Abstract
Cancer-related fatigue is a common and often long lasting symptom for many breast cancer survivors. Fatigued survivors show evidence of elevated inflammation, but the physiological mechanisms driving inflammatory activity have not been determined. Alterations in the autonomic nervous system, and particularly parasympathetic nervous system activity, are a plausible, yet understudied contributor to cancer-related fatigue. The goal of this study was to replicate one previous study showing an association between lower parasympathetic activity and higher fatigue in breast cancer survivors (Fagundes et al., 2011), and to examine whether inflammation mediates this association. Study participants were drawn from two samples and included 84 women originally diagnosed with early-stage breast cancer prior to age 50. Participants completed questionnaires, provided blood samples for determination of interleukin (IL)-6 and C-reactive protein (CRP), and underwent electrocardiography (ECG) assessment for evaluation of resting heart rate variability (HRV), a measure of parasympathetic activity. Results showed that lower HRV was associated with higher fatigue (p < .05), as predicted. In bivariate analyses, HRV was also correlated with circulating concentrations of IL-6 and CRP. However, path analyses did not support inflammation as a mediator of the association between HRV and fatigue; instead, associations among these variables appeared to be driven by age and BMI. These findings identify HRV as a potential contributor to cancer-related fatigue, but suggest that inflammation does not mediate this association in younger, healthy breast cancer survivors who are several years post-treatment. The autonomic nervous system merits additional attention in research on the etiology of cancer-related fatigue.
Keywords: symptoms, autonomic nervous system, autonomic functioning, parasympathetic activity, inflammation
1. Introduction
Fatigue is one of the most common and distressing symptoms reported by breast cancer patients and survivors. Cancer-related fatigue is experienced by 30–60% of patients undergoing cancer treatment (Jacobsen et al., 1999; Servaes et al., 2002; Lawrence et al., 2004; Bower, 2008), and can persist for up to ten years post treatment (Bower et al., 2006). Cancer patients and survivors describe their fatigue as more severe, pervasive, and debilitating than “normal” fatigue caused by lack of sleep or overexertion (Poulson, 2003). Empirical evidence confirms that the intensity and duration of cancer-related fatigue is significantly greater than fatigue experienced by healthy controls (Andrykowski et al., 1998). Cancer-related fatigue negatively impacts quality of life, and may predict shorter survival (Andrykowski et al., 1998; Bower et al., 2000; Groenvold et al., 2007).
Given the prevalence and impact of cancer-related fatigue, there has been considerable interest in the biological mechanisms underlying this symptom. One proposed mechanism is elevated inflammation. Indeed, studies have shown an association between elevated inflammatory markers and fatigue during and after treatment (Bower & Lamkin, 2013). However, the processes that initiate and sustain inflammatory activity in the aftermath of cancer treatment have not been determined.
One possible driver of increased inflammation in fatigued cancer survivors may be the autonomic nervous system (ANS). The ANS is a key regulator of the immune system, including the inflammatory cytokine network. In general, activation of the sympathetic branch of the ANS leads to increased inflammation and activation of the parasympathetic branch leads to decreased inflammation (Thayer & Sternberg, 2006; Irwin & Cole, 2011), although these effects are complex and highly contextual (Sanders & Straub, 2002). Thus, increased activity in the sympathetic branch or decreased activity in the parasympathetic branch may play a role in inflammation and associated symptoms of cancer-related fatigue. In the current study, we focus exclusively on the role of parasympathetic activity. It has been hypothesized that parasympathetic nervous system stimulation (via the vagus nerve) leads to decreases in production of pro-inflammatory cytokines through the release of the neurotransmitter acetylcholine (Tracey, 2002; 2009). Indeed, studies have documented cross-sectional associations between higher parasympathetic activity, as indexed with heart rate variability (HRV), and lower levels of inflammation (e.g. Sajadieh et al., 2004; Sloan et al., 2007). Parasympathetic activity is typically measured by capturing HRV, which is the fluctuation of time between consecutive heartbeats (Task Force, 1996; Appelhans & Luecken, 2006). Although HRV is influenced by both branches of the autonomic nervous system, data processing techniques allow for the extraction of the unique influence of the parasympathetic branch on HRV.
Previous research examining the association between autonomic activity and fatigue in non-cancer samples has supported the importance of the parasympathetic nervous system. Studies have shown that individuals with chronic fatigue syndrome have reduced HRV compared to healthy controls (Boneva et al., 2007; Beaumont et al., 2012), as do women reporting stress-related fatigue (Olsson et al., 2009). Of note, a meta-analysis comparing HRV in healthy controls to individuals with functional somatic disorders (which are characterized by high levels of fatigue) found that the poor quality of studies and publication bias limits the ability to draw conclusions (Tak et al., 2009). To our knowledge, only one previous study has examined the association between HRV and cancer-related fatigue. In a sample of 109 breast cancer survivors who were 17 months post diagnosis on average, Fagundes and colleagues (2011) found that lower resting HRV was associated with higher levels of fatigue. Associations with inflammation were not examined in that study.
