Abstract
Purpose
To characterize the determinants of variability for oxaliplatin pharmacokinetics including age, renal function, and hepatic function in children and adults.
Methods
Oxaliplatin pharmacokinetic data were combined from phase I and II clinical trials: 3 pediatric trials (Peds1–3) and 2 adult NCI organ dysfunction studies (Hepatic, Renal). A population pharmacokinetic model was developed utilizing platinum ultrafiltrate concentrations to characterize changes in oxaliplatin disposition with age and organ dysfunction along with other potential sources of oxaliplatin pharmacokinetic variability.
Results
A total of 1508 concentrations from 186 children and adults were used in the study. The data were well described by a three-compartment model. Serum creatinine was an independent predictor of clearance while age was an independent predictor of volume of distribution. While age was a significant covariate on clearance in the univariate analysis, age effects on clearance were entirely accounted for by serum creatinine. Gender, hepatic function, and race had no effect on clearance or volume of distribution. Median clearance values were 0.58 (Hepatic), 0.34 (Renal), 0.78 (Peds1), 0.74 (Peds2), and 0.81 (Peds3) (L/hr/kg0.75). Monte Carlo simulations of the final model with 130 mg/m2 yielded median AUC values of: 14.2 (2–6 yr), 16.8 (6–12 yr), 16.5 (12–18 yr), and 17.3 (>18 yr) (μg*hr/mL).
Conclusions
Renal function had the greatest effect on clearance with a small age effect seen on the distribution of oxaliplatin. Young pediatric patients had higher clearance values than adults as a result of better renal function.
Keywords: oxaliplatin, population pharmacokinetics, renal dysfunction, hepatic dysfunction, pediatric cancer
Introduction
The design of clinical trials of novel oncology therapeutics provides unique challenges to develop optimal regimens. Dosages for drugs used for adult indications are often estimated from in vitro or animal data; thus clinical trials may not be optimally designed to allow drugs to have significant clinical impact. Most therapeutics are less extensively studied in pediatrics as compared to adults due to a limited patient population. Pediatric dosages are often extrapolated from adult studies without taking into account differences in maturation on pharmacokinetics [3,8,10]. As such, many promising drugs may appear to lack efficacy or have excessive toxicity when tested in early phase clinical trials in children with cancer.
Population pharmacokinetic modeling allows for the integration of data from multiple studies and has been recommended by the FDA as an approach to understand age and other effects on pharmacokinetic parameters [18]. Oncology therapeutics can have complicated pharmacokinetics, which requires thorough sampling regimens to appropriately characterize pharmacokinetic parameters. Pharmacokinetic modeling can allow for the evaluation of drugs that previously failed in clinical trials to assess future potential in new regimens [10].
Oxaliplatin is a cytotoxic chemotherapeutic agent, which inhibits DNA synthesis. It is in the same family as the widely used cisplatin and carboplatin, however lacks the significant dose limiting ototoxicity and nephrotoxicity associated with these compounds. Oxaliplatin has been studied in children and adults for a variety of solid tumors including brain, lung, breast, liver, and gastric tumors over a wide range of dosages (60–160 mg/m2) [11], but currently is only approved for use by the United States FDA [13] for adult colorectal cancer in combination with 5-fluorouracil in the FOLFOX regimen [12]. While multiple pharmacokinetic studies have been conducted in children and adults [2,6,15–17], the data have never been combined in a comprehensive population pharmacokinetic model and pharmacokinetic differences based on patient characteristics have not been fully explored. The prolonged elimination kinetics of oxaliplatin necessitates a thorough sampling regimen [7], however most prior studies did not sample for sufficient amount of time or obtain an adequate number of samples throughout the necessary interval.
We developed a comprehensive population pharmacokinetic model to assess pharmacokinetic differences between pediatric and adult patients. Our model combined five pediatric and adult clinical trials of oxaliplatin. This unified analysis aimed to characterize the determinants of variability for oxaliplatin pharmacokinetics including age, renal function, and hepatic function in children and adults.
Methods
Patient population
Oxaliplatin data were combined from 5 prior published clinical trials of children and adults. The adult data was comprised of National Cancer Institute studies of patients with hepatic [16] and renal dysfunction [17]. Pediatric studies were conducted by St. Jude Children’s Hospital (Peds1) [15], the Children’s Oncology Group (Peds2) [2], and the Pediatric Brain Tumor Consortium (Peds3) [6]. Informed consent was obtained from patients, parents, or guardians for all participants in the studies. Individual study characteristics are described below and presented in Table 1.
Table 1.
