Abstract
Curcumin, a well-known dietary pigment derived from Curcuma longa, has been shown to be a potent anti-inflammatory, antioxidant, and anticarcinogenic compound. The present study was designed to investigate the cytotoxic potential of curcumin against a range of human tumor cell lines in an attempt to understand its mechanism of action, which may lead to its possible therapeutic applications. We have shown that different cancer cell lines differ in their sensitivity to curcumin. Cell lines established from malignancies like leukemia, breast, colon, hepatocellular, and ovarian carcinomas underwent apoptosis in the presence of curcumin, whereas cell lines from lung, kidney, prostate, cervix, CNS malignancies, and melanomas showed resistance to the cytotoxic effects of curcumin. Sensitivity of the cancer cell lines to curcumin correlated with the generation of superoxide radicals as determined by the reduction of ferricytochrome C. Curcumin-resistant tumor cell lines showed significantly higher production of Hsp70, thus mounting a stress response and protecting the cells from the apoptotic cell death. These observations yield clues toward understanding the regulation of the cell death machinery by the stress proteins. Interestingly, curcumin had no effect on nontransformed cell lines, which showed neither superoxide generation nor the induction of a stress response. These observations demonstrate that curcumin is an interesting molecule with varied actions, depending on the cell type.
INTRODUCTION
Curcumin (1,7-bis [4-hydroxy phenyl]-1,6-heptadiene-3,5-dione), the biologically active compound from the rhizomes of Curcuma longa, has been known in the traditional Indian medicine for centuries. Curcumin has been shown to possess anti-inflammatory, antimutagenic, and anticarcinogenic effects (Pachuari and Mukherjee 1956; Sharma 1976; Kuttan et al 1985). Curcumin also exhibits an antiproliferative capability and is therefore identified as a potent anticancer agent (Mehta et al 1997; Jee et al 1998; Kawamori et al 1999). The cytotoxic activity of curcumin has been observed in a wide range of cell lines like NIH3T3, mouse sarcoma S180, human kidney cancer cells 293 (Jiang et al 1996), and human basal cell carcinoma (Jee et al 1998). Dietary administration of curcumin significantly suppressed development of chemically induced tumors in mice (Huang et al 1994). Its chemopreventive activity was observed when it was administered during the promotion/progression stages of colon cancer (Kawamori et al 1999). Very little is known about the exact mechanism of action of curcumin. Some of its reported biological activities are an inducer of heat shock proteins (Kato et al 1998), a modulator of Ca++ ion flux and p53 (Chen et al 1996), an inhibitor of nitric oxide synthase gene expression (Chan et al 1998), and an inducer of apoptosis in cancer cells (Jiang et al 1996).
Heat shock response or stress response is among the most highly conserved cellular responses across the species (Welch 1990). Cells respond to external stress, resulting in the expression of certain genes and their products, which are called heat shock proteins (Hsps) (Kiang and Tsokos 1998). Heat shock proteins consist of both stress inducible and constitutive family members which perform housekeeping functions and act as molecular chaperones by helping nascent polypeptides assume their proper conformation (Kiang and Tsokos 1998; Sharp et al 1999). Hsps have been shown to participate in antigen presentation, intracellular trafficking and apoptosis (Kiang and Tsokos 1998; Sharp et al 1999). Hsp70 plays an important role during the apoptotic process, and overexpression studies have shown that Hsp70 induces a protective response against apoptosis (Mosser et al 2000). Under the conditions of stress, Hsp70, once synthesized, binds to denatured proteins in an ATP-dependent fashion to prevent further denaturation and help in proper refolding into native conformation (Freeman et al 1995), and the cell lines lacking the heat shock response were triggered into apoptotic process (Sreedhar et al 1999).
We have recently demonstrated cytotoxic activity of curcumin against a rat histiocytoma (Khar et al 1999). Curcumin-mediated tumor cell death is through apoptosis, which involves the generation of reactive oxygen species and the specific activation of caspase-3 (Bhaumik et al 1999). In the present study, we investigated the sensitivity of different human tumor cell lines towards the cytotoxic activity of curcumin in vitro. Our studies implicate differential behavior of various cell lines to the apoptotic potential of curcumin. The ability of curcumin to induce apoptosis in sensitive cell lines depends, at least in part, on its ability to generate superoxide radicals. In addition, our results strongly suggest the overexpression of Hsp70 and the mounting of stress response protects cells from the cytotoxic effects of curcumin. Moreover, curcumin had no effect on primary cells and nontransformed cell lines. Curcumin being a dietary component, these observations may be useful in the generation of long-term prophylactic effects for certain types of malignancies.
MATERIALS AND METHODS
Tumor cell lines
All the human tumor cell lines used in this study were from the National Cancer Institute/American type culture collection and have been adapted to grow in DMEM containing 10% FCS and a mixture of penicillin (100 U/mL) and streptomycin (50 μg/mL). The adhered cell lines were subcultured after mild trypsinization.
Nontransformed cell lines and primary cultures
Rat skin fibroblasts (RSF), CHO, L-929, F111, and human corneal epithelial cells (HCE) were maintained in DMEM containing 10% FCS. RSF cells were obtained after enzymatic (trypsin and collagenase) dispersion of neonatal rat skin. Lymphocytes and hepatocytes were produced after teasing the spleen and liver, respectively, by flushing sterile DMEM with the help of a syringe. Primary cells were maintained in DMEM-FCS.
