioned responses attained by each odour component in the CS test) are shown in the electronic supplementary material, figure S2 both for 1- and 3-trial conditioning. Responses differed between odourants (F5,5 = 16.49, p < 0.0001) but were consistent after 1- and 3-trial conditioning (F1,5 = 0.08, p = 0.80). Interestingly, the salience of elements carried over to the binary mixtures as mixtures of more salient odours were more salient than mixtures of less salient odours (see the electronic supplementary material, figure S1c,d).
(b). Odourant components are accessible to bees within an olfactory mixture
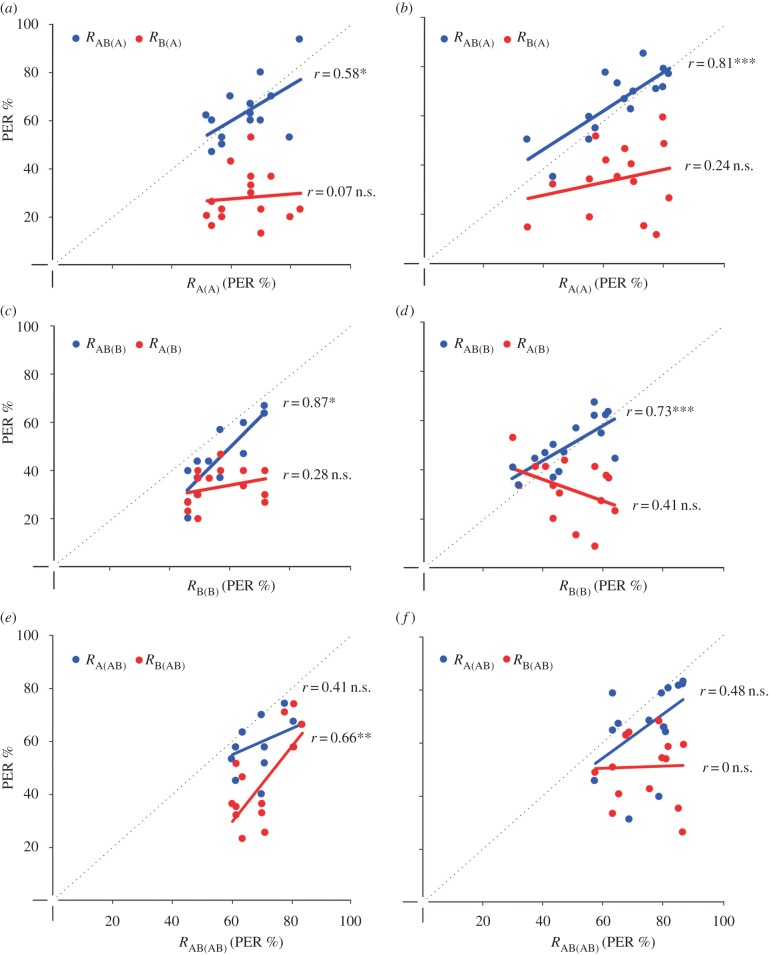
To determine whether bees recognize the presence of odour components in an olfactory mixture, we focused on the performance of the groups trained with either A or B alone (Ctrl A and Ctrl B) and analysed whether their responses to the mixture AB (RAB(A) and RAB(B), respectively, where RY(X) is the response to stimulus Y after training to stimulus X) correlated with those to the trained element (RA(A) or RB(B)).
Responses to the mixture after odourant-component learning were always positively correlated with responses to the learned odourant component. This was true after 1-trial conditioning both for the more salient (figure 2a, RAB(A), Pearson correlation, p < 0.05) and the less salient odour (figure 2c, RAB(B), p < 0.01) and after 3-trial conditioning (figure 2b, RAB(A), p < 0.01; figure 2d, RAB(B), p < 0.01). No correlation was found between the responses to the trained and the non-trained odourant components (1-trial conditioning: figure 2a,c, RB(A) and RA(B), non-significant in both cases; 3-trial conditioning: figure 2b,d, RB(A) and RA(B), non-significant in both cases). In other words, after having learned a single odourant, bees recognize it in the mixture and respond to the latter as if it were similar to the learned odourant. The presence of a non-learned odourant component in the mixture is irrelevant as the mixture contains the component that is sufficient to predict the reward.
Figure 2.
Correlation between the responses to a conditioned odourant (abscissa) and to non-conditioned odourants (ordinate) after 1-trial (a,c,e) and 3-trial conditioning (b,d,f). (a,b) Correlation between the responses to the conditioned odourant A (RA(A)) and the responses to the non-conditioned odourants B (RB(A); red dots) and AB (RAB(A); blue dots) after conditioning to A. (c,d) Correlation between the responses to the conditioned odourant B (RB(B)) and the responses to the non-conditioned odourants A (RA(B); red dots) and AB (RAB(B); blue dots) after conditioning to B. (e,f) Correlation between the responses to the conditioned mixture AB (RAB(AB)) and the responses to the non-conditioned odourants A (RA(AB); blue dots) and B (RB(AB); red dots) after conditioning to AB. The diagonal dotted line indicates equal responses on each axis. Owing to identical measurement values some correlation plots show less than 15 data points per experimental group. n.s., non-significant; *p < 0.05, **p < 0.01, ***p < 0.005.
