Abstract
Small variations in signalling pathways have been linked to phenotypic diversity and speciation. In vertebrates, teeth represent a reservoir of adaptive morphological structures that are prone to evolutionary change. Cyprinid fish display an impressive diversity in tooth number, but the signals that generate such diversity are unknown. Here, we show that retinoic acid (RA) availability influences tooth number size in Cyprinids. Heterozygous adult zebrafish heterozygous for the cyp26b1 mutant that encodes an enzyme able to degrade RA possess an extra tooth in the ventral row. Expression analysis of pharyngeal mesenchyme markers such as dlx2a and lhx6 shows lateral, anterior and dorsal expansion of these markers in RA-treated embryos, whereas the expression of the dental epithelium markers dlx2b and dlx3b is unchanged. Our analysis suggests that changes in RA signalling play an important role in the diversification of teeth in Cyprinids. Our work illustrates that through subtle changes in the expression of rate-limiting enzymes, the RA pathway is an active player of tooth evolution in fish.
Keywords: retinoic acid, tooth, cyprinids, development, evolution
1. Introduction
Small variations in signalling molecules linked to species variation were first demonstrated in Darwin's finches with bmp4 demonstrated to be a major actor in beak shape variation [1]. To identify additional signalling molecules that play a role during evolution, a number of criteria are likely to be important: (i) that a particular tissue or organ displays sufficient variation in closely related species and (ii) that the developmental biology of the organ or tissue development is sufficiently known and can be manipulated. Teeth represent an ideal candidate tissue for such studies as they display an impressive diversity in terms of size, shape, number and localization [2]. With more than 3000 species, Cypriniformes is the most diverse order of freshwater fishes with an impressive diversity in pharyngeal dentition [3]. Retinoic acid (RA) is a morphogen molecule with pleiotropic roles during embryonic development in vertebrates [4] and has long been proposed to play an important role during evolution [5,6]. RA levels are tightly controlled in the developing embryo, mainly by the expression of specific synthetizing (aldh1a) and degrading (cyp26) enzymes. These provide the ability to precisely govern the local levels of RA during development, alteration in which could impact developmental processes. We have previously established that RA is required for tooth induction in the Cypriniform zebrafish [7]. Here, we investigate how local levels of RA signalling in the tooth-forming region influence tooth number, providing a mechanism to underpin dentition evolution in Cyprinids.
2. Results
(a). Cyprinid's dentition is highly diverse
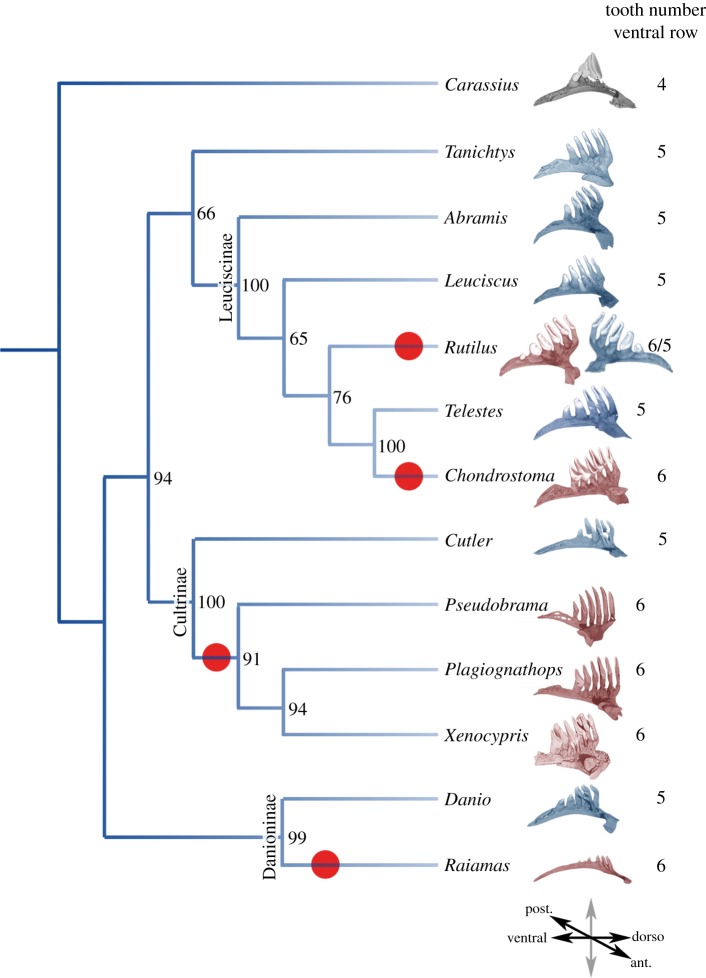
We previously characterized the diversity of tooth row organization and tooth shape in Cyprinids [8,9]. Whereas in most species, the main tooth row contains five teeth, we found at least four independent cases in which six teeth are present on the fifth ceratobranchial bone (red circles, figure 1): in Cultrinae, in which at least three genera form a monophyletic group [10]; in Leuciscinae, in which Rutilus and Chondrostoma represents two independent lineages [11] harbouring six teeth; and in Raiamas, a Danioninae distantly related to the zebrafish. The case of Rutilus is particularly interesting as it displays an asymmetric organization with the left ceratobranchial harbouring six teeth, whereas the right one contains only five (see the electronic supplementary material, figure S1 for more details). This suggests that there were convergent gains of one tooth during evolution in these various clades.
