Abstract
IMPORTANCE
Recent large-scale genome-wide association studies have discovered several genetic variants associated with Alzheimer disease (AD); however, the extent to which DNA methylation in these AD loci contributes to the disease susceptibility remains unknown.
OBJECTIVE
To examine the association of brain DNA methylation in 28 reported AD loci with AD pathologies.
DESIGN, SETTING, AND PARTICIPANTS
Ongoing community-based clinical pathological cohort studies of aging and dementia (the Religious Orders Study and the Rush Memory and Aging Project) among 740 autopsied participants 66.0 to 108.3 years old.
EXPOSURES
DNA methylation levels at individual CpG sites generated from dorsolateral prefrontal cortex tissue using a bead assay.
MAIN OUTCOMES AND MEASURES
Pathological diagnosis of AD by National Institute on Aging–Reagan criteria following a standard postmortem examination.
RESULTS
Overall, 447 participants (60.4%) met the criteria for pathological diagnosis of AD. Brain DNA methylation in SORL1, ABCA7, HLA-DRB5, SLC24A4, and BIN1 was associated with pathological AD. The association was robustly retained after replacing the binary trait of pathological AD with 2 quantitative and molecular specific hallmarks of AD, namely, Aβ load and paired helical filament tau tangle density. Furthermore, RNA expression of transcripts of SORL1 and ABCA7 was associated with paired helical filament tau tangle density, and the expression of BIN1 was associated with Aβ load.
CONCLUSIONS AND RELEVANCE
Brain DNA methylation in multiple AD loci is associated with AD pathologies. The results provide further evidence that disruption of DNA methylation is involved in the pathological process of AD.
Alzheimer disease (AD) is a complex age-related neuro-degenerative illness and is considered highly heritable.1,2 Besides the 3 causal genes of familial AD (ie, APP, PSEN1, and PSEN2) and the well-known apolipoprotein E (APOE) gene, large-scale genome-wide association studies3-7 have identified and replicated additional common variants that are associated with susceptibility of AD, including CR1, BIN1, CD33, CLU, ABCA7, CD2AP, PICALM, EPHA1, MS4A6A, and MS4A4A. The latest meta-analysis8 to date (with almost 75 000 individuals) further expands the list to include HLA-DRB5, PTK2B, SORL1, SLC24A4, DSG2, INPP5D, MEF2C, NME8, ZCWPW1, CELF1, FERMT2, and CASS4. Studies9,10 report the association of a rare variant in TREM2 with AD risk. In addition, a large hexanucleotide repeat expansion in C9oborf72 is implicated in AD.11,12
Genetic marks are tagged by epigenetic information, such as DNA methylation and histone modification, and these epigenetic mechanisms are essential in regulating gene expression and maintaining genomic homeostasis. Aberrant epigenetic alterations are associated with various complex human diseases, including AD.13-16 The existence of cis methylation quantitative trait loci raises the possibility that alteration of DNA methylation in AD loci likely has an important role in affecting the disease susceptibility.17 We are unaware of any other studies that have systematically assessed the association of brain DNA methylation in these loci with AD pathologies.
In this study, we target brain DNA methylation in 28 known susceptibility loci for AD and examine the global association of methylation in each locus. Our study uses a unique collection of data that integrates pathological measures with brain DNA methylation from 740 autopsied participants in 2 ongoing clinical pathological studies of aging and dementia.
Methods
Study Participants
Participants were from the Religious Orders Study18 and the Rush Memory and Aging Project.19 Both studies were approved by the Institutional Review Board of Rush University Medical Center, and each participant signed a consent form and an Anatomical Gift Act. Participants enroll without known dementia and agree to annual clinical evaluations and organ donation at the time of death. By January 2010, when the methylation data were generated, more than 2400 participants had been enrolled, with the follow-up rate among survivors exceeding 90%. More than 800 autopsies had been performed, and the autopsy rate exceeds 90%.
Neuropathological Assessment of AD
Postmortem brains were processed and examined following a standard procedure.20 Multiple AD pathology measures were examined, including neuropathological AD diagnosis, Aβ load, and paired helical filament tau immunoreactive neurofibrillary tangle (tau tangle) density. Neuropathological AD diagnosis follows the National Institute on Aging–Reagan criteria, which require an intermediate likelihood of AD (ie, at least Braak stage 3 or 4 and Consortium to Establish a Registry for Alzheimer's Disease [CERAD] moderate plaques) or a high likelihood of AD (ie, at least Braak stage 5 or 6 and CERAD frequent plaques).21-23 Further details of the brain autopsy procedures and quantification of these pathology measures are provided in the eMethods in the Supplement.
Brain DNA Methylation and RNA Expression
Frozen tissues of the dorsolateral prefrontal cortex of deceased participants were thawed on ice, and DNA was extracted from gray matter following a mini-protocol (QIAamp DNA 51306; Qiagen). DNA methylation was interrogated using a bead assay (Infinium Human Methylation450; Illumina). Raw data were processed following a rigorous pipeline for data quality control, as detailed in the eMethods in the Supplement. At the end of the quality-control pipeline, we obtained distinct DNA methylation values for 420 132 autosomal CpG sites for 740 samples. In this study, we restricted our analysis to the sites that cover both genic and 100-kilobase flanking areas around each of 28 reported AD loci.
