Abstract
The chaperonin-containing t-complex polypeptide 1 (CCT) is a hetero-oligomeric molecular chaperone that assists in the folding of actin, tubulin, and other cytosolic proteins. We recently reported that the expression level of CCT is closely correlated with growth rates of mammalian cultured cells. Here we examine the levels of CCT subunits and other molecular chaperones in tumor tissues of patients with hepatocelluar and colonic carcinoma, and compare them with nontumor tissues in the same patients. Expression levels of CCTβ in tumor tissues was significantly higher than in nontumor tissues in all patients with hepatocellular carcinoma (n = 15) and 83% of patients with colonic carcinoma (n = 17). The increased level of CCT expression in colonic cancer cells was confirmed by immunohistochemistry with anti-CCTβ antibody. The levels of CCTβ were highly correlated (r = 0.606) with those of the proliferating cell nuclear antigen (PCNA), which was used as an indicator of cell growth. CCTα gave similar results, although the correlation with PCNA levels was weaker. Other cytosolic and endoplasmic reticulum chaperones also showed higher expression in significant numbers of tumor tissues but less frequently than that observed with CCT. These results suggest that CCT is up-regulated in rapidly proliferating tumor cells in vivo to effectively produce proteins required for growth, and may serve as a useful tumor marker because it is widely distributed in the cytosol.
INTRODUCTION
The chaperonin-containing t-complex polypeptide 1 (CCT), also called TRiC or c-cpn, mediates the folding of proteins in the cytosol (reviewed by Kubota et al 1995; Lewis et al 1996; Willison and Horwich 1996). CCT is a member of the chaperonin family that includes mitochondrial 60-kDa heat shock protein (HSP60), bacterial GroEL, plastid Rubisco subunit binding protein, and archea group II chaperonins. CCT has been shown to assist in the folding of actin and tubulin in the presence of ATP in vitro (Tian et al 1995; Frydman and Hartl 1996; Farr et al 1997) and to bind newly synthesized actin and tubulin in vivo (Thulasiraman et al 1999). Several other proteins were also reported as possible CCT substrates. CCT is required for the maturation of cyclin E (Won et al 1998); and myosin II (Srikakulam and Winkelmann 1999), α-transducin (Farr et al 1997), von Hippel Lindau protein (Feldman et al 1999) and firefly luciferase (Frydman and Hartl 1996; Gebauer et al 1998) can be folded with the assistance of CCT. In addition, more than 70 unidentified newly synthesized proteins can be immunoprecipitated with CCT in mammalian cells (Thulasiraman et al 1999). These observations indicate that CCT plays essential roles in protein folding in eukaryotic cytosol.
Electron microscopic analysis revealed that CCT has a double-torus-like structure with 8-fold rotational symmetry assembled from 16 subunits (Llorca et al 1999). In mammalian somatic cells, CCT is composed of 8 different subunits of approximately 60 kDa each: α, β, γ, δ, ε, ζ-1, η, and θ (Kubota et al 1995). These subunits are encoded by independent genes and show approximately 30% identity at the amino acid sequence level (Kubota et al 1994, 1999).
Recently, we found that CCT expression levels correlate closely with growth rates in mammalian cultured cells, and that the expression of CCT is markedly up-regulated around early S phase of the cell cycle (Yokota et al 1999). Because cell cycle-dependent proteins such as proliferating cell nuclear antigen (PCNA) and MIB-1 (Ki-67) antigen are frequently used as tumor markers (Risio 1994; Elias 1997; Schipper et al 1998), we postulated that CCT could also be useful as a tumor marker. Here we analyze CCT levels in primary tumor tissues obtained from patients with hepatocellular and colonic carcinoma and compare them with nontumor tissues from the same patients. We also examined the expression levels in tumor and nontumor tissues of other chaperone proteins, including the 70-kDa heat shock protein (HSP70), cognate of HSP70 (HSC70), and the 78-kDa and 94-kDa endoplasmic reticulum (ER) glucose-regulated proteins (GRP78 and GRP94, respectively). Of all the molecular chaperones tested, CCT was the most frequently up-regulated in tumor tissues.
