Abstract
Objective
To determine the durability over 96 weeks of safety and efficacy of lopinavir/ritonavir (LPV/r) and raltegravir (RAL) which was demonstrated to have non-inferior efficacy relative to a regimen of LPV/r with nucleoside/nucleotide reverse transcriptase inhibitors (N(t)RTIs) (Control) in primary analysis at 48 weeks.
Design
Open label, centrally randomised trial.
Setting
Recruitment was from 37 primary and secondary care sites from Africa, Asia, Australia, Europe and Latin America.
Subjects
541 HIV-1 infected adults virologically failing first-line non-NRTI + 2N(t)RTI, with no previous exposure to protease inhibitors or integrase strand transfer inhibitors were analysed, 425 completed 96 weeks follow up on randomised therapy.
Intervention
Randomisation was 1:1 to Control or RAL.
Main outcome measures
Differences between the proportion of participants with plasma HIV-1 RNA (VL) <200 copies/mL by intention to treat were compared with a non-inferiority margin of −12%. Differences in biochemical, haematological and metabolic changes were assessed using T-tests.
Results
VL <200 copies/mL at 96 weeks was: RAL 80.4%, Control 76.0% (difference: 4.4 [95%CI −2.6, 11.3]) and met non-inferiority criteria. The RAL arm had a significantly higher mean change (difference Control-RAL; 95%CI) in haemoglobin (−2.9; −5.7, −1.1), total lymphocytes (−0.2; −0.3, −0.0), total cholesterol (−0.5; −0.8, −0.3), HDL cholesterol (−0.1; −0.1, −0.0) and LDL cholesterol (−0.3; −0.5, −0.2).
Conclusion
At 96 weeks, both RAL and Control maintained efficacy greater than 75% and continued to demonstrate similar safety profiles. These results support the use of a combination LPV/r and RAL regimen as an option following failure of 1st line NNRTI + 2N(t)RTIs.
Trial Registration
ClinicalTrials.gov NCT00931463
Introduction
An estimated 9.7 million people in low- and middle-income countries are now receiving antiretroviral therapy (ART) with the number of people receiving therapy globally tripling over the last five years [1]. With this increase in the numbers of people initiating therapy, so too there is inevitably an increase in the number failing first-line therapy. The choice and rationale for selection of second-line therapy is limited.
A recent Cochrane review suggests that there is little evidence to inform treatment guidelines for second-line therapy recommendations [2]. Within this setting of an acknowledged dearth of information, the World Health Organization (WHO) recommends a boosted-protease inhibitor plus two nucleoside analogues (N(t)RTIs) as the first option for second-line therapy [1]. This approach maximises the use of more commonly available combination antiretroviral therapies (cART); however it does not address the need for simple and tolerable regimens in order to maintain long term suppressive therapy.
The Week 48 report of the SECOND-LINE trial and the Week 96 results of the EARNEST study have reported outcomes of randomised trials that examined the safety and efficacy of experimental regimens of cART for treatment of people who were failing first line therapy[3,4]. Both studies demonstrated non-inferior outcomes for an experimental regimen of cART containing raltegravir plus ritonavir-boosted lopinavir (LPV/r), a very simple regimen, compared to 2–3N(t)RTI + LPV/r.
Here we report the 96 week follow-up data from the SECOND-LINE trial in order to assess the durability of treatment effects in terms of safety and efficacy of the randomised treatment groups.
Methods
The protocol for this trial and supporting CONSORT checklist are available as supporting information; see S1 Protocol and S1 CONSORT Checklist.
A full description of the methods for the SECOND-LINE trial has been previously published [3]. In summary, SECOND-LINE was a parallel group, randomised, open-label multi-centre international trial conducted at 37 sites across Africa, Asia, Central and South America, Australia and Europe.
Eligible participants were adults (≥16 years or as per local legal requirements) positive for HIV- 1 antibody, failing first-line therapy (non-nucleoside reverse transcriptase inhibitor [NNRTI] plus two nucleoside/nucleotide reverse transcriptase inhibitors [N(t)RTIs]). Failure was defined as two consecutive (>7 days apart) plasma HIV-1 RNA (VL) measures of >500 copies/mL. Patients were excluded if they had prior exposure to a protease inhibitor, an integrase strand transfer inhibitor or had evidence of active viral hepatitis B infection (defined as the presence of HBV surface antigen in serum) [3].
