Abstract
Recent evidence suggests that heat shock proteins (Hsps) may have an important systemic role as a signal to activate the immune system. Since acute exercise is known to induce Hsp72 (the inducible form of the 70-kDa family of Hsp) in a variety of tissues including contracting skeletal muscle, we hypothesized that such exercise would result in the release of Hsp72 from stressed cells into the blood. Six humans (5 males, 1 female) ran on a treadmill for 60 minutes at a workload corresponding to 70% of their peak oxygen consumption. Blood was sampled from a forearm vein at rest (R), 30 minutes during exercise, immediately postexercise (60 minutes), and 2, 8, and 24 hours after exercise. These samples were analyzed for serum Hsp72 protein. In addition, plasma creatine kinase (CK) was measured at these time points as a crude marker of muscle damage. With the exception of the sample collected at 30 minutes, muscle biopsies (n = 5 males) were also obtained from the vastus lateralis at the time of blood sampling and analyzed for Hsp72 gene and protein expression. Serum Hsp72 protein increased from rest, both during and after exercise (0.13 0.10 vs 0.87 ± 0.24 and 1.02 ± 0.41 ng/mL at rest, 30 and 60 minutes, respectively, P < 0.05, mean SE). In addition, plasma CK was elevated (P < 0.05) 8 hours postexercise. Skeletal muscle Hsp72 mRNA expression increased 6.5-fold (P < 0.05) from rest 2 hours postexercise, and although there was a tendency for Hsp72 protein expression to be elevated 2 and 8 hours following exercise compared with rest, results were not statistically significant. The increase in serum Hsp72 preceded any increase in Hsp72 gene or protein expression in contracting muscle, suggesting that Hsp72 was released from other tissues or organs. This study is the first to demonstrate that acute exercise can increase Hsp72 in the peripheral circulation, suggesting that during stress these proteins may indeed have a systemic role.
INTRODUCTION
Heat shock proteins (Hsps) are a group of highly conserved proteins present in the cells of all living organisms. Although expressed in low concentrations in the basal state, they are highly inducible by a variety of pathological, physiological, and environmental stressors (Kiang and Tsokos 1998). Specifically, stressors such as increased temperature (Mizzen and Welch 1988), ischemia (Marber et al 1995), protein degradation (Chiang et al 1989), hypoxia (Guttman et al 1980), acidosis (Weitzel et al 1985), free radical formation (Adrie et al 2000), increased cytosolic Ca2+ (Welch et al 1983), endotoxemia (Ofenstein et al 2000), and reduced glucose availability (Sciandra and Subjeck 1983), are all known to increase Hsp in variety of tissues and cell lines. Of note, these stressors are often, if not always, characteristic of contracting mammalian skeletal muscle cells (for review, see Febbraio 2000). It is not surprising, therefore, that physical exercise has been demonstrated to increase Hsp72, the inducible form of the 70-kDa family of Hsp, in a variety of tissues in several mammalian species. Specifically, acute exercise up-regulates Hsp72 gene and protein expression in the heart (Salo et al 1991; Skidmore et al 1995), liver (Salo et al 1991), brain (Walters et al 1998), and skeletal muscle (Locke et al 1990; Salo et al 1991; Skidmore et al 1995) of rodents. In addition, increased gene (Puntschart et al 1996; Febbraio and Koukoulas 2000; Febbraio et al 2001) and protein (Febbraio et al 2001) expression has been observed in human skeletal in response to acute exercise.
It has been known for some time that the primary role of the Hsp is to act as molecular chaperones by binding to denatured proteins and acting as catalysts in the assembly of protein complexes (Gething and Sambrook 1992; Morimoto 1993). In addition, it has been suggested that Hsp72 may be synthesized in cells to act as a force-generating motor, capable of pulling proteins across organellular membranes similar to conventional force-generating proteins, such as myosin and kinesin (Glick 1995). However, evidence is emerging that Hsp may have another important role in immune defense (Moseley 2000). In a recent study, Asea et al (2000) demonstrated that exogenous Hsp72 bound specifically to the cell surface of human monocytes in vitro. In addition, these (Asea et al 2000) and other (Breloer et al 1999; Chen et al 1999; Multhoff et al 1999) authors have demonstrated that Hsp can stimulate cytokine production in immune cells. It has been suggested that Hsp can be released from cells into the extracellular milieu to bind to membranes of other cells (Hightower and Guidon 1989; Asea et al 2000). Indeed, Hsps have been demonstrated to be released from stressed human diabetic islet cells (Child et al 1995) and cultured rat embryo cells (Hightower and Guidon 1989). To our knowledge, no previous studies have determined whether physical exercise, which results in a marked increase in Hsp72 protein expression within tissues, also results in the release of this protein into the circulation. Given its potential systemic role and the fact that exercise results in marked changes in immune function (Pedersen and Hoffman-Goetz 2000), we performed such an experiment. We hypothesized that an acute bout of physical exercise would increase Hsp72 protein expression in contracting skeletal muscle, which would then be released resulting in an increase in Hsp72 within the blood.
