Abstract
The small heat shock proteins Hsp12.2 and αB-crystallin differ in that the former occurs as tetramers, without chaperonelike activity, whereas the latter forms multimers and is a good chaperone. To investigate whether the lack of chaperone activity of Hsp12.2 is primarily due to its tetrameric structure or rather to intrinsic sequence features, we engineered chimeric proteins by swapping the N-terminal, C-terminal, and tail regions of Hsp12.2 and αB-crystallin, designated as n-c-t and N-C-T, respectively. Three of the chimeric sHsps, namely N-c-T, n-c-T, and N-C-t, showed nativelike secondary and quaternary structures as measured by circular dichroism and gel permeation chromatography. Combining the conserved α-crystallin domain of Hsp12.2 with the N-terminal and tail regions of αB-crystallin (N-c-T) resulted in multimeric complexes, but did not restore chaperonelike activity. Replacing the tail region of Hsp12.2 with that of αB-crystallin (n-c-T) did not alter the tetrameric structure and lack of chaperone activity. Similarly, providing αB-crystallin with the tail of Hsp12.2 (N-C-t) did not substantially influence the multimeric complex size, but it reduced the chaperoning ability, especially for small substrates. These results suggest that the conserved α-crystallin domain of Hsp12.2 is intrinsically unsuitable to confer chaperonelike activity and confirms that the tail region in αB-crystallin modulates chaperonelike capacity in a substrate-dependent manner.
INTRODUCTION
The ubiquitous small heat shock proteins (sHsps) are evolutionarily related on the basis of a conserved C-terminal domain of 80–100 amino acids, the α-crystallin domain (de Jong et al 1998). Their monomeric masses vary from 12 to 43 kDa, although more typically being between 17 and 30 kDa. Most sHsps form large complexes of 150–800 kDa. The only known sHsp crystal structure, that of Methanococcus jannaschii Hsp16.5, reveals a highly ordered spherical array of 24 subunits around a central cavity, with dimeric building blocks (Kim et al 1998). The C-terminal domain of Hsp16.5 has an immunoglobulinlike fold, composed of 9 β-strands. Secondary structure predictions (de Jong et al 1998) and spin-labeling studies (Koteiche and Mchaourab 1999) indicate that other sHsps possess a similar fold. However, the complexes formed by various eukaryotic sHsps might be less ordered than that of M jannaschii Hsp16.5, as indicated by cryoelectron microscopic analyses (Haley et al 2000).
Most sHsps function in vitro as molecular chaperones by binding unfolding proteins and keeping them in a folding competent state (Horwitz 1992; Ehrnsperger et al 1997; Lee et al 1997). Substrates appear to be bound to exposed hydrophobic sites (Raman and Rao 1994; Das and Surewicz 1995), mostly located in the α-crystallin domain (Smith et al 1996; Lee et al 1997; Sharma et al 1997; Sharma et al 2000). Mutation analyses indicate that also the N-terminal domain (Leroux et al 1997a; Derham et al 2001) and the flexible C-terminal tail (Smulders et al 1996; Fernando and Heikkila 2000; Lindner et al 2000) are involved in the chaperonelike activity.
The nematode Caenorhabditis elegans has 16 sHsps (Ding and Candido 2000), 4 of which are approximately 12 kDa, which are the smallest known sHsps, consisting of little more than the α-crystallin domain. The N-terminal domains are a mere 25–26 residues in length, whereas C-terminal tails are very short or not present at all. Three of these 12-kDa sHsps—Hsp12.2, Hsp12.3, and Hsp12.6—have been characterized and were found to form up to tetramers and lack chaperonelike activity (Leroux et al 1997b; Kokke et al 1998). The absence of chaperone activity was suggested to be due to the inability to form multimeric complexes. However, it has recently been reported that yeast Hsp26 dimers and tetramers of mouse Hsp25 and C elegans Hsp25 can have chaperonelike activity (Ehrnsperger et al 1998; Haslbeck et al 1999; Ding and Candido 2000). It, therefore, is questionable whether the oligomeric form of the Hsp12s per se is responsible for their lack of chaperone activity. It might rather relate to the reduced N-terminal domain and C-terminal tail or to sequence features in the α-crystallin domain itself.
To distinguish between these alternatives, we made domain-swapped mutants of Hsp12.2 and the well-characterized sHsp αB-crystallin, and examined their secondary structures, complex sizes, stabilities, and chaperonelike activities. These data indicate that in addition to the near absence of a tail region, it notably is the α-crystallin domain that is responsible for the lack of chaperonelike activity.
