Abstract
Cell-cell contact-mediated Notch signaling is essential for mesenchymal stem cell (MSC) chondrogenesis during development. However, subsequent deactivation of Notch signaling is also required to allow for stem cell chondrogenic progression. Recent literature has shown that Notch signaling can also influence Wnt/β-catenin signaling, critical for MSC differentiation, through perturbations in cell-cell contacts. Traditionally, abundant cell-cell contacts, consistent with development, are emulated in vitro using pellet cultures for chondrogenesis. However, cells are often encapsulated within biomaterials-based scaffolds, such as hydrogels, to improve therapeutic cell localization in vivo. To explore the role of Notch and Wnt/β-catenin signaling in the context of hydrogel-encapsulated MSC chondrogenesis, we compared signaling and differentiation capacity of MSCs in both hydrogels and traditional pellet cultures. We demonstrate that encapsulation within poly(ethylene glycol) (PEG) hydrogels reduces cell-cell contacts, and both Notch (7.5-fold) and Wnt/β-catenin (84.7-fold) pathway activation. Finally, we demonstrate that following establishment of cell-cell contacts and transient Notch signaling in pellet cultures, followed by Notch signaling deactivation, resulted in a 1.5-fold increase in MSC chondrogenesis. Taken together, these findings support that cellular condensation, and the establishment of initial cell-cell contacts is critical for MSC chondrogenesis, and this process is inhibited by hydrogel encapsulation.
Keywords: mesenchymal stem cells, hydrogels, wnt signaling, notch signaling, cell-cell contacts
1. Introduction
Mesenchymal stem cells (MSCs) are widely used in regenerative medicine approaches to restore, maintain, and enhance tissue functions [1–3]. MSCs are multipotent, have the capacity to self-renew, and are easily isolated from various tissues such as fat, muscle, and bone marrow [1, 3, 4]. In particular, MSCs have demonstrated efficacy in the regeneration of musculoskeletal tissues such as bone, tendon, muscle, and cartilage [5–8]. Direct tissue injection of MSCs suffers from poor cell localization, resulting in limited therapeutic efficacy. Biomaterials are a commonly employed strategy to enhance localization of MSCs in vivo for regenerative medicine applications [2, 9, 10]. In particular, hydrogels are a popular choice due to their similar biophysical properties as compared to native tissue [2, 10–14]. Specifically, PEG hydrogels have been employed to encapsulate neonatal articular chondrocytes and adipose-derived stem cells in cartilage regeneration approaches [15]. PEG hydrogels can be easily modified with biological motifs and synthetic extracellular matrix (ECM) mimetics to control cell adhesion, proliferation, and differentiation [10–14]. Furthermore, degradable functionalities can be incorporated into PEG macromers to provide control over network degradation kinetics and promote host tissue ingrowth and remodeling [12, 13].
While PEG hydrogels have been used with marked success in clinical trials [8], the therapeutic efficacy of this strategy is limited by our incomplete understanding of how biomaterials can alter cell-cell interactions and influence cell function and behavior. Specifically, when MSCs are encapsulated in 3D biomimetic microenvironments, a stark reduction in cell-cell interactions occurs [14, 16, 17]. However, developmentally, cell-cell adhesion plays an essential role in initiating chondrogenesis in early cell condensation. The expression of immunoglobin proteins such as N-Cadherin and neural cell adhesion molecule (N-CAM) are indicative of the formation of cell-cell interactions, and have been demonstrated to be essential in both in vitro and in vivo limb chondrogenesis [18]. While these adhesive events are characteristic of cartilage development, the subsequent activation of signal transduction pathways, specifically Notch and Wnt/β-catenin, facilitated through the formation of cell-cell interactions in biomaterial microenvironments are complex and not yet fully understood. Thus, in this study we focus on temporal regulation of the Notch signaling pathway through modulation of cell-cell contacts [19].
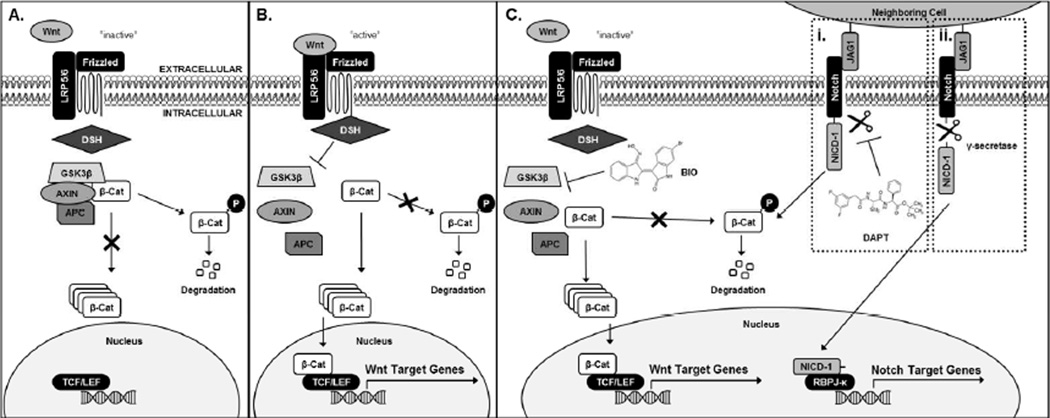
Mechanistically, cartilage development from stem cell to mature chondrocyte is controlled by signaling pathways such as Notch and Wnt/β-catenin. Previous findings demonstrate that initial cell-cell contacts, followed by the subsequent deactivation of Notch signaling after 3 days, are necessary for MSC chondrogenesis [20, 21]. Pellet culture conditions have commonly been used to induce MSC chondrogenesis in vitro, as it facilitates the formation of cell-cell contacts, resulting in the activation of Notch signaling [21]. Thus, controlling cell-cell contacts and subsequent Notch signaling is critical toward developing effective regenerative therapies for cartilage [19]. Additionally, the Wnt/β-catenin signaling pathway, which is a known regulator of MSC fate, interacts with many other regulatory pathways, including Notch [22]. Recent literature has demonstrated that Notch post-transcriptionally regulates β-catenin activity and therefore acts to control Wnt/β-catenin gene activation [22]. In addition, Wnt/β-catenin signaling is regulated by the cytosolic destruction complex [23]. The destruction complex is composed of glycogen synthase kinase 3 β (GSK3β), Axin-1 (AXIN), and adenomatosis polyposis coli (APC), and when intact, degrades β-catenin (Fig. 1A) [23]. Conversely, Wnt/β-catenin activation results via inhibition of one of the proteins of the destruction complex, which enables β- catenin translocation to the nucleus and binding of β-catenin to transcriptional T-cell factor/lymphoid enhancer-binding factor (TCF/LEF), leading to Wnt target gene activation (Fig. 1B) [23]. We have previously demonstrated that the small molecule agonist of Wnt/β-catenin signaling, 6-bromoindirubin-3’-oxime (BIO), promotes β-catenin accumulation and Wnt target gene transcription (Fig. 1C) [17]. In comparison to Wnt signaling, activation of Notch signaling is due to cell-cell contact-mediated extracellular Jagged (JAG1)-Notch Extracellular Domain (NECD1) interactions [22]. JAG1-NECD1 interaction leads to cleavage of Notch1 Intracellular Domain (NICD1) by γ-secretase (Fig. 1Ci) [22]. NICD1 then translocates to the nucleus, where it binds to Recombination Binding Protein for immunoglobulin kappa J region (RBPJ-κ) transcription factor and induces expression of Notch target genes [22]. NICD1 cleavage and subsequent Notch gene expression can be downregulated by treatment with the γ-secretase inhibitor, N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT) (Fig. 1Cii). Crosstalk between these pathways occurs when Notch is inactive and NICD1 remains membrane-bound, enabling it to degrade β-catenin and block Wnt gene expression (Fig. 1C) [22].
Figure 1.