MATERIALS AND METHODS
Subjects
Five active, but not specifically trained, males of mean (±SD) age 26.4 (4.1) years, weight 75.2 (8.7) kg, and VO2peak 3.6 (0.38) L/min and 1 female age 30 years, weight 71 kg, and VO2peak 3.2 L/min volunteered to participate in the study. Subjects were made fully aware of the experimental procedure and possible risks involved in the study prior to giving their written informed consent to take part in the investigation, which was approved by the University of Melbourne Human Research Ethics Committee.
Experimental procedures
Each subject's peak oxygen consumption (VO2peak) was determined via an incremental running test on a treadmill, and a running speed corresponding to 70% VO2peak was calculated from this test. Subjects attended the laboratory at least 7 days after this test to perform the experimental trial in the morning (between 0700 and 0930 hours) after an overnight fast. In the 24 hours prior to the experimental trial, subjects were instructed to abstain from strenuous exercise, alcohol, tobacco, and caffeine. Each subject was required to run for 60 minutes on a motorized treadmill at the predetermined speed in ambient conditions set at 20°C with a relative humidity <40%. Oxygen uptake was measured via expired pulmonary gas collection every 15 minutes to verify the exercise intensity. During exercise, subjects were permitted to drink water ad libitum, but no food was consumed until after exercise. Blood and muscle were sampled immediately before exercise (described in the following section). Blood was also sampled after 30 and 60 minutes of exercise and then 2, 8, and 24 hours after exercise. Muscle samples were also obtained at the completion of exercise (60 minutes) and 2, 8, and 24 hours after exercise.
Tissue sampling
Blood samples were drawn from an in-dwelling intravenous catheter (Terumo, Tokyo, Japan) positioned in the antecubital fossa at rest, 30 minutes and immediately postexercise (60 minutes). The catheter was flushed with 0.9% saline following each collection to ensure patency. Subsequent collections at 2, 8, and 24 hours after exercise were collected by venepuncture from a forearm vein. At each collection time, 4 mL of whole blood were placed in a tube containing lithium heparin, mixed, and spun in a centrifuge for plasma collection. Plasma was stored at −20°C prior to analysis for creatine kinase (CK). A further 4 mL of blood were placed in a tube containing a clot-inducing plug (Vacutainer Systems Europe, Cedex, France). This tube was inverted 6 times, stored on ice for 30 minutes, then spun in a centrifuge at 1200 × g at 4°C. The separated serum was collected and stored at −20°C prior to analysis for Hsp72 protein. Muscle biopsies (n = 5 men) were obtained at midthigh level from the vastus lateralis using the percutaneous needle biopsy technique (Bergstrom 1975) modified to include suction. Although it may be argued that the vastus lateralis is not the most appropriate muscle to sample for a running study (Costill et al 1973), we have recently demonstrated that the rate of glycogen use, and hence the degree of muscle recruitment, was not different when comparing 60 minutes of running with 60 minutes of cycling (Starkie et al 2001a). On collection, muscle samples were immediately (<5 seconds) frozen in liquid nitrogen. The immediate postexercise and 8-hour samples were taken from the same leg as the resting sample. The 2- and 24-hour biopsies were taken from the opposite limb. Samples were obtained from separate incisions ∼5 cm apart (distal to proximal) and ∼2.5 cm apart (medial to lateral). This precaution was taken to ensure that different motor units were sampled and that Hsp72 expression was not due to the trauma of the biopsy technique. We have previously shown that such a precaution does not result in Hsp72 expression in noncontracting muscle (Febbraio et al 2001).
Tissue analysis
Gene expression
Total RNA was extracted from a portion (10–40 mg) of frozen muscle using the acid guanidium thiocyanate–phenol–chloroform extraction method (Chomczynski and Sacchi 1987) and modified according to methods described elsewhere (Febbraio and Koukoulas 2000). For each total RNA sample, 0.1 μg was reverse transcribed in a 10-μL reaction containing 1 × TaqMan® RT buffer, 5.5 mM MgCl2, 500 μM each 2′-deoxynucleoside 5′-triphosphate, 2.5 μM random hexamers, 0.4 U/μL RNase inhibitor, and 1.25 U/μL MultiScribe™ reverse transcriptase (Applied Biosystems, Foster City, CA, USA). Control samples were analyzed where total RNA samples received all reagents except the reverse transcriptase (rt−). The reverse transcription reactions were performed using a GeneAmp PCR system 9600 (Applied Biosystems) with conditions at 25°C for 10 minutes, 48°C for 30 minutes, and 95°C for 5 minutes. Each reaction was then diluted 1/10 in 0.01 M EDTA pH 8.0 and stored at −80°C until further use.