MATERIALS AND METHODS
Cloning of C elegans Hsp12.2 and domain swap mutants
Mutant N-C-t (see Fig 1B for clarification of notation) was obtained by polymerase chain reaction (PCR) using human αB complementary DNA (cDNA) as a template with the primers N-FOR (5′-GGAATTCCATATGGACATCGCCATCCACCACCC-3′; NdeI site underlined) and C-t-REV (5′-ATCTGAGATCTTTATGCTTTCCTTGGTCCATT-3′; BglII site underlined). In these and the following primers, the αB-crystallin part is in roman typeface and the Hsp12.2 part in italic typeface. To obtain mutant n-c-T, 2 independent PCRs were performed: one to synthesize the T fragment, containing a partial overlap with the n-c part, using human αB cDNA as template and the primers T-FOR (5′-CCATCACTGCTTCCAAGAAACAGGTCTCTGGCCCT-3′) and T-REV (5′-ATCTGAGATCTTTATTTCTTGGGGGCTGCGG-3′; BglII site underlined), and a second PCR using Hsp12.2 cDNA and the T fragment as templates together with the n-FOR (5′-GGAATTCCATATGTCCGCTATCGAGGTGAC-3′; NdeI site underlined) and T-REV primers. Mutant n-C-T was synthesized by 3 independent PCRs: one to synthesize fragment n with an overlap with the C-T part, using Hsp12.2 cDNA as a template and the primers n-FOR and n-REV (5′-CTGTCCTTCTCCAGGCGCATCTTGACAACTCCGTCGTTGT-3′) and another to obtain the C-T fragment with an overlap with n, using human αB-crystallin cDNA as a template and the primers C-FOR (5′-ACAACGACGGAGTTGTCAAGATGCGCCTGGAGAAGGACAG-3′) and T-REV. Finally, a PCR was performed using fragment n and fragment C-T as templates and n-FOR and T-REV as primers to obtain the n-C-T product. Mutant N-c-t was synthesized by first amplifying separately the domains N and c-t. The N fragment was amplified using the primers N-FOR and N-REV (5′-TTTTCCTTGGTGTTGTGTACCTCTGAGAGTCCAGTGTCAA-3′) and has a partial overlap with the c-domain. A second PCR was performed using the primers c-FOR (5′-TTGACACTGGACTCTCAGAGGTACACAACACCAAGGAAAA-3′) and c-t-REV (5′-ATCTGGGATCCTTAAGCCTTCTTGGAAGCAG-3′; BamHI site underlined), resulting in the c-t domain with a partial overlap of the N part. Next, the N-c-t product was amplified using the primers N-FOR and c-t-REV. To synthesize the N-c-T mutant, the N-c-t cDNA and the T fragment (with c overlap) were used as templates in a PCR using the primers N-FOR and T-REV. The PCR products of the mutants were ligated in the NdeI-BamHI sites of the pET3a expression vector and checked by sequencing.
Fig. 1.
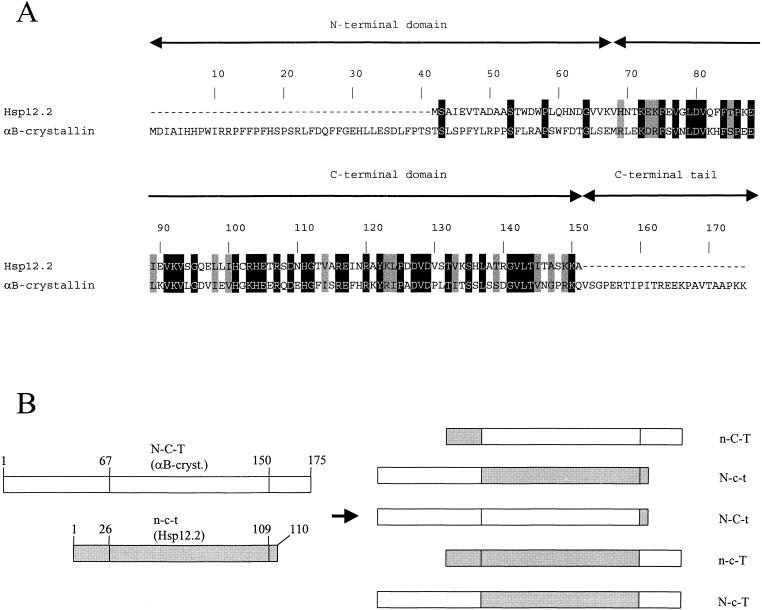
Alignment and swap mutants of C elegans Hsp12.2 and human αB-crystallin. (A) Alignment of Hsp12.2 and αB-crystallin. Identical residues are in black and similar residues in gray. The demarcation of domains is according to Leroux et al (1997b) and de Jong et al (1998). (B) Schematic representation of domains in αB-crystallin (open bars, uppercase N-C-T), Hsp12.2 (gray bars, lowercase n-c-t), and their swap mutants. Numbers indicate residue positions in the proteins. N,n: N-terminal domain; C,c: C-terminal domain; T,t: C-terminal tail. Note that t consists of only 1 residue
Expression and purification of recombinant proteins
Proteins were expressed in Escherichia coli BL21(DE3) as described previously (Kokke et al 1998). After lysis and centrifugation, αB-crystallin, n-C-T, n-c-T, and N-c-T were largely present in the soluble fraction, whereas Hsp12.2, N-C-t, and N-c-t were mainly in the pellet.
