Abstract
Background
From 1958–70, >100,000 people in northern Chile were exposed to a well-documented, distinct period of high drinking water arsenic concentrations. We previously reported ecological evidence suggesting that early-life exposure in this population resulted in increased mortality in adults from several outcomes including lung and bladder cancer.
Methods
We have now completed the first study ever assessing incident cancer cases after early-life arsenic exposure, and the first study on this topic with individual participant exposure and confounding factor data. Subjects included 221 lung and 160 bladder cancer cases diagnosed in northern Chile from 2007–2010, and 508 age and gender-matched controls.
Results
Odds ratios (ORs) adjusted for age, sex, and smoking in those only exposed in early-life to arsenic water concentrations of ≤110, 110–800, and >800 μg/L were 1.00, 1.88 (95% confidence interval (CI), 0.96–3.71), and 5.24 (3.05–9.00) (p-trend<0.001) for lung cancer, and 1.00, 2.94 (1.29–6.70), and 8.11 (4.31–15.25) (p-trend<0.001) for bladder cancer. ORs were lower in those not exposed until adulthood. The highest category (>800 μg/L) involved exposures which started 49–52 years before, and ended 37–40 years before the cancer cases were diagnosed.
Conclusion
Lung and bladder cancer incidence in adults was markedly increased following exposure to arsenic in early-life, even up to 40 years after high exposures ceased. Findings like these have not been identified before for any environmental exposure, and suggest that humans are extraordinarily susceptible to early-life arsenic exposure.
Impact
Policies aimed at reducing early-life exposure may help reduce the long-term risks of arsenic-related disease.
Keywords: Arsenic, lung cancer, bladder cancer, early-life exposure, Chile
Introduction
Children and fetuses may be particularly susceptible to environmental carcinogens (1), but to date the evidence for this is mostly indirect or based on animal studies with inconsistent results (2). Few human data are available, especially for common exposures like arsenic, or common cancers like lung and bladder cancer. Most human data suggesting that early-life events may cause adult cancer involve exposures that are rare (e.g., atomic bomb radiation or diethylstilbestrol) or difficult to assess historically (e.g., secondhand tobacco smoke) (3–5). This paucity of research has important public health implications, since almost all current environmental regulations are based on animal or occupational studies where exposures occurred in adults (6). The failure to incorporate effects from exposures in young children and fetuses (“early-life”), not only for arsenic but for any harmful agent, could lead to standards that are not sufficiently protective.
Millions of people worldwide are exposed to naturally-occurring arsenic in their drinking water (7), and ingested arsenic is an established cause of lung, bladder, and skin cancer (8). The major problem in studying the long-term carcinogenic impacts of early-life exposure to arsenic, or any chemical agent, is the difficulty in following study subjects and their exposure patterns beginning in early life and into those ages where adult cancer risks are high, usually a period of 50 years or more. Accurate exposure data over this many years is rarely available. However, a unique scenario in Region II of northern Chile offers a rare opportunity to investigate the long-term effects of arsenic with good data on past exposure. In the late 1950s, river water from the nearby Andes Mountains containing high concentrations of naturally-occurring arsenic was diverted to the largest city in the area (Antofagasta) to supply drinking water (9). This resulted in a 13-year period (1958–70) during which >100,000 people were exposed to arsenic concentrations >800 μg/L. Treatment plants installed since 1970 reduced concentrations to <10 μg/L today (Table 1). Several other cities in this area had arsenic water concentrations between 110–800 μg/L, at these also declined at about the same time. Another set of cities has continuously had arsenic water concentrations at much lower levels. Region II lies in the Atacama Desert, the driest inhabited place on earth. There are very few water sources and essentially everyone lives in one of the cities and drinks water from one of the few large public water supplies in each city. In addition, historical records of arsenic concentrations are available for all cities in this area, including Antofagasta, with records dating back >40 years. Consequently, retrospective assessments of lifetime arsenic exposure can be estimated in this area with good accuracy simply by knowing the cities in which a person lived.
Table 1.
Historical concentrations of arsenic in drinking water (μg/L) in northern Chile by year
| Average Arsenic Concentration (μg/L)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Years
|
|||||||||
| Region | City or Town | Populationa | 1930–57 | 1958–70 | 1971–77 | 1978–79 | 1980–87 | 1988–2005 | 2005+ |
| I | Arica | 168,594 | 10 | 10 | 10 | 10 | 10 | 10 | 9 |
| Putre | 1,799 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Iquique | 196,941 | 60 | 60 | 60 | 60 | 60 | 60 | 10 | |
| Huara | 2,365 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | |
| Pica | 5,622 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| Pozo Almonte | 9,855 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | |
| II | Tocopilla | 21,827 | 250 | 250 | 636 | 110 | 110 | 40 | 10 |
| Maria Elena | 6,852 | 250 | 250 | 636 | 110 | 110 | 39 | 39 | |
| Calama | 125,946 | 150 | 150 | 287 | 110 | 110 | 40 | 38 | |
| San Pedro | 4,522 | 600 | 600 | 600 | 600 | 600 | 600 | 600 | |
| Antofagasta | 270,184 | 90 | 860 | 110 | 110 | 70 | 40 | 10 | |
| Mejillones | 7,660 | 90 | 860 | 110 | 110 | 70 | 37 | 10 | |
| Taltal | 10,101 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | |
| Recent migrants | 82,312 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | |
Population data are based on the 2002 Chile census (41).
The scenario in Region II, with its well-documented exposure, occurring 4–5 decades ago (i.e., with an appropriate latency), good records on exposure, large numbers of people exposed, and a distinct rise and decline in exposure is incredibly rare in epidemiology and provides a rare opportunity to examine the long-term cancer risks of a common in utero or childhood exposure.