Study demographics with median (range): Age (years), SCR (serum creatinine mg/dL), weight (kg), gender (percentage of male patients), dosage (mg/m2), median and range of number of samples per subject, planned sample collection times for studies (hr), and number of subjects per study.
| Study | Age (yr) | SCR (mg/dL) | Weight (kg) | Gender (% male) | Dosage (mg/m2) | Samples per subject | Time points (hr) | Subjects |
|---|---|---|---|---|---|---|---|---|
| Hepatic | 61 (28–80) | 0.8 (0.5–1.6) | 67 (40–115) | 45 | 130 (60–130) |
8 (3–10) | 2, 2.25, 2.75, 3, 5, 8, 24, 48, 168 | 55 |
| Renal | 65 (32–86) | 1.6 (0.6–3.4) | 71 (40–165) | 68 | 130 (60–130) |
11 (2–13) | 0, 2, 2.25, 2.50, 2.75, 3, 5, 8, 24, 48, 168, 336, 504 | 34 |
| St. Jude (Peds1) | 11 (5–21) | 0.7 (0.3–1) | 27 (16–123) | 65 | 130 (85–160) |
9 (4–11) | 0.5, 1, 2, 4, 6, 24, 48, 168, 336, 504 | 26 |
| COG (Peds2) | 12 (1.3–23) | . | 37 (8–140) | 65 | 130 (130–130) |
3 (1–3) | 2.5, 6, 168 | 46 |
| PBTC (Peds3) | 7 (0.6–19) | 0.5 (0.2–0.9) | 22 (7–108) | 80 | 130 (82–130) |
3 (1–3) | 0.5, 4, 168 | 25 |
| Overall | 20 (0.6–86) | 0.8 (0.2–3.4) | 59 (7–165) | 62 | 130 (60–160) |
8 (1–13) | 0.5–504 | 186 |
Drug administration and pharmacokinetic sampling
The hepatic dysfunction study (Hepatic) was a National Cancer Institute Organ Dysfunction Working Group study of patients with solid tumors and variable hepatic function including normal controls. Patients were greater than 18 years of age with advanced malignancy. Oxaliplatin was administered every 3 weeks with 60 to 130 mg/m2 administered over a 2 hour infusion. Oxaliplatin was supplied by the Cancer Therapy Evaluation Program of the National Cancer Institute. Intensive PK data included up to 10 samples collected during the dosing interval of therapy cycles 1 and 2 [16].
The renal dysfunction study (Renal) was a National Cancer Institute Organ Dysfunction Working Group study of patients with solid tumors and variable renal function including normal controls. Patients were greater than 18 years of age with advanced malignancy. Oxaliplatin was administered every 3 weeks between 60 and 130 mg/m2 over a 2 hour infusion and was supplied by the Cancer Therapy Evaluation Program of the National Cancer Institute. Intensive PK data included up to 13 samples collected during the dosing interval for cycles 1 and 2 of therapy [17].
St. Jude Children’s Research Hospital (Peds1) performed a phase I dose-escalation clinical trial of oxaliplatin in children and adolescents less than 21 years of age with refractory solid tumors. Oxaliplatin was administered every 2 or 3 weeks as a 100, 130, or 160 mg/m2 2 hour infusion. A subset of patients received daily carbamazepine to test potential for greater dose escalation. Oxaliplatin was provided by Sanofi-Aventis (Malvern, PA). Intensive PK sampling included up to 10 samples collected during the dosing interval [15].
The Children’s Oncology Group (Peds2) performed a phase II clinical trial of oxaliplatin in children aged 21 years or younger with refractory solid tumors. Oxaliplatin was administered over 2 hours at a dose of 130 mg/m2 or 4.3 mg/kg in patients 12 months of age or younger every 3 weeks. Oxaliplatin was supplied by the Cancer Therapy Evaluation Program of the National Cancer Institute. PK data included up to 3 samples after the first dose of cycle 1 [2].
The Pediatric Brain Tumor Consortium (Peds3) performed an open-label phase II study of oxaliplatin in children and adolescents less than 22 years of age with recurrent or refractory brain tumors including medulloblastoma, supratentorial primitive neuroectodermal tumors, and atypical teratoid rhabdoid tumors. Oxaliplatin was administered over 2 hours at a dose of 130 mg/m2 or 4.3 mg/kg in patients 12 months of age or younger every 3 weeks. Oxaliplatin was supplied by the Cancer Therapy Evaluation Program of the National Cancer Institute. PK sampling included up to 3 samples in the dosing interval [6].
Platinum ultrafiltrate analytic assay
Plasma ultrafiltrate platinum represents unbound, biologically active drug and thus was used to assess pharmacokinetics for the studies [7]. For all samples except those from the Hepatic study, platinum ultrafiltrate concentrations were measured by a validated inductively coupled plasma mass spectrometry assay performed with a lower limit quantitation of 1 ng/mL. For the Hepatic study, platinum ultrafiltrate was determined by a validated atomic absorption assay at the Analysis Pharmacology Core facility at the City of Hope (Duarte, CA) with a lower limit of quantitation of 10 ng/mL. In five subjects from the Renal study, the platinum ultrafiltrate concentrations increased more than 20% between the first and second measurements, which was felt to be secondary to drug left in the infusion line rather than a true platinum ultrafiltrate concentration, thus the first measurement from 5 patients was excluded from the analysis.
