Abstract
Autoantibodies against certain stress or heat shock proteins (Hsps) may play a role in the pathogenesis and/or prognosis of some diseases. Using immunoblotting with human recombinant Hsps and univariate and multivariate logistic regression models, we have investigated the presence of antibodies against Hsp70, the inducible member of the 70-kDa family of heat shock proteins, and analyzed its possible association with hypertension and working conditions. Plasma and serum were collected from 764 steel mill workers from 6 work sites exposed to (1) severe noise; (2) severe noise and dust; (3) noise, dust, and heat; (4) noise and heat; (5) severe noise and heat; and (6) office conditions (control). Workers with prolonged exposure to stresses such as noise, dust, and high temperature and a combination of these in the workplace had a high incidence (26.6% to 40.2%) of antibodies to Hsp70 compared to the lowest incidence (18.6%) of antibodies to Hsp70 in the control group of office workers. Moreover, there was a statistical association of antibodies against Hsp70 with hypertension. The statistical correlation between the presence of antibodies to Hsp70 and hypertension is higher in the group of workers with blood pressure of 160/95 mmHg than in the 140/90-mmHg group after excluding possible effects of the workplace stresses. These results suggest that harsh workplace conditions can increase the production of antibodies against Hsp70 and that the presence of antibodies to this stress protein may be associated with hypertension. The precise mechanism for the elevation of antibodies against Hsps by environmental and workplace stresses and their relation to hypertension remains to be established.
INTRODUCTION
Stress or heat shock proteins (Hsps) are a group of highly conserved proteins that are induced by heat and a variety of noxious stimuli, including abnormal physiological stresses such as ischemia, fever, viral infection, and environmental xenobiotics or chemical stressors such as heavy metals, free radicals, and carbon monoxide (Lindquist and Craig 1988; Morimoto et al 1994). Hsps are usually grouped into 4 main families (Hsp90–110, Hsp/Hsc70, Hsp60, and the small Hsps [Hsp10–30]) on the basis of their apparent molecular masses in sodium dodecyl sulfate polyacrylamide gels. The best-known Hsp is the highly inducible member of the Hsp/Hsc70 family, with apparent molecular mass of 71 and 72 kDa in rat and human, respectively, and referred to here as Hsp70. Overexpression of Hsp70 provides cells with resistance to harmful or stressful conditions, and this acquired resistance is known as thermotolerance (Laszlo 1988; Li et al 1991; Parsell and Lindquist 1994). Similarly, elevated levels of Hsp70 in organs such as the heart and brain can transiently protect the whole organ from ischemic injury (Currie et al 1993; Marber et al 1995; Plumier et al 1995, 1997). Hsps of the Hsp/Hsc70, Hsp60, and Hsp90 families have also been shown to function as molecular chaperones, facilitating the synthesis, folding, assembly, and intracellular transport of many proteins (Hightower 1991; Gething 1992; Muchowski et al 2000). In addition, Hsps may also play important roles in the processes of growth, differentiation, and development (Tanguay et al 1993; Michaud et al 1997).
Hypertension is a disease with a genetic component. The severity of hypertension may be modified by many environmental factors, both in humans as well as in genetic and experimental rodent models of hypertension (Hamet et al 1990). It is also generally accepted that stress contributes to human high blood pressure. Some stresses also have the ability to induce Hsp70 in vascular tissue and may contribute to the development of hypertension in chronically stressed animals. The genetic basis of environmental susceptibility to hypertension may also involve an abnormal control of heat shock genes. As the biological role of the Hsps and molecular chaperones in a multitude of cellular processes unfolds, it is of little surprise that they should be implicated in human diseases. The scientific literature is replete with observations that underscore the potential link between the aberrant expression of heat shock or stress proteins and disease states (Burdon 1993; Morimoto et al 1994). In addition, some of the Hsps can present as self-antigens to the immune system, resulting in the production of autoantibodies (to Hsps) in patients with inflammatory diseases or autoimmune disorders; after various infections caused by viruses, bacteria, mycobacteria, and parasites; or with atherosclerosis (Xu et al 1993, 1999; Wu et al 1996, 1998). Direct evidence links antibodies against mycobacterial Hsp65 and carotid wall atherosclerosis, and the most severe degree of atherosclerosis was demonstrated to predict 5-year mortality (Xu et al 1993, 1999; Schett et al 1995). In addition, serum antibodies to mycobacterial Hsp65 and human Hsp60 have been associated with borderline hypertension (Frosttegard et al 1997; Pockley et al 2000). However, until now, there are few investigations on the possible association of autoantibodies against the inducible Hsp70 and hypertension. We therefore investigated whether the presence of antibodies to human Hsp70 might be a risk factor in the development of hypertension in 764 steel mill workers using Western blot analysis and a logistic regression model of analysis.