RNA-Seq expression data were generated from frozen dorsolateral prefrontal cortex tissues following the construction of complementary DNA libraries. The paired-end reads were mapped using the Ensemble human genome transcriptomic database (http://www.ensembl.org). RNA expression of the associated AD genes was queried and examined for an association with AD pathologies. Details on the RNA-Seq expression profiling are provided in the eMethods in the Supplement.
Statistical Analysis
The primary analysis examined the association of DNA methylation with pathological AD diagnosis, which was done in 2 steps. First, parallel logistic regression models were conducted with AD diagnosis as the binary outcome and individual CpG site as the predictor. Because age has a large effect on AD pathology and may affect DNA methylation, we controlled for age at death in all models. Additional covariates included sex, batch, and bisulfite conversion efficiency. Additional analyses controlled for potential confounding due to the presence of macroscopic and microscopic infarcts and cortical Lewy bodies. Second, significance values (P values) of individual CpGs in a specific AD locus were combined following the Fisher product method,24 denoted by ψ = −2Σ log pi. Here, pi refers to the significance value of ith CpG. This omnibus statistic ψ assesses a hypothesis that none of the CpGs in the interrogated locus were associated with the outcome. Statistical significance of ψ was tested using random permutations (eMethods in the Supplement).
Associated loci identified in the primary analysis were subjected to further interrogation in which we repeated the analyses by replacing the binary outcome of AD diagnosis with continuous measures of Aβ load and separately tau tangle density in linear regression models. Because both pathology measures are right skewed, we applied square root transformation before the analyses. Similar regression analyses were performed to explore the association of RNA transcript expression in the associated loci with AD pathologies. Postmortem intervals and RNA degradation could potentially have an effect on RNA expression; therefore, we adjusted for postmortem intervals and RNA degradation scores in these analyses, in addition to age and sex.
We corrected for multiple testing using the procedure by Benjamini and Hochberg.25 In identifying associated loci, correction was applied at the locus level. In assessing the top CpG sites and RNA transcripts within each locus, experimentwise correction was applied. Statistical analyses were performed using software programs (SAS, version 9.3; SAS Institute and R, version 3.0.1; http://www.r-project.org).
Results
Characteristics of the Study Participants
DNA methylation and neuropathology data were available from 740 study participants. The mean (SD) age at death was 88.0 (6.7) years (age range, 66.0-108.3 years); 471 (63.6%) were female, and the mean (SD) years of education was 16.4 (3.6) years (range, 3-28 years). Neuropathological evaluations showed that 471 (63.6%) had moderate or frequent neuritic plaques as defined by CERAD and 369 (49.9%) had Braak stage 4 or higher. Overall, 447 study participants (60.4%) met the criteria for pathological diagnosis of AD. The median Aβ load was 2.2% (interquartile range, 0.4%-5.5%), and the median tau tangle density was 3.7/mm2 (interquartile range, 1.2-8.2/mm2).
Association of Methylation in Target Loci With Pathological AD
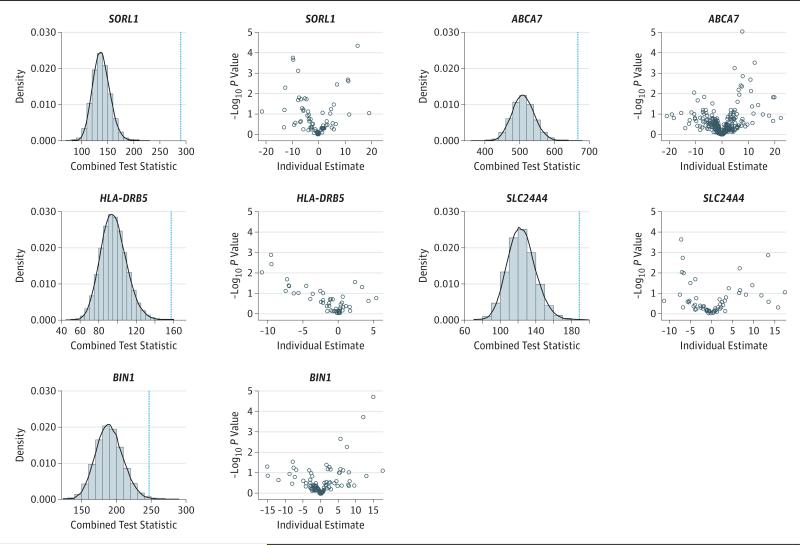
For each of 28 AD loci, parallel logistic regression analyses were performed, and significance values of individual CpGs were combined for assessment of the global association at the locus level. DNA methylation in SORL1, ABCA7, HLA-DRB5, SLC24A4, and BIN1 was associated with pathological AD diagnosis (Table 1 and Figure 1). The results for SORL1, ABCA7, HLA-DRB5, and SLC24A4 also survived more conservative Bonferroni correction. The results were unchanged after controlling for infarcts and Lewy bodies (Table 2). Subsequent analyses focused on these 5 associated loci. Their Online Mendelian Inheritance in Man accession numbers are SORL1 (602005), ABCA7 (605414), HLA-DRB5 (604776), SLC24A4 (609840), and BIN1 (601248).
Table 1.