Pharmacokinetic analysis
Using the computer program NONMEM (version 7.2) with a GNU Fortran G77 Compiler concentration time data were fitted using first-order conditional estimation method (FOCE) with interaction. A three-compartment pharmacokinetic structural model (ADVAN11, TRANS4 subroutine) with first order absorption was used to describe the data. The three compartment model had a central compartment and two peripheral compartments with the following parameters: inter-compartmental clearance for peripheral compartments (Q2, Q3), clearance of central compartment (CL), volume of distribution for the central compartment (V1), and volume of peripheral compartments (V2, V3). An exponential-normal distribution error model was used for inter-subject variability.
Pharmacokinetic parameters were scaled by subject size before evaluation of other potential covariates. An allometric approach was used with clearance (CL) scaled by allometric weight (WT0.75) and volume of distribution (Vd) scaled by weight (WT1.0). Serum creatinine (SCR), age, liver function (AST, total bilirubin), race (African American, white), and female gender were evaluated as potential covariates for clearance and volume of distribution. AST and total bilirubin values were unavailable for patients in the Peds1, Peds2, and Renal studies, thus patients were assigned the median AST and total bilirubin values from patients with normal hepatic function in the Peds3 and Hepatic data sets. Serum creatinine values were unavailable for all patients in the Peds2 data set and all patients were assigned the median SCR value from the Peds1 and Peds3 studies. Potential covariates were added to the model one at a time as a linear function, with covariates that improved the model fitting by a change in the objective function of at least 4.0 (p<~0.05) being retained in the initial covariate screen. A backward elimination approach was utilized in the multivariate assessment. Covariates found to improve the objective function by 8.0 (p<~0.005) or greater were retained in the final model.
Separate residual errors were considered for each data set (Renal, Hepatic, Peds1–3). Empiric Bayesian estimates of individual pharmacokinetic parameters were generated from the final model using the POSTHOC subroutine. The final model was cross–validated to assess pharmacokinetic model performance. The data were divided into six sets with subjects from each study randomly assigned to one of the six sets, which were used to generate model parameters. These model parameters were then used to predict the concentrations of the subjects excluded from the model. This approach was repeated for each of the six data sets until every subject’s concentrations were predicted with a model that was developed independent of their own data. Median absolute error and median error were calculated as measures of precision and bias. Both of these measures were expressed as percentages of population-predicted concentrations. A 1000 sample bootstrap assessment of the final model was performed using Wings for NONMEM.
Monte Carlo simulations were performed using the final population PK model to compare different age groups with standard dosing. Concentration profiles were generated for 1,000 virtual male and female patients from 2 to 75 years of age. Weight distributions for simulations were derived from CDC 50th percentile weights [9]. Median serum creatinine values for each age group and gender for patients with normal renal function from the studies were used with a 25% variance. A dosage of 130 mg/m2 was used for all subjects.
Results
Patients
Pharmacokinetic data were available for 186 children and adults (1508 platinum ultrafiltrate concentrations). 31 subjects had two visits; 2 in the Hepatic study and 29 in the Renal study. Table 1 summarizes patient characteristics at first PK visit. Serum creatinine values for patients in the Peds2 data set were unavailable for the analysis, thus these patients were assigned the median serum creatinine value from Peds1 and Peds3 data sets. A summary of the median platinum ultrafiltrate concentrations for each study normalized to a dose of 130 mg/m2 is shown in Figure 1. Patients in the Renal study appear to have higher median concentrations than the Hepatic or pediatric studies.
Figure 1.
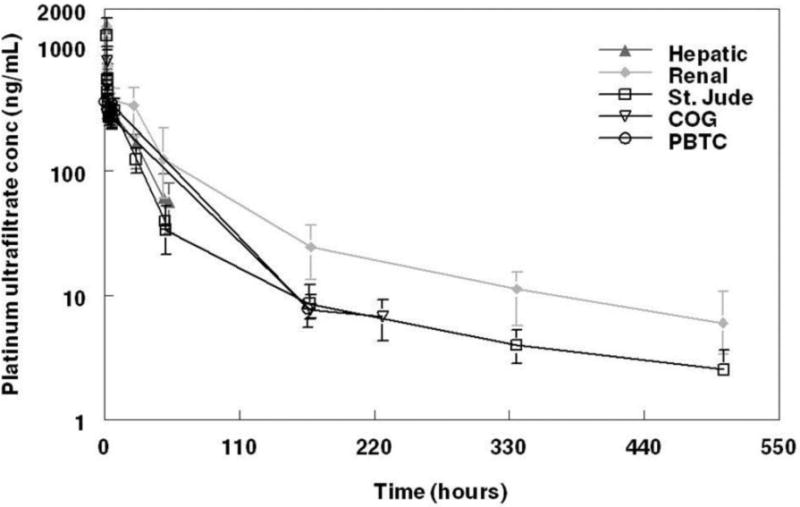
Summary of platinum ultrafiltrate concentrations. Median concentrations by study normalized to a dosage of 130 mg/m2. Error bars represent interquartile range (25–75%). Renal dysfunction patients appear to have higher median concentrations than Hepatic or pediatric patients.