MATERIALS AND METHODS
Tissue sampling
Between July 1997 and August 1999, 32 patients were enrolled in this study at the Department of Gastroenterological Surgery, Kyoto University Graduate School of Medicine. Informed consent was obtained from all patients, and 15 primary hepatocellular carcinomas and 17 colonic cancers (2 ascending, 2 transverse, 2 descending, 7 sigmoid, and 4 rectal cancers) were included. Samples of primary tumors and vicinal nonneoplastic tissues were obtained after excision of surgical specimens. Tissue samples were immediately frozen and stored at −80°C until analysis.
Antibodies
Rat monoclonal antibodies to CCTα (clone 84a) and HSC70 (1B5), and mouse monoclonal antibodies to HSP70 (C92F3A-5) and KDEL (10C3) were purchased from StressGen (Victoria, British Columbia, Canada). The anti-KDEL antibody was used to detect GRP78 and GRP94. Mouse monoclonal antibodies to PCNA (PC10) and actin (C4) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and Chemicon (Temecula, CA, USA), respectively. Rabbit polyclonal antibody against CCTβ (BC-1; Hynes et al 1995) was kindly provided by Prof K.R. Willison.
Western blotting
Tissue specimens were homogenized in a buffer containing 1% Triton X-100, 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, 2 mM ethylenediamine tetraacetic acid, 0.1 IU/mL aprotinin, and 10 mM Hepes-NaOH (pH 7.5), and centrifuged at 25 000 × g for 20 minutes at 4°C. The protein concentration of the resulting supernatant was determined by the Bradford method (1976) using bovine serum albumin as a standard. The extracted proteins were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) using 10% gels (for PCNA) or 7.5% gels (for other proteins; Laemmli 1970), and blotted onto polyvinylidene difluoride filters according to the method of Towbin et al. (Towbin et al 1979). Detection and quantitation of proteins on the filters using specific antibodies was carried out as described previously (Yokota et al 1999). Briefly, the filters were incubated with an appropriate primary antibody and then with alkaline phosphatase-conjugated goat antibody against rabbit, rat, or mouse immunoglobulins. Immunoreactive bands were visualized by developing with the solution containing tetrazolium bromochloroindolylphosphate and nitrobluetetrazolium. Digital images of the resulting blots were obtained with a flatbed scanner and analyzed using the public domain NIH Image program (U.S. National Institutes of Health, Bethesda, MD, USA). Experiments were carried out three times, and mean values and standard deviations were calculated.
Immunohistochemistry
Tissue samples were fixed in 4% formaldehyde and immunohistochemical staining of paraffin sections (4 μm) was carried out using an LSAB2/HRP kit (Dako, Via Real Carpinteria, CA, USA) according to the manufacturer's instructions. Briefly, after blocking endogenous peroxidase activity and nonspecific protein binding, sections were incubated with anti-CCTβ antibody (1:100). Sections were then incubated with biotinylated anti-rabbit immunoglobulin and peroxidase-conjugated streptavidin, and developed with 3-amino-9-ethyl carbasol. Developed sections were counterstained with hematoxylin.