METHODS
Study subjects and environmental conditions
The study subjects were workers at the Maansan Steel & Iron Limited Co in Anhui, China. They worked in 6 work sites and were exposed to different levels of several stresses. Group 1 consisted of 175 persons working at the supply and drain unit and exposed to severe noise (98 dB). Group 2 consisted of 91 persons working at the material unit and exposed to severe noise and dust (13 mg/m3). Group 3 comprised 107 persons working at the electric heat unit and exposed to noise, dust, heat, and other stresses, such as carbon monoxide. Group 4 consisted of 158 persons working at the steel rolling mill and exposed to noise and heat. Group 5 comprised 115 workers in another steel rolling mill and exposed to severe noise and heat. Group 6 is the control group consisting of 118 office workers not exposed on a regular basis to the stressful environmental factors of the other 5 groups. Dry and wet ventilated thermometers and radiation thermometers were used to determine the dry and wet temperatures and radiant heat in the workplaces. Noise levels were determined with a sound pressure audiometer (SPL, Jiangxi, China). The concentrations of dust in the air were determined by collecting dust onto a filter with an air sampling pump and by weighing the dust accumulation on the filter. The measurement of noise, dust, and temperature was performed 9 times at 10 AM, 3 PM, and 5 PM for 3 consecutive days.
Evaluation of health status
Health status was evaluated in all workers using a questionnaire and by clinical and laboratory examination. The questionnaire was used to obtain the personal and family history, including risk factors for hypertension, for each worker. An industrial hygienist filled in the questionnaire with each worker before the medical examination. The clinical examination included weight, height, pulse, oral temperature, blood pressure, and electrocardiography (ECG) (Nurminen 1986; Hickey and Graham 1988; Levy and Wegman 1988). Body mass index was calculated as weight (kilograms) divided by height (meters squared). Systolic and diastolic blood pressures were taken with a standard mercury sphygmomanometer after >10 minutes of rest while the subject was in a sitting position. The values used in the present analysis were means of 3 measurements taken during 1 to 2 hours by several specially trained nurses or physicians. Hypertension in this study was defined by 2 criteria: whether the person was currently using any antihypertensive drugs and blood pressure thresholds >160/95 or >140/90 mmHg (Frosttegard et al 1997). Venous blood was also collected and divided into 2 parts, one of which was heparinized to separate plasma for the detection of antibodies against Hsp70. The second part was used for determining the concentration of triglycerides and total cholesterol using kits from Bioengineering Reagents Company (Wenzhou, China).
Determination of the dilution of antibodies to the inducible Hsp70 in plasma of subjects
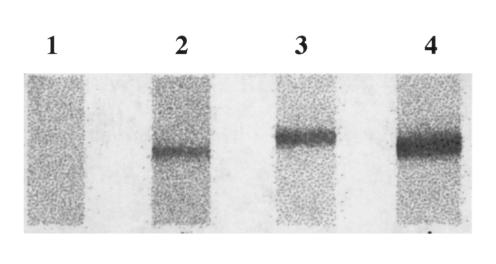
Recombinant human Hsp70 was obtained through the expression of the human cDNA coding for an inducible member of the HSP70 family, Hsp72 (hereafter referred to as Hsp70), in NaCl-induced Escherichia coli GJ1168 cells using pET30 as expression vector (Tanguay et al 1993; Bhandari and Gowrishnkar 1997). Approximately 10–15 μg of recombinant human Hsp70 was loaded on each SDS-PAGE gel without comb and transferred electrophoretically to nitrocellulose membranes (NC) (Wu et al 1998). The transfer was monitored by staining with Ponceau Rouge. The NC band containing Hsp70 was cut into 2 × 3-mm pieces and marked with a small red dot on the protein side of the membrane. The NC pieces were placed in individual wells of an ELISA plate, rinsed with PBS, and saturated with 100 μL of blocking buffer (PBS containing 5% skim milk powder) for 1 hour at 37°C with gentle agitation and washed with PBS-0.05% Tween 80 for 5 minutes. The plasma diluted 1:10, 1:20, and 1:40 in 100 μL PBS containing 5% skim milk powder was incubated with the NC pieces at 37°C for 2 hours with gentle agitation. After washing the membranes 6 times (10 minutes each time) with 200 μL PBS-0.05% Tween 80, 100 μL of HRP labeled goat anti-human IgG (Sigma) in blocking buffer (1:2500) were added, and this was incubated at 37°C for 1 hour. Membranes were washed 6 times (10 minutes each time) with 200 μL PBS-0.05% Tween 80. The presence of antibodies to Hsp70 was revealed using DAB (3,3-diaminobenzidine tetrahydrochloride) buffer for 3–5 minutes. The brown band on NC is regarded as the positive and no color on NC as negative (Wu et al 1998). An example of a Western blot is shown in Figure 1. The technique used is inexpensive and, while not being as sensitive as an immunoassay, gives reliable and reproducible results under the laboratory conditions available.