Can bees recognize an odourant component after having learned a mixture? How does element salience affect this response? To answer these questions we analysed the responses of the OVS groups (trained with the mixture AB). When presented with the single odourants, bees of the OVS groups responded in some cases significantly more to one odourant than to the other, thus revealing an overshadowing effect (see red cells in the electronic supplementary material, table S1). For example, bees trained to the mixture of 2-octanol and 2-octanone (see figure 1e) had a response of 58% to 2-octanol (A) and 36% to 2-octanone (B) after one conditioning trial, and of 67% and 37%, respectively, after three conditioning trials, showing that 2-octanol overshadowed 2-octanone.
After one conditioning trial, responses to the mixture did not correlate with responses to the stronger component A (figure 2e, RA(AB), Pearson correlation: non-significant) but rather correlated with responses to the weaker component B (figure 2e, RB(AB), p < 0.01, respectively). Thus, when experience was low, odourant salience was not the decisive factor guiding the bees’ responses. After three conditioning trials, the response to the weaker component lost its predictive power (figure 2f, RB(AB), non-significant) while that to the stronger component induced a marginally non-significant correlation (figure 2f, RA(AB), 0.05 < p < 0.1). In other words, after learning an olfactory mixture, bees responded to the components but this generalization was, at the beginning, not based on component salience. With increasing experience, however, generalization from the mixture to the less salient component disappeared.
Taken together, the results of figure 2 show that both odourants A and B, are accessible to bees in a binary olfactory mixture, either after having learned a single odourant, A or B, or a mixture AB. Yet performances in these two situations are not symmetric: when a bee has learned a single odourant, she identifies its presence in a blend and bases her responses to the blend on this known odourant (figures 2a–d); reciprocally, when a bee has learned a blend and is afterwards confronted with the incomplete information of a single odourant, her choices are less clear as they do not seem to strictly follow element salience, at least with less experience (figure 2e,f).
(c). Increased mixture experience induces more overshadowing
How does experience affect overshadowing? After one conditioning trial, we found overshadowing in six out of 15 groups trained with the mixtures (see red cells and statistics in the electronic supplementary material, table S1; McNemar test, p < 0.05 in all six cases). After three conditioning trials, nine cases showed overshadowing in the 15 OVS groups (see red cells and statistics in the electronic supplementary material, table S1; McNemar test, p < 0.05 in all nine cases). These included the same six mixtures that showed overshadowing after 1-trial conditioning plus three novel cases. Thus, 3-trial conditioning induced more cases of overshadowing than single-trial conditioning. However, experience did not change odourant dominance: whenever a tendency to respond more to one odour component was found after 1-trial conditioning, it was accentuated after 3-trial conditioning. Close examination of dominant odourants showed a prevalence of 9-carbon odourants over 8-carbon odourants (see the electronic supplementary material, table S1, 1-trial conditioning: cases I, IX, X and XI; and 3-trial conditioning: cases I, II, III, IX, X and XI). More specifically, 2-nonanol generally dominated over the other odourants (electronic supplementary material, table S1, 1-trial conditioning: cases X and XI; and 3-trial conditioning: cases II, X, XI and XV).
These results indicate that when bees search for an olfactory mixture containing a more salient odourant, they focus more on this odourant with increasing experience.
(d). Odourant salience does not fully account for overshadowing
Is overshadowing fully explained by the presence of an odourant with higher salience? After 1-trial conditioning, we found no direct relationship between the occurrence of overshadowing (see red cells in the electronic supplementary material, table S1) and the fact that one odourant was learned significantly better than the other when trained alone (see blue cells in the electronic supplementary material, table S1). From the six overshadowing cases found, only one corresponded to a difference in salience between the odourants (2-nonanol and octanal; χ2 = 11.92, p < 0.025; see the electronic supplementary material, table S1). Two other cases were found in which differences in salience existed between odourants (nonanal and 2-octanone, nonanal and octanal; and χ2 = 10.00 and χ2 = 5.55, respectively, p < 0.025 in both cases); yet, no overshadowing occurred when these odourants were presented in a mixture (χ2 = 0.07 and χ2 = 2.29, respectively, non-significant in both cases). After 3-trial conditioning, from the nine overshadowing cases found, only two corresponded to a difference in odourant salience (2-nonanol and 2-octanone, and 2-nonanol and 2-octanol; χ2 = 15.43 and χ2 = 9.54, respectively, p < 0.025 in both cases). Thus, significant differences in odourant salience do not always support overshadowing. A correspondence between both situations was only found in 1 of 6 (1-trial conditioning) and 2 of 9 (3-trial conditioning) overshadowing cases.