Previously, we reported that arsenic-related odds ratios (ORs) of lung, bladder, and kidney cancer were high in this area, but analyses of early-life exposure were not reported (10, 11). We have also reported ecologic findings linking early-life arsenic to high lung and bladder cancer mortality, but data on cancer incidence or individual data on exposure, migration, and smoking were not available (12). Here we report the first findings ever to link an early-life environmental chemical exposure to high risks of adult cancer incidence and the first study on this topic with individual data on life-long exposure and potential confounders.
Materials and Methods
Participants
The study area comprised two neighboring regions (Regions I, II) in northern Chile with a population of about one million people (Table 1) (13). Study design details are reported elsewhere (11). Briefly, lung and bladder cancer cases were ascertained from all pathologists, hospitals, and radiologists in the area and included people who: 1. Had primary lung or bladder cancer first diagnosed between October 2007 and December 2010; 2. Lived in the study area at the time of diagnosis; 3. Were >25 years old when diagnosed; and 4. Were able to provide interview data or had a close relative who could. Seventy-two percent were histologically confirmed, with the remaining diagnoses based on radiologic (computed tomography) and physician’s clinical findings. Controls without lung, bladder or kidney cancer were randomly selected from the 2007–9 Chilean Electoral Registry for the study area, frequency matched to cases by gender and five-year age group. Our analyses showed that the Electoral Registry contained >95% of people over age 50 years compared to the national census.
Interviews
After obtaining informed consent, participants were interviewed in person using a standardized questionnaire. For deceased subjects, we interviewed the nearest relative (“proxy”). Participants were asked to provide all residences lived at and all jobs held for ≥6 months. This included the residence the parents lived at when the child was born, and thus included in utero exposure. Questions regarding tobacco covered age when smoking began, periods quit, total years smoked, cigarettes smoked per day, and secondhand smoke exposure. Subjects were asked about their typical drinking water intake currently and in the past, but these data had small impacts on classifying exposure in this study so were not used here. Other questions asked about race, occupational exposures, and height and weight (e.g., body mass index (BMI)) currently, 20, and 40 years ago.
Arsenic exposure
For each subject, each residence was linked to an arsenic water concentration measurement for that city or town for the relevant time period so that an arsenic concentration could be assigned to each year of each subject’s life. Details on the arsenic water measurements are provided elsewhere (14, 15). Most records were obtained from municipal water companies, who supply essentially all water in the study area and are required to perform chemical testing at least yearly. Additional measurements were collected from government agencies, research studies, and other sources (9, 16–20). Arsenic measurements were also available for all large cities in Chile outside the study area, and these were also linked to residences. Arsenic water concentrations were available for >95% of all residences for both cases and controls. Residences for which water records were not available were in areas not known to have high arsenic levels so were assigned a value of zero. Bottled water and water filtered with reverse osmosis were also assigned a value of zero but were rarely used until recently. Cumulative (μg/L-years) and average exposures were calculated as the sum and mean, respectively, of subject’s yearly arsenic concentrations.
Statistical analyses
Cancer ORs were calculated using unconditional logistic regression. Variables entered into logistic regression models included sex, age (year), and smoking (three categories of average cigarettes per day while smoking: 0, 1–9, >10). Additional models included mining work (yes or no), obesity (recent body-mass index (BMI) ±30 kg/m2), socioeconomic status (SES) scores (lower vs. upper two tertiles), or self-reported exposure to a known carcinogen at work including asbestos, silica, or arsenic (yes or no). SES scores were based on self-reports of 12 items, including ownership of household appliances, car, computer, and domestic help (one point for each household item and two points each for a car or domestic help). Local researchers advised that these items are a better way to assess SES in this area than education or income. Adjusting for smoking pack-years or 10-year age categories had little impact on results.
To assess the impacts of early-life exposure, cancer ORs were calculated for subjects who were exposed to arsenic water concentrations of 111–800 μg/L or >800 μg/L at birth or as children ≤age 15 but not exposed >110 μg/L as adults (≥25 years old), using subjects who were never exposed >110 μg/L at any time as the reference. Category cut-off points were based on the distribution of arsenic water concentrations in the major cities: Arica and Iquique, ≤110 μg/L; Calama and Tocopilla 111–800 μg/L; and Antofagasta and Mejillones, >800 μg/L (Table 1). Setting the lower cut-off point at 10 or 60 μg/L greatly reduced sample sizes since several of the higher exposure cities had arsenic water concentrations near 110 μg/L for a few years after their higher exposures ended. Defining adults as ≥age 16 did not substantially change ORs but resulted in smaller sample sizes since many children who were highly exposed at age 15 were also highly exposed for a few years after. Because most of the highest exposures in Region II didn’t begin until 1958, all subjects exposed to water concentrations >800 μg/L as children were age 70 or under during our study, so these analyses were restricted to subjects ≤70 years old.
ORs were also calculated for subjects exposed to arsenic water concentrations of 111–800 μg/L or >800 μg/L as adults (≥age 20) but not before (“adult only exposure”), using subjects who were never exposed >110 μg/L at any time as the reference. All subjects exposed to arsenic water concentrations >800 μg/L only as adults were ≥60 years old, so these analyses were restricted to subjects ≥ age 60.
In most analyses, arsenic exposure was based on the highest known arsenic water concentration to which the subject was exposed during the relevant ages, although cumulative exposure was also assessed. This was entered as a continuous variable and ORs are presented for a cumulative exposure of 10 mg/L-years, roughly the level associated with living in Antofagasta for the 13-year high exposure period. Dose-response trends were assessed using the Cochrane-Armitage test for linear trend, and analyses were done in SAS version 9.2 (SAS Institute Inc., Cary NC).