The primary goal of the current study was to examine the association between HRV and fatigue in a sample of younger female breast cancer survivors. This group was of interest because they are at elevated risk for fatigue and other negative consequences of cancer treatment (Howard-Anderson et al., 2012). Based on studies that show an association between HRV and fatigue in other disorders (Boneva et al., 2007; Beaumont et al., 2012) and in breast cancer survivors (Fagundes et al., 2011), we hypothesized that cancer-related fatigue would be associated with lower HRV. Our second goal was to examine inflammation as a potential mediating pathway between HRV and fatigue. Low HRV is associated with elevated levels of circulating proinflammatory cytokines (Sajadieh et al., 2004; Sloan et al., 2007), and inflammation in turn is correlated with elevated symptoms of fatigue in cancer survivors (Bower & Lamkin, 2013). Thus, we hypothesized that the association between HRV and fatigue would be mediated by elevations in circulating markers of inflammation.
2. Methods
2.1 Participants
The study data were drawn from two samples of younger breast cancer survivors. Sample 1 comes from an observational study of the psychological and physiological characteristics of younger breast cancer survivors (n=50) conducted from August 2010 to July 2011. Sample 2 comes from a randomized controlled trial of mindfulness meditation for younger breast cancer survivors (n=71) conducted from April 2011 to June 2012. The current study focuses on a subset of women from each of these studies (n=24 from Sample 1; n=60 from Sample 2; total n=84) for whom we have electrocardiogram (ECG) data. ECG data were collected to assess resting autonomic nervous system activity using identical protocols for data collection and data processing in each study.
Participants from the original studies were identified through several sources: the UCLA Health System tumor registry, offices of medical oncologists, and a listserv of women who had previously participated in research conducted by our group and agreed to be re-contacted for future studies. Eligibility criteria for both studies were as follows: (1) diagnosed with early-stage breast cancer at age 50 years or less; (2) no current evidence of disease; (3) at least 1 year post initial cancer diagnosis and at least 3 months post treatment with radiation or chemotherapy (for Sample 1, the criteria was at least 6 months post treatment); (4) able to give informed consent; (5) able to read and write English. Women who had active medical conditions that were not controlled by medication were considered ineligible. If they had an acute illness on the day of their appointment, their assessment was rescheduled. All participants gave informed consent prior to completing the study, which was approved by the UCLA Institutional Review Board.
2.2 Procedures
Participants for both studies completed an in-person assessment at our research laboratory between 0800h and 1000h to control for potential diurnal variations in immune measures. Prior to the visit, participants completed a questionnaire battery that included information about demographics, cancer history, and fatigue. At the visit, height and weight were assessed, and blood was drawn via venipuncture. Participants had fasted prior to coming in to the lab to ensure no influence of caffeine or food intake on immune measurements. After the blood draw, spot electrodes were placed on each forearm and chest for ECG. Participants were asked to rest comfortably in their chair with both legs on the floor and arms in their laps or on the arm rests. They were asked to refrain from speaking and moving. They were not given reading material or other distraction. Resting ECG data was recorded for 15 minutes. Note that data from the baseline assessment (pre-intervention) for Sample 2 was used in all analyses.
2.3 Measures
2.3.1. Heart rate variability
HRV was computed from the continuous ECG recordings by analyzing the variability in the intervals between heart beats (R-R intervals; Task Force, 1996). A modified lead II electrode placement using three electrodes was used. The ECG was sampled at 1000 Hz and stored for off-line processing using AcqKnowledge (Biopac Systems Inc., USA) and Kubios (University of Kuopio, Finland). During the data processing, average HRV was calculated across 2–3 minute segments of time, which were then averaged across the 15 minutes to come up with single indices of resting HRV.
HRV is influenced by both sympathetic and parasympathetic nervous system activity. Because of our focus on the parasympathetic nervous system, we chose two measures of HRV that are recommended as preferential measures of parasympathetic activity (Task Force, 1996). Through time domain analysis, the intervals of subsequent normal R-R waves are measured over a period of time. The root mean square of the successive differences in R-R intervals (RMSSD) is calculated and reported in milliseconds. RMSSD is thought to represent parasympathetically mediated HRV (Task Force, 1996). Low RMSSD at rest is a proposed index of poor autonomic regulation (Appelhans & Luecken, 2006).
The second HRV outcome was calculated through frequency domain analysis in which R-R time intervals are separated into frequency bands. Some heart rate oscillations are faster than others and thus frequency deconstruction enables the transformation of R-R intervals into high (0.15 – 0.40 Hz) and low frequency bands (0.04–0.15 Hz). High frequency HRV (HF-HRV) is a specific marker of parasympathetic activity. In our sample, RMSSD and HF-HRV were highly correlated, r=.915.
2.3.2. Inflammatory markers
Blood was obtained via venipuncture and stored in EDTA tubes at −80 C for subsequent batch testing. We focused on two inflammatory markers, IL-6 and CRP, which have been associated with cancer-related fatigue (Schubert et al., 2007; Alfano et al., 2012) and with HRV (Sajadieh et al., 2004; Sloan et al., 2007). Plasma levels of IL-6 were determined by the Quantikine high-sensitivity ELISA kit (R&D Systems, Minneapolis, MN), with a lower detection limit of 0.039 pg/mL. Plasma CRP levels were determined by a high-sensitivity ELISA (Immundiagnostik; ALPCO Immunoassays, Salem, NH), with a lower limit of detection of 0.4 μg/L. All samples were run in duplicate, and intra- and inter-assay precision for both tests were less than or equal to 10%.