Omnibus Test for DNA Methylation in Associated Alzheimer Disease Loci With Pathological Alzheimer Disease
| Locus | Chromosome | Covered Region | No. of CpGs | Observed Test Statistic | P Value | |
|---|---|---|---|---|---|---|
| Permuted | Adjusteda | |||||
| SORL1 | 11 | 121222911:121604471 | 69 | 289.6763 | <1.0 × 10−6 | <.0000280 |
| ABCA7 | 19 | 940101:1165570 | 255 | 666.5471 | 6.0 × 10−6 | .0000840 |
| HLA-DRB5 | 6 | 32385153:32598006 | 48 | 157.3848 | 5.0 × 10−5 | .0004667 |
| SLC24A4 | 14 | 92688924:93067825 | 62 | 188.9191 | .00013 | .0009100 |
| BIN1 | 2 | 127705598:127964903 | 95 | 247.3020 | .0032 | .0179200 |
| INPP5D | 2 | 233825035:234216549 | 87 | 214.4398 | .0188 | .0877333 |
| FERMT2 | 14 | 53223988:53517815 | 54 | 138.7177 | .0239 | .0917000 |
| TREM2 | 6 | 41026245:41230922 | 74 | 182.7488 | .0262 | .0917000 |
| CLU | 8 | 27354433:27572328 | 70 | 173.0821 | .0316 | .0983111 |
| PTK2B | 8 | 27068998:27416908 | 101 | 235.4268 | .0517 | .1476000 |
| MS4A6A | 11 | 59839079:60050674 | 23 | 61.24 | .0651 | .1657091 |
| CELF1 | 11 | 47387488:47674792 | 11 | 357.3677 | .0847 | .1976333 |
| APP | 21 | 27152860:27643446 | 21 | 53.82021 | .1005 | .2164615 |
| PSEN1 | 14 | 73503142:73790399 | 56 | 129.3102 | .1241 | .2415467 |
| MS4A4A | 11 | 59948013:60176445 | 31 | 74.74408 | .1294 | .2415467 |
| CR1 | 1 | 207569472:207915110 | 49 | 109.341 | .1991 | .3484250 |
| PSEN2 | 1 | 226958272:227183804 | 68 | 143.9049 | .3032 | .4383400 |
| EPHA1 | 7 | 142988204:143205985 | 89 | 186.9672 | .3033 | .4383400 |
| CASS4 | 20 | 54887167:55134396 | 84 | 176.5964 | .3070 | .4383400 |
| CD2AP | 6 | 47345524:47694996 | 35 | 75.1397 | .3131 | .4383400 |
| APOE | 19 | 45309038:45512650 | 104 | 214.1933 | .3683 | .4910667 |
| PICALM | 11 | 85568213:85880923 | 32 | 66.19298 | .4035 | .5135455 |
| ZCWPW1 | 7 | 99898494:100126302 | 123 | 237.6435 | .6369 | .7753565 |
| C9orf72 | 9 | 27446543:27673842 | 19 | 32.78186 | .7097 | .8279833 |
| NME8 | 7 | 37788198:38040002 | 48 | 84.99657 | .7830 | .8740308 |
| MEF2C | 5 | 87914057:88299922 | 146 | 270.7734 | .8116 | .8740308 |
| CD33 | 19 | 51628334:51843274 | 56 | 94.70939 | .8789 | .8963000 |
| DSG2 | 18 | 28978026:29228814 | 21 | 31.04398 | .8963 | .8963000 |
P values are adjusted using the Benjamini-Hochberg procedure.25
Figure 1. DNA Methylation With Pathological Alzheimer Disease Diagnosis in Associated Loci.
For each locus, the left panel shows the histogram and density function of the global test statistic generated from randomly permuted data, with a blue dashed line superimposed to represent the value of the same statistic observed from the actual data. The right panel is the volcano plot showing the significance level vs the regression estimates for individual CpG sites.
Table 2.
Omnibus Test for DNA Methylation in Associated Alzheimer Disease Loci With Pathological Alzheimer Disease, Adjusted for Other Pathologiesa
| Locus | Model A | Model B | ||
|---|---|---|---|---|
| Observed Test Statistic | Permuted P Value | Observed Test Statistic | Permuted P Value | |
| SORL1 | 290.3979 | <1.0 × 10−5 | 277.8277 | <1.0 × 10−5 |
| ABCA7 | 665.9884 | <1.0 × 10−5 | 643.6079 | 8.0 × 10−5 |
| HLA-DRB5 | 159.832 | 5.0 × 10−5 | 156.2942 | .00012 |
| SLC24A4 | 190.1051 | .0001 | 180.3139 | .00068 |
| BIN1 | 249.7376 | .00248 | 251.4047 | .00179 |
Model A is adjusted for the presence of macroscopic and microscopic infarcts, in addition to age, sex, batch, and bisulfite conversion efficiency. Model B is further adjusted for the presence of cortical Lewy bodies.