Schematic of Wnt/β-catenin and Notch signaling pathways and pathway crosstalk as illustrated herein. Inactive Wnt signaling leads to β-catenin degradation (A). Active Wnt signaling allows for cytosolic accumulation and subsequent translocation of β-catenin to the nucleus and transcription of Wnt target genes (B). Concurrent cell-cell contact-activated Notch signaling with downstream Notch inhibition through DAPT treatment leads to degradation of β-catenin (Ci). Without DAPT and in the presence of cell-cell contacts, Notch-Wnt crosstalk occurs and leads to downstream activation of both Wnt and Notch Target Genes (Cii).
In this work, we examined the influence of hydrogel encapsulation on MSC cell-cell contacts, subsequent activation of Notch and Wnt/β-catenin signaling pathways, and MSC chondrogenic differentiation. Specifically, we utilized common cell culture conditions to study cell-cell contact-mediated Notch and Wnt/β-catenin signaling activation. Cell-cell contacts were promoted using MSC pellet culture, a method commonly used to induce chondrogenic differentiation of MSCs by emulating developmental cell condensation [21, 24, 25]. These cell-cell contacts are essential for Notch signaling because they facilitate the formation of JAG1-NECD1 interactions, which leads to the subsequent activation of Notch [26]. In contrast, cell-cell contacts are significantly limited by cellular encapsulation within PEG hydrogels lacking cell-adhesive ligands, thus reducing JAG1-NECD1. Finally, to elucidate the importance of Notch inhibition in MSC chondrogenic differentiation, cell pellets were treated with a Notch inhibitor thereby emulating the effects of hydrogel encapsulation.
2. Materials & Methods
All materials were purchased from Sigma-Aldrich unless otherwise noted.
2.1 Chemical Synthesis
2.1.1 Synthesis of Small Molecule Drug 6-Bromoindirubin-3’-Oxime (BIO)
The small molecule Wnt/β-catenin agonist BIO was synthesized as previously described [17, 27]. The final product was verified with 1H-NMR and matrix-assisted laser desorption/ionization time of flight (MALDI-TOF) mass spectroscopy (m/z=356 g/mol).
2.1.2 Synthesis of Poly(ethylene glycol) (PEG) Macromolecular Monomers (Macromers)
Linear PEG (Alfa Aesar, MW 10 kDa) and methacrylic anhydride (MA) at a molar ratio of 1:5 (PEG:MA) was microwaved (1100 W; Sharp) in a glass scintillation vial for 5 minutes [28, 29]. The reaction was cooled and the resulting product, PEG dimethacrylate (PEGDM), was dissolved in dichloromethane, precipitated in chilled diethyl ether, and collected by vacuum filtration. The resulting product was dialyzed against deionized water (molecular weight cut-off = 6–8000 kDa; Spectrum Labs, Rancho Dominguez, USA). 1H-NMR analysis (Bruker Avance 400 MHz, CDCl3) was used to determine the methacrylate functionality (>95%) per PEG macromer. (-CH2CH2O-(PEG), 908H, 3.2–3.8 ppm, multiplet; CH2=C(CH3)-, 4H/macromer, 5.6 and 6.3 ppm, singlets; CH2=C(CH3)-, 6H/macromer, 1.9 ppm, singlet).
2.2 Effect of 3D Culture Conditions on Cellular Notch-Wnt/β-catenin Crosstalk
2.2.1 Cell Culture
Human bone marrow MSCs were isolated (Lonza, Walkerville, MD) as described previously [30] and maintained at 37 °C with 5% CO2 in low-glucose Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies) with 10% fetal bovine serum (FBS; Atlanta Biologicals), 100 U/ml penicillin, 100 µg/ml streptomycin, and 0.25 µg/ml amphotericin B (Hyclone).
Mouse embryonic fibroblasts (C3H10T1/2, Clone 8) were obtained from American Type Culture Collection (ATCC; Manassas, VA) and maintained at 37 °C with 5% CO2 in Basal Eagle’s Medium (BME; Cellgro) with 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, and 0.25 µg/ml amphotericin B. C3H10T1/2 cells were used as a surrogate for MSCs due to their superior transfection efficiencies and similar differentiation capacities [17, 31–33].
2.2.2 Photoencapsulation of C3H10T1/2s in Poly(ethylene glycol) (PEG) Hydrogels
A 10 wt% solution of PEGDM was prepared in phosphate-buffered saline (PBS). The photoinitiator phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) [34], was added at a final concentration of 0.05 wt%. Trypsinized cells were added to PEGDM macromer solution to achieve a final concentration of 50 million cells/mL. PEG-cell solutions were added to sterile 1.5 mL microcentrifuge tubes as molds. Solutions were exposed to long-wavelength 365 nm light (5 mW/cm2) for 10 minutes at room temperature (2.5 × 105 cells/gel; 5 µL) [35, 36].
2.2.3 C3H10T1/2 and MSC Pellet Culture Formation
To form pellets, trypsinized cells (2.5×105 cells/pellet; C3H10T1/2 cells or MSCs where specified) were pelleted by centrifugation at 1000 RPM for 5 minutes in 15 mL conical tubes or in 96-well V-bottom plates (Greiner Bio-One). Cell pellets were then incubated for 3 weeks in growth media containing chondrogenic supplements [37], or BIO and/or DAPT. Pellet cultures were fixed in 4% paraformaldehyde, cryosectioned, stained for glycosaminoglycan production (toluidine blue, chondrogenic).
2.2.4 Perturbing Notch and Wnt/β-catenin Signaling Using DAPT and BIO
Cell-laden hydrogels and pellets were cultured in growth media with continuous 5 µM DAPT and/or 5 µM BIO for 24 hours and then analyzed for either Notch or Wnt/β-catenin signaling activity with untreated cells as controls (see Section 2.2.5).
2.2.5 Analyzing β-catenin and NICD1 Activity in C3H10T1/2 cells
To quantify Notch and Wnt/β-catenin signaling activity, cells were transfected with RBPJ-κ, or TOPFlash and FOPFlash (negative control) plasmids (Addgene plasmids 12456 and 12457) [38], respectively. RBPJ-κ is a luminescent reporter plasmid specific to nuclear RBPJ-κ-NICD1 activity. Similarly, TOPFlash is specific to nuclear TCF/LEF-β-catenin activity; FOPFlash plasmid is a scrambled, inactive consensus reporter for TOPFlash. 72 hours after seeding in 48-well plates at 10,000 cells/cm2, cells were transfected with 0.4 µg/well of reporter plasmid using Lipofectamine® LTX and PlusTM Reagent (Invitrogen) for 3 hours at 37 °C with 5% CO2. Immediately after transfection, cells were either photoencapsulated in PEGDM hydrogels or centrifuged to form pellet cultures with untreated cells as controls. To analyze Notch or Wnt/β-catenin signaling activity, total luminescence was measured (BioTek Synergy MX plate-reader) after 24 hours using the Luciferase Assay Kit (lysis reagent and luciferase reagent; Promega, Madison, WI). Luciferase signal was normalized to cellular DNA concentration (Quant-iT PicoGreen® dsDNA Assay Kit; Invitrogen) (Tecan Infinite plate-reader) to account for experimental variation across samples.
2.2.6 Assessment of Hydrogel-Encapsulated Cell-Cell Contacts
24 hours after hydrogel encapsulation or pellet culture formation, cells were stained using the LIVE/DEAD cell viability assay (Life Technologies). Samples were imaged using fluorescent confocal laser scanning microscopy (FV1000 Olympus). Cell viability was assessed using computer-assisted, colorimetric-based quantification methods.
Additionally, 5 µm sections of frozen cell-laden hydrogels and pellets were immunostained using a goat polyclonal anti-N-cadherin antibody (#sc-31030, Santa Cruz Biotechnology, Inc., Dallas, TX) and an immunoperoxidase detection kit (Vectastain ABC Kit, Vector Laboratories, Burlingame, CA). Frozen samples were warmed to room temperature, quenched of endogenous peroxidase activity (DAKO Dual Endogenous Enzyme Blocking Reagent; DAKO S2003), and blocked with Vectastain 5% normal rabbit serum (NRS). Slides were then incubated with a 1:50 dilution of goat polyclonal anti-N-Cadherin antibody in 2.5% NRS in phosphate buffered saline with Tween-20 (PBST) overnight at 4 °C. Slides were washed and then incubated in Vectastain biotinylated rabbit anti-goat secondary antibody in PBST, washed, and incubated with Vectastain ABC reagent. Slides were developed using the Vectastain ImmPACT DAB peroxidase reagent and counter-stained for 1 min using haematoxylin (Invitrogen).