RESULTS
Up-regulation of molecular chaperones in tumor tissues
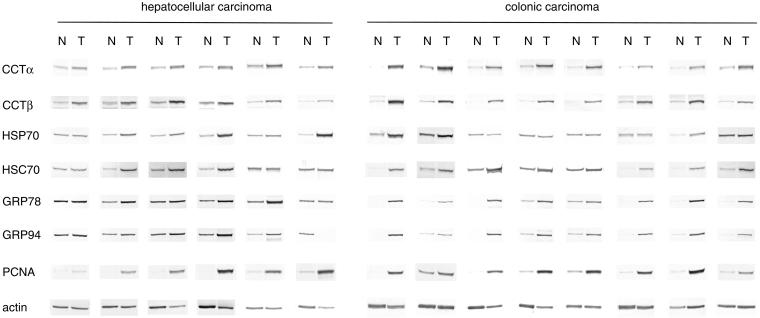
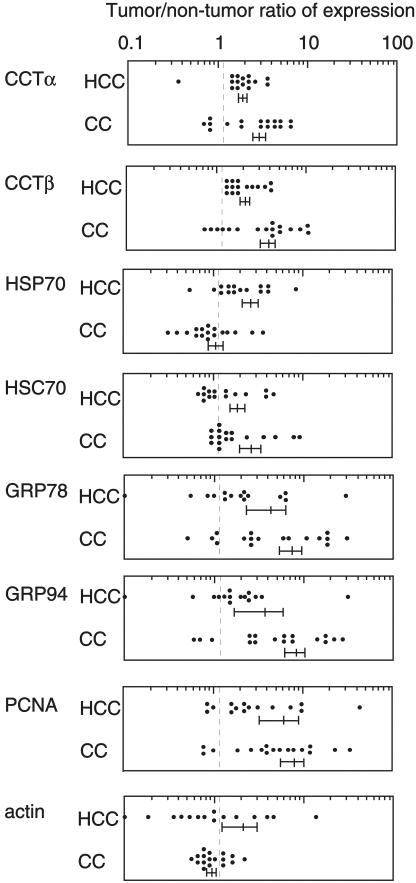
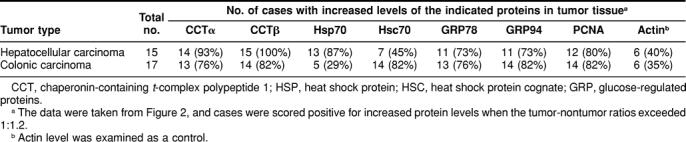
Tumor tissues and surrounding nontumor tissues from the same patients with hepatocellular (n = 15) or colonic (n = 17) carcinoma were obtained at the time of surgery, and the protein expression levels of cytosolic molecular chaperones CCT, HSP70, and HSC70, and ER molecular chaperones GRP78 and GRP94 in these tissues were analyzed by Western blot analysis. In addition, the levels of PCNA (a marker of rapid cell growth) and actin (a control for intracellular protein) were determined; representative results are shown in Figure 1. The intensity of each band was quantified, and tumor:nontumor ratios of individual proteins expressed in the same patients were determined (Fig. 2 and Table 1). In all patients with hepatocellular and colonic carcinoma, the expression levels of CCT (α and β subunits), GRP78, and GRP94 were frequently (73%–100%) enhanced in tumor, as was the expression level of PCNA (80%–82%). Of the molecular chaperone proteins examined, CCTβ was the most frequently up-regulated in tumor tissue (82%–100%), closely followed by CCTα (76%–93%). HSP70 was frequently up-regulated in hepatocellular carcinoma (87%) but not in colonic carcinoma (29%). In contrast, HSC70 levels were frequently increased in colonic carcinoma (82%), but much less often in hepatocellular carcinoma (45%). Actin expression levels were was not up-regulated in tumor tissues from a significant number of patients (only 35%–40% of cases showed actin up-regulation).
Fig. 1. .
Protein expression levels of CCT (α and β subunits), HSP70, HSC70, GRP78, GRP94, PCNA, and actin in tumor and nontumor tissues derived from patients with hepatocellular and colonic carcinoma. Soluble proteins were extracted from tumor and nontumor tissues from the same patients and separated by SDS-PAGE (total protein laded: 10 μg/lane for CCTα, CCTβ, HSP70, and HSC70; and 5 μg/lane for GRP78, GRP94, PCNA, and actin). These proteins were then analyzed by Western blotting using specific antibodies. Tissue samples from 15 patients with hepatocellular carcinoma and 17 patients with colonic carcinoma were tested, and representative data are shown. N, nontumor tissue; T, tumor tissue
Fig. 2. .