The soluble fractions containing αB-crystallin, n-C-T, n-c-T, and N-c-T were dialyzed against 20 mM Tris-HCl (pH 6.9, pH 7.5, pH 7.5, and pH 8.5, respectively) and purified over a Fast Flow DEAE-Sepharose column (Pharmacia-LKB) using the same buffers. The sHsps eluted in the flow-through were pooled, freeze-dried, and stored at −20°C. To further purify N-c-T, the freeze-dried protein was resolubilized in 4 mL of water, dialyzed against 20 mM Tris-HCl (pH 8.0), 1 mM EDTA, and 50 mM sodium chloride, and fractionated on a Superose 6 column (HR 30/10, Amersham-Pharmacia) in the same buffer. Fractions containing the purified protein were pooled, freeze-dried, and stored at −20°C.
Pellets containing Hsp12.2, N-C-t, and N-c-t were dissolved in DE-52 starting buffer (6 M urea, 0.02% β-mercaptoethanol, 5 mM Tris-HCl [pH 8.0]), dialyzed against the same buffer, and centrifugated. The supernatant was fractionated on a DE-52 column using a gradient from 5 to 100 mM Tris-HCl (pH 8.0) (de Jong et al 1984). Fractions containing purified proteins were dialyzed against water, freeze-dried, and stored at −20°C. Before performing structural and functional studies, N-c-t, N-C-t, and Hsp12.2 were refolded by dissolving 1 mg of protein in 1 mL of denaturing buffer, which contained 6 M urea in TGN (20 mM Tris-HCl [pH 8.0], 6% glycerol, 200 mM NaCl), incubating overnight at 4°C, and slowly removing the urea by dialysis against a gradient from denaturing buffer to TGN buffer throughout 72 hours. The refolded proteins were finally dialyzed against the particular buffer that was required for the subsequent analysis.
Secondary structure measurement
Circular dichroism (CD) spectra were recorded on a Jasco J-810 spectropolarimeter. Spectra shown are the average of 4 scans, with a scan rate of 20 nm/min and a quartz cell length of 1 mm. Experiments were performed at a protein concentration of 250 μg/mL in 20 mM Tris-HCl (pH 8.0) and 20 mM NaCl at 20°C.
Heat stability
Heat-induced aggregation was measured on a Perkin-Elmer Lambda 2 UV/Vis spectrophotometer connected to a thermostatted circulating water bath and a thermocouple to register the sample temperature. Temperature was raised at 1°C/min and absorption was measured at 360 nm with intervals of 30 seconds. Protein concentration was 250 μg/mL in 20 mM Tris-HCl (pH 8.0) and 100 mM NaCl.
Heat-induced unfolding was measured on a Jasco J-810 spectropolarimeter and performed with a temperature range from 20°C to 70°C, using a quartz cell with 1-mm path length. Samples were heated at a rate of 1°C/min and were left to incubate for 4 minutes at each temperature before measuring the ellipticity at 205 nm. Concentration of the proteins was 250 μg/mL in phosphate buffer (20 mM NaPi [pH 6.9], 100 mM Na2SO4).
Gel permeation chromatography
An LKB Bromma high-performance liquid chromatography system was used in conjunction with a Superose 12 HR 10/30 prepacked column (Amersham-Pharmacia) for analysis of complex size. Samples containing 250 μg of protein in 1 mL of TGN buffer were incubated for 15 minutes at room temperature before being applied and eluted with TGN buffer at a flow rate of 0.5 mL/min. Absorbance was measured at 280 nm. High and low molecular mass standards (Amersham-Pharmacia) were used for calibration.
Chaperone assays
The capacities of the studied proteins to prevent aggregation of unfolding substrates were studied in the insulin assay, in which aggregation of the insulin B-chain is induced by reduction with DTT (Farahbakhsh et al 1995), and the citrate synthase assay, in which thermally unfolding citrate synthase is used as a substrate (Ehrnsperger et al 1997).