Results
Overall, 370 lung and 289 bladder cancer cases were ascertained. Of these, 46 lung and 23 bladder cancer cases were ineligible based on age and residential criteria. Of the remaining, 4 lung (1.2%) and 12 (4.5 percent) bladder cancer cases could not be located, moved outside the study area, or provided insufficient residential information. Of the remaining, 14 lung (4.4%) and 22 (8.7%) bladder cancer cases or their next-of-kin declined participation. The large majority of cases were interviewed within 4–5 months of diagnosis, and 39.6% and 17.7% of lung and bladder cancer cases had died prior to interview so proxy interviews were performed. Among 872 initially selected controls with viable addresses, 78 (8.9%) no longer lived at the address and could not be located, were ineligible due to illness, or gave insufficient information. Of the remaining 794, 154 (19.4%) declined to participate. An additional 72 bladder, 85 lung cancer cases, and 132 controls were exposed >110 μg/L both in early-life and as adults and were excluded. Demographic variables were similar in these subjects compared to the included subjects, although these excluded subjects were older (median age 69 vs. 65 in included subjects, p<0.001) and had higher overall arsenic exposures (Table S1). Potential controls who did not participate were younger (63.7 vs. 66.0 years, respectively) and more likely male (72.5 vs. 67.3%) than those who did, but inclusion rates were similar among the major exposure areas: 75.5% in Antofagasta, 71.3% in Iquique and Calama, and 74.5% in Arica. The participating control’s cities of residence at the time of ascertainment was similar to the population distribution of the 2002 Chile census (Table S2).
Sociodemographic characteristics are shown for those with early-life (Table 2) and adult-only exposure (Table 3). Cases and controls were similar for most variables, although both bladder and lung cancer cases were more likely to be heavy smokers than controls. Cancer ORs were not elevated for those smoking <10 cigarettes per day although the median cigarettes smoked per day while smoking in this group was low (3.0 cigs/day) and the majority were former smokers (60.7%). Cases also had higher average, cumulative, and maximum arsenic exposures.
Table 2.
Demographic characteristics of subjects never highly exposed or only exposed in utero or as children
| Controls
|
Bladder cancer cases
|
Lung cancer cases
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | ORa | (95% CI) | N | (%) | ORa | (95% CI) | |
| Total | 286 | (100) | 90 | (100) | 139 | (100) | ||||
| Sex | ||||||||||
| Female | 101 | (35.3) | 16 | (17.8) | 40 | (28.8) | ||||
| Male | 185 | (64.7) | 74 | (82.2) | 99 | (72.2) | ||||
| Age (years) | ||||||||||
| ≥ 60 | 128 | (44.8) | 31 | (34.4) | 61 | (43.9) | ||||
| 50–59 | 112 | (39.2) | 36 | (40.0) | 64 | (46.0) | ||||
| <50 | 46 | (16.1) | 23 | (25.6) | 14 | (10.1) | ||||
| Smoking: daily average | ||||||||||
| Never | 91 | (31.8) | 20 | (22.2) | 1.00 | Ref | 26 | (18.7) | 1.00 | Ref |
| 0–9 cigs/day | 126 | (44.1) | 29 | (32.2) | 1.05 | (0.56–1.97) | 29 | (20.9) | 0.81 | (0.44–1.46) |
| ≥10 cigs/day | 69 | (24.1) | 41 | (45.6) | 2.70 | (1.46–5.02) | 84 | (60.4) | 4.26 | (2.48–7.31) |
| Mining work | ||||||||||
| No | 239 | (83.6) | 73 | (81.1) | 1.00 | Ref | 116 | (83.4) | 1.00 | Ref |
| Yes | 47 | (16.4) | 17 | (18.9) | 1.18 | (0.64–2.19) | 23 | (16.6) | 1.01 | (0.58–1.74) |
| Body-mass index >30 kg/m2b | ||||||||||
| No | 278 | (97.2) | 85 | (94.4) | 1.00 | Ref | 132 | (95.0) | 1.00 | Ref |
| Yes | 8 | (2.8) | 5 | (5.6) | 2.04 | (0.65–6.41) | 7 | (5.0) | 1.84 | (0.65–5.19) |
| Socioeconomic status (tertiles) | ||||||||||
| High | 103 | (36.0) | 38 | (42.2) | 1.00 | Ref | 42 | (30.2) | 1.00 | Ref |
| Medium | 112 | (39.2) | 20 | (22.2) | 0.48 | (0.26–0.89) | 53 | (38.1) | 1.16 | (0.71–1.88) |
| Low | 71 | (24.8) | 32 | (35.6) | 1.22 | (0.70–2.17) | 44 | (31.7) | 1.52 | (0.90–2.56) |
|
| ||||||||||
| Mean | (SD) | Mean | (SD) | p-values | Mean | (SD) | p-values | |||
|
| ||||||||||
| Drinking water arsenic exposurec | ||||||||||
| Maximum (μg/L) | 207.5 | (294.5) | 506.7 | (387.0) | <0.001 | 431.9 | (384.8) | <0.001 | ||
| Cumulative (mg/L-yr) | 3.48 | (4.12) | 7.45 | (5.56) | <0.001 | 6.91 | (5.58) | <0.001 | ||
| Average (μg/L) | 66.8 | (78.6) | 147.9 | (106.3) | <0.001 | 130.1 | (104.9) | <0.001 | ||
| Drinking water intake (L/day)c | ||||||||||
| Current | 1.66 | (1.00) | 2.01 | (1.28) | 0.003 | 1.87 | (0.88) | 0.002 | ||
| 20 years ago | 1.89 | (1.25) | 2.04 | (1.21) | 0.003 | 1.98 | (0.83) | 0.002 | ||
| Municipal (%)d | 89.6 | (19.4) | 93.6 | (12.3) | 0.32 | 91.1 | (0.17) | 0.89 | ||
| Residencesc | ||||||||||
| Average number | 3.2 | (2.0) | 2.8 | (1.9) | 0.04 | 2.9 | (1.8) | 0.10 | ||
| Average length (years) | 25.8 | (16.3) | 30.2 | (18.4) | 0.12 | 29.1 | (17.6) | 0.09 | ||
| In study area (%)e | 77.7 | (28.6) | 82.0 | (29.3) | 0.32 | 87.0 | (23.5) | 0.89 | ||
Abbreviations: CI, confidence interval; OR, odds ratio; Ref, reference; SD, standard deviation.
