Abstract
The stigma attached to HIV is a major public health problem. HIV-associated morbidity, the specter of impending premature mortality, and reduced capacity to reciprocate within networks of mutual aid are key contributors to status loss and the social exclusion of persons with HIV in sub-Saharan Africa. The pooled dataset used in my analysis, which includes 4,314 persons with HIV surveyed in 12 different sub-Saharan African countries, represents the largest study to date of internalized stigma among persons with HIV. My findings indicate that nearly one-fifth of study participants provided survey responses consistent with internalization of stigmatizing beliefs. Furthermore, striking socioeconomic gradients in internalized stigma were observed. A clear implication of my findings is that the adverse health and psychosocial impacts of HIV stigma are likely concentrated among those with the fewest socioeconomic resources for managing and resisting it.
Keywords: social stigma, social discrimination, HIV, Africa South of the Sahara, social class
INTRODUCTION
In many countries throughout sub-Saharan Africa, HIV is highly stigmatized [1]. The reasons driving these stigmatizing attitudes toward persons with HIV likely vary across cultural contexts, but several core instrumental and symbolic motivations have been described [2]. An important driver of HIV-related stigma is the association between HIV infection and disability, economic incapacity, and death [3]. Another important driver of stigma is the way in which HIV has been associated with marginalized groups perceived to be deviant or engaged in behaviors thought to be morally problematic [4]. In the noxious environment of uncharitable and stigmatizing attitudes, persons with HIV may come to accept these perceptions as valid and thereby develop self-defacing beliefs about themselves, a phenomenon commonly referred to as internalized stigma [5].
Internalized stigma is typically assessed through direct survey questions designed to assess the extent to which the study participant reports having negative self-perceptions such as self-hatred, worthlessness, and shame. In the recently published systematic review by Pantelic et al. [6], no single instrument dominated the literature. However, the different instruments identified in that review all followed a similar archetype, including the 9-item negative self-image subscale of the HIV Stigma Scale [7], the 5-item negative self-perception subscale of the HIV/AIDS Stigma Instrument-People Living with HIV/AIDS [8], a 12-item internalized stigma scale [9], or the 6-item Internalized AIDS-Related Stigma Scale [10,11]. The data supporting the reliability and validity of these instruments were all derived from persons with HIV who were interviewed in contexts in which their seropositivity was known to both the study participants as well as to the interviewers (e.g., persons recently diagnosed as HIV-positive either were identified in an HIV-related services venue such as a support group or clinic, or were referred to the study investigators from these venues). Unique to the study by Visser et al. [9] was the administration of scales with parallel wording both to persons with HIV as well as to persons of unknown serostatus living in the general community.
Although several studies have surveyed general population samples throughout sub-Saharan Africa to assess the extent of stigmatizing attitudes towards persons with HIV [12–16], less work has focused on systematically assessing the extent of internalized stigma among persons with HIV. This is an important gap in the literature because of the adverse effects of stigma on health and wellbeing. Consistent with insights derived from social stress theory [17] and modified labeling theory [5,18,19], internalized stigma among persons with HIV has been associated with isolation and depression [20–23], as well as failure to link to care [24]. These findings are consistent with the elevated prevalence of depression in this vulnerable population [25] and are extremely problematic given the well-known relationships between depression, treatment adherence, and poor health outcomes [26–28]. Among persons with HIV engaged in care, stigma has also been described as a major barrier to treatment adherence and retention in care [29,30]. For these reasons, improved understanding about the extent of internalized stigma among persons with HIV could have substantial public health implications.
Conceptual Framework
As argued by Gilmore & Somerville [31], stigma has historically served to reinforce inequalities and power imbalances that existed even prior to the HIV epidemic. Economic status represents one such fault line, particularly in sub-Saharan African settings of generalized poverty where subsistence agriculture is the norm [32] and where social protection schemes are limited [33]. Based on their programmatic experience in rural Haiti, Castro & Farmer [34] described how poverty -- and other large-scale social forces conceptualized under the rubric of structural violence (such as racism, sexism, and political violence) -- “already representing an almost universal stigma, will be the primary reason that poor people living with HIV suffer from greater AIDS-related stigma” (p.55).
Summarizing a diverse body of literature drawn from anthropology, economics, psychology, and sociology, in collaboration with my colleagues I recently elaborated a conceptual model of HIV stigma describing how several interrelated factors are key contributors to status loss and social exclusion in sub-Saharan Africa: HIV-associated morbidity, the specter of impending premature mortality, and reduced capacity to reciprocate within networks of mutual aid [3]. For example, in one qualitative study, a man from a community sample in Zimbabwe described how persons with HIV are perceived as a drain on their communities: “Right now those who are infected are not treated as fellow human beings. They are already declared dead, and regarded as useless as a grave… They mean that these people are no longer able to do anything useful. They say they are just waiting for the day of their death” (p.2275) [16]. These empirical observations are consistent with functions of stigma as described by evolutionary psychologists [35,36] and the instrumental vs. altruistic distinctions raised by social capital theorists [37] -- as well as with the modified labeling perspective, which theorizes that when persons with a stigma (such as HIV or mental illness) internalize the expectations and assumptions imposed on them by the majority, the label becomes a part of their identity and behavior [5,18,19]. Persons who are spoiled with the label of HIV infection are treated differently on this basis -- or they may preemptively anticipate the differential treatment and adopt defensive, isolating, and potentially maladaptive responses that undermine their life chances. While there may be other drivers of stigma, such as the association between HIV and promiscuity, much of the recent qualitative literature dealing with HIV in sub-Saharan Africa has identified the above factors as the main source of its stigma in this particular context [3,38].
In our conceptual paper, we provided further triangulating evidence on importance of these factors in explaining the stigma of HIV by reviewing literature about the psychosocial impacts of HIV treatment [3]. Numerous studies have shown that the increasing availability of HIV treatment reduces stigmatizing attitudes in the general population [15,39–41]. Among persons with HIV, improved health and economic productivity directly resulting from HIV treatment has been shown to reduce internalized stigma and improve psychological wellbeing [42–47]. Taken together, these findings provide robust empirical support for the conceptual model of HIV stigma and predict that socioeconomic gradients in internalized stigma will be observed among persons with HIV. To formally test this hypothesis, I analyzed nationally representative data from Demographic and Health Surveys (DHS) conducted in 12 different sub-Saharan African countries. My study had two primary aims: (1) to estimate the prevalence of internalized stigma among persons with HIV who were aware of their seropositivity; and (2) to assess the extent to which socioeconomic gradients in internalized stigma were observed.
METHODS
Data Source
The data for this analysis were drawn from the DHS. The DHS are publicly available, population-level surveys implemented by host country governments with funding and technical assistance from ICF Macro and the U.S. Agency for International Development. Each survey employed a multistage stratified design with probabilistic sampling, with each household having an equal probability of selection, and was designed to be nationally representative of all women of reproductive age (i.e., 15–49 years). The men’s questionnaire was briefer than the women’s questionnaire and was administered to a smaller sample of men in each country, with a wider range of age eligibility (i.e., 15–59 years for most surveys).
In 2001, ORC Macro introduced population-based HIV testing in selected DHS, generally using dried blood spot samples of capillary blood collected by finger prick. Details of specimen collection, laboratory testing, and quality assurance have been published previously [48]. Importantly, at the time of consent, participants were informed that they would not be provided with access to their test results. Specimen data were anonymized to ensure confidentiality and were transported to a central laboratory for HIV testing. This meant that HIV test results conducted as part of the DHS could not have influenced the survey responses, and that only study participants who had previously obtained an HIV test (on their own) could potentially have been aware of their serostatus at the time of the survey. The extent to which such a study participant could have accurately inferred his or her HIV serostatus at the time of the survey would have depended on the timing of the prior HIV test in relation to any potential exposures and in relation to the survey itself (Figure 1).
Figure 1. Possible chronologies of HIV infection, HIV testing, and survey administration in the Demographic and Health Surveys.
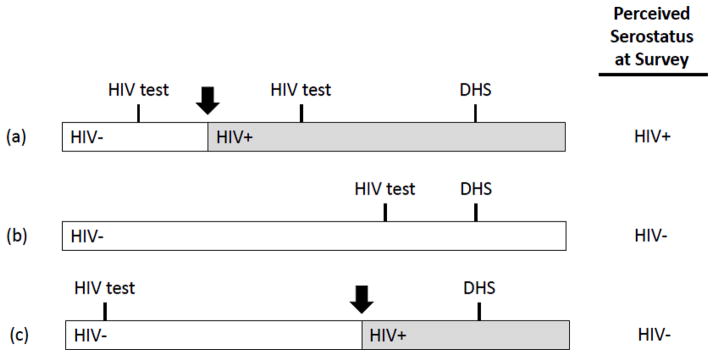
(a) The participant tests negative for HIV, then acquires HIV (black arrow) and tests positive for HIV prior to the survey. At the time of the survey, this participant accurately perceives himself to be HIV-positive. (b) The participant tests negative for HIV and remains HIV-negative. At the time of the survey, this participant accurately perceives himself to be HIV-negative. (c) The participant tests negative for HIV once in the past, then acquires HIV but does not have another HIV test prior to the survey. At the time of the survey, the participant inaccurately perceives himself to be HIV-negative.