Figure 1.
Cyprinidae dentition diversity. Phylogeny of several species of Cyprinidae based on cytb, coi and rag1 markers. Phylogenetic analyses were performed using RaxML, with a GTR + G + I partitioned model, with 1000 bootstraps (values indicated for each branch). For each species, the fifth ceratobranchial bearing pharyngeal teeth is pictured and the number of teeth on the ventral row is indicated. Species with six teeth on the ventral row are red. Red dots indicate the events of tooth gain on the ventral row, based on parsimonious hypotheses. The orientation of the ceratobranchial arch is indicated at the bottom of the tree. Colour code: grey, four teeth; blue, five teeth; red, six teeth in the ventral part of the fifth ceratobranchial arch.
(b). Retinoic acid signalling in the tooth-forming region
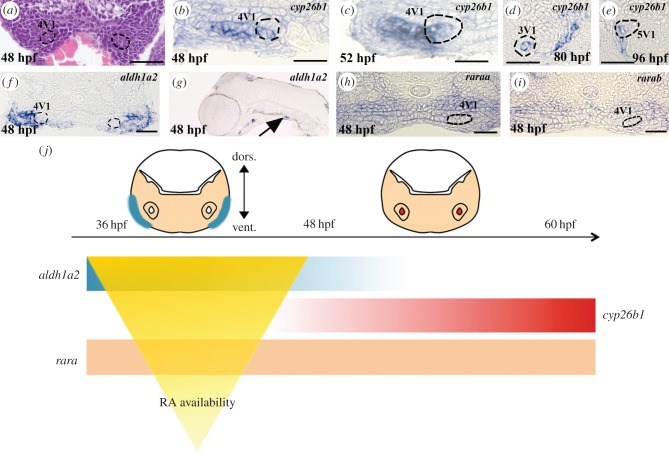
To test if RA signalling plays a role in the diversification of the tooth row in Cyprinids, we first studied the expression of RA signalling pathway genes during the development of the first three teeth (4V1, which appeared first, and then 3V1 and 5V1), and the first replacement tooth 4V2 [12,13]. aldh1a2, the rate-limiting enzyme of RA formation required for tooth induction, is expressed in the posterior ventral pharynx from 24 to 72 hpf [7,14], but excluded from the tooth bud itself (figure 2a,f,g). We found that cyp26b1, an RA degrading enzyme [15,16], is expressed at 48 hpf, where the first tooth 4V1 will be formed, but also in the entire lateral length of the ventral pharynx, where other teeth will subsequently emerge from 48 to 52 hpf (figure 2b,c). Later in development, cyp26b1 is expressed in the mesenchyme of the second forming tooth 3V1, whereas expression remains detected in a broader region that contains more than the tooth buds (figure 2d). At 96 hpf, expression is detected in the pharyngeal epithelium surrounding half of the first replacement tooth germ 4V2 (figure 2e; the same pictures without any annotation are available in the electronic supplementary material, figure S2). We also checked the expression pattern of the other cyp26 enzymes (cyp26a1, cyp26c1) and showed that they are not expressed in the tooth-forming region at these stages (see the electronic supplementary material, figure S3). In zebrafish, the RA receptors α (RARα-A and α-B) are implicated in 4V1 induction [7,17]. We monitored their expression at the time of 4V1 (43 hpf) and 3/5V1 (48 hpf) induction [18], and found them expressed in the entire ventralmost part of the pharynx, not restricted to the region of 4V1 or 3/5V1 induction (figure 2h,i). These expression patterns suggest that RA is produced broadly in the posterior ventral pharynx, but its availability is tightly regulated by cyp26b1 expression, which prevents RA being present outside of its domain of action in both a spatial and temporal manner (figure 2j).
Figure 2.