Participants were randomised to one of two antiretroviral regimens containing a total daily dose of ritonavir (200 mg)-boosted lopinavir (800 mg) (LPV/r) (administered either once or twice daily) plus either: i) two or three NtRTIs (Control); or ii) raltegravir 400 mg twice daily (RAL). The dosing schedule (once or twice daily) for LPV/r was determined by the treating physician. Trial visits were scheduled at Weeks 0 (randomisation) 4, 8, 12 and then every 12 weeks to Week 96. At each visit vital signs, physical health and adverse events were assessed and blood was collected for T-cell subset counts, haematology, serum biochemistry including renal function markers, liver function tests, metabolic markers including cholesterol fractions (Weeks 0, 24, 48, 72 and 96 only) and plasma viral load (VL) assessment. Local assessment of VL was used for patient management. Centrally analysed VL was used for assessment of efficacy. Serum collected after fasting was used for assessment of metabolic measures. Estimated glomerular filtration rate (eGFR) was calculated using the modification of diet in renal disease (MDRD) equation [5]. Adherence was measured at Weeks 4, 48 and 96 using the Adult AIDS Clinical Trials Group adherence instrument [6].
Outcomes
All outcomes in this report were assessed at Week 96. The primary endpoint of this analysis was the proportion of participants with VL <200 copies/mL at 96 weeks. Secondary endpoints were proportion with VL <50 copies/mL and <400 copies/mL, time to loss of virological response and mean change from baseline in CD4+ T-cell count/mm3. Virologic failure was also assessed and defined as a VL >200 copies/mL at any time during the trial. Clinical events (adverse events, AIDS, serious non-AIDS [SNAEs], and death), mean changes in laboratory measures and adherence were also assessed. SNAEs were independently reviewed by a physician at the Kirby Institute (Sydney, Australia) according to INSIGHT criteria [7]. Ten-year risk of coronary heart disease (CHD) was determined according to the Framingham equation and metabolic syndrome according to National Cholesterol Education Program Adult Treatment Panel III [8,9]. Both these composite endpoints were only calculated for individuals who had all data components available. There were no imputations for missing data.
Sample size
The sample size was based on estimates expected at Week 48 of no difference between arms and a predicted efficacy of 80% (VL <200 copies/mL). Two hundred and forty eight participants per arm gave 90% power to show non-inferiority using a margin of 12% (two sided α = 0.05) of the RAL arm compared to the Control arm in the modified intention to treat analysis. The recruited sample size was inflated to allow for adequate power in the Week 48 per protocol population, resulting in 541 participants being randomly assigned. This population will retain 90% power to detect non- inferiority (margin of 12%, two sided α = 0.05) if efficacy over 96 week above 71.5% is maintained.
Randomisation
Treatment arm allocation, stratified by clinical site and VL (≤ vs > 100,000 copies/mL), was automated with randomisation lists built in to the electronic clinical record form. Randomisation was 1:1 with a blocking factor of four. Allocation could only be triggered by the investigator after completion of participant consent and entry of screening and eligibility data. After random allocation, participants and investigators were not masked to treatment arm allocation.
Statistical analysis
All binary virologic endpoints were assessed in three analytical populations: i) a modified intention to treat (ITT) population consisting of all randomised participants who commenced study drug and attended at least one follow-up visit- in this population deaths, loss to follow-up and treatment change due to virologic failure were imputed as failures; ii) a per protocol (PP); and iii) a non-completer imputed as failure (NC = F), equivalent to the FDA snapshot analysis [10].
Change in CD4+ T cells/mm3 was assessed in the ITT population (last observation carried forward). For other variables, summaries are provided of available data with no imputation.
All analyses were pre-specified. VL threshold analyses were assessed for non-inferiority on the basis of 95% confidence intervals (CI) at a pre-specified margin of the lower confidence bound of −12%. All other endpoints were tested for superiority (two side α = 0.05). The Cochran-Mantel-Haenszel test was used to check for consistency of associations between treatment arms and virological outcomes across screening VL strata.
We used χ2 and Fisher’s exact tests to analyse categorical variables; t-test for continuous variables; and Cox regression for time-to-event outcomes and calculation of hazard ratios (HR). Assessment of residual showed no evidence of violation of proportional hazards assumptions. Logistic regression was used to test for interaction of categorical variables. SAS version 9.2 was used for data analysis.
The study was approved by each site’s Ethics Committee under the auspices and approval of the lead ethics/IRB of St Vincent's Hospital Sydney (Reference HREC/09/SVH/89). All participants provided direct written informed consent by signing the Participant Information and Consent Form approved by the IRB. Where a participant was considered a minor written informed consent was obtained from both the participant and their parent/guardian, the ethics committees approved this procedure. Hard copies of the consent form are filed at the participant's relevant clinical site and a copy was given to each participant.