Treatment with curcumin
Different human tumor cell lines (1 × 106 cells) were incubated with different concentrations (10–75 μM) of curcumin for 24 and 48 hours to arrive at optimal concentration and incubation time. Stock solution of curcumin (Sigma, St Louis, MO, USA) in ethanol was diluted in the culture medium to obtain the required concentrations keeping the final ethanol concentration below 1%.
Immunofluorescence and flow cytometry
Washed tumor cells were treated with anti-Fas-L (1:100, Santa Cruz Biotechnology [clone SC-834]/Calbiochem, La Jolla, CA, USA) for 1 hour. The cells were washed and treated with FITC-conjugated anti-rabbit Ig (1:250) (Amersham International, Amersham, UK) for 30 minutes. The cells were washed thoroughly, and the percentage of positive cells was quantified by flow cytometry using FACStar Plus (Becton Dickinson, Mountain View, CA, USA).
Western blotting
Cell lysates from curcumin-treated and untreated cells were run on SDS-polyacrylamide gel and transferred to nitrocellulose membrane. The blots were probed with anticytochrome C (Pharmingen, San Diego, USA) and anti-Hsp70 (Stressgen Biotech Corporation, Victoria, BC, Canada) antibodies. The bound primary antibody was detected with anti-mouse IgG coupled to alkaline phosphatase (1:5000) and developed with BCIP substrate and NBT.
Isolation of RNA and Northern hybridization
Total cellular RNA was isolated from different cell lines by the guanidinium thiocyanate–phenol–chloroform method. RNA samples were subjected to electrophoresis on 1% agarose gel containing 2% formaldehyde and 3-(N-morpholino)propanesulfonic acid/EDTA buffer. The fractionated RNA was transferred to Hybond N+ membrane (Amersham) in 50 mM NaOH. The blots were prehybridized for 6 hours at 65°C in hybridization buffer. The cDNA probe for Hsp70 was labeled with [32P]dATP by the random primer labeling method. The labeled probe (106 cpm/mL) was added, and the hybridization was carried out overnight. The blots were washed to a final stringency of 0.2 × SSPE, 0.1% SDS at 65°C and exposed to x-ray film at −70°C.
Generation of superoxide
Superoxide-anion-induced reduction of ferricytochrome C to ferrocytochrome C was monitored spectrophotometrically at 550 nm (Pick 1986); 5 × 104 cells were suspended in complete phenol red-free DMEM and plated in 96-well plates. The cells were treated with curcumin (50 μM), and the superoxide anions released were estimated in the presence of 80 μM cytochrome C with and without SOD (300 U/mL).
In a parallel experiment, NAc (1 mM) inhibitable cytochrome C reduction was estimated after 1 hour of curcumin treatment. The total cellular proteins were estimated with Bradford reagent using BSA as the standard.
Propidium iodide staining and flow cytometry
Tumor cells after the incubation were washed with phosphate-buffered saline (PBS) and fixed in 70% ethanol. Cells were stained with propidium iodide reagent (propidium iodide, Calbiochem, 50 μg/mL in 0.1% sodium citrate containing 0.1% Triton X-100) and analyzed by flow cytometry. The pre-G0/G1 peak represented the apoptotic population of cells.
DNA extraction and electrophoresis
Fixed tumor cells were washed, then suspended in citrate-phosphate buffer, and the fragmented DNA was extracted as described earlier (Bright et al 1995). Fragmented DNA was electrophoresed on 0.8% agarose gel at 2 V/cm for 16 hours and visualized under UV light after staining with 5 μg/mL ethidium bromide. In addition to the extraction of fragmented DNA, the total cellular DNA was also extracted separately by the phenol-chloroform extraction method and analyzed after electrophoresis.
Release of cytochrome C from mitochondria
In order to detect the release of cytochrome C from mitochondria during curcumin-mediated apoptosis, tumor cells (5 × 106) after treatment with curcumin (50 μM) for 4 hours were washed in cold PBS, suspended in 0.5 mL ice-cold cytoplasmic extraction buffer (20 mM HEPES, pH 7.5, 10 mM KCl, 2 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 0.1 mM phenylmethylsulfonylfluoride, 1 mM DTT, and a mixture of protease inhibitors), and incubated on ice for 10 minutes (Yang et al 1997). The cells were disrupted with 40 strokes in a Dounce homogenizer and centrifuged at 3000 × g for 10 minutes at 4°C. The supernatant was further centrifuged at 100 000 × g for 30 minutes and used in Western blotting experiments.
RESULTS
Differential response of tumor cells to the apoptotic effects of curcumin
We tested the sensitivity of different human tumor cell lines to the apoptotic activity of curcumin. Cell lines of the reticuloendothelial origin were sensitive to curcumin (Table 1). Also, ovarian carcinoma, one of the colon carcinoma cell lines, hepatocellular carcinoma, and breast carcinoma cell lines underwent apoptosis on treatment with curcumin. However, one of the cell lines of colon, ovarian, and breast carcinomas was resistant to the effects of curcumin. In addition, cell lines of lung, kidney, prostate, cervix, CNS origin, and melanomas showed total resistance to curcumin even at a higher dose and for a longer incubation time (data not shown). All subsequent experiments were done with a few representative curcumin-resistant and -sensitive cell lines selected randomly.
Table 1.