2.3.3. Fatigue
Items from the Fatigue Symptom Inventory (FSI) were used to assess subjective fatigue (Hann et al., 1998). This scale was developed for use in cancer patients and has strong psychometric properties (Donovan & Jacobsen, 2010). We used three items to capture specific aspects of cancer-related fatigue – amount, severity, and duration. Women rated their average level of fatigue in the past week (FSI average) and their level of fatigue on the day they felt most fatigued in the past week (FSI most) on an 11-point scale ranging from 0 (not at all fatigued) to 10 (as fatigued as I could be). Participants also indicated the number of days they felt fatigued in the past week (FSI days). On all dimensions, higher scores indicate worse fatigue.
2.3.4. Physical Activity
Godin & Shepard’s (1985) Leisure Time Exercise Questionnaire was used to capture amount of physical activity women participated in during a typical 7-day period. Women indicated how many times a week and for how long they engaged in mild (e.g. gentle yoga), moderate (e.g. fast walking), or strenuous (e.g. running) exercise. A composite score capturing the metabolic equivalent (MET) hours of physical activity per week was calculated (Jones et al., 2005). This scale has demonstrated concurrent validity and reliability (Jacobs et al., 1993), and is frequently used in cancer samples (e.g. Jones et al., 2005).
2.3.5. Demographics and cancer-treatment history
Demographic and cancer treatment information was determined from self-report questionnaires, which assessed racial/ethnic status, marital status, education, employment, income, and whether or not they currently smoked. Body mass index (BMI) was calculated from the in-person assessment of height and weight. Cancer-treatment variables included whether or not the women had received chemotherapy and/or radiation, what type of surgery they underwent (lumpectomy or mastectomy), and whether or not they were currently receiving endocrine therapy.
3. Statistical Analyses
First, we assessed whether participants in Sample 1 and Sample 2 differed from each other in any systematic way. We conducted t-tests and chi-square analyses on all demographic, treatment-related, fatigue, HRV, and inflammation variables to examine whether there were significant differences between groups. There were no systematic differences between the two samples (see Tables 1 and 2) and thus for all of the following analyses the samples were combined.2 All HRV and inflammatory markers were normalized using log transformations prior to any analyses. Raw values are provided in Table 2 for descriptive purposes.
Table 1.
Baseline Characteristics for Total Sample and Individual Samples
| Total (N = 84) | Sample 1 (n = 24) | Sample 2 (n = 60) | p valuea | |
|---|---|---|---|---|
| Age, M (SD) | 46.5 (6.8) | 47.1 (5.7) | 46.5 (7.2) | 0.739 |
| Years since diagnosis, M (SD) | 3.7 (2.2) | 3.6 (1.8) | 3.7 (2.3) | 0.768 |
| Ethnicity, N (%) | 0.406 | |||
| White | 62 (73.2) | 16 (66.7) | 46 (76.7) | |
| African American | 2 (2.4) | 1 (4.2) | 1 (1.7) | |
| Asian | 10 (11.9) | 4 (16.7) | 6 (10.0) | |
| Other | 10 (11.9) | 3 (12.5) | 7 (11.7) | |
| Marital Status, N (%) | 0.174 | |||
| In committed relationship | 59 (70.2) | 21 (87.5) | 38 (63.3) | |
| Other | 25 (29.8) | 3 (12.5) | 22 (36.7) | |
| Family Yearly Income, N (%) | 0.337 | |||
| Under $60,000 | 15 (18.3) | 3 (13.0) | 12 (20.3) | |
| $60,001 – $100,000 | 19 (23.2) | 8 (34.8) | 11 (18.6) | |
| Over $100,000 | 48 (58.5) | 12 (52.2) | 36 (61.0) | |
| Employment, N (%) | 0.386 | |||
| Employed full time | 46 (54.8) | 15 (62.5) | 31 (51.7) | |
| Employed part time | 16 (19.1) | 3 (12.5) | 13 (21.7) | |
| Home maker | 8 (9.5) | 3 (12.5) | 5 (8.3) | |
| Unemployed | 6 (7.1) | 0 (0) | 6 (10.0) | |
| Other | 8 (9.52) | 3 (12.5) | 5 (8.3) | |
| Cancer treatments received, N (%) | ||||
| Chemotherapy | 62 (73.8) | 19 (79.1) | 43 (71.7) | 0.48 |
| Radiation therapy | 55 (65.5) | 14 (58.3) | 41 (68.3) | 0.384 |
| Surgery Type, N (%) | 0.018* | |||
| Lumpectomy only | 33 (39.3) | 4 (16.7) | 29 (48.3) | |
| Mastectomy | 50 (59.5) | 19 (79.2) | 31 (51.7) | |
| None | 1 (1.2) | 1 (4.1) | 0 (0) | |
| Current endocrine therapy | 56 (66.7) | 16 (66.8) | 40 (66.7) | 1 |
Comparison between studies was tested with t-tests or chi square.
p<.05.
Table 2.
Means of Key Study Variables for Total Sample and by Individual Sample
| Variable | Total (N = 84) | Sample 1 (n = 24) | Sample 2 (n = 60) | |
|---|---|---|---|---|
|
| ||||
| M (SD) | M (SD) | M (SD) | p valuea | |
| Fatigue | ||||
| FSI average | 4.04 (1.94) | 4.29 (1.90) | 3.93 (1.96) | 0.448 |
| FSI most | 6.11 (2.41) | 6.25 (2.03) | 6.05 (2.56) | 0.734 |
| FSI days | 4.24 (2.28) | 4.02 (2.09) | 4.33 (2.36) | 0.573 |
| Heart Rate Variability | ||||
| RMSSD | 29.01 (17.23) | 25.40 (14.09) | 30.47 (18.25) | 0.226 |
| HF-HRV (power ms2) | 397.74 (400.83) | 339.40 (290.55) | 421.88 (438.43) | 0.4 |
| Inflammation | ||||
| IL-6 (pg/mL) | 1.27 (.76) | 1.35 (.75) | 1.15 (.69) | 0.217 |
| CRP (pg/mL) | 1.43 (1.74) | 1.35 (1.51) | 1.21 (1.48) | 0.425 |
Comparison between studies was tested with t-tests or chi square. All units reported in original scale, not log transformed.