We quantitated Hsp72 mRNA by real-time PCR using an ABI PRISM 7700 Sequence Detector (Applied Biosystems) using a multiplex comparative critical threshold (CT) method. With this method, a CT value reflects the cycle number at which DNA amplification is first detected. In each multiplex reaction, both human Hsp72 and 18S mRNA were detected in the one tube, where primers were limited for 18S. This was possible because of the different reporter dyes attached to each TaqMan® probe for Hsp72 and 18S, which fluoresce at different emission wavelength maxima. In preliminary experiments, we demonstrated no effect on CT values when we compared multiplex to nonmultiplex Hsp72 reactions as well as primer-limited multiplex to non-primer-limited 18S single-tube reactions. For the comparative CT method, a validation experiment was performed where we demonstrated approximately equal efficiencies of both human Hsp72 and 18S amplifications over different initial template concentrations.
To perform real-time PCR, human Hsp72 probe and primers were designed using Primer Express™ Version 1.0 (Applied Biosystems) from the human heat shock protein (Hsp72) gene sequence (GenBank/EMBL accession nos. M11717 and M15432). A 72-bp Hsp72 fragment was amplified using the forward primer, 5′-ACCAAGCAGACGCAGATCTTC-3′ and the reverse primer, 5′-GCCCTCGTACACCTGGATCA-3′ (Sigma Genosys, Castle Hill, New South Wales, Australia). A TaqMan fluorescent probe, 5′-FAM (6-carboxy fluorescein)-CCTACTCCGACAACCAACCCGGG-3′ TAMRA (6-carboxy-tetramethylrhodamine) (Applied Biosystems) was included with the primers in each reaction. The TaqMan probe and primers for 18S were supplied in a control reagent kit (Applied Biosystems).
PCR reactions were carried out in 25-μL volumes consisting of 1 × TaqMan Universal PCR Master Mix (including passive reference), 50 nM TaqMan 18S probe, 20 nM 18S forward primer, 80 nM 18S reverse primer, 100 nM TaqMan human Hsp72 probe, and 900 nM human Hsp72 forward and reverse primers. The concentrations of the Hsp72 probe and primers were chosen on the basis of pilot analyses where optimal concentrations were determined. cDNA (5 ng) and rt− preparations were amplified using the following conditions: 50°C for 2 minutes and 95°C for 10 minutes followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Comparative CT calculations for the expression of Hsp72 were all relative to the resting sample for each subject. For each sample, 18S CT values were subtracted from Hsp72 CT values to derive a delta CT value. The resting value for each subject was then subtracted from the exercise samples for each subject to derive a delta-delta CT value. The expression of human Hsp72 relative to the resting sample was then evaluated using the expression 2-delta-deltaCT.
Protein expression
An enzyme-linked immunosorbent assay (EIA) method (#EKS-700 StressGen Biotechnologies, Victoria, British Columbia, Canada) was used to determine the relative expression of Hsp72 protein in both muscle and serum. This assay has the ability to detect extremely low concentrations of Hsp72 protein in tissue extracts and serum. The sensitivity of this method was as low as 200 pg/mL and a standard curve range from 780–50 000 pg/mL. The traditional method for Hsp72 detection and quantitation is accomplished in 2 steps: immunoblotting followed by densitometry scanning. We performed an experiment where we quantitated the immunosorbent method against this traditional method and demonstrated approximate equal levels of Hsp72 in basal muscle samples by both methods (1–4 ng/μg protein). The intra- and interassay coefficients of variation were determined to be less than 10%.
The EIA method employs a quantitative sandwich immunoassay. The wells of the provided immunoassay plate are precoated with a mouse monoclonal antibody specific for inducible Hsp72 for a number of mammalian species including human. The antibody recognizes recombinant and native Hsp72 and has no reactivity with Hsp73, GRP78, E. coli DnaK, M. tuberculosis Hsp71 or Hsp60. Hsp72 is captured by the antibody and then detected with an Hsp72-specific, biotinylated rabbit polyclonal antibody. An avidin/horseradish-peroxidase conjugate binds the biotinylated antibody, and color development is achieved by the addition of tetramethylbenzidine (TMB) substrate. The development of a blue stain is proportional to captured Hsp72. Color development is halted by the addition of an acid stop solution, which converts the color to yellow. Absorbance is measured at 450 nm, and a standard curve constructed from known dilutions of Hsp72 protein allows quantitative assessment of Hsp72. Samples of tissue protein were extracted from skeletal muscle using the method advised for the Hsp72 EIA technique (StressGen). Hsp72 EIA Extraction Reagent was treated with a protease inhibitor, 0.1 mM phenylmethylsulfonylfluoride (PMSF). A prescribed volume of this reagent (14 μL/tissue) was added to tissue samples. Samples were homogenized with an electrical mixer (AG Polytron PT3100 Kinematica, Lucerne, Switzerland) and then spun at 20 200 × g for 10 minutes in a 4°C refrigerated microcentrifuge (5417 C/R Eppendorf, Hamburg, Germany). The supernatants, forming the tissue extracts, were collected, and the total protein content of each was determined by the Bradford method (Bradford 1976). Tissue extracts and known amounts of a protein standard (bovine serum albumin) were incubated with a protein-binding dye (Bio-Rad Laboratories, Hercules, CA, USA). Absorbance was measured at 600 nm (EIA Reader, Model 2550, Bio-Rad) and the total protein concentration of samples interpolated from the linear relationship between the protein standards and absorbance. Extracts were frozen at −70°C prior to EIA Hsp72 analysis.