2.3 Evaluation of MSC Gene Expression
MSCs were treated with DAPT and/or BIO and allowed to proliferate for 21 days in pellet culture as previously described. At day 0, 14, and 21, quantitative reverse-transcriptase polymerase chain reaction (RT-PCR) was used to assess gene expression. RNA was isolated and purified using the E.Z.N.A. Total RNA Kit (Bio-Tek); RNA concentration was normalized across samples to account for experimental variation. RT was performed using the iScript™ cDNA Synthesis Kit (Bio-Rad). PCR was performed using SybrGreen (SsoFastEvaGreen Master Mix, Bio-Rad) and the CFX96 Real-Time PCR Detection System (Bio-Rad). Primers for a housekeeping gene (β-actin), sex-determining region Y-box 9 (Sox9), type II collagen (Col2), and aggrecan (ACAN) were used (Table S1). The PCR thermal cycle parameters used were as follows: hold at 95 °C for 5 minutes, followed by 40 cycles of 15 seconds at 95 °C for denaturation, 60 seconds at the specified annealing temperature (Table S1), and 20 seconds at 72 °C for extension. Threshold cycle (CT) analysis was used to quantify PCR products relative to a housekeeping gene using the Pfaffl method [39].
2.4 Statistical Analysis
Data is presented as mean with error bars depicting standard error of measurement with at least three independent experiments (n=3–9 as indicated in figure legends) for each data point. Statistics were assessed using either one-way or two-way ANOVA with Bonferroni post-hoc analysis. A p-value of α<0.05 was considered significant.
3. Results
3.1 Hydrogel Encapsulation Inhibits MSC Chondrogenesis
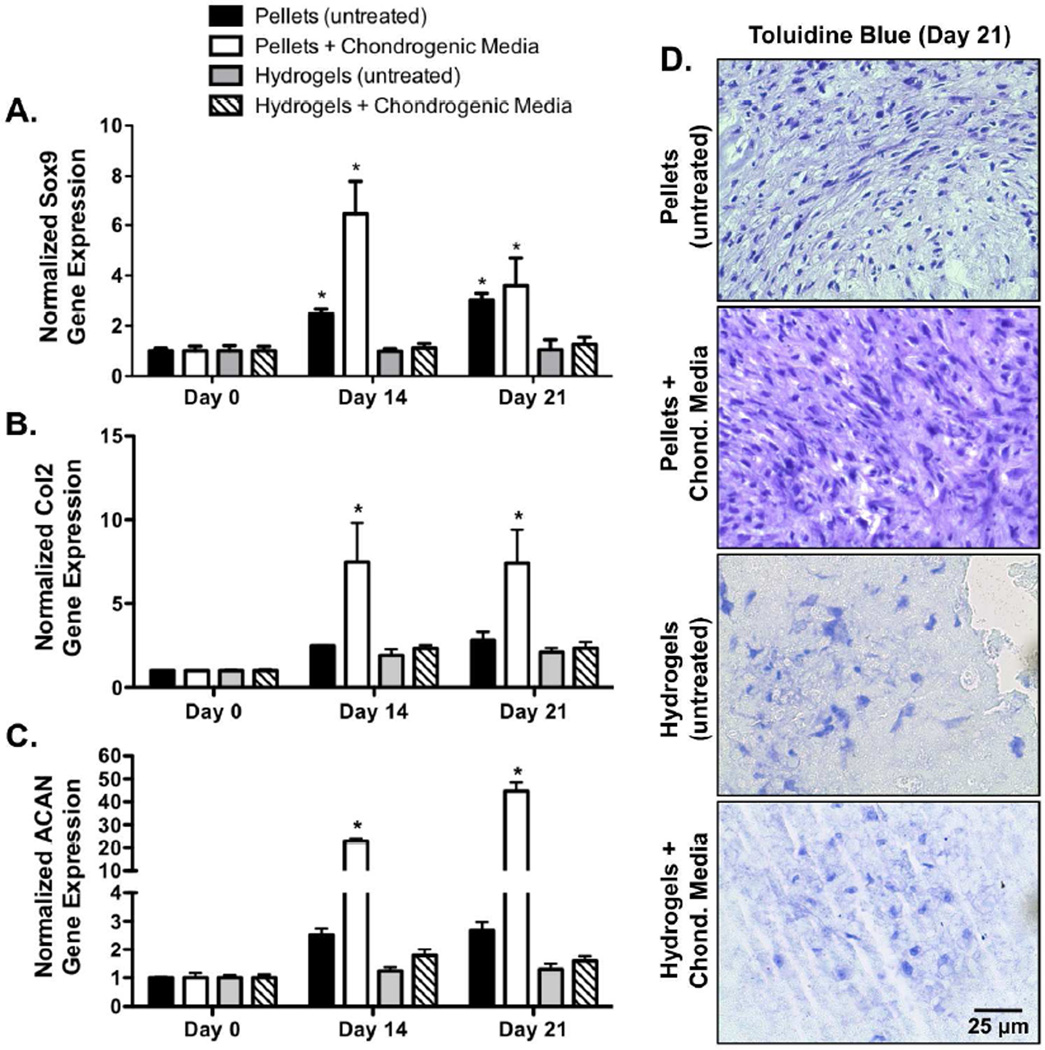
To investigate the effect of cell-cell contacts on chondrogenesis, MSCs were pelleted, or encapsulated in PEG hydrogels, and cultured for 21 days. Compared to MSC pellets cultured in growth media absent of chondrogenic supplements, MSC pellets cultured in chondrogenic media exhibited statistically significant 2.6- and 1.2-fold increases in Sox9 gene expression at 14 and 21 days of culture, respectively (Fig. 2A). Similarly, MSC pellets cultured in chondrogenic media exhibited significant 3.0- and 2.6-fold increases in Col2 gene expression (Fig. 2B), and 9.0- and 16.6-fold increases in ACAN gene expression at 14 and 21 days (Fig. 2C), respectively, as compared to MSC pellets cultured in growth media absent of chondrogenic supplements. Although statistical significance was not reached, hydrogel-encapsulated MSCs cultured in chondrogenic media exhibited a 1.1- and 1.2-fold increase in Sox9 gene expression at 14 and 21 days, as compared to hydrogel-encapsulated MSCs cultured in growth media alone (Fig. 2A). Similarly, hydrogel-encapsulated MSCs cultured in chondrogenic media exhibited 1.2- and 1.1-fold increases in Col2 gene expression (Fig. 2B), and 1.4- and 1.2-fold increases in ACAN gene expression at 14 and 21 days (Fig. 2C), respectively, as compared to hydrogel-encapsulated MSCs cultured in growth media alone. However, compared to MSC pellets cultured in chondrogenic media, hydrogel encapsulated MSCs cultured in chondrogenic media exhibited a significant 5.8- and 2.9-fold reduction in Sox9 gene expression at 14 and 21 days, respectively (Fig. 2). Similarly, hydrogel encapsulated MSCs cultured in chondrogenic media exhibited significant 3.2- and 3.2-fold decreases in Col2 gene expression (Fig. 2B), and 12.8- and 28.1-fold decreases in ACAN gene expression at 14 and 21 days (Fig. 2C), respectively, as compared to MSC pellets cultured in chondrogenic media. Furthermore, hydrogel encapsulated MSCs cultured in chondrogenic media exhibited qualitatively decreased glycosaminoglycan staining as indicated by lighter toluidine blue stained histological sections (Fig. 2D). Taken together, these results demonstrate that hydrogel encapsulation, as compared to traditional pellet culture, reduces MSC chondrogenesis, both in the presence of basal and chondrogenic media.