RESULTS
Construction and structural characterization of domain swap mutants
To study whether a gain of chaperone function can be achieved for C elegans Hsp12.2, the N-terminal region, the C-terminal domain, and the C-terminal tail residue were exchanged with the homologous regions of human αB-crystallin. αB-crystallin was chosen because it is well characterized and its α-crystallin domain is very similar to that of Hsp12.2 (43% identity; Fig 1A). Five swap mutants were constructed (Fig 1B), expressed in E coli, and purified to more than 95% as estimated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (data not shown). The purified chimeras N-c-t and n-C-T were very unstable and could not be further analyzed.
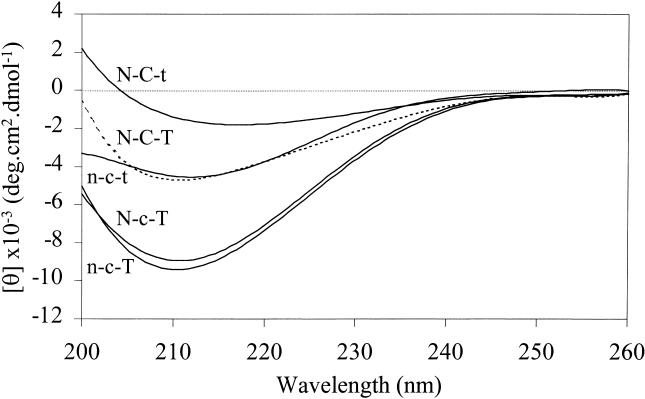
Correct folding of the other recombinant proteins was assessed by far-UV CD spectroscopy (Fig 2). The spectra for Hsp12.2 (n-c-t) and αB-crystallin (N-C-T) show, as expected, negative ellipticities with minima around 213 and 211 nm, respectively (Leroux et al 1997b; Kokke et al 1998; Kumar and Rao 2000). The chimeras n-c-T and N-c-T show an increased ellipticity compared with N-C-T and n-c-t, but the overall spectra are characteristic for β-sheet proteins, suggesting that they, too, are properly folded. The ellipticity of N-C-t is less negative than that of N-C-T, and the minimum is at 217 nm. This latter difference may reflect the decreased content of random coil due to the loss of the flexible C-terminal tail.
Fig. 2.
Secondary structure determination. Far-UV CD spectra of αB-crystallin (N-C-T), Hsp12.2 (n-c-t), and the domain swap mutants N-C-t, N-c-T, and n-c-T
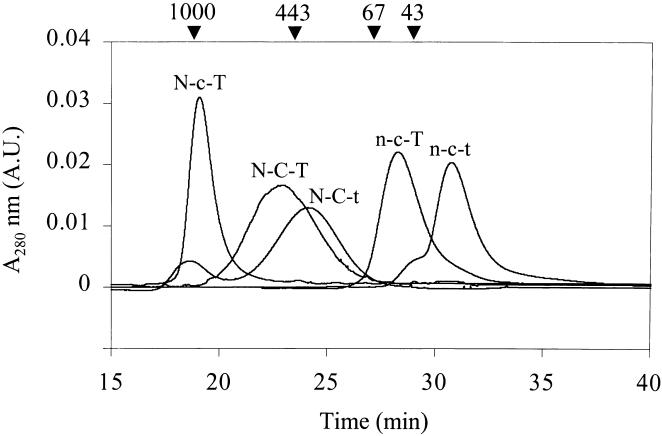
As shown in Figure 3, Superose 12 gel permeation revealed that Hsp12.2 (n-c-t) and αB-crystallin (N-C-T) elute as 39- and 500-kDa complexes, corresponding with tetramers and multimers of about 24 subunits, respectively (Kokke et al 1998; Sun et al 1998). The swap mutant n-c-T gives a peak of 56 kDa, also indicative of a tetramer, whereas N-C-t elutes as a multimer of around 400 kDa. Taking into account that N-C-t is approximately 15% smaller than N-C-T, the mass of 400 kDa may still correspond with a 24-mer. Thus, changing the length of the C-terminal tail region of αB-crystallin (N-C-T to N-C-t) and Hsp12.2 (n-c-t to n-c-T) does not seem to affect the number of subunits in the complex. The chimera N-c-T elutes at the void volume of the column, ie, forming complexes larger than 1 MDa. The large complex size of N-c-T seems to relate to the N-terminal region of αB-crystallin, because the mutant n-c-T forms tetramers. However, the α-crystallin domain must be involved, too, because exchanging this domain (N-C-T to N-c-T) increases the complex size from 500 to more than 1000 kDa.