Retinoic acid signalling in the tooth-forming region. (a) Transverse sections of the posterior ventral pharynx of a 48 hpf Danio rerio embryo stained with haematoxylin and eosin. The 4V1 tooth germs are marked by dashed lines. (b–e) Transverse sections of the posterior ventral pharynx of embryos post in situ hybridization embryos stained with cyp26b1 at (b) 48 hpf, (c) 52 hpf, (d) 80 hpf and (e) 96 hpf. The 4V1 tooth bud is marked by dashed lines in (b,c). The 3V1 and 4V2 tooth buds are marked by dashed lines in (d) and (e), respectively. (f) Transverse and (g) longitudinal sections of the posterior ventral pharynx of an embryo post in situ hybridization embryos stained with aldh1a2 at 48 hpf. 4V1 is marked in (f), whereas expression in the ventral posterior pharynx in the vicinity of the forming tooth bud is indicated by an arrow in (g). Note that aldh1a2 expression is excluded from the developing tooth bud in (f). (h) raraa and (i) rarab expression in transverse sections of the posterior ventral pharynx at 48 hpf. The position of the 4V1 tooth bud is indicated. Note that both raraa and rarab are expressed within the tooth bud but also in the entire posterior ventral pharynx. (j) Summary of the temporal and regional expression pattern of RA signalling actors: at 36 hpf, aldh1a2 is expressed in the ventral pharynx until 48 hpf. cyp26b1 expression starts at 50 hpf in the tooth bud mesenchyme. raraa and rarab are expressed through the entire ventral pharynx. The RA availability within the tooth-forming region is temporally sharpened by the expressions of both synthetizing (aldh1a2) and degrading (cyp26b1) enzymes. Scale bars, 25 µm.
(c). Retinoic acid signalling controls tooth number
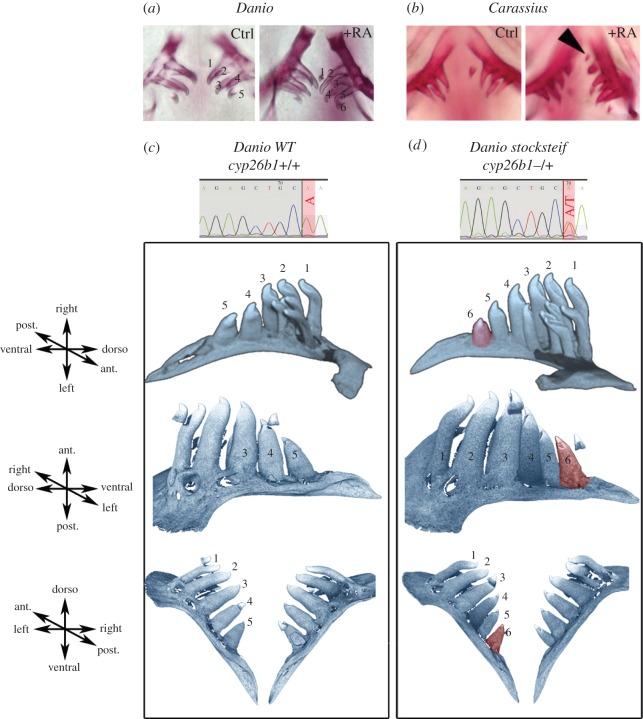
Based on this tight regulation of RA availability in the ventral posterior pharynx, we then studied the effect of exogenous RA on the development of the tooth row. Under RA exposure at 10−8 M from 56 hpf to 12 days post fertilization (dpf), embryos often (55% of observed cases, n = 18) display six rather than five teeth in each pharyngeal jaw at 14 dpf detected either by Alcian blue or by Alizarin red staining (figure 3a). The presence of this extra tooth is difficult to interpret as it could correspond to an extra tooth in the row or, alternatively, to an earlier appearance of the first replacing tooth 4V2 [19]. As it is very difficult to discriminate between these possibilities, we studied this effect in adult fishes in which the amount of RA varies within a more physiological range. For this, we used the stocksteif (sst) mutant in which the cyp26b1 gene is mutated, therefore compromising RA degradation and leading to more endogenous RA [16], especially in the ventral posterior pharynx where cyp26b1 is highly expressed (figure 2b). Using computed microtomography, we observed a high penetrance (51%, n = 45) of tooth rows harbouring six teeth in the adult sst heterozygous mutants, whereas five were observed in wild-type fishes (figure 3c,d). Among the heterozygous fish that had a sixth tooth, 65% had unilateral extra tooth, whereas 35% had bilateral formation of an extra tooth. This sixth tooth is present in an extra position in the normal row as it is present in wild species harbouring six teeth in the first row (e.g. Rutilus or Pseudobrama; figure 1; electronic supplementary material, figure S1). In many cases, we observed, as in Rutilus, asymmetric distribution with, respectively, five and six teeth on each side. However, in contrast to Rutilus, in about one-third of the sample, the affected side varies randomly, exhibiting six teeth on both sides. When we compare the six-tooth row with the five-tooth row in the same individuals, we observed that the six-tooth row is greater (113% the size of the five-tooth row) and that the teeth are slightly shifted medially. We observed the same increase in size (112%) when comparing the right five-tooth row with the left six-tooth row in Rutilus (see the electronic supplementary material, figure S4). Interestingly, we observed that RA also induces the formation of an extra tooth in another cyprinid species: the goldfish Carassius carassius. In goldfish, four teeth are present in a single row, but after RA treatment (5×10−8 M, 2–14 dpf or 3–16 dpf), we observed a fifth tooth in about 50% (n = 15) of the treated embryos (figure 3b). This result demonstrates that RA has the same effect of increasing number of teeth in distant cyprinid species. We noted, however, that the goldfish appears to develop its extra tooth laterally, whereas the zebrafish develops it mesial to the tooth closest to the midline. This may suggest that there is an interesting difference to explore between the way these two species form their extra teeth. We concluded that a tight regulation of RA levels restricts tooth number in Cyprinids and that small local variations in RA are sufficient for the formation of an extra tooth.