Relative expression levels of CCTα, CCTβ, HSP70, HSC70, GRP78, GRP94, and actin in tumor tissues. Expression levels of proteins in tumor and nontumor tissues were analyzed by Western blotting as described in Figure 1 and quantified by digital image analysis after scanning the blots. Tumor:nontumor ratios were calculated using the data derived from the same patients with hepatocellular (n = 15) and colonic (n = 17) carcinoma. Experiments were carried out in triplicate and the results are presented as mean values (standard deviations were less than 15%). Dotted lines indicate a tumor:nontumor ratio of 1.2. Mean values with standard errors for each group are indicated under the plots. HCC, hepatocellular carcinoma; CC, colonic carcinoma
Table 1.
Number of patients with increased expression of molecular chaperones and proliferating cell nuclear antigen (PCNA) in hepatocellular and colonic carcinomas
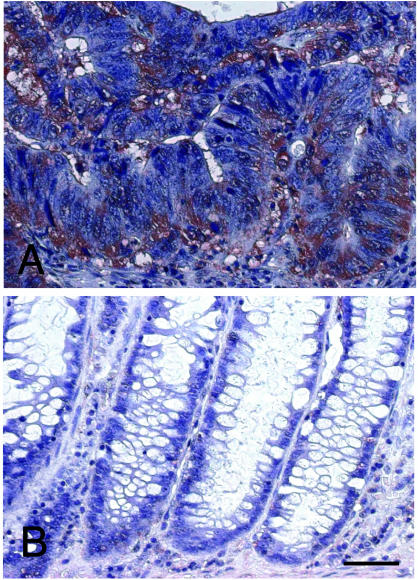
Immunohistochemical staining of CCTβ in colonic carcinona and surrounding normal tissues indicated that CCTβ protein is abundant in cytosolic portions of malignant epithelial tissue (Fig. 3A). In contrast, the degree of CCTβ staining in normal epithelial tissue (Fig. 3B) or connective tissues was much weaker than that in colonic carcinoma tissue. Immunohistochemical staining of microwave-treated sections with anti-CCTγ antibody (GC-1; Hynes et al 1995) exhibited similar staining patterns (data not shown). These results confirmed that the increased expression of CCT occurred primarily in the cytosol of tumor cells.
Fig. 3. .
Immunohistochemical staining of CCTβ in colonic carcinoma. Sections of colonic carcinoma (A) and surrounding normal colon tissue (B) from the same patient were incubated with anti-CCTβ antibody (BC-1). CCTβ protein specifically recognized by the antibody was stained red by peroxidase reaction with 3-amino-9-ethyl carbasol. Sections were counterstained with hematoxylin for nuclear staining in blue. Bar = 50 μm
Correlation between expression levels of different proteins
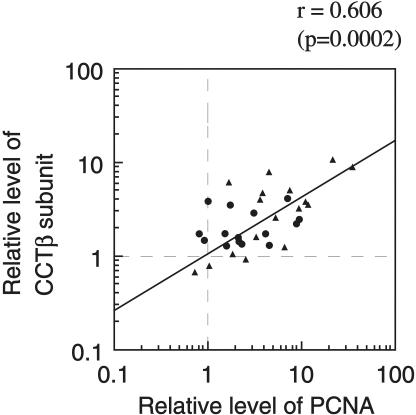
The degree of up-regulation of PCNA in tumor tissue correlated strongly (r = 0.606, P = 0.0002) with that of CCTβ (Fig. 4), and to a lower but significant degree (r = 0.367, P = 0.038) with that of CCTα. The degree of up-regulation of HSC70 showed significant correlation (r =0.589, P = 0.0003) with that of PCNA, whereas that of HSP70 did not (r = −0.020). Up-regulation of GRP78 showed a weak but significant correlation with that of PCNA (r = 0.354, P = 0.046), whereas the degree of up-regulation of GRP94 did not show significant correlation with that of PCNA (r = 0.218). Consistent with the fact that CCTα and CCTβ are components of the same complex (Kubota et al 1995), the up-regulation of these 2 proteins correlated very strongly (r = 0.686, P < 0.0001). The up-regulation of GRP78 and GRP94 also correlated very strongly (r = 0.690, P < 0.001), suggesting that the “unfolded protein response,” by which ER chaperones are induced (McMillan et al 1994), occurred in an appreciable number of tumor tissues. Actin expression did not show any significant correlation with the other proteins tested. The cases examined contained 15 moderately differentiated and 3 well-differentiated colonic carcinomas, and 7 poorly differentiated, 3 moderately differentiated, and 5 well-differentiated hepatocellular carcinomas. However, no significant correlation was observed between the degree of differentiation and the expression levels of molecular chaperones. Larger numbers of tumors should be examined to address whether expression of molecular chaperones correlate with the degree of differentiation in hepatocellular or colon cancer. These results indicate that CCT subunit proteins are frequently up-regulated in hepatocellular and colonic carcinoma tissues. The up-regulation of CCT in these tumor tissues may be related to the rapid growth of tumor cells.