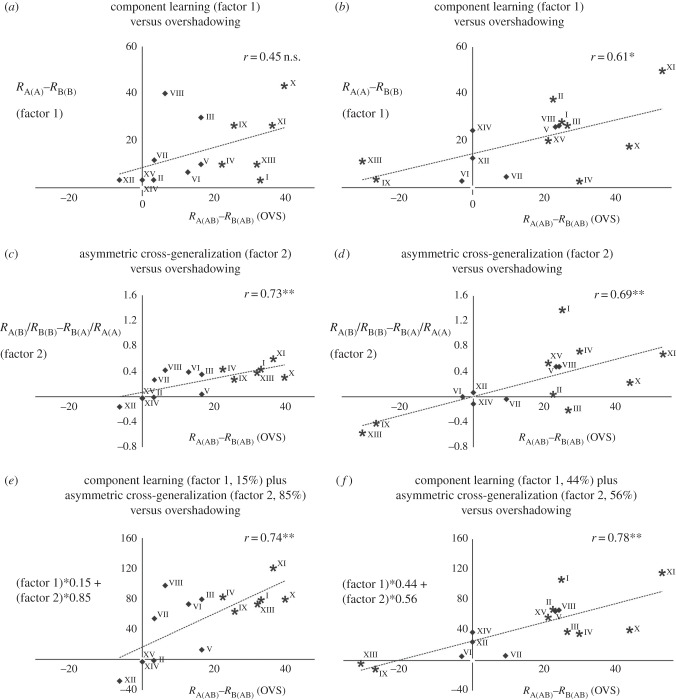
To provide a finer analysis of the power of component salience for predicting overshadowing, we quantified the amount of overshadowing within each of the 15 OVS groups as RA(AB)−RB(AB) (figure 3a, left). The difference in component salience, measured in the respective Ctrl groups, was quantified as RA(A)−RB(B) (figure 3a, right). After one conditioning trial, no significant correlation was found between both variables (r = 0.45, p > 0.05, non-significant, figure 4a); conversely, a significant correlation was found after three conditioning trials (r = 0.61, p < 0.05, figure 4b). These results show that differences in odourant salience do not fully predict overshadowing, especially when experience is reduced. Yet, they become more predictive with increasing experience, thus showing that learning contributes to overshadowing.
Figure 3.
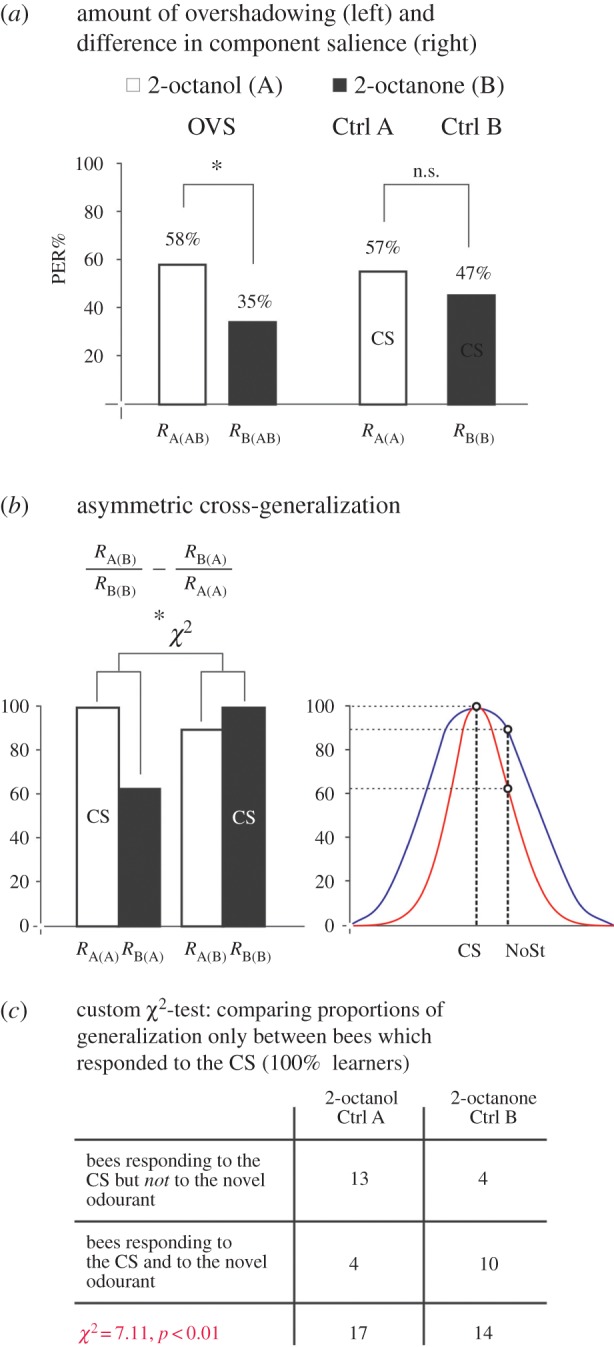
Quantitative analysis of the overshadowing effect (a) and of asymmetric cross-generalization (b,c) after a 1-trial conditioning. (a) The overshadowing effect. Test responses to the single odourants (A: 2-octanol, B: 2-octanone) after mixture conditioning in the OVS group (RA(AB) and RB(AB)) and test responses to the component after component conditioning in Ctrl A (RA(A)) and Ctrl B (RB(B)). The results show that the significant overshadowing effect (OVS group) is not correlated with a significant difference between the learning levels of the two odourants. n.s., non-significant; *p < 0.05. (b) The asymmetric cross-generalization effect. The asymmetric cross-generalization was calculated as RA(B)/RB(B) − RB(A)/RA(A). Only bees responding to the CS (A in Ctrl A and B in Ctrl B) were used for this analysis; thus, their response levels are set to 100% (left panel, CS bars). The asymmetric cross-generalization found could be explained by differences in the generalization profiles of the two odourants (right panel). The element with a narrow generalization profile (red line) show less generalization to a novel stimulus (NoSt) than the element with a broader generalization profile (blue line). (c) The 2 × 2 table used for statistical analysis (χ2) of the asymmetric cross-generalization. The table presents in the first row the number of bees responding to the CS but not to the novel odourant and in the second row the number of bees responding to both the CS and the novel odourant in the Ctrl A and Ctrl B groups.