SORL1
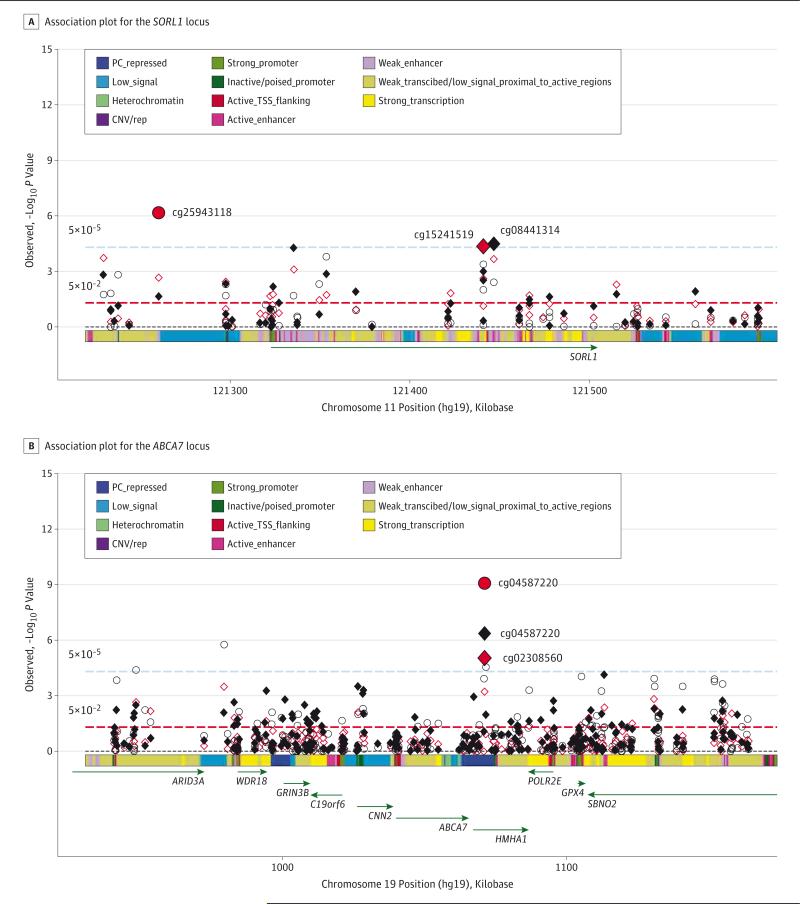
We interrogated 69 CpG sites in the SORL1 locus (sortilin-related receptor, L [DLR class] A repeats containing), of which 8 showed associations with pathological AD diagnosis (eTable 1 in the Supplement). The top CpG (cg15241519) was observed in a weakly transcribed region in the gene body, where methylation was associated with greater odds for pathological AD.
Methylation at 7 CpG sites in SORL1 was associated with Aβ load and 7 CpG sites with tau tangle density (eTable 2 and eTable 3 in the Supplement). Of these, 4 CpG sites (cg15241519, cg08441314, cg11606444, and cg22136098) were associated with both indexes. The regional association plot (Figure 2A) highlights the location of top hits for the 3 pathological AD traits.
Figure 2. Association Plot for the SORL1 and ABCA7 Loci.
A, Association plot for the SORL1 locus. P values of individual CpG sites for each of the 3 Alzheimer disease pathological indexes (red diamond indicates pathological Alzheimer disease; black diamond, Aβ load; and red circle, tau tangle density) were plotted against their genomic positions. The color band on the x-axis represents the chromatic state of the region. The smallest P value for each outcome is highlighted with larger symbols. B, Association plot for the ABCA7 locus. CNV/rep indicates copy number variation/repetitive; PC, Polycomb; and TSS, transcription start site.
We examined the expression of 13 SORL1 transcripts in relation to AD pathologies. We found little association with Aβ load. By contrast, upregulated expression of the SORL1 transcript (ENST00000524873.1) was associated with higher tau tangle density (mean [SE] β coefficient, 2.676 [0.687]; adjusted P = .0015). Methylation and expression in the locus were only weakly correlated (r range, −0.15 to 0.15) (eFigure 1 in the Supplement).
ABCA7
We analyzed 255 CpG sites in the ABCA7 locus (adenosine tri-phosphate [ATP]-binding cassette, sub-family A [ABC1], member 7). Nineteen sites showed nominal association with pathological AD dagnosis, and the result was enriched for positively associated CpGs (eTable 4 in the Supplement). Specifically, the regression coefficients for 15 of these 19 CpGs (78.9%) had positive signs such that methylation was associated with greater odds for AD diagnosis. Of these, 3 CpG sites remained significant after adjusting for multiple testing, among which cg02308560 and cg04587220 (30 base pairs apart) were observed in a polycomb-repressed region in the HMHA1 gene proximate to the 3’ untranslated region of ABCA7.
Methylation at 12 CpG sites in the ABCA7 locus was associated with Aβ load and 18 CpG sites with tau tangle density (eTable 5 and eTable 6 in the Supplement). Figure 2B shows that the methylation sites in the HMHA1 gene demonstrate the strongest signals in relation to AD pathologies. Similar to pathological AD, the results were enriched for positive association such that methylation at all but one of these CpG sites was associated with increased burden of AD pathologies.
We examined 17 transcripts of ABCA7 for the association of RNA expression with AD pathologies. We observed that ENST00000525073.2 expression was associated with higher tau tangle density (mean [SE] β coefficient, 0.062 [0.018]; adjusted P = .0138). Correlations of DNA methylation at interrogated CpG sites with the expression of ABCA7 transcripts were weak (r range, −0.2 to 0.2) (eFigure 2 in the Supplement).
HLA-DRB5
Eight of forty-eight CpG sites in the HLA-DRB5 locus (major histocompatibility complex, class II, DR beta 5) showed nominal association with pathological AD diagnosis; however, none survived multiple testing (eTable 7 in the Supplement). Methylation in the locus was associated with Aβ load and with tau tangle density. At the CpG level, 3 CpG sites were associated with Aβ load and 9 were associated with tau tangles (eTable 8 and eTable 9 in the Supplement). An association plot shows that methylation association with AD pathologies peaked in the nearby HLA-DRA gene (Figure 3A). We found no association of HLA-DRB5 RNA expression with any of the 3 pathological AD traits.