Because direct questions about a participant’s awareness of his or her HIV serostatus (e.g., “Are you HIV positive?”) were not included in the DHS, I used data obtained from several different sources to identify persons who had previously tested positive for HIV and who were aware of their seropositivity at the time of the survey. Study participants had to meet the following four conditions in order to be classified as “HIV-positive and aware of their seropositivity”:
A positive HIV test obtained during the DHS
An affirmative response to the question, “I don’t know want to know the results, but have you ever been tested to see if you have HIV?”
A lag of 12 months or less, given in response to the question, “How many months ago was your most recent HIV test?”
An affirmative response to the question, “I don’t want to know the results, but did you get the results of the test?”
Mishra et al. [49] employed a similar algorithm to identify persons with HIV who were aware of their seropositivity but employed a broader testing window. As described below, I conducted a sensitivity analysis using a narrower time window to confirm the robustness of my findings.
Measures
The primary outcome of interest was internalized stigma. Because the DHS do not contain direct questions about awareness of seropositivity or negative self-perceptions, internalized stigma must be inferred from participants’ responses to the three questions about HIV-stigmatizing attitudes that were included in the DHS: “Would you buy fresh vegetables from a shopkeeper or vendor if you knew that this person had HIV?” “If a member of your family became sick with AIDS, would you be willing to care for her or him in your own household?” “In your opinion, if a female teacher has HIV but is not sick, should she be allowed to continue teaching in the school?” (These questions were administered only to persons who responded affirmatively to the question, “Have you ever heard of an illness called AIDS?”) Among persons who did not know their serostatus or who knew that they were HIV-negative -- which comprised the majority of respondents in the DHS surveys -- negative responses to these questions were interpreted as reflecting a desire for social distance from persons with HIV (motivated often, but not always, by distaste for the symbolic meaning of HIV in a given cultural context or by instrumental concerns about casual transmission [2]). Consequently these questions, which have been proposed as core indicators for monitoring the HIV epidemic by the Joint United Nations Programme on HIV/AIDS [50], are also described in the DHS as measuring “accepting attitudes” towards persons with HIV. Among persons with HIV who were aware of their seropositivity, however, negative responses to these same exact questions were interpreted as reflecting self-defacing beliefs and perceptions about the self.
This manner of operationalizing internalized stigma -- whereby responses to the same survey question are employed to measure conceptually different constructs depending on one’s serostatus -- is consistent with the succinct description offered by Burris [51], who wrote that stigma exerts its adverse effects by “triggering disgust in the normal and shame in the stigmatized” (p.474). It is also consistent with the approach used by Visser et al. [9], who fielded nearly identical questions (but phrased slightly differently) to persons with HIV as well as to persons in the general community, with the aim of developing parallel scales to measure HIV-related stigma that could be compared across both samples. For example, a community member might be asked to respond to the item, “I think less of someone because they have HIV,” while a person with HIV might be administered the item, “I think less of myself because I have HIV.”
In the context of the DHS questions, an HIV-negative person may refuse to purchase goods from an HIV-positive vendor, with a negative response to the question betraying his or her disgust of persons with HIV. In contrast, an HIV-positive person may also refuse to purchase goods from an HIV-positive vendor, with a negative response to the question instead betraying a sense of shame or self-hatred. Based on the responses to these three questions, an omnibus binary variable for internalized stigma was created, with a value equal to 1 if the participant responded negatively to any of the three questions. If a study participant provided a “don’t know” response to all of these questions, the value of the variable was set to missing and the observation was excluded from the complete-case analysis.
Statistical Analysis
I selected all recent DHS that were administered in continental sub-Saharan Africa and for which HIV testing data were available. Twenty-three DHS conducted between 2003 and 2012 met these criteria. Details on staff training, pretesting, and other survey procedures are detailed in the DHS final reports for each country, available from the ICF Macro web site at http://www.measuredhs.com. I excluded 11 DHS in which there were (as determined using the method described above) fewer than 100 persons with HIV who were aware of their seropositivity: Burkina Faso (2010) (N=24), Burundi (2010) (N=59), the Democratic Republic of the Congo (2007) (N=9), Ghana (2008) (N=9), Guinea (2005) (N=2), Côte d’Ivoire (2011–12) (N=66), Liberia (2007) (N=10), Mali (2006) (N=4), Niger (2006) (N=3), Senegal (2010–11) (N=18), and Sierra Leone (2008) (N=7). Therefore 12 DHS were used in the pooled analyses: Cameroon (2011), Ethiopia (2011), Gabon (2012), Kenya (2008), Lesotho (2009), Malawi (2010), Rwanda (2010), Swaziland (2006–07), Tanzania (2011–12), Uganda (2011), Zambia (2007), and Zimbabwe (2010–11). Appendix A provides greater detail on characteristics of these DHS, including sample sizes, rates of survey response and HIV testing, and population-level estimates of acceptance of persons with HIV. All point estimates and variance estimates were obtained by using the survey weights and clustering variables provided by ICF Macro.
Appendix A.
Demographic and Health Surveys included in this analysis †
| Country | Year | Women
|
Men
|
HIV Prevalence | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Survey Response Rate | Number of Interviews | HIV Testing Rate | Has Heard of HIV | Accepts Persons with HIV | Survey Response Rate | Number of Interviews | HIV Testing Rate | Has Heard of HIV | Accepts Persons with HIV | |||
| Cameroon | 2011 | 97 | 15,426 | 94 | 96 | 12 | 96 | 7,191 | 92 | 98 | 18 | 4.3 |
| Ethiopia | 2011 | 95 | 16,515 | 89 | 97 | 17 | 89 | 14,110 | 82 | 99 | 18 | 1.5 |
| Gabon | 2012 | 98 | 8,422 | 96 | 99 | 21 | 96 | 5,654 | 94 | 100 | 33 | 4.1 |
| Kenya | 2008 | 96 | 8,444 | 86 | 99 | 33 | 89 | 3,465 | 79 | 100 | 48 | 6.3 |
| Lesotho | 2009 | 98 | 7,624 | 94 | 97 | 24 | 95 | 3,317 | 88 | 95 | 20 | 23.0 |
| Malawi | 2010 | 97 | 23,020 | 91 | 99 | 20 | 92 | 7,175 | 84 | 99 | 36 | 10.6 |
| Rwanda | 2010 | 99 | 13,671 | 99 | 100 | 53 | 99 | 6,329 | 98 | 100 | 64 | 3.0 |
| Swaziland | 2006–07 | 94 | 4,987 | 87 | 100 | 43 | 89 | 4,156 | 78 | 99 | 47 | 25.9 |
| Tanzania | 2011–12 | 96 | 10,967 | 90 | 99 | 25 | 89 | 8,352 | 79 | 100 | 40 | 5.1 |
| Uganda | 2011 | 98 | 12,153 | 97 | 99 | 20 | 96 | 9,588 | 94 | 98 | 31 | 7.3 |
| Zambia | 2007 | 97 | 7,146 | 77 | 99 | 26 | 91 | 6,500 | 72 | 99 | 33 | 14.3 |
| Zimbabwe | 2010–11 | 93 | 9,171 | 80 | 98 | 17 | 86 | 7,480 | 69 | 98 | 11 | 15.2 |
All estimates obtained from ICF Macro country reports
As described above, persons with HIV who were aware of their seropositivity were the focus of my analysis. Importantly, these participants were inferred to be aware of their seropositivity at the time of the survey because they had recently obtained the results of an HIV test on their own prior to the survey. This assumption cannot be directly tested, as data on self-reported HIV status were not obtained in the DHS. As an indirect test of this assumption, I estimated the weighted proportion of participants in each of the four categories of serostatus and awareness (i.e., HIV-positive and aware, HIV-positive and unaware, HIV-negative and aware, HIV-negative and unaware) who endorsed at least one of the stigmatizing attitudes described above. My expectation was that such an analysis would demonstrate a different response pattern among persons with HIV who were aware of their seropositivity compared to participants in the other three categories.