Figure 2.
While human MSC pellets cultured in growth media containing chondrogenic supplements exhibit a significant increase in Sox9 (A), Col2 (B), and ACAN (C) gene expression human MSC-laden hydrogels do not undergo a detectable increase in Sox9 (A), Col2 (B), and ACAN (C) expression, even when treated with chondrogenic growth media (n=3; error bars represent standard error of the mean; p-value of <0.05 indicates significance compared to day 0 (*)). Similarly, MSC-laden hydrogels exhibited qualitatively decreased glycosaminoglycan staining as indicated by toluidine blue stained histological sections (D) (Bar = 25 µm).
3.2 Hydrogel Encapsulation Reduces Cell-Cell Contacts Compared to Pellet Culture
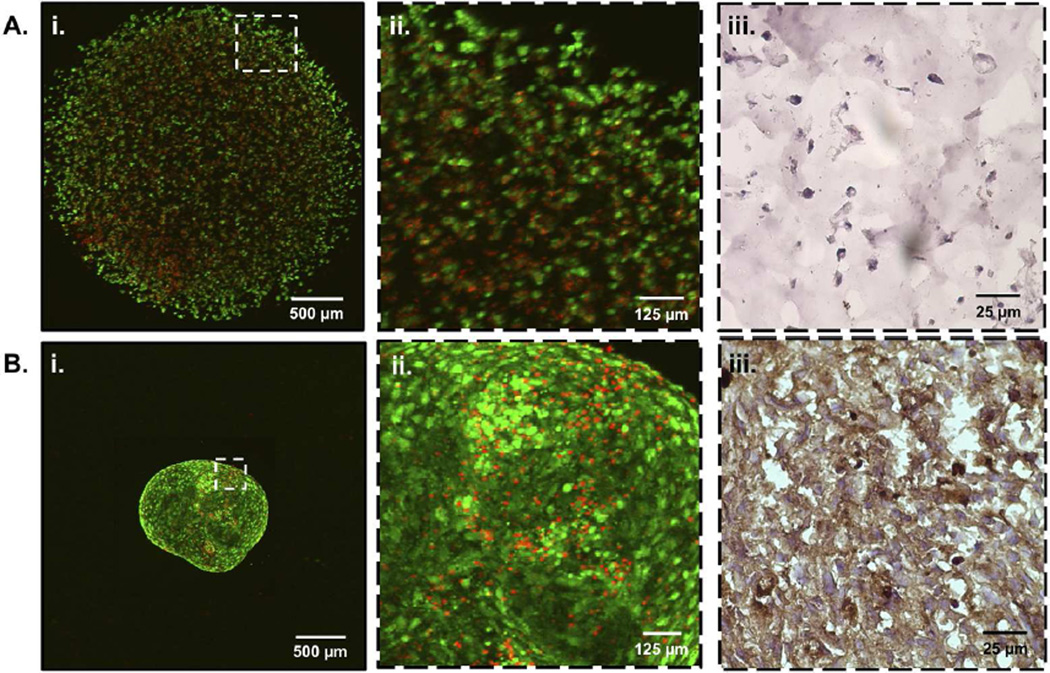
Having demonstrated that hydrogel encapsulation reduces MSC chondrogenic differentiation (Fig. 2) we sought to investigate the effect of hydrogel encapsulation on regulating the formation of cell-cell contacts. 24 hours after hydrogel encapsulation or pellet culture formation, cells were assessed for viability and morphology using confocal microscopy (Fig. 3). Hydrogel encapsulated C3H10T1/2s (Fig. 3Ai) appeared rounded and isolated, with few cell-cell interactions (Fig. 3Aii). As previously noted, C3H10T1/2 cells have superior transfection efficiencies and similar differentiation capacities to MSCs and were therefore used as an MSC-surrogate for investigation of cell-cell contact formation and subsequent signaling pathway analysis [17, 31–33]. In contrast to hydrogel encapsulated cells, C3H10T1/2 pellets (Fig. 3Bi) appeared densely packed, with numerous cell-cell contacts (Fig. 3Bii). Additionally, quantification of images was performed to verify a high degree of cell viability (> 90%) for both hydrogels and pellet cultures after 24 hours (Fig. 3). Furthermore, hydrogels and pellet cultures were immunostained for N-Cadherin, a prominent cell adhesion molecule implicated in early stages of chondrogenesis [40]. In comparison to hydrogel encapsulated MSCs (Fig. 3Aiii), cell peripheries of MSC pellet cultures (Fig. 3Biii) stained more intensely for N-Cadherin (brown), thus confirming the relative abundance of cell-cell interactions in pellet cultures over hydrogel encapsulations. Taken together, these results demonstrate that hydrogel encapsulation reduces cell-cell contacts as compared to traditional pellet culture techniques.
Figure 3.
LIVE/DEAD confocal microscopy of C3H10T1/2s encapsulated in PEG hydrogels (Ai) and traditional pellet cultures (Bi) both exhibit > 90% cell viability (live cells; calcein AM (green), dead cells; ethidium homodimer (red)). Cell-cell contacts are reduced in PEG hydrogel encapsulations (Aii) as compared to pellet cultures (Bii). Representative images of frozen cell-laden hydrogels (Aiii) and pellets (Biii) were immunohistochemically stained for N-Cadherin (brown) to further illustrate decreased cell-cell contact in hydrogel encapsulated MSCs.
3.3 Inhibiting Cell-Cell Contacts via Hydrogel Encapsulation Downregulates Notch and Wnt/β-Catenin Signaling
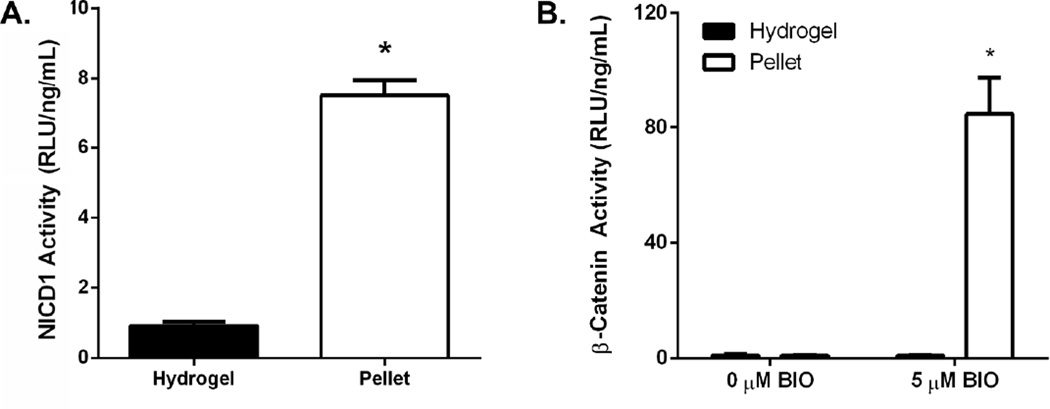
It has been well established that cell-cell contacts are critical for the initiation of Notch signaling [19, 40]. Furthermore, it has been demonstrated that in the absence of Notch signaling, membrane bound NICD1 can post-transcriptionally regulate β-catenin and subsequently Wnt/β-catenin signaling [22]. As hydrogel encapsulation reduces cell-cell contacts (Fig. 3), subsequent Notch and Wnt/β-catenin signaling was quantified via NICD1 and β-catenin activity. As shown in Figure 4A, hydrogel encapsulation, previously shown to reduce cell-cell contacts, resulted in a 7.5-fold reduction in NICD1 activity as compared to cells grown in pellet cultures.
Figure 4.
C3H10T1/2s encapsulated in PEG hydrogels exhibit reduced NICD1 activity compared with pellet-cultured cells (A) (n=3; average ± standard error; *p<0.05 vs. hydrogel). C3H10T1/2s exhibit decreased β-catenin activity as compared to pellet-cultured cells following treatment with 5 µM BIO (B) (n=3–9; average ± standard error; *p<0.05 vs. 0 µM BIO).