Results
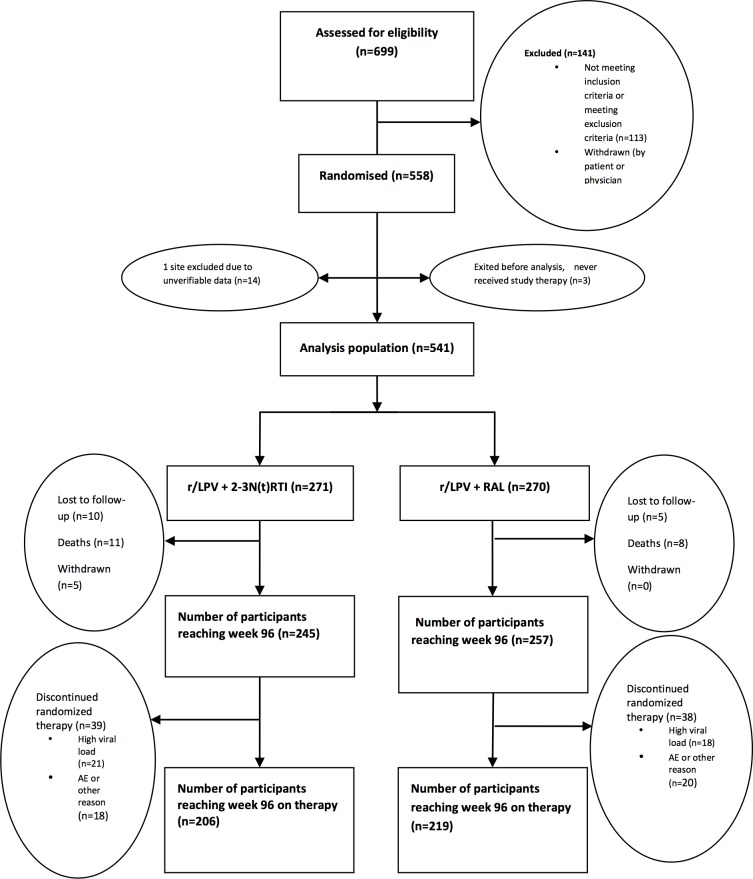
The study commenced recruitment in March 2010 with last participant through 96 weeks of follow up in July 2013. The baseline characteristics of the study population were balanced between arms [3]. Participants were 55% male, 42% Asian, 36% African and 14% Hispanic, with a mean (sd) age of 39 (8.8) years, duration of first line ART was 4.1 (2.8) years, VL was 4.2 (0.9) log copies/mL, CD4+ T cell count was 211 (157) cells/mm3. Of the study population randomised to the Control and RAL arms respectively, 245/271(90%) and 257/270(95%) reached 96 weeks of follow up, of whom 206 (84%) and 219 (85%) were on randomised therapy (Fig. 1). At Week 96 there was no difference between arms in self-reported adherence with more than 85% of participants in each arm reporting taking “All” or “Most” of their pills.
Fig 1. Participant disposition over 96 weeks.
At Week 96, 76% (206/271) in the Control and 80% (217/270) in the RAL arm reported a VL <200 copies /mL (difference RAL-Control = 4.4%, 95%CI −2.6, 11.3) thereby satisfying our a priori criteria for non-inferiority. Results were consistent in the PP and NC = F populations, <50 and <400 copy thresholds, and by screening VL strata (Fig. 2). Time to loss of virologic response did not differ between arms (Control 26.9/100 person years (py), RAL 28.1/100 py; HR 1.1 [95% CI 0.8, 1.4]; p = 0.68). Over the first 48 weeks of the trial 55 participants in the Control group and 49 in the RAL group had experienced virologic failure; this increased to 82 and 83 respectively over the entire 96 weeks of follow-up. The frequency of emergent resistance among those who failed with an amplifiable sequence, in the Control and RAL arm, was as follows: NtRTI 8/64 (12.5%) and 2/65 (3.1%); protease inhibitor 2/64 (3.1%) and 1/65 (1.5%); integrase strand inhibitor 1/72 (1.4%) and 20/79 (25.3%).
Fig 2. Virologic response at Week 96, by randomised arm, study population and screening strata.
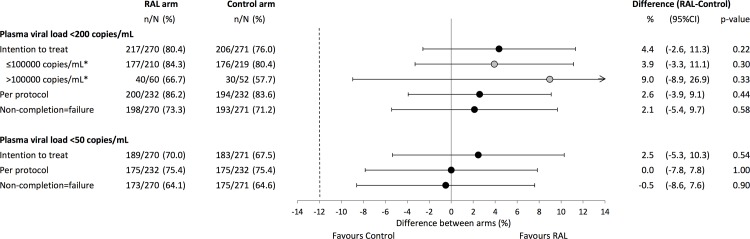
RAL = lopinavir/ritonavir+raltegravir Control = lopinavir/ritonavir+2/3 nucleoside/nucleotide reverse transcriptase inhibitors. *grey circle = screening plasma viral load strata, P interaction = 0.81, bars are 95%confidence intervals (CI).
