Abstract
Background
The brain mechanisms of cognitive-behavioral therapy (CBT), a highly effective treatment for pediatric obsessive-compulsive disorder (OCD), are unknown. Neuroimaging in adult OCD indicates that CBT is associated with metabolic changes in striatum, thalamus, and anterior cingulate cortex. We therefore probed putative metabolic effects of CBT on these brain structures in pediatric OCD using proton magnetic resonance spectroscopic imaging (1H MRSI).
Method
Five unmedicated OCD patients (4 ♀, 13.5±2.8) and 9 healthy controls (7 ♀, 13.0±2.5) underwent MRSI (1.5 T, repetition-time/echo-time=1500/30 ms) of bilateral putamen, thalamus and pregenual anterior cingulate cortex (pACC). Patients were rescanned after 12 weeks of exposure-based CBT. The Children's Yale-Brown Obsessive-Compulsive Scale (CY-BOCS) of OCD symptoms was administered before and after CBT.
Results
Four of 5 patients responded to CBT (mean 32.8% CY-BOCS reduction). Multiple metabolite effects emerged. Pre-CBT, N-acetyl-aspartate+N-acetyl-aspartyl-glutamate (tNAA) in left pregenual anterior cingulate cortex (pACC) was 55.5% higher in patients than controls. Post-CBT, tNAA (15.0%) and Cr (23.9%) in left pACC decreased and choline compounds (Cho) in right thalamus increased (10.6%) in all 5 patients. In left thalamus, lower pre-CBT tNAA, glutamate+glutamine (Glx), and myo-inositol (mI) predicted greater post-CBT drop in CY-BOCS (r = 0.98) and CY-BOCS decrease correlated with increased Cho.
Conclusions
Interpretations are offered in terms of the Glutamatergic Hypothesis of Pediatric OCD. Similar to 18FDG-PET in adults, objectively measurable regional MRSI metabolites may indicate pediatric OCD and predict its response to CBT.
Keywords: Child OCD CBT, Pregenual anterior cingulate, Thalamus, N-acetyl-aspartate, Glutamate
1. Introduction
Cognitive-behavioral therapy (CBT) is highly effective against pediatric obsessive-compulsive disorder (OCD) (Piacentini et al., 2006), a chronic, debilitating condition affecting 0.5-2% of children and adolescents (Rapoport et al., 2000; Zohar, 1999), but the brain bases of CBT are poorly understood. Several 18Fluorodeoxyglucose positron emission tomography (18FDG-PET) studies of adult OCD (Baxter et al., 1987, 1988; Kwon et al., 2003; Nordahl et al., 1989; Perani et al., 1995; Sawle et al., 1991; Saxena et al., 2001; Swedo et al., 1989) found above-normal pre-treatment glucose metabolic rate (GMR) in brain structures such as the caudate, thalamus, or cingulate cortex. These structures form functional fronto-striato-thalamic loops thought to be hyperactive in OCD (reviewed in Middleton, 2009). Four studies (Baxter et al., 1992; Brody et al., 1998; Schwartz et al., 1996) including ours (Saxena et al., 2009a), found, according to region, increase or decrease in GMR in these structures after CBT. Hence, CBT may change regional brain energetic metabolism. Due to its mildly invasive nature and use of radiotracers, however, PET is not performed in children in the absence of medical indications which are rarely present in OCD. Therefore, it is unknown if abnormally elevated GMR exists in frontosubcortical circuits in children with OCD or if such putative elevation would change in response to OCD therapy.
Proton magnetic resonance spectroscopy (1H MRS) is a safer, non-invasive in vivo neuroimaging alternative to PET for addressing questions of regional brain energy metabolism. MRS records a series of peaks or resonances each representing the local concentration of a different neurometabolite or small family of chemically related neurometabolites. These metabolites include the two most abundant CNS amino acids: N-acetyl-aspartate (NAA) and glutamate (Glu). In vivo in humans, NAA is almost always measured together with spectrally-overlapping N-acetyl-aspartyl-glutamate (NAAG) and Glu is often measured together with overlapping glutamine (Gln), in which case NAA+NAAG is abbreviated ―tNAA‖ (total NAA) and Glu+Gln is abbreviated ―Glx‖. Other MRS peaks include those for creatine+phosphocreatine (Cr+PCr), choline-compounds (Cho), and myo-inositol (mI). 1H MRS has linked tNAA (O'Neill et al., 2000) and Glx (Pfund et al., 2000) to GMR, while the usually invasive, technically more challenging, and much less widely available 13C MRS has done likewise for NAA (Moreno et al., 2001) and Glu (Sibson et al., 1998) proper. Cr buffers the ATP yield of energy catabolism (Erecinska and Silver, 1989; Miller, 1991) and may reflect energy supply by the mitochondrion (Petroff et al., 2003). Cho (Stork and Renshaw, 2005) and mI (Cecil et al., 2006) are intermediates in membrane synthesis and thus represent carbon diverted from the cell-energy stream. Other metabolic interactions concern the vast quantities of water generated by energetic catabolism. NAA and NAAG are metabolic water export carriers (Baslow, 2003; Baslow et al., 2005) while NAA, Glu, PCr, Cho, and mI all serve as osmolytes (Ross and Blüml, 2001) that regulate cell water content. Thus, it is conceivable that elevated GMR in OCD, particularly if it diminishes membrane synthesis and/or enhances water production, leads to abnormal levels of multiple MRS metabolites, likewise for CBT-assiciated GMR changes.
MRS studies of adult OCD (reviewed in O'Neill and Schwartz, 2005; Saxena et al., 2009b) have, in fact, found abnormal tNAA, Glu or Glx, Cr, Cho, or mI, or ratios of these compounds, at baseline in cingulate cortex, subcortical loop nuclei, or their interconnecting white matter (Bartha et al., 1998; Ebert et al., 1997; Jang et al., 2006; Kitamura et al., 2006; Whiteside et al., 2006; Yücel et al., 2007, 2008; Zurowski et al., 2007). Baseline tNAA/Cr in right pregenual anterior cingulate cortex (pACC)(for cingulate subregions nomenclature see Vogt, 2009; O'Neill et al., 2009) correlated negatively with Yale-Brown Obsessive-Compulsive Scale score (YBOCS) (Ebert et al., 1997) and tNAA in left and right posterior middle cingulate cortex (pMCC) correlated negatively with the fMRI blood oxygenation level-dependent (BOLD) effect (Yücel et al., 2007). In Jang et al. (2006), tNAA/Cr in frontal white matter increased after treatment with serotonin reuptake inhibitors (SRIs) and in Zurowski et al. (2007) above-normal Glu in midline pregenual anterior cingulate cortex (pACC) and above-normal Cho in right ―ventral striatum‖ decreased after CBT. Our study of CBT for adult OCD (O'Neill et al., 2007), using the magnetic resonance spectroscopic imaging (MRSI) variant of MRS, yielded several findings including baseline effects of OCD on tNAA, Cr, Cho, or mI in pACC and thalamus; effects of CBT on Glx, Cr, or mI in anterior middle cingulate cortex (aMCC) and thalamus; and correlations between neurometabolite levels and Y-BOCS scores. Thus, there is evidence from adult OCD that regional MRS neurometabolites are abnormal in OCD, respond to CBT, and vary with clinical symptoms. These adult OCD results, however, may not apply to early-onset OCD, which may be a distinct subtype of the disease (Geller et al., 1998) and for which the patient is more often treatment-naïve.
Pediatric OCD (reviewed in MacMaster et al., 2008) has been less intensively studied with MRS. At baseline, elevated Glx was found in caudate (Rosenberg et al., 2000) and below-normal Glx was found in midline pACC (Rosenberg et al., 2004), as were effects involving NAA, Cr, and Cho in mesial thalamus (Fitzgerald et al., 2000; Rosenberg et al., 2001; Smith et al., 2003; Mirza et al., 2006). The pACC Glx effect was associated with the GRIN2B gene coding for the N-methyl-D-aspartate (NMDA) Glu receptor (Arnold et al., 2009). Caudate Glx dropped in response to the SRI paroxetine (Bolton et al., 2001; Moore et al., 1998; Rosenberg et al., 2000). One published study (Benazon et al., 2003) did not find any effects of CBT on MRS metabolites in pediatric OCD. That study, however, examined only the left caudate, leaving open the possibility of effects of CBT on metabolites in other brain regions.