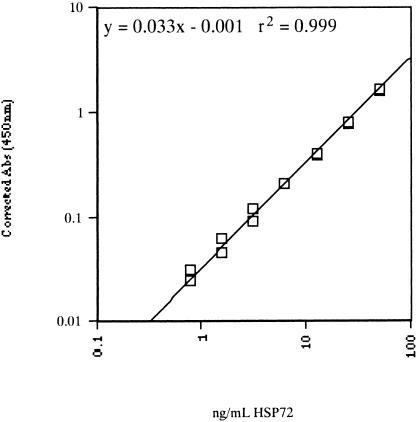
One hundred microliters of prepared Hsp72 standard (0.78–50 ng/mL), undiluted serum samples, and tissue protein samples (diluted between 1/50 and 1/80 with sample diluent) were added to the microassay plate in duplicate. Wells were covered and incubated at room temperature for 2 hours, with gentle rocking. After incubation, liquid was aspirated from all wells, and the wells were washed with Wash Buffer (StressGen) 6 times. One hundred microliters of Anti-Hsp70: Biotin Antibody (diluted 1/500) were added to all wells and incubated for 1 hour. After incubation, wells were washed a further 6 times before the addition of 100 μL Avidin-HRP conjugate (diluted 1/10 000). After a 1-hour incubation, wells were washed as described previously, and TMB substrate was added and incubated for 10 minutes at room temperature. One hundred microliters of Acid Stop Solution were added to all wells, and absorbance was measured at 450 nm (EIA Reader, Model 2550, Bio-Rad). The absorbence of the assay blank (0 ng/mL Hsp72) was subtracted from all other standards and samples to account for the background absorbence of reagents. The Hsp72 concentrations of each sample were then interpolated from the constructed standard curve plotted on a log-log scale (Fig 1). The concentrations of Hsp72 in each tissue sample were expressed as a percentage of the total protein content, and then Hsp72 expression of each sample was represented as a fold change from rest. As subjects were assayed on different plates, a semiquantitative method of reporting was used. This eliminated error being introduced by variations in the concentration of Hsp72 standards between assays. The concentration of Hsp72 in serum samples was expressed as weight of the protein per milliliter of serum (ng/mL). All serum samples were run with the same standard curve.
Fig 1.
Typical standard curve for the Hsp72 protein enzyme-linked immunosorbent assay. Hsp72 concentration (ng/mL) vs corrected absorbance at 450 nm. Hsp72 standard concentrations: 0, 0.78, 1.56, 3.13, 6.25, 12.50, 25.00, and 50.00 ng/mL. Log-log axis, linear fit, r2 = 0.999
Creatine kinase
Plasma CK concentration (U/L) was determined by automated enzyme reactions using the International Federation of Clinical Chemistry (IFCC) recommended method (automated analysis for AU5000 Olympus, Japan).
Statistical analysis
A 1-way analysis of variance with repeated measures on the time factor was used to analyze the data. Newman-Keuls multimean comparison tests were used to locate differences when a significant F-value was obtained. A Statistica software package was used to compute these statistics. The level of significance for all tests was set at P < 0.05.
RESULTS
Serum Hsp72 protein concentration
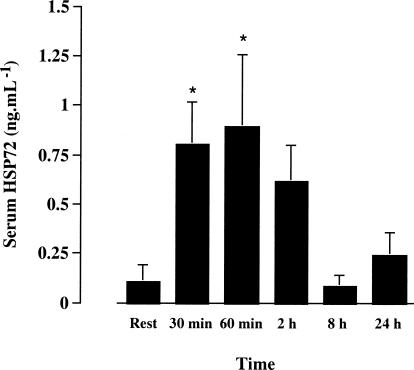
We detected very small amounts of Hsp72 in the serum of 2 of the 6 subjects at rest. However, after 30 and 60 minutes of exercise, we detected Hsp72 in all subjects, such that values at these times were greater than rest (0.13 ± 0.10 vs 0.87 ± 0.24 and 1.02 ± 0.41 ng/mL at rest, 30 and 60 minutes, respectively, P < 0.05, mean ± SE). At 8 and 24 hours after exercise, serum Hsp72 had returned to resting levels (Fig 2).
Fig 2.