Curcumin-induced apoptosis in different human tumor cell lines
Oligonucleosomal DNA fragmentation
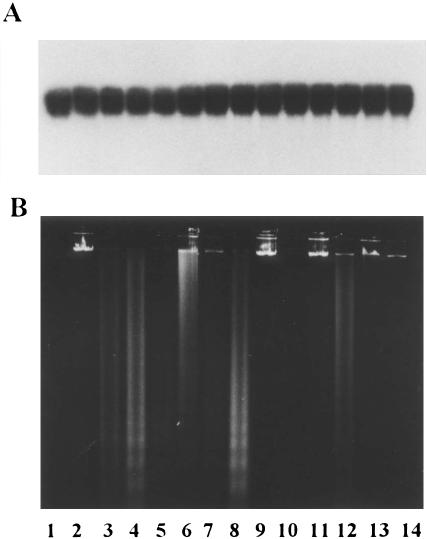
Apoptosis in curcumin-sensitive cell lines was characterized by nuclear fragmentation and formation of apoptotic bodies (data not shown). Oligonucleosomal DNA fragmentation, a hallmark of apoptosis (Wyllie 1980), was also observed in curcumin-sensitive cell lines (Fig 1B). DNA laddering pattern was seen in JURKAT, HL-60, K-562, and MDAMB cells after treatment with curcumin, MCF-7 cell line being an exception. This cell line was earlier shown to lack caspase-3 and hence to be resistant to DNA fragmentation (Janicke et al 1998). On the contrary, the cell lines like SW620 and PA-1, which were resistant to curcumin, did not display DNA fragmentation (Fig 1B). We also looked at the cleavage of high-molecular-weight DNA after curcumin treatment. None of the cell lines tested showed any such cleavage (Fig 1A).
Fig 1.
DNA fragmentation analysis of curcumin-treated human tumor cell lines. (A) High-molecular-weight DNA. (B) Internucleosomal DNA fragments. Lanes 1 and 2: MCF-7 control and treated; lanes 3 and 4: JURKAT control and treated; lanes 5 and 6: HL-60 control and treated; lanes 7 and 8: K-562 control and treated; lanes 9 and 10: SW620 control and treated; lanes 11 and 12: MDAMB control and treated; lanes 13 and 14: PA-1 control and treated. The cell lines were treated with 50 μM curcumin for 24 hours
Curcumin-induced apoptosis involves superoxide generation
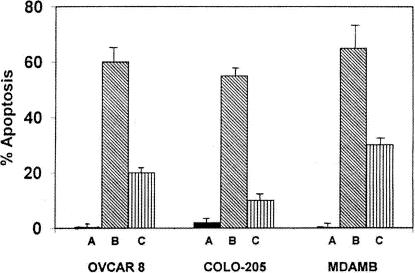
Increase in the generation of ROS has been implicated in some forms of apoptotic cell death where they function as intracellular signaling molecules (Jacobson 1996). In order to understand the mechanism of action of curcumin-induced apoptosis in tumor cells, we studied the generation of superoxide after the treatment of cells with curcumin. Significantly higher levels of superoxide radicals were produced by OVCAR-8, COLO-205, and MDAMB cells in the presence of curcumin (Table 2). Incidentally, all these cell lines are sensitive to curcumin. Cell lines like PA-1 and SW620, which produce low quantities of O2−, are resistant to curcumin-induced apoptosis (Table 2). These observations were confirmed by using NAc, which is an O2− scavenger. NAc and curcumin, when added together, inhibited significantly the apoptotic process in OVCAR-8, COLO-205, and MDAMB cells (Fig 2). These observations indicate an important role for O2− in the induction of apoptosis, produced after treatment of cells with curcumin. Curcumin-induced apoptosis was abrogated by treatment with z-VAD-fmk, a broad-spectrum caspase inhibitor, suggesting the role of caspases in curcumin-induced apoptosis (data not shown).
Table 2.
Curcumin-induced generation of superoxide anions and apoptosis in different tumor cell lines
Fig 2.
Curcumin-induced apoptosis in OVCAR-8, COLO-205, and MDAMB cells. (A) Control. (B) Curcumin (50 μM) treatment. (C) Curcumin and NAc (1 mM) treatment
Release of cytochrome C from mitochondria
Apoptosis in the majority of cells studied proceeds with the translocation of cytochrome C from mitochondria to cytosol (Bossy-Wetzel et al 1998), where it forms a complex with Apaf-1 and caspase-9, leading to the activation of caspase-3 (Li et al 1997). We have analyzed the release of cytochrome C in curcumin-treated cells by Western blotting. A significant release of cytochrome C was observed when HL-60 cells were treated with curcumin for 4 hours (Fig 3B, lane 4). However, we could not observe any significant release of cytochrome C in curcumin-treated COLO-205, OVCAR-8, and HepG2 cells, suggesting that the release of cytochrome C may not occur during curcumin-induced apoptosis.
Fig 3.