The present pilot study sought effects of CBT on neurometabolites in pediatric OCD in additional brain regions. We used MRSI to allow simultaneous bilateral sampling of multiple brain sites at high spatial resolution. Bilateral sampling can investigate possible lateralized effects of CBT on OCD brain metabolism (Baxter et al., 1992; Brody et al., 1998; Nakatani et al., 2003; Schwartz et al., 1996). We also studied relations of MRSI metabolites to clinical symptoms of OCD and their response to CBT. We particularly anticipated effects involving tNAA and Glx. In addition to helping identify the neuroanatomic loci and neurochemical bases of OCD and CBT, MRSI may also yield objective predictors or indices of treatment response.
2. Methods and Materials
2.1. Subjects, clinical assessment, cognitive behavioral therapy
Five medication-free patients with DSM-IV OCD (4 girls; mean age 13.5 ± 2.8 years; Table 1) without prior history of CBT were recruited from our hospital-based specialty clinic. OCD diagnosis was determined via semi-structured diagnostic interview. The patients were compared to 9 healthy control subjects (7 girls; 13.0 ± 2.5) contemporaneously examined at our center as part of the NIH National Pediatric MRI Database project (Brain Development Cooperative Group and Evans, 2006). Each patient underwent once weekly sessions of standard exposure-based CBT for 12 weeks as prescribed in our treatment manual (Piacentini et al., 2007). Within 1 week before starting and after completing CBT, patients underwent clinical assessment with the Children's Yale-Brown Obsessive-Compulsive Scale (CY-BOCS)(Scahill et al., 1997), which served as a principal measure of core OCD symptom severity. (A blind independent evaluator conducted the assessments under the supervision of a doctoral-level psychologist.) The study was approved by the UCLA Human Subjects Committee. Informed assent or consent was obtained from each subject respectively his or her parents prior to participation.
Table 1.
Clinical characteristics of study pediatric OCD patients
| CY-BOCS Score | ||||
|---|---|---|---|---|
| Patient | Sex | Age, yr | pre-CBT | post-CBT |
| N001 | female | 9.2 | 20 | 6 |
| N002 | female | 16.2 | 30 | 35 |
| N003 | female | 14.8 | 29 | 11 |
| N004 | male | 14.8 | 28 | 20 |
| N005 | female | 12.4 | 24 | 16 |
| OCD mean | 4 ♀/1 ♂ | 13.5 ± 2.8 | 26.2 ± 4.1 | 17.6 ± 11.1 |
| control mean | 7 ♀/2 ♂ | 13.0 ± 2.5 | -- | -- |
OCD, obsessive-compulsive disorder; CY-BOCS, Children's Yale-Brown Obsessive-Compulsive Scale; CBT, cognitive-behavioral therapy
2.2. Proton magnetic resonance spectroscopic imaging
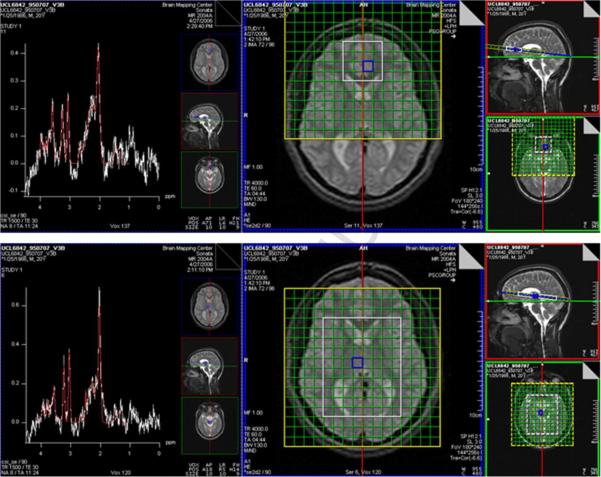
Whole-brain structural MRI and water-suppressed 1H MRSI (PRESS, repetition-time/echo-time = 1500/30 ms, 8 excitations) were acquired in 1.5-hr sessions at 1.5 T on a Siemens Sonata scanner using a quadrature headcoil within 1 week before starting and then within 1 week after completing CBT for patients and at baseline only for controls. MRSI was acquired from two bilateral 9 mm-thick arrays (―slabs‖; Fig. 1) of 11 x 11 mm2 voxels. One slab sampled pACC, the other putamen and thalamus. The caudate nuclei were also sampled but not analyzed due to insufficient data passing quality control. Acquisition was immediately repeated for each slab without water-suppression (1 excitation).
Fig. 1.
PRESS magnetic resonance spectroscopic imaging (MRSI) slabs (white boxes) sampling bilateral pregenual anterior cingulate cortex (pACC; top) and putamen and thalamus (bottom). The center and right panels depict the slabs on various structural MRI sections. Sample spectra (left panels) are from voxels selected in blue.
MR spectra were fit automatically with LCModel (Provencher, 2001) yielding levels of tNAA, Glx, Cr, Cho, and mI referenced to unsuppressed water expressed in Institutional Units (IU). After segregation of the whole-brain MRI into gray-matter, white-matter, and CSF binary masks (Shattuck et al., 2001), the MRSI Voxel Picker (MVP) package (O'Neill et al., 2006; Seese et al., in press) was used for MRI/MRSI co-processing. For each MRSI slab, MVP reconstructed the MRI and the binary masks into the space of the corresponding MRSI PRESS volume; computed the volume percent (vol%) gray matter, white matter, and CSF in each MRSI voxel; corrected the LCModel-derived levels of each metabolite for voxel CSF content; and automatically rejected spectra not meeting quality control criteria; and displayed results on a guided user interface (GUI). Quality control criteria were linewidth ≤ 0.1 ppm and signal-to-noise ratio ≥ 3. Additionally, within spectra, individual metabolite peaks were rejected that were not considered reliable by LCModel (standard deviation of metabolite signal > 20%). Using the GUI, voxels were selected by a blinded operator on the MVP GUI in left and right pACC, putamen, and thalamus. Within each region, MVP averaged the values for all MRSI voxels together that satisfied the above criteria and for which tissue content was ≥ 40 vol% gray matter for the putamina or ≥ 60 vol% for the thalami and pACC.
2.3. Statistical analyses
A non-parametric approach to statistics was taken. For between-group comparisons of baseline metabolite levels and voxel tissue composition (vol% gray matter, vol% white matter) and for post-pre comparisons of these endpoints within the OCD group, data were rank-transformed prior to analysis with the independent T-test (baseline comparisons) or the paired T-test (post-pre comparisons). For brain regions where significant baseline or post-pre differences in tissue composition were found, univariate and repeated-measures analysis-ofcovariance (ANCOVA) respectively were performed in lieu of T-test with vol% gray and/or white matter as covariates. No separate analyses were performed for vol% CSF since it is a linear combination of vol% gray and white matter. Spearman correlations were performed on the untransformed OCD data between baseline metabolite levels and baseline CY-BOCS scores, between baseline metabolites and post-pre change in CY-BOCS (―predictors of response‖), and between post-pre change in metabolites and post-pre change in CY-BOCS. Criterion for statistical significance was p<0.05, Bonferroni corrected for multiple comparisons (uncorrected for comparisons of tissue-composition). Bonferroni correction was effected by multiplying p values by 6, the number of independent brain regions sampled with MRSI.
Potential lateral asymmetry of metabolite effects was assessed for major findings. To do this, the laterality index (LI) was computed for ranked baseline or post-pre-CBT metabolite levels. For a given brain region and metabolite, LI = (L-R)/(L+R) with L being the metabolite level in the left brain and R the metabolite level in the right brain. LI = +1 implies full left-sided while LI = -1 means full right-sided lateralization of the baseline metabolite or metabolite response to CBT. LI was compared between the OCD and controls groups at baseline using an independent T-Test; LI across the OCD group was compared to the value “0” (no lateralization) for post-pre effects using a one-sample T-test, both tests being non-parametric due to rank-transform.