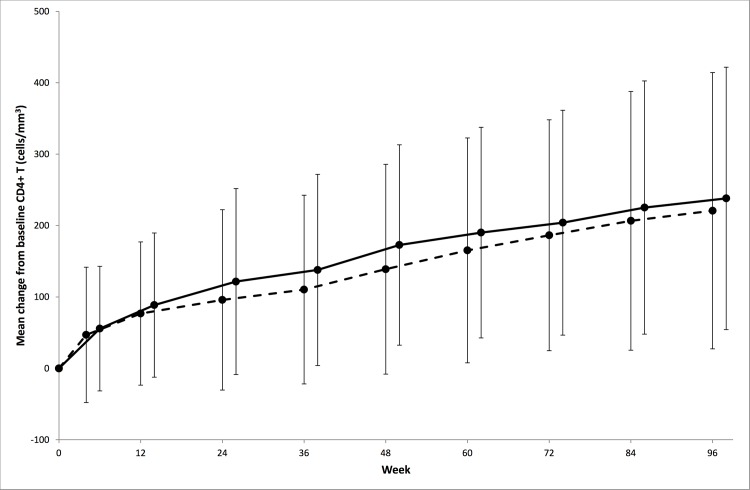
CD4+ T cells/mm3 increased over the whole 96 weeks in both arms (mean change from baseline [95%CI]: 201.7 [178.6, 224.9] and 228.7 [206.6, 250.8] respectively) (Fig. 3). There was no significant difference between arms in mean change from baseline to Week 96 (Control-RAL −27.0 95%CI −59.0, 5.0; p = 0.10).
Fig 3. Mean change from baseline in CD4+ T cells/mm3 over 96 weeks by randomised arm.
RAL = lopinavir/ritonavir+raltegravir Control = lopinavir/ritonavir+2/3 nucleoside/nucleotide reverse transcriptase inhibitors. (Control- - - RAL ---, bars indicate standard deviation).
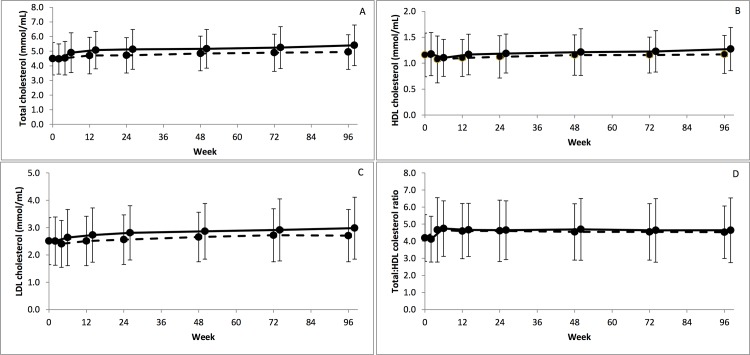
The differences between arms for mean change from baseline to Week 96 were statistically significant, with greater increases in the RAL arm, for total lymphocytes, haemoglobin, total HDL and LDL cholesterol (Table 1). Mean total cholesterol after 96 weeks of therapy was 4.9 (1.2) in the Control arm and 5.4 (1.4) in the RAL arm, with total:HDL cholesterol ratio increasing up to week 4 (4.7 in both arms) before stabilizing through 96 weeks (Fig. 4). There was a significant difference for mean change from baseline in ten-year CHD risk between arms (mean [sd] baseline Control: 4.1 [4.7], RAL: 3.8 [4.5]) to Week 96 (Control: 4.4[4.4], RAL: 4.6 [5.1]; difference in change −0.6 95%CI −1.2, −0.1; p = 0.02).
Table 1. Differences in mean change from baseline to Week 96 in laboratory markers by randomised arm.