Serum Hsp72 before (rest), at 30 and 60 minutes of treadmill running at 70% of maximal oxygen uptake and 2, 8, and 24 hours postexercise. * denotes difference (P < 0.05) compared with rest. Data expressed as means ± SE (n = 6)
Skeletal muscle Hsp72 mRNA and protein expression
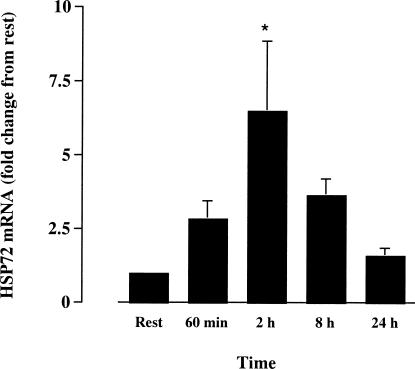
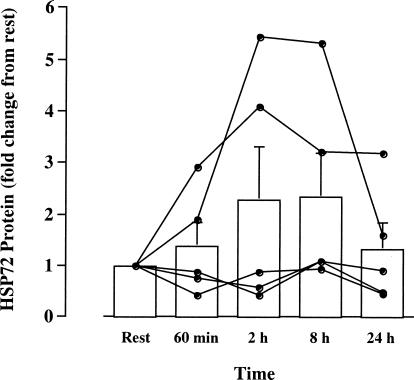
Hsp72 mRNA expression was not significantly elevated by the end of exercise (60 minutes) yet had increased 6.5-fold (P < 0.05, n = 5) 2 hours after exercise. Hsp72 mRNA was no different from rest at 8 and 24 hours after exercise (Fig 3). Expression of the ribosomal 18S gene did not differ between time points, demonstrating that this gene was constitutively expressed and that changes in Hsp72 gene expression were not due to changes in the expression of ribosomal 18S. Hsp72 protein content was not significantly different when comparing any time point with rest. However, 2 subjects showed marked increases from rest at 2 and 8 hours after exercise (Fig 4).
Fig 3.
Hsp72 gene expression in vastus lateralis before (rest), after 60 minutes of treadmill running at 70% of maximal oxygen uptake, and 2, 8, and 24 hours postexercise. * denotes difference (P < 0.05) compared with rest. Data (mean ± SE) expressed relative to the resting sample for each subject (n = 5)
Fig 4.
Hsp72 protein expression in vastus lateralis before (rest), after 60 minutes of treadmill running at 70% of maximal oxygen uptake, and 2, 8, and 24 hours postexercise. Data expressed for each individual and as mean ± SE. Data expressed relative to the resting sample for each subject (n = 5)
Creatine kinase
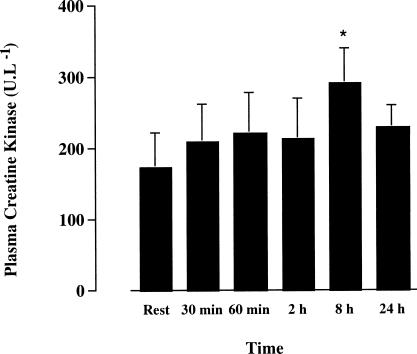
The concentration of plasma CK was elevated from rest (P < 0.05) 8 hours after exercise. However, the plasma concentration of this enzyme was not different compared with rest at all other time points (Fig 5). Of note, 2 subjects who had marked Hsp72 protein expression in the skeletal muscle samples also had the highest postexercise CK concentration.
Fig 5.
Plasma creatine kinase before (rest), at 30 and 60 minutes of treadmill running at 70% of maximal oxygen uptake, and 2, 8, and 24 hours postexercise. * denotes difference (P < 0.05) compared with rest. Data expressed as means ± SE (n = 6)
DISCUSSION
We have demonstrated, for the first time, a stress-induced increase in serum Hsp72 in humans. However, contrary to our hypothesis, Hsp72 protein was not significantly elevated in the contracting skeletal muscle, suggesting that Hsp72 was released into the circulation by other tissues or organs. Given that Hsp72 binds and activates human monocytes (Asea et al 2000), our in vivo data suggest that these proteins possibly have a systemic function, including mediation of the effects of exercise on immune function.
As previously stated, we could not detect Hsp72 in the serum of 4 of 6 subjects at rest. To our knowledge, this is only the second time expression of Hsp72 protein in the circulation of humans has been examined. Pockley et al (1998) measured Hsp72 protein expression in the serum of healthy, unstressed individuals. Levels of this protein were reported to be 1131 ± 254 and 2543 ± 3808 ng/mL for males and females, respectively (mean ± SD). However, the median values for males and females were 280 and 740 ng/mL, indicating a large variability in the expression of this protein in the circulation. While highly inducible by stress, Hsp72 is present only in relatively lower concentrations in normal cells. For this reason, it is somewhat surprising that such high circulating levels of Hsp72 were found in healthy, rested individuals.