Fig 1. .
Purified recombinant Hsp70 was electrophoresed in SDS-PAGE, transferred to nitrocellulose membranes, and cut into 2–3-mm-wide strips. These were incubated with the plasma and the presence of antibodies to Hsp70 detected as described in Methods. Lane 1: negative; lanes 2–4: positive
Statistical analyses
The database and its pretreatment were carried out using the statistical analysis software (EPI Info 6.0) package. The analyses were carried out using SPSS in univariate and multivariate logistic regression models. Multivariate logistic regression models were again built for a forward stepwise selection procedure (P values for entry and removal, 0.10 and 0.15, respectively). Other analyses were carried out on the basis of the chi-square test and Student's t-test. Statistical inferences are based on the different levels of significance (P < 0.05 or P < 0.01).
RESULTS
The environmental conditions
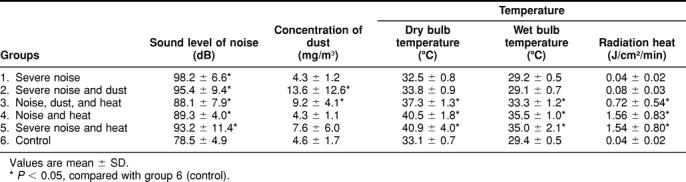
The workplace noise levels, dust concentrations, and temperatures are presented in Table 1 for each group of workers. This table also summarizes the stressful working conditions for each group of workers: (1) severe noise; (2) severe noise and dust; (3) noise, dust, and heat; (4) noise and heat; (5) severe noise and heat; and (6) control. Group 1 had no other stresses than severe noise levels (98 dB). Group 2 had the highest level of dust in the air (13.6 mg/m3), and groups 4 and 5 had the highest exposure to heat with dry bulb temperatures over 40°C. Overall, each of the 6 groups of workers was exposed to different environmental working conditions.
Table 1.
Environmental working conditions in 6 groups of workers
Detection of autoantibodies against Hsp70
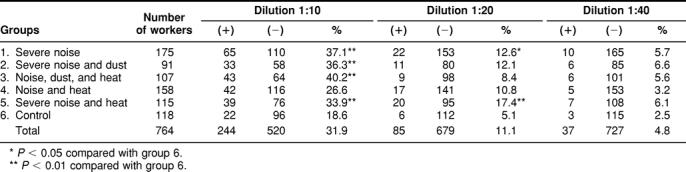
Here we have extended our earlier investigations (Wu et al 1996, 1998) on antibodies to human inducible Hsp70 to include workplace stressors such as noise, dust, and heat. Plasma from workers were tested at different dilutions from 1:10 to 1:40 for immunoreactivity against NC-bound human Hsp70. As shown in Table 2, at a dilution of 1:10, the incidence of plasma samples having Hsp70 antibodies is higher in the workers of group 3 (noise, dust, and heat and other stresses, such as carbon monoxide), group 1 (severe noise), group 2 (severe noise and dust), and group 5 (severe noise and heat) than in the other 2 groups (group 4 and the control, group 6). At a dilution of 1:20, the percent of workers having immunoreactive Hsp70 antibodies is statistically higher in groups 5 and 1. In groups 2 and 4, the percent of workers with a positive immunoreaction is slightly higher than in the control group but is not statistically different. The percent of workers having a positive immunoreaction for Hsp70 antibodies at a dilution of 1:40 is similar in all groups.