Our primary hypothesis was that lower levels of resting HRV would be associated with higher levels of cancer-related fatigue. We first looked at the bivariate correlations between fatigue and HRV indices. Then we conducted regression analyses predicting three fatigue variables (FSI average, FSI most, FSI days) from HRV (RMSSD and HF-HRV), controlling for BMI and age because of their known associations with HRV and fatigue (e.g. Byrne et al., 1996; Britton et al., 2007; Zhang, 2007; Donovan et al., 2007). We also examined physical activity as a potential confound because of previous work linking physical activity to lower HRV (e.g. De Meersman, 1993) and lower inflammation (e.g. Reuben et al., 2003), and evidence that exercise interventions moderately reduce cancer-related fatigue (e.g. Brown et al., 2011).Smoking status may also be a confound in the relationship between HRV, fatigue, and inflammation. Seven women reported being current smokers. We ran all analyses with and without these women; results remained the same, thus they were left in the sample in the analyses reported here to maintain our sample size.
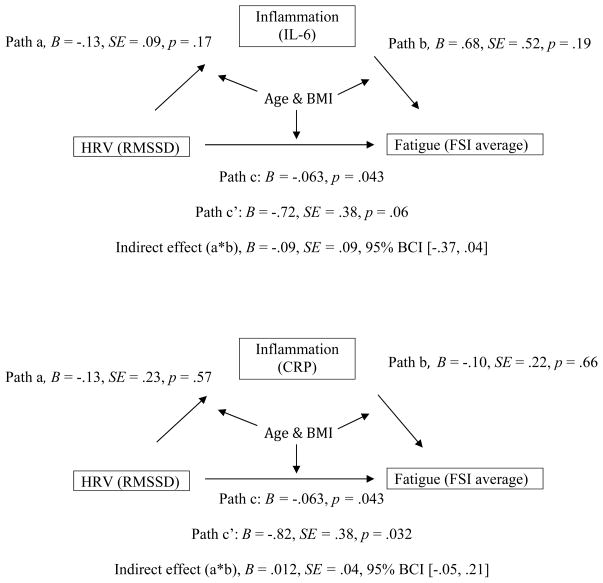
We then tested whether the association between HRV and fatigue was mediated by inflammation using path analysis with the sem command in STATA 12. Mediation is tested by evaluating the significance of the indirect effect, which is determined by comparing the total and direct effects (see Figure 1; Iacobucci et al., 2007). Because the distribution of indirect effects is known to not be normally distributed, significance of the indirect effect was tested using 95% bias-corrected confidence intervals (BCI) based on 1,000 replicates (MacKinnon et al., 2004; Fritz & MacKinnon, 2007). BMI and age were included as covariates in all paths in the model.
Figure 1.
Path model examining the indirect effect of HRV on fatigue through IL-6 and CRP controlling for age and BMI in all paths. Path a represents the relationship between HRV and either IL-6 or CRP. Path b represents the relationship between IL-6 or CRP and average level of fatigue. Path c is the total effect of HRV on fatigue (parallel to the regression analyses), and path c′ is the direct effect of HRV on fatigue after controlling for either IL-6 or CRP. The difference between the total and direct effects, or indirect effect, was not significant in the models for either IL-6 or CRP, indicating that neither marker is a significant mediator of the relationship between HRV and average fatigue. BCI = bias corrected confidence interval.
4. Results
4.1 Participant characteristics
Table 1 displays the demographic and treatment-related characteristics of participants from whom we collected ECG data. For the total sample (across Sample 1 and Sample 2), women were on average 46 years old, the majority were Caucasian (73%), in a committed relationship (70%), employed at least part time (74%), and were 3.7 years from diagnosis. The majority of women had received chemotherapy (74%), radiation therapy (66%), and were currently receiving endocrine therapy (67%). Differences between the two samples in treatment received were only present for type of surgery. A smaller percentage of women in Sample 1 received a lumpectomy only (17%) compared to Sample 2 (48%).
Table 2 displays the mean fatigue, HRV, and inflammatory values for each sample. The mean FSI average score was 4.04 (SD=1.94), which is above the clinically significant cutoff of 3 on this scale (Donovan & Jacobsen, 2010). The mean levels of RMSSD, HF-HRV, IL-6, and CRP, are comparable to those reported for healthy adults (Marsland et al., 2007; Gruenewald et al., 2009; Gordon et al., 2012).
We next examined the association between HRV and demographic, treatment-related, and other potential confounds. Consistent with previous research, women had lower HRV if they were older (RMSSD, r = −.26, p = .016; HF-HRV, r = −.32, p = .003) and had a higher BMI (RMSSD, r = −.25, p = .025; HF-HRV, r = −.15, p < .1). No other demographic or treatment related variables were associated with HRV. Physical activity was not associated with HRV (RMSSD, r = .15, p = .178; HF-HRV, r = .079, p = .483).