Fig. 3.
Determination of complex size. Superose 12-gel permeation chromatography of αB-crystallin (N-C-T), Hsp12.2 (n-c-t), and the mutants N-C-t, N-c-T, and n-c-T. Arrowheads indicate elution times of molecular mass markers
Thermostability of chimeras
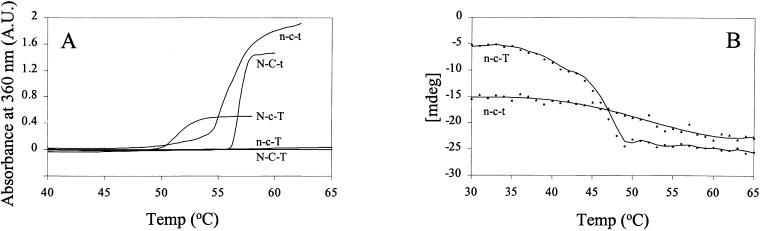
The thermostabilities of the chimeras were compared with those of their “parent” proteins αB-crystallin and Hsp12.2 by incubating them at increasing temperatures in a thermostatted UV/Vis-spectrophotometer. Figure 4A shows that αB-crystallin (N-C-T) remained soluble up to 65°C. As shown before, Hsp12.2 (n-c-t) started to aggregate at 48°C (Kokke et al 1998). The chimeras N-C-t and N-c-T aggregated rapidly at 56°C and 48°C, respectively, showing a reduced thermostability compared with αB-crystallin (N-C-T). Interestingly, n-c-T did not noticeably aggregate, even after a 1-hour incubation at 60°C (data not shown). Thus, the mutant n-c-T would seem to be more stable than Hsp12.2 (n-c-t), but it is possible that n-c-T unfolds without aggregating because of the presence of the hydrophilic tail T of αB-crystallin. To assess this latter possibility, CD studies of Hsp12.2 (n-c-t) and n-c-T were performed at increasing temperatures (Fig 4B). Ellipticity at 205 nm is a measure for random coil structure and thus for unfolding. The midpoints of the unfolding curves are around 50°C and 47°C, respectively, indicating that n-c-T is actually even less stable than Hsp12.2 (n-c-t). The inability of n-c-T to aggregate at high temperatures is thus apparently due to the presence of the flexible C-terminal tail.
Fig. 4.
Thermostability measurements. (A) Turbidity at increasing temperatures measured at 360 nm for αB-crystallin (N-C-T), Hsp12.2 (n-c-t), and the mutants N-C-t, N-c-T, and n-c-T. (B) Temperature dependence of ellipticity at 205 nm in CD spectra of n-c-t and n-c-T
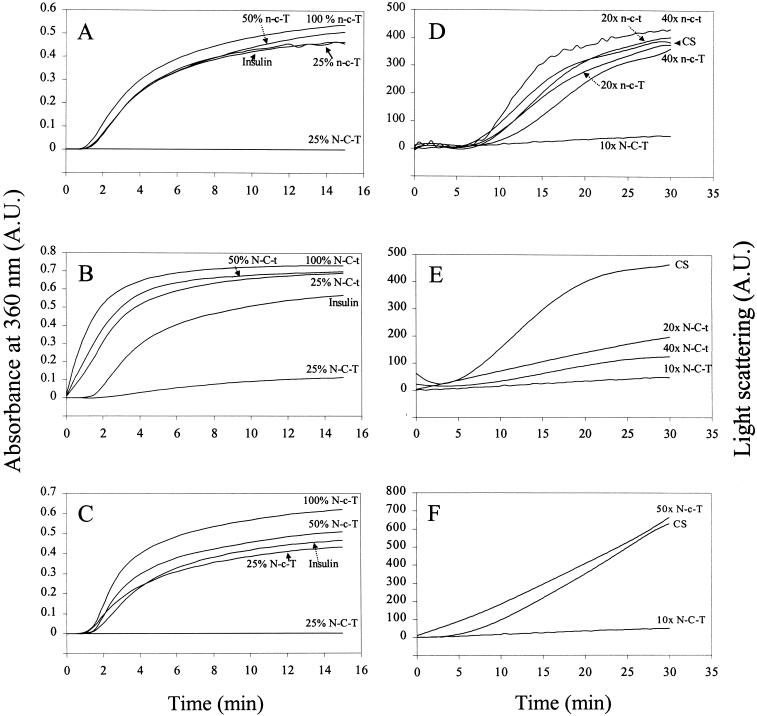
Chaperonelike activity
To determine the chaperonelike capacity of the chimeras, we used 2 different assays. In the insulin assay, none of the chimeras showed any ability to prevent the aggregation of the unfolding substrate (Fig 5, A–C). For n-c-T and N-c-T this apparent lack of chaperonelike activity was confirmed in the citrate synthase assay (Fig 5, D,F). N-C-t, however, was able to inhibit the precipitation of citrate synthase, although to a considerably lesser extent than αB-crystallin (N-C-T) (Fig 5E). The lack of chaperone activity of n-c-T and N-c-T might suggest that the α-crystallin domain of Hsp12.2 is unable to confer chaperonelike activity. The limited activity of N-C-t indicates that the C-terminal 25 amino acids of αB-crystallin have an important role in the chaperonelike activity.