3. Results
3.1. Baseline clinical OCD symptoms and treatment response
Group mean pre-treatment CY-BOCS score was 26.2±4.1 (Table 1), indicating moderate symptom severity. Post-CBT, CY-BOCS declined 8-18 points in 4 patients and increased by 5 points in a fifth. This yielded a post-CBT group mean of 17.6±11.1, a mean decline of 32.8%, which however was not statistically significant (t=-2.4, p=0.073, uncorrected).
3.2. MRSI regional voxel tissue composition
Table 2 lists group-mean vol% gray matter, white matter, and CSF in all six brain regions for healthy controls and for OCD patients before and after CBT. At baseline, vol% white matter in left pACC was significantly lower (t=-3.3, p<0.01, uncorrected) and vol% gray matter in right pACC was significantly higher (t=2.7, p<0.05, uncorrected) in the OCD than the control group. No other between-group comparisons of baseline tissue composition were significant. Nor were any post-pre-CBT differences in voxel tissue composition within the OCD group significant.
Table 2.
Regional group-mean ± standard deviation MRSI voxel tissue compositions
| left hemisphere | right hemisphere | |||||
|---|---|---|---|---|---|---|
| tissue | control | pre-CBT | post-CBT | control | pre-CBT | post-CBT |
| pregenual anterior cingulate cortex |
||||||
| vol% GM | 69.6 ± 5.1 | 73.4 ± 7.3 | 75.4 ± 6.3 | 68.5 ± 4.8 | 76.9 ± 5.7* | 77.1 ± 1.5 |
| vol% WM | 22.2 ± 9.7 | 7.8 ± 3.9** | 7.2 ± 2.2 | 15.7 ± 8.0 | 8.2 ± 3.2 | 10.4 ± 3.3 |
| vol% CSF | 7.9 ± 6.3 | 18.5 ± 7.8 | 17.1 ± 5.1 | 15.4 ± 9.8 | 14.8 ± 8.8 | 12.3 ± 3.9 |
|
putamen |
||||||
| vol% GM | 67.6 ± 8.5 | 60.6 ± 15.7 | 64.2 ± 9.1 | 64.1 ± 11.4 | 64.7 ± 5.1 | 68.4 ± 17.1 |
| vol% WM | 32.2 ± 8.4 | 39.3 ± 15.6 | 35.6 ± 9.0 | 35.7 ± 11.3 | 35.1 ± 5.2 | 31.5 ± 17.1 |
| vol% CSF | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
|
thalamus |
||||||
| vol% GM | 76.5 ± 6.2 | 76.0 ± 6.2 | 73.9 ± 3.8 | 79.8 ± 6.5 | 74.6 ± 7.3 | 73.3 ± 5.3 |
| vol% WM | 12.6 ± 6.9 | 14.5 ± 3.4 | 13.3 ± 6.7 | 12.0 ± 7.1 | 16.3 ± 4.4 | 17.6 ± 4.2 |
| vol% CSF | 10.1 ± 6.4 | 9.2 ± 5.3 | 12.3 ± 3.8 | 7.2 ± 4.0 | 8.9 ± 5.4 | 8.7 ± 5.7 |
MRSI, magnetic resonance spectroscopic imaging; CBT, cognitive-behavioral therapy; pre-CBT, OCD patients before CBT; post-CBT, OCD patients after CBT; vol%, volume percent; GM, gray matter; WM, white matter; CSF, cerebrospinal fluid.
p<0.05
0.01 for OCD v. control (non-parametric analyses of rank-transformed data; uncorrected).
3.3. MRSI regional neurometabolite levels
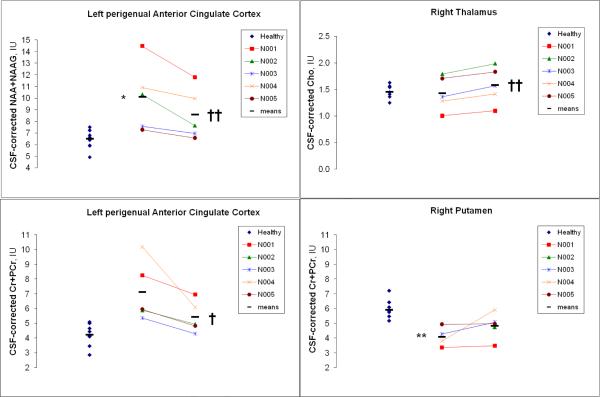
Table 3 lists group-mean levels of the five MRSI metabolite endpoints in all brain regions for healthy controls and for OCD patients before and after CBT. At baseline, tNAA in left pACC was 55.5% higher in the OCD than the control group (ANCOVA covarying vol% white matter, F=13.3, df=1,11, p<0.05, Bonferroni corrected). Thereby, all 5 patients had tNAA levels above the control mean (Fig. 2). Cr in right putamen was 31.8% lower in patients than controls (t=-5.3, p<0.01, Bonferroni corrected). Four of 5 patients had Cr below the control mean at this site (Fig. 2). Several other effects of OCD on regional baseline metabolite levels were observed but these were not significant after Bonferroni correction. Within the OCD group, tNAA (15.0%; t=-9.0, p<0.01, Bonferroni corrected) and Cr (23.9%; t=-5.1, p<0.05, Bonferroni corrected) in left pACC decreased after CBT, these decreases being seen in all 5 patients (Fig. 2). In right thalamus, Cho increased 10.6% (t=9.0, p<0.01, Bonferroni corrected) after CBT; an increase was seen in all 5 patients (Fig. 2). Other effects of CBT on regional metabolites in the OCD group were observed but were not significant after Bonferroni correction.
Table 3.
Regional group-mean ± standard deviation MRSI neurometabolite levels, IU
| left hemisphere | right hemisphere | |||||
|---|---|---|---|---|---|---|
| metabolite | control | pre-CBT | post-CBT | control | pre-CBT | post-CBT |
| pregenual anterior cingulate cortex |
||||||
| tNAA | 6.5 ± 0.8 | 10.1 ± 2.9* | 8.6 ± 2.2†† | 6.9 ± 0.9 | 8.5 ± 1.6 | 8.1 ± 1.3 |
| Glx | 11.8 ± 1.4 | 15.8 ± 6.0 | 16.2 ± 2.2 | 14.4 ± 2.8 | 13.8 ± 3.8 | 14.4 ± 0.6 |
| Cr | 4.2 ± 0.8 | 7.1 ± 2.0 | 5.4 ± 1.1† | 4.5 ± 1.0 | 6.0 ± 1.9 | 5.3 ± 0.8 |
| Cho | 1.2 ± 0.3 | 1.9 ± 0.7 | 1.7 ± 0.5 | 1.2 ± 0.3 | 1.5 ± 0.4 | 1.4 ± 0.2 |
| mI | 3.6 ± 0.7 | 5.1 ± 1.5 | 4.6 ± 1.1 | 3.4 ± 1.0 | 3.9 ± 0.5 | 4.0 ± 0.2 |
|
putamen |
||||||
| tNAA | 7.2 ± 1.1 | 6.5 ± 1.4 | 6.9 ± 1.0 | 7.6 ± 0.6 | 6.0 ± 0.8 | 6.6 ± 1.3 |
| Glx | 12.8 ± 2.9 | 11.0 ± 1.8 | 12.6 ± 1.9 | 12.8 ± 1.4 | 11.0 ± 1.0 | 10.3 ± 2.6 |
| Cr | 5.6 ± 0.8 | 4.8 ± 0.9 | 5.4 ± 0.9 | 6.0 ± 0.7 | 4.1 ± 0.7** | 4.8 ± 0.9 |
| Cho | 1.2 ± 0.1 | 1.1 ± 0.3 | 1.3 ± 0.2 | 1.3 ± 0.2 | 1.1 ± 0.2 | 1.3 ± 0.1 |
| mI | 2.6 ± 0.2 | 2.7 ± 0.9 | 2.8 ± 0.2 | 2.8 ± 0.4 | 2.5 ± 0.7 | 2.9 ± 0.9 |
|
thalamus |
||||||
| tNAA | 7.4 ± 1.8 | 7.7 ± 1.3 | 7.2 ± 0.7 | 7.4 ± 1.5 | 7.1 ± 1.5 | 7.2 ± 1.0 |
| Glx | 10.8 ± 2.5 | 11.6 ± 1.7 | 9.9 ± 2.2 | 11.2 ± 2.6 | 11.9 ± 1.7 | 10.8 ± 2.3 |
| Cr | 4.7 ± 1.4 | 4.4 ± 0.8 | 4.4 ± 0.7 | 4.6 ± 1.3 | 4.0 ± 1.1 | 4.6 ± 1.1 |
| Cho | 1.6 ± 0.4 | 1.4 ± 0.3 | 1.5 ± 0.2 | 1.4 ± 0.1 | 1.4 ± 0.3 | 1.6 ± 0.4†† |
| mI | 3.5 ± 0.9 | 3.2 ± 0.8 | 3.2 ± 0.2 | 3.4 ± 0.8 | 3.2 ± 0.7 | 3.1 ± 0.8 |
IU, water-referenced, CSF-corrected Institutional Units; tNAA, N-acetyl-aspartate+N-acetyl-aspartylglutamate; Glx, glutamate+glutamine; Cr, creatine+phosphocreatine; Cho, choline-containing compounds; mI, myo-inositol; other abbreviations as in Table 2.