After confirming that hydrogel encapsulation-induced reductions in cell-cell contacts results in impaired Notch signaling, the effect of hydrogel encapsulation on Wnt/β-catenin signaling was assessed. In this study, BIO treatment was used to activate Wnt/β-catenin signaling to a detectable level [17, 27], while having no influence on Notch signaling (Fig. S1). Compared to untreated C3H10T1/2 pellets, pellets treated with BIO exhibited a significant 84.7-fold increase in Wnt/β-catenin signaling (Fig. 4B). In contrast, BIO treatment of encapsulated C3H10T1/2s resulted in no detectable increases in Wnt/β-catenin signaling (Fig. 4B). Compared to BIO treated pellets, hydrogel encapsulated C3H10T1/2s treated with BIO exhibit a significant 95.1-fold reduction in Wnt/β-catenin signaling (Fig. 4B). These results demonstrate that cellular encapsulation results in a reduction in both Notch and Wnt/β-catenin signaling.
3.4 Inhibition of Notch Signaling Downregulates Wnt/β-catenin Signaling
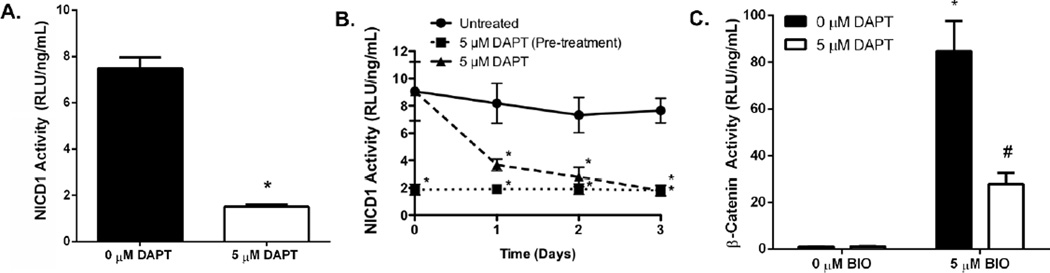
As inhibition of cell-cell contacts reduces Wnt/β-catenin signaling in hydrogel-encapsulated cells, the role of Notch signaling in the regulation of Wnt/β-catenin was further explored within pellet cultures (Fig. 5A). DAPT, a γ-secretase inhibitor that prevents NICD1 cleavage, was used to prevent cell-cell contact-mediated downstream Notch signaling. Untreated C3H10T1/2s were shown to exhibit constant temporal NICD1 activity for a period of 3 days following formation of pellets (Fig. 5B). In contrast, when C3H10T1/2s were treated with DAPT following pellet formation a significant 2.2- and 2.6-fold decrease in NICD1 activity was observed 1 and 2 days after pellet formation, respectively, as compared to untreated control pellets (Fig. 5A & 5B; 5 µM DAPT). In contrast, pellets formed from C3H10T1/2s pre-treated with DAPT for 24 hrs prior to pellet formation (as well as constant DAPT treatment following pellet formation) were shown to exhibit a persisting 4.9-fold loss in NICD1 activity as compared to untreated pellets for 3 days following formation of pellets (Fig. 5B; 5 µM DAPT pre-treatment).
Figure 5.
Pre-treatment of C3H10T1/2s with DAPT results in a significant reduction in NICD1 activity immediately following pellet formation (A) (n=3; average ± standard error; *p<0.05 vs. 0 µM DAPT). Treatment of C3H10T1/2s with DAPT following pellet formation resulted in a gradual reduction in NICD1 activity over ~48 hrs (B) (n=3; average ± standard error; *p<0.05 vs. Untreated). Subsequent treatment of C3H10T1/2s with both DAPT and BIO results in a significant reduction in β-catenin activity as compared to treatment with BIO alone (C) (n=3–9; average ± standard error; *p<0.05 vs. 0 µM BIO, #p<0.05 vs. 5 µM BIO + 0 µM DAPT).
As illustrated in Figure 5A, 24 hr DAPT pre-treatment of C3H10T1/2 pellets resulted in a significant 4.9-fold reduction in NICD1 activity, as compared to untreated C3H10T1/2 pellets, a result similar to the effect of limiting cell-cell contacts via hydrogel encapsulations (Fig. 4A). In addition, and as previously noted, BIO-treated C3H10T1/2 pellets exhibited a significant 84.7-fold increase in Wnt/β-catenin signaling as compared to untreated controls (Fig. 4B & 5A). However, simultaneous treatment of C3H10T1/2 pellets with both BIO and DAPT resulted in a significant 3.0-fold decrease in β-catenin activity when compared to BIO-treated pellet culture controls (Fig. 5A). It should be noted, these results support previous findings that Notch inactivation regulates Wnt/β-catenin signaling presumably through post-transcriptional degradation of β-catenin by inactive membrane-bound NICD1 [22].
3.5 In the Presence of Cell-Cell Contacts, Notch Inhibition Enhances Long-Term Chondrogenic Differentiation
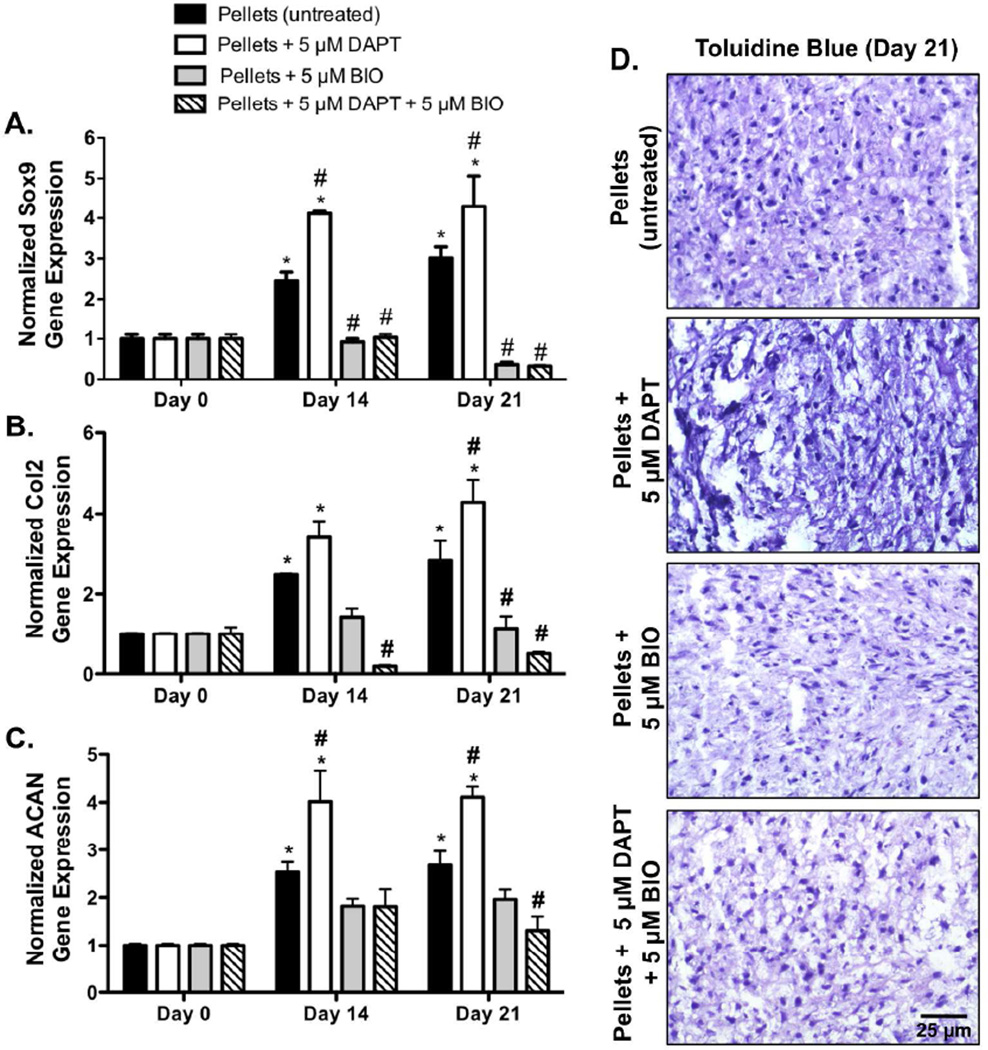
Having demonstrated that a failure to form cell-cell contacts (Fig. 3) inhibits Notch activation and subsequent downstream Wnt/β-catenin signaling (Fig. 4 & 5), the effect of cell-cell contact on MSC chondrogenesis was further examined via gene expression analysis. Pellet cultures were formed and immediately treated with a Notch antagonist (DAPT) to emulate initial cell-cell contact formation and subsequent Notch inhibition (as observed developmentally, but not in hydrogel encapsulation). In addition, pellets were treated with or without an agonist of Wnt/β-catenin signaling (BIO) in the absence of standard chondrogenic induction media (to isolate the effects of cell-cell interactions). Pellets treated with DAPT in the absence of chondrogenic media exhibited significant 1.7- and 1.4-fold increases in Sox9 gene expression at days 14 and 21, respectively, as compared to untreated MSC pellets (Fig. 6A). Similarly, DAPT treated MSC pellets exhibited significant 1.4- and 1.5-fold increases in Col2 gene expression (Fig. 6B), and 1.6- and 1.5-fold increases in ACAN gene expression at days 14 and 21 (Fig. 6C), respectively, as compared to untreated MSC pellets. In addition, DAPT treated MSC pellets exhibited qualitatively increased glycosaminoglycan staining, as compared to untreated MSC pellets, as indicated by darker toluidine blue stained histological sections (Fig. 6D). Furthermore, pellets treated with DAPT in the absence of chondrogenic media exhibited significant 3.7- and 3.4-fold increases in Sox9 gene expression at days 14 and 21, respectively, as compared