Figure 4.
Correlation analyses between the overshadowing effect (OVS; abscissa) and the component-learning effect (a,b), the asymmetric cross-generalization effect (c,d) and the combination of both effects (e,f) (ordinates) for all odour combinations used (I-XV; see the electronic supplementary material, table S1, for more details) after 1-trial conditioning (a,c,e) and 3-trial conditioning (b,d,f). (a,b) Correlation between the overshadowing effect (RA(AB)−RB(AB); OVS) and the differences in component learning (RA(A)−RB(B); factor 1). (c,d) Correlation between the overshadowing effect and the differences in asymmetric cross-generalization (RA(B)/RB(B)−RB(A)/RA(A); factor 2). (e,f) Correlation between the overshadowing effect and a combination of both factors 1 and 2 differentially weighted (best models in multiple regressions). Diamonds represent non-significant data points; stars represent significant cases of overshadowing. n.s., non-significant; *p < 0.05, **p < 0.01.
(e). The generalization profile of an odourant affects odourant dominance
We reasoned that the strength of an odourant could result from its generalization profile. In particular, by analysing the responses of the Ctrl groups trained with the single odourants, we noticed that bees sometimes responded more to odour A after learning odour B (RA(B)) than to B after learning A (RB(A)). This asymmetric cross-generalization [32], which can be quantified through the difference RA(B)/RB(B) − RB(A)/RA(A), appeared even though odour concentrations were equalized according to their vapour pressures, and even when both odourants were learned at the same level (figure 3b). It could constitute a different form of salience as stimuli that are learned equally well but that differ in their generalization profiles induce responses varying in specificity [34,35]: narrow generalization profiles result in more precise odourant identification and may enhance odourant strength while broader generalization profiles would have the opposite effect (figure 3b).
We thus hypothesized that differences in cross-generalization between the single odourants could contribute to overshadowing. To discard influences of odourant salience (i.e. learning level; see above), only generalization responses of those bees that learned to respond to their respective conditioned odourant (i.e. which responded to the CS in the tests) were considered and compared between Ctrl groups (figure 3b). Contrarily to all prior analyses, which used all data available, we restricted our dataset for this analysis, in order to answer the specific question of whether the generalization profile of an odourant influences overshadowing. Figure 3c shows a 2 × 2 contingency table and a χ2 analysis applied to the case of the odourants 2-octanol and 2-octanone. After 1-trial conditioning, 17 bees trained to 2-octanol responded to this alcohol while 14 bees trained to 2-octanone responded to this ketone. Bees trained to 2-octanone generalized more to 2-octanol (10 animals) than bees trained to 2-octanol did it for 2-octanone (four animals), thus resulting in a significant asymmetric cross-generalization (2 × 2 χ12 = 7.11; p < 0.01).
After one conditioning trial, we found no direct relationship between the occurrence of overshadowing (see red cells in the electronic supplementary material, table S1) and asymmetric cross-generalization (see green cells in the electronic supplementary material, table S1). From the six overshadowing cases found, only one exhibited a significant difference in cross-generalization (2-octanol, 2-octanone; χ2 = 7.11, p < 0.01 see the electronic supplementary material, table S1). However, after three conditioning trials, from the nine overshadowing cases found, five showed asymmetric cross-generalization (p < 0.025 in all five cases; see green cells and χ2 values in the electronic supplementary material, table S1). Thus, significant differences in cross-generalization alone do not always result in overshadowing as a correspondence between both situations was only found in one of six (1-trial conditioning) and five of nine (3-trial conditioning) overshadowing cases.
To achieve a finer analysis of the relationship between overshadowing and asymmetric cross-generalization, we quantified this variable in all 15 odourant pairs as RA(B)/RB(B)−RB(A)/RA(A) and analysed whether it correlated or not with the occurrence of overshadowing (calculated as RA(AB)− RB(AB); see above) both for 1- and 3-trial conditioning. In both conditions, the correlation between overshadowing and asymmetric cross-generalization was significant (1-trial conditioning: Pearson's correlation, r = 0.73, p < 0.01, figure 4c; 3-trial conditioning: r = 0.69, p < 0.01, figure 4d), thus showing that asymmetric cross-generalization played a significant role for overshadowing irrespective of experience. We thus conclude that the generalization profiles of odourants play a significant role in mixture learning and processing. Narrower profiles make an odour more discriminable and thus enhance its dominance within an olfactory mixture.
(f). Salience and asymmetric cross-generalization together provide a better account of overshadowing
We performed multiple-regression analyses to define the weight of odourant salience and asymmetric cross-generalization in the best model predicting overshadowing. In the 1-trial conditioning experiment, differences in odourant salience (i.e. learning level) contributed a relative weight of 15% to the best model while asymmetric cross-generalization contributed 85% to the overshadowing effect (based on standardized beta values; see figure 4e: r = 0.74, p < 0.01). Owing to the overruling weight of asymmetric cross-generalization, the combined model does not constitute a significant improvement compared with the model that takes only asymmetric cross-generalization into account (figure 4c: r = 0.73). When the same analysis was performed for the 3-trial conditioning experiment, asymmetric cross-generalization was still the dominant factor as it contributed 56% to the overshadowing effect while differences in odourant salience contributed the remaining 44% (figure 4f: r = 0.78, p < 0.001). In this case, the combined model was better than the one taking only differences in element salience into account (figure 4b: r = 0.61) but was also slightly better than the one considering only asymmetric cross-generalization (figure 4d: r = 0.69).