p<0.05
0.01 for OCD v. control
p<0.05
0.01 for post-pre OCD (non-parametric analyses of rank-transformed data; Bonferroni corrected for multiple comparisons).
Fig. 2.
Effects of obsessive-compulsive disorder (OCD) and its treatment with cognitive-behavioral therapy (CBT) on regional MRSI metabolites in 5 pediatric patients (N001-N005). Pre-CBT values are in the center column, post-CBT values in the right column of each panel. At left are baseline levels for 9 age- and sex-matched healthy controls (blue diamonds). Horizontal bars denote group means. p<*0.05, **0.01 for baseline OCD v. control, p<†0.05, ††0.01 post-pre OCD (non-parametric analyses of rank-transformed data; Bonferroni corrected for multiple comparisons).
At baseline, the laterality index, LI, for baseline tNAA in the pACC did not differ significantly between OCD (LI = 0.10±0.19) and control (-0.05±0.41) groups (t=-1.0, p=0.36, ns). But LI for baseline Cr in the putamen did differ significantly (OCD: 0.44±0.26; control: -0.14±0.15; t=-4.1, p<0.05) favoring higher Cr on the left in patients and on the right in controls. Post-pre-CBT LI for tNAA in pACC (t=-0.80, p=0.47, ns) and for Cho in thalamus (t=-1.5, p=0.20, ns) did not differ significantly from 0 (no net lateralization of CBT effect).
3.4. Correlations between MRSI metabolite levels and CY-BOCS
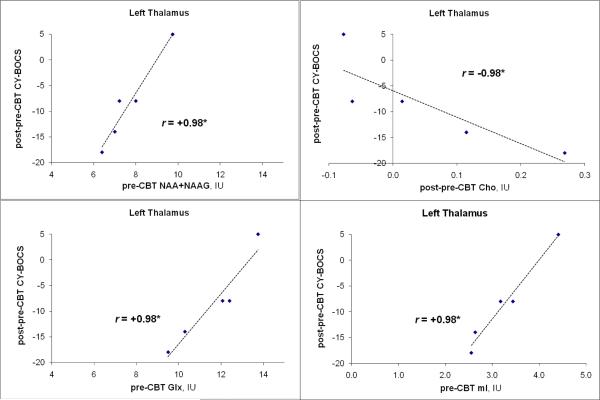
Within the OCD group, there were several correlations between pre-CBT regional metabolite levels and pre-CBT CY-BOCS scores, but these were not significant after Bonferroni correction. In left thalamus, baseline levels of tNAA, Glx, and mI each (all p<0.05, r= 0.98, Bonferroni corrected) predicted post-CBT decline in CY-BOCS (Fig. 3). Also, post-pre increase in Cho in this nucleus correlated significantly with post-pre decrease in CY-BOCS (r=0.98, p<0.05, Bonferroni corrected). Post-pre CBT changes in other regional metabolites also correlated with post-pre CBT change in CY-BOCS, but these were not significant after Bonferroni correction.
Fig. 3.
MRSI left thalamic neurometabolite predictors and correlates of response to CBT in 5 pediatric OCD patients (blue diamonds). Three panels (upper and lower left, lower right) indicate relations between baseline metabolites (tNAA, Glx, mI) and post-pre-CBT change in OCD symptom severity (CY-BOCS); one panel (upper right) shows correlation between post-pre-CBT change in Cho metabolite and change in CY-BOCS. p< *0.05 (Spearman; Bonferroni corrected for multiple comparisons).
4. Discussion
This pilot MRSI study of CBT for pediatric OCD found that multiple metabolites in cingulostriato-thalamic brain structures are abnormal in untreated OCD or may predict or change with clinical response to CBT. Tentative interpretations of our findings—in line with the Glutamatergic Hypothesis of Pediatric OCD (Rosenberg and Keshavan, 1998) -- implicate glutamatergic proteins, such as the neuronal NMDA receptor and the astrocyte-bound enzyme glutamate carboxypeptidase II (GCP-II) that hydrolyzes NAAG into NAA and Glu (Robinson et al., 1987). Other explanations, similar to PET studies in adult OCD (Baxter et al., 1987,1988; Kwon et al., 2003; Nordahl et al., 1989; Perani et al., 1995; Sawle et al., 1991; Saxena et al., 2001; Swedo et al., 1989), suggest abnormal regional energetic metabolism in OCD that changes in response to CBT (Baxter et al., 1992; Brody et al., 1998; Schwartz et al., 1996; Saxena et al., 2009a).
Elevated baseline tNAA, which decreased after CBT, was observed in left pACC in OCD. These effects were seen in all 5 patients and were accompanied by post-CBT drop in Cr in 4/5 patients. Higher baseline tNAA in our patient than in our control sample may be driven by lower voxel white-matter content in the patients. White matter, however, made-up < 25% of mean voxel volume and the between-group tNAA difference remained significant after covarying for white-matter content. Also, the significant post-CBT decreases in tNAA and Cr within the OCD group occurred in the absence of significant change in white matter. These facts argue against explanations in terms of tissue content.
An alternative, glutamatergic explanation involves NAAG metabolism. On the astrocyte membrane, NAAG is bound on its glutamyl moiety by the metabotropic glutamatergic R3 (mGluR3) receptor which poises NAAG for lysis into NAA and Glu by the membrane-adjacent GCP-II molecule (Baslow, 2000). As is suspected in cerebral cortex of patients with schizophrenia (Ghose et al., 2004), underactivity of GCP-II and/or of mGluR3 could therefore lead to a surfeit of NAAG and thence to an increase in tNAA, the sum of NAA and NAAG. NAAG normally constitutes 6-25% of tNAA (Edden et al., 2007; Pouwels and Frahm, 1997), hence 1-5-fold changes in normal NAAG could account for the elevated baseline tNAA and post-CBT tNAA drop seen in OCD patients in our study. Reduced NAAG lysis could also contribute to below-normal baseline Glx, which we did not see in our sample but which was documented by the Rosenberg Group (Arnold et al., 2009; Rosenberg et al., 2004).
NAAG effects could also mediate clinical OCD symptoms and their remediation with CBT as follows. Glu stimulates neuronal long-term potentiation (LTP)(Meador, 2007), enabling formation of associative memory links (Meador, 2007; Tsien, 2000), by acting at the NR2 subunits of the NMDA receptor (Stephenson, 2006). NAAG modulates NMDA receptor activity (Bergeron et al., 2005; Puttfarcken et al., 1993) at the co-agonist glycine modulatory site (GMS)(Coyle and Tsai, 2004) on the NR1 subunits (Stephenson, 2006). Therefore, elevated NAAG in OCD may disturb normal LTP formation. Overinduction of LTP could, for example, exaggerate formation of associative links in long-term memory. If, consistent with the affective functions of the pACC (Middleton, 2009; Vogt, 2009), some of these excessive links have anxious, idiosyncratic, or trivial character, they could manifest as clinical symptoms of OCD.