Table 2.
Incidence of antibodies against Hsp70 in plasma of workers
Association of anti-Hsp70 with hypertension
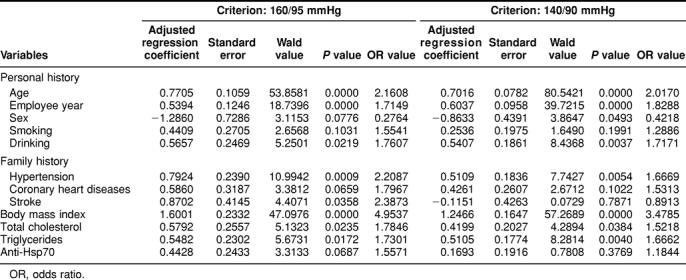
A univariate logistic regression analysis shows that age, employee working years, drinking, family history in cardiovascular diseases, body mass index, total cholesterol, and triglycerides are the risk factors of hypertension (Table 3; odds ratio [OR] value > 1.7). This table also shows a statistical association of antibodies against Hsp70 with hypertension (P < 0.1) if hypertension is defined as 160/95 mmHg (P = 0.0687) but not if a value of 140/90 mmHg is used (P = 0.3769).
Table 3.
Univariate logistic regression analysis of risk factors in hypertension
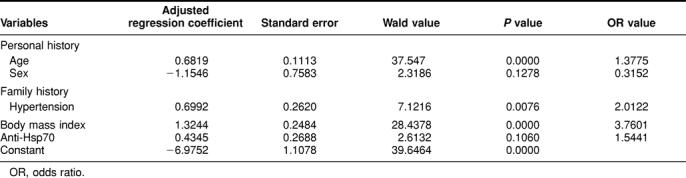
Further analysis of these data was done using a multivariate logistic regression model built with a forward stepwise selection procedure (P values for entry and removal, 0.10 and 0.15, respectively). While this analysis did not reach statistical significance, it does suggest that the presence of Hsp70 antibodies in the serum of workers may be associated with hypertension when the criterion of 160/95 mmHg is used (Table 4). The same analysis carried out with a criterion of 140/90 mmHg does not show such an association (criterion of 140/90 mmHg = 0.6260 × age + 0.4052 × family history in hypertension + 0.9952 × body mass index − 0.7696 × sex − 5.1335).
Table 4.
Multivariate logistic regression analysis of the risk factors of hypertension (160/95 mmHg)
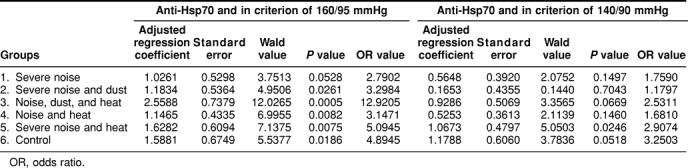
To determine the effects of the workplace environment (noise, dust, heat, and so on) on the association of Hsp70 antibodies with hypertension, a multivariate logistic regression model was built using group 6 (control, minimal exposure to these workplace stresses) as a reference. As shown in Table 5, the increased frequency of Hsp70 antibodies is associated with hypertension independently of the stresses in the workplace environment. There is a stronger statistical correlation between the presences of Hsp70 antibodies and hypertension using the criterion of 160/95 mmHg than 140/90 mmHg. In other words, environmental stresses (noise, dust, and heat) in the workplace do not modify the established association between hypertension and the presence of Hsp70 antibodies.
Table 5.