Increased Hsp72 gene transcription indicated that the exercise stress caused sufficient intracellular disruption to trigger an Hsp response. Despite the marked uniform increase in Hsp72 mRNA in contracting muscle (Fig 3), increases in Hsp72 protein expression were observed in only 2 subjects (Fig 4). The observation of an increase in mRNA in the absence of any uniform increase in protein supports previous findings in humans. Puntschart et al (1996) observed an increase in Hsp72 gene expression 4 minutes, 30 minutes, and 3 hours after running exercise. However, at these time points, Hsp72 protein was not increased. Two possible explanations were suggested by these authors to account for this observation. First, the increase in Hsp72 protein was delayed beyond 3 hours following exercise and/or, second, the amount of newly synthesized protein was small compared with the preexisting level. The findings of this study make the latter suggestion probable. Protein content was not elevated at 8 or 24 hours postexercise, and 2 subjects displayed marked elevation of Hsp72 protein within 2 hours of exercise. The absence of increased protein content in the remaining subjects is, therefore, best explained by only a minor amount of new Hsp72 protein being synthesized, or that translation was completely inhibited.
Although it is clear that exercise resulted in an increase in circulating Hsp72, our data cannot determine the tissue of origin or the process of release. Since the appearance of Hsp72 in the serum both preceded any increase in either Hsp72 gene or protein expression in contracting skeletal muscle and was seen in all subjects, it is unlikely that contracting muscle was the tissue of origin. Of note, since CK levels were slightly elevated, suggesting that there may have been some muscle damage, we cannot rule out the possibility that Hsp72 was released from the skeletal muscle prior to any Hsp72 synthesis in these muscles. However, given that the CK levels were only slightly elevated above resting levels, this scenario seems unlikely. It is likely, therefore, that the origin of the serum Hsp72 was not from the contracting muscle. A variety of other cells and tissues, such as leukocytes (Ryan et al 1991; Fehrenbach et al 2000), heart (Salo et al 1991, Skidmore et al 1995), liver (Salo et al 1991), and brain (Walters et al 1998), have been demonstrated to increase Hsp72 expression in response to exercise. It is possible, therefore, that one or many of these cells or tissues contributed to the increase in serum Hsp72 in the present study. It is also not known whether Hsp72 was released via a transporter mechanism through the plasma membrane or simply released through cell death or cell membrane rupture. Of note, the 70-kDa family of Hsps lacks a conventional signal peptide sequence that would allow them to be transported through cytosolic or plasma membranes (Gunter and Walter 1994), suggesting that intracellular Hsp72 can be released only following cell death or increased cell membrane permeability. In support of this, we recently conducted a study where we had humans perform prolonged knee-extensor kicking, a model of exercise that involves purely concentric contractions (Febbraio et al 2001). In this previous study, CK was not increased during or after exercise, indicating an absence of muscle damage. Despite a 2-fold increase in Hsp72 protein expression within contracting muscle, we were unable to detect any Hsp72 release into the circulation from this muscle, using a femoral arteriovenous balance technique (Febbraio et al 2001), providing evidence that Hsp72 is not released when the tissue membranes are intact.
Recent evidence suggests that Hsp can stimulate cytokine production in immune cells (Breloer et al 1999; Chen et al 1999; Multhoff et al 1999; Asea et al 2000). Specifically, Asea et al (2000) demonstrated that the proinflammatory cytokines IL-1β, TNF-α, and IL-6 were activated by a very small quantity of Hsp72. More important, this previous study (Asea et al 2000) has demonstrated that the activation of IL-1β and IL-6 was via a CD14-dependent pathway. This glycosylphosphatidylinositol-anchored protein is situated on the plasma membrane. Therefore, these data suggest that in order to activate IL-1β and IL-6 within monocytes, Hsp72 must act via binding to the plasma membrane. Studies examining the effect of exercise on monocyte function support this hypothesis. It has been demonstrated that acute exercise increases Hsp72 production within monocytes (Ryan et al 1991; Fehrenbach et al 2000). However, we have recently shown that very stressful exercise either does not affect (Starkie et al 2000) or indeed decreases (Starkie et al 2001b) intracellular monocyte IL-6 production in vivo. It appears, therefore, that in order for Hsp72 to activate an IL-6 response in monocytes, they must first be released from the monocyte before adhering to the surface of another to act via the CD14-dependent pathway. Given that exercise results in many immunological changes (Pedersen and Hoffman-Goetz 2000), this scenario seems likely.
In summary, we have demonstrated that exercise-induced stress results in a marked increase in Hsp72 within the circulation. This increase is rapid and precedes production within the contracting skeletal muscles. Further work examining the effect of exercise on Hsp72 surface expression on monocytes is warranted to confirm Hsp72 as a central signal to the immune system in vivo.