Expression of CD95-L (A) and the release of cytochrome C (B) after treatment of cells with curcumin. (A) Lane 1: control; lanes 2–6 represent incubation time of 2, 4, 6, 8, and 24 hours, respectively, of MDAMB cells with curcumin (50 μM). CD95-L expression is maximum at the 6-hour time point. (B) Lanes 1 and 2: AK-5 cells (control); lanes 3 and 4: HL-60; lanes 5 and 6: COLO-205; lanes 7 and 8: HepG2; lanes 9 and 10: OVCAR-8 cells, where the odd lanes represent uninduced and the even lanes represent induced cytosols. Cytochrome C release is shown by HL-60 cells
Induction of CD95-ligand expression by curcumin
We also studied the expression of CD95-ligand by different human tumor cell lines after treatment with curcumin for 24 hours. It was interesting to note that cell lines that were sensitive to curcumin were induced to express CD95-L, whereas the curcumin-resistant cell lines failed to show such induction (Table 3). Treatment of cells with NAc inhibited the expression of CD95-L, thereby suggesting a possible correlation between O2− generation and CD95-L expression. These results were confirmed by Western blotting using MDAMB cells (Fig 3A). MDAMB cells showed CD95-L expression after 6 hours of treatment with curcumin. However, curcumin did not induce the expression of CD95 receptor in these cells. Moreover, anti-CD95-L antibody was unable to block curcumin-induced apoptosis in sensitive cell lines (data not shown). Therefore, the exact role of CD95-L induction after curcumin treatment remains to be investigated.
Table 3.
Curcumin-induced expression of CD95-ligand by tumor cell lines
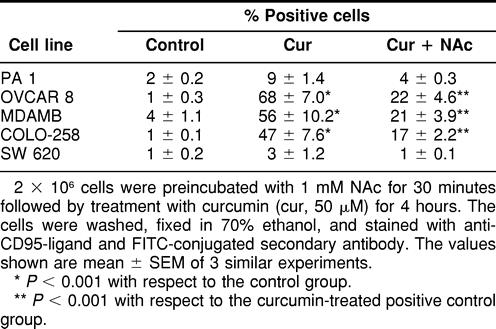
Curcumin-induced stress response
In order to understand the differential activity of curcumin on different cell lines and to investigate the mechanisms involved in imparting either sensitivity or resistance toward curcumin, we studied the expression profile of Hsp70 in tumor cells. It was interesting to note that curcumin-resistant cell lines showed significantly higher expression of Hsp70 after treatment with curcumin, as compared to the curcumin-sensitive cell lines (Fig 4). These observations were confirmed by Northern hybridization where PA-1 cells (resistant to curcumin) produced higher levels of Hsp70 transcripts after 3 hours of treatment with curcumin (Fig 5). There was some expression of Hsp70 in COLO-205 and MDAMB cells after 4 hours of incubation with curcumin (Fig 4); however, this expression may not be sufficient to make these cells resistant to curcumin.
Fig 4.
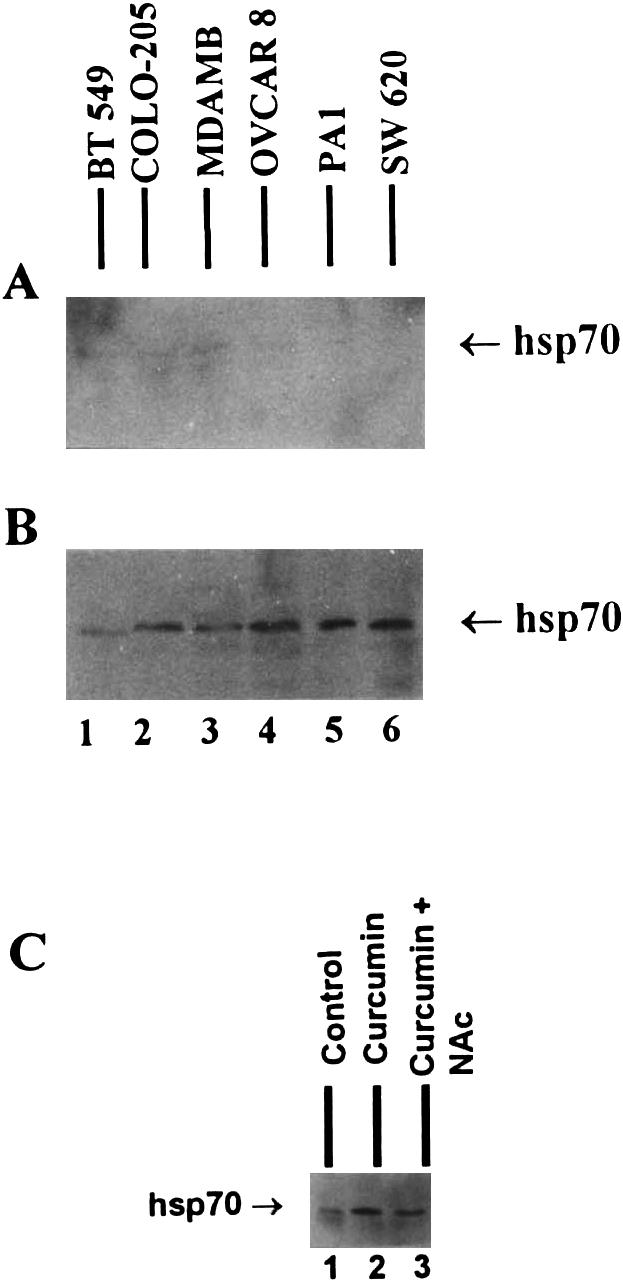
Expression of Hsp70 protein by different tumor cell lines after treatment with curcumin. (A) Untreated control cells. (B) Cells treated with 50 μM curcumin for 4 hours. (C) Partial inhibition of Hsp70 expression by Nac (1 mM) after treatment of SW620 cells with curcumin (50 μM)
Fig 5.