Unadjusted odds ratios (OR) comparing bladder or lung cancer cases to controls. Odds ratios are not reported for age and sex since subjects were frequency matched on these factors.
Body mass index 20 years before cancer diagnosis (cases) or subject ascertainment (controls).
Means, standard deviations, and p-values comparing bladder or lung cancer cases to controls.
Percent of all drinking water supplied by municipal sources (versus bottled, private well, or other source). Includes sources for residences outside the study area.
Percent total person-time in Regions 1 and 2 in northern Chile.
Table 3.
Demographic characteristics of subjects never highly exposed or only exposed as adults
| Controls
|
Bladder cancer cases
|
Lung cancer cases
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | ORa | (95% CI) | N | (%) | ORa | (95% CI) | |
| Total | 332 | 84 | 115 | |||||||
| Sex | ||||||||||
| Female | 105 | (31.6) | 24 | (28.6) | 38 | (33.0) | ||||
| Male | 227 | (68.4) | 60 | (71.4) | 77 | (67.0) | ||||
| Age (years) | ||||||||||
| >80 | 51 | (15.4) | 18 | (21.4) | 20 | (17.4) | ||||
| 70–80 | 157 | (47.3) | 40 | (47.6) | 53 | (46.1) | ||||
| <70 | 124 | (37.3) | 26 | (31.0) | 42 | (36.5) | ||||
| Smoking: highest daily average | ||||||||||
| Never | 140 | (42.2) | 25 | (29.8) | 1.00 | Ref | 28 | (24.3) | 1.00 | Ref |
| 0–9 cigs/day | 110 | (38.2) | 27 | (35.7) | 1.37 | (0.76–2.50) | 16 | (20.9) | 0.73 | (0.37–1.41) |
| ≥10 cigs/day | 82 | (19.6) | 32 | (34.5) | 2.19 | (1.21–3.94) | 71 | (54.8) | 4.33 | (2.59–7.25) |
| Mining work | ||||||||||
| No | 273 | (82.2) | 64 | (76.2) | 1.00 | Ref | 93 | (80.9) | 1.00 | Ref |
| Yes | 59 | (17.8) | 20 | (23.8) | 1.45 | (0.81–2.57) | 22 | (19.1) | 1.09 | (0.64–1.88) |
| Body-mass index >30 kg/m2b | ||||||||||
| No | 311 | (93.7) | 78 | (92.9) | 1.00 | Ref | 106 | (92.2) | 1.00 | Ref |
| Yes | 21 | (6.3) | 6 | (7.1) | 1.14 | (0.44–2.92) | 9 | (7.8) | 1.26 | (0.56–2.83) |
| Socioeconomic status (tertiles) | ||||||||||
| High | 88 | (26.5) | 27 | (32.1) | 1.00 | Ref | 20 | (17.4) | 1.00 | Ref |
| Medium | 100 | (30.1) | 26 | (31.0) | 0.85 | (0.46–1.56) | 31 | (27.0) | 1.36 | (0.73–2.56) |
| Low | 144 | (43.4) | 31 | (36.9) | 0.70 | (0.39–1.25) | 64 | (55.6) | 1.96 | (1.11–3.45) |
|
| ||||||||||
| Mean | (SD) | Mean | (SD) | p-values | Mean | (SD) | p-values | |||
|
| ||||||||||
| Drinking water arsenic exposurec | ||||||||||
| Maximum (μg/L) | 237.7 | (323.7) | 490.1 | (387.2) | <0.001 | 275.9 | (346.7) | 0.50 | ||
| Cumulative (mg) | 4.18 | (4.79) | 7.67 | (6.29) | <0.001 | 4.54 | (5.03) | 0.52 | ||
| Average (μg/L) | 58.8 | (64.5) | 105.7 | (83.1) | <0.001 | 64.9 | (70.3) | 0.51 | ||
| Drinking water intake (L/day)c | ||||||||||
| Current | 1.63 | (0.80) | 1.98 | (0.80) | <0.001 | 1.68 | (0.57) | 0.08 | ||
| 20 years ago | 1.86 | (1.18) | 1.92 | (1.15) | <0.001 | 1.81 | (0.78) | 0.08 | ||
| Municipal (%)d | 89.6 | (18.6) | 92.6 | (16.5) | 0.48 | 85.8 | (23.0) | 0.16 | ||
| Residencesc | ||||||||||
| Average number | 3.7 | (2.1) | 3.2 | (1.9) | 0.07 | 3.8 | (2.1) | 0.70 | ||
| Average length (years) | 28.5 | (20.0) | 35.2 | (24.5) | 0.04 | 28.0 | (19.4) | 0.73 | ||
| In study area (%)e | 74.8 | (26.1) | 78.7 | (23.1) | 0.48 | 75.4 | (27.9) | 0.16 | ||
Abbreviations: CI, confidence interval; OR, odds ratio; Ref, reference; SD, standard deviation.
Unadjusted odds ratios (OR) comparing bladder or lung cancer cases to controls. Odds ratios are not reported for age and sex since subjects were frequency matched on these factors.
Body mass index 20 years before cancer diagnosis (cases) or subject ascertainment (controls).
Means, standard deviations, and p-values comparing bladder or lung cancer cases to controls.
Percent of all drinking water supplied by municipal sources (versus bottled, private well, or other source). Includes sources for residences outside the study area.
Percent total person-time in Regions 1 and 2 in northern Chile.