Multivariate logistic regression analysis of the association of antibodies to Hsp70 with hypertension
Association of anti-Hsp70 with harsh working conditions
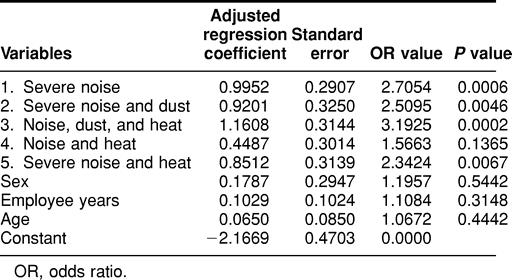
Finally, the association between antibodies to Hsp70 and working conditions was assessed using the multivariate regression analysis. As shown in Table 6, the group exposed to low noise and dust (group 4) shows the lowest correlation, but the other groups, especially those exposed to a combination of multiple stresses (groups 1, 2, 3, and 5), do show a correlation between antibodies to Hsp70 and harsh working conditions. However, as noted previously, a significant correlation between hypertension and Hsp70 antibody levels is not dependent on workplace environmental stresses. Furthermore, levels of environmental stress in the workplace did not correlate significantly with hypertension in this study (analysis not shown), consistent with the broadly held hypothesis that hypertension results from multiple genetic and environmental factors.
Table 6.
Multivariate logistic regression analysis of the association of antibodies to Hsp70 with harsh working conditions
DISCUSSION
Our findings support the idea that prolonged exposure to workplace stresses, such as noise, dust, or heat, can affect the production of antibodies against Hsp70. Some possible reasons for the production of antibodies against Hsps are (1) genetic factors, (2) viral and bacterial infection, (3) the denaturation and release of Hsps as a result of cell damage, and (4) the presence of antigen-specific lymphocytes (Wu et al 1996).
As a molecular chaperone, Hsp70 is involved in many important basic biological processes, including inflammation, the acquisition of cell tolerance to chemical and physical stresses (heat exposure), and cell survival following ischemia and other stresses in heart and brain (Currie et al 1993; Marber et al 1995; Plumier et al 1995, 1997). Thus, once Hsps are expressed at elevated levels, they may become the target of humoral and T cell–mediated immune responses and may provide a link between the immune response to infection and autoimmunity caused by T lymphocyte cross-reactivity among Hsps of different origins (Xu et al 1993; Schett et al 1995; Xu et al 1999).
Our analysis included separation of all workers into one of 2 groups: positive or negative according to the presence of Hsp70 antibodies. There were no significant differences in the incidence of chronic diseases such as chronic bronchitis, diabetes, hepatitis, renal diseases, or tumor; in smoking and drinking habits; or in family history of genetic diseases and cardiovascular diseases between these 2 groups (data not shown). However, the incidence of family history with cardiovascular diseases and abnormal electrocardiogram was slightly higher in the Hsp70 antibody positive group than in the negative group. Our present results suggest that neither the genetic background nor previous infections are directly responsible for the higher occurrence of Hsp70 antibodies in several groups of workers. We previously reported the presence of antibodies to Hsp70 in workers experiencing long-term exposure to high temperature, carbon monoxide, and benzene and in patients with benzene poisoning and suggested that the presence of such antibodies might potentially be useful biomarkers to assess if workers are experiencing abnormal stress within their living and working environment (Wu et al 1995, 1996, 1998). On the other hand, the presence of antibodies to various Hsps is thought to be of significance in the generation, formation, and prognosis of diseases (Jarjour et al 1991; Bongrazio et al 1994; Kaufmann and Schoel 1994; Minowada and Welch 1995; Xu et al 1996; Wu et al 1998). For example, Jarjour et al (1991) suggested that the difference in the level of anti-HSP antibodies seen in sera of patients with various rheumatoid and other inflammatory diseases relative to normal controls might reflect disease-associated polyclonal B cell activation. Shingai et al (1995) reported the presence of antibodies to Hsps in patients with autoimmune liver diseases and suggested that the presence of anti-Hsc70 antibodies was an indicator of primary biliary cirrhosis. Work from Schett et al (1995) and Xu et al (1999) has implicated mycobacterial Hsp65 as an antigen involved in instigating the chronic immune response characteristic of human atherosclerosis. Their cross-sectional data have shown a direct relationship between antibodies against Hsp65 and carotid wall atherosclerosis. These antibodies are sustained among those with the most severe degree of underlying atherosclerosis and were demonstrated to predict 5-year mortality (Xu et al 1993, 1999; Schett et al 1995). An association between the presence of soluble human Hsp60 and anti-Hsp60 antibodies in the serum and atherosclerosis has also been recently reported (Pockley et al 2000; Xu et al 2000). While Hsp autoantibodies are associated with a chronic disease, our detection of Hsp70 antibodies in workers free of chronic disease might be explained by the relative abundance of the antibodies. In the present study, antibodies to Hsp70 were detected at serum dilutions of 1:10 to 1:40 and at such low concentration appear to be below a clinical threshold.