4.2 Associations between HRV and fatigue
Our primary analyses examined the association between measures of HRV and fatigue. Bivariate correlations between variables are presented in Table 3. As predicted, RMSSD was significantly correlated with FSI average and FSI days, and marginally correlated with FSI most. Women with lower RMSSD scores, indicating lower HRV, reported higher average levels of fatigue, more days fatigued, and marginally greater fatigue severity. In regression analyses controlling for physical activity, the associations remained the same (for FSI average, B = −.075, p = .02; for FSI days, B = −.056, p = .043; for FSI most, B = −.045, p = .084). After controlling for age and BMI, the association between RMSSD and FSI average remained significant (B = −.063, p = .043), though the associations with FSI days and FSI most were attenuated (B = −.042, p = .125 and B = −.036, p = .158, respectively).
Table 3.
Correlations Between Fatigue, HRV, and Inflammatory Markers
| 1. | 2. | 3. | 4. | 5. | 6. | |
|---|---|---|---|---|---|---|
| 1. FSI average | - | |||||
| 2. FSI most | .816*** | - | ||||
| 3. FSI days | .734*** | .638*** | - | |||
| 4. RMSSD (ln) | −.269* | −.197+ | −.229* | - | ||
| 5. HRV-HF (ln) | −.204+ | −.167 | −.172 | .915*** | - | |
| 6. IL-6 (ln) | .213+ | .203+ | −.178 | −.310** | −.322** | - |
| 7. CRP (ln) | .028 | .044 | −.090 | −.231* | −.244* | .609*** |
p < .10.
p < .05.
p < .01.
p <.001.
HF-HRV was marginally correlated with FSI average, but not with the other two fatigue indices. When controlling for physical activity, the association with FSI average remained marginally significant, B = −.11, p = .077. After controlling for age and BMI, this association was attenuated (B = −.092, p = .121).
4.3 Associations between HRV and inflammation
In bivariate analyses, RMSSD was significantly correlated with IL-6 and CRP (see Table 3). After controlling for physical activity, the association between RMSSD and IL-6 remained significant, B = −.344, p = .012, while the association between RMSSD and CRP was slightly attenuated, B = −.105, p = .061. After controlling for age and BMI, the associations were no longer significant (B = −.193, p = .193 for IL-6; B = −.033, p = .596 for CRP).
HF-HRV was also significantly correlated with IL-6 and CRP. After controlling for physical activity, these associations remained significant (for IL-6, B = −.709, p = .006; for CRP, B = −.216, p = .041). After controlling for age and BMI, the associations were attenuated (B = −.496, p = .078 for IL-6; B = −.109, p = .364 for CRP).
4.4 Associations between inflammation and fatigue
IL-6 was marginally correlated with fatigue (see Table 3). Women with higher IL-6 values reported marginally greater average levels of fatigue and fatigue severity. The associations between IL-6 and fatigue indices remained marginally significant when physical activity was added to the model (all p’s <.15). After controlling for age and BMI, the associations between IL-6 and fatigue were not significant (for FSI average, B = .038, p = .133; for FSI days, B = .015, p = .498; for FSI most, B = .03, p = .148). CRP was not associated with any indices of fatigue.
4.5 Path analyses testing inflammation as a mediator of HRV and fatigue
Next, we conducted path analyses to test whether the association between HRV and fatigue was mediated by IL-6 or CRP. The models are illustrated in Figure 1. We selected RMSSD as the predictor variable and FSI average as the dependent variable for these analyses based on results from the correlation and regression analyses. Note that mediational effects may still be present when the relationship between the independent and dependent variable is weak (Stanton et al., 2013). Age and BMI were included as covariates in all models. Results showed that neither IL-6 nor CRP were significant mediators of the association between RMSSD and average fatigue.
5. Discussion
Cancer-related fatigue is a significant problem for many cancer survivors, and particularly younger women diagnosed with premenopausal breast cancer (Howard-Anderson et al., 2012). The drivers of persistent post-treatment fatigue remain largely unknown. The current project tested an understudied contributor to cancer-related fatigue – parasympathetic nervous system activity. Results supported the hypothesis that lower parasympathetic activity, as measured by resting HRV, was associated with higher levels of fatigue. Specifically, breast cancer survivors who had lower levels of RMSSD reported higher levels of average fatigue in the past week, controlling for age, BMI, and physical activity. These findings are consistent with an earlier report that found an association between HRV and fatigue in breast cancer survivors within one year post treatment (Fagundes et al., 2011), and extend these results to demonstrate an association with fatigue in longer-term (and younger) survivors.
Our second goal was to test inflammation as a mediator of the association between HRV and fatigue. Bivariate analyses supported the possibility of inflammation as a mediator, as RMSSD was correlated with plasma levels of IL-6 and CRP, and IL-6 was marginally correlated with fatigue measures. However, these bivariate associations were attenuated after controlling for age and BMI. Moreover, a path model that included age and BMI as control variables did not support either inflammatory marker as a mediator in this young, relatively healthy sample. Together, these findings suggest that BMI may be playing an important role in driving inflammatory activity in this young, healthy sample, and may account in part for links between inflammation and cancer-related fatigue, as seen in previous research (Alfano et al., 2012).