Figure 3.
Retinoic acid controls tooth number. (a) Alizarin red staining of 12 dpf untreated and treated zebrafish (Danio rerio) embryos with 10−8 M of RA from 56 hpf onwards. (b) Alizarin red staining of 14 dpf untreated and treated goldfish (Carassius auratus) embryos with 5 × 10−8 M of RA from 48 hpf onwards. A black arrowhead indicates the extra tooth induced by RA treatment. (c,d) Computed microtomography scans of (c) wild-type zebrafish adults (cyp26b1+/+) and of (d) stocksteif (sst) heterozygous mutants (cyp26b1+/−). Teeth on the ventral row are numbered and the sixth extra tooth observed in sst mutants is highlighted in red. Chromatograms of genotyping showing the A > T nonsense mutation in heterozygous is provided.
(d). Exogenous exposure expands the domain of expression of lateral pharyngeal mesenchyme markers
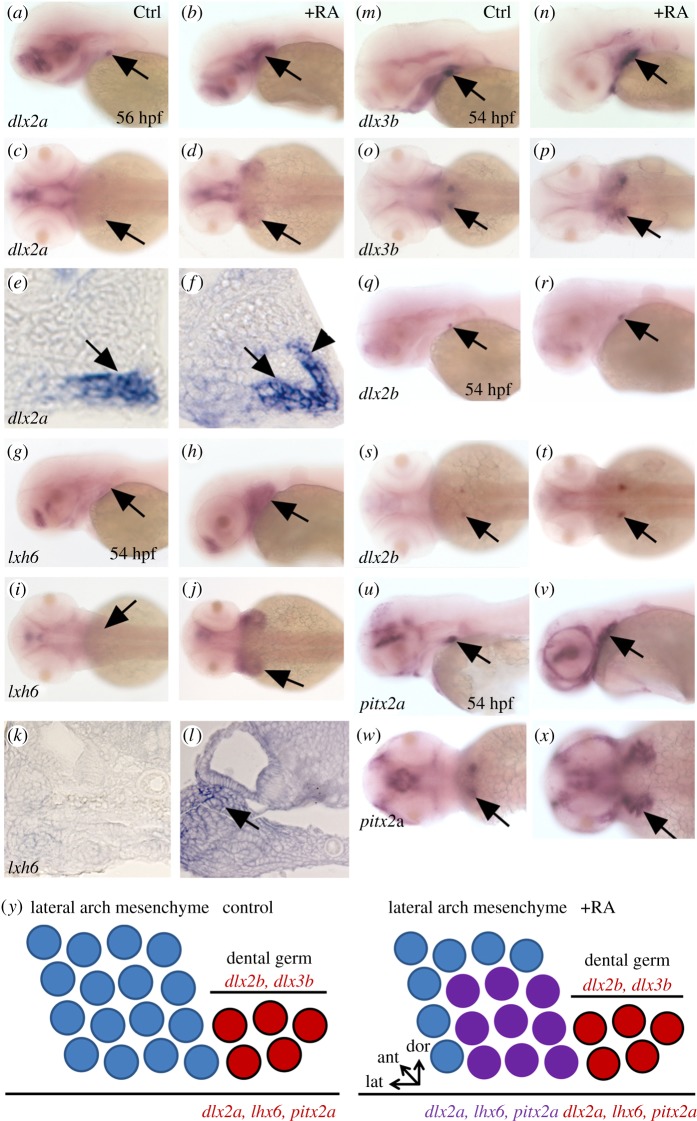
We investigated the expression of the dental and pharyngeal mesenchyme markers in RA-treated zebrafish embryos. Under RA exposure, dlx2a expression is detected in a larger and wider expression domain in the pharyngeal arch mesenchyme (figure 4a–f). This expansion of dlx2a expression is specific to the tooth region as its expression in the forebrain is unaffected. Similarly, lhx6 expression, which is normally confined to the dental mesenchyme and the lateral pharyngeal mesenchyme at this developmental stage, is expressed throughout the entire pharyngeal mesenchyme and part of the epithelium under RA exposure (figure 4g–l). pitx2a, which is normally restricted to the dental epithelium, has its expression expanded to the pharyngeal epithelium under RA treatments (figure 4u–x). The dental epithelium-specific markers dlx3b and dlx2b do not display such a dramatic expansion of their respective domain of expression under RA treatments (figure 4m–r). At most, we note a slightly increase of expression in the dental epithelium of the 4V1 tooth bud (especially for dlx3b) under RA exposure. We therefore concluded from these experiments that exogenous RA exposure can expand the expression of the pharyngeal mesenchyme markers such as dlx2a, lhx6 and dental epithelium pitx2a, providing a broader domain for tooth induction that cannot be compensated by cyp26b1 expression owing to an excess of RA signalling in this region. However, it remains to be demonstrated that this expansion of pharyngeal markers may reflect an expansion of tooth-competent mesenchyme.
Figure 4.
Expansion of pharyngeal mesenchyme markers in RA treated embryos. (a–f) expression of dlx2a in the tooth bud of 4V1 and lateral arch mesenchyme marked by an arrow in (a,c,e) control and (b,d,f) RA-treated embryos. Note the lateral, anterior and dorsal expansion of dlx2a expression in RA-treated embryos (arrowhead in (f)). (g–l) Expression of lhx6 in the lateral pharyngeal mesenchyme marked by an arrow in (g,i,k) control and (h,j,l) RA-treated embryos. As for dlx2a, note the lateral, anterior