4. Discussion
This paper shows that bees process and learn binary olfactory mixtures following what can be interpreted as an analytic strategy. They detected the presence of odourant components in the binary mixture and were able to respond to them after mixture training. This response was driven by dominant odours, which induced overshadowing. Odourant salience, measured in terms of learning efficiency, was not the main determinant of this phenomenon. Rather, the effect of cumulative experience and the generalization profile of an odourant appear to play a larger role in this process. When taken together, these two features provide a compelling account of overshadowing.
(a). A novel account of odourant strength
Odourant dominance within complex olfactory blends has been reported in several studies of olfactory processing in bees [9,10,31,36,37]. Yet, the features that define this dominance remained unclear until now so that this phenomenon was thought to be mixture-specific [31,38]. Odourant concentration was decisive in experiments in which bees were trained with ternary mixtures in which one odourant was more concentrated than the other two [31]; the concentrated odourant was more likely to become dominant but this dominance was visible only when the three odourants were presented at low concentrations [31]. In our case, we tried to diminish the impact of odourant concentration by equating vapour pressure between odourants. Odourant dominance was still present and supported the occurrence of overshadowing.
In accordance with a previous study [31], the learning success of an odourant, which is usually considered as a direct readout of its salience [26], could not predict overshadowing when experience was reduced (figure 4a; 1-trial conditioning) but became predictive when experience increased from one to three conditioning trials (figure 4b; 3-trial conditioning). However, when this factor was normalized by considering only the responses of bees that effectively learned to respond to their CS, asymmetric cross-generalization between Ctrl groups emerged as a more reliable predictor of overshadowing in the OVS group.
This finding led us to the discovery that asymmetries in the generalization profiles of the odourants that integrate a mixture are an important factor for the occurrence of overshadowing, irrespectively of the amount of experience (figure 4c,d). We suggest that the coexistence of odourants with narrow and broad generalization profiles within a mixture favours that the former become dominant odourants through an enhancement of their distinctiveness and thus of their recognition within the mixture. This hypothesis may apply to highly complex mixtures made of many components for which neither learning efficiency nor odour structural features could account for odourant dominance [31].
(b). Cognitive and associative interpretations of overshadowing are compatible with our explanation of this phenomenon
Overshadowing has been explained either in purely associative terms or in more cognitive terms. For the associative account [26], there is a limit to the associative strength conditionable by a given reinforcer, and this fixed total must be shared between all stimuli integrating a conditioned mixture. Thus, a salient element will capture the major part of associative strength, and will interfere with conditioning to the other elements. For the cognitive account [39], conditioning requires attention, and if one element of a conditioned mixture captures more attention, it will decrease conditioning to the less salient elements. Both theories provide a straightforward account of overshadowing and are compatible with our findings. If we consider that the associative strength and/or the attentional power of an odourant is affected by its generalization profile, we can explain why certain odourants dominate within a mixture, either by being more effectively associated with the reward or by eliciting more attention. It would be interesting to analyse the validity of our conclusions for other sensory modalities and behavioural contexts by determining to what extent asymmetries in stimulus generalization provide an efficient, general account of overshadowing.
(c). A neural-based explanation of overshadowing
Asymmetries in odourant cross-generalization seem to be a frequent feature of the honeybee olfactory system [32,40] even when vapour pressures and learning levels are normalized as in our work. Asymmetries in olfactory generalization could arise at the level of the molecular olfactory receptors located in single-odourant receptor neurons. These receptors differ in their tuning to specific olfactory ligands [41–43]; some are narrowly tuned to specific odourants while others exhibit a broader response profile [42]. Competition for a binding site can occur in the case of a mixture with structurally similar odourants. Accordingly, syntopic interactions at a single-receptor binding site have been proposed as an explanation for some within-mixture interactions occurring in olfactory mixtures [44].
Differences in odourant specificity can translate from the periphery to the antennal lobe, the primary olfactory centre in the insect brain. Each antennal lobe is composed of glomeruli and odours are encoded as specific spatio-temporal glomerular activation patterns [45]. Glomeruli constitute convergence sites for olfactory receptor neurons, inhibitory and excitatory local interneurons connecting glomeruli laterally, and efferent projection neurons conveying the olfactory message to higher order brain centres. Because there is a correspondence between the number of molecular olfactory receptors and the number of glomeruli in the bee (approx. 166) [46], and each glomerulus receives input from only one class of olfactory receptor neurons expressing the same molecular receptor [47], the difference in responses at the receptor level (see above) may translate to the glomerular level where some odourants may induce glomerular activation patterns narrowly tuned around a main glomerulus whilst other odourants may induce broader activation patterns including more glomeruli. Higher odour concentrations activate more glomeruli than lower concentrations [48,49]. The fact that odourant dominance is more likely in the case of concentrated odourants but only when both overshadowing and overshadowed odourants are presented at lower concentrations [31] indicates that there is an interplay between odourant concentration and the neural activation profile of odourants that needs to be elucidated.
Calcium imaging experiments have shown that inhibition between glomeruli can be asymmetric [50]. In our case, glomeruli activated by odourant A may inhibit glomeruli coding for odourant B, whereas glomeruli coding for odourant B may not inhibit or even excite those coding for odourant A.