| Control | RAL | Difference (Control-RAL) | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | 95%CI | p-value | ||
| Immunology and haematology | |||||||
| CD4+ T (cells/mm3) | 201.7 | 193.7 | 228.7 | 184.6 | −27.0 | (−59.0, 5.0) | 0.100 |
| Total lymphocytes (cells/mm3) | 0.5 | 0.7 | 0.7 | 0.7 | −0.2 | (−0.3, −0.0) | 0.018 |
| Neutrophils(cells/mm3) | 0.8 | 1.4 | 0.9 | 1.4 | −0.1 | (−0.3, 0.2) | 0.452 |
| Haemoglobin (g/L) | 5.5 | 17.2 | 8.3 | 14.7 | −2.9 | (−5.7, −0.1) | 0.046 |
| Biochemistry | |||||||
| ALT (U/L) | −11.1 | 25.4 | −6.6 | 32.9 | −4.5 | (−9.7, 0.7) | 0.091 |
| Creatine kinase (U/L) | −13.7 | 203.3 | −14.9 | 193.1 | 1.3 | (−33.8, 36.4) | 0.943 |
| eGFR (mL/min/1.73m2) | −5.5 | 16.5 | −5.2 | 14.7 | −0.3 | (−3.1, 2.5) | 0.834 |
| Metabolic parameters | |||||||
| Total cholesterol (mmol/L) | 0.4 | 1.2 | 0.9 | 1.2 | −0.5 | (−0.8, −0.3) | <0.001 |
| HDL choletersol (mmol/L) | 0.0 | 0.4 | 0.1 | 0.4 | −0.1 | (−0.1, −0.0) | 0.003 |
| Total:HDL cholesteol ratio | 0.4 | 1.3 | 0.5 | 1.5 | −0.2 | (−0.4, 0.1) | 0.204 |
| LDL cholesterol (mmol/L) | 0.2 | 0.8 | 0.5 | 0.9 | −0.3 | (−0.5, −0.2) | <0.001 |
| Triglycerides (mmol/L) | 0.8 | 1.9 | 1.0 | 1.8 | −0.1 | (−0.5, 0.2) | 0.417 |
| Glycaemic parameters | |||||||
| Glucose (mmol/L) | −0.1 | 1.4 | 0.2 | 1.8 | −0.3 | (−0.5, 0.0) | 0.090 |
| Insulin (mU/L) | −0.9 | 16.3 | 0.6 | 14.4 | −1.6 | (−4.3, 1.2) | 0.267 |
| HOMA | −0.3 | 5.5 | 0.4 | 4.6 | −0.7 | (−1.6, 0.2) | 0.128 |
eGFR: estimated glomerular filtration rate, LDL: low density lipoprotein, HDL: high density lipoprotein, HOMA: homeostasis model assessment
Fig 4. Absolute mean cholesterol fractions and ratio over 96 weeks by randomised arm.
(A) total cholesterol, (B) high density lipoprotein (HDL) cholesterol, (C) low density lipoprotein (LDL) cholesterol, (D) Total:HDL cholesterol ratio. RAL = lopinavir/ritonavir+raltegravir Control = lopinavir/ritonavir+2/3 nucleoside/nucleotide reverse transcriptase inhibitors. (Control - - - RAL ---, bars indicate standard deviation).
There were no significant differences in any biochemistry, including eGFR (mean difference −0.3 95%CI −3.1, 2.5; p = 0.834), glycaemic parameters (Table 1), nor in the proportion developing metabolic syndrome (Control: 22/238, RAL 34/253; difference −0.0% 95%CI−0.1, 0.0; p = 0.17).
There were no differences between arms in rates of individuals ever reporting an adverse event AIDS or death (Table 2). Of the 11 (Control) and 8 (RAL) deaths, 3 and 4 respectively occurred in the last 48 weeks of follow up. A total of 66 AIDS events were reported by 53 individuals. The most commonly occurring AIDS event was tuberculosis (Control 16/30, RAL 18/36) followed by Pneumocystis jirovecii pneumonia (Control 2/30, RAL 4/36). Serious non-AIDS events were reported by three participants in the Control arm and four in the RAL arm. Having any clinical event (AIDS, SNAE or death) was associated with never achieving virologic suppression (Odds ratio 5.8 95%CI 3.2–10.8; p = <0.001).
Table 2. Clinical outcomes by randomised arm.
| Control (N = 271) | RAL (N = 270) | ||||||
|---|---|---|---|---|---|---|---|
| n | Rate/100 py | n | Rate/100 py | Hazard Ratio | 95%CI | p-value | |
| Deaths | 11 | 2.4 | 8 | 1.6 | 0.7 | (0.3, 1.8) | 0.456 |
| AIDS or death | 29 | 6.6 | 34 | 7.6 | 1.2 | (0.7, 1.9) | 0.538 |
| Serious adverse events | 33 | 7.4 | 40 | 8.8 | 1.2 | (0.8, 1.9) | 0.456 |
| Adverse events | 1391 | 297.2 | 1387 | 285.0 | 0.9 | (0.8, 1.1) | 0.238 |
| Number of participants reporting Grade 3/4 adverse events* | n | % | n | % | |||
| Number of Grade 3/4 adverse events | |||||||
| Total | 45 | 16.6 | 36 | 13.3 | |||
| 55 | 46 | ||||||
| Infections and infestations | 11 | 4.1 | 7 | 2.6 | |||
| 12 | 13 | ||||||
| General disorders and administration site conditions | 4 | 1.5 | 5 | 1.9 | |||
| 4 | 5 | ||||||
| Gastrointestinal disorders | 7 | 2.6 | 1 | 0.4 | |||
| 9 | 1 | ||||||
| Blood and lymphatic system disorders | 4 | 1.5 | 3 | 1.1 | |||
| 10 | 3 | ||||||
| Metabolism and nutrition disorders | 2 | 0.7 | 3 | 1.1 | |||
| 2 | 5 | ||||||
| Pregnancy, puerperium and perinatal conditions | 2 | 0.7 | 3 | 1.1 | |||
| 2 | 4 | ||||||
| Ceased antiretroviral therapy due to any grade adverse event | 12/46 | 26.1 | 9/45 | 20.0 | |||
*Grade 3/4 adverse events summarised by MedDRA System Organ Class which were reported by 5 or more people
py: person years
Grade 3/4 events were reported by 45/271 (17%) and 36/270 (13%) (p = 0.29) of individuals in the Control and RAL arms respectively (Table 2). The greatest disparity between arms for grade 3/4 events as summarized by system organ class was for gastrointestinal disorders which were reported by 7/271 (3%) and 1/270 (<0.1%) individuals in each arm respectively. Of the grade 3/4 adverse events 8/55 in the Control arm and 13/46 in the RAL arm were attributed as being possibly, probably or definitely related to study drugs. There was no difference in the proportion of participants reporting adverse events associated with skeletal muscle toxicity (Control 49/271, RAL 61/270; difference −4.5%, 95%CI −11.3, 2.13; p = 0.19). Similar proportions of participants reported ceasing antiretroviral therapy due to an adverse event (Control 12/46, RAL 9/45).