Population pharmacokinetic analysis – covariate analysis
After allometric scaling for weight, SCR (Figure 2a, base model) and age (Figure 2b, base model) had effects on CL, while age (Figure 2c, base model), AST, and total bilirubin had effects on V1. SCR and age were thus identified as potential covariates for CL while age, AST, and total bilirubin were identified as potential covariates for V1 in the univariate screen. SCR on CL and age on V1 were significant covariates retained in the final model. Inter-subject variability was assessed on CL and a single inter-subject variability was used for V1, V2, and V3. The Renal data was assigned a separate error from the other four studies with an improvement in the objective function.
Figure 2.
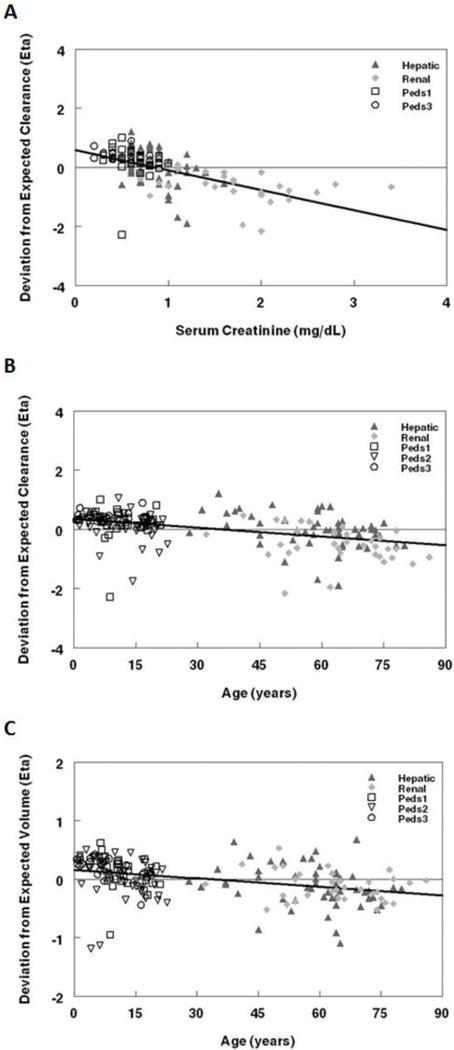
Population pharmacokinetic base model. The deviation from expected clearance was plotted against: a. Age and b. Serum creatinine. c. The deviation from expected volume (V1) was plotted against age. Regression lines are represented by solid black lines on plots.
The final population pharmacokinetic model described the data without significant bias as shown in Figure 3a–b. Shrinkage estimates for inter-subject variability were low, 5.2% (CL) and 10.8% (V1, V2, V3) while values for the error terms were 13.9% (non-Renal) and 3.6% (Renal). Final model parameter and variance estimates are shown in Table 2. The residual error estimates for the Renal data set was significantly more than that for the other data. Cross-validation of the final model demonstrated a median absolute error similar to the residual error of the model for non-Renal data, 26.2% (11.2% to 47.2%, 25th–75th percentile). Precision was similar across the population age continuum. While positive bias was present for pediatric data with a median error of 13.2% (−8.5% to 46.8%, 25th–75th percentile), but not adult data which had a median error of −1.08% (−22.4% to 32.2%, 25th–75th percentile), there was minimal bias in the composite model with a median error of 5.0% (−17.9% to 26.2%, 25th–75th percentile). Bootstrap evaluation of the final model successfully converged 86% percent of the time and estimation results are summarized in Table 2. The final parameter estimates of the model fall well within the 95% confidence interval and are close in value to the median bootstrap estimates demonstrating that the final model represents the population well.
Figure 3.