to MSCs encapsulated in hydrogels and cultured with chondrogenic supplements (Fig. 2). In addition, DAPT treated MSC pellets exhibited significant 1.5- and 1.8-fold increases in Col2 gene expression (Fig. 6B), and 2.3- and 2.6-fold increases in ACAN gene expression at days 14 and 21 (Fig. 6C), respectively, as compared to MSCs encapsulated in hydrogels and cultured with chondrogenic supplements (Fig. 2). In contrast, pellets treated with BIO alone, or BIO and DAPT, exhibited significant reductions in Sox9, Col2, and ACAN gene expression at days 14 and 21, as compared to untreated MSC pellets, or MSC pellets treated with DAPT alone (Fig. 6A). Specifically, pellets treated with BIO alone exhibited significant 8.4-, 2.5-, and 1.4-fold reductions in Sox9, Col2, and ACAN gene expression, as compared to untreated MSC pellets at day 21 (Fig. 6A). Similarly, BIO treated pellets exhibited 11.9-, 3.8-, and 2.1-fold reductions in Sox9, Col2, and ACAN gene expression, as compared to DAPT treated MSC pellets at day 21 (Fig. 6A). In addition, pellets concurrently treated with BIO and DAPT exhibited 9.4-, 5.6-, and 2.0-fold reductions in Sox9, Col2, and ACAN gene expression, as compared to untreated MSC pellets at day 21 (Fig. 6A). Furthermore, BIO and DAPT treatment resulted in 13.5-, 8.5-, and 3.1-fold reductions in Sox9, Col2, and ACAN gene expression, as compared to DAPT treated MSC pellets at day 21 (Fig. 6A). Taken together, these results demonstrate that in pellet cultures, specifically in the presence of cell-cell contacts, Notch inhibition enhances long-term MSC chondrogenesis.
Figure 6.
In the presence of cell-cell contacts (pellet cultures) Notch inhibition enhances chondrogenic differentiation as measured by Sox9 (A), Col2 (B), and ACAN (C) gene expression. After 14 and 21 days, human MSC pellet culture Sox9 (A), Col2 (B), and ACAN (C) expression is upregulated with DAPT treatment alone but reduced to negligible levels with BIO treatment (n=3; error bars represent standard error of the mean; p-value of <0.05 indicates significance compared to day 0 (*) and time matched untreated pellet). Similarly, pellet cultures treated with either BIO, or BIO and DAPT exhibited qualitatively decreased glycosaminoglycan staining as indicated by toluidine blue stained histological sections (D) (Bar = 25 µm).
4. Discussion
Much emphasis within cartilage tissue engineering approaches has been placed on identifying the optimal combination of cells, growth factors, and scaffolds [41–44]. However, cell-cell contact-dependent signaling pathways, such as Notch, which are heavily implicated in MSC fate and function [19–21], have been largely neglected. In our work, we have shown that, in contrast to pellet cultures, PEG hydrogel encapsulation reduces MSC chondrogenenic potential due to limited cell-cell contacts (Fig. 2). Hydrogel encapsulation starkly reduces the amount of cell-cell contacts, thus reducing both Notch and Wnt/β-catenin signaling activation (Figs. 4 & 5). Furthermore, our results support the importance of cellular condensation and the establishment of cell-cell contacts in promoting chondrogenic differentiation, as assessed by glycosaminoglycan staining and gene expression analysis of Sox9, Col2, and ACAN (Fig. 2 & 6). Together these results support findings that initial cell-cell contacts are critical for chondrogenic differentiation, and without them chondrogenic differentiation is significantly impaired, as observed in hydrogel encapsulations [19, 21, 40].
Membrane-bound NICD1 has been shown to negatively regulate β-catenin protein levels and control MSC behavior [22]. Our results demonstrate that loss of cell-cell contacts through PEG hydrogel encapsulations and DAPT-mediated inhibition of Notch signaling activity in pellet cultures have direct effects on β-catenin activity and MSC chondrogenesis (Figs. 5–7). Without activation of Notch signaling via cellular condensation in pellet cultures, MSC chondrogenesis is reduced, as demonstrated by decreased glycosaminoglycan staining, as well as the lack of Sox9, Col2, and ACAN upregulation at day 21 (Fig. 2A–C). However, if Notch is subsequently inhibited with DAPT, membrane-bound NICD1 becomes persistent and negatively regulates Wnt/β-catenin by degrading cytoplasmic β-catenin, as previously reported (Fig. 4 & 5) [22]. The reduction in β-catenin via Notch potentiates the progression of chondrogenesis, whereas the continued upregulation of β-catenin with BIO blocks chondrogenesis (Fig. 6). In summary, our in vitro experiments demonstrate that MSC pellet cultures, which establish cellular condensation, undergo enhanced chondrogenesis when subsequently treated with the Notch antagonist DAPT (Fig. 6). In contrast, MSCs encapsulated within PEG hydrogels fail to establish initial cellular condensation and undergo significantly reduced chondrogenic differentiation (Fig. 2).
Figure 7.
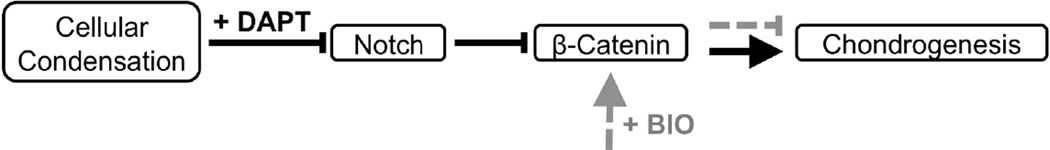
In vivo cellular condensation was emulated in vitro via MSC pellet culture. As demonstrated via Sox9, Col2, and ACAN gene expression (Fig. 6), treatment of pellets with DAPT replicates the effects seen in MSC hydrogel encapsulation. Reduced Wnt/β-catenin signaling through membrane-bound NICD1-mediated degradation of β-catenin promotes MSC chondrogenesis (black solid). However, treatment with the Wnt agonist, BIO, inhibits MSC chondrogenesis (grey dashed).
