Every scientist, clinician, and patient seeks that holy grail that will cure disease. Nowhere is this need more acute than in the field of neurodegeneration. In neurogenetic diseases, in the large majority of cases, aberrant code is embedded in the DNA in every cell, and the blood–brain barrier is an obstacle to drug delivery. Treatments must therefore involve regular intrathecal injections or use an alternative delivery mechanism. For spinal muscular atrophy, one of the common and devastating motor neuron disorders, the advancement of new therapeutic candidates to clinical trials, including an antisense oligonucleotide-based therapy and, more recently, an adeno-associated virus serotype 9–based gene therapy, has provided hope and optimism for the entire field of neurodegeneration. However, this excitement has been tempered by the increasing realization that synergistic approaches will almost certainly be required to ensure sufficiently broad and early correction of the survival-of-motor-neuron 2 (SMN2) mRNA splicing, especially for the most common and severe infantile form of the disease, spinal muscular atrophy type 1. Recent work by Naryshkin and colleagues1 points to the possibility that a small-molecule therapy may provide an effective and orally available therapy for spinal muscular atrophy. These investigators used methods to screen for and identify small molecules that target RNA splicing with high efficacy and, more importantly, specificity.
RNA splicing patterns drive cell biology at the most basic level. The RNA transcribed from a gene template is processed to remove noncoding sequences (called introns) before becoming a mature messenger RNA (mRNA) capable of directing the synthesis of proteins. This process requires the complex cellular machinery known as the spliceosome.
A process known as alternative mRNA splicing can result in dozens of different mRNA species from a single pre-mRNA template. Alternative splicing results in the exclusion of one or more exons from the mature mRNA and facilitates the expression of different protein isoforms (all derived from the same gene) at different stages of development, in different tissues, and in response to environmental cues, such as stress or cellular injury.2,3 Identifying small molecules that modify splicing and in turn alter the quantity or quality of transcripts in which too much or too little of a given mRNA species contributes to disease pathogenesis provides an attractive pathway for the development of targeted therapeutics. The dynamic nature of the transcriptome (the full complement of RNA molecules in a given cell) allows for a rapid effect on a select target, and cell lines from patients can help investigators identify potential off-target effects before advancing to phase 1 studies.
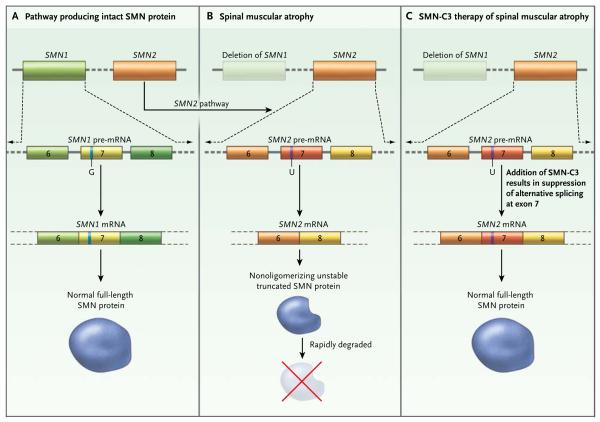
Spinal muscular atrophy has long been an attractive target for therapeutic splicing. It is caused by homozygous disruption of the gene SMN1; both copies of SMN1 in a person must be disrupted for the disease to occur. However, humans have two paralogous SMN genes. The other SMN gene, SMN2, is highly similar to SMN1, with only a handful of sequence differences. One of these lies at nucleotide position 840; the thymidine residue in SMN2 activates alternative splicing and excludes exon 7 from the majority of SMN2 transcripts generated and, in turn, results in the generation of a truncated SMN protein that is rapidly degraded (Fig. 1).
Figure 1. Alternative SMN2 Messenger RNA (mRNA) Splicing as a Target for Small-Molecule Therapies.
SMN1 and SMN2 are paralogous genes encoding the survival-of-motor-neuron (SMN) protein (Panel A). SMN2 differs from SMN1 in having a T (U in the pre-mRNA) instead of a C (G in the pre-mRNA) at nucleotide 840, creating an alternative splice site in SMN2 mRNA that results in exclusion of exon 7 from the majority of transcripts. This results in an unstable form of the SMN protein, which is rapidly degraded. In the disease state, SMN1 produces little or no transcript, and thus virtually no full-length SMN protein is produced (Panel B). The addition of SMN-C3, a small molecule that modifies SMN2 splicing, results in robust levels of full-length SMN (Panel C).