and dorsal expansion of lhx6 expression in RA-treated embryos (arrow in transverse section in (l)). (m–t) Expression of dlx2b and dlx3b in the dental epithelium marked by an arrow in (m,o,q,s) control and (n,p,r,t) RA-treated embryos. RA exposure has only a minor effect on the expression of these dental epithelium markers. (u–x) Expression of pitx2a in the pharyngeal and dental epithelium marked by an arrow in (u,w) control and (v,x) RA-treated embryos. Note the lateral, anterior and dorsal expansion of pitx2a expression in RA treated embryos. (y) Diagram of a longitudinal section of the posterior ventral pharyngeal epithelium and mesenchyme of the 4V1 tooth germ during early morphogenesis, dorsal up, anterior to the left of a control embryo (top) and RA-treated embryo (bottom). In red, dlx2b and dlx3b are restricted to the folding dental epithelium, whereas dlx2a is expressed in both the developing tooth (red) germ and the lateral pharyngeal mesenchyme (purple); lhx6 is expressed in both the dental mesenchyme (red) and in lateral arch mesenchyme (purple); pitx2a is expressed in the dental epithelium (red) and the pharyngeal epithelium (purple). Under RA exposure, expression of dlx2a, lhx6 and pitx2a is expanded in three directions (laterally, dorsally and anteriorly), whereas expression of dlx2b and dlx3b remains confined in the dental epithelium, but their expression domains are slightly enlarged compared with control.
3. Discussion
Taken together, our results suggest that the RA pathway has a dramatic effect on tooth row formation in zebrafish and that modulation of RA level in zebrafish resembles what is observed in wild species: RA controls the number of teeth in the dental row and a decrease in cyp26b1 level in sst heterozygous mutant shows that this effect can be observed for a relatively narrow range of concentrations. Interestingly, the variation of tooth number (from five to six teeth) has occurred several times during cyprinid evolution. Indeed, we previously characterized the diversity of tooth row organization and tooth shape in cyprinids [8]. Whereas in most species, the main tooth row contains five teeth, we found at least four independent cases in which six teeth are present on the fifth ceratobranchial bone (red circles, figure 1). This is confirmed by the analysis of tooth evolution in Leuciscinae that we are currently performing and that will be published separately (E.P-V. and L.V. 2015, unpublished data). The case of Rutilus is particularly interesting as it displays an asymmetric organization with the left ceratobranchial harbouring six teeth, whereas the right one contains only five. The most parsimonious explanation of this pattern is effectively a convergent gain of one tooth during evolution in these various clades. Unfortunately, we cannot test directly whether RA was implicated in these convergent changes as we cannot have access to the embryos of these species.