Fig. 5.
Chaperone assays of domain swap mutants. (A-C) Reduction-induced aggregation of insulin B chain at 40°C as a function of time in the absence and presence of various amounts of n-c-T, N-C-t, and N-c-T (top to bottom). N-C-T (αB-crystallin) is used as a positive control. sHsp to insulin ratios (wt/wt) are indicated. (D-F) Temperature-induced aggregation of citrate synthase at 43°C in the absence and presence of domain swap mutants and Hsp12.2 (n-c-t) and αB-crystallin (N-C-T) as a control. Molar ratios of sHsp to citrate synthase are shown
DISCUSSION
The sHsps all share a conserved C-terminal domain of 80–100 amino acids, which is thought to be responsible for the common features of this family. When making swap mutants to study the properties of this domain, it is important to choose the proper exchange sites. The demarcation between the N- and C-terminal domains is generally taken to correspond with the boundary between the exons encoding these domains in α-crystallin (de Jong et al 1998). However, in the crystal structure of M jannaschii Hsp16.5, 2 β-strands are located N-terminally of this position and are part of the immunoglobulinlike structure of the C-terminal domain (Kim et al 1998). The sequence of this region, as well as the predicted presence of the 2 β-strands, is not conserved in other sHsps (Koteiche and Mchaourab 1999; Kappé, Leunissen, and de Jong, unpublished results). Therefore, our choice to swap at the classic border between the N- and C-terminal domain (Leroux et al 1997b; de Jong et al 1998) is somewhat arbitrary. However, the studied chimeras, N-c-T, N-C-t and n-c-T, appeared well folded as judged from their far-UV CD spectra (Fig 2).
Mutant N-c-T is unable to protect substrate proteins from heat- or reduction-induced aggregation (Fig 5 C,F). This is unlikely to be due to its large complex size (Fig 3), since sHsps mutants with similar sizes have been reported as having chaperonelike activity (Horwitz et al 1998; Kumar and Rao 2000). Also the somewhat lower thermostability of N-c-T (Fig 4A) is unlikely to be the reason for its lack of chaperonelike activity, since the unfolding temperature is above the temperature used in the 2 chaperone assays. The most likely explanation then is that the α-crystallin domain of Hsp12.2 is intrinsically unsuitable to confer chaperonelike activity, even when fitted with the N-terminal domain and C-terminal tail of a good chaperone like αB-crystallin. Although sequences in the C-terminal domain of αA- and αB-crystallin that are supposed to be involved in substrate binding (Sharma et al 2000) are reasonably conserved in Hsp12.2, this is apparently not sufficient to warrant chaperonelike activity.
However, it is most likely that the chaperonelike functioning of sHsps also depends on the synergistic interaction of the α-crystallin domain with the N-terminal domain and C-terminal tail. We, therefore, cannot exclude that the lack of chaperonelike activity of N-c-T is due to the inability of the N- and C-terminal regions of αB-crystallin to function in conjunction with the α-crystallin domain of Hsp12.2. The finding that swapping the N-terminal domains of αA- and αB-crystallin, both good chaperones, abolishes chaperone activity in one chimera and enhances it in the other (Kumar and Rao 2000) indicates how subtle the requirements for interaction between the N- and C-terminal domains of different sHsps may be.