Post-CBT decrease in tNAA (Fig. 2) may result from CBT-associated increase in mGluR3-GCPII activity in pACC reversing the above pathological effects. (The extracellular NAA arising from NAAG hydrolysis by GCP-II is normally rapidly degraded by the oligodendrocyte-bound enzyme aspartoacylase; Baslow, 2000; Chakraborty et al., 2001; Tsai and Coyle, 1995.) Stabilized Glu and NAAG then reduce NMDA receptor activity and inhibit LTP, decreasing formation of dysfunctional associative links and decreasing OCD symptom severity. The recently observed D-cycloserine enhancement of CBT efficacy for OCD (Rothbaum, 2008; Wilhelm et al., 2008) already suggests action at the GMS, where both NAAG and D-cycloserine dock, in the mechanism of CBT. (There are also several negative trials of D-cycloserine, e.g., Storch et al., 2010, though these may be due to issues of dose and timing of administration.) How could CBT change mGluR3-GCPII activity? We speculate that the willful sustained attention to anxiogenic stimuli without ritualization prescribed in CBT is attended by a tonic stream of incoming post-synaptic Glu and NAAG received by the astrocyte. In symptomatic OCD, this stream is interrupted by the avoidant and ritual-seeking behavior of the patient before it can induce compensatory neurophysiologic changes. But when exposure-based CBT techniques are adhered to, the tonic stream acts long enough, perhaps by saturating receptor and transporter capacities, to signal the astrocyte nucleus, e.g., through elevated Ca2+, to re-regulate mGluR3 and/or GCP-II. Over the 12 week course of therapy, sufficient re-regulation is achieved to reach a new, more balanced astrocyte-neuron steady state. What about the post-CBT decrease in Cr? The glycine that competes with NAAG at the GMS is excreted by astrocytes that also synthesize Cr from glycine (Dringen et al., 1998). Thus, lower NAAG may increase demand for astrocyte export of glycine, diminishing intra-astrocyte Cr synthesis, hence lowering MRS Cr. Similar actions may explain the post-CBT drop in Cr observed in right putamen in our OCD sample.
Above-normal tNAA in pACC was not measured in the untreated OCD subjects studied by the Rosenberg Group (Arnold et al., 2009; Rosenberg et al., 2004), who instead observed below-normal Glx, which we did not see. Three possible explanations are as follows. First, these authors advise us that they did not determine the tissue content of their pACC MRS voxels. Thus, differences in voxel tissue content between their patient and/or control groups and ours may have contributed to the disparity in results. Second, we did not determine the genetic status of our OCD patients. Arnold et al. (2009) found that the diminished Glx effect correlated with the GG haplotype of the rs1019385 polymorphism of the GRIN2B gene and not with the GT or TT haplotypes. If patients in our sample have the GT or TT haplotypes, it could perhaps be the reason why they did not exhibit depressed baseline Glx. Finally, the Rosenberg Group sampled left and right pACC together in a single MRS voxel, while we sampled them separately in smaller MRSI voxels. Thus, if effects such as elevated tNAA in OCD are lateralized, they may have been missed by combined left-right sampling.
In our OCD sample in left thalamus, lower pre-CBT levels of tNAA, Glx, and Cr predicted greater post-CBT decrease in CY-BOCS. Post-pre-CBT increase in Cho correlated with post-pre-CBT decrease in CY-BOCS. Also, Cho in right thalamus increased after CBT. These effects may be explained in part by abnormal thalamic energetic metabolism in pediatric OCD, some aspects of which are sensitive to CBT. If above-normal thalamic GMR falls in response to CBT in pediatric OCD as it does in adult OCD, that would reduce diversion of neuronal and astrocyte carbon from membrane synthesis to energetic oxidation, allowing the membrane intermediate Cho to rise post-CBT, perhaps correlating with the post-CBT drop in OCD symptoms (CY-BOCS scores). Initially more severely impaired OCD patients present with greater drops in CY-BOCS, which correlated with lower baseline thalamic tNAA, Glx, and Cr. As mentioned, NAA, PCr, and mI are osmolytes that regulate cell water content (Ross and Blüml, 2001). Elevated glycolysis represented by GMR and subsequent elevated Krebs Cycle and oxidative phosphorylation activity would generate above-normal quantities of cell water in untreated OCD patients which would then reduce following CBT. Excess cell water would drive down the intracellular concentrations of the osmolytes NAA, PCr, and mI in order to keep the cell membrane from bursting. Such a greater effect in more severely impaired patients would lead to the observed correlations between low pre-CBT tNAA, Cr, and mI and high post-pre-CBT drop in CY-BOCS.
Our MRSI effects were only significant in one brain hemisphere. And for Cr in the putamen a significantly abnormal lateralization index was observed in patients. This follows a trend for lateralized effects of CBT on OCD brain metabolism seen with PET in adult OCD (Baxter et al., 1992; Brody et al., 1998; Nakatani et al., 2003; Schwartz et al., 1996). The notion of lateralized function in the cingulate is also supported by a recent high-resolution fMRI investigation (Lütcke and Frahm, 2008).
Limitations of this study include small sample size and MRSI acquisition at 1.5 T. Control subjects had only a baseline and not a follow-up MR scan. Acquisition at 3 T or higher might have enabled us to quantitate the NAA and NAAG resonances separately (rather than combined as tNAA) with the aid of specialized pulse sequences (Edden et al., 2007) and to quantitate the Glu and Gln resonances separately (rather than combined as Glx). Higher field strength would also render MRSI acquisition with smaller voxel sizes more feasible, enabling better matching of voxel tissue content between patients and controls. Bearing these limitations in mind, the study lends support to the emphasis placed on glutamatergic systems, including the NMDA receptor, in OCD (Arnold et al., 2009; Rosenberg and Keshavan, 1998; Rosenberg et al., 2004) and CBT (Rothbaum, 2008; Wilhelm et al., 2008). It also suggests that effects of OCD and of CBT on neuroenergetic metabolism in the thalamus seen in adult OCD may also exist in pediatric OCD. Finally, regional MRSI metabolite levels may have some clinical utility in predicting patient core symptom response to CBT.
Highlights.
The brain mechanisms of cognitive-behavioral therapy (CBT), a highly effective treatment for pediatric obsessive-compulsive disorder (OCD), are unknown
Since neuroimaging in adult OCD indicates that CBT is associated with metabolic changes in striatum, thalamus, and anterior cingulate cortex, we acquired proton magnetic resonance spectroscopic imaging (MRSI) of the brain in children with OCD before and after CBT
Multiple metabolite levels changed in the cingulate cortex and thalamus of patients after CBT
Some of these metabolite changes correlated with symptomatic response to CBT
Results were interpreted in terms of the Glutamatergic Hypothesis of Pediatric OCD
Acknowledgements
We would like to thank John C. Mazziotta, MD, Fawzy Fawzy, MD, and Edythe D. London, PhD, for supporting this project in its early stages. This study was funded by an NIMH grant (R01 MH081864-01A1) to Drs. O'Neill and Piacentini. For generous support, we thank the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, Ahmanson Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family, William M and Linda R Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Northstar Fund, the National Center for Research Resources grants RR12169, RR13642 and RR08655 and the Wallis Foundation.