Developmentally, embryogenesis and limb chondrogenesis is initiated by MSC condensation and the formation of cell-cell contacts (Fig. 7) [19, 45]. These events are correlated with the expression of the chondrogenic marker, Sox9, which has been shown to regulate the expression of genes that encode cartilage structural proteins such as ACAN and Col2; this protein-based ECM provides the characteristic mechanics of cartilage tissue [40]. Consistent with our findings (Fig. 6), transient, but not sustained, Notch signaling has been shown to be instrumental in MSC chondrogenesis [19, 20, 46–49]. In only early stages of stem cell commitment to the chondrogenic lineage, Notch, JAG1 and Notch target genes Hes (Hairy and Enhancer of Split) and Hey (Hairy/enhancer-of-split related with YRPW motif-like protein) are detected in vivo [40, 48, 49]. Furthermore, both sustained expression of Notch over 2 weeks and inhibition of Notch (DAPT) in the first five days in vitro was shown to completely block chondrogenesis, thus confirming the temporal relevance of Notch activation [48, 49]. Similarly, we show that Notch inhibition with DAPT resulted in qualitatively increased glycosaminoglycan staining, and enhanced Sox9, Col2, and ACAN gene expression, markers of chondrogenesis, at days 14 and 21 (Fig. 6A–C). These findings confirm that manipulation of Notch signaling through genetic models, small molecule treatment, and cell culture conditions can be used to achieve temporal regulation of Notch signaling to alter MSC chondrogenesis. However, it should be noted that the relationship between Notch signaling and other orchestrated cellular behaviors is complex [50–55]. In addition to its role in chondrogenesis, Notch has been implicated as a key regulator of bone homeostasis via osteoblast differentiation [50–55]. The Notch pathway, in association with Wnt/β-catenin signaling has also been shown to be a key regulator of proliferation [53, 56], further highlighting the complexity of cell signaling cascades in regulating cell function.
While Wnt/β-catenin signaling-mediated regulation of MSC chondrogenesis is complex and still not fully understood, the Wnt agonist, BIO, which was previously used to expand MSCs for transplantation [17], has also been shown to inhibit MSC chondrogenesis through increased and perpetual Wnt/β-catenin signaling (Fig. 5 & 6) [17]. The observed inverse relationship between Wnt/β-catenin signaling and MSC chondrogenesis is also consistent with reports of correlation between Wnt inhibition and stem cell chondrogenesis both in vivo and in vitro [57, 58]. Treatment of MSC pellets with Wnt inhibitors for 21 days was shown by Im et al. to increase gene expression of the chondrocyte marker Col2 [57]. This correlation is also reflected in our own work, where it can be observed that in the presence of DAPT and associated decreases in Notch signaling (Fig. 5A), Wnt/β-catenin signaling is reduced (Fig. 5C), and associated MSC chondrogenic potential is enhanced (Fig. 6). While numerous pathways may contribute, inhibition of MSC chondrogenesis through Wnt agonism is thought to be due to a Notch-independent effect on Wnt/β-catenin activity, which results in the expression of certain Hox (homeobox) genes (Hoxa11 and Hoxd11) and suppression of cartilage development [59, 60]. Furthermore, recent work has highlighted the non-canonical role of Notch in the downregulation of Wnt/β-catenin signaling [22, 62]. As Notch and Wnt/β-catenin signaling are both implicated in MSC differentiation, developing a rigorous understanding of Notch-dependent regulation of Wnt/β-catenin will provide insight useful for development of new MSC-based approaches for cartilage tissue engineering. This work demonstrates that cell hydrogel encapsulation disrupts cell-cell contact formation and reduces MSC chondrogenic potential through unidirectional cell-cell contact-mediated Notch-Wnt/β-catenin crosstalk.
Although our data points towards the importance of cell-cell interactions for chondrogenesis, there have been numerous reports of MSC chondrogenic differentiation and subsequent cartilage elaboration within a variety of hydrogel chemistries [1, 14, 41–43, 63]. Many of these approaches modify hydrogels with adhesive ligands to control differentiation [14, 41–43, 63]. For example, PEG hydrogels that controlled the temporal presentation of the ubiquitous adhesive peptide RGD enhanced MSC chondrogenesis, compared with persistent RGD presentation [64]. RGD peptides were cleaved by cellular matrix metalloproteinase 13 (MMP-13) activity, thus establishing a system with cell-dictated release of adhesion sites. These findings underscore the importance of temporal relevance of cell-material interactions during MSC chondrogenesis. More recently, hyaluronic acid hydrogels functionalized with N-cadherin peptides to mimic cell-cell adhesion within a hydrogel have been shown to enhance MSC chondrogenesis with simultaneous treatment of TGFβ3 [63]. These studies demonstrate the effects of cell-cell interactions and cell-material interactions for stem cell mediated cartilage regeneration, though the underlying signaling events responsible for chondrogenesis were not explicitly studied.
Going forward, special considerations should be taken in the design of biomaterials constructs for MSC chondrogenesis. For example, increases in cell density can enhance chondrogenesis, potentially through increasing cell-cell contacts. When encapsulated within hyaluronic acid and agarose gels, MSC-generated cartilage tissue is markedly increased with greater cell densities [65, 66]. Together, these findings and our work suggest that a simple hydrogel system is not sufficient to support cartilage regeneration; rather, proper recapitulation of developmentally relevant signal transduction events via the combination of cell-cell and cell-material interactions are critical for the engineering of functional cartilage tissue.
5. Conclusion
Cell-cell contacts and early cell condensation have previously been demonstrated to be critically important in promoting chondrogenesis. Reduction of initial cell-cell contacts via biomaterials-based transplantation methods such as PEG encapsulation results in decreased Notch signaling as well as a reduction in chondrogenic differentiation. Furthermore, as previously demonstrated in literature, inhibition of Notch signaling following initial cell-cell contacts is critical for execution of MSC chondrogenic programs. Together the results presented herein demonstrate that hydrogel encapsulation, and the failure to establish initial cellular condensation and Notch signaling activation significantly reduced chondrogenic differentiation. Through this improved understanding of the role that cell-cell contacts play in regulating cell fate and function, more effective biomaterials-based cell transplantation and tissue regenerative strategies can be realized through the utilization of synergistic, instructive biochemical and biophysical signals that emulate not only the cell microenvironment, but the underlying cellular signaling activity.
Supplementary Material
After 24 hours of treatment with BIO, there was no significant change in NICD1 activity. Thus, Wnt/β-catenin signaling activation does not have an effect on Notch signaling activity (n=3; average ± standard error; no significance detected between groups).
Primer Sequences for RT-PCR Gene Expression Analysis.
Continuous DAPT and BIO treatment of MSC pellet cultures did not have significant cytotoxic effects over a period of 3 weeks. After 21 days, MSC pellet cultures retain high cell viability (> 90%) as illustrated by confocal LIVE/DEAD imaging (green, calcein AM (live cells); red, ethidium homodimer (dead cells)).
Acknowledgements
Funding for this research was provided by the NIH (R01-AR064200 and R01-DE022949), Orthopaedic Research and Education Foundation/Musculoskeletal Transplant Foundation (OREF/MTF), and the Rochester/Finger Lakes Eye & Tissue Bank (RETB/FLETB). Fellowship support to MDH was provided through the NIH (T32-AR053459) and to CSC and DSR through the Xerox Corporation. Equipment was purchased through funds provided by the NIH (S10-RR026542-01, P30-AR061307, and S10-RR027340-01). The authors wish to thank Teresa Sherman and Marina Feigenson for their help in plasmid isolation, Sarah Mack and Ashish Thomas for histology assistance, and Dr. James McGrath for use of laboratory equipment.