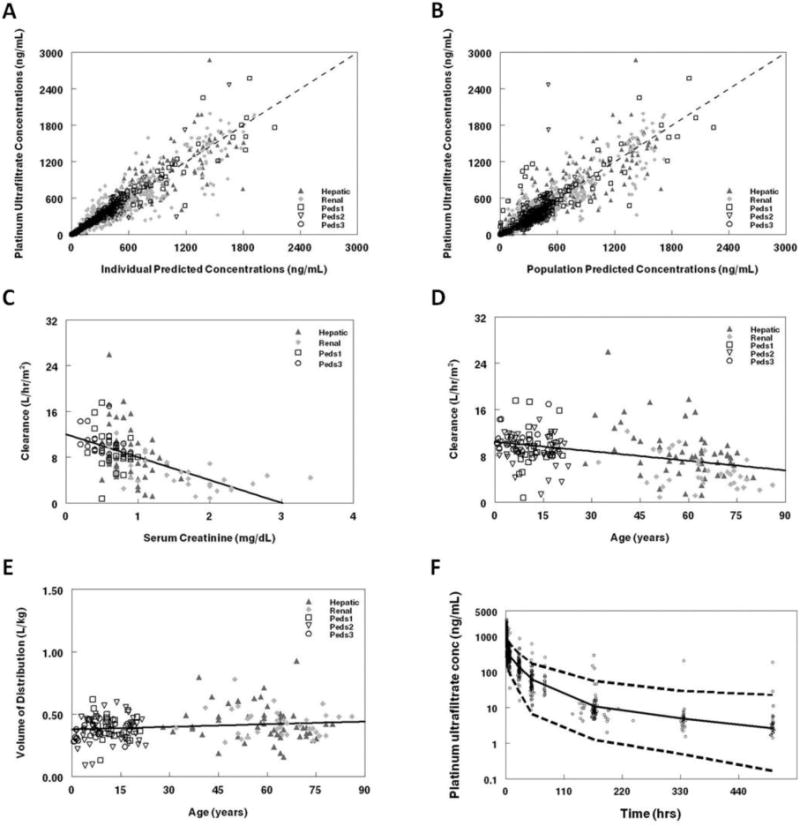
Population pharmacokinetic final model. Platinum ultrafiltrate concentrations from the study are compared with: a. Individual predictions for platinum ultrafiltrate and b. Population pharmacokinetic predicted levels. The line of unity on the goodness of fit plots demonstrates that the model describes the data without bias. Clearance (L/hr/m2) was plotted against c. Serum creatinine and d. Age, while volume of distribution (L/kg) was plotted against e. age. Regression lines are represented by solid black lines on plots. f. Visual predictive plot for the population pharmacokinetic model with concentration data adjusted to reflect a dosage of 130 mg/m2. Median concentrations (solid lines) with the 97.5 and 2.5 percentiles (dashed lines) from the Monte Carlo simulation of the final model are presented on the graph. The serum creatinine distribution used for the simulation was identical to that of patients with normal renal function from the data set.
Table 2.
Population pharmacokinetic final model parameter estimates: Thetas, variability, and error with standard errors. Bootstrap estimates with 95% confidence intervals. Equations for clearance (CL), volume of distribution of central compartment (V1), volume of distribution of peripheral compartments (V2, V3), and inter-compartmental clearances for peripheral compartments (Q2, Q3) used for the final model. WTKG represents weight in kilograms.
| Final Parameter | Standard Error | Bootstrap Estimates (Median and 95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Θ1 (Cl) | 0.67 | 0.033 | 0.67 (0.60–0.74) | ||||||
| Θ2 (Q2) | 2.95 | 0.149 | 2.94 (2.63–3.27) | ||||||
| Θ3 (Q3) | 0.22 | 0.023 | 0.22 (0.17–0.27) | ||||||
| Θ4 (V1) | 0.39 | 0.028 | 0.39 (0.34–0.44) | ||||||
| Θ5 (V2) | 6.22 | 0.28 | 6.23 (5.77–6.84) | ||||||
| Θ6 (V3) | 16.9 | 1.91 | 17.1 (12.8–21.5) | ||||||
| Θ7 (Age on V1, yr) | 0.16 | 0.043 | 0.16 (0.01–0.27) | ||||||
| Θ8 (SCR on CL, mg/dL) | 0.67 | 0.12 | 0.67 (0.44–0.91) | ||||||
| Variability | |||||||||
| Inter-subject (CL) | 49.4% | 6.13% | 48.4 (37.3–61.0)% | ||||||
| Inter-subject (V1–V3) | 36.7% | 3.33% | 36.6 (29.6–43.9)% | ||||||
| Inter-subject interaction (CL-V) | 20.2% | 6.37% | 23.1 (−0.05–51.9)% | ||||||
| Error | |||||||||
| Proportional (Renal Data) |
40.1% | 2.70% | 39.9 (34.9–45.9)% | ||||||
| Proportional (Non-renal Data) |
22.8% | 1.19% | 22.8 (20.6–25.7)% | ||||||
|
|
|
||||||||
SCR modeled as a linear function of clearance accounted for the age effect on clearance (Figure 3c–d, final model) observed in the univariate screen (see Table 2, equations) and had a much greater effect than age on the volume of distribution (Figure 3e, final model). After age was modeled as a linear function of volume of distribution, total bilirubin and AST were no longer significant covariates. Clearance varied 6.7-fold over the range of SCR values seen in the study, whereas volume of distribution ranged 2.2-fold over the age range seen in the study. Median (IQR) clearance values by study were: 0.58 (0.47–0.79) Hepatic, 0.34 (0.23–0.53) Renal, 0.78 (0.63–0.94) Peds1, 0.74 (0.64–0.82) Peds2, and 0.81 (0.75–0.94) Peds3 (L/hr/kg0.75). Volume of distribution (L/kg) increased by 32% between children and adults.