Next, focusing on persons with HIV who were aware of their seropositivity, I fitted three sets of regression models to the pooled data, specifying internalized stigma as the binary dependent variable. Three variables related to socioeconomic status were the primary explanatory variables of interest: educational attainment, professional occupation (defined as being employed in a professional, clerical, or sales capacity [52]), and household asset wealth [53]. These estimates were adjusted for potential confounding by the following variables: sex, age, marital status, household headship, recent sexual activity (in the 4 weeks prior to the survey), and HIV-related knowledge. HIV-related knowledge was assessed with a series of eight questions about different aspects of HIV prevention and/or transmission, such as “Can people reduce their chance of getting HIV by using a condom every time they have sex?” and “Is it possible for a healthy-looking person to have HIV?” An HIV knowledge score was defined as the total sum of correct responses (out of eight maximum). Because the pooled estimates could potentially mask heterogeneity between countries, I also estimated country-specific socioeconomic gradients in stigma. I assumed a Poisson distribution for the binary dependent variables. The exponentiated regression coefficients were interpreted as risk ratios [54,55]. For ease of exposition, the risk ratios were converted to average marginal effects [56], which provide direct representation of how changes in the explanatory variables affect the probability of the outcome.
Sensitivity Analyses
To assess the robustness of my findings, I conducted several sensitivity analyses. First, as discussed above, the extent to which an HIV-positive study participant may accurately perceive his or her seropositivity at the time of the survey may depend on the timing of his or her prior HIV test. The 12-month window used in my analysis to identify persons with HIV aware of their seropositivity may inadvertently classify some HIV-positive study participants as being unaware of their seropositivity if any negative HIV test(s) obtained prior to the study were remote enough in time to have been obtained prior to HIV acquisition [exemplified in Figure 1(c)]. By permitting HIV-positive study participants unaware of their seropositivity to be included in the analytic sample (along with the HIV-positive study participants aware of their seropositivity, who are the intended focus of the analysis) this would likely inflate the sample size and reduce the estimated prevalence of internalized stigma. To reduce this possibility, I employed an alternate definition in which HIV-positive study participants needed to have had obtained an HIV test within six months prior to the survey in order to be considered aware of their seropositivity. All regression models were then re-fitted to the data. Second, my decision to remove from the complete-case analysis any “don’t know” and missing responses to the stigma questions could have resulted in biased estimates. To address this possibility, I constructed alternate measures by imputing missing outcomes as “yes” or “no,” respectively, and then refitted the pooled regression models to the data.
Ethical Review
The data collection procedures for the DHS were approved by the ICF Macro institutional review board as well as by the relevant ethical review boards in the host country for each survey. Participants provided oral informed consent separately for the survey and for the HIV test. The specific analysis described in this article was reviewed by the Partners Human Research Committee and was considered exempt from full review.
RESULTS
Across all 12 DHS included in this analysis, there were a total of 167,002 study participants who had heard of HIV and who consented to HIV testing: 108,416 participants who tested negative for HIV and who had not obtained the results of an HIV test on their own during the 12 months prior to the survey (“HIV-negative and unaware”); 9,401 participants who tested positive for HIV and who had no prior test (“HIV-positive and unaware”); 41,953 participants who tested negative for HIV and who had a prior test (“HIV-negative and aware”); and 4,314 participants who tested positive for HIV and who had a prior test (“HIV-positive and aware”). Participants categorized as HIV-positive and aware of their seropositivity were, as expected, much less likely to endorse one or more HIV-stigmatizing attitudes: among those who were HIV-negative and unaware, 42.7 percent (95% confidence interval [CI], 42.1–43.3); HIV-positive and unaware, 26.2 percent (95% CI, 25.1–27.3); HIV-negative and aware, 29.9 percent (95% CI, 29.1–30.7); and HIV-positive and aware, 18.6 percent (95% CI, 17.2–20.0).
Study participants categorized as “HIV-positive and aware of their seropositivity” formed the analytic sample for this analysis (weighted prevalence, 2.6 percent; 95% CI, 2.5–2.7). Their respective country population prevalence rates ranged from 0.6 percent in Ethiopia to 9.5 percent in Lesotho (Appendix B). Summary characteristics for the pooled sample are shown in Table 1.
Appendix B.
Proportion of DHS participants who tested positive for HIV and were aware of their seropositivity, among all who were tested for HIV, by country (N=167,002)
| Sample Size | HIV-Positive and Aware of Seropositivity †
|
||
|---|---|---|---|
| Number of Cases | Weighted Proportion | ||
| Cameroon | 13,844 | 185 | 0.013 (0.011–0.016) |
| Ethiopia | 27,819 | 205 | 0.006 (0.004–0.008) |
| Gabon | 10,819 | 191 | 0.017 (0.013–0.021) |
| Kenya | 6,867 | 182 | 0.024 (0.018–0.031) |
| Lesotho | 6,542 | 603 | 0.095 (0.087–0.104) |
| Malawi | 13,834 | 159 | 0.013 (0.010–0.017) |
| Rwanda | 13,243 | 188 | 0.014 (0.012–0.017) |
| Swaziland | 8,151 | 499 | 0.060 (0.054–0.067) |
| Tanzania | 17,656 | 341 | 0.023 (0.020–0.026) |
| Uganda | 21,130 | 554 | 0.027 (0.025–0.030) |
| Zambia | 10,813 | 327 | 0.027 (0.024–0.032) |
| Zimbabwe | 16,284 | 880 | 0.060 (0.055–0.065) |
Tested positive for HIV through the DHS and had obtained the results of an HIV test on their own during the 12 months prior to the survey
Table 1.
Summary statistics for the pooled sample of persons with HIV who were aware of their seropositivity (N=4,314)
| Variable | Number of Cases | Weighted Mean or Proportion (95% CI) |
|---|---|---|
| Internalized stigma | 855 | 0.19 (0.17–0.20) |
| Formal education | ||
| None | 339 | 0.07 (0.06–0.08) |
| Primary | 2,030 | 0.47 (0.45–0.49) |
| Secondary | 1,706 | 0.40 (0.38–0.42) |
| Higher | 239 | 0.06 (0.05–0.07) |
| Professional occupation | 918 | 0.21 (0.19–0.22) |
| Household asset wealth | ||
| Poorest | 647 | 0.13 (0.12–0.14) |
| Poorer | 722 | 0.15 (0.14–0.17) |
| Middle | 741 | 0.17 (0.16–0.19) |
| Less Poor | 1,003 | 0.24 (0.23–0.26) |
| Least Poor | 1,201 | 0.30 (0.28–0.32) |
| Female | 2,859 | 0.64 (0.62–0.66) |
| Age, years | 4,314 | 33.5 (33.2–33.8) |
| Married or Cohabiting | 2,606 | 0.61 (0.60–0.63) |
| Head of household | 2,027 | 0.49 (0.47–0.51) |
| Sexually active | 2,224 | 0.53 (0.51–0.54) |
| HIV knowledge score (out of 8 maximum) | 4,314 | 6.89 (6.84–6.93) |
CI, confidence interval
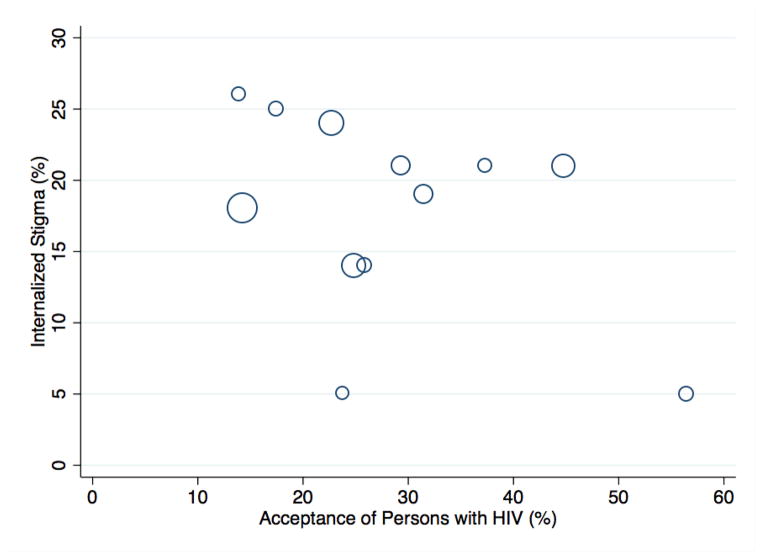
The pooled prevalence of internalized stigma was 18.6 percent (95% CI, 17.2–20.0). The country-level prevalence of internalized stigma ranged from 5.5 percent in Malawi to 26.2 percent in Cameroon (Appendix C). Across the 12 countries, there was an inverse correlation between internalized stigma and the percentage of persons in the general population who expressed accepting attitudes towards persons with HIV, but the correlation was not statistically significant (Spearman’s rho = −0.39; 95% CI, −0.79 to 0.24) (Figure 2).
Appendix C.