Northern hybridization of Hsp70 transcripts expressed by COLO-205 (lanes 1–4), MDAMB (lanes 5–8), and PA-1 cells (lanes 9–12). Lanes 1, 5, and 9 are controls; lanes 2, 6, and 10 represent 1-hour treatment with 50 μM curcumin; lanes 3, 7, and 11 represent 2-hour treatment, and lanes 4, 8, and 12 represent 3-hour treatment with curcumin. Only 3-hour time points show expression of Hsp70 (A), PA-1 cells showing the maximum expression. (B) GAPDH control showing RNA integrity and loading
We also studied the expression of Hsp70 after treatment of SW620 (resistant to curcumin) cells with NAc. NAc (1 mM) was able to reduce curcumin-induced expression of Hsp70 in these cells (Fig 4C), thereby indicating the involvement of superoxide radicals in the induction of Hsp70 expression.
These observations lead us to suggest that human tumor cell lines produced superoxide radicals on treatment with curcumin, which in turn induced the stress response. Cell lines that generated significantly higher levels of Hsp70 and perhaps other Hsps survived and were resistant to curcumin-induced cytotoxicity, whereas cell lines that could not generate significant stress response were triggered into apoptotic pathway by superoxide radicals (Fig 6).
Fig 6.
Flowchart showing curcumin-mediated O2− generation and the induction of stress response leading to either survival or death of the tumor cells
Effect of curcumin on nontransformed cells
We also studied the effect of curcumin on primary cultures and nontransformed cell lines. Curcumin was ineffective in inducing apoptosis in these cells (Table 4). Nontransformed cells when treated with curcumin produced very low concentrations of O2− (Fig 7A) and therefore did not exhibit any stress response (Fig 7B). Human corneal epithelial cells (HCE) produced slightly higher amounts of O2− and Hsp70, which could be due to its transformed state induced by SV40 T-antigen. These observations suggest that curcumin behaves differently when added to nontransformed cells and that the protection in these cells is not through the induction of stress response as observed in curcumin-resistant tumor cell lines.
Table 4.
Effect of curcumin on primary and nontransformed cells
Fig 7.
Effect of curcumin on nontransformed cells. Primary cultures of splenocytes and hepatocytes and established cell lines were treated with curcumin (50 μM) for 24 hours and evaluated for the generation of superoxide anions (A) and the expression of Hsp70 (B)
DISCUSSION
Curcumin has been reported to be a potent antiproliferative agent for many tumor types (Rao et al 1995; Sikora et al 1997). It has also been shown to act as a proapoptotic agent (Kuo et al 1996; Khar et al 1999). However, curcumin inhibited apoptosis induced by either dexamethasone or UV irradiation (Sikora et al 1997). Curcumin suppressed the activity of AP-1 transcription factor in the cells that were stimulated to proliferate (Sikora et al 1997), and it also suppressed c-jun and NF-kB activation in breast carcinoma cells (Mehta et al 1997).
Recently, we demonstrated antitumor activity of curcumin in a rat histiocytoma AK-5 (Khar et al 1999). In addition, curcumin induced apoptosis through the generation of reactive oxygen species (Bhaumik et al 1999). In the present investigation, we studied the effect of curcumin on human tumor cell lines of different origin. Our present studies indicate sensitivity of cell lines, established from certain types of malignancies, to the apoptotic activity of curcumin. However, cell lines from melanomas, lung, kidney, prostate, CNS, and cervical carcinomas were resistant to the apoptotic effects of curcumin (Table 1). Recently, it has become clear that suppression of apoptosis by tumor-promoting agents in preneoplastic cells is an important mechanism for tumor promotion (Bayly et al 1994), and the disappearance of nodular lesions as induced by the administration of chemopreventive agents like s-adenosyl-l-methionine has been associated with the apoptotic process (Garcea et al 1989). Thus, the anti-tumor-promoting effects of curcumin, at least in part, could be contributed to its apoptosis-inducing activity.
The apoptotic activity of curcumin as reported earlier (Bayly et al 1994; Jiang et al 1996; Khar et al 1999) involves activation of caspases, generation of ROS, and loss of mitochondrial transmembrane potential. Using the caspase inhibitor z-VAD and the inhibitor for ROS generation NAc, we have been able to inhibit apoptosis induced by curcumin. These observations suggest that the curcumin-induced apoptosis in sensitive human cancer cell lines involves the generation of ROS and the activation of caspases.
The release of cytochrome C from mitochondria has been regarded as an important step in the initiation of apoptosis (Reed 1997); however, its release has not been demonstrated in some types of apoptotic processes (Tang et al 1998). Cytochrome C is involved in the activation of caspases and thus initiates the apoptotic pathway. Curcumin seems to induce cytochrome C release in only HL-60 cells; other tumor cell lines tested do not show such a release (Fig 3). Thus, it is plausible to assume that curcumin-induced apoptosis in some of the cell lines tested is independent of cytochrome C release from the mitochondria. The involvement of mitochondria in apoptosis, and its being cause or consequence, is a complex phenomenon. The release of cytochrome C may critically depend on the type of stimulus, its intensity, and the differential cascade it may induce. The hydrophobic property of curcumin enables the molecule to pass easily through the plasma membrane. Thus, curcumin-induced apoptosis is not mediated through a specific receptor and therefore may result in the activation of a different signaling pathway in the apoptotic cascade.