Monte Carlo Simulations
A visual predictive plot (Figure 3f) with the results of the Monte Carlo simulation plotted against the data normalized to a dosage of 130 mg/m2 had 94.4% of concentrations for patients with normal renal function within the 95% confidence interval of the simulated values demonstrating that the model represented the data well. The Monte Carlo simulation utilized a dosage of 130 mg/m2 and showed increasing AUC values with age. Median (interquartile range (IQR)) AUC (μg*hr/mL) values were: 14.2 (9.3–19.6) 2–6 years, 16.8 (11.8–25.0) 6–12 years, 16.5 (12.8–24.7) 12–18 years, and 17.3 (11.8–25.0) adults.
Discussion
The current study provides a comprehensive examination of key patient factors associated with altered drug clearance – age, gender, race, renal and hepatic function. Previously published pharmacokinetic studies of oxaliplatin had suggested an effect of age [5,6], gender [1,5,6], and renal function [1,5,6,17] on oxaliplatin clearance, although several of these factors were not found to correlate with oxaliplatin clearance in other studies [7]. Gender was not found to affect clearance in the current analysis. In two studies where it was found to be significant, it was integrated into a creatinine clearance estimation equation [5] or only marginally associated with clearance [1]. The current analysis had more subjects and did not find gender to significantly affect clearance during the univariate screen with the estimated female value 86% (0.0796, standard error) that of males.
While age appeared to have an effect on clearance, the multivariate analysis demonstrated the age effect was entirely accounted for by differences in renal function (e.g., serum creatinine), which was confounded with age. Oxaliplatin is predominantly renally cleared which is consistent with the results found in the current study. A small age effect was noted on the primary volume of distribution which had not previously been described and was likely secondary to changes in body composition including total body water, adipose tissue, and circulating plasma proteins [4]. While AST and total bilirubin appeared to have an effect on the volume of distribution, the multivariate analysis demonstrated that this effect was entirely accounted for by age, as the majority of adults with available AST and total bilirubin values had elevated levels in the Hepatic study. Hepatic function as represented by AST and total bilirubin was not found to affect clearance over the range of values seen in the study consistent with previously published results.
Oxaliplatin is cleared by glomerular filtration and irreversible plasma protein binding [7]. The drug has relatively complex pharmacokinetic distribution behavior with triphasic kinetics, however, prior studies either lacked a complete sampling regimen or had too few subjects to appropriately characterize pharmacokinetic disposition. The subset analyses of the current data set and previously published population pharmacokinetic studies of oxaliplatin utilized non-compartmental methods, modeling of individual subject data, or two compartment models [1, 2, 5, 6, 15–17]. Our composite analysis comprised more than three times the number of subjects of any prior analysis and included subjects with more extensive sampling to develop a three compartment population pharmacokinetic model, thereby providing a more complete picture of oxaliplatin pharmacokinetics. The median clearance values obtained from the Peds2–3 and Hepatic subset analyses were higher than those seen in the composite analysis while the Renal values were lower. While minimal differences would be expected amongst the pediatric data sets, the prior subset analyses showed differences in the median clearance between the Peds1 data set and other pediatric data sets with values of 9.4 (Peds1) as compared to 14.0 (Peds2) and 13.5 (Peds3) L/hr/m2. The composite analysis yielded similar median clearance values among studies with 9.1 (Peds1), 9.4 (Peds2), and 10.1 (Peds3) L/hr/m2.
A prior population pharmacokinetic study by Delord et al [5] developed a two compartment model utilizing 24 hour sampling for patients receiving oxaliplatin treatment for advanced colorectal cancer. Renal clearance was found to account for 45% of total clearance while the incorporation of a 5 hour urine collection only yielded a 34% renal contribution. The renal effect was thus less dramatic than in the current study likely due to limited plasma and urine sampling with a smaller range of serum creatinine values used. Bastian et al [1] developed a two compartment model that incorporated enterohepatic recycling in a separate elimination/storage compartment for 56 patients in a phase I/II clinical trial. While this study only sampled out to 28 hours, it incorporated 15 patients with renal impairment resulting in a similar effect of renal function on clearance as the current study.
Pediatric dosages for studies are often extrapolated from adult trial values, but pharmacokinetics can vary substantially with maturation which can have a large impact on dosing and pharmacologic effect (e.g., toxicity) [3,10]. In pediatric populations, the ability to conduct comprehensive pharmacokinetic studies may be limited due to the amount of blood and samples that can be obtained given lower total body blood volumes. This can make the determination of age, organ dysfunction, and other variables that alter pharmacokinetics challenging [8]. With population pharmacokinetic modeling techniques, studies with limited concentration-time data may be combined with small numbers of subjects with intensive data as was done in the current study to accurately determine these pharmacokinetic parameters. These models lend to Monte Carlo simulations to assess alternative dosing regimens for different age groups, which can inform better dosing decisions for subsequent clinical trials to attain desired goals such as improving systemic exposures associated with antitumor effects [18].