Prevalence of internalized stigma, among people with HIV who were aware of their seropositivity, by country (N=4,314)
| Sample Size | Internalized Stigma †
|
||
|---|---|---|---|
| Number of Cases | Weighted Proportion | ||
| Central Africa | |||
| Cameroon | 185 | 47 | 0.26 (0.20–0.34) |
| Gabon | 191 | 46 | 0.14 (0.10–0.20) |
| Eastern Africa | |||
| Ethiopia | 205 | 49 | 0.25 (0.15–0.39) |
| Kenya | 182 | 37 | 0.21 (0.14–0.30) |
| Rwanda | 188 | 10 | 0.05 (0.03–0.10) |
| Uganda | 554 | 80 | 0.14 (0.11–0.17) |
| Southern Africa | |||
| Lesotho | 603 | 160 | 0.24 (0.20–0.28) |
| Malawi | 159 | 11 | 0.05 (0.03–0.12) |
| Swaziland | 499 | 104 | 0.21 (0.18–0.26) |
| Tanzania | 341 | 72 | 0.19 (0.14–0.25) |
| Zambia | 327 | 70 | 0.21 (0.16–0.27) |
| Zimbabwe | 880 | 169 | 0.18 (0.16–0.21) |
Provided a negative response to any of the three questions about attitudes towards persons with HIV
Figure 2. Cross-country correlation between acceptance of persons with HIV in the general population vs. internalized stigma among persons with HIV (N=12).
The x-axis denotes the percentage of persons in that country who provided survey responses consistent with accepting attitudes towards persons with HIV. The y-axis denotes the percentage of persons with HIV in that country who provided survey responses consistent with internalized stigma. Circle sizes are directly proportional to the sample size of persons with HIV in that country.
Unadjusted and multivariable-adjusted correlates of internalized stigma are presented in Table 2. Gradients in internalized stigma were observed for all three measures of socioeconomic status: namely, higher socioeconomic status was associated with lower internalized stigma. Expressed in terms of average marginal effects, the estimated associations were large in magnitude. For example, compared to persons in the poorest quintile of asset wealth, persons in the highest quintile had a 19.9 percentage point (95% CI, 14.2–25.5) lower probability of reporting internalized stigma.
Table 2.
Socioeconomic gradients in internalized stigma, among persons with HIV who were aware of their seropositivity (N=4,314)
| Internalized Stigma, ARR (95% CI)
|
||
|---|---|---|
| Unadjusted | Adjusted for Covariates † | |
| Formal education | ||
| None | Ref | Ref |
| Primary | 0.70 (0.56–0.86) *** | 0.82 (0.66–1.01) |
| Secondary | 0.46 (0.36–0.57) *** | 0.61 (0.48–0.78) *** |
| Higher | 0.24 (0.12–0.47) *** | 0.36 (0.18–0.71) ** |
| Occupation | ||
| Other | Ref | Ref |
| Professional | 0.73 (0.59–0.91) ** | 0.79 (0.64–0.97) * |
| Quintiles of household asset wealth | ||
| Poorest | Ref | Ref |
| Poorer | 0.84 (0.68–1.03) | 0.91 (0.74–1.11) |
| Middle | 0.68 (0.55–0.84) *** | 0.76 (0.62–0.94) * |
| Less Poor | 0.56 (0.45–0.69) *** | 0.66 (0.53–0.81) *** |
| Least Poor | 0.33 (0.25–0.42) *** | 0.41 (0.32–0.53) *** |
ARR, adjusted risk ratio; CI, confidence interval
, **, and *** indicate statistical significance at the level of P<0.05, P<0.01, and P<0.001, respectively
Adjusted for sex, age, marital status, household headship, recent sexual activity, and HIV knowledge score
The sensitivity analyses did not yield substantive changes in these findings. When the estimation sample was restricted to persons with HIV who had obtained the results of an HIV test during the six months prior to the survey, the sample size was reduced to 3,514 persons and the same educational, occupational, and economic gradients in internalized stigma were observed (Appendix D). Fewer than four percent of responses to the individual stigma questions consisted of missing or “don’t know” responses. Alternately coding these responses as “yes” or “no” yielded no substantive changes in the estimated gradients (data not shown).
Appendix D.
Socioeconomic gradients in internalized stigma, among persons with HIV who were aware of their seropositivity, using a six-month HIV testing window (N=3,514)
| Internalized Stigma, ARR (95% CI)
|
||
|---|---|---|
| Unadjusted | Adjusted for Covariates † | |
| Formal education | ||
| None | Ref | Ref |
| Primary | 0.70 (0.56–0.88) ** | 0.82 (0.64–1.06) |
| Secondary | 0.48 (0.37–0.61) *** | 0.67 (0.50–0.88) ** |
| Higher | 0.28 (0.14–0.57) *** | 0.44 (0.21–0.91) * |
| Occupation | ||
| Other | Ref | Ref |
| Professional | 0.78 (0.63–0.98) * | 0.84 (0.67–1.05) |
| Quintiles of household asset wealth | ||
| Poorest | Ref | Ref |
| Poorer | 0.82 (0.65–1.04) | 0.88 (0.70–1.11) |
| Middle | 0.66 (0.52–0.83) *** | 0.73 (0.58–0.92) ** |
| Less Poor | 0.52 (0.41–0.66) *** | 0.61 (0.48–0.78) *** |
| Least Poor | 0.34 (0.26–0.45) *** | 0.43 (0.32–0.56) *** |
ARR, adjusted risk ratio; CI, confidence interval
, **, and *** indicate statistical significance at the level of P<0.05, P<0.01, and P<0.001, respectively
Adjusted for sex, age, marital status, household headship, recent sexual activity, and HIV knowledge score
Due to the much smaller sample sizes in the country-specific regression models, the associations with the socioeconomic explanatory variables of interest were less precisely estimated. In the country-specific analyses, educational and economic -- but not occupational -- gradients in internalized stigma were consistently observed. Of the 14 adjusted relative risks for the secondary and higher education categories, 12 estimates were less than one; and the estimates for higher education were generally greater in magnitude than the estimates for secondary education (Appendix E). Similarly, of the 22 adjusted relative risks for the uppermost household asset wealth quintiles, 20 estimates were less than one; and the estimates for less poor quintiles were greater in magnitude compared to the estimates for poorer quintiles (Appendix F).
Appendix E.
Educational and occupational gradients in internalized stigma, among persons with HIV who were aware of their seropositivity, by country (N=4,314)†
| Educational Attainment, ARR (95% CI) ‡
|
Professional Occupation, ARR (95% CI)¶ | |||
|---|---|---|---|---|
| Primary | Secondary | Higher | ||
| Central Africa | ||||
| Cameroon | § | 1.24 (0.67–2.28) | ||
| Gabon | 1.40 (0.51–3.88) | 0.51 (0.17–1.53) | || | 1.32 (0.62–2.82) |
| Eastern Africa | ||||
| Ethiopia | 0.26 (0.11–0.61) ** | 0.26 (0.05–1.45) | 0.01 (0.00–0.13) *** | 1.44 (0.64–3.25) |
| Kenya | 4.06 (0.74–22.2) | 2.58 (0.34–19.5) | 3.97 (0.45–35.2) | 0.64 (0.23–1.75) |
| Rwanda | § | 1.61 (0.38–6.76) | ||
| Uganda | 1.04 (0.55–1.97) | 0.62 (0.25–1.53) | 0.34 (0.04–2.87) | 0.61 (0.31–1.22) |
| Southern Africa | ||||
| Lesotho | 0.57 (0.38–0.86) ** | 0.52 (0.31–0.87) * | 0.49 (0.16–1.47) | 0.94 (0.58–1.52) |
| Malawi | § | 0.24 (0.03–2.13) | ||
| Swaziland | 0.89 (0.56–1.41) | 0.52 (0.31–0.89) * | 0.43 (0.14–1.31) | 0.68 (0.38–1.22) |
| Tanzania | 1.22 (0.55–2.73) | 0.44 (0.12–1.62) | || | 0.97 (0.30–3.18) |
| Zambia | 0.70 (0.33–1.50) | 0.22 (0.09–0.52) *** | || | 0.94 (0.58–1.54) |
| Zimbabwe | 0.93 (0.45–1.91) | 0.72 (0.35–1.49) | || | 0.34 (0.19–0.61) *** |
ARR, adjusted risk ratio; CI, confidence interval
, **, and *** indicate statistical significance at the level of P<0.05, P<0.01, and P<0.001, respectively
All estimates adjusted for sex, age, marital status, household headship, recent sexual activity, and HIV knowledge score
No formal education is the reference category
Any occupation other than professional status (professional, clerical, or sales) is the reference category
Could not be estimated
Secondary and higher education categories collapsed into a single category to enable estimation
Appendix F.