Acknowledgments
We acknowledge the technical assistance and advice of Erik Wallen of the Department of Biochemistry, Royal Melbourne Hospital, for performing the creatine kinase analyses. We also thank the subjects for their participation. This study was supported by the Australian Research Council and Stressgen Biotechnologies.
REFERENCES
- Adrie C, Richter C, and Bachelet M. et al. 2000 Contrasting effects of NO and peroxynitrites on HSP70 expression and apoptosis in human monocytes. Am J Physiol. 279:C452–C460. [DOI] [PubMed] [Google Scholar]
- Asea A, Kraeft S, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependent pathway, demonstrating its dual role as a chaperone and cytokine. Nature Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Bergstrom J. Percutaneous needle biopsy of skeletal muscle for physiological and clinical research. Scand J Clin Invest. 1975;35:609–616. [PubMed] [Google Scholar]
- Bradford M. A refined sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Breloer B, Fleisher B, Von Bonin A. In vivo and in vitro activation of T cells after administration of Ag-negative heat shock proteins. J Immunol. 1999;162:3141–3147. [PubMed] [Google Scholar]
- Chen W, Saldath U, Bellman K, Burkart V, Kolb H. Human 60-kDa heat shock protein: a danger signal to the innate immune system. J Immunol. 1999;162:3212–3219. [PubMed] [Google Scholar]
- Chiang H-L, Terlecky SR, Plant CP, Dice JF. A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science. 1989;246:382–385. doi: 10.1126/science.2799391. [DOI] [PubMed] [Google Scholar]
- Child DF, Williams CP, Jones RP, Hudson PR, Jones M, Smith CJ. Heat shock protein studies in type 1 and type 2 diabetes and human islet cell culture. Diab Med. 1995;12:595–599. doi: 10.1111/j.1464-5491.1995.tb00548.x. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Costill DL, Jansson ED, Gollnick PD, Saltin B. Glycogen utilization in leg muscles of men during level and uphill running. Acta Physiol Scand. 1974;91:475–481. doi: 10.1111/j.1748-1716.1974.tb05703.x. [DOI] [PubMed] [Google Scholar]
- Febbraio MA. Does skeletal muscle function and metabolism affect exercise performance in the heat? Exerc Sport Sci Rev. 2000;28:171–177. [PubMed] [Google Scholar]
- Febbraio MA, Koukoulas I. HSP72 gene expression progressively increases in human skeletal muscle during prolonged, exhaustive exercise. J Appl Physiol. 2000;89:1055–1060. doi: 10.1152/jappl.2000.89.3.1055. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Steensberg A, Walsh R, Koukoulas I, Hall G, Saltin B, and Klarlund 0B 2001 Reduced muscle glycogen availability is associated with elevated HSP72 in contracting human skeletal muscle. J Physiol, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbach E, Niess AM, Schlotz E, Passek F, Dickhuth H-H, Northoff H. Transcriptional and translational regulation of heat shock proteins in leukocytes of endurance runners. J Appl Physiol. 2000;89:704–710. doi: 10.1152/jappl.2000.89.2.704. [DOI] [PubMed] [Google Scholar]
- Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Glick BS. Can Hsp70 proteins act as force-generating motors? Cell. 1995;80:11–14. doi: 10.1016/0092-8674(95)90444-1. [DOI] [PubMed] [Google Scholar]
- Gunter E, Walter L. Genetic aspects of the hsp70 multigene family in vertebrates. Experientia. 1994;50:987–1001. doi: 10.1007/BF01923453. [DOI] [PubMed] [Google Scholar]
- Guttman SD, Glover CVC, Allis CD, Gorovsky MA. Heat shock, deciliation and release from anoxia induce the synthesis of the same polypeptides in starved T. pyriformis. Cell. 1980;22:299–307. doi: 10.1016/0092-8674(80)90177-4. [DOI] [PubMed] [Google Scholar]
- Hightower LE, Guidon PT. Selective release from cultured mammalian cells of heat-shock (stress) proteins that resemble glia-axon transfer proteins. J Cell Physiol. 1989;138:257–266. doi: 10.1002/jcp.1041380206. [DOI] [PubMed] [Google Scholar]
- Kiang JG, Tsokos GC. Heat shock protein 70kDa: molecular biology, biochemistry and physiology. Pharmacol Ther. 1998;80:183–201. doi: 10.1016/s0163-7258(98)00028-x. [DOI] [PubMed] [Google Scholar]
- Locke M, Noble EG, Atkinson BG. Exercising mammals synthesise stress proteins. Am J Physiol. 1990;258:C723–C729. doi: 10.1152/ajpcell.1990.258.4.C723. [DOI] [PubMed] [Google Scholar]