Figure 3. Association Plot for the HLA-DRB5 and SLC24A4 Loci.
A, Association plot for the HLA-DRB5 locus. P values of individual CpG sites for each of the 3 Alzheimer disease pathological indexes (red diamond indicates pathological Alzheimer disease; black diamond, Aβ load; and red circle, tau tangle density) were plotted against their genomic positions. B, Association plot for the SLC24A4 locus. CNV/rep indicates copy number variation/repetitive; PC, Polycomb; and TSS, transcription start site.
SLC24A4
We interrogated 62 CpG sites in the SLC24A4 locus (solute carrier family 24, member 4) (Figure 3B). Three CpGs showed associations with pathological AD and 8 with Aβ load (eTable 10 and eTable 11 in the Supplement). We found weaker association with tau tangle pathology (eTable 12 in the Supplement). We examined the expression of 9 transcripts of SLC24A4 and found no association with any of the 3 pathological AD traits.
BIN1
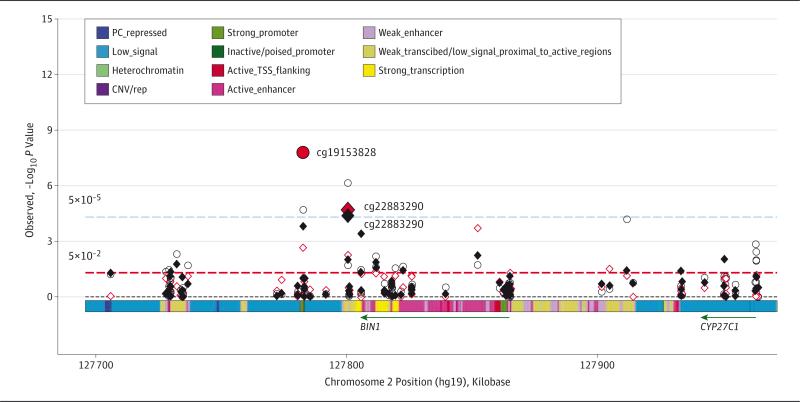
In total, 95 CpG sites in the BIN1 locus (bridging integrator 1) were interrogated. Two CpG sites showed associations with pathological AD (eTable 13 in the Supplement). The cg22883290 site was located in a weakly transcribed region proximate to the 3’ untranslated region of BIN1, and cg04019522 was in an active enhancer region in the gene body. Methylation at both sites was associated with greater odds for pathological AD diagnosis.
Methylation at 3 CpG sites in BIN1 was associated with Aβ load and 5 CpG sites with tau tangle density (eTable 14 and eTable 15 in the Supplement). An association plot shows that the strongest signals came from the sites proximate to the 3’ untranslated region of BIN1, as well as the downstream CpG island harboring cg19153828 (Figure 4).
Figure 4. Association Plot for the BIN1 Locus.
P values of individual CpG sites for each of the 3 Alzheimer disease pathological indexes (red diamond indicates pathological Alzheimer disease; black diamond, Aβ load; and red circle, tau tangle density) were plotted against their genomic positions. CNV/rep indicates copy number variation/repetitive; PC, Polycomb; and TSS, transcription start site.
We examined the expression of 14 BIN1 transcripts. Higher expression of 3 BIN1 transcripts (ENST00000393040.3, ENST00000409400.1, and ENST00000462958.1) was associated with greater Aβ load (eTable 16 in the Supplement). Surprisingly, we observed that another transcript (ENST00000316724.5) showed an association in the opposite direction such that higher expression was associated with less amyloid. The pairwise correlations between methylation of individual CpG sites and BIN1 expression showed a weak correlation, with Pearson r between −0.27 and 0.23 (eFigure 3 in the Supplement).
Mediation Analysis
In the final set of analyses, we explored potential mediation of amyloid on the association of DNA methylation with tau tangle pathology. To do so, we repeated the analysis of tau tangle density by further controlling for Aβ load for the 4 loci in which CpGs were strongly associated with both amyloid and tangles. An attenuated association of DNA methylation would be evidence of mediation.26 We found that DNA methylation associations with tau tangle density were essentially retained for all the loci after controlling for Aβ load (Table 3). These data suggest that methylation has an independent effect on these 2 molecular processes.
Table 3.
Omnibus Test for DNA Methylation in Associated Alzheimer Disease Loci With Aβ Load and Tau Tangle Density
| Aβ Loada | Tau Tangle Densitya | Tau Tangle Densityb | ||||
|---|---|---|---|---|---|---|
| Locus | Observed Test Statistic | Permuted P Value | Observed Test Statistic | Permuted P Value | Observed Test Statistic | Permuted P Value |
| SORL1 | 285.6039 | <1.0 × 10−5 | 242.0672 | <1.0 × 10−5 | 203.1709 | .00039 |
| ABCA7 | 1035.527 | <1.0 × 10−5 | 1017.743 | <1.0 × 10−5 | 992.4105 | <1.0 × 10−5 |
| HLA-DRB5 | 159.6211 | 5.0 × 10−5 | 221.1747 | <1.0 × 10−5 | 176.9269 | 1.0 × 10−5 |
| BIN1 | 291.9307 | <1.0 × 10−5 | 368.237 | <1.0 × 10−5 | 289.7298 | 1.0 × 10−5 |
The models are adjusted forage, sex, batch, and bisulfite conversion efficiency.