Oxaliplatin had a poor response rate in prior pediatric studies, which may be due to many factors including the use of single agent therapy, inherent resistance to oxaliplatin, and insufficient oxaliplatin systemic exposure. In adult studies of colorectal cancer, response rates of 9–24% were seen for single agent therapy in patients with previously untreated colon cancer, but improved to 34–67% in combined therapy with 5-fluoruracil [11]. The Peds2 and Peds3 studies had significantly worse responses with a combined 3 partial responses in 156 patients. The current study performed Monte Carlo simulations demonstrating lower AUC values for younger patients as compared to adults despite receiving the same BSA based dosage of 130 mg/m2. While higher pediatric dosing could be considered to achieve similar exposure to adults, it is unclear if this would result in an improved response rate.
Unlike adult cancer patients, pediatric oncology patients rarely have pharmacokinetic studies performed in subsets of patients with organ dysfunction (i.e., liver or kidney). While the pediatric data sets in the current study were of patients with normal liver and renal function, we developed a comprehensive population pharmacokinetic model, which can be helpful in extrapolating clearance values in children with organ dysfunction. Such models can allow for more personalized drug regimens in pediatric patients. The current study used serum creatinine to represent renal function, but the relationship between glomerular filtration rate and serum creatinine varies between children and adults. Creatinine clearance may be a more reliable marker of renal function for a combined study of pediatric and adult patients [14], however, creatinine clearance values were not available for the majority of the pediatric studies. In addition, the Peds2 study had missing values for serum creatinine and while pediatric patients with elevated serum creatinine or reduced glomerular filtration rate were excluded, it is unclear if any subjects had any mild renal dysfunction present.
The current study was able to compare exposures across a wide variety of patient populations including children, adults, and organ dysfunction patients. However, it was unable to describe oxaliplatin pharmacodynamics as the clinical output information needed to construct pharmacodynamic models was not consistently available for the analysis. In addition, platinum ultrafiltrate was measured as a surrogate for oxaliplatin concentrations and active therapeutic exposure. While the use of platinum ultrafiltrate may not be a direct measure of the active drug moiety, it is the most common analyte used in prior oxaliplatin pharmacokinetic studies. However, the pharmacokinetic model is still useful to assess alternative dosing regimens for different age groups with varying renal and hepatic function which can help design better future clinical trials of combination regimens involving oxaliplatin.
In conclusion, oxaliplatin pharmacokinetics were well described by a three compartment population pharmacokinetic model which demonstrated that predicted renal function had the greatest effect on clearance and a small age effect was seen on distribution of the drug. Pediatric patients had higher clearance values than adults as a result of better renal function. Population pharmacokinetics can allow for better design of clinical trials in children and adults along with re-evaluation of previously tested drugs to broaden applicability in future regimens.
Acknowledgments
The authors would like to thank Dr. Percy Ivy from the National Institutes of Health and Dr. Paul Juniewicz from Sanofi-Aventis for assistance with the project. We would like to thank the Children’s Oncology Group and the Pediatric Brain Tumor Consortium for providing the ADVL0421 and PBTC-010 data sets respectively. This study was supported by grants from the National Institutes of Health (EVC: U54 HD071600-01, TWS: U01 CA062505, P30 CA033572), St. Jude Cancer Center Support Grant CA21765, the American Lebanese Syrian Associated Charities (ALSAC), and Kaiser Santa Clara.
Edmund Capparelli served in a consultant/advisory role at Trius Pharmaceuticals, The Medicines Company, and Cempra. Maryam Fouladi received funding from Genentech and Roche.
Footnotes
Conflicts of Interest/Disclosures: There are no additional disclosures or potential conflicts of interest to report.
Ethical standards: The previously completed clinical trials from which data was utilized for the current analysis were approved by ethics committees at each respective institution and were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Informed consent was obtained from patients, parents, or guardians for all participants in the studies.