Economic gradients in internalized stigma, among persons with HIV who were aware of their seropositivity, by country (N=4,314) †
| Household Asset Wealth, ARR (95% CI) ‡
|
||||
|---|---|---|---|---|
| Poorer | Middle | Less Poor | Least Poor | |
| Central Africa | ||||
| Cameroon | 0.39 (0.21–0.74) ** | 0.24 (0.11–0.51) *** | 0.33 (0.17–0.62) *** | 0.40 (0.20–0.76) ** |
| Gabon | 0.71 (0.37–1.35) | 0.40 (0.15–1.08) | 0.16 (0.05–0.50) ** | ¶ |
| Eastern Africa | ||||
| Ethiopia | 3.09 (0.73–13.1) | 1.71 (0.39–7.48) | 1.11 (0.28–4.32) | 0.41 (0.09–1.89) |
| Kenya | 0.73 (0.27–1.96) | 0.22 (0.06–0.80) * | 0.61 (0.16–2.33) | 0.16 (0.04–0.63) ** |
| Rwanda | 2.27 (0.68–7.62) | 0.46 (0.03–8.12) | 0.44 (0.06–3.02) | ¶ |
| Uganda | 0.94 (0.48–1.82) | 0.84 (0.43–1.62) | 0.74 (0.43–1.29) | 0.37 (0.18–0.77) ** |
| Southern Africa | ||||
| Lesotho | 0.85 (0.56–1.29) | 0.74 (0.51–1.08) | 0.46 (0.27–0.76) ** | 0.71 (0.45–1.12) |
| Malawi | 1.79 (0.28–11.5) | 0.53 (0.06–4.73) | 0.09 (0.01–1.29) | 0.84 (0.08–2.19) |
| Swaziland | 1.26 (0.66–2.44) | 1.71 (0.97–3.03) | 1.52 (0.86–2.67) | 0.79 (0.39–1.62) |
| Tanzania | 1.04 (0.57–1.90) | 0.67 (0.31–1.44) | 0.51 (0.24–1.09) | 0.36 (0.15–0.88) * |
| Zambia | 0.83 (0.42–1.58) | 0.73 (0.39–1.35) | 0.63 (0.33–1.19) | 0.23 (0.08–0.65) ** |
| Zimbabwe | 0.68 (0.44–1.05) | 0.80 (0.51–1.25) | 0.71 (0.48–1.05) | 0.24 (0.11–0.51) *** |
ARR, adjusted risk ratio; CI, confidence interval
, **, and *** indicate statistical significance at the level of P<0.05, P<0.01, and P<0.001, respectively
All estimates adjusted for sex, age, marital status, household headship, recent sexual activity, and HIV knowledge score
Poorest household asset wealth quintile is the reference category
Less poor and least poor household asset wealth quintiles collapsed into a single category to enable estimation
DISCUSSION
In this cross-sectional analysis of population-based data collected in 12 different sub-Saharan African countries, I found that internalized stigma was highly prevalent among persons with HIV who were aware of their seropositivity. Nearly one-fifth of persons with HIV provided survey responses consistent with internalized shame or self-hatred. Except for Malawi and Rwanda, where the prevalence of internalized stigma was 5 percent, the prevalence of internalized stigma was largely consistent across countries (ranging from 14–26 percent). With the caveat that drawing valid comparisons across studies can be difficult given differences in study design and implementation, these estimates are comparable to, or potentially slightly lower than, those based on studies conducted in sub-Saharan Africa prior to HIV treatment scale-up. Between one-tenth and one-half of HIV-positive participants in studies conducted in 2003–2006 responded affirmatively to statements like “I am ashamed that I am HIV positive” and “I sometimes feel worthless because I am HIV positive” [8,21,57]. Thus, it does not appear that internalized stigma has declined appreciably among persons with HIV in most countries in sub-Saharan Africa. This is an important public health issue given the relatively flat trend in CD4 count at treatment initiation observed in sub-Saharan Africa over the past decade [58].
One potential reason for the stubbornly high rate of internalized stigma among persons with HIV in sub-Saharan Africa may be that HIV-stigmatizing attitudes remain widely held in the general population. At the country level, there was an inverse, but not statistically significant, correlation between HIV-stigmatizing attitudes among persons in the general population and internalized stigma among persons with HIV. As described in the ICF Macro reports summarizing findings from the DHS for each country, only a minority of persons in the general population expressed accepting attitudes towards persons with HIV. A comparison of these estimates with large-sample studies conducted a decade earlier in Botswana and South Africa suggest comparable levels of social distancing and high levels of support for coercive measures [13,40,59–62]. Most recently, Chan et al. [63] showed that internalized stigma among persons with HIV has increased in rural Uganda, in the context of concomitant increases in anticipated stigma in the general population. Thus, although the scale-up of HIV treatment appears to have lessened fears about HIV [3,15,39,40] and there is some evidence that stigmatizing attitudes have even declined in some countries [64], my estimates suggest that persons with HIV have yet to feel widespread acceptance in sub-Saharan Africa.
A second important finding of my analysis is that gradients in internalized stigma were observed along the usual fault lines of educational attainment, occupational status, and household wealth. When disaggregated by country, these gradients were less precisely estimated but were most consistently observed for household wealth: persons with HIV from the poorest households were at more than twice the risk of internalized stigma compared to persons with HIV from the least poor households. These socioeconomic gradients are consistent with those described in the literature on smoking, stigma, and social class [65,66]. In a qualitative study of 40 British smokers and non-smokers, Farrimond & Joffe [67] showed that smokers of lower occupational status were more likely to endorse the negative aesthetic associated with smoking and to accept the labels of their marginalized social status, while smokers of higher occupational status were better able to resist stigmatization and were more likely to distance themselves from these negative aspects of their group identity. These gradients should be of concern to public health advocates because the adverse psychosocial and health impacts of HIV stigma are well known [20–22,29,30,68–72]. A clear implication of my findings is that the adverse impacts of HIV stigma are likely concentrated among those with the fewest socioeconomic resources for managing and resisting it.
Concern about these gradients might arguably be tempered by the fact that the prevalence of HIV in sub-Saharan Africa is known to be higher among wealthier persons in the general population [49,73,74,75]. However, emerging data suggest that this counterintuitive association is changing over time. Hargreaves and colleagues [76–78] have theorized that current trends in new HIV infections may be following the predictions of Julian Tudor-Hart’s “inverse care law” [79]. Restated more recently as the “inverse equity hypothesis” [80] and the “inequality paradox” [81], the inverse care law suggests that large-scale HIV prevention programs could paradoxically reinforce social inequalities in HIV infection through selective advantage to persons who are most able to respond to them [82–84] -- namely, the wealthier and more educated.
Limitations
Interpretation of my findings is subject to three important limitations. First, only participants who consented to HIV testing were included in the analyses. The rates of HIV test refusal ranged from as low as 1 percent among women in Rwanda to more than 30 percent among men in Zimbabwe. Persons who did not provide consent for HIV testing were wealthier and more educated [85–87]. HIV stigma is also a well known barrier to uptake of HIV testing in general [88–90]. Furthermore, longitudinal data from the Malawi Diffusion and Ideational Change Project (MDICP) showed that persons who tested positive for HIV and received their results were less likely to consent to repeat testing in subsequent waves of the survey [91], and failure to adjust for selection bias has been shown to yield underestimates of national HIV prevalence [87,92]. These various lines of evidence suggest that my analytic sample was likely comprised of less wealthy, less educated, and less stigmatized persons. These factors would have therefore biased the estimated prevalence of internalized stigma towards zero and would have also biased the estimated socioeconomic gradient in internalized stigma towards the null.
Second, awareness of seropositivity was inferred but not directly measured. Thus, my sample could have included participants who (contrary to fact) did not perceive themselves to be HIV-positive, either because they had obtained a negative HIV test prior to acquiring HIV sometime before participating in the DHS or because they had obtained a positive HIV test but simply disbelieved it. The sensitivity analysis presented earlier in this article suggests that the former is unlikely to be a major concern, as narrowing the testing window from 12 months to six months did not appreciably shift the estimated socioeconomic gradients. The latter cannot be directly tested but is not entirely implausible. In South Africa, Katz et al. [93] documented a surprisingly high rate of treatment refusals among persons with HIV who were eligible for treatment, even after two months of continued counseling; and the most common reason given for refusing treatment was “feeling healthy.” Among study participants in the MDICP who tested positive for HIV and received their results, when resurveyed two years later and asked to estimate their HIV risk nearly one-half reported that they thought they had a zero probability of being infected with HIV [94].
To address these concerns, in my initial analyses I demonstrated that the sample is unlikely to be comprised of such denialists: study participants categorized as “HIV-positive and aware of their seropositivity” were much less likely to endorse HIV-stigmatizing attitudes compared to other participants. While this is not a direct test of validity, such a strategy has been employed in other contexts to demonstrate different patterns of attitudes and behaviors among “known positives” and other study participants (tested positive but unaware, tested negative but aware/unaware), including studies of people testing for HIV [95] and Huntington’s disease [96]. Even if my analysis is not accepted as valid, however, it is still possible to anticipate the direction of the bias. HIV-positive persons who falsely believed themselves to be cured of HIV would have been less educated, and their responses to the stigma questions would have been more similar to those of HIV-negative persons who were aware of their seronegativity. Their inclusion in the sample likely would have biased the estimated prevalence of internalized stigma towards zero and would have also biased the estimated socioeconomic gradient in internalized stigma towards the null.