Replacing the C-terminal tail of αB-crystallin by that of Hsp12.2 (N-C-T to N-C-t), which is in fact a truncation of 24 amino acids (Fig 1A), resulted in complete loss of protective activity in the insulin aggregation assay, whereas a reasonably good protection was found in the citrate synthase assay. Interestingly, also C-terminally truncated mouse Hsp25 has been shown to have reduced activity in the reduction-based α-lactalbumin assay, whereas being completely active in the citrate synthase assay (Lindner et al 2000). It thus seems that the hydrophilic tail of the sHsps plays a role in substrate specificity. This might relate to the size of the substrate. Insulin and α-lactalbumin are both small peptides of 7 and 14 kDa, respectively, whereas citrate synthase is a dimer of 50-kDa subunits. Since sHsps bind unfolding proteins in their molten globule state (Rajaraman et al 1996; Lindner et al 1997), in which a protein is only slightly unfolded, it is likely that citrate synthase is already bound after minor unfolding. Most of the citrate synthase molecule will still be intact and capable of keeping itself in solution, thus being less dependent on the hydrophilic C-terminal tails in the sHsp complex. Moreover, the large size of citrate synthase might limit the number of substrate molecules that can bind to the sHsp complex, thus avoiding overloading it with substrates. In contrast, denaturing insulin B and α-lactalbumin become relatively more unfolded because of their smaller size. Moreover, being small, many peptides can bind to the sHsp complex and depend much more on the flexible sHsp tail to keep the chaperone-substrate complex in solution. If the tail is not there, as in N-C-t, the complex becomes unstable and precipitates.
In summary, the α-crystallin domain of Hsp12.2, and not its tetrameric structure, is probably responsible for the inability of this protein to function as a molecular chaperone, because addition of the N-terminal and C-terminal regions of αB-crystallin, which have been shown to be important for increasing the complex size, does not restore chaperonelike activity.
Acknowledgments
This investigation was carried out under auspices of the Netherlands Organization for Scientific Research.
REFERENCES
- Das KP, Surewicz WK. Temperature-induced exposure of hydrophobic surfaces and its effect on the chaperone activity of α-crystallin. FEBS Lett. 1995;369:321–325. doi: 10.1016/0014-5793(95)00775-5. [DOI] [PubMed] [Google Scholar]
- de Jong WW, Caspers GJ, Leunissen JA. Genealogy of the α-crystallin-small heat-shock protein superfamily. Int J Biol Macromol. 1998;22:151–162. doi: 10.1016/s0141-8130(98)00013-0. [DOI] [PubMed] [Google Scholar]
- de Jong WW, Zweers A, Versteeg M, Nuy-Terwindt EC. Primary structures of the α-crystallin A chains of twenty-eight mammalian species, chicken and frog. Eur J Biochem. 1984;141:131–140. doi: 10.1111/j.1432-1033.1984.tb08167.x. [DOI] [PubMed] [Google Scholar]
- Derham BK, van Boekel MAM, Muchowski PJ, Clark JI, Horwitz J, Hepburne-Scott HW, Crabbe JC, Harding JJ. Chaperone function of mutant versions of αA- and αB-crystallin prepared to pinpoint chaperone binding sites. Eur J Biochem. 2001;268:713–721. doi: 10.1046/j.1432-1327.2001.01929.x. [DOI] [PubMed] [Google Scholar]
- Ding L, Candido EPM. HSP25, a small heat shock protein associated with dense bodies and M-lines of body wall muscle in Caenorhabditis elegans. J Biol Chem. 2000;275:9510–9517. doi: 10.1074/jbc.275.13.9510. [DOI] [PubMed] [Google Scholar]
- Ehrnsperger M, Gräber S, Gaestel M, Buchner J. Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J. 1997;16:221–229. doi: 10.1093/emboj/16.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrnsperger M, Hergersberg C, Wienhues U, Nichtl A, Buchner J. Stabilization of proteins and peptides in diagnostic immunological assays by the molecular chaperone Hsp25. Anal Biochem. 1998;259:218–225. doi: 10.1006/abio.1998.2630. [DOI] [PubMed] [Google Scholar]
- Farahbakhsh ZT, Huang QL, Ding LL, Altenbach C, Steinhoff HJ, Horwitz J, Hubbell WL. Interaction of α-crystallin with spin-labeled peptides. Biochemistry. 1995;34:509–516. doi: 10.1021/bi00002a015. [DOI] [PubMed] [Google Scholar]