References
- 1.Bastian G, Barrail A, Urien S. Population pharmacokinetics of oxaliplatin in patients with metastatic cancer. Anticancer Drugs. 2003;14:817–824. doi: 10.1097/00001813-200311000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Beaty O, 3rd, Berg S, Blaney S, Malogolowkin M, Krailo M, Knight R, Schaiquevich P, Stewart C, Chen Z, Nelson M, Voss S, Ivy SP, Adamson PC. A phase II trial and pharmacokinetic study of oxaliplatin in children with refractory solid tumors: a Children’s Oncology Group study. Pediatr Blood Cancer. 2010;55:440–5. doi: 10.1002/pbc.22544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capparelli E, Williams P. Pharmacometrics in pharmacotherapy and drug development: pediatric application. In: Ette E, Williams P, editors. Pharmacometrics: the science of quantitative pharmacology. John Wiley and Sons, Inc; 2007. [Google Scholar]
- 4.Ceja M, Christensen A, Yang S. Dosing considerations in pediatric oncology. US Pharmacist. 2013;38:8–11. [Google Scholar]
- 5.Delord JP, Umlil A, Guimbaud R, Gregoire N, Lafont T, Canal P, Bugat R, Chatelut E. Population pharmacokinetics of oxaliplatin. Cancer Chemother Pharmacol. 2003;51:127–131. doi: 10.1007/s00280-002-0550-3. [DOI] [PubMed] [Google Scholar]
- 6.Fouladi M, Blaney SM, Poussaint TY, Freeman BB, 3rd, McLendon R, Fuller C, Adesina AM, Hancock ML, Danks MK, Stewart C, Boyett JM, Gajjar A. Phase II study of oxaliplatin in children with recurrent or refractory medulloblastoma, supratentorial primitive neuroectodermal tumors, and atypical teratoid rhabdoid tumors: a pediatric brain tumor consortium study. Cancer. 2006;107:2291–7. doi: 10.1002/cncr.22241. [DOI] [PubMed] [Google Scholar]
- 7.Graham MA, Lockwood GF, Greenslade D, Brienza S, Bayssas M, Gamelin E. Clinical pharmacokinetics of oxaliplatin: a critical review. Clin Cancer Res. 2000;6:1205–18. [PubMed] [Google Scholar]
- 8.Laughon MM, Benjamin DK, Jr, Capparelli EV, Kearns GL, Berezny K, Paul IM, Wade K, Barrett J, Smith PB, Cohen-Wolkowiez M. Innovative clinical trial design for pediatric therapeutics. Expert Rev Clin Pharmacol. 2011;4:643–52. doi: 10.1586/ecp.11.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDowell MA, Fryar CD, Hirsch R, Ogden CL. Anthropometric reference data for children and adults: U.S. population, 1999–2002. Adv Data. 2005:1–5. [PubMed] [Google Scholar]
- 10.Pastoor D, Lala M, Gobburu J. Pharmacometrics applications to pediatric trials. In: Mulberg A, Murphy D, Dunne J, Mathis L, editors. Pediatric drug development: concepts and applications. John Wiley and Sons Ltd; Chichester, United Kingdom: 2013. pp. 332–341. [Google Scholar]
- 11.Raymond E, Chaney SG, Taamma A, Cvitkovic E. Oxaliplatin: a review of preclinical and clinical studies. Ann Oncol. 1998;9:1053–71. doi: 10.1023/a:1008213732429. [DOI] [PubMed] [Google Scholar]
- 12.Rokkam A. Colorectal cancer. In: Cashen A, Van Tine BA, editors. Hematology and Oncology Subspecialty Consult Wolters Kluwer. Lippincott Williams and Wilkins; Philadelphia: 2012. pp. 239–245. [Google Scholar]
- 13.Sanofi-Aventis. Eloxatin (oxaliplatin) prescribing information: injection for intravenous use 2012 [Google Scholar]
- 14.Schwartz GJ, Work DF. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol. 2009;4:1832–43. doi: 10.2215/CJN.01640309. [DOI] [PubMed] [Google Scholar]
- 15.Spunt SL, Freeman BB, 3rd, Billups CA, McPherson V, Khan RB, Pratt CB, Stewart CF. Phase I clinical trial of oxaliplatin in children and adolescents with refractory solid tumors. J Clin Oncol. 2007;25:2274–80. doi: 10.1200/JCO.2006.08.2388. [DOI] [PubMed] [Google Scholar]
- 16.Synold TW, Takimoto CH, Doroshow JH, Gandara D, Mani S, Remick SC, Mulkerin DL, Hamilton A, Sharma S, Ramanathan RK, Lenz HJ, Graham M, Longmate J, Kaufman BM, Ivy P. Dose-escalating and pharmacologic study of oxaliplatin in adult cancer patients with impaired hepatic function: a National Cancer Institute Organ Dysfunction Working Group study. Clin Cancer Res. 2007;13:3660–6. doi: 10.1158/1078-0432.CCR-06-2385. [DOI] [PubMed] [Google Scholar]
- 17.Takimoto CH, Graham MA, Lockwood G, Ng CM, Goetz A, Greenslade D, Remick SC, Sharma S, Mani S, Ramanathan RK, Synold TW, Doroshow JH, Hamilton A, Mulkerin DL, Ivy P, Egorin MJ, Grem JL. Oxaliplatin pharmacokinetics and pharmacodynamics in adult cancer patients with impaired renal function. Clin Cancer Res. 2007;13:4832–9. doi: 10.1158/1078-0432.CCR-07-0475. [DOI] [PubMed] [Google Scholar]
- 18.Zisowsky J, Krause A, Dingemanse J. Drug development for pedatric populations: regulatory aspects. Pharmaceutics. 2010;2:364–388. doi: 10.3390/pharmaceutics2040364. [DOI] [PMC free article] [PubMed] [Google Scholar]