A third limitation is that my measure of internalized stigma did not rely on the conventional method of asking the participant to directly attribute his or her negative feelings of shame or guilt to HIV infection, e.g., “Tell me if you agree or disagree with this statement: ‘Being HIV positive makes me feel dirty’” [10]. Instead, internalized stigma was indirectly inferred based on the participant’s response and seropositivity. The primary advantage of this approach is that the interviewer is able to inquire about an extremely sensitive topic without directly personalizing them or requiring the respondent to explicitly acknowledge his or her seropositivity. It is also consistent with previously validated methods to generate parallel scales for measuring HIV-related stigma among persons with HIV as well as among persons in the general community [9]. However, there are two disadvantages of this approach. (a) The questions described hypothetical situations that some participants could have simply misunderstood without further clarifying information [97,98]. (b) Some participants’ responses may have been subject to a form of attribution error related to actor-observer bias [99]. Namely, participants may have attributed their own seropositivity as being caused by a specific situation while viewing another person’s seropositivity as being caused by his or her personality. If these participants did not fully identify with other persons with HIV, their responses to the questions about HIV-stigmatizing attitudes may not have fully reflected internalized stigma.
Conclusions
Despite these limitations, my findings suggest that internalized stigma is highly prevalent among persons with HIV in sub-Saharan Africa, with nearly one-fifth of participants providing survey responses consistent with internalized shame or self-hatred. Furthermore, socioeconomic gradients in internalized stigma were observed across countries. These estimates suggest that the adverse health and psychosocial impacts of HIV stigma are concentrated among the most disadvantaged.
Acknowledgments
I received no specific funding for the conduct of this study. I acknowledge salary support from U.S. National Institutes of Health K23MH096620 and the Robert Wood Johnson Health and Society Scholars Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Disclosures: None
References
- 1.Rankin WW, Brennan S, Schell E, Laviwa J, Rankin SH. The stigma of being HIV-positive in Africa. PLoS Med. 2005;2(8):e247. doi: 10.1371/journal.pmed.0020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pryor JB, Reeder GD, Vinacco R, Kott TL. The instrumental and symbolic functions of attitudes towards persons with AIDS. J Appl Soc Psychol. 1989;19(5):377–404. [Google Scholar]
- 3.Tsai AC, Bangsberg DR, Weiser SD. Harnessing poverty alleviation to reduce the stigma of HIV in sub-Saharan Africa. PLoS Med. 2013;10(11):e1001557. doi: 10.1371/journal.pmed.1001557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cassens BJ. Social consequences of the acquired immunodeficiency syndrome. Ann Intern Med. 1985;103(5):768–771. doi: 10.7326/0003-4819-103-5-768. [DOI] [PubMed] [Google Scholar]
- 5.Link BG, Cullen FT, Struening E, Shrout PE. A modified labeling theory approach to mental disorders: an empirical assessment. Am Sociol Rev. 1989;54(3):400–423. [Google Scholar]
- 6.Pantelic M, Shenderovich Y, Cluver L, Boyes M. Predictors of internalised HIV-related stigma: a systematic review of studies in sub-Saharan Africa. Health Psychol Rev. doi: 10.1080/17437199.2014.996243. in press. [DOI] [PubMed] [Google Scholar]
- 7.Berger BE, Ferrans CE, Lashley FR. Measuring stigma in people with HIV: psychometric assessment of the HIV stigma scale. Res Nurs Health. 2001;24(6):518–529. doi: 10.1002/nur.10011. [DOI] [PubMed] [Google Scholar]
- 8.Holzemer WL, Uys LR, Chirwa ML, et al. Validation of the HIV/AIDS Stigma Instrument - PLWA (HASI-P) AIDS Care. 2007;19(8):1002–1012. doi: 10.1080/09540120701245999. [DOI] [PubMed] [Google Scholar]
- 9.Visser MJ, Kershaw T, Makin JD, Forsyth BW. Development of parallel scales to measure HIV-related stigma. AIDS Behav. 2008;12(5):759–771. doi: 10.1007/s10461-008-9363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalichman SC, Simbayi LC, Cloete A, Mthembu PP, Mkhonta RN, Ginindza T. Measuring AIDS stigmas in people living with HIV/AIDS: the Internalized AIDS-Related Stigma Scale. AIDS Care. 2009;21(1):87–93. doi: 10.1080/09540120802032627. [DOI] [PubMed] [Google Scholar]
- 11.Tsai AC, Weiser SD, Steward WT, et al. Evidence for the reliability and validity of the internalized AIDS-related stigma scale in rural Uganda. AIDS Behav. 2013;17(1):427–433. doi: 10.1007/s10461-012-0281-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nyblade L, Pande R, Mathur S, et al. Disentangling HIV and AIDS stigma in Ethiopia, Tanzania, and Zambia. Washington, D.C: International Center for Research on Women; 2003. [Google Scholar]
- 13.Kalichman SC, Simbayi LC, Jooste S, et al. Development of a brief scale to measure AIDS-related stigma in South Africa. AIDS Behav. 2005;9(2):135–143. doi: 10.1007/s10461-005-3895-x. [DOI] [PubMed] [Google Scholar]
- 14.Mishra V, Agrawal P, Alva S, Gu Y, Wang S. Changes in HIV-related knowledge and behaviors in sub-Saharan Africa. Calverton: ICF Macro; 2009. DHS Comparative Reports No. 24. [Google Scholar]
- 15.Genberg BL, Hlavka Z, Konda KA, et al. A comparison of HIV/AIDS-related stigma in four countries: negative attitudes and perceived acts of discrimination towards people living with HIV/AIDS. Soc Sci Med. 2009;68(12):2279–2287. doi: 10.1016/j.socscimed.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maman S, Abler L, Parker L, et al. A comparison of HIV stigma and discrimination in five international sites: the influence of care and treatment resources in high prevalence settings. Soc Sci Med. 2009;68(12):2271–2278. doi: 10.1016/j.socscimed.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearlin LI. The sociological study of stress. J Health Soc Behav. 1989;30(3):241–256. [PubMed] [Google Scholar]
- 18.Link BG. Understanding labeling effects in the area of mental disorders: an assessment of the effects of expectations of rejection. Am Sociol Rev. 1987;52(1):96–112. [Google Scholar]
- 19.Link BG, Mirotznik J, Cullen FT. The effectiveness of stigma coping orientations: can negative consequences of mental illness labeling be avoided? J Health Soc Behav. 1991;32(3):302–320. [PubMed] [Google Scholar]
- 20.Takada S, Weiser SD, Kumbakumba E, et al. The dynamic relationship between social support and HIV stigma in rural Uganda. Ann Behav Med. 2014;48(1):26–37. doi: 10.1007/s12160-013-9576-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simbayi LC, Kalichman S, Strebel A, Cloete A, Henda N, Mqeketo A. Internalized stigma, discrimination, and depression among men and women living with HIV/AIDS in Cape Town, South Africa. Soc Sci Med. 2007;64(9):1823–1831. doi: 10.1016/j.socscimed.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai AC, Bangsberg DR, Frongillo EA, et al. Food insecurity, depression and the modifying role of social support among people living with HIV/AIDS in rural Uganda. Soc Sci Med. 2012;74(12):2012–2019. doi: 10.1016/j.socscimed.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fife BL, Wright ER. The dimensionality of stigma: a comparison of its impact on the self of persons with HIV/AIDS and cancer. J Health Soc Behav. 2000;41(1):50–67. [PubMed] [Google Scholar]
- 24.Govindasamy D, Ford N, Kranzer K. Risk factors, barriers and facilitators for linkage to antiretroviral therapy care: a systematic review. AIDS. 2012;26(16):2059–2067. doi: 10.1097/QAD.0b013e3283578b9b. [DOI] [PubMed] [Google Scholar]