Stresses in the workplace appear to be associated with the higher occurrence of Hsp70 antibodies (Tables 2 and 6). The Hsp70 antibodies are likely in response to previous elevation of Hsp70, possibly due to the psychological stress of a challenging workplace. Interestingly, Hsp70 (but not Hsp30, Hsp60, or Hsp90) is found in hearts of Japanese quails exposed to loud noise, inescapable irritation, cold temperature, or isolation in darkness (Hoekstra et al 1998). Similarly, Hsp70 is elevated in rat aorta and adrenal glands after restraint stress (Udelsman et al 1993). It was suggested that the adrenal response was adrenocorticotropin dependent and that the vascular response was adrenergic. Such responses suggest that increased sympathetic tone is involved in the expression of Hsp70. In addition, elevated sympathetic tone is associated with increased vascular tone (diastolic blood pressure) and hypertension in people (Eliasson 1985; Adams et al 1998).
The present statistical analysis supports an association between the presence of antibodies against Hsp70 and hypertension. The association is higher if the blood pressure criterion of 160/95 mmHg rather than 140/90 mmHg is applied. Interestingly, enhanced expression of Hsp70 has been detected in both cultured cells and organs from hypertensive experimental animals (Hamet et al 1992; Xu et al 1995, 1996).
In summary, we have shown a relationship between workplace stress and the presence of Hsp70 antibodies and an association between Hsp70 antibodies and hypertension. It remains to be determined whether there is a relationship between the induction of Hsp70 by workplace stresses, possibly mediated by increased sympathetic tone, and production of plasma antibodies against Hsp70 and whether Hsp70 or antibodies against Hsp70 play a role in the pathogenesis of hypertension.
Acknowledgments
We are particularly grateful to all individuals who voluntarily accepted to participate in the study and to the many members of the medical personnel of Maansan Hospital, to Maansan Steel & Iron Limited Co in Anhui and their generous help in the examination and sampling of subjects, and to Dr Frank B. Hu (Harvard University, Boston) for his careful reading of our manuscript. This work was supported by research funds from the National Natural Science Foundation of China (NNSFC) and from the Health Ministry of China (TW) and a joint exchange program grant between the NNSFC and the Medical Research Council of Canada (TW and RMT). Work in R.M.T.'s laboratory is supported by the Canadian Institutes of Health Research (CIHR). Work in R.W.C.'s laboratory is supported by the Heart and Stroke Foundation of New Brunswick and the Canadian Stroke Network.
REFERENCES
- Adams SL, Roxe DM, Weiss J, Zhang F, Rosenthal JE. Ambulatory blood pressure and Holter monitoring of emergency physicians before, during, and after a night shift. Acad Emerg Med. 1998;5:871–877. doi: 10.1111/j.1553-2712.1998.tb02816.x. [DOI] [PubMed] [Google Scholar]
- Bhandari P, Gowrishnkar J. An Escherichia coli host strain useful for efficient overproduction of cloned gene products with NaCl as the inducer. J Bacteriol. 1997;179:4403–4406. doi: 10.1128/jb.179.13.4403-4406.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongrazio M, Comini, Gaia G, Bachetti T, Ferrari R. Hypertension, aging, and myocardial synthesis of heat-shock protein 72. Hypertension. 1994;24:620–624. doi: 10.1161/01.hyp.24.5.620. [DOI] [PubMed] [Google Scholar]
- Burdon RH. Heat shock proteins in relation to medicine. Mol Aspects Med. 1993;14:83–165. doi: 10.1016/0098-2997(93)90020-e. [DOI] [PubMed] [Google Scholar]
- Currie RW, Tanguay RM, Kingma JG. Heat-response and limitation of tissue necrosis during occusion/reperfusion in rabbit hearts. Circulation. 1993;87:963–971. doi: 10.1161/01.cir.87.3.963. [DOI] [PubMed] [Google Scholar]
- Eliasson K. Borderline hypertension: circulatory, sympatho-adrenal and psychological reactions to stress. Acta Med Scand Suppl. 1985;692:1–90. [PubMed] [Google Scholar]