Experience-induced modifications of these central responses could also account for asymmetric cross-generalization. Associative olfactory learning induces changes in antennal lobe responses in different insects (e.g. honeybee [51,52], fruit fly [53], moth [54]). If, after learning two different odourants A and B, the glomerular pattern of A becomes more distinct from that of B after A training, but the opposite occurs after B training (i.e. glomerular patterns become more similar), then bees would exhibit less generalization from A to B than from B to A, irrespective of odourant salience, measured in terms of the number and intensities of activated glomeruli.
Finally, higher order processes and structures may play a significant role for the occurrence of overshadowing. For example, the innate salience of an odourant may not be determined at the sensory coding level (i.e. not at the level of the antennal lobe) but may be assigned by the mushroom bodies, which have been proposed to act as an experience-dependent recoding device transforming the highly dimensional sensory coding space into a low-dimensional coding space of value-based information [55].
In conclusion, our study shows that the phenomenon of olfactory overshadowing can be efficiently accounted for by the salience of an odourant (element-learning effect) and by its generalization profile (asymmetric cross-generalization effect). Together, both features explain why some odourants can better compete than others for a major share of attention or associative strength in a mixture and both set the basis for odourant dominance. Future investigations should focus on how neural activity at the peripheral and the central levels accounts for generalization, stimulus dominance and overshadowing within and across sensory modalities.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We are very grateful to four anonymous reviewers for helpful comments and criticisms to the previous version of the manuscript. We also thank O. Najyb and N. Yahi for help with the experiments. M.S. thanks Jochen Erber for support.
Data accessibility
Data available from the Dryad Digital Repository: http://doi.org/10.5061/dryad.fd128.
Funding statement
M.G. acknowledges the support of the Institut Universitaire de France, the French Research Council (CNRS) and the University Paul Sabatier. J.-C.S. thanks the CNRS. M.S. was supported by the Graduiertenkolleg 120 ‘Signal cascades in living systems' (Freie Universität Berlin).
References
- 1.Mackintosh NJ. 1971. An analysis of overshadowing and blocking. Q. J. Exp. Psychol. 23, 118–125. ( 10.1080/00335557143000121) [DOI] [Google Scholar]
- 2.Pavlov IP, Anrep GV. 1927. Conditioned reflexes: an investigation of the physiological activity of the cerebral cortex, vol. xv, 430 p [432] p. of plates p London, UK: Oxford University Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamin LJ. 1968. Attention-like processes in classical conditioning. In Miami symposium predictability, behavior and aversive stimulation (ed Jones MR.), pp. 9–32. Miami, FL: University Miami Press. [Google Scholar]
- 4.Kamin LJ. 1969. Selective association and conditioning. In Fundamental issues in associative learning (ed Honig WK.), pp. 42–64. Halifax, Canada: Dalhousie University Press. [Google Scholar]
- 5.Derby CD, Hutson M, Livermore BA, Lynn WH. 1996. Generalization among related complex odorant mixtures and their components: analysis of olfactory perception in the spiny lobster. Physiol. Behav. 60, 87–95. ( 10.1016/0031-9384(95)02237-6) [DOI] [PubMed] [Google Scholar]
- 6.Brembs B, Heisenberg M. 2001. Conditioning with compound stimuli in Drosophila melanogaster in the flight simulator. J. Exp. Biol. 204, 2849–2859. [DOI] [PubMed] [Google Scholar]
- 7.Couvillon PA, Klosterhalfen S, Bitterman ME. 1983. Analysis of overshadowing in honeybees. J. Comp. Psychol. 97, 154–166. ( 10.1037/0735-7036.97.2.154) [DOI] [Google Scholar]
- 8.Couvillon P, Bitterman M. 1989. Reciprocal overshadowing in the discrimination of color-odor compounds by honeybees: further tests of a continuity model. Learn. Behav. 17, 213–222. ( 10.3758/bf03207637) [DOI] [Google Scholar]
- 9.Pelz C, Gerber B, Menzel R. 1997. Odorant intensity as a determinant for olfactory conditioning in honeybees: roles in discrimination, overshadowing and memory consolidation. J. Exp. Biol. 200, 837–847. [DOI] [PubMed] [Google Scholar]
- 10.Smith BH. 1998. Analysis of interaction in binary odorant mixtures. Physiol. Behav. 65, 397–407. ( 10.1016/S0031-9384(98)00142-5) [DOI] [PubMed] [Google Scholar]
- 11.Couvillon P, Mateo E, Bitterman M. 1996. Reward and learning in honeybees: analysis of an overshadowing effect. Learn. Behav. 24, 19–27. ( 10.3758/bf03198950) [DOI] [Google Scholar]