These findings suggest that there may be other pathways linking HRV to cancer-related fatigue. Some investigators have suggested that sluggish autonomic responses to environmental demands or an imbalance between sympathetic and parasympathetic branches may contribute to physical fatigability and avoidance of physically demanding tasks (Pagani & Lucini, 1999), leading to reduced physical activity, deconditioning, and increased fatigue. Indeed, patients with chronic fatigue syndrome show reduced parasympathetic and increased sympathetic responsiveness to standard lab stimuli tests (i.e. walking, paced breathing) and mental stress tasks (Pagani et al., 1994; Sisto et al., 1995; Cordero, et al., 1996). To our knowledge, only one study has tested the association between fatigue and autonomic responses to challenge in cancer survivors. Fagundes et al. (2011) found that in response to the Trier Social Stress Test, fatigued breast cancer survivors had significantly lower HRV and higher norepinephrine during the baseline and recovery periods than less fatigued women, though the magnitude of changes in HRV throughout the task did not differ based on fatigue level. There is also evidence that breast cancer survivors with persistent fatigue show blunted HPA responses to acute stress (Bower et al., 2005). Future studies should examine autonomic and endocrine responses to challenge among individuals with cancer-related fatigue. Perhaps more important, longitudinal studies are needed that assess ANS activity at the beginning of the cancer trajectory, to determine whether HRV predicts the development and persistence of cancer-related fatigue and underlying mechanisms for this association.
There are several limitations to our study. First, we used a cross-sectional design to examine associations between fatigue, HRV, and inflammation. Although this design can provide insight into factors that contribute to cancer-related fatigue, longitudinal studies are required to determine whether these factors play an etiological role in fatigue onset or persistence, or are instead a consequence of fatigue. Second, we choose to focus on resting HRV given evidence linking resting HRV with fatigue and inflammation in non-cancer samples. Future research should include more comprehensive assessment of ANS function both at rest and in response to acute stress. Third, we focused on two inflammatory markers that have been associated with fatigue and HRV in previous research; evaluation of other markers (e.g., soluble TNF receptor type II) would provide a more comprehensive assessment of the pro-inflammatory cytokine network and may have enhanced our ability to identify associations with cancer-related fatigue (Bower et al., 2002, 2011). In addition, associations between cancer-related fatigue and biological processes may be more evident in samples specifically selected for reporting severe and persistent fatigue (e.g., Bower et al., 2002; Collado-Hidalgo et al., 2006; Alexander et al., 2009). Additionally, we did not carefully capture medication use, which, despite the overall good health of the sample, may influence levels of inflammation. Finally, our study was not specifically powered to detect mediation. Mediation effects can be difficult to find in small samples, and thus the results from our model should be considered preliminary (Fritz & MacKinnon, 2007).
In conclusion, HRV has been linked to a variety of psychological and physical illnesses (Thayer & Sternberg, 2006), including depression, anxiety disorders, and all-cause mortality (Tsuji et al., 1994; Gorman & Sloan, 2000). Our findings suggest that HRV may also play a role in fatigue experienced in the aftermath of cancer treatment. Given the prevalence and persistence of this symptom in the growing population of cancer survivors, future work should continue to explore the role of HRV as a correlate and potential contributor to this debilitating symptom.
Footnotes
We also tested whether there were any significant differences in demographic and treatment-related variables between the women from the parent studies who underwent ECG assessment and those who did not undergo ECG assessment. Women who underwent ECG assessment were significantly more likely to have received a mastectomy as opposed to a lumpectomy; there were no other significant differences.
Contributor Information
Alexandra D. Crosswell, UCLA Department of Psychology.
Kimberly G. Lockwood, University of Pittsburgh Department of Psychology, 210 S. Bouquet Street, Sennott Square 3rd Floor, Pittsburgh, PA, USA, 15260
Patricia A. Ganz, UCLA Schools of Medicine & Public Health & the Jonsson Comprehensive Cancer Center, 650 Charles Young Drive South, Room A2-125 CHS, Los Angeles, CA, USA, 90095-6900
Julienne E. Bower, UCLA Department of Psychology and Department of Psychiatry and Behavioral Sciences, 1285 Franz Hall, Box 951563, Los Angeles, CA, USA, 90095-1563
References
- Alexander S, Minton O, Andrews P, Stone P. A comparison of the characteristics of disease-free breast cancer survivors with or without cancer-related fatigue syndrome. Eur J Cancer. 2009;45:384–392. doi: 10.1016/j.ejca.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfano CM, Imayama I, Neuhouser ML, Kiecolt-Glaser JK, Wilder Smith A, Meeske K, McTiernan A, Bernstein L, Baumgartner KB, Ulrich CM, Ballard-Barbash R. Fatigue, inflammation, and w-3 and w-6 fatty acid intake among breast cancer survivors. J Clin Oncol. 2012;30:1280–1287. doi: 10.1200/JCO.2011.36.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrykowski MA, Curran SL, Lightner R. Off-treatment fatigue in breast cancer survivors: a controlled comparison. J Behav Med. 1998;21:1–18. doi: 10.1023/a:1018700303959. [DOI] [PubMed] [Google Scholar]