- 25.Tsai AC. Reliability and validity of depression assessment among persons with HIV in sub-Saharan Africa: systematic review and meta-analysis. J Acquir Immune Defic Syndr. 2014;66(5):503–511. doi: 10.1097/QAI.0000000000000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai AC, Weiser SD, Petersen ML, Ragland K, Kushel MB, Bangsberg DR. A marginal structural model to estimate the causal effect of antidepressant medication treatment on viral suppression among homeless and marginally housed persons with HIV. Arch Gen Psychiatry. 2010;67(12):1282–1290. doi: 10.1001/archgenpsychiatry.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsai AC, Karasic DH, Hammer GP, et al. Directly observed antidepressant medication treatment and HIV outcomes among homeless and marginally housed HIV-positive adults: a randomized controlled trial. Am J Public Health. 2013;103(2):308–315. doi: 10.2105/AJPH.2011.300422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez P, Tsai AC, Muzoora C, et al. Reversal of the kynurenine pathway of tryptophan catabolism may improve depression in ART-treated HIV-infected Ugandans. J Acquir Immune Defic Syndr. 2014;65(4):456–462. doi: 10.1097/QAI.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katz IT, Ryu AE, Onuegbu AG, et al. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J Int AIDS Soc. 2013;16(Suppl 2):18640. doi: 10.7448/IAS.16.3.18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinsler JJ, Wong MD, Sayles JN, Davis C, Cunningham WE. The effect of perceived stigma from a health care provider on access to care among a low-income HIV-positive population. AIDS Patient Care STDs. 2007;21(8):584–592. doi: 10.1089/apc.2006.0202. [DOI] [PubMed] [Google Scholar]
- 31.Gilmore N, Somerville MA. Stigmatization, scapegoating and discrimination in sexually transmitted diseases: overcoming ‘them’ and ‘us’. Soc Sci Med. 1994;39(9):1339–1358. doi: 10.1016/0277-9536(94)90365-4. [DOI] [PubMed] [Google Scholar]
- 32.World Bank. World development report 2008: agriculture for development. Washington, D.C: The International Bank for Reconstruction and Development/The World Bank; 2007. [Google Scholar]
- 33.Kalusopa T, Dicks R, Osei-Boateng C, editors. Social protection schemes in Africa. Windhoek: African Labour Research Network; 2012. [Google Scholar]
- 34.Castro A, Farmer P. Understanding and addressing AIDS-related stigma: from anthropological theory to clinical practice in Haiti. Am J Public Health. 2005;95(1):53–59. doi: 10.2105/AJPH.2003.028563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neuberg SL, Smith DM, Asther T. Why people stigmatize: toward a biocultural framework. In: Heatherton TF, Kleck RE, Hebl MR, Hull JG, editors. The social psychology of stigma. New York: The Guilford Press; 2000. pp. 31–61. [Google Scholar]
- 36.Kurzban R, Leary MR. Evolutionary origins of stigmatization: the functions of social exclusion. Psychol Bull. 2001;127(2):187–208. doi: 10.1037/0033-2909.127.2.187. [DOI] [PubMed] [Google Scholar]
- 37.Portes A, Sensenbrenner J. Embeddedness and immigration: notes on the social determinants of economic action. Am J Sociol. 1993;98(6):1320–1350. [Google Scholar]
- 38.Niehaus I. Death before dying: understanding AIDS stigma in the South Africa lowveld. J S Afr Stud. 2007;33(4):845–860. [Google Scholar]
- 39.Farmer P, Leandre F, Mukherjee JS, et al. Community-based approaches to HIV treatment in resource-poor settings. Lancet. 2001;358(9279):404–409. doi: 10.1016/s0140-6736(01)05550-7. [DOI] [PubMed] [Google Scholar]
- 40.Wolfe WR, Weiser SD, Leiter K, et al. The impact of universal access to antiretroviral therapy on HIV stigma in Botswana. Am J Public Health. 2008;98(10):1865–1871. doi: 10.2105/AJPH.2007.122044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maman S, van Rooyen H, Stankard P, et al. NIMH Project Accept (HPTN 043): results from in-depth interviews with a longitudinal cohort of community members. PLoS One. 2014;9(1):e87091. doi: 10.1371/journal.pone.0087091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thirumurthy H, Zivin JG, Goldstein M. The economic impact of AIDS treatment: labor supply in western Kenya. J Hum Resources. 2008;43(3):511–552. [PMC free article] [PubMed] [Google Scholar]
- 43.Nyanzi-Wakholi B, Lara AM, Watera C, Munderi P, Gilks C, Grosskurth H. The role of HIV testing, counselling, and treatment in coping with HIV/AIDS in Uganda: a qualitative analysis. AIDS Care. 2009;21(7):903–908. doi: 10.1080/09540120802657498. [DOI] [PubMed] [Google Scholar]
- 44.Wagner GJ, Ghosh-Dastidar B, Garnett J, Kityo C, Mugyenyi P. Impact of HIV antiretroviral therapy on depression and mental health among clients With HIV in Uganda. Psychosom Med. 2012;74(9):883–890. doi: 10.1097/PSY.0b013e31826629db. [DOI] [PubMed] [Google Scholar]
- 45.Tsai AC, Bangsberg DR, Bwana M, et al. How does antiretroviral treatment attenuate the stigma of HIV? Evidence from a cohort study in rural Uganda. AIDS Behav. 2013;17(8):2725–2731. doi: 10.1007/s10461-013-0503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mbonye M, Nakamanya S, Birungi J, King R, Seeley J, Jaffar S. Stigma trajectories among people living with HIV (PLHIV) embarking on a life time journey with antiretroviral drugs in Jinja, Uganda. BMC Public Health. 2013;13(1):804. doi: 10.1186/1471-2458-13-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Venkataramani AS, Haberer JE, Thirumurthy H, et al. CD4+ cell count at antiretroviral therapy initiation and economic restoration in rural Uganda. AIDS. 2014;28(8):1221–1226. doi: 10.1097/QAD.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mishra V, Vaessen M, Boerma JT, et al. HIV testing in national population-based surveys: experience from the Demographic and Health Surveys. Bull World Health Organ. 2006;84(7):537–545. doi: 10.2471/blt.05.029520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mishra V, Assche SB, Greener R, et al. HIV infection does not disproportionately affect the poorer in sub-Saharan Africa. AIDS. 2007;21 (Suppl 7):S17–28. doi: 10.1097/01.aids.0000300532.51860.2a. [DOI] [PubMed] [Google Scholar]
- 50.UNAIDS. National AIDS programmes: a guide to monitoring and evaluation. UNAIDS/00.17E. Geneva: Joint United Nations Programme on HIV/AIDS; 2000. [Google Scholar]
- 51.Burris S. Stigma, ethics and policy: a commentary on Bayer’s “Stigma and the ethics of public health: Not can we but should we”. Soc Sci Med. 2008;67(3):473–475. doi: 10.1016/j.socscimed.2008.03.020. discussion 476–477. [DOI] [PubMed] [Google Scholar]
- 52.Pallitto CC, O’Campo P. Community level effects of gender inequality on intimate partner violence and unintended pregnancy in Colombia: testing the feminist perspective. Soc Sci Med. 2005;60(10):2205–2216. doi: 10.1016/j.socscimed.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 53.Filmer D, Pritchett LH. Estimating wealth effects without expenditure data -- or tears: an application to educational enrollments in states of India. Demography. 2001;38(1):115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 54.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 55.Yelland LN, Salter AB, Ryan P. Performance of the modified Poisson regression approach for estimating relative risks from clustered prospective data. Am J Epidemiol. 2011;174(8):984–992. doi: 10.1093/aje/kwr183. [DOI] [PubMed] [Google Scholar]
- 56.Williams R. Using the margins command to estimate and interpret adjusted predictions and marginal effects. Stata J. 2012;12(2):308–331. [Google Scholar]
- 57.Yebei VN, Fortenberry JD, Ayuku DO. Felt stigma among people living with HIV/AIDS in rural and urban Kenya. Afr Health Sci. 2008;8(2):97–102. [PMC free article] [PubMed] [Google Scholar]
- 58.Siedner MJ, Ng CK, Bassett IV, Katz IT, Bangsberg DR, Tsai AC. Trends in CD4 count at presentation to care and treatment initiation in sub-Saharan Africa, 2002–2013: a meta-analysis. Clin Infect Dis. doi: 10.1093/cid/ciu1137. in press. Epub 16 Dec 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shinsasa O, Simbayi L. Nelson Mandela/HSRC study of HIV/AIDS: South African national HIV prevalence, behavioral risks and mass media, household survey 2002. Cape Town: Human Sciences Research Council; 2002. [Google Scholar]
- 60.Visser MJ, Makin JD, Vandormael A, Sikkema KJ, Forsyth BW. HIV/AIDS stigma in a South African community. AIDS Care. 2009;21(2):197–206. doi: 10.1080/09540120801932157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kalichman SC, Simbayi LC. HIV testing attitudes, AIDS stigma, and voluntary HIV counselling and testing in a black township in Cape Town, South Africa. Sex Transm Infect. 2003;79(6):442–447. doi: 10.1136/sti.79.6.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kalichman SC, Simbayi L. Traditional beliefs about the cause of AIDS and AIDS-related stigma in South Africa. AIDS Care. 2004;16(5):572–580. doi: 10.1080/09540120410001716360. [DOI] [PubMed] [Google Scholar]
- 63.Chan BT, Weiser SD, Boum Y, et al. Persistent HIV-related stigma in rural Uganda during a period of increasing HIV incidence despite treatment expansion. AIDS. 2015;29(1):83–90. doi: 10.1097/QAD.0000000000000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mall S, Middelkoop K, Mark D, Wood R, Bekker LG. Changing patterns in HIV/AIDS stigma and uptake of voluntary counselling and testing services: the results of two consecutive community surveys conducted in the Western Cape, South Africa. AIDS Care. 2013;25(2):194–201. doi: 10.1080/09540121.2012.689810. [DOI] [PubMed] [Google Scholar]