- Fernando P, Heikkila JJ. Functional characterization of Xenopus small heat shock protein, Hsp30C: the carboxyl end is required for stability and chaperone activity. Cell Stress Chaperones. 2000;5:148–159. doi: 10.1379/1466-1268(2000)005<0148:fcoxsh>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley DA, Bova MP, Huang QL, Mchaourab HS, Stewart PL. Small heat-shock protein structures reveal a continuum from symmetric to variable assemblies. J Mol Biol. 2000;298:261–272. doi: 10.1006/jmbi.2000.3657. [DOI] [PubMed] [Google Scholar]
- Haslbeck M, Walke S, Stromer T, Ehrnsperger M, White HE, Chen S, Saibil HR, Buchner J. Hsp26: a temperature-regulated chaperone. EMBO J. 1999;18:6744–6751. doi: 10.1093/emboj/18.23.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J. α-Crystallin can function as a molecular chaperone. Proc Natl Acad Sci U S A. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J, Bova M, Huang QL, Ding L, Yaron O, Lowman S. Mutation of αB-crystallin: effects on chaperone-like activity. Int J Biol Macromol. 1998;22:263–269. doi: 10.1016/s0141-8130(98)00024-5. [DOI] [PubMed] [Google Scholar]
- Kim KK, Kim R, Kim S-H. Crystal structure of a small heat-shock protein. Nature. 1998;394:595–599. doi: 10.1038/29106. [DOI] [PubMed] [Google Scholar]
- Kokke BPA, Leroux MR, Candido EPM, Boelens WC, de Jong WW. Caenorhabditis elegans small heat-shock proteins Hsp12.2 and Hsp12.3 form tetramers and have no chaperone-like ability. FEBS Lett. 1998;433:228–232. doi: 10.1016/s0014-5793(98)00917-x. [DOI] [PubMed] [Google Scholar]
- Koteiche HA, Mchaourab HS. Folding pattern of the α-crystallin domain in αA-crystallin determined by site-directed spin labeling. J Mol Biol. 1999;294:561–577. doi: 10.1006/jmbi.1999.3242. [DOI] [PubMed] [Google Scholar]
- Kumar LVS, Rao ChM. Domain swapping in human αA and αB crystallins affects oligomerization and enhances chaperone-like activity. J Biol Chem. 2000;275:22009–22013. doi: 10.1074/jbc.M003307200. [DOI] [PubMed] [Google Scholar]
- Lee GJ, Roseman AM, Saibil HR, Vierling E. A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state. EMBO J. 1997;16:659–671. doi: 10.1093/emboj/16.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux MR, Ma BJ, Batelier G, Melki R, Candido EPM. Unique structural features of a novel class of small heat shock proteins. J Biol Chem. 1997b;272:12847–12853. doi: 10.1074/jbc.272.19.12847. [DOI] [PubMed] [Google Scholar]
- Leroux MR, Melki R, Gordon B, Batelier G, Candido EPM. Structure-function studies on small heat shock protein oligomeric assembly and interaction with unfolded polypeptides. J Biol Chem. 1997a;272:24646–24656. doi: 10.1074/jbc.272.39.24646. [DOI] [PubMed] [Google Scholar]
- Lindner RA, Carver JA, and Ehrnsperger M. et al. 2000 Mouse Hsp25, a small heat shock protein. Eur J Biochem. 267:1923–1932. [DOI] [PubMed] [Google Scholar]
- Rajaraman K, Raman B, Rao ChM. Molten-globule state of carbonic anhydrase binds to the chaperone-like α-crystallin. J Biol Chem. 1996;271:27595–27600. doi: 10.1074/jbc.271.44.27595. [DOI] [PubMed] [Google Scholar]
- Raman B, Rao ChM. Chaperone-like activity and quaternary structure of α-crystallin. J Biol Chem. 1994;269:27264–27268. [PubMed] [Google Scholar]
- Sharma KK, Kaur H, Kester K. Functional elements in molecular chaperone α-crystallin: identification of binding sites in αB-crystallin. Biochem Biophys Res Comm. 1997;239:217–222. doi: 10.1006/bbrc.1997.7460. [DOI] [PubMed] [Google Scholar]
- Sharma KK, Kumar RS, Kumar GS, Quinn PT. Synthesis and characterization of a peptide identified as a functional unit in αA-crystallin. J Biol Chem. 2000;275:3767–3771. doi: 10.1074/jbc.275.6.3767. [DOI] [PubMed] [Google Scholar]
- Smith JB, Liu Y, Smith DL. Identification of possible regions of chaperone activity in lens α-crystallin. Exp Eye Res. 1996;63:125–128. doi: 10.1006/exer.1996.0100. [DOI] [PubMed] [Google Scholar]
- Smulders RHPH, Carver JA, Lindner RA, Van Boekel MAM, Bloemendal H, de Jong WW. Immobilization of the C-terminal extension of bovine αA-crystallin reduces chaperone-like activity. J Biol Chem. 1996;271:29060–29066. doi: 10.1074/jbc.271.46.29060. [DOI] [PubMed] [Google Scholar]
- Sun TX, Liang JJN. Intermolecular exchange and stabilization of recombinant human αA- and αB-crystallin. J Biol Chem. 1998;273:286–290. doi: 10.1074/jbc.273.1.286. [DOI] [PubMed] [Google Scholar]