Abbreviations
- ANCOVA
analysis of covariance
- aMCC
anterior middle cingulate cortex
- BOLD
blood oxygenation level-dependent
- CBT
cognitive-behavioral therapy
- Cho
choline-containing compounds
- Cr+PCr
creatine+phosphocreatine
- CY-BOCS
Children's Yale-Brown Obsessive-Compulsive Scale
- 18FDG-PET
18Fluorodeoxyglucose positron emission tomography
- Glx
glutamine+glutamate
- GMR
glucose metabolic rate
- mI
myo-inositol
- GMS
glycine modulatory site
- 1H MRS
proton magnetic resonance spectroscopy
- 1H MRSI
proton magnetic resonance spectroscopic imaging
- NMDA
N-methyl-D-aspartate
- OCD
obsessive-compulsive disorder
- pACC
pregenual anterior cingulate cortex
- pMCC
posterior middle cingulate cortex
- tNAA
N-acetyl-aspartate+N-acetyl-aspartyl-glutamate
- SRI
serotonin reuptake inhibitor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold PD, MacMaster FP, Richter MA, Hanna GL, Sicard T, Burroughs E, Mirza Y, Easter PC, Rose M, Kenendy JL, Rosenberg DR. Glutamate receptor gene (GRIN2B) associated with reduced anterior cingulate glutamatergic concentration in obsessive-compulsive disorder. Psychiatry Res. 2009;172(2):136–139. doi: 10.1016/j.pscychresns.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartha R, Stein MB, Williamson PC, Drost DJ, Neufeld RW, Carr TJ, Canaran G, Densmore M, Anderson G, Siddiqui AR. A short echo 1H spectroscopy and volumetric MRI study of the corpus striatum in patients with obsessive-compulsive disorder and comparison subjects. Am J Psychiatry. 1998;155:1584–1591. doi: 10.1176/ajp.155.11.1584. [DOI] [PubMed] [Google Scholar]
- Baslow MH. Functions of N-acetyl-L-aspartate and N-acetyl-L-aspartylglutamate in the vertebrate brain. Role in glial cell-specific signaling. J Neurochem. 2000;75:453–459. doi: 10.1046/j.1471-4159.2000.0750453.x. [DOI] [PubMed] [Google Scholar]
- Baslow MH. N-acetylaspartate in the vertebrate brain: metabolism and function. Neurochem Res. 2003;28(6):941–953. doi: 10.1023/a:1023250721185. [DOI] [PubMed] [Google Scholar]
- Baslow MH, Dyakin VV, Nowak KL, Hungund BL, Guilfoyle DN. 2-PMPA, a NAAG peptidase inhibitor, attenuates magnetic resonance BOLD signals in brain of anesthesized mice. J Molec Neurosci. 2005;26:1–15. doi: 10.1385/JMN:26:1:001. [DOI] [PubMed] [Google Scholar]
- Baxter LR, Phelps ME, Mazziotta JC, Guze BH, Schwartz JM, Selin CE. Local cerebral glucose metabolic rates in obsessive-compulsive disorder - a comparison with rates in unipolar depression and in normal controls. Arch Gen Psychiatry. 1987;44:211–218. doi: 10.1001/archpsyc.1987.01800150017003. [DOI] [PubMed] [Google Scholar]
- Baxter LR, Jr, Schwartz JM, Mazziotta JC, Phelps ME, Pahl JJ, Guze BH, Fairbanks L. Cerebral glucose metabolic rates in non-depressed obsessive-compulsives. Am J Psychiatry. 1988;145:1560–1563. doi: 10.1176/ajp.145.12.1560. [DOI] [PubMed] [Google Scholar]
- Baxter LR, Schwartz JM, Bergman KS, Szuba MP, Guze BH, Mazziotta JC, Alazraki A, Selin CE, Ferng HK, Munford P, Phelps ME. Caudate glucose metabolic rate changes with both drug and behavior therapy for obsessive-compulsive disorder. Arch Gen Psychiatry. 1992;49:681–689. doi: 10.1001/archpsyc.1992.01820090009002. [DOI] [PubMed] [Google Scholar]
- Benazon NR, Moore GJ, Rosenberg DR. Neurochemical analyses in pediatric obsessive-compulsive disorder in patients treated with cognitive-behavioral therapy. JAACAP. 2003;42(11):1279–1285. doi: 10.1097/01.chi.0000087562.01900.de. [DOI] [PubMed] [Google Scholar]
- Bergeron R, Coyle J, Tsai G, Greene R. NAAG reduces NMDA receptor current in CA1 hippocampal pyramidal neurons of acute slices and dissociated neurons. Neuropsychopharmacology. 2005;30:7–16. doi: 10.1038/sj.npp.1300559. [DOI] [PubMed] [Google Scholar]
- Bolton J, Moore GJ, MacMillan S, Stewart CM, Rosenberg DR. Case study: caudate glutamatergic changes with paroxetine persist after medication discontinuation in pediatric OCD. JAACAP. 2001;40:903–906. doi: 10.1097/00004583-200108000-00011. [DOI] [PubMed] [Google Scholar]
- Brain Development Cooperative Group. Evans AC. The NIH MRI study of normal brain development. NeuroImage. 2006;30:184–202. doi: 10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Brody AL, Saxena S, Schwartz JM Stoessel PW, Maidment K, Phelps ME, Baxter LR., Jr FDG-PET predictors of response to behavioral therapy versus pharmacotherapy in obsessive-compulsive disorder. Psychiatry Res: Neuroimaging. 1998;84:1–6. doi: 10.1016/s0925-4927(98)00041-9. [DOI] [PubMed] [Google Scholar]
- Cecil KM, Patel NC, DelBello MP. Inositol metabolism in pediatric bipolar disorder. Clin Neuropsychiatry. 2006;3(3):177–183. [Google Scholar]
- Chakraborty G, Mekala P, Yahya D, Wu G, Ledeen RW. Intraneuronal N-acetyl aspartate supplies acetyl groups for myelin lipid synthesis: evidence for myelin-associated aspartoacylase. J Neurochem. 2001;78:736–745. doi: 10.1046/j.1471-4159.2001.00456.x. [DOI] [PubMed] [Google Scholar]
- Ebert D, Speck O, König A, Berger M, Hennig J, Hohagen F. 1H-magnetic resonance spectroscopy in obsessive-compulsive disorder: evidence for neuronal loss in the cingulate gyrus and the right striatum. Psychiatry Res: Neuroimaging. 1997;74:173–176. doi: 10.1016/s0925-4927(97)00016-4. [DOI] [PubMed] [Google Scholar]
- Edden RA, Pomper MG, Barker PB. In vivo differentiation of N-acetyl aspartyl glutamate from N-acetyl aspartate at 3 Tesla. Magn Reson Med. 2007;57(6):977–982. doi: 10.1002/mrm.21234. [DOI] [PubMed] [Google Scholar]
- Erecinska M, Silver IA. ATP and brain function. JCBFM. 1989;9:2–19. doi: 10.1038/jcbfm.1989.2. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KD, Moore GJ, Paulson LA, Stewart CM, Rosenberg DR. Proton spectroscopic imaging of the thalamus in treatment-naive pediatric obsessive-compulsive disorder. Biol Psychiatry. 2000;47:174–182. doi: 10.1016/s0006-3223(99)00286-3. [DOI] [PubMed] [Google Scholar]
- Geller DA, Biederman J, Jones J, Shapiro S, Schwartz S, Park KS. Obsessive-compulsive disorder in children and adolescents: A review. Harvard Rev Psychiat. 1998;5(5):260–273. doi: 10.3109/10673229809000309. [DOI] [PubMed] [Google Scholar]
- Ghose S, Weickert CS, Colvin SM, Coyle JT, Herman MM, Hyde TM, Kleinman JE. Glutamate carboxypeptidase II gene expression in the human frontal and temporal lobe in schizophrenia. Neuropsychopharmacology. 2004;29:117–125. doi: 10.1038/sj.npp.1300304. [DOI] [PubMed] [Google Scholar]
- Jang JH, Kwon JS, Jang DP, Moon W-J, Lee J-M, Ha TH, Chung EC, Kim IY, Kim SI. A proton MRSI study of brain N-acetylaspartate level after 12 weeks of citalopram treatment in drug-naïve patients with obsessive compulsive disorder. Am J Psychiatry. 2006;163:1202–1207. doi: 10.1176/ajp.2006.163.7.1202. [DOI] [PubMed] [Google Scholar]