The model is further adjusted for Aβ load.
Discussion
In this study, we targeted brain DNA methylation in 28 previously reported AD loci. We assessed at the locus level the global association of DNA methylation. We found that DNA methylation in 5 of 28 AD loci was associated with pathological AD diagnosis, including SORL1, ABCA7, HLA-DRB5, SLC24A4, and BIN1.
Increasing evidence exists for AD-related alterations in DNA methylation. Evaluations of immunoreactivity of DNA methylation markers suggest that levels of global DNA methylation are reduced in entorhinal cortex27 and hippocampus28 in AD. However, this result is inconclusive. Increased global DNA methylation is observed in frontal cortex of AD brain,29 and findings from another genome-wide DNA methylation association study14 showed hypermethylated and hypomethylated sites in AD brain. In this study, not all interrogated loci demonstrated evidence of methylation alterations with AD. Most of the top CpG sites in ABCA7 and BIN1 showed increased burden of AD pathologies with more methylation. These results suggest that the pathogenesis of AD affects the brain's epigenome in a strong but specific manner and hint at a greater level of complexity in the role of the epigenome in the disease.
Our findings provide new evidence that brain DNA methylation in AD loci might be involved in the pathological process of AD. Specifically, a recent genome-wide DNA methylation study30 reported 71 discrete CpG sites that are associated with neuritic amyloid plaques, including cg02308560 in ABCA7 and cg22883290 in BIN1. Herein, we confirm the signals in ABCA7 and BIN1 with Aβ load. Furthermore, we show that methylation in these 2 loci is also related to tau tangle density. In addition, we observed associations in 3 additional AD loci. Of these, the SORL1 locus shows the most significant association with AD. To our knowledge, only one prior study31 has examined brain DNA methylation in SORL1 in relation to AD. The study targeted thegene's promoter sequences using much fewer brain samples, and it demonstrated no differences in methylation between AD and controls. Separately, we observed associations of methylation in HLA-DRB5 and SLC24A4, and we are unaware of any previous report on these associations.
Our results suggest that altered methylation in these loci might involve both Aβ and tau tangle pathologies. SORL1 and ABCA7 have previously been implicated in the Aβ process. SORL1 encodes sortilin-related receptor LR11 or SorLA, which controls Aβ production such that reduced expression of SORL1 tends to increase Aβ production and hence promote AD.32 ABCA7 encodes an ATP–binding cassette transporter that regulates processing of amyloid precursor protein (APP) in vitro. A 2008 study33 on cultured cells reported that ABCA7 inhibits Aβ secretion. A more recent study34 on transgenic mice showed that ABCA7 regulates Aβ homeostasis in the brain and deletion of ABCA7 doubles cerebral Aβ accumulation. Nonetheless, the relationship between DNA methylation and Aβ is complex. One study35 showed that Aβ reduces global DNA methylation but increases neprilysin DNA methylation, which subsequently suppresses neprilysin expression, leading to further Aβ degradation. BIN1 is thought to be involved in endocytosis, which could serve as a pathway that leads to APP production and release.36,37 The gene was also shown to mediate AD risk by modulating tau pathology.38 Little is known about functional consequences of HLA-DRB5 and SLC24A4 in relation to AD. The HLA-DRB5 locus encodes a major histocompatibility complex class II protein involved in immune responses,8 and SLC24A4 may be involved in neural development.39 In addition, the SLC24A4 gene is located next to the RIN3 (Ras and Rab interactor 3) gene, which interacts with BIN1 in the early endocytic pathway.40
Our RNA expression data also reveal some notable results. We find little evidence that SORL1 or ABCA7 expression is associated with Aβ load. Instead, higher expression of the SORL1 transcript, and similarly that of ABCA7, is associated with more tangle pathology. One plausible explanation is that these genes are overexpressed in response to the neuronal inflammation or degeneration that is induced by AD pathologies, such as tau pathology. We observe that higher expression of 3 BIN1 transcripts is associated with more Aβ,consistent with the finding thatBIN1 expression is elevated in AD brain.38 Our analysis also reveals another transcript in the BIN1 locus, of which higher expression is associated with less amyloid. Recent evidence suggests that the BIN1 protein level is lower in brains of sporadic AD cases.41
Separately, our data show that pairwise correlations between methylation of individual CpGs and expression of the associated genesareweak.Large-scale data sets examining the relation ship between methylation status and gene expression level sinhuman brain are just beginning to emerge. Therefore, their relationship at this time remains poorly understood. It is likely that several factors contribute to the poor associations. First, our methylation scan only captures a fraction of the CpGs across the genome. Second, DNA methylation is just one of many epigenetic changes that contributeto the expression, and other factors, such as micro RNA, might be at play.42 Third, while there are sparse data from brain regarding these associations at the density measured in our study, weak associations have been reported for other tissues.43 Fourth, we are specifically interrogating genes that harbor genetic variants associated with AD. Genomic variants are known to affect methylation quantitative traitloci and expression quantitative trait loci, both cis and trans, in human brain and other tissues.43,44 Additional studies are warranted to elucidate the relationship between DNA methylation and RNA expression in these target genes.