Fig. 4. .
Correlation between CCTβ and PCNA expression levels in tumor tissues from the same patients with hepatocellular (circle) or colonic (triangle) carcinoma. Data are taken from Figure 2
DISCUSSION
In this study, we presented evidence that levels of the cytosolic chaperonin, CCT, are frequently up-regulated in hepatocellular and colonic carcinomas relative to corresponding normal tissues in the same patients. We recently reported that the expression level of CCT is dependent on cell growth rate, regardless of cell type, as a result of preferential synthesis at the G1/S transition through early S phase in the cell cycle (Yokota et al 1999). CCT is a hetero-oligomeric complex composed of 8 or 9 subunit species (Kubota et al 1995), of which 2, the α and β subunits, were examined in the present study. The levels of up-regulation of CCTα and CCTβ in tumor tissues correlated strongly with each other, although they were not identical. CCTβ showed more frequent up-regulation in tumors and a higher correlation with the up-regulation of PCNA than did CCTα. The observed difference between the α and β subunits may be related to our recent observation that the turnover rates of these subunits are significantly different, and that CCTα is less stable than CCTβ in the cell (submitted for publication). These results suggest that CCT is up-regulated in tumor cells to meet the increased requirement for assistance in the folding of proteins that are actively synthesized during cell growth. Such target proteins may include tubulins (Yokota et al 1999) and cyclin E (Won et al 1998).
We also examined several other molecular chaperones and observed frequent up-regulation of GRP78, GRP94, HSP70 (only in hepatocellular carcinoma), and HSC70 (only in colonic carcinoma) in tumor tissues. GRP78 and GRP94 mediate protein folding in the ER, and are induced by glucose starvation or accumulation of abnormal proteins in the ER (the so-called “unfolded protein response”; Lee 1992; McMillan et al 1994). HSP70 is the major cytosolic protein induced by heat shock and chemical stress, and assists in the refolding of denatured proteins (Hightower 1991; Morimoto 1993). HSC70 (cognate of HSP70) is a cytosolic molecular chaperone that is important for the folding of newly synthesized polypeptides; HSC70 cooperates with CCT for folding proteins in vitro (Gebauer et al 1998). Several molecular chaperones, including HSPs (Ferrarini et al 1992; Gress et al 1994; Kaur and Ralhan 1995; Ralhan and Kaur 1995) and GRPs (Bini et al 1997; Gazit et al 1999), have been shown to be up-regulated in malignant cancers or in cell lines derived from them, which has led to a discussion of their etiological roles (Soti and Csermely 1998; Jäättelä 1999). Increased expression of these chaperones in tumor cells may contribute to survival and growth under stressful conditions in the tumor (Soti and Csermely 1998; Jäättelä 1999), which is in agreement with our results showing increased expression of GRP78 and GRP94. However, the observed up-regulation of these chaperones was less frequent than that of CCT. The levels of up-regulation of CCTβ and HSC70 in tumor tissue correlated strongly with that of PCNA, which is used as a marker of cell growth (Woods et al 1991; Yu et al 1991), suggesting that a number of molecular chaperones are up-regulated in tumor cells not only to cope with stressful conditions but also to actively produce proteins for cell growth.
In conclusion, our present results suggest that the cytosolic chaperonin, CCT, may be useful as a cytosolic protein marker of rapidly growing tumors (including hepatocellular and colonic cancers), in addition to conventional tumor markers localized in the nucleus such as PCNA and MIB-1 (Ki-67; Risio 1994; Elias 1997; Schipper et al 1998).