- Kitamura H, Shioiri T, Kimura T, Ohkubo M, Nakada T, Someya T. Parietal white matter abnormalities in obsessive–compulsive disorder: a magnetic resonance spectroscopy study at 3-Tesla. Acta Psychiatr Scand. 2006;114:101–108. doi: 10.1111/j.1600-0447.2006.00858.x. [DOI] [PubMed] [Google Scholar]
- Kwon JS, Kim JJ, Lee DW, Lee JS, Lee DS, Kim MS, Lyoo IK, Cho MJ, Lee MC. Neural correlates of clinical symptoms and cognitive dysfunctions in obsessive-compulsive disorder. Psychiatry Res: Neuroimaging. 2003;122:37–47. doi: 10.1016/s0925-4927(02)00104-x. [DOI] [PubMed] [Google Scholar]
- Lütcke H, Frahm J. Lateralized anterior cingulate function during error processing and conflict monitoring as revealed by high-resolution fMRI. Cerebral Cortex. 2008;18:508–515. doi: 10.1093/cercor/bhm090. [DOI] [PubMed] [Google Scholar]
- MacMaster FP, O'Neill J, Rosenberg DR. Brain imaging in pediatric obsessive compulsive disorder. JAACAP. 2008;47(11):1262–1272. doi: 10.1097/CHI.0b013e318185d2be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador KJ. The basic science of memory as it applies to epilepsy. Epilepsia. 2007;48(Suppl. 9):23–25. doi: 10.1111/j.1528-1167.2007.01396.x. [DOI] [PubMed] [Google Scholar]
- Middleton FA. The contribution of anterior cingulate-basal ganglia circuitry to complex behavior and psychiatric disorders. In: Vogt BA, editor. Cingulate neurobiology and disease. Oxford University Press; New York: 2009. pp. 633–652. [Google Scholar]
- Miller BL. A review of chemical issues in 1H NMR spectroscopy: N-acetyl-L-aspartate, creatine and choline. NMR Biomed. 1991;4(2):47–52. doi: 10.1002/nbm.1940040203. [DOI] [PubMed] [Google Scholar]
- Mirza Y, O'Neill J, Smith EA, Russell A, Smith JM, Banerjee SP, Bhandari R, Boyd C, Rose M, Ivey J, Renshaw PF, Rosenberg DR. Increased medial thalamic creatine/phosphocreatine found by proton magnetic resonance spectroscopy in children with obsessive-compulsive disorder versus major depression and healthy controls. J Child Neurol. 2006;21(2):106–111. doi: 10.1177/08830738060210020201. [DOI] [PubMed] [Google Scholar]
- Moore GJ, MacMaster FP, Stewart C, Rosenberg D. Case study: Glutamatergic changes with paroxetine therapy for pediatric obsessive-compulsive disorder. JAACAP. 1998;37:663–667. doi: 10.1097/00004583-199806000-00017. [DOI] [PubMed] [Google Scholar]
- Moreno A, Ross BD, Bluml S. Direct determination of the N-acetyl-L-aspartate synthesis rate in the human brain by (13)C MRS and (1-(13)C)glucose infusion. J Neurochem. 2001;77(1):347–50. doi: 10.1046/j.1471-4159.2001.t01-1-00282.x. [DOI] [PubMed] [Google Scholar]
- Nakatani E, Nakgawaa A, Oharab Y, Gotob S, Uozumib N, Iwakirib M, Yamamotob Y, Motomuraa K, Iikurab Y, Yamagamib T. Effects of behavior therapy on regional cerebral blood flow in obsessive–compulsive disorder. Psychiatry Res: Neuroimaging. 2003;124:113–120. doi: 10.1016/s0925-4927(03)00069-6. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, Benkelfat C, Semple WE. Cerebral glucose metabolic rates in obsessive-compulsive disorder. Neuropsychopharmacol. 1989;2(1):23–28. doi: 10.1016/0893-133x(89)90003-1. [DOI] [PubMed] [Google Scholar]
- O'Neill J, Eberling JL, Schuff N, Jagust WJ, Reed B, Soto G, Ezekiel F, Klein GJ, Weiner MW. Correlation of 1H MRSI and 18FDG-PET. Magn Reson Med. 2000;43:244–50. doi: 10.1002/(sici)1522-2594(200002)43:2<244::aid-mrm11>3.0.co;2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill J, Gorbis E, Feusner J, Maidment KM, Yip JC, Chang S, Salamon N, Saxena S. ISMRM 15th Scientific Meeting and Exhibition. Berlin: May 19-25, 2007. 1H MRSI study of effects of cognitive-behavioral therapy on obsessive-compulsive disorder. [Google Scholar]
- O'Neill J, Schwartz JM. OCD Treatment: The role of volition in OCD therapy: Neurocognitive, neuroplastic, and neuroimaging aspects. Clin Neuropsychiatry. 2005;1(1):10–18. [Google Scholar]
- O'Neill J, Sobel TL, Vogt BA. Localizing cingulate subregions of interest in magnetic resonance images guided by cytological parcellations. In: Vogt BA, editor. Cingulate neurobiology and disease. Oxford University Press; New York: 2009. pp. 3–30. [Google Scholar]
- Perani D, Colombo C, Bressi S, Binfanti A, Grassi F, Scarone S, Bellodi L, Smeraldi E, Fazio F. (18F)-FDG-PET study in obsessive-compulsive disorder: A clinical/metabolic correlation study after treatment. Br J Psychiatry. 1995;166:244–250. doi: 10.1192/bjp.166.2.244. [DOI] [PubMed] [Google Scholar]
- Petroff OAC, Errante LD, Kim JH, Spencer DD. N-acetyl-aspartate, total creatine, and myo-inositol in the epileptogenic human hippocampus. Neurology. 2003;60:1646–1651. doi: 10.1212/01.wnl.0000068020.85450.8b. [DOI] [PubMed] [Google Scholar]
- Pfund Z, Chugani DC, Juhasz C, Muzik O, Chugani HT, Wilds IB, Seraji-Bozorgzad N, Moore GJ. Evidence for coupling between glucose metabolism and glutamate cycling using FDG PET and 1H magnetic resonance spectroscopy in patients with epilepsy. J Cereb Blood Flow Metab. 2000;20(5):871–878. doi: 10.1097/00004647-200005000-00014. [DOI] [PubMed] [Google Scholar]
- Piacentini J, Langley A, Roblek T. Cognitive-behavioral treatment of childhood OCD: It's only a false alarm. Oxford University Press; Oxford: 2007. [Google Scholar]
- Piacentini J, March J, Franklin M. Cognitive-behavioral therapy for youngsters with obsessivecompulsive disorder. In: Kendall P, editor. Child and adolescent therapy: Cognitive-behavioral procedures. 3rd ed. Guilford; New York: 2006. pp. 297–321. [Google Scholar]
- Pouwels PJW, Frahm J. Differential distribution of NAA and NAAG in human brain as determined by quantitative localized Proton MRS. NMR Biomed. 1997;10:73–78. doi: 10.1002/(sici)1099-1492(199704)10:2<73::aid-nbm448>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14:260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- Puttfarcken PS, Handen JS, Montgomery DT, Coyle JT, Werling LL. N-Acetylaspartylglutamate modulation of N-methyl-D-aspartate-stimulated (3H)norepinephrine release from rat hippocampal slices. J Pharmacol Exp Ther. 1993;266:796–803. [PubMed] [Google Scholar]
- Rapoport J, Inoff-Germain G, Weissman MM, Greenwald S, Narrow WE, Jensen PS, Lahey BB, Canino G. Childhood obsessive-compulsive disorder in the NIMH MECA Study: Parent versus child identification of cases. J Anxiety Disord. 2000;14:535–548. doi: 10.1016/s0887-6185(00)00048-7. [DOI] [PubMed] [Google Scholar]
- Robinson MB, Blakely RD, Couto R, Coyle JT. Hydrolysis of the brain dipeptide N-acetyl-L-aspartyl-L-glutamate. Identification and characterization of a novel N-acetylated alpha-linked acidic dipeptidase activity from rat brain. J Biol Chem. 1987;262:14498–14506. [PubMed] [Google Scholar]