Conclusions
In summary, investigating the association of DNA methylation in target loci with AD pathology is a first step to better understand potential functional pathways that link epigenetic disruptions to the disease. By leveraging genome-wide DNA methylation profiles and neuropathological data from 740 autopsied older persons, this is the first and largest study to our knowledge that has interrogated brain DNA methylation in AD loci for associations with multiple indexes for AD pathology. Limitations are noted. Brain tissue came from a single region of the dorsolateral prefrontal cortex. Our present data are not mature enough to derive potential effect due to cellular composition, and future work needs to collect additional data and develop novel methods to identify target cell types that drive the association of DNA methylation in each locus.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by grants P30AG10161, R01AG15819, R01AG17917, R01AG36042, R01AG36836, and U01AG46152 from the National Institutes of Health.
Role of the Funder/Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Yu had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Yu, Chibnik, Pochet, Meissner, De Jager, Bennett.
Acquisition, analysis, or interpretation of data: Yu, Chibnik, Srivastava, Pochet, Xu, Kozubek, Obholzer, Schneider, Meissner, De Jager, Bennett.
Drafting of the manuscript: Yu, Pochet.
Critical revision of the manuscript for important intellectual content: Chibnik, Srivastava, Pochet, Yang, Xu, Kozubek, Obholzer, Leurgans, Schneider, Meissner, De Jager, Bennett.
Statistical analysis: Yu, Yang, Leurgans.
Obtained funding: Schneider, De Jager, Bennett. Administrative, technical, or material support: All authors.
Study supervision: Pochet, Bennett.
Conflict of Interest Disclosures: None reported.
Additional Contributions: We thank all the participants of the Religious Orders Study and the Rush Memory and Aging Project, as well as the staff at the Rush Alzheimer's Disease Center, for this work.
REFERENCES
- 1.Gatz M, Pedersen NL, Berg S, et al. Heritability for Alzheimer's disease: the study of dementia in Swedish twins. J Gerontol A Biol Sci Med Sci. 1997;52(2):M117–M125. doi: 10.1093/gerona/52a.2.m117. [DOI] [PubMed] [Google Scholar]
- 2.Wilson RS, Barral S, Lee JH, et al. Heritability of different forms of memory in the Late Onset Alzheimer's Disease Family Study. J Alzheimers Dis. 2011;23(2):249–255. doi: 10.3233/JAD-2010-101515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollingworth P, Harold D, Sims R, et al. Alzheimer's Disease Neuroimaging Initiative; CHARGE Consortium; EADI1 Consortium. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat Genet. 2011;43(5):429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambert JC, Heath S, Even G, et al. European Alzheimer's Disease Initiative Investigators. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41(10):1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 5.Naj AC, Jun G, Beecham GW, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nat Genet. 2011;43(5):436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seshadri S, Fitzpatrick AL, Ikram MA, et al. CHARGE Consortium; GERAD1 Consortium; EADI1 Consortium. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010;303(18):1832–1840. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harold D, Abraham R, Hollingworth P, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat Genet. 2009;41(10):1088–1093. doi: 10.1038/ng.440. [published corrections appear in Nat Genet. 2009;41(10):1156 and 2013;45(6):712].
- 8.Lambert JC, Ibrahim-Verbaas CA, Harold D, et al. European Alzheimer’s Disease Initiative (EADI); Genetic and Environmental Risk in Alzheimer's Disease; Alzheimer's Disease Genetic Consortium; Cohorts for Heart and Aging Research in Genomic Epidemiology. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45(12):1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guerreiro R, Wojtas A, Bras J, et al. Alzheimer Genetic Analysis Group. TREM2 variants in Alzheimer's disease. N Engl J Med. 2013;368(2):117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonsson T, Stefansson H, Steinberg S, et al. Variant of TREM2 associated with the risk of Alzheimer's disease. N Engl J Med. 2013;368(2):107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harms M, Benitez BA, Cairns N, et al. NIA-LOAD/NCRAD Family Study Consortium. C9orf72 hexanucleotide repeat expansions in clinical Alzheimer disease. JAMA Neurol. 2013;70(6):736–741. doi: 10.1001/2013.jamaneurol.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohli MA, John-Williams K, Rajbhandary R, et al. Repeat expansions in the C9ORF72 gene contribute to Alzheimer disease in Caucasians. Neurobiol Aging. 2013;34(5):1519.e5–1519.e12. doi: 10.1016/j.neurobiolaging.2012.10.003. doi:10.1016/j.neurobiolaging.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005;6(8):597–610. doi: 10.1038/nrg1655. [DOI] [PubMed] [Google Scholar]