We previously hypothesized that early RA exposure can initiate homeotic transformation of anterior and dorsal pharyngeal tissue in the vicinity of tooth bud formation to a more posterior and ventral identity. This could explain why early exogenous RA exposure is able to induce the formation of anterior ectopic teeth in zebrafish [20]. This was exemplified by the anterior expansion of pitx2a expression in the pharyngeal epithelium under RA treatments. Our novel results indicate that pharyngeal mesenchyme markers also experience an expansion of their domain of expression in the three directions: laterally, anteriorly and dorsally under RA exposure (figure 4y). We therefore speculate that the new availability of cells expressing pharyngeal mesenchymal markers in a broader domain of the pharynx in the proximodistal axis under RA treatments is sufficient to induce an extra tooth in the ventral most part of the fifth ceratobrachial arch. This change in cell identity, unlike what we proposed for ectopic anterior tooth induction, does not need to be linked to a homeotic transformation for the following reasons.
First, the timing of treatments does not fit anymore with a homeotic transformation: when we begin our RA treatments after 24 hpf, we no longer observed the induction of anterior ectopic teeth, as expression of the dental epithelium markers dlx2b and dl3xb remain restricted to the 4V1 tooth bud in the posterior pharynx. Second, the formation of an extra tooth in the ventralmost row of the fifth ceratobranchial arch does not require a dramatic change such as would be needed to induce anterior ectopic teeth. A simple lateral expansion of the competent domain of cells that can form a tooth is sufficient. Although in our RA treatment, we observed an expansion of pharyngeal markers in three directions, and we speculate that the lateral expansion of these markers is enough. The nature of signals and genes responsive to the higher number of cells expressing dlx2a and lhx6 that are needed to induce the extra tooth remain to be determined.
In addition, we also consistently note that exogenous RA has an effect on the shape of individual teeth, and this may be linked to a stronger variation in RA level as this is not observed in adult sst heterozygous but only after high and/or early treatment with exogenous RA. We are currently analysing this effect in more detail to better understand its developmental basis and to see if it may corresponds to change in wild species. It will be particularly interesting to study if there is co-occurrence of changes in tooth number and tooth shape during cyprinids evolution, and if change in RA signalling is the mechanism that has been recruited to promote tooth elongation in such cases.
Our analysis reveals that in addition to its action on body plan evolution in vertebrates [5,6,21], RA signalling can also intervene in the fine-tuning of tooth number at a taxonomically restricted level. Our findings provide a link, at a microevolutionary scale, between a specific change in RA signalling and a novel morphology. This is reminiscent of the situation of Hox genes, which, after having been considered solely as regulators of major body plan transitions, were demonstrated to be micromanagers involved in morphological differences between Drosophila species [22,23]. Our observation offers a coherent framework to explore the implication of such a signalling pathway in evolutionary adaptations.
4. Experimental procedures
(a). Zebrafish handling
Zebrafish and their embryos were handled according to standard protocols [24] and in accordance with the animal welfare committees of the Ecole Normale Supérieure de Lyon and Deakin University. The stocksteif zebrafish mutant was used as previously presented [16,25].
(b). Zebrafish assays
RA and DEAB treatments were performed as previously described [7]. Whole mount in situ hybridizations were performed as described [26]. Pictures were taken on an Axioimager (Zeiss) and processed using Adobe Photoshop. Alcian blue and Alizarin red stainings were performed as previously described [7].
(c). Phylogenetic analysis
The phylogenetic tree was built using three markers: cytochrome b (cytb), cytochrome oxidase 1 (coI) and recombination activating gene (rag1). Sequences were downloaded from GenBank (references available upon request). Sequences were aligned and concatenated with Seaview. Phylogenetic reconstruction was performed using RaxML, with a partitioned GTR + G + I model. Bootstrap support was calculated with 1000 repetitions. Carassius auratus was taken as outgroup.
(d). Microtomography
Specimens all came from the collections from the Museum of Natural History in Paris (MNHN), except Danio rerio and Carassius auratus, which came from our breeding facilities. All specimens were scanned by conventional microtomography using a nanotom Phoenix X-ray (General Electrics) with the following parameters: 70 kV tension, 100 mA tension, 3000 images, 500 ms exposure time per image and three images for average per position. After scanning, reconstruction was performed with the software attached to the machine (data rec). Three-dimensional volumes were then analysed with VGStudioMax in order to virtually extract the pharyngeal bones.