Discussion
We have shown that over 96 weeks participants who have previously failed first line ART consisting of NNRTI + 2 N(t)RTIs and then commence a regimen of LPV/r with RAL or LPV/r + 2–3N(t)RTI, achieve and maintain VL suppression rates greater than 75%. This finding, as it relates to the N(t)RTI-containing arm, supports therapy guidelines as stated by the WHO. We also demonstrate that a regimen containing LPV/r + RAL provides a non-inferior alternative to currently recommended therapy in a second-line setting [1].
Our study was conducted in diverse settings across high, middle and low income countries, with a high proportion of females and included homosexual and heterosexual participants. Thus the findings from our study are likely to be highly generalizable across similar populations. While our study was not blinded, the primary endpoint, VL, was objective and centrally analysed and thus unlikely to be subject to biased assessment. Randomisation was also central and electronic and the well-balanced characteristics between arms suggests that allocation was random and there is no indication of confounding from measured covariates [3]. However, it is possible that patient management or participant reporting of self-assessed outcomes may have been influenced by the open label nature of this trial.
At Week 48, a statistically significant difference in mean change from baseline CD4+ T-cells/mm3 was noted between treatment arms [3], however this difference abated by 96 weeks. This finding may have resulted from sicker people leaving the trial. Alternatively there may have been a further improvement in patient health, with both arms showing a greater than 50 cell/mm3 increase in the latter 48 weeks of the trial.
Both trial regimens were well tolerated with the vast majority of participants staying in follow-up, on randomised therapy and adherent. Comparable tolerance is supported by the equal frequency of virologic failure in each arm. The predictors of failure and the role of emergent resistance, particularly integrase strand inhibitor resistance in the RAL arm, require further investigation. In the Control arm, mean change in haemoglobin and total lymphocyte count were significantly lower than in the RAL arm. Associations between thymidine-containing regimens and haematological changes have previously been reported [11], we therefore conducted a pre-specified exploratory analysis restricted to those not on zidovudine-containing regimens in the Control arm compared to the RAL arm. This analysis showed substantial attenuation of the difference in change from baseline in haemoglobin and lymphocytes, such that there was no significant difference between groups. This finding should, however, be interpreted with caution as it is not a true randomised comparison. We found no decrement in the Control arm in terms of eGFR. 253 of 271 (93%) of participants in the Control arm were tenofovir containing regimens, which have been found to result in greater decreases in cohort studies and some but not all switch studies [12–15].
The elevations in total LDL and HDL cholesterol in the RAL arm reported here are consistent with the findings reported at Week 48. It appears that total cholesterol in the RAL arm may be continuing to marginally increase at a higher rate than the Control arm. This may be partially due to high exposure to tenofovir in the Control arm which has been shown to have a beneficial impact on lipids [3,16]. However these findings occur in the presence of no difference between arms in total:HDL ratio. How the lipid profiles translate to clinical risk is difficult to ascertain. The mean total cholesterol in the RAL population (5.4mmol/L) is within the moderate risk estimation for an individual in the general population [8]. While those in the RAL arm were at significantly greater risk for CHD than in the Control arm, their mean absolute ten year risk at the end of study was 4.6%. Recent guidelines, in contrast to Framingham, are also moving away from threshold based assessment of risk to a more holistic individual based risk assessment [8,17]. Poorer lipid outcomes have not been reported in other extended follow up randomised trials of RAL containing regimens [18,19].