- Frosttegard J, Lemne C, Andersson B, Zee RVD, Liessling R, Faire U. Association of serum antibodies to heat shock protein 65 with borderline hypertension. Hypertension. 1997;29:40–44. doi: 10.1161/01.hyp.29.1.40. [DOI] [PubMed] [Google Scholar]
- Gething MJ. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Hamet P, Kong D, Pravenec M, Kunes J, Kren V, Klir P, Sun YL, Tremblay J. Restriction fragment length polymorphism of hsp70 gene, localized in the RT1 complex, is associated with hypertension in spontaneously hypertensive rats. Hypertension. 1992;19:611–614. doi: 10.1161/01.hyp.19.6.611.a. [DOI] [PubMed] [Google Scholar]
- Hamet P, Malo D, Tremblay J. Increased transcription of a major stress gene in spontaneously hypertensive mice. Hypertension. 1990;15:904–908. doi: 10.1161/01.hyp.15.6.904. [DOI] [PubMed] [Google Scholar]
- Hickey N, Graham IM 1988 Factors related to hypertension. In: Hypertension. Croom Helm, London, Sydney, 29–70. [Google Scholar]
- Hightower LE. Heat shock, stress protein, chaperones, and proteotoxicity. Cell. 1991;66:191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- Hoekstra KA, Iwama GK, Nichols CR, Godin DV, Cheng KM. Increased heat shock protein expression after stress in Japanese quail. Stress. 1998;2:265–272. doi: 10.3109/10253899809167290. [DOI] [PubMed] [Google Scholar]
- Jarjour WN, Jeffries BD, Davis JS, Welch WJ, Mimura T, Winfield JD. Autoantibodies to human stress proteins. Arthritis Rheum. 1991;34:1133–1138. doi: 10.1002/art.1780340909. [DOI] [PubMed] [Google Scholar]
- Kaufmann SHE, Schoel B 1994 Heat shock proteins as antigens in immunity against infection and self. In: The Biology of Heat Shock Proteins and Molecular Chaperones, ed Morimoto RI, Tissières A, Georgopoulos C. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 495–531. [Google Scholar]
- Laszlo A. Evidence for two states of thermotolerance in mammalian cells. Int J Hyperth. 1988;4:513–526. doi: 10.3109/02656738809027695. [DOI] [PubMed] [Google Scholar]
- Levy BS, Wegman DH 1988 Recognizing occupational diseases. In: Occupational Health—Recognizing and Preventing Work-Related Diseases, ed Levy BS, Wegman DH. Little, Brown and Company, Boston, 29–43. [Google Scholar]
- Li GC, Li LY, Liu K, Mak JK, Chen L, Lee WMF. Thermal response of rat fibroblasts stably transfected with the human 70kDa heat shock protein encoding gene. Proc Natl Acad Sci USA. 1991;88:1681–1685. doi: 10.1073/pnas.88.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Marber MS, Mestril R, Chi S, Sayen MR, Yellon DM, Dillmann WH. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest. 1995;96:1446–1456. doi: 10.1172/JCI117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud S, Marin R, Tanguay RM. Regulation of heat shock gene induction and expression during Drosophila development. Cell Mol Life Sci. 1997;53:104–113. doi: 10.1007/PL00000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minowada G, Welch WJ. Clinical implications of the stress response. J Clin Invest. 1995;95:3–12. doi: 10.1172/JCI117655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI, Tissières A, and Georgopoulos C 1994 The Biology of Heat Shock Proteins and Molecular Chaperones. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Muchowski PJ, Schaffar G, Sittler A, Wanker EE, Hayer-Hartl MK, Hartl FU. Hsp70 and hsp40 chaperones can inhibit self-assembly of polyglutamine proteins into amyloid-like fibrils. Proc Natl Acad Sci U S A. 2000;97:7841–7846. doi: 10.1073/pnas.140202897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurminen M 1986 Reappraisal of an epidemiological study. In: Epidemiology of Occupational Health, Ed Karvonen M, Mikheev MI. WHO regional publications European series no 20, Copenhagen, 392 p. [Google Scholar]