- 12.Tennant WA, Bitterman ME. 1975. Blocking and overshadowing in two species of fish. J. Exp. Psychol. Anim. Behav. Process. 1, 22–29. ( 10.1037/0097-7403.1.1.22) [DOI] [PubMed] [Google Scholar]
- 13.Daneri MF, Muzio RN. 2013. Blocking and overshadowing in a phylogenetically ancient vertebrate group: amphibians. Rev. Latinoam. Psicol. 45, 185–200. ( 10.14349/rlp.v45i2.1138) [DOI] [Google Scholar]
- 14.Urcuioli PJ, Honig WK. 1980. Control of choice in conditional discriminations by sample-specific behaviors. J. Exp. Psychol. Anim. Behav. Process. 6, 251–277. ( 10.1037/0097-7403.6.3.251) [DOI] [PubMed] [Google Scholar]
- 15.Leising KJ, Garlick D, Blaisdell AP. 2011. Overshadowing between landmarks on the touchscreen and in arena with pigeons. J. Exp. Psychol. Anim. Behav. Process. 37, 488–494. ( 10.1037/a0023914) [DOI] [PubMed] [Google Scholar]
- 16.Gould-Beierle KL, Kamil AC. 1999. The effect of proximity on landmark use in Clark's nutcrackers. Anim. Behav. 58, 477–488. ( 10.1006/anbe.1999.1185) [DOI] [PubMed] [Google Scholar]
- 17.Cole MR, Gibson L, Pollack A, Yates L. 2011. Potentiation and overshadowing of shape by wall color in a kite-shaped maze using rats in a foraging task. Learn. Motiv. 42, 99–112. ( 10.1016/j.lmot.2010.11.001) [DOI] [Google Scholar]
- 18.Horne MR, Iordanova MD, Pearce JM. 2010. Spatial learning based on boundaries in rats is hippocampus-dependent and prone to overshadowing. Behav. Neurosci. 124, 623–632. ( 10.1037/a0020824) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kosaki Y, Austen JM, McGregor A. 2013. Overshadowing of geometry learning by discrete landmarks in the water maze: effects of relative salience and relative validity of competing cues. J. Exp. Psychol. Anim. Behav. Process. 39, 126–139. ( 10.1037/a003.1199) [DOI] [PubMed] [Google Scholar]
- 20.Kehoe EJ. 1982. Overshadowing and summation in compound stimulus conditioning of the rabbit's nictitating membrane response. J. Exp. Psychol. Anim. Behav. Process. 8, 313–328. ( 10.1037/0097-7403.8.4.313) [DOI] [PubMed] [Google Scholar]
- 21.Cook M, Mineka S. 1987. Second-order conditioning and overshadowing in the observational conditioning of fear in monkeys. Behav. Res. Ther. 25, 349–364. ( 10.1016/0005-7967(87)90013-1[pii]) [DOI] [PubMed] [Google Scholar]
- 22.Liljeholm M, Balleine BW. 2009. Mediated conditioning versus retrospective revaluation in humans: the influence of physical and functional similarity of cues. Q. J. Exp. Psychol. 62, 470–482. ( 10.1080/17470210802008805) [DOI] [PubMed] [Google Scholar]
- 23.Robinson CW, Sloutsky VM. 2007. Visual processing speed: effects of auditory input on visual processing. Dev. Sci. 10, 734–740. ( 10.1111/j.1467-7687.2007.00627.x) [DOI] [PubMed] [Google Scholar]
- 24.Prados J. 2011. Blocking and overshadowing in human geometry learning. J. Exp. Psychol. Anim. Behav. Process. 37, 121–126. ( 10.1037/a0020715) [DOI] [PubMed] [Google Scholar]
- 25.Redhead ES, Hamilton DA, Parker MO, Chan W, Allison C. 2013. Overshadowing of geometric cues by a beacon in a spatial navigation task. Learn. Behav. 41, 179–191. ( 10.3758/s13420-012-0096-0) [DOI] [PubMed] [Google Scholar]
- 26.Rescorla RA, Wagner AR. 1972. A theory of classical conditioning: variations in the effectiveness of reinforcement and non-reinforcement. In Classical conditioning II: current research and theory (eds Black AH, Prokasy WF.), pp. 64–99. New York, NY: Appleton-Century-Crofts. [Google Scholar]
- 27.Sandoz JC. 2011. Behavioral and neurophysiological study of olfactory perception and learning in honeybees. Front. Syst. Neurosci. 5, 98 ( 10.3389/fnsys.2011.00098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandoz JC, Deisig N, de Brito Sanchez MG, Giurfa M. 2007. Understanding the logics of pheromone processing in the honeybee brain: from labeled-lines to across-fiber patterns. Front. Behav. Neurosci. 1, 5 ( 10.3389/neuro.08.005.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bitterman ME, Menzel R, Fietz A, Schafer S. 1983. Classical conditioning of proboscis extension in honeybees (Apis mellifera). J. Comp. Psychol. 97, 107–119. ( 10.1037/0735-7036.97.2.107) [DOI] [PubMed] [Google Scholar]
- 30.Giurfa M, Sandoz JC. 2012. Invertebrate learning and memory: fifty years of olfactory conditioning of the proboscis extension response in honeybees. Learn. Mem. 19, 54–66. ( 10.1101/lm.024711.111) [DOI] [PubMed] [Google Scholar]
- 31.Reinhard J, Sinclair M, Srinivasan MV, Claudianos C. 2010. Honeybees learn odour mixtures via a selection of key odorants. PLoS ONE 5, e9110 ( 10.1371/journal.pone.0009110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guerrieri F, Schubert M, Sandoz JC, Giurfa M. 2005. Perceptual and neural olfactory similarity in honeybees. PLoS Biol. 3, 60–74. ( 10.1371/journal.pbio.0030060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rescorla RA, Holland PC. 1982. Behavioral studies of associative learning in animals. Annu. Rev. Psychol. 33, 265–308. ( 10.1146/annurev.ps.33.020182.001405) [DOI] [Google Scholar]