The release of cytochrome C from mitochondria and the inhibition of the mitochondrial respiratory chain was assumed to result in the overproduction of ROS, which would act as mediators of the death signaling pathway (Schulze-Osthoff et al 1992). Studies have shown that the addition of ROS or the depletion of endogenous antioxidants can induce programmed cell death (Buttke and Sandstrom 1994).
Curcumin has been shown to induce expression of stress proteins in certain cell types. In addition, the stress response induced by curcumin has been ascribed to its inhibitory activity during the metabolism of arachidonic acid (Kato et al 1998). In order to understand the mechanism of resistance shown by several human tumor cell lines in our study, we investigated the expression of Hsp70, which has been previously shown to protect cells from undergoing apoptosis (Yenari et al 1999). It was interesting to observe a higher expression of Hsp70 in the presence of curcumin by the resistant tumor cell lines (Fig 4), whereas the sensitive cell lines produced only basal levels of Hsp70, which may not be sufficient to impart protection during apoptosis. The expression of Hsp70 was reduced when NAc was added along with curcumin (Fig 4C). These observations suggest that the cells are subjected to stress by the production of ROS after curcumin treatment and that the cell lines that respond to this stress by producing Hsp70 and possibly other Hsps are protected and therefore are resistant to curcumin. On the other hand, the curcumin-sensitive cell lines undergo apoptosis because of their inability to produce sufficient Hsp70. The protective mechanism of Hsp70 in the apoptotic process is not well understood. It is believed to perform a chaperone function, thereby preventing protein malfolding and aggregation. Recently, Hsp70, because of its chaperone function, has been shown to affect the apoptotic process at the levels of cytochrome C release as well as at initiator caspase activation (Mosser et al 2000). It may also function in the inhibition of effector caspases or in delaying their activation, probably by binding to the inactive (pro) form of these proteins. During oxidative injury, Hsp70 may be protecting the cells through an antioxidant mechanism, probably by stabilizing the endogenous antioxidants. Increased levels of glutathione have been observed in Hsp70-overproducing glial cells, which protect the cells from glucose deprivation or H2O2 exposure (Xu and Gifferd 1997).
CD95-ligand (CD95-L), after interaction with CD95 receptor, induced apoptosis in the target cells (Krammer 1999). CD95-L has also been shown to induce inflammatory response in several studies (Miwa et al 1998) in addition to its role in immune evasion (Khar et al 1998). We observed higher expression of CD95-L in the curcumin-sensitive cell lines, whereas the cell lines that are not killed by curcumin do not express CD95-L (Table 3). Reactive oxygen intermediates have been shown to induce CD95-L mRNA in HepG2 cells (Hug et al 1997). Although the exact role of CD95-L during curcumin treatment remains to be investigated, our studies do suggest an important role for CD95-L during the apoptotic process in curcumin-sensitive cell lines; however, we have not been able to demonstrate induction of CD95 receptor expression after treatment of the cells with curcumin (data not shown).
Recently, CD95-L has been shown to act as a costimulatory receptor in CD8+ T cells (Suzuki et al 2000). In addition, CD95-L has been shown to interact with the extracellular matrix proteins, which in turn potentiated its proapoptotic activity (Aoki et al 2001). Thus, induction of CD95-L expression by curcumin in tumor cells may have tremendous implications in vivo, where it may augment costimulatory function during an immune response against the cancer.
Although curcumin has been shown to reduce the proliferation rate of human colon cancer cells HT-29 and HCT-15, it was unable to induce apoptosis in these cells (Hanif et al 1997). In our studies, we investigated the apoptotic potential of curcumin on different human tumor cell lines. The possibility that curcumin-resistant cell lines may not undergo apoptosis but may get arrested in the cell cycle during division should be studied further.
Thus, curcumin is a highly potent molecule possessing anticancer activity. In addition, its being ineffective towards nontransformed cells (Table 4) makes it more interesting from the therapeutic point of view, as it may not generate any side effects that are normally associated with other anticancer compounds.
Our studies suggest that curcumin, which is an active component of the Indian spice, may have many beneficial effects, especially in preventing some of the malignancies that are sensitive to curcumin activity. In order to obtain more insights into establishing antitumor activity of curcumin against different types of human malignancies, it may be important to study the in vivo effects of curcumin using an athymic mouse model.
Acknowledgments
The authors thank Dr L.A. Weber for providing Hsp70 cDNA. We are also grateful to Dr Usha K. Srinivas for helpful discussions. Mr K. Kennady helped us in flow cytometric analysis. Ms T. Hemalatha typed the manuscript. R.A. is the recipient of a CSIR fellowship. Financial support was provided by the Indian Council of Medical Research.