Acknowledgments
We thank Prof K.R. Willison for the antibody to CCTβ. We also thank Drs H. Shirane, T. Tanaka, and K. Sugita for helpful comments on immunohistochemical experiments.
REFERENCES
- Bini L, Magi B, and Marzocchi B. et al. 1997 Protein expression profiles in human breast ductal carcinoma and histologically normal tissue. Electrophoresis. 18:2832–2841. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Elias JM. Cell proliferation indexes: a biomarker in solid tumors. Biotech Histochem. 1997;72:78–85. doi: 10.3109/10520299709082216. [DOI] [PubMed] [Google Scholar]
- Farr GW, Scharl EC, and Schumacher RJ. et al. 1997 Chaperonin-mediated folding in the eukaryotic cytosol proceeds through rounds of release of native and nonnative forms. Cell. 89:927–937. [DOI] [PubMed] [Google Scholar]
- Feldman DE, Thulasiraman V, and Ferreyra RG. et al. 1999 Formation of the VHL-elongin BC tumor suppressor complex in mediated by the chaperonin TRiC. Mol Cell. 4:1051–1061. [DOI] [PubMed] [Google Scholar]
- Ferrarini M, Heltai S, and Zocchi MR. et al. 1992 Unusual expression and localization of heat-shock proteins in human tumor cells. Int J Cancer. 51:613–619. [DOI] [PubMed] [Google Scholar]
- Frydman J, Hartl FU. Principles of chaperone-assisted protein folding: difference between in vitro and iv vivo mechanisms. Science. 1996;272:1497–1502. doi: 10.1126/science.272.5267.1497. [DOI] [PubMed] [Google Scholar]
- Gazit G, Lu J, Lee AS. De-regulation of GRP stress protein expression in human breast cancer cell lines. Berast Cancer Res Treat. 1999;54:135–146. doi: 10.1023/a:1006102411439. [DOI] [PubMed] [Google Scholar]
- Gebauer M, Melki R, Gehring U. The chaperone cofactor Hop/p60 interacts with the cytosolic chaperonin-containing TCP-1 and affects its nucleotide exchange and protein folding activities. J Biol Chem. 1998;273:29475–29480. doi: 10.1074/jbc.273.45.29475. [DOI] [PubMed] [Google Scholar]
- Gress TM, Muller-Pillasch F, and Weber C. et al. 1994 Differential expression of heat shock proteins in pancreatic carcinoma. Cancer Res. 54:547–551. [PubMed] [Google Scholar]
- Hightower LE. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991;66:191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- Hynes G, Kubota H, Willison KR. Antibody characterisation of two distinct conformations of the chaperonin-containing TCP-1 from mouse testis. FEBS Lett. 1995;358:129–132. doi: 10.1016/0014-5793(94)01408-s. [DOI] [PubMed] [Google Scholar]
- Jäättelä M. Escaping cell death: survival proteins in cancer. Exp Cell Res. 1999;248:30–43. doi: 10.1006/excr.1999.4455. [DOI] [PubMed] [Google Scholar]
- Kaur J, Ralhan R. Differential expression of 70-kDa heat shock-protein in human oral tumorigenesis. Int J Cancer. 1995;63:774–779. doi: 10.1002/ijc.2910630604. [DOI] [PubMed] [Google Scholar]
- Kubota H, Hynes G, and Carne A. et al. 1994 Identification of six Tcp-1-related genes encoding divergent subunits of the TCP-1-containing chaperonin. Curr Biol. 4:89–99. [DOI] [PubMed] [Google Scholar]
- Kubota H, Hynes G, Willison K. The chaperonin containing t-complex polypeptide 1 (TCP-1): multisubunit machinery assisting in protein folding and assembly in the eukaryotic cytosol. Eur J Biochem. 1995;230:3–16. doi: 10.1111/j.1432-1033.1995.tb20527.x. [DOI] [PubMed] [Google Scholar]