- 34.Shepard RN. 1987. Toward a universal law of generalization for psychological science. Science 237, 1317–1323. ( 10.1126/science.3629243) [DOI] [PubMed] [Google Scholar]
- 35.Spence KW. 1937. The differential response in animal to stimuli varying within a single dimension. Psychol. Rev. 44, 430–444. ( 10.1037/h0062885) [DOI] [Google Scholar]
- 36.Laloi D, Bailez O, Roger B, Pham-Delègue MH, Wadhams LJ. 2000. Recognition of complex odors by restrained and free-flying honeybees, Apis mellifera. J. Chem. Ecol. 26, 2307–2319. ( 10.1023/a:1005522826673) [DOI] [Google Scholar]
- 37.Laloi D, Roger B, Blight MM, Wadhams LJ, Pham-Delegue MH. 1999. Individual learning ability and complex odor recognition in the honey bee, Apis mellifera L. J. Insect Behav. 12, 585–597. ( 10.1023/a:1020919501871) [DOI] [Google Scholar]
- 38.Smith BH, Cobey S. 1994. The olfactory memory of the honeybee Apis mellifera. II Blocking between odorants in binary mixtures. J. Exp. Biol. 195, 91–108. [DOI] [PubMed] [Google Scholar]
- 39.Sutherland NS, Mackintosh NJ. 1971. Mechanisms of animal discriminatlon learning, 559 p New York, NY: Academic Press. [Google Scholar]
- 40.Smith BH, Menzel R. 1989. The use of electromygram recordings to quantify odourant discrimination in the honey bee, Apis mellifera. J. Insect. Physiol. 35, 369–375. ( 10.1016/0022-1910(89)90110-8) [DOI] [Google Scholar]
- 41.Araneda RC, Kini AD, Firestein S. 2000. The molecular receptive range of an odorant receptor. Nat. Neurosci. 3, 1248–1255. ( 10.1038/81774) [DOI] [PubMed] [Google Scholar]
- 42.Hallem EA, Carlson JR. 2006. Coding of odors by a receptor repertoire. Cell. 125, 143–160. ( 10.1016/j.cell.2006.01.050) [DOI] [PubMed] [Google Scholar]
- 43.Krautwurst D, Yau KW, Reed RR. 1998. Identification of ligands for olfacatory receptors by functional expression of a receptor library. Cell 95, 917–926. ( 10.1016/S0092-8674(00)81716-X) [DOI] [PubMed] [Google Scholar]
- 44.Munch D, Schmeichel B, Silbering AF, Galizia CG. 2013. Weaker ligands can dominate an odor blend due to syntopic interactions. Chem. Senses 38, 293–304. ( 10.1093/chemse/bjs138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joerges J, Küttner A, Galizia CG, Menzel R. 1997. Representation of odours and odour mixtures visualized in the honeybee brain. Nature 387, 285–288. ( 10.1038/387285a0) [DOI] [Google Scholar]
- 46.Robertson HM, Wanner KW. 2006. The chemoreceptor superfamily in the honey bee, Apis mellifera: expansion of the odorant, but not gustatory, receptor family. Genome Res. 16, 1395–1403. ( 10.1101/gr.5057506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murthy VN. 2011. Olfactory maps in the brain. Annu. Rev. Neurosci. 34, 233–258. ( 10.1146/annurev-neuro-061010-113738) [DOI] [PubMed] [Google Scholar]
- 48.Carlsson MA, Hansson BS. 2003. Dose-response characteristics of glomerular activity in the moth antennal lobe. Chem. Senses 28, 269–278. ( 10.1093/chemse/28.4.269) [DOI] [PubMed] [Google Scholar]
- 49.Sachse S, Galizia CG. 2003. The coding of odour-intensity in the honeybee antennal lobe: local computation optimizes odour representation. Eur. J. Neurosci. 18, 2119–2132. ( 10.1046/j.1460-9568.2003.02931.x) [DOI] [PubMed] [Google Scholar]
- 50.Sachse S, Galizia CG. 2002. The role of inhibition for temporal and spatial odor representation in olfactory output neurons: a calcium imaging study. J. Neurophysiol. 87, 1106–1117. [DOI] [PubMed] [Google Scholar]
- 51.Faber T, Joerges J, Menzel R. 1999. Associative learning modifies neural representations of odors in the insect brain. Nat. Neurosci. 2, 74–78. ( 10.1038/4576) [DOI] [PubMed] [Google Scholar]
- 52.Rath L, Galizia CG, Szyszka P. 2011. Multiple memory traces after associative learning in the honey bee antennal lobe. Eur. J. Neurosci. 34, 352–360. ( 10.1111/j.1460-9568.2011.07753.x) [DOI] [PubMed] [Google Scholar]
- 53.Yu D, Ponomarev A, Davis RL. 2004. Altered representation of the spatial code for odors after olfactory classical conditioning: memory trace formation by synaptic recruitment. Neuron 42, 437–449. ( 10.1016/S0896-6273(04)00217-X) [DOI] [PubMed] [Google Scholar]
- 54.Daly KC, Christensen TA, Lei H, Smith BH, Hildebrand JG. 2004. Learning modulates the ensemble representations for odors in primary olfactory networks. Proc. Natl Acad. Sci. USA 101, 10 476–10 481. ( 10.1073/pnas.0401902101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Menzel R. 2014. The insect mushroom body, an experience-dependent recoding device. J. Physiol. 108, 84–95. ( 10.1016/j.jphysparis.2014.07.004) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: http://doi.org/10.5061/dryad.fd128.