(e). Histology
Whole-mount hybridized embryos stored in glycerol were dehydrated through several baths of absolute ethanol, then in butanol, and finally embedded in paraplast for 7 µm cross-sections. Sections were mounted in eukitt, observed and photographed under bright field. H&E staining was performed as previously described [10].
(f). sst genotyping
We amplified the part of the cyp26b1 gene that is targeted in the sstsa2 mutant by genomic PCR using custom primers (F: CACTCTCCTAATTTTAGGTTTAACCAC, R: GGAAAGGCACAAGGAGAATG). The A–T mutation introduces a new NheI restriction site, so we checked the genotype by digestion of the amplicon (data not shown). We confirmed the result by sequencing the amplicon and looking at the raw chromatogram.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
The authors thank the staff members of the Deakin University Zebrafish Facility for providing ongoing husbandry care. We thank Megan Ellis and Isabelle Germon for technical help and Patrick Prunet for support. We thank Michalis Averof, Samir Merabet, François Leulier and Joanne Burden for critical reading of the manuscript. We thank Stefan Schulte-Merker for providing the cyp26b1 mutant fish.
Authors' contributions
Y.G., E.S. and V.L. designed all experiments. R.A. treatments, in situ hybridization, Alcian blue and Alizarin red staining were performed by Y.G., E.S. and L.B. Micro-CT scan were done and interpreted by E.P.-V. and L.V. Goldfish experiments were done by C.L. and E.S. Histology analysis was done by V.B.-B. We thank Stefan Schulte-Merker for providing the cyp26b1 mutant fish. Data were analysed by Y.G., E.S., L.V. and V.L. Y.G., V.L. and E.S. wrote the manuscript.
Funding statement
This research was supported by an ARC fellowship, an Alfred Deakin Fellowship, a Central Research Grant from Deakin University and funding from the Molecular & Medical Research Strategic Research Centre at Deakin University (to Y.G.). E.S. is supported by the French Ministry of Research and La Ligue contre le cancer, ENS Lyon, the ANR programme (Bouillabaisse ANR-09-BLAN_0127) and the French Ministry of Research (to V.L.).
Competing interests
The authors declare they have no conflict of interest.
References
- 1.Abzhanov A, Protas M, Grant BR, Grant PR, Tabin CJ. 2004. Bmp4 and morphological variation of beaks in Darwin's finches. Science 305, 1462–1465. ( 10.1126/science.1098095) [DOI] [PubMed] [Google Scholar]
- 2.Jernvall J, Salazar-Ciudad I. 2007. The economy of tinkering mammalian teeth. Novartis Found. Symp. 284, 207–216; discussion 216–224 ( 10.1002/9780470319390.ch14) [DOI] [PubMed] [Google Scholar]
- 3.Stock DW. 2007. Zebrafish dentition in comparative context. J. Exp. Zool. B Mol. Dev. Evol. 308, 523–549. ( 10.1002/jez.b.21187) [DOI] [PubMed] [Google Scholar]
- 4.Rhinn M, Dolle P. 2012. Retinoic acid signalling during development. Development 139, 843–858. ( 10.1242/dev.065938) [DOI] [PubMed] [Google Scholar]
- 5.Fujiwara S, Kawamura K. 2003. Acquisition of retinoic acid signaling pathway and innovation of the chordate body plan. Zool. Sci. 20, 809–818. ( 10.2108/zsj.20.809) [DOI] [PubMed] [Google Scholar]
- 6.Marletaz F, Holland LZ, Laudet V, Schubert M. 2006. Retinoic acid signaling and the evolution of chordates. Int. J. Biol. Sci. 2, 38–47. ( 10.7150/ijbs.2.38) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibert Y, et al. 2010. Formation of oral and pharyngeal dentition in teleosts depends on differential recruitment of retinoic acid signaling. FASEB J. 24, 3298–3309. ( 10.1096/fj.09-147488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasco-Viel E, Charles C, Chevret P, Semon M, Tafforeau P, Viriot L, Laudet V. 2010. Evolutionary trends of the pharyngeal dentition in Cypriniformes (Actinopterygii: Ostariophysi). PLoS ONE 5, e11293 ( 10.1371/journal.pone.0011293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasco-Viel E, Yang L, Veran M, Balter V, Mayden RL, Laudet V, Viriot L. 2014. Stability versus diversity of the dentition during evolutionary radiation in cyprinine fish. Proc. R. Soc. B 281, 20132688 ( 10.1098/rspb.2013.2688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao W, Zhang Y, Liu H. 2001. Molecular systematics of Xenocyprinae (Teleostei: Cyprinidae): taxonomy, biogeography, and coevolution of a special group restricted in East Asia. Mol. Phylogenet. Evol. 18, 163–173. ( 10.1006/mpev.2000.0879) [DOI] [PubMed] [Google Scholar]