Footnotes
Disclosure: all authors state that they have no conflicts of interest
References
- 1.Elisseeff J, Puleo C, Yang F, Sharma B. Advances in skeletal tissue engineering with hydrogels. Orthod Craniofac Res. 2005;8(3):150–161. doi: 10.1111/j.1601-6343.2005.00335.x. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman MD, Xie C, Zhang X, Benoit DS. The effect of mesenchymal stem cells delivered via hydrogel-based tissue engineered periosteum on bone allograft healing. Biomaterials. 2013;34(35):8887–8898. doi: 10.1016/j.biomaterials.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 4.Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. Journal of cellular biochemistry. 1997;64(2):295–312. [PubMed] [Google Scholar]
- 5.Long T, Zhu Z, Awad HA, Schwarz EM, Hilton MJ, Dong Y. The effect of mesenchymal stem cell sheets on structural allograft healing of critical sized femoral defects in mice. Biomaterials. 2014 doi: 10.1016/j.biomaterials.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie C, Reynolds D, Awad H, Rubery PT, Pelled G, Gazit D, et al. Structural bone allograft combined with genetically engineered mesenchymal stem cells as a novel platform for bone tissue engineering. Tissue engineering. 2007;13(3):435–445. doi: 10.1089/ten.2006.0182. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Xie C, Lin AS, Ito H, Awad H, Lieberman JR, et al. Periosteal progenitor cell fate in segmental cortical bone graft transplantations: implications for functional tissue engineering. Journal of Bone and Mineral Research. 2005;20(12):2124–2137. doi: 10.1359/JBMR.050806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selmi T, Verdonk P, Chambat P, Dubrana F, Potel J-F, Barnouin L, et al. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: outcome at two years. Journal of Bone & Joint Surgery, British Volume. 2008;90(5):597–604. doi: 10.1302/0301-620X.90B5.20360. [DOI] [PubMed] [Google Scholar]
- 9.Elisseeff J, Puleo C, Yang F, Sharma B. Advances in skeletal tissue engineering with hydrogels. Orthodontics & craniofacial research. 2005;8(3):150–161. doi: 10.1111/j.1601-6343.2005.00335.x. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman MD, Van Hove AH, Benoit DS. Degradable hydrogels for spatiotemporal control of mesenchymal stem cells localized at decellularized bone allografts. Acta Biomater. 2014;10(8):3431–3441. doi: 10.1016/j.actbio.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benoit DSW, Collins SD, Anseth KS. Multifunctional hydrogels that promote osteogenic human mesenchymal stem cell differentiation through stimulation and sequestering of bone morphogenic protein 2. Adv Funct Mater. 2007;17(13):2085–2093. doi: 10.1002/adfm.200700012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benoit DSW, Durney AR, Anseth KS. Manipulations in hydrogel degradation behavior enhance osteoblast function and mineralized tissue formation. Tissue Eng. 2006;12(6):1663–1673. doi: 10.1089/ten.2006.12.1663. [DOI] [PubMed] [Google Scholar]
- 13.Lin CC, Anseth KS. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharm Res. 2009;26(3):631–643. doi: 10.1007/s11095-008-9801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salinas CN, Anseth KS. The enhancement of chondrogenic differentiation of human mesenchymal stem cells by enzymatically regulated RGD functionalities. Biomaterials. 2008;29(15):2370–2377. doi: 10.1016/j.biomaterials.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai JH, Kajiyama G, Smith RL, Maloney W, Yang F. Stem cells catalyze cartilage formation by neonatal articular chondrocytes in 3D biomimetic hydrogels. Scientific reports. 2013;3 doi: 10.1038/srep03553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salinas CN, Cole BB, Kasko AM, Anseth KS. Chondrogenic differentiation potential of human mesenchymal stem cells photoencapsulated within poly (ethylene glycol)-arginine-glycine-aspartic acid-serine thiol-methacrylate mixed-mode networks. Tissue engineering. 2007;13(5):1025–1034. doi: 10.1089/ten.2006.0126. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman MD, Benoit DSW. Agonism of Wnt-beta-catenin signalling promotes mesenchymal stem cell (MSC) expansion. J Tissue Eng Regen Med. 2013 doi: 10.1002/term.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oberlender SA, Tuan RS. Expression and functional involvement of N-cadherin in embryonic limb chondrogenesis. Development. 1994;120(1):177–187. doi: 10.1242/dev.120.1.177. [DOI] [PubMed] [Google Scholar]
- 19.Karlsson C, Lindahl A. Notch Signaling in Chondrogenesis. International Review of Cell and Molecular Biology. 2009:65–88. doi: 10.1016/S1937-6448(09)75003-8. [DOI] [PubMed] [Google Scholar]
- 20.Hardingham TE, Oldershaw RA, Tew SR. Cartilage, SOX9 and Notch signals in chondrogenesis. Journal of anatomy. 2006;209(4):469–480. doi: 10.1111/j.1469-7580.2006.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoo JU, Barthel TS, Nishimura K, Solchaga L, Caplan AI, Goldberg VM, et al. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am. 1998;80(12):1745–1757. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Kwon C, Cheng P, King IN, Anderson P, Shenje L, Nigam V, et al. Notch Post-translationally Regulates Beta-Catenin Protein in Stem and Progenitor Cells. Nature Cell Biology. 2011;13(10):1244–1251. doi: 10.1038/ncb2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sineva GS, Pospelov VA. Inhibition of GSK3β enhances both adhesive and signalling activities of @@β-catenin in mouse embryonic stem cells. Biology of the Cell. 2010;102(10):549–564. doi: 10.1042/BC20100016. [DOI] [PubMed] [Google Scholar]
- 24.Lee JY, Hall R, Pelinkovic D, Cassinelli E, Usas A, Gilbertson L, et al. New use of a three-dimensional pellet culture system for human intervertebral disc cells: initial characterization and potential use for tissue engineering. Spine. 2001;26(21):2316–2322. doi: 10.1097/00007632-200111010-00005. [DOI] [PubMed] [Google Scholar]
- 25.Bosnakovski D, Mizuno M, Kim G, Ishiguro T, Okumura M, Iwanaga T, et al. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells in pellet cultural system. Experimental hematology. 2004;32(5):502–509. doi: 10.1016/j.exphem.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Chillakuri CR, Sheppard D, Lea SM, Handford PA, editors. Seminars in cell & developmental biology. Elsevier; 2012. Notch receptor–ligand binding and activation: Insights from molecular studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polychronopoulos P, Magiatis P, Skaltsounis AL, Myrianthopoulos V, Mikros E, Tarricone A, et al. Structural basis for the synthesis of indirubins as potent and selective inhibitors of glycogen synthase kinase-3 and cyclin-dependent kinases. J Med Chem. 2004;47(4):935–946. doi: 10.1021/jm031016d. [DOI] [PubMed] [Google Scholar]
- 28.Lin-Gibson S, Bencherif S, Cooper JA, Wetzel SJ, Antonucci JM, Vogel BM, et al. Synthesis and characterization of PEG dimethacrylates and their hydrogels. Biomacromolecules. 2004;5(4):1280–1287. doi: 10.1021/bm0498777. [DOI] [PubMed] [Google Scholar]
- 29.Van Hove A, Wilson B, Benoit D. Microwave-assisted Functionalization of Poly (ethylene glycol) and On-resin Peptides for Use in Chain Polymerizations and Hydrogel Formation. Journal of visualized experiments: JoVE. 2012;(80) doi: 10.3791/50890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pittenger M, Mackay A, Beck S, Jaiswal R, Douglas R, Mosca J, et al. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 31.Zhao L, Li G, Chan K-M, Wang Y, Tang P-F. Comparison of multipotent differentiation potentials of murine primary bone marrow stromal cells and mesenchymal stem cell line C3H10T1/2. Calcif Tissue Int. 2009;84:56–64. doi: 10.1007/s00223-008-9189-3. [DOI] [PubMed] [Google Scholar]
- 32.Shea CM, Edgar CM, Einhorn TA, Gerstenfeld LC. BMP treatment of C3H10T1/2 mesenchymal stem cells induces both chondrogenesis and osteogenesis. J Cell Biochem. 2003;90(6):1112–1127. doi: 10.1002/jcb.10734. [DOI] [PubMed] [Google Scholar]
- 33.