The SMN protein plays an integral role in the spliceosomal assembly and processing of premRNA species in all cells. Studies have also implicated it in the processes of transcription, the cellular stress response, apoptosis, cytoskeletal dynamics, and axonal transport.
Since all patients with spinal muscular atrophy have at least one intact copy of SMN2, a single, targeted small molecule that suppresses alternative splicing of SMN2 mRNA, thus “rescuing” the full-length mRNA and increasing SMN protein levels, has broad therapeutic potential. Unfortunately, the early promise of histone deacetylase inhibitors (e.g., valproic acid) provided by cell cultures derived from patients with spinal muscular atrophy and animal models of spinal muscular atrophy has not been realized.4 Off-target toxic effects present a critical major hurdle for these and many other promising small-molecule therapies.
Naryshkin and colleagues used a human embryonic kidney-cell line containing an SMN2 minigene (a gene fragment containing both regulatory and coding regions of SMN2 that are sufficient to retain select functions of the non-mutated gene) to screen a library of small molecules for chemical classes of compounds that promoted the inclusion of exon 7 into SMN2 mRNA transcripts. They identified three orally available compounds that they designated SMN-C1, SMN-C2, and SMN-C3. They subsequently found that all three compounds modified SMN2 splicing and increased SMN protein biosynthesis in fibroblasts from patients with spinal muscular atrophy type 1, type 2, or type 3 and from controls (asymptomatic persons with a single SMN1 deletion) in a dose-dependent manner. They found a similar effect in cultured motor neuron–like as well as neuronlike and glia-like patient-derived induced pluripotent stem cells. Finally, they characterized the selectivity of these compounds using RNA sequence analysis to compare treated cells with control cells. They identified only 6 genes (out of 11,714) in which transcription was up-regulated or down-regulated by more than a factor of 2, suggesting a high level of specificity. Most important, they found a substantial benefit of these compounds in two different animal models of spinal muscular atrophy, across a variety of outcomes relevant to disease pathogenesis, including improved survival, improved motor function, and preservation of motor-unit circuitry.
Time will tell whether the apparent promise of these and related compounds will be realized for patients with spinal muscular atrophy to the same extent as has been shown in cultured cells and animal models. Emerging data suggest that a radically altered transcriptome precedes motor neuron degeneration and loss5: reversing downstream effects in symptomatic patients will undoubtedly present a considerable therapeutic challenge. The animal data described by Naryshkin et al. and by others underscore the need for early, even presymptomatic, treatment intervention. Nonetheless, small-molecule therapies remain potential tools to modify the transcriptome in a discrete and targeted fashion, and in so doing, ameliorate if not cure some types of disease.
Footnotes
Disclosure forms provided by the author are available with the full text of this article at NEJM.org.
References
- 1.Naryshkin NA, Weetall M, Dakka A, et al. Motor neuron disease. SMN2 splicing modifiers improve motor function and longevity in mice with spinal muscular atrophy. Science. 2014;345:688–93. doi: 10.1126/science.1250127. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Chen K, Sloan SA, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–47. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lah GJ, Li JS, Millard SS. Cell specific alternative splicing of Drosophila Dscam2 is crucial for proper neuronal wiring. Neuron. 2014;83:1376–88. doi: 10.1016/j.neuron.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Riessland M, Ackermann B, Förster A, et al. SAHA ameliorates the SMA phenotype in two mouse models for spinal muscular atrophy. Hum Mol Genet. 2010;19:1492–506. doi: 10.1093/hmg/ddq023. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Z, Pinto AM, Wan L, et al. Dysregulation of synapto-genesis genes antecedes motor neuron pathology in spinal muscular atrophy. Proc Natl Acad Sci U S A. 2013;110:19348–53. doi: 10.1073/pnas.1319280110. [DOI] [PMC free article] [PubMed] [Google Scholar]