Lung cancer ORs in those only exposed in early life for arsenic water concentrations of ≤110, 111–800, and >800 μg/L were 1.00, 1.88 (95% confidence interval (CI), 0.96–3.71), and 5.24 (3.05–9.00) (Table 4). Corresponding ORs for adult-only exposure were 1.00, 0.95 (0.46–1.97), and 1.32 (0.75–2.34). Bladder cancer ORs in those only exposed in early life for these same arsenic water concentrations were 1.00, 2.94 (1.29–6.70), and 8.11 (4.31–15.25). Corresponding bladder cancer ORs for adult-only exposure were 1.00, 2.21 (1.03–4.74), and 4.71 (2.61–8.48). ORs for early-life exposure were similar when other age categorizations were used (Table S3).
Table 4.
Lung and bladder cancer ORs in subjects only exposed in utero and as children and in subjects only exposed as adults
| Arsenic | Controls | Cases | OR | Unadjusted 95% CI |
p-trend | OR | Adjusteda 95% CI |
p-trend | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LUNG CANCER | |||||||||||
| Exposed only in utero or as children:b | |||||||||||
| ≤110 μg/L | 201 | 59 | 1.00 | Ref | 1.00 | Ref | |||||
| 111–800 μg/L | 41 | 20 | 1.66 | (0.90–3.05) | 1.88 | (0.96–3.71) | |||||
| >800 μg/L | 44 | 60 | 4.65 | (2.86–7.55) | <0.001 | 5.24 | (3.05–9.00) | <0.001 | |||
| Exposed only as adults:c | |||||||||||
| ≤110 μg/L | 226 | 74 | 1.00 | Ref | 1.00 | Ref | |||||
| 111–800 μg/L | 41 | 13 | 0.97 | (0.49–1.91) | 0.95 | (0.46–1.97) | |||||
| >800 μg/L | 65 | 28 | 1.32 | (0.79–2.20) | 0.34 | 1.32 | (0.75–2.34) | 0.35 | |||
| BLADDER CANCER | |||||||||||
| Exposed only in utero or as children:b | |||||||||||
| ≤110 μg/L | 201 | 29 | 1.00 | Ref | 1.00 | Ref | |||||
| 111–800 μg/L | 41 | 13 | 2.19 | (1.05–4.58) | 2.94 | (1.29–6.70) | |||||
| >800 μg/L | 44 | 48 | 7.56 | (4.30–13.30) | <0.001 | 8.11 | (4.31–15.25) | <0.001 | |||
| Exposed only as adults:c | |||||||||||
| ≤110 μg/L | 226 | 30 | 1.00 | Ref | 1.00 | Ref | |||||
| 111–800 μg/L | 41 | 12 | 2.20 | (1.04–4.66) | 2.21 | (1.03–4.74) | |||||
| >800 μg/L | 65 | 42 | 4.87 | (2.83–8.38) | <0.001 | 4.71 | (2.61–8.48) | <0.001 | |||
Abbreviations: CI, confidence interval; OR, odds ratio; Ref, reference
Adjusted for age, sex, and smoking.
Average arsenic water concentrations in the three exposure categories were 49.6, 254.6, and 860 μg/L.
Average arsenic water concentrations in the three exposure categories were 45.8, 313.2, and 860 μg/L.
ORs for early-life exposure were similar in males, in non-proxy subjects, and in analyses adjusted for occupational exposures, SES, and obesity (Figure 1). ORs in females were slightly lower but the differences compared to males were not statistically significant. Lung cancer ORs in those aged 60–70 who were exposed only in early life were 1.00, 3.58 (95% confidence interval (CI), 1.06–12.1), and 5.17 (2.14–12.5) (p-trend<0.001) for arsenic water concentrations of ≤110, 111–800, and >800 μg/L (not in tables). Corresponding bladder cancer ORs for this age group were 1.00, 2.72 (0.47–15.7), and 8.01 (2.88–22.2).
Figure 1.
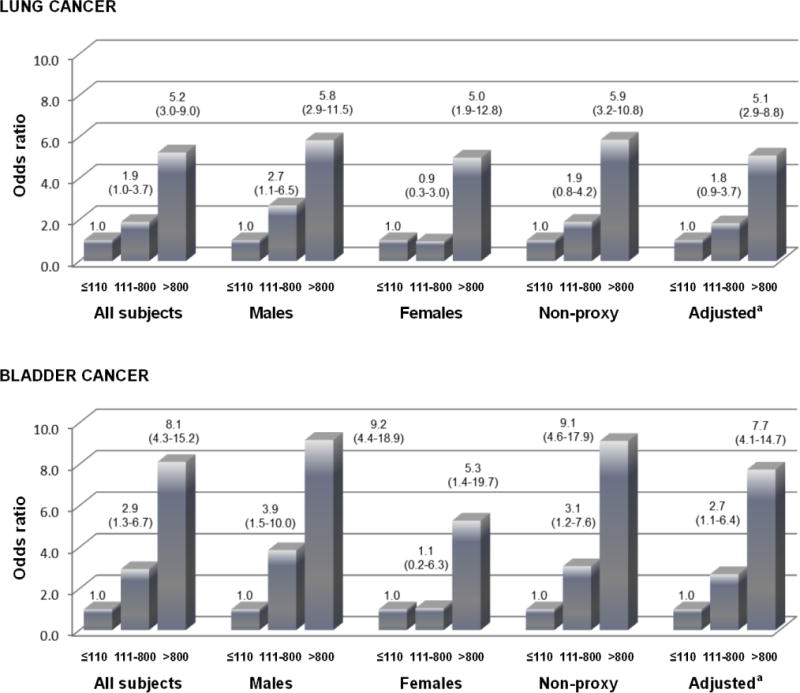
Cancer odds ratios for in utero and childhood exposure by categories of arsenic concentrations (μg/L) in males, females, non-proxy subjects, and in additionally adjusted analyses.
aAdjusted for age, sex, smoking, mining work, occupational carcinogen exposure, socioeconomic status, and obesity.