- Rosenberg DR, Amponsah A, Sullivan A, MacMillan S, Moore GJ. Increased medial thalamic choline in pediatric obsessive-compulsive disorder as detected by quantitative in vivo spectroscopic imaging. J Child Neurol. 2001;16:636–641. doi: 10.1177/088307380101600902. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Keshavan MS. Toward a neurodevelopmental model of obsessive–compulsive disorder. Toward a neurodevelopmental model of obsessive–compulsive disorder. Biol Psychiatry. 1998;43:623–640. doi: 10.1016/s0006-3223(97)00443-5. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, MacMaster FP, Keshavan MS, Fitzgerald KD, Stewart CM, Moore GJ. Decrease in caudate glutamatergic concentrations in pediatric obsessive-compulsive disorder patients taking paroxetine. JAACAP. 2000;39(9):1096–1103. doi: 10.1097/00004583-200009000-00008. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Mirza Y, Russell A, Tang J, Smith JM, Banarjee SP, Bhandari R, Rose M, Ivey J, Boyd C, Moore GJ. Reduced anterior cingulate glutamatergic concentrations in childhood OCD and major depression versus healthy controls. JAACAP. 2004;43(9):1146–1153. doi: 10.1097/01.chi.0000132812.44664.2d. [DOI] [PubMed] [Google Scholar]
- Ross BD, Blüml S. Magnetic resonance spectroscopy of the human brain. The Anat Record (New Anat) 2001;265:54–84. doi: 10.1002/ar.1058. [DOI] [PubMed] [Google Scholar]
- Sawle GV, Hymas NF, Lees AJ, Frackowiak RS. Obsessional slowness: Functional studies with positron emission tomography. Brain. 1991;114(Pt 5):2191–2202. doi: 10.1093/brain/114.5.2191. [DOI] [PubMed] [Google Scholar]
- Saxena S, Bota RG, Brody AL. Brain-behavior relationships in obsessive-compulsive disorder. Sem Clin Neuropsychiatry. 2001;6:82–101. doi: 10.1053/scnp.2001.21833. [DOI] [PubMed] [Google Scholar]
- Saxena S, Gorbis E, O'Neill J, Baker SK, Mandelkern MA, Maidment KM, Chang S, Salamon N, Brody AL, Schwartz JM, London ED. Rapid effects of brief intensive cognitive-behavioral therapy on brain glucose metabolism in obsessive-compulsive disorder. Molec Psychiatry. 2009a;14(2):197–205. doi: 10.1038/sj.mp.4002134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S, O'Neill J, Rauch SL. The role of cingulate cortex dysfunction in obsessive-compulsive disorder. In: Vogt BA, editor. Cingulate neurobiology and disease. Oxford University Press; New York: 2009b. pp. 587–618. [Google Scholar]
- Scahill L, Riddle MA, McSwiggin-Hardin M, Ort SI, King RA, Goodman WK, Cicchetti D, Leckman JF. Children's Yale-Brown Obsessive Compulsive Scale: reliability and validity. JAACAP. 1997;36:844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- Schwartz JM, Stoessel PW, Baxter LR, Jr, Martin KM, Phelps ME. Systematic changes in cerebral glucose metabolic rate after successful behavior modification treatment of obsessive-compulsive disorder. Arch Gen Psychiatry. 1996;53:109–113. doi: 10.1001/archpsyc.1996.01830020023004. [DOI] [PubMed] [Google Scholar]
- Seese RR, O'Neill J, Hudkins M, Siddarth P, Levitt J, Tseng B, Wu KN, Caplan R. In press. Proton magnetic resonance spectroscopy and thought disorder in childhood schizophrenia. Schiz Res. doi: 10.1016/j.schres.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck D, Sandor-Leahy S, Schaper K, Rottenberg D, Leahy R. Magnetic resonance image tissue classification using a partial volume model. Neuroimage. 2001;13:856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- Sibson NR, Shen J, Mason GF, Rothman DL, Behar KL, Shulman RG. Functional energy metabolism: in vivo 13C-NMR spectroscopy evidence for coupling of cerebral glucose consumption and glutamatergic neuronal activity. Dev Neurosci. 1998;20(4-5):321–330. doi: 10.1159/000017327. [DOI] [PubMed] [Google Scholar]
- Smith EA, Russell A, Lorch E, Banerjee SP, Rose M, Ivey J, Bhandari R, Moore GJ, Rosenberg DR. Increased medial thalamic choline found in pediatric patients with obsessive-compulsive disorder versus major depression or healthy control subjects: A magnetic resonance spectroscopy study. Biol Psychiatry. 2003;54:1399–1405. doi: 10.1016/s0006-3223(03)00474-8. [DOI] [PubMed] [Google Scholar]
- Stephenson FA. Structure and trafficking of NMDA and GABAA receptors. Biochem Soc Trans. 2006;34(5):877–881. doi: 10.1042/BST0340877. [DOI] [PubMed] [Google Scholar]
- Storch EA, Murphy TK, Goodman WK, Geffken GR, Lewin AB, Henin A, Micco JA, Sprich S, Wilhelm S, Bengtson M, Geller DA. A preliminary study of D-cycloserine augmentation of cognitive-behavioral therapy in pediatric obsessive-compulsive disorder. Biol Psychiatry. 2010;68(11):1073–6. doi: 10.1016/j.biopsych.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork C, Renshaw PF. Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Molec Psychiatry. 2005;10:900–919. doi: 10.1038/sj.mp.4001711. [DOI] [PubMed] [Google Scholar]
- Swedo SE, Schapiro MG, Grady CL, Cheslow DL, Leonard HL, Kumar A, Friedland R, Rapoport SI, Rapoport JL. Cerebral glucose metabolism in childhood onset obsessive-compulsive disorder. Arch Gen Psychiatry. 1989;46:518–523. doi: 10.1001/archpsyc.1989.01810060038007. [DOI] [PubMed] [Google Scholar]
- Tsai G, Coyle JT. N-acetyl aspartate in neuropsychiatric disorders. Prog Neurobiol. 1995;46:531–540. doi: 10.1016/0301-0082(95)00014-m. [DOI] [PubMed] [Google Scholar]
- Tsien JZ. Linking Hebb's coincidence-detection to memory formation. Curr Opin Neurobiol. 2000;10:266–273. doi: 10.1016/s0959-4388(00)00070-2. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Regions and subregions of the cingulate gyrus. In: Vogt BA, editor. Cingulate neurobiology and disease. Oxford University Press; New York: 2009. pp. 3–30. [Google Scholar]
- Whiteside SP, Port JD, Deacon BJ, Abramowitz JS. A magnetic resonance spectroscopy investigation of obsessive–compulsive disorder and anxiety. Psychiatry Res: Neuroimaging. 2006;146:137–147. doi: 10.1016/j.pscychresns.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Yücel M, Harrison BJ, Wood SJ, Fornito A, Wellard RM, Pujol J, Clarke K, Phillips ML, Kyrios M, Velakoulis D, Pantelis C. Functional and biochemical alterations of the medial frontal cortex in obsessive-compulsive disorder. Arch Gen Psych. 2008;64(8):946–955. doi: 10.1001/archpsyc.64.8.946. [DOI] [PubMed] [Google Scholar]
- Yücel M, Wood SJ, Wellard RM, Harrison BJ, Fornito A, Pujol J, Clarke K, Velakoulis D, Pantelis C. Anterior cingulate glutamate-glutamine levels predict symptom severity in women with obsessive-compulsive disorder. Aust NZ J Psych. 2008;42(6):467–477. doi: 10.1080/00048670802050546. [DOI] [PubMed] [Google Scholar]
- Zohar AH. The epidemiology of obsessive-compulsive disorder in children and adolescents. Child Adolesc Psychiatr Clin N Am. 1999;8(3):445–460. [PubMed] [Google Scholar]
- Zurowski B, Weber-Fahr W, Freyer T, Wahli K, Büchert M, Kuelz AK, Hohagen F, Braus DF, Voderholzer U, Kordoni A. Neurochemical abnormalities in patients with obsessive-compulsive disorder diminish in the course of behavior therapy. Soc Neurosci Abs. 2007 Nov 5; [Google Scholar]