- 14.Bakulski KM, Dolinoy DC, Sartor MA, et al. Genome-wide DNA methylation differences between late-onset Alzheimer's disease and cognitively normal controls in human frontal cortex. J Alzheimers Dis. 2012;29(3):571–588. doi: 10.3233/JAD-2012-111223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rakyan VK, Down TA, Balding DJ, Beck S. Epigenome-wide association studies for common human diseases. Nat Rev Genet. 2011;12(8):529–541. doi: 10.1038/nrg3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu H, Liu X, Deng Y, Qing H. DNA methylation, a hand behind neurodegenerative diseases. Front Aging Neurosci. 2013;5:85. doi: 10.3389/fnagi.2013.00085. doi:10.3389/fnagi.2013.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang D, Cheng L, Badner JA, et al. Genetic control of individual differences in gene-specific methylation in human brain. Am J Hum Genet. 2010;86(3):411–419. doi: 10.1016/j.ajhg.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett DA, Schneider JA, Arvanitakis Z, Wilson RS. Overview and findings from the Religious Orders Study. Curr Alzheimer Res. 2012;9(6):628–645. doi: 10.2174/156720512801322573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the Rush Memory and Aging Project. Curr Alzheimer Res. 2012;9(6):646–663. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66(12):1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 21.The National Institute on Aging and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. Neurobiol Aging. 1997;18(4 suppl 1):S1–S2. [PubMed] [Google Scholar]
- 22.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 23.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), part II: standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 24.Fisher RA. Questions and answers #14. Am Stat. 1948;2(5):30–31. [Google Scholar]
- 25.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 26.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 27.Mastroeni D, Grover A, Delvaux E, Whiteside C, Coleman PD, Rogers J. Epigenetic changes in Alzheimer's disease: decrements in DNA methylation. Neurobiol Aging. 2010;31(12):2025–2037. doi: 10.1016/j.neurobiolaging.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chouliaras L, Mastroeni D, Delvaux E, et al. Consistent decrease in global DNA methylation and hydroxymethylation in the hippocampus of Alzheimer's disease patients. Neurobiol Aging. 2013;34(9):2091–2099. doi: 10.1016/j.neurobiolaging.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao JS, Keleshian VL, Klein S, Rapoport SI. Epigenetic modifications in frontal cortex from Alzheimer's disease and bipolar disorder patients. Transl Psychiatry. 2012;2:e132. doi: 10.1038/tp.2012.55. doi:10.1038/tp.2012 .55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Jager PL, Srivastava G, Lunnon K, et al. Alzheimer's disease: early alterations in brain DNA methylation at ANK1, BIN1, RHBDF2 and other loci. Nat Neurosci. 2014;17(9):1156–1163. doi: 10.1038/nn.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furuya TK, da Silva PN, Payão SL, et al. SORL1 and SIRT1 mRNA expression and promoter methylation levels in aging and Alzheimer's Disease. Neurochem Int. 2012;61(7):973–975. doi: 10.1016/j.neuint.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 32.Rogaeva E, Meng Y, Lee JH, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39(2):168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan SL, Kim WS, Kwok JB, et al. ATP-binding cassette transporter A7 regulates processing of amyloid precursor protein in vitro. J Neurochem. 2008;106(2):793–804. doi: 10.1111/j.1471-4159.2008.05433.x. [DOI] [PubMed] [Google Scholar]
- 34.Kim WS, Li H, Ruberu K, et al. Deletion of Abca7 increases cerebral amyloid-β accumulation in the J20 mouse model of Alzheimer's disease. J Neurosci. 2013;33(10):4387–4394. doi: 10.1523/JNEUROSCI.4165-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen KL, Wang SS, Yang YY, Yuan RY, Chen RM, Hu CJ. The epigenetic effects of amyloid-β1-40 on global DNA and neprilysin genes in murine cerebral endothelial cells. Biochem Biophys Res Commun. 2009;378(1):57–61. doi: 10.1016/j.bbrc.2008.10.173. [DOI] [PubMed] [Google Scholar]
- 36.Itoh T, De Camilli P. BAR, F-BAR (EFC) and ENTH/ANTH domains in the regulation of membrane-cytosol interfaces and membrane curvature. Biochim Biophys Acta. 2006;1761(8):897–912. doi: 10.1016/j.bbalip.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 37.Koo EH, Squazzo SL. Evidence that production and release of amyloid β-protein involves the endocytic pathway. J Biol Chem. 1994;269(26):17386–17389. [PubMed] [Google Scholar]
- 38.Chapuis J, Hansmannel F, Gistelinck M, et al. GERAD Consortium. Increased expression of BIN1 mediates Alzheimer genetic risk by modulating tau pathology. Mol Psychiatry. 2013;18(11):1225–1234. doi: 10.1038/mp.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larsson M, Duffy DL, Zhu G, et al. GWAS findings for human iris patterns: associations with variants in genes that influence normal neuronal pattern development. Am J Hum Genet. 2011;89(2):334–343. doi: 10.1016/j.ajhg.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kajiho H, Saito K, Tsujita K, et al. RIN3: a novel Rab5 GEF interacting with amphiphysin II involved in the early endocytic pathway. J Cell Sci. 2003;116(pt 20):4159–4168. doi: 10.1242/jcs.00718. [DOI] [PubMed] [Google Scholar]
- 41.Glennon EB, Whitehouse IJ, Miners JS, et al. BIN1 is decreased in sporadic but not familial Alzheimer's disease or in aging. PLoS One. 2013;8(10):e78806. doi: 10.1371/journal.pone.0078806. doi:10.1371/journal.pone.0078806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van den Hove DL, Kompotis K, Lardenoije R, et al. Epigenetically regulated microRNAs in Alzheimer's disease. Neurobiol Aging. 2014;35(4):731–745. doi: 10.1016/j.neurobiolaging.2013.10.082. [DOI] [PubMed] [Google Scholar]
- 43.van Eijk KR, de Jong S, Boks MP, et al. Genetic analysis of DNA methylation and gene expression levels in whole blood of healthy human subjects. BMC Genomics. 2012;13:636. doi: 10.1186/1471-2164-13-636. doi:10.1186/1471-2164-13-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gibbs JR, van der Brug MP, Hernandez DG, et al. Abundant quantitative trait loci exist for DNA methylation and gene expression in human brain. PLoS Genet. 2010;6(5):e1000952. doi: 10.1371/journal.pgen.1000952. doi:10.1371 /journal.pgen.1000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.