Figure 2 shows the lung and bladder cancer ORs comparing subjects exposed >800 μg/L to subjects exposed ≤110 μg/L at each individual age of exposure, ignoring exposures at any other age. For both cancers, ORs are highest for earlier ages of exposure. Lung and bladder cancer ORs adjusted for age, sex, and smoking for each 10 mg/L-year increase in cumulative exposure in those highly exposed in early life but not as adults were 4.49 (2.84–7.11) and 5.21 (3.11–8.73), respectively. Corresponding ORs in those with adult-only exposure were 1.20 (0.74–1.94) and 3.23 (2.02–5.18).
Figure 2.
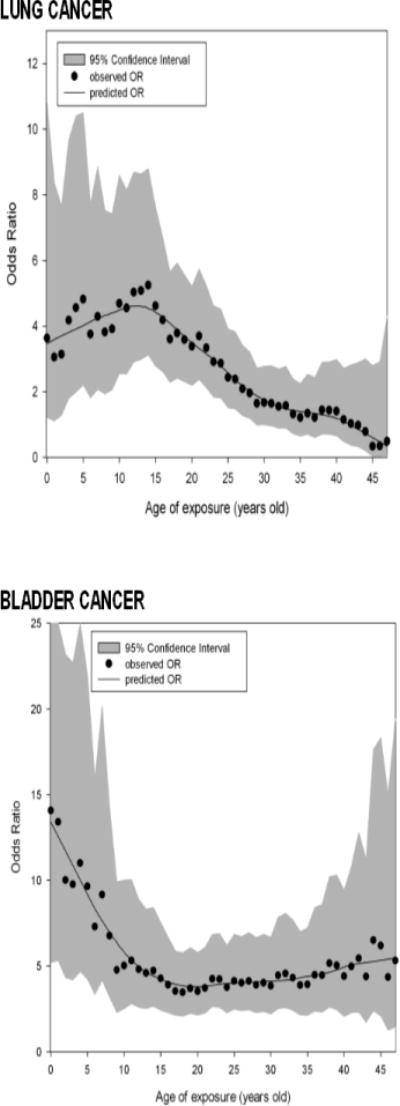
Cancer odds ratios (OR) comparing subjects exposure >800 μg/L to subjects exposed ≤110 μg/L by age of exposure.a
aFor example, the lung cancer odds ratio comparing those exposed >800 μg/L at age 10 to those exposed ≤110 μg/L at age 10 is 4.7 (95% CI, 2.6–8.6). Odds ratios are adjusted for age, sex, and smoking.
Discussion
These findings provide rare human evidence that an early-life environmental exposure can be associated with very high risks of cancer in adults. The presence of dose-response relationships and low p-values suggest that these findings are unlikely due to chance. The particularly novel aspect of this study is the unique exposure situation in northern Chile which allowed us to assess early-life exposure impacts of over a period of >50 years with accurate data on past exposure, and this is the first analytic study ever to link an early-life or in utero environmental chemical exposure to high risks of cancer for such a long period after the exposures occurred.
Other research supports the plausibility of our findings. Ingested arsenic is an established cause of bladder and lung cancer (8), and is known to cross the placenta (21). Studies of low birth weight, smoking, lung infections, and air pollution all provide evidence that early-life events can lead to lung damage manifested later in life (22–24). Our studies in Chile have linked early-life arsenic exposure to respiratory symptoms, lung function decrements, and mortality from lung cancer, bladder cancer, and bronchiectasis (12, 25, 26). In rodents, although arsenic-caused tumors are difficult to induce when arsenic is given in adulthood (27), prenatal exposures have been shown to induce adult tumors much more readily (28).
There are several reasons why in utero or childhood exposures may confer high cancer risks. The fetal and early childhood periods are times of rapid organogenesis and cell proliferation which may allow for mutagenic, epigenetic, or other permanent carcinogenic alterations. These are also periods when metabolism, detoxification, and excretion pathways are undeveloped, and when intake of air and water (and the contaminants in them) are higher on a body weight basis (1). In laboratory experiments, gestational arsenic exposure has been linked to overexpression of estrogen receptor and epidermal growth factor genes (29), carcinogenic changes in stem cells (30), and increased tumorgenicity of other agents (28). Arsenic has been linked to epigenetic effects such as altered DNA methylation, histone modification, and miRNA expression, and these might also increase long-term cancer risks (31). These later findings may be especially relevant to in utero exposures since the embryonic period is a time of significant reprogramming of DNA methylation (32, 33).
Early-life exposure has been unequivocally linked to adult cancer in human studies for only a few other agents: asbestos, high-dose radiation, and diethylstilbestrol (34). However, these exposures are rare and their relevance to lower chronic exposures is uncertain (35). In our study, the large majority of exposures >100 μg/L ended around 1970, so latency patterns were the same in those with childhood and adult only exposures. We found higher ORs in those with early-life exposure compared to those exposed only as adults. But, because subjects in the latter group were older, the relative impacts of earlier vs. later-life exposure on absolute risks can’t be determined from these data. It could be hypothesized that early-life arsenic exposure is only increasing cancer in younger age groups where absolute risks are low. However, we found that lung cancer ORs for early-life exposures were high in adults aged 60–70. Since these are the ages where lung cancer is most common in Chile, early-life exposure likely had a major impact on absolute risks in this study area. Consistent associations between lung cancer and adult exposure were not seen in this study, although a small increase in risk or the roll of chance can not be ruled out. Further evaluations involving larger sample sizes and a broader number of years of case ascertainment may help elucidate the risks from adult only exposure.