- 11.Perea S, Bohme M, Zupancic P, Freyhof J, Sanda R, Ozulug M, Abdoli A, Doadrio I. 2010. Phylogenetic relationships and biogeographical patterns in Circum-Mediterranean subfamily Leuciscinae (Teleostei, Cyprinidae) inferred from both mitochondrial and nuclear data. BMC Evol. Biol. 10, 265 ( 10.1186/1471-2148-10-265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borday-Birraux V, Van der Heyden C, Debiais-Thibaud M, Verreijdt L, Stock DW, Huysseune A, Sire JY. 2006. Expression of Dlx genes during the development of the zebrafish pharyngeal dentition: evolutionary implications. Evol. Dev. 8, 130–141. ( 10.1111/J.1525-142x.2006.00084.X) [DOI] [PubMed] [Google Scholar]
- 13.Huysseune A, Van der heyden C, Sire JY. 1998. Early development of the zebrafish (Danio rerio) pharyngeal dentition (Teleostei, Cyprinidae). Anat. Embryol. (Berl.) 198, 289–305. ( 10.1007/s004290050185) [DOI] [PubMed] [Google Scholar]
- 14.Grandel H, et al. 2002. Retinoic acid signalling in the zebrafish embryo is necessary during pre-segmentation stages to pattern the anterior–posterior axis of the CNS and to induce a pectoral fin bud. Development 129, 2851–2865. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Q, Dobbs-McAuliffe B, Linney E. 2005. Expression of cyp26b1 during zebrafish early development. Gene Express. Patterns 5, 363–369. ( 10.1016/j.modgep.2004.09.011) [DOI] [PubMed] [Google Scholar]
- 16.Spoorendonk KM, Peterson-Maduro J, Renn J, Trowe T, Kranenbarg S, Winkler C, Schulte-Merker S. 2008. Retinoic acid and Cyp26b1 are critical regulators of osteogenesis in the axial skeleton. Development 135, 3765–3774. ( 10.1242/dev.024034) [DOI] [PubMed] [Google Scholar]
- 17.Bertrand S, et al. 2007. Unexpected novel relational links uncovered by extensive developmental profiling of nuclear receptor expression. PLoS Genet. 3, e188 ( 10.1371/journal.pgen.0030188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huysseune A, Witten PE. 2006. Developmental mechanisms underlying tooth patterning in continuously replacing osteichthyan dentitions. J. Exp. Zool. B Mol. Dev. Evol. 306, 204–215. ( 10.1002/jez.b.21091) [DOI] [PubMed] [Google Scholar]
- 19.Huysseune A. 2006. Formation of a successional dental lamina in the zebrafish (Danio rerio): support for a local control of replacement tooth initiation. Int. J. Dev. Biol. 50, 637–643. ( 10.1387/ijdb.052098ah) [DOI] [PubMed] [Google Scholar]
- 20.Seritrakul P, Samarut E, Lama TT, Gibert Y, Laudet V, Jackman WR. 2012. Retinoic acid expands the evolutionarily reduced dentition of zebrafish. FASEB J. 26, 5014–5024. ( 10.1096/fj.12-209304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mark M, Lohnes D, Mendelsohn C, Dupe V, Vonesch JL, Kastner P, Rijli F, Bloch-Zupan A, Chambon P. 1995. Roles of retinoic acid receptors and of Hox genes in the patterning of the teeth and of the jaw skeleton. Int. J. Dev. Biol. 39, 111–121. [PubMed] [Google Scholar]
- 22.Stern DL. 1998. A role of Ultrabithorax in morphological differences between Drosophila species. Nature 396, 463–466. ( 10.1038/24863) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akam M. 1998. Hox genes: from master genes to micromanagers. Curr. Biol 8, R676–R678. ( 10.1016/S0960-9822(98)70433-6) [DOI] [PubMed] [Google Scholar]
- 24.Westerfield M. 1993. The zebrafish book: a guide for the laboratory use of zebrafish (Brachydanio rerio), 2nd edn Corvallis, OR: University of Oregon Press. [Google Scholar]
- 25.Jackman WR, Stock DW. 2006. Transgenic analysis of Dlx regulation in fish tooth development reveals evolutionary retention of enhancer function despite organ loss. Proc. Natl Acad. Sci. USA 103, 19 390–19 395. ( 10.1073/Pnas.0609575103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thisse C, Thisse B. 2008. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 3, 59–69. ( 10.1038/Nprot.2007.514) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.