- Appelhans BM, Luecken LJ. Heart rate variability as an index of regulated emotional responding. Rev Gen Psychol. 2006;10:229–240. [Google Scholar]
- Beaumont A, Burton AR, Lemon J, Bennett BK, Lloyd A, Vollmer-Conna U. Reduced cardiac vagal modulation impacts on cognitive performance in chronic fatigue syndrome. PLoS One. 2012;7:e49518. doi: 10.1371/journal.pone.0049518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boneva RS, Decker MJ, Maloney EM, Lin J, Jones JF, Helgason HG, Heim CM, Rye DB, Reeves WC. Higher heart rate and reduced heart rate variability persist during sleep in chronic fatigue syndrome: a population-based study. Auton Neurosci. 2007;137:94–101. doi: 10.1016/j.autneu.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Bower JE. Behavioral symptoms in patients with breast cancer and survivors. J Clin Oncol. 2008;26:768–777. doi: 10.1200/JCO.2007.14.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Aziz N. Altered cortisol response to psychological stress in breast cancer survivors with persistent fatigue. Psychosom Medicine. 2005;67:277–280. doi: 10.1097/01.psy.0000155666.55034.c6. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64:604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Desmond KA, Bernaards C, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer. 2006;106:751–758. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18:743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29:3517–3522. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav Immun. 2013;30:S48–57. doi: 10.1016/j.bbi.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton A, Shipley M, Malik M, Hnatkova K, Hemingway H, Marmot M. Changes in heart rate and heart rate variability over time in middle-aged men and women in the general population (from Whitehall II Cohort Study) Am J Cardiol. 2007;100:524–527. doi: 10.1016/j.amjcard.2007.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JC, Huedo-Medina TB, Pescatello LS, Pescatello SM, Ferrer RA, Johnson BT. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20:123–133. doi: 10.1158/1055-9965.EPI-10-0988. [DOI] [PubMed] [Google Scholar]
- Byrne EA, Fleg JL, Vaitkevicius PV, Wright J, Porges SW. Role of aerobic capacity and body mass index in the age-associated decline in heart rate variability. J Appl Physiol. 1996;81:743–750. doi: 10.1152/jappl.1996.81.2.743. [DOI] [PubMed] [Google Scholar]
- Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006;12:2759–2766. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- Cordero DL, Sisto SA, Tap WN, LaManca JJ, Pareja JG, Natelson BH. Decreased vagal power during treadmill walking in patients with chronic fatigue syndrome. Clin Auton Res. 1996;6:329–333. doi: 10.1007/BF02556303. [DOI] [PubMed] [Google Scholar]
- De Meersman RE. Heart rate variability and aerobic fitness. Am Heart J. 1993;125:726–731. doi: 10.1016/0002-8703(93)90164-5. [DOI] [PubMed] [Google Scholar]
- Donovan KA, Jacobsen PB. The Fatigue Symptom Inventory: a systematic review of its psychometric properties. Support Care in Cancer. 2010;19:169–185. doi: 10.1007/s00520-010-0989-4. [DOI] [PubMed] [Google Scholar]
- Donovan KA, Small BJ, Andrykowski MA, Munser P, Jacobsen PB. Utility of a cognitive-behavioral model to predict fatigue following breast cancer treatment. Health Psychol. 2007;26:464–467. doi: 10.1037/0278-6133.26.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes CP, Murray DM, Hwang SB, Gouin JP, Thayer JF, Sollers JJ, Shapiro CL, Malarkey WB, Kiecolt-Glaser JK. Sympathetic and parasympathetic activity in cancer-related fatigue: more evidence for a physiological substrate in cancer survivors. Psychoneuroendocrinology. 2011;36:1137–1147. doi: 10.1016/j.psyneuen.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz MW, MacKinnon DP. Required sample size to detect the mediated effect. Psychological Science. 2007;18:233–239. doi: 10.1111/j.1467-9280.2007.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin G, Shepard RJ. A simple method to assess exercise behavior in the community. Canadian Journal of Applied Sport Sciences. 1985;10:141–146. [PubMed] [Google Scholar]
- Gordon JL, Ditto B, D’Antono B. Cognitive depressive symptoms associated with delayed heart rate recovery following interpersonal stress in healthy men and women. Psychophysiology. 2012;49:1082–1089. doi: 10.1111/j.1469-8986.2012.01397.x. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Sloan RP. Heart rate variability in depressive and anxiety disorders. American Heart Journal. 2000;140:S77–S83. doi: 10.1067/mhj.2000.109981. [DOI] [PubMed] [Google Scholar]
- Groenvold M, Petersen MA, Idler E, Bjorner JB, Fayers PM, Mouridsen HT. Psychological distress and fatigue predicted recurrence and survival in primary breast cancer patients. Breast Cancer Res Treat. 2007;105:209–219. doi: 10.1007/s10549-006-9447-x. [DOI] [PubMed] [Google Scholar]
- Gruenewald TL, Cohen S, Matthews KA, Tracy R, Seeman TE. Association of socioeconomic status with inflammation markers in black and white men and women in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Soc Sci Med. 2009;69:451–9. doi: 10.1016/j.socscimed.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hann DM, Jacobsen PB, Azzarello LM, Martin SC, Curran SL, Fields KK, Greenberg H, et al. Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Qual Life Res. 1998;7:301–310. doi: 10.1023/a:1024929829627. [DOI] [PubMed] [Google Scholar]