After 96 weeks of follow up, our study showed both continued efficacy of the RAL-containing regimen, and non-inferiority compared to Control, in the treatment of HIV following first-line failure. This outcome and the overall adverse events profile are consistent with the preliminary 96 week data from comparative arms in the EARNEST trial[4]. While EARNEST was set in low income countries and SECOND LINE in predominantly middle income countries, both trials demonstrate non-inferior efficacy of a simple RAL based therapy with high tolerability. Given the broad setting of these studies the results bode well for the effectiveness of Control and RAL regimens. However detailed results from other trials are required to determine whether RAL consistently results in an increase in cholesterol and if so the consequential long term risks. There were very low levels of emergent PI resistance in either arm of the trial and moderate NtRTI and integrase strand inhibitor resistance in the Control and RAL arms respectively. Further investigations are currently underway exploring the relationships between resistance, virological failure and adherence.
At current prices RAL containing regimens are unlikely to provide a cost effective alternative to standard of care in low income countries. This is likely to be the case until price reductions and generic options become available. However RAL use may provide a viable and durable alternative for second-line therapy in middle and high income countries, and for patients unable to receive NRTI-based regimens.
Supporting Information
(DOC)
(PDF)
(DOCX)
Acknowledgments
Thank you to the participants of the Second Line study.
The study was designed by the protocol steering committee who took responsibility for the general overview of project conduct. The site investigators and staff collected data under the supervision of the project team. The data were reviewed and interpreted by the protocol steering committee. The writing committee wrote the report.
SECOND-LINE Study Group
Consortium authors—: J Amin, MA Boyd, J-M Molina, CL Moore, N Kumarasamy, C Nwizu, MH Losso, L Mohapi, S Kerr, AH Sohn, H Teppler, B Renjifo, S Emery, DA Cooper. Protocol steering committee—W Belloso, MA Boyd, DA Cooper, J Elliott, S Emery, B Gazzard, E Gotuzzo, A Kamarulzaman, E Kedem, N Kumarasamy, P Li, P-L Lim, MH Losso, JS Madero, P Mallon, L Mohapi, J-M Molina, C Nwizu, P Phanuphak, S Kerr, H Teppler, B Renjifo, C Woodward, M Wolff. Project team—C Abela, J Amin, M Arriaga, N Berthon-Jones, MA Boyd, DA Cooper, K Courtney-Vega, S Emery, N Espinosa, H Haskelberg, S Hough, W Lee, CL Moore, K Passadee, J Taylor, M Valdovinos, J Zhou. Site investigators—W Belloso, C Benites, V Bittar, A Casiro, P Chetchotisakd, J Contarelli, S Foulkes, B Gazzard, E Gotuzzo, A Kamarulzaman, E Kedem, N Kumarasamy, C Lee, N Leerattanapecth, P Li, P-L Lim, G Lopardo, MH Losso, M Luna, S Lupo, JS Madero, OG Messina, L Mohapi, J-M Molina, C Nwizu, C Perez, P Phanuphak, R Salazar, J Sanchez, D Smith, CT Soo, K Supparatpinyo, JFA Villanueva, MJ Wolff, R Wood. Site staff—E Betina Angel, R Aramburo, JV Bazalar, SR Borja, R Cabello, A Clarke, G Copertari, DO David, M Delfino, J Echevarria, S Ferret, N Grace, LAG Hernandez, R Kaplan, T Khotphuwieng, S Kumar, A La Rosa, AL Loh, S Man, P Mootsikapun, LK Nim, RG Northland, SFS Omar, S Poongulali, A Rodriguez, A Rojas, D Salami, M Sanchez, J Sarangapany, P Sugandhavesa, C Maor, C Higgs, M Tan, L Trape, W-Y Chung, J Aploon, R Lourens, C Lai Fong, M Valdovinos, G Viloria, C Wongvoranet. Data and safety monitoring board—R Gulick, D Dunn, M Dolan. Endpoint reviewer—S Pett.
Data Availability
Due to patient confidentiality requirements, requests for SECOND-LINE data may be sent to Professor Sean Emery, semery@kirby.unsw.edu.au
Funding Statement
University of New South Wales, Merck and AbbVie funded the study. The sponsors participated in the study design, data collection, data analysis, data interpretation, and review and approval of the report and supplied the study drug. Merck provided support in the form of salaries for author HT and AbbVie for BR. The specific roles of these authors are articulated in the 'author contributions' section.
References
- 1.WHO (2010) Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach: 2010 Revision. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach: 2010 Revision. Geneva.