- 65.Bayer R, Stuber J. Tobacco control, stigma, and public health: rethinking the relations. Am J Public Health. 2006;96(1):47–50. doi: 10.2105/AJPH.2005.071886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stuber J, Galea S, Link BG. Smoking and the emergence of a stigmatized social status. Soc Sci Med. 2008;67(3):420–430. doi: 10.1016/j.socscimed.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Farrimond HR, Joffe H. Pollution, peril and poverty: a British study of the stigmatization of smokers. J Comm Appl Social Psychol. 2006;16(6):481–491. [Google Scholar]
- 68.Tsai AC, Bangsberg DR, Kegeles SM, et al. Internalized stigma, social distance, and disclosure of HIV seropositivity in rural Uganda. Ann Behav Med. 2013;46(3):285–294. doi: 10.1007/s12160-013-9514-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Medley A, Garcia-Moreno C, McGill S, Maman S. Rates, barriers and outcomes of HIV serostatus disclosure among women in developing countries: implications for prevention of mother-to-child transmission programmes. Bull World Health Organ. 2004;82(4):299–307. [PMC free article] [PubMed] [Google Scholar]
- 70.Musheke M, Bond V, Merten S. Individual and contextual factors influencing patient attrition from antiretroviral therapy care in an urban community of Lusaka, Zambia. J Int AIDS Soc. 2012;15 (Suppl 1):17366. doi: 10.7448/IAS.15.3.17366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gourlay A, Birdthistle I, Mburu G, Iorpenda K, Wringe A. Barriers and facilitating factors to the uptake of antiretroviral drugs for prevention of mother-to-child transmission of HIV in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2013;16(1):18588. doi: 10.7448/IAS.16.1.18588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Musheke M, Ntalasha H, Gari S, et al. A systematic review of qualitative findings on factors enabling and deterring uptake of HIV testing in sub-Saharan Africa. BMC Pub Health. 2013;13:220. doi: 10.1186/1471-2458-13-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shelton JD, Cassell MM, Adetunji J. Is poverty or wealth at the root of HIV? Lancet. 2005;366(9491):1057–1058. doi: 10.1016/S0140-6736(05)67401-6. [DOI] [PubMed] [Google Scholar]
- 74.Lachaud JP. HIV prevalence and poverty in Africa: micro- and macro-econometric evidences applied to Burkina Faso. J Health Econ. 2007;26(3):483–504. doi: 10.1016/j.jhealeco.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 75.Msisha WM, Kapiga SH, Earls F, Subramanian SV. Socioeconomic status and HIV seroprevalence in Tanzania: a counterintuitive relationship. Int J Epidemiol. 2008;37(6):1297–1303. doi: 10.1093/ije/dyn186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hargreaves JR, Glynn JR. Educational attainment and HIV-1 infection in developing countries: a systematic review. Trop Med Int Health. 2002;7(6):489–498. doi: 10.1046/j.1365-3156.2002.00889.x. [DOI] [PubMed] [Google Scholar]
- 77.Hargreaves JR, Bonell CP, Boler T, et al. Systematic review exploring time trends in the association between educational attainment and risk of HIV infection in sub-Saharan Africa. AIDS. 2008;22(3):403–414. doi: 10.1097/QAD.0b013e3282f2aac3. [DOI] [PubMed] [Google Scholar]
- 78.Hargreaves JR, Davey C, White RG. Does the ‘inverse equity hypothesis’ explain how both poverty and wealth can be associated with HIV prevalence in sub-Saharan Africa? J Epidemiol Community Health. 2013;67(6):526–529. doi: 10.1136/jech-2012-201876. [DOI] [PubMed] [Google Scholar]
- 79.Hart JT. The inverse care law. Lancet. 1971;1(7696):405–412. doi: 10.1016/s0140-6736(71)92410-x. [DOI] [PubMed] [Google Scholar]
- 80.Victora CG, Vaughan JP, Barros FC, Silva AC, Tomasi E. Explaining trends in inequities: evidence from Brazilian child health studies. Lancet. 2000;356(9235):1093–1098. doi: 10.1016/S0140-6736(00)02741-0. [DOI] [PubMed] [Google Scholar]
- 81.Frohlich KL, Potvin L. Transcending the known in public health practice: the inequality paradox: the population approach and vulnerable populations. Am J Public Health. 2008;98(2):216–221. doi: 10.2105/AJPH.2007.114777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Link BG, Northridge ME, Phelan JC, Ganz ML. Social epidemiology and the fundamental cause concept: on the structuring of effective cancer screens by socioeconomic status. The Milbank quarterly. 1998;76(3):375–402. doi: 10.1111/1468-0009.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.de Walque D. How does the impact of an HIV/AIDS information campaign vary with educational attainment? Evidence from rural Uganda. J Dev Econ. 2007;84(2):686–714. [Google Scholar]
- 84.Tsai AC. A typology of structural approaches to HIV prevention: a commentary on Roberts and Matthews. Soc Sci Med. 2012;75(9):1562–1567. doi: 10.1016/j.socscimed.2012.06.033. discussion 1568–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Garcia Calleja JM, Marum LH, Carcamo CP, Kaetano L, Muttunga J, Way A. Lessons learned in the conduct, validation, and interpretation of national population based HIV surveys. AIDS. 2005;19 (Suppl 2):S9–S17. doi: 10.1097/01.aids.0000172872.88347.f3. [DOI] [PubMed] [Google Scholar]
- 86.Mishra V, Barrere B, Hong R, Khan S. Evaluation of bias in HIV seroprevalence estimates from national household surveys. Sex Transm Infect. 2008;84 (Suppl 1):i63–i70. doi: 10.1136/sti.2008.030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barnighausen T, Bor J, Wandira-Kazibwe S, Canning D. Correcting HIV prevalence estimates for survey nonparticipation using Heckman-type selection models. Epidemiology. 2011;22(1):27–35. doi: 10.1097/EDE.0b013e3181ffa201. [DOI] [PubMed] [Google Scholar]
- 88.Pettifor A, MacPhail C, Suchindran S, Delany-Moretlwe S. Factors associated with HIV testing among public sector clinic attendees in Johannesburg, South Africa. AIDS Behav. 2010;14(4):913–921. doi: 10.1007/s10461-008-9462-5. [DOI] [PubMed] [Google Scholar]
- 89.Corno L, de Walque D. Socioeconomic determinants of stigmatization and HIV testing in Lesotho. AIDS Care. 2013;25 (Suppl 1):S108–113. doi: 10.1080/09540121.2012.736937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pitpitan EV, Kalichman SC, Eaton LA, et al. AIDS-related stigma, HIV testing, and transmission risk among patrons of informal drinking places in Cape Town, South Africa. Ann Behav Med. 2012;43(3):362–371. doi: 10.1007/s12160-012-9346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reniers G, Eaton J. Refusal bias in HIV prevalence estimates from nationally representative seroprevalence surveys. AIDS. 2009;23(5):621–629. doi: 10.1097/QAD.0b013e3283269e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hogan DR, Salomon JA, Canning D, Hammitt JK, Zaslavsky AM, Barnighausen T. National HIV prevalence estimates for sub-Saharan Africa: controlling selection bias with Heckman-type selection models. Sex Transm Infect. 2012;88 (Suppl 2):i17–23. doi: 10.1136/sextrans-2012-050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Katz IT, Essien T, Marinda ET, et al. Antiretroviral therapy refusal among newly diagnosed HIV-infected adults. AIDS. 2011;25(17):2177–2181. doi: 10.1097/QAD.0b013e32834b6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de Paula A, Shapira G, Todd PE. How beliefs about HIV status affect risky behaviors: evidence from Malawi. J Appl Econometrics. 2014;29(6):944–964. [Google Scholar]
- 95.Thornton RL. The demand for, and impact of, learning HIV status. Am Econ Rev. 2008;98(5):1829–1863. doi: 10.1257/aer.98.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oster E, Shoulson I, Dorsey ER. Optimal expectations and limited medical testing: evidence from Huntington Disease. Am Econ Rev. 2013;103(2):804–830. doi: 10.1257/aer.106.6.1562. [DOI] [PubMed] [Google Scholar]
- 97.Yoder PS, Nyblade L. Comprehension of questions in the Tanzania AIDS Indicator Survey. Calverton: ORC Macro; 2004. [Google Scholar]
- 98.Nyblade L, MacQuarrie K, Phillip F, et al. Measuring HIV stigma: results of a field test in Tanzania. Washington, D.C: U.S. Agency for International Development; 2005. [Google Scholar]
- 99.Jones EE, Nisbett RE. The actor and the observer: divergent perceptions of the causes of behavior. In: Jones EE, Kanhouse DE, Kelley HH, Nisbett RE, Valins S, Weiner B, editors. Attribution: perceiving the causes of behavior. Morristown: General Learning Press; 1972. pp. 79–94. [Google Scholar]