Exposure misclassification could have resulted from missing exposure data; inaccurate recall of residential history, water sources, or water consumption; or arsenic from non-drinking water sources. Because exposure was assessed similarly in cases and controls, most of this was likely non-differential and biased ORs towards the null. And, because exposure was primarily based on the cities in which the subjects lived, and errors in recalling this information are likely minimal, the impact of recall errors are probably small. Proxy interviews were more common among cases than controls. However, previous research has shown that proxy respondents can provide reasonably accurate residential histories (36). In addition, the fact that results were similar when proxy subjects were excluded suggests that including these subjects caused little bias. Arsenic may come from food, occupations, or dust from mine tailings. However, adjustments for arsenic or other carcinogen exposure at work had little effect (Figure 1), and analyses done in Regions I and II have shown that arsenic exposures from food or mine tailings are small compared to the intake associated with consuming water with arsenic concentrations of 110–850 μg/L (37, 38). Errors in identifying cases may have occurred but cases were ascertained using the same procedures throughout the study area, and hospital cancer committees and death certificates were used to locate missed cases. Confounding is also possible but unlikely, given the fact that findings changed little with adjustments.
Overall, we found evidence that lung and bladder cancer incidence in adults was markedly increased following exposure to arsenic in early life up to 40 years after high exposures ceased providing evidence that humans are extraordinarily susceptible to lifelong effects from early-life arsenic exposure. In Chile and elsewhere, many of the highest exposures have ended, but our results suggest that high cancer risks from early-life exposures are likely to continue decades after the exposures are stopped. Public awareness campaigns aimed at reducing important co-exposures might help reduce arsenic-related mortality in these areas (39). Also, routine screening with low-dose lung computed tomography has been shown to reduce mortality in heavy smokers (40), raising the possibility that this may also be effective in people with past arsenic exposure.
Supplementary Material
Acknowledgments
None
Financial Support: C. Steinmaus, C. Ferreccio, J. Acevedo, Y. Yuan, J. Liaw, V. Villagra, A.H. Smith received support from grant R01ES014032 from the National Institute of Environmental Health Sciences. Y. Yuan, J. Liaw, and A.H. Smith received support from grant P42 ES04705 from the National Institute of Environmental Health Sciences.
Footnotes
Conflicts of interest: None
References
- 1.Miller MD, Marty AM, Arcus A, Brown J, Morry D, Sandy S. Differences between children and adults: implications in risk assessment at California EPA. Int J Toxicol. 2001;21:403–18. doi: 10.1080/10915810290096630. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Environmental Protection Agency. Supplemental Guidance for Assessing Susceptibility from Early-Life Exposure to Carcinogens. Washington DC: 2005. [Google Scholar]
- 3.Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med. 1971;284:878–81. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- 4.Nilsson R. Environmental tobacco smoke revisited: the reliability of the data used for risk assessment. Risk Anal. 2001;21:737–60. doi: 10.1111/0272-4332.214147. [DOI] [PubMed] [Google Scholar]
- 5.Preston DL, Cullings H, Suyama A, Funamoto S, Nishi N, Soda M, et al. Solid cancer incidence in atomic bomb survivors exposed in utero or as young children. J Natl Cancer Inst. 2008;100:428–36. doi: 10.1093/jnci/djn045. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Environmental Protection Agency. Integrated Risk Information System. 2013 Accessed 07/10/13. http://www.epa.gov/IRIS/
- 7.Ravenscroft P. Predicting the global distribution of natural arsenic contamination of groundwater. Symposium on arsenic: the geography of a global problem, Royal Geographical Society; London. 2007; Accessed 03/16/12. http://www.geog.cam.ac.uk/research/projects/arsenic/symposium/S1.2_P_Ravenscroft.pdf. [Google Scholar]
- 8.International Agency for Research on Cancer. Some drinking-water disinfectants and contaminants, including arsenic. Vol. 84. Lyon: 2004. [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreccio C, Gonzalez C, Milosavjlevic V, Marshall G, Sancha AM, Smith AH. Lung cancer and arsenic concentrations in drinking water in Chile. Epidemiology. 2000;11:673–79. doi: 10.1097/00001648-200011000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Ferreccio C, Smith AH, Duran V, Barlaro T, Benitez H, Valdes R, et al. Case-control study of arsenic in drinking water and kidney cancer in uniquely exposed Northern Chile. Am J Epidemiol. 2013;178:813–8. doi: 10.1093/aje/kwt059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinmaus CM, Ferreccio C, Acevedo Romo J, Yuan Y, Cortes S, Marshall G, et al. Drinking water arsenic in northern Chile: high cancer risks 40 years after exposure cessation. Cancer Epidemiol Biomarkers Prev. 2013;22:623–30. doi: 10.1158/1055-9965.EPI-12-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith AH, Marshall G, Liaw J, Yuan Y, Ferreccio C, Steinmaus C. Mortality in young adults following in utero and childhood exposure to arsenic in drinking water. Environ Health Perspect. 2012;120:1527–31. doi: 10.1289/ehp.1104867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Instituto Nacional de Estadisticas. Resultados Preliminares del Censo 2012. Santiago, Chile: 2012. Accessed 07/14/13. http://www.censo.cl/ [Google Scholar]
- 14.Ferreccio C, Gonzalez Psych C, Milosavjlevic Stat V, Marshall Gredis G, Sancha AM. Lung cancer and arsenic exposure in drinking water: a case-control study in northern Chile. Cad Saude Publica. 1998;14(Suppl 3):193–8. doi: 10.1590/s0102-311x1998000700021. [DOI] [PubMed] [Google Scholar]