- 2.Humphreys EH, Chang LW, Harris J (2010) Antiretroviral regimens for patients with HIV who fail first-line antiretroviral therapy. Cochrane Database Syst Rev: CD006517. [DOI] [PubMed]
- 3. Boyd MA, Kumarasamy N, Moore CL, Nwizu C, Losso MH, et al. (2013) Ritonavir-boosted lopinavir plus nucleoside or nucleotide reverse transcriptase inhibitors versus ritonavir-boosted lopinavir plus raltegravir for treatment of HIV-1 infection in adults with virological failure of a standard first-line ART regimen (SECOND-LINE): a randomised, open-label, non-inferiority study. Lancet 381: 2091–2099. 10.1016/S0140-6736(13)61164-2 [DOI] [PubMed] [Google Scholar]
- 4. Paton NI, Kityo C, Hoppe A, Reid A, Kambugu A, et al. (2014) Assessment of second-line antiretroviral regimens for HIV therapy in Africa. N Engl J Med 371: 234–247. 10.1056/NEJMoa1311274 [DOI] [PubMed] [Google Scholar]
- 5. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, et al. (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470. [DOI] [PubMed] [Google Scholar]
- 6. Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, et al. (2000) Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG). AIDS Care 12: 255–266. [DOI] [PubMed] [Google Scholar]
- 7. Lifson AR, Group IERCW, Belloso WH, Davey RT, Duprez D, et al. (2010) Development of diagnostic criteria for serious non-AIDS events in HIV clinical trials. HIV Clin Trials 11: 205–219. 10.1310/hct1104-205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. D'Agostino RB Sr, Grundy S, Sullivan LM, Wilson P, Group CHDRP (2001) Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA 286: 180–187. [DOI] [PubMed] [Google Scholar]
- 9. Wilson PW, D'Agostino RB, Parise H, Sullivan L, Meigs JB (2005) Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 112: 3066–3072. [DOI] [PubMed] [Google Scholar]
- 10. Smith F, Hammerstrom T, Soon G, Zhou S, Chen BB, et al. (2011) A Meta-analysis to Assess the FDA DAVP's TLOVR Algorithm in HIV Submissions. Drug Information Journal 45: 291–300. [Google Scholar]
- 11. Moyle G, Sawyer W, Law M, Amin J, Hill A (2004) Changes in hematologic parameters and efficacy of thymidine analogue-based, highly active antiretroviral therapy: a meta-analysis of six prospective, randomized, comparative studies. Clin Ther 26: 92–97. [DOI] [PubMed] [Google Scholar]
- 12. Goicoechea M, Liu S, Best B, Sun S, Jain S, et al. (2008) Greater tenofovir-associated renal function decline with protease inhibitor-based versus nonnucleoside reverse-transcriptase inhibitor-based therapy. J Infect Dis 197: 102–108. 10.1086/524061 [DOI] [PubMed] [Google Scholar]
- 13. Morlat P, Vivot A, Vandenhende MA, Dauchy FA, Asselineau J, et al. (2013) Role of traditional risk factors and antiretroviral drugs in the incidence of chronic kidney disease, ANRS CO3 Aquitaine cohort, France, 2004–2012. PLoS One 8: e66223 10.1371/journal.pone.0066223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nishijima T, Gatanaga H, Shimbo T, Komatsu H, Endo T, et al. (2013) Switching tenofovir/emtricitabine plus lopinavir/r to raltegravir plus Darunavir/r in patients with suppressed viral load did not result in improvement of renal function but could sustain viral suppression: a randomized multicenter trial. PLoS One 8: e73639 10.1371/journal.pone.0073639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Campo R, DeJesus E, Bredeek UF, Henry K, Khanlou H, et al. (2013) SWIFT: prospective 48-week study to evaluate efficacy and safety of switching to emtricitabine/tenofovir from lamivudine/abacavir in virologically suppressed HIV-1 infected patients on a boosted protease inhibitor containing antiretroviral regimen. Clin Infect Dis 56: 1637–1645. 10.1093/cid/cis1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Behrens G, Maserati R, Rieger A, Domingo P, Abel F, et al. (2012) Switching to tenofovir/emtricitabine from abacavir/lamivudine in HIV-infected adults with raised cholesterol: effect on lipid profiles. Antivir Ther 17: 1011–1020. 10.3851/IMP2305 [DOI] [PubMed] [Google Scholar]
- 17.Stone NJ, Robinson J, Lichtenstein AH, Bairey Merz CN, Lloyd-Jones DM, et al. (2013) 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. [DOI] [PubMed]
- 18. Gotuzzo E, Markowitz M, Ratanasuwan W, Smith G, Prada G, et al. (2012) Sustained efficacy and safety of raltegravir after 5 years of combination antiretroviral therapy as initial treatment of HIV-1 infection: final results of a randomized, controlled, phase II study (Protocol 004). J Acquir Immune Defic Syndr 61: 73–77. [DOI] [PubMed] [Google Scholar]
- 19. Reynes J, Trinh R, Pulido F, Soto-Malave R, Gathe J, et al. (2013) Lopinavir/ritonavir combined with raltegravir or tenofovir/emtricitabine in antiretroviral-naive subjects: 96-week results of the PROGRESS study. AIDS Res Hum Retroviruses 29: 256–265. 10.1089/AID.2011.0275 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(PDF)
(DOCX)
Data Availability Statement
Due to patient confidentiality requirements, requests for SECOND-LINE data may be sent to Professor Sean Emery, semery@kirby.unsw.edu.au