REFERENCES
- Aoki K, Kurooka M, Chen J-J, Petryniak J, Nabel EG, Nabel GJ. Extracellular matrix interacts with soluble CD95L: retention and enhancement of cytotoxicity. Nature Immunol. 2001;2:333–337. doi: 10.1038/86336. [DOI] [PubMed] [Google Scholar]
- Bayly AC, Roberts RA, Dive C. Suppression of liver cell apoptosis in vitro by the non-genotoxic hepatocarcinogen and peroxisome proliferator nafenopin. J Cell Biol. 1994;125:197–203. doi: 10.1083/jcb.125.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaumik S, Anjum R, Rangaraj N, Pardhasaradhi BVV, Khar A. Curcumin mediated apoptosis in AK-5 cells involves the production of reactive oxygen intermediates. FEBS Lett. 1999;456:311–314. doi: 10.1016/s0014-5793(99)00969-2. [DOI] [PubMed] [Google Scholar]
- Bossy-Wetzel E, Newmeyer DD, Green DR. Mitochondrial cytochrome C release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J. 1998;17:37–49. doi: 10.1093/emboj/17.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright JJ, Kausalya S, Khar A. Natural killer cell activation associated induction of target cell DNA fragmentation in a spontaneously regressing rat histiocytoma. Immunology. 1995;85:638–644. [PMC free article] [PubMed] [Google Scholar]
- Buttke TM, Sandstrom PA. Oxidative stress as a mediator of apoptosis. Immunol Today. 1994;15:7–10. doi: 10.1016/0167-5699(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Chan MM, Huang HI, Fenton MR, Fong D. In vivo inhibition of nitric oxide synthase gene expression by curcumin, a cancer preventive natural product with anti-inflammatory properties. Biochem Pharmacol. 1998;55:1955–1962. doi: 10.1016/s0006-2952(98)00114-2. [DOI] [PubMed] [Google Scholar]
- Chen YC, Kuo TC, Lin-Shiau SY, Lin JK. Induction of HSP70 gene expression by modulation of Ca(+2) ion and cellular p53 protein by curcumin in colorectal carcinoma cells. Mol Carcinog. 1996;17:224–234. doi: 10.1002/(SICI)1098-2744(199612)17:4<224::AID-MC6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Freeman BC, Myers MP, Schumacher R, Morimoto RI. Identification of a regulatory motif in Hsp70 that affects ATPase activity, substrate binding and interaction with HDJ-1. EMBO J. 1995;14:2281–2292. doi: 10.1002/j.1460-2075.1995.tb07222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcea R, Daino R, and Pascale MM. et al. 1989 Inhibition of promotion and persistent nodule growth by s-adenosyl-l-methionine in rat liver carcinogenesis: role of remodeling and apoptosis. Cancer Res. 49:1850–1859. [PubMed] [Google Scholar]
- Hanif R, Qiao L, Shiff SJ, Rigas B. Curcumin, a natural plant phenolic food additive, inhibits cell proliferation and induces cell cycle changes in colon adenocarcinoma cell lines by a prostaglandin-independent pathway. J Lab Clin Med. 1997;130:576–584. doi: 10.1016/s0022-2143(97)90107-4. [DOI] [PubMed] [Google Scholar]
- Huang MT, Lou YR, Ma W, Newmark H, Rewhl K, Conney AH. Inhibitory effects of dietary curcumin on forestomach, duodenal, and colon carcinogenesis in mice. Cancer Res. 1994;54:5841–5847. [PubMed] [Google Scholar]
- Hug H, Strand S, Grambihler A, Galle J, Hack V, Stremmel W, Krammer PH, Galle PR. Reactive oxygen intermediates are involved in the induction of CD95 ligand mRNA expression by cytostatic drugs in hepatoma cells. J Biol Chem. 1997;272:28191–28193. doi: 10.1074/jbc.272.45.28191. [DOI] [PubMed] [Google Scholar]
- Jacobson MD. Reactive oxygen species and programmed cell death. Trends Biochem Sci. 1996;21:83–86. [PubMed] [Google Scholar]
- Janicke RU, Sprengart ML, Wati MR, Porter AG. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem. 1998;273:9357–9360. doi: 10.1074/jbc.273.16.9357. [DOI] [PubMed] [Google Scholar]
- Jee SH, Shen SC, Tseng CR, Chiu HC, Kuo ML. Curcumin induces a p53-dependent apoptosis in human basal cell carcinoma cells. J Invest Dermatol. 1998;111:656–661. doi: 10.1046/j.1523-1747.1998.00352.x. [DOI] [PubMed] [Google Scholar]
- Jiang MC, Yang-Yen HF, Yen JJ, Lin JK. Curcumin induces apoptosis in immortalized NIH 3T3 and malignant cancer cell lines. Nutr Cancer. 1996;26:111–120. doi: 10.1080/01635589609514468. [DOI] [PubMed] [Google Scholar]
- Kato K, Ito H, Kamei K, Iwamoto I. Stimulation of the stress-induced expression of stress proteins by curcumin in cultured cells and in rat tissues in vivo. Cell Stress Chaperones. 1998;3:152–160. doi: 10.1379/1466-1268(1998)003<0152:sotsie>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamori T, Lubet R, Steele VE, Kelloff GJ, Kaskey RB, Rao CV, Reddy BS. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res. 1999;5:597–601. [PubMed] [Google Scholar]
- Khar A, Ali AM, Pardhasaradhi BVV, Begum Z, Anjum R. Antitumor activity of curcumin is mediated through the induction of apoptosis in AK-5 tumor cells. FEBS Lett. 1999;445:165–168. doi: 10.1016/s0014-5793(99)00114-3. [DOI] [PubMed] [Google Scholar]
- Khar A, Varalakshmi Ch, Pardhasaradhi BVV, Ali AM, Kumari AL. Depletion of the effector cell population in the peritoneum by tumor cells overexpressing Fas-ligand: a mechanism of immune evasion. Cell Immunol. 1998;189:85–91. doi: 10.1006/cimm.1998.1367. [DOI] [PubMed] [Google Scholar]