- 15.Smith AH, Goycolea M, Haque R, Biggs ML. Marked increase in bladder and lung cancer mortality in a region of Northern Chile due to arsenic in drinking water. Am J Epidemiol. 1998;147:660–69. doi: 10.1093/oxfordjournals.aje.a009507. [DOI] [PubMed] [Google Scholar]
- 16.Borgono JM, Venturino H, Vicent P. [Clinical and epidemiologic study of arsenicism in northern Chile (author’s transl)] Revista Medica de Chile. 1980;108:1039–48. [PubMed] [Google Scholar]
- 17.CONAMA. Technical Information Sheet: Analysis of Human Exposure to Arsenic in Large Cities (Study No. 21-0022-002) Santiago: Comisión Nacional del Medio Ambiente; 2000. [Google Scholar]
- 18.Rivara MI, Cebrian M, Corey G, Hernandez M, Romieu I. Cancer risk in an arsenic-contaminated area of Chile. Toxicol Ind Health. 1997;13:321–38. doi: 10.1177/074823379701300217. [DOI] [PubMed] [Google Scholar]
- 19.Sancha AM, Frenz P. Estimate of current exposure of the urban population of northern Chile to arsenic. Interdisciplinary Perspectives on Drinking Water Risk Assessment and Management Proceedings of the Santiago (Chile) Symposium; September 1998; IAHS Publ 260 2000. [Google Scholar]
- 20.Zaldivar R. Arsenic contamination of drinking water and foodstuffs causing endemic chronic poisoning. Beitr Pathol. 1974;151:384–400. doi: 10.1016/s0005-8165(74)80047-8. [DOI] [PubMed] [Google Scholar]
- 21.Concha G, Vogler G, Lezcano D, Nermell B, Vahter M. Exposure to inorganic arsenic metabolites during early human development. Toxicol Sci. 1998;44:185–90. doi: 10.1006/toxs.1998.2486. [DOI] [PubMed] [Google Scholar]
- 22.Gauderman WJ, Avol E, Gilliland F, Vora H, Thomas D, Berhane K, et al. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med. 2004;351:1057–67. doi: 10.1056/NEJMoa040610. [DOI] [PubMed] [Google Scholar]
- 23.Svanes C, Omenaas E, Jarvis D, Chinn S, Gulsvik A, Burney P. Parental smoking in childhood and adult obstructive lung disease: results from the European Community Respiratory Health Survey. Thorax. 2004;59:295–302. doi: 10.1136/thx.2003.009746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barker DJ, Godfrey KM, Fall C, Osmond C, Winter PD, Shaheen SO. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ. 1991;303:671–5. doi: 10.1136/bmj.303.6804.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dauphine DC, Ferreccio C, Guntur S, Yuan Y, Hammond SK, Balmes J, et al. Lung function in adults following in utero and childhood exposure to arsenic in drinking water: preliminary findings. Int Arch Occup Environ Health. 2011;84:591–600. doi: 10.1007/s00420-010-0591-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith AH, Yunus M, Khan AF, Ercumen A, Yuan Y, Hira Smith M, et al. Chronic respiratory symptoms in children following in utero and early life exposure to arsenic in drinking water in Bangladesh. Int J Epidemiol. 2013 doi: 10.1093/ije/dyt120. [In press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waalkes MP, Keefer LK, Diwan BA. Induction of proliferative lesions of the uterus, testes, and liver in swiss mice given repeated injections of sodium arsenate: possible estrogenic mode of action. Toxicol Appl Pharmacol. 2000;166:24–35. doi: 10.1006/taap.2000.8963. [DOI] [PubMed] [Google Scholar]
- 28.Waalkes MP, Liu J, Diwan BA. Transplacental arsenic carcinogenesis in mice. Toxicol Appl Pharmacol. 2007;222:271–80. doi: 10.1016/j.taap.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller MD, Marty MA. Impact of environmental chemicals on lung development. Environ Health Perspect. 2010;118:1155–64. doi: 10.1289/ehp.0901856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tokar EJ, Diwan BA, Waalkes MP. Arsenic exposure transforms human epithelial stem/progenitor cells into a cancer stem-like phenotype. Environ Health Perspect. 2010;118:108–15. doi: 10.1289/ehp.0901059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren X, McHale CM, Skibola CF, Smith AH, Smith MT, Zhang L. An emerging role for epigenetic dysregulation in arsenic toxicity and carcinogenesis. Environ Health Perspect. 2011;119:11–9. doi: 10.1289/ehp.1002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dean W, Santos F, Reik W. Epigenetic reprogramming in early mammalian development and following somatic nuclear transfer. Semin Cell Dev Biol. 2003;14:93–100. doi: 10.1016/s1084-9521(02)00141-6. [DOI] [PubMed] [Google Scholar]
- 33.Hitchler MJ, Domann FE. An epigenetic perspective on the free radical theory of development. Free Radic Biol Med. 2007;43:1023–36. doi: 10.1016/j.freeradbiomed.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Birnbaum LS, Fenton SE. Cancer and developmental exposure to endocrine disruptors. Environ Health Perspect. 2003;111:389–94. doi: 10.1289/ehp.5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Preston RJ. Children as a sensitive subpopulation for the risk assessment process. Toxicol Appl Pharmacol. 2004;199:132–41. doi: 10.1016/j.taap.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 36.Nelson L, Longstrentch W, Koepsell T, Checkoway H, van Belle G. Completeness and accuracy of interview data from proxy respondents: demographics, medical, and lifestyle factors. Epidemiology. 1994;5:204–17. doi: 10.1097/00001648-199403000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Ferreccio C, Sancha AM. Arsenic exposure and its impact on health in Chile. J Health Popul Nutr. 2006;24:164–75. [PubMed] [Google Scholar]
- 38.Gobierno Regional Arica y Parincota. Programa Maestro de Intervencion Zonas Con Presencia de Polimetales en Arica. Arica: Sep, 2009. [Google Scholar]
- 39.Ferreccio C, Yuan Y, Calle J, Benitez H, Parra RL, Acevedo J, et al. Arsenic, tobacco smoke, and occupation: associations of multiple agents with lung and bladder cancer. Epidemiology. 2013;24:898–905. doi: 10.1097/EDE.0b013e31829e3e03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. NEJM. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