- Marber MS, Mestril R, Chi S-H, Sayen MR, Yellon DM, Dillman WH. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest. 1995;95:1446–1456. doi: 10.1172/JCI117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizzen LA, Welch WJ. Characterization of the thermotolerant cell. I. effects of protein synthesis activity and the regulation of heat-shock protein 70 expression. J Cell Biol. 1988;106:1105–1116. doi: 10.1083/jcb.106.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI. Cells in stress: transcriptional activation of heat shock genes. Science. 1993;259:1409–1410. doi: 10.1126/science.8451637. [DOI] [PubMed] [Google Scholar]
- Moseley PL. Exercise, stress and the immune conversation. Exerc Sport Sci Rev. 2000;28:128–132. [PubMed] [Google Scholar]
- Multhoff G, Mizzen L, and Winchester CC. et al. 1999 Heat shock protein 70 (Hsp70) stimulates proliferation and cytolytic activity of natural killer cells. Exp Hematol. 27:1627–1636. [DOI] [PubMed] [Google Scholar]
- Ofenstein JP, Heidemann S, Juett-Wilstermann A, Sarnaik A. Expression of stress proteins HSP 72 & HSP 32 in response to endotoxemia. Ann Clin Lab Sci. 2000;30:92–98. [PubMed] [Google Scholar]
- Pedersen BK, Hoffman-Goetz L. Exercise and the immune system: regulation, integration and adaptation. Physiol Rev. 2000;80:1055–1081. doi: 10.1152/physrev.2000.80.3.1055. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Shepherd J, Corton JM. Detection of heat shock protein 70 (HSP70) and anti-HSP70 antibodies in the serum of normal individuals. Immunol Invest. 1998;27:367–377. doi: 10.3109/08820139809022710. [DOI] [PubMed] [Google Scholar]
- Puntschart A, Vogt M, Widmer HR, Hoppeler H, Billeter R. HSP70 expression in human skeletal muscle after exercise. Acta Physiol Scand. 1996;157:411–417. doi: 10.1046/j.1365-201X.1996.512270000.x. [DOI] [PubMed] [Google Scholar]
- Ryan AJ, Gisolfi CV, Moseley PL. Synthesis of 70K stress protein by human leukocytes: effect of exercise in the heat. J Appl Physiol. 1991;70:466–471. doi: 10.1152/jappl.1991.70.1.466. [DOI] [PubMed] [Google Scholar]
- Salo DC, Donovan CM, Davies KJA. HSP70 and other possible heat shock or oxidative stress proteins are induced in skeletal muscle, heart, and liver during exercise. Free Radic Biol Med. 1991;11:239–246. doi: 10.1016/0891-5849(91)90119-n. [DOI] [PubMed] [Google Scholar]
- Sciandra JJ, Subjeck JR. The effect of glucose on protein synthesis and thermosensitivity in Chinese hamster ovary cells. J Biol Chem. 1983;258:12091–12093. [PubMed] [Google Scholar]
- Skidmore R, Gutierrez JA, Guerriro V, Kregal KC. HSP70 induction during exercise and heat stress in rats: role of internal temperature. Am J Physiol. 1995;268:R92–R97. doi: 10.1152/ajpregu.1995.268.1.R92. [DOI] [PubMed] [Google Scholar]
- Starkie RL, Angus DJ, Rolland J, Hargreaves M, Febbraio MA. Effect of prolonged submaximal exercise and carbohydrate ingestion on monocyte intracellular cytokine production in humans. J Physiol. 2000;528:647–655. doi: 10.1111/j.1469-7793.2000.t01-1-00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkie RL, Arkinstall MJ, Koukoulas I, Hawley JA, Febbraio MA. Carbohydrate ingestion attenuates the increase in plasma interleukin-6 but not skeletal muscle interleukin-6 mRNA, during exercise in humans. J Physiol. 2001a;533:585–591. doi: 10.1111/j.1469-7793.2001.0585a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkie RL, Rolland J, Angus DJ, Anderson MJ, Febbraio MA. Circulating monocytes are not the source of elevations in plasma IL-6 and TNF-α levels after prolonged running. Am J Physiol. 2001b;280:C769–C774. doi: 10.1152/ajpcell.2001.280.4.C769. [DOI] [PubMed] [Google Scholar]
- Walters TJ, Ryan KL, Tehrany MR, Jones MB, Paulus LA, Mason PA. HSP70 expression in the CNS in response to exercise and heat stress in rats. J Appl Physiol. 1998;84:1269–1277. doi: 10.1152/jappl.1998.84.4.1269. [DOI] [PubMed] [Google Scholar]
- Weitzel G, Pilatus U, Rensing L. Similar dose response of heat shock protein synthesis and intracellular pH change in yeast. Exp Cell Res. 1985;159:252–256. doi: 10.1016/s0014-4827(85)80054-9. [DOI] [PubMed] [Google Scholar]
- Welch WJ, Garrels JI, Thomas GP, Lin JJ, Feramisco JR. Biochemical characterization of the mammalian stress proteins and identification of two stress proteins as glucose and Ca2+-ionophore-regulated proteins. J Biol Chem. 1983;258:7102–7111. [PubMed] [Google Scholar]