- Kiang JG, Tsokos GC. Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol Ther. 1998;80:183–201. doi: 10.1016/s0163-7258(98)00028-x. [DOI] [PubMed] [Google Scholar]
- Krammer PH. CD95(APO-1/Fas)-mediated apoptosis: live and let die. Adv Immunol. 1999;71:163–210. doi: 10.1016/s0065-2776(08)60402-2. [DOI] [PubMed] [Google Scholar]
- Kuo ML, Huang TS, Lin JK. Curcumin, an antioxidant and anti-tumor promoter, induces apoptosis in human leukemia cells. Biochim Biophys Acta. 1996;1317:95–100. doi: 10.1016/s0925-4439(96)00032-4. [DOI] [PubMed] [Google Scholar]
- Kuttan R, Bhanumathy P, Nirmala K, George MC. Potential anticancer activity of turmeric (Curcuma longa) Cancer Lett. 1985;29:197–202. doi: 10.1016/0304-3835(85)90159-4. [DOI] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmed M, Alnemri ES, Wang X. Cytochrome C and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Mehta K, Pantazis P, McQueen T, Aggarwal BB. Antiproliferative effect of curcumin (diferuloylmethane) against human breast tumor cell lines. Anticancer Drugs. 1997;8:470–481. doi: 10.1097/00001813-199706000-00010. [DOI] [PubMed] [Google Scholar]
- Miwa K, Asano M, Horai R, Iwakura Y, Nagata S, Suda T. Caspase 1-independent IL-1beta release and inflammation induced by the apoptosis inducer Fas ligand. Nature Med. 1998;4:1287–1292. doi: 10.1038/3276. [DOI] [PubMed] [Google Scholar]
- Mosser DD, Caron AW, Bourget L, Meriin AB, Sherman MY, Morimoto RI, Massie B. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biol. 2000;20:7146–7159. doi: 10.1128/mcb.20.19.7146-7159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachuari SP, Mukherjee SK. Effect of Curcuma longa and Curcuma amanda on the cholesterol levels in experimental hyper cholesterolemia of rabbits. J Res Indian Med. 1956;6:27–31. [Google Scholar]
- Pick E. Microassays for superoxide and hydrogen peroxide production and nitroblue tetrazolium reduction using an enzyme immunoassay microplate reader. Methods Enzymol. 1986;132:407–421. doi: 10.1016/s0076-6879(86)32026-3. [DOI] [PubMed] [Google Scholar]
- Rao CV, Rivenson A, Simi B, Reddy BS. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res. 1995;55:259–266. [PubMed] [Google Scholar]
- Reed JC. Cytochrome c: can't live with it—can't live without it. Cell. 1997;91:559–562. doi: 10.1016/s0092-8674(00)80442-0. [DOI] [PubMed] [Google Scholar]
- Schulze-Osthoff K, Bakker AC, Vanhacsebroeck B, Beyaert R, Jacob WA, Fiers W. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions: evidence for the involvement of mitochondrial radical generation. J Biol Chem. 1992;267:5317–5323. [PubMed] [Google Scholar]
- Sharma OP. Antioxidant activity of curcumin and related compounds. Biochem Pharmacol. 1976;25:1811–1812. doi: 10.1016/0006-2952(76)90421-4. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Massa SM, Swanson RA. Heat-shock protein protection. Trends Neurosci. 1999;22:97–99. doi: 10.1016/s0166-2236(98)01392-7. [DOI] [PubMed] [Google Scholar]
- Sikora E, Bielak-Zmijeswka A, Piwocka K, Skierski J, Radziszewska E. Inhibition of proliferation and apoptosis of human and rat T lymphocytes by curcumin, a curry pigment. Biochem Pharmacol. 1997;54:899–907. doi: 10.1016/s0006-2952(97)00251-7. [DOI] [PubMed] [Google Scholar]
- Sreedhar AS, Pardhasaradhi BVV, Begum Z, Khar A, Srinivas UK. Lack of heat shock response triggers programmed cell death in a rat histiocytic cell line. FEBS Lett. 1999;456:339–342. doi: 10.1016/s0014-5793(99)00970-9. [DOI] [PubMed] [Google Scholar]
- Suzuki I, Martin S, Boursalian TE, Beers C, Fink PJ. Fas ligand costimulates the in vivo proliferation of CD8+ T cells. J Immunol. 2000;165:5537–5543. doi: 10.4049/jimmunol.165.10.5537. [DOI] [PubMed] [Google Scholar]
- Tang DG, Li L, Zhu Z, Joshi B. Apoptosis in the absence of cytochrome c accumulation in the cytosol. Biochem Biophys Res Commun. 1998;242:380–384. doi: 10.1006/bbrc.1997.7969. [DOI] [PubMed] [Google Scholar]
- Welch WJ 1990 Mammalian stress response: cell physiology and biochemistry of stress proteins. In: Stress Proteins in Biology and Medicine, ed Morimoto RI, Tissieres A, Georgopoulos C. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 223–278. [DOI] [PubMed] [Google Scholar]
- Wyllie AH. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980;284:555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Xu L, Gifferd RG. HSP70 protects murine astrocytes from glucose deprivation injury. Neurosci Lett. 1997;224:9–12. doi: 10.1016/s0304-3940(97)13444-9. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu X, and Bhalla K. et al. 1997 Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 275:1129–1132. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Gifferd RG, Sapolsky RM, Steinberg GK. The neuroprotective potential of heat shock protein 70 (HSP70) Mol Med Today. 1999;5:525–531. doi: 10.1016/s1357-4310(99)01599-3. [DOI] [PubMed] [Google Scholar]