- Kubota H, Yokota S, and Yanagi H. et al. 1999 Structures and coregulated expression of the genes encoding mouse cytosolic chaperonin CCT subunits. Eur J Biochem. 262:492–500. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee AS. Mammalian stress response: induction of the glucose-regulated protein family. Curr Opin Cell Biol. 1992;4:267–273. doi: 10.1016/0955-0674(92)90042-b. [DOI] [PubMed] [Google Scholar]
- Lewis SA, Tian G, and Vainberg IE. et al. 1996 Chaperonin-mediated folding of actin and tubulin. J Cell Biol. 132:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorca O, McCormack EA, and Hynes G. et al. 1999 Eukaryotic type II chaperonin CCT interacts with actin through specific subunits. Nature. 402:693–696. [DOI] [PubMed] [Google Scholar]
- McMillan DR, Gething MJ, Sambrook J. The cellular response to unfolded proteins: intercompartmental signaling. Curr Opin Biotech. 1994;5:540–545. doi: 10.1016/0958-1669(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Morimoto RI. Cells in stress: transcriptional activation of heat shock genes. Science. 1993;259:1409–1410. doi: 10.1126/science.8451637. [DOI] [PubMed] [Google Scholar]
- Ralhan R, Kaur J. Differential expression of Mr 70,000 heat shock protein in normal, premalignant, and malignant human uterine cervix. Clin Cancer Res. 1995;1:1217–1222. [PubMed] [Google Scholar]
- Risio M. Methodological aspects of using immunohistochemical cell proliferation biomarkers in colorectal carcinoma chemoprevention. J Cell Biochem Suppl. 1994;19:61–67. [PubMed] [Google Scholar]
- Schipper DL, Wagenmans MJM, and Peters WHM. et al. 1998 Significance of cell proliferation measurement in gastric cancer. Eur J Cancer. 34:781–790. [DOI] [PubMed] [Google Scholar]
- Soti C, Csermely P. Molecular chaperones in the etiology and therapy of cancer. Pathol Oncol Res. 1998;4:316–321. doi: 10.1007/BF02905225. [DOI] [PubMed] [Google Scholar]
- Srikakulam R, Winkelmann DA. Myosin II folding is mediated by a molecular chaperonin. J Biol Chem. 1999;274:27265–27273. doi: 10.1074/jbc.274.38.27265. [DOI] [PubMed] [Google Scholar]
- Thulasiraman V, Yang C-F, Frydman J. In vivo newly translated polypeptides are sequestered in a protected folding environment. EMBO J. 1999;18:85–95. doi: 10.1093/emboj/18.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian G, Vainberg IE, and Tap WD. et al. 1995 Specificity in chaperonin-mediated protein folding. Nature. 375:250–253. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willison KR, Horwich AL 1996 Structure and function of chaperonins in archaebacteria and eukaryotic cytosol. In: The Chaperonins, ed Ellis RJ. Academic Press, San Diego, 107–136. [Google Scholar]
- Won K-A, Schumacher RJ, and Farr GW. et al. 1998 Maturation of human cyclin E requires the function of eukaryotic chaperonin CCT. Mol Cell Biol. 18:7584–7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods AL, Hall PA, and Shepherd NA. et al. 1991 The assessment of proliferating cell nuclear antigen (PCNA) immunostaining in primary gastrointestinal lymphomas and its relationship to histological grade, S+G2+M phase fraction (flow cytometric analysis) and prognosis. Histopathology. 19:21–27. [DOI] [PubMed] [Google Scholar]
- Yokota S, Yanagi H, and Yura T. et al. 1999 Cytosolic chaperonin is upregulated during cell growth: preferential expression and binding to tubulin at G1/S transition through early S phase. J Biol Chem. 274:37070–37078. [DOI] [PubMed] [Google Scholar]
- Yu CC, Hall PA, and Fletcher CD. et al. 1991 Haemangiopericytomas: the prognostic value of immunohistochemical staining with a monoclonal antibody to proliferating cell nuclear antigen (PCNA). Histopathology. 19:29–33. [DOI] [PubMed] [Google Scholar]