- Parsell DA, Lindquist S 1994 Heat shock proteins and stress tolerance. In: The Biology of Heat Shock Proteins and Molecular Chaperones, ed Morimoto RI, Tissières A, Georgopoulos C. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 457–494. [DOI] [PubMed] [Google Scholar]
- Plumier C, Krueger AM, Currie RW, Kontoyiannis D, Kollias G, Pagoulatos GN. Transgenic mice expressing the human inducible Hsp70 have hippocampal neurons resistant to ischemic injury. Cell Stress Chaperones. 1997;2:162–167. doi: 10.1379/1466-1268(1997)002<0162:tmethi>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumier C, Ross BM, Currie RW, Angelidis CE, Kazlaris H, Kollias G, Pagoulatos GN. Transgenic mice expression of the human heat shock protein 70 have improved post-ischemic myocardial recovery. J Clin Invest. 1995;95:1854–1860. doi: 10.1172/JCI117865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockley AG, Wu R, Lemne C, Kiessling R, de Faire U, Frosttegard J. Circulating heat shock protein 60 is associated with early cardiovascular disease. Hypertension. 2000;36:303–307. doi: 10.1161/01.hyp.36.2.303. [DOI] [PubMed] [Google Scholar]
- Schett G, Xu Q, Amberger A, Van Der Zee R, Recheis H, Willeit J, Wick G. Autoantibodies against heat shock protein 65 mediate endothelial cytotoxicity. J Clin Invest. 1995;96:2569–2577. doi: 10.1172/JCI118320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingai R, Maeda T, Onishi S, Yamamoto Y. Autoantibody against 70 kD heat-shock protein in patients with autoimmune liver diseases. J Hepatol. 1995;23:382–390. doi: 10.1016/0168-8278(95)80195-2. [DOI] [PubMed] [Google Scholar]
- Tanguay RM, Wu Y, Khandjian EW. Tissue-specific expression of heat shock stress proteins of the mouse in the absence of stress. Dev Genet. 1993;14:112–118. doi: 10.1002/dvg.1020140205. [DOI] [PubMed] [Google Scholar]
- Udelsman R, Blake MJ, Stagg CA, Li DG, Putney DJ, Holbrook NJ. Vascular heat shock protein expression in response to stress: endocrine and autonomic regulation of this age-dependent response. J Clin Invest. 1993;91:465–473. doi: 10.1172/JCI116224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, He H, and Tanguay RM. et al. . 1995 The combined effects of high temperature and carbon monoxide on heat stress response. J Tongji Med Univ. 15:178–183. [DOI] [PubMed] [Google Scholar]
- Wu T, Tanguay RM, Wu Y, He H, Xu D, Feng J, Shi W, Zhang G. Presence of antibodies to heat stress proteins and its possible significance in workers exposed to high temperature and carbon monoxide. Biomed Environ Sci. 1996;9:370–379. [PubMed] [Google Scholar]
- Wu T, Yuan Y, Wu Y, He H, Zhang G, Tanguay RM. Presence of antibodies to heat stress proteins in workers exposed to benzene and in patients with benzene poisoning. Cell Stress Chaperones. 1998;3:161–167. doi: 10.1379/1466-1268(1998)003<0161:poaths>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Fawcett TW, Udelsman R, Holbrook NJ. Activation of heat shock transcription factor 1 in rat aorta in response to high blood pressure. Hypertension. 1996;28:53–57. doi: 10.1161/01.hyp.28.1.53. [DOI] [PubMed] [Google Scholar]
- Xu Q, Kiechl S, Mayr M, Metzler B, Egger G, Oberhollenzer F, Willeit J, Wick G. Association of serum antibodies to heat shock protein 65 with carotid atherosclerosis: clinical significance determined in a follow-up study. Circulation. 1999;100:1169–1174. doi: 10.1161/01.cir.100.11.1169. [DOI] [PubMed] [Google Scholar]
- Xu Q, Li D, Holbrook NJ, Udelsman R. Acute hypertension induces heat-shock protein 70 gene expression in rat aorta. Circulation. 1995;92:1223–1229. doi: 10.1161/01.cir.92.5.1223. [DOI] [PubMed] [Google Scholar]
- Xu Q, Schett G, and Perschinka H. et al. . 2000 Serum soluble heat shock protein 60 is elevated in subjects with atherosclerosis in a general population. Circulation. 102:14–20. [DOI] [PubMed] [Google Scholar]
- Xu Q, Willeit J, and Marosi M. et al. . 1993 Association of serum antibodies to heat shock protein 65 with carotid atherosclerosis. Lancet. 341:255–259. [DOI] [PubMed] [Google Scholar]