- Howard-Anderson J, Ganz PA, Bower JE, Stanton AL. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104:386–405. doi: 10.1093/jnci/djr541. [DOI] [PubMed] [Google Scholar]
- Iacobucci D, Saldanha N, Deng X. A meditation on mediation: evidence that structural equations models perform better than regressions. J Consum Psychol. 2007;17:140–154. [Google Scholar]
- Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11:625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs DR, Ainsworth BE, Hartman TJ, Leon AS. A simultaneous evaluation of ten commonly used physical activity questionnaires. Med Sci Sport Exerc. 1993;25:81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- Jacobsen PB, Hann DM, Azzarello LM, Horton J, Balducci L, Lyman GH. Fatigue in women receiving adjuvant chemotherapy for breast cancer: characteristics, course, and correlates. J Pain Symptom Manage. 1999;18:233–242. doi: 10.1016/s0885-3924(99)00082-2. [DOI] [PubMed] [Google Scholar]
- Jones LW, Courneya S, Fairey AS, Mackey JR. Does the theory of planned behavior mediate the effects of an oncologist’s recommendation to exercise in newly diagnosed breast cancer survivors? Results from a randomized controlled trial. Health Psych. 2005;24:189–197. doi: 10.1037/0278-6133.24.2.189. [DOI] [PubMed] [Google Scholar]
- Lawrence DP, Kupelnick B, Miller K, Devine D, Lau J. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. J Natl Cancer Inst. 2004;32:40–50. doi: 10.1093/jncimonographs/lgh027. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: distribution of the product and resampling methods. Multivariate Behav Res. 2004;39:99–128. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland A, Gianaros PJ, Prather AA, Jennings R, Neumann SA, Manuck SB. Stimulated production of proinflammatory cytokines covary inversely with heart rate variability. Psychosom Med. 2007;69:709–16. doi: 10.1097/PSY.0b013e3181576118. [DOI] [PubMed] [Google Scholar]
- Olsson EM, Roth WT, Melin L. Psychophysiological characteristics of women suffering from stress-related fatigue. Stress and Health. 2009;26:113–126. [Google Scholar]
- Pagani M, Lucini D, Mela GS, Langewitz W, Malliani A. Sympathetic overactivity in subjects complaining of unexplained fatigue. Clin Sci. 1994;87:655–661. doi: 10.1042/cs0870655. [DOI] [PubMed] [Google Scholar]
- Pagani M, Lucini D. Chronic fatigue syndrome: a hypothesis focusing on the autonomic nervous system. Clin Sci. 1999;96:117–125. [PubMed] [Google Scholar]
- Poulson MJ. Not just tired. J Clin Oncol. 2003;21:112–113. doi: 10.1200/JCO.2003.01.191. [DOI] [PubMed] [Google Scholar]
- Reuben DB, Judd-Hamilton L, Harris TB, Seeman TE. The associations between physical activity and inflammatory markers in high-functioning older persons: MacArthur Studies of Successful Aging. J Am Geriatr Soc. 2003;51:1125–1130. doi: 10.1046/j.1532-5415.2003.51380.x. [DOI] [PubMed] [Google Scholar]
- Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Abedini S, Hansen JF. Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. Eur Heart J. 2004;25:363–370. doi: 10.1016/j.ehj.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Sanders VM, Straub RH. Norepinephrine, the B-adrenergic receptor, and immunity. Brain Behav Immun. 2002;16:290–332. doi: 10.1006/brbi.2001.0639. [DOI] [PubMed] [Google Scholar]
- Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav Immun. 2007;21:413–427. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Servaes P, Verhagen C, Bleijenberg G. Fatigue in cancer patients during and after treatment: prevalence, correlates, and interventions. Eur J Cancer. 2002;38:27–43. doi: 10.1016/s0959-8049(01)00332-x. [DOI] [PubMed] [Google Scholar]
- Sisto SA, Tapp W, Drastal S, Bergen M, DeMasi I, Cordero D, Nateloseon B. Vagal tone is reduced during paced breathing in patients with chronic fatigue syndrome. Clin Auton Res. 1995;5:139–143. doi: 10.1007/BF01826195. [DOI] [PubMed] [Google Scholar]
- Sloan RP, McCreath H, Tracey KJ, Sidney S, Liu K, Seeman T. RR interval variability is inversely related to inflammatory markers: the CARDIA study. Mol Med. 2007;13:178–184. doi: 10.2119/2006-00112.Sloan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton AL, Luecken LJ, MacKinnon DP, Thompson EH. Mechanisms in psychosocial interventions for adults living with cancer: opportunity for integration of theory, research, and practice. J Consult Clin Psychol. 2013;81:318–335. doi: 10.1037/a0028833. [DOI] [PubMed] [Google Scholar]
- Tak LM, Riese H, de Bock GH, Manoharan A, Kok IC, Rosmalen JGM. As good as it gets? A meta-analysis and systematic review of methodological quality of heart rate variability studies in functional somatic disorders. Biological Psychology. 2009;82:101–110. doi: 10.1016/j.biopsycho.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Thayer JF, Sternberg E. Beyond heart rate variability: vagal regulation of allostatic systems. Ann N Y Acad Sci. 2006;1088:361–372. doi: 10.1196/annals.1366.014. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. Reflex control of immunity. Nature Reviews Immunology. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji H, Venditti FJ, Manders ES, Evans JC, Larson MG, Feldman CL, Levy D. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation. 1994;90:878–883. doi: 10.1161/01.cir.90.2.878. [DOI] [PubMed] [Google Scholar]
- Zhang J. Effects of age and sex on heart rate variability in health subjects. Journal of Manipulative and Physiological Therapeutics. 2007;30:374–379. doi: 10.1016/j.jmpt.2007.04.001. [DOI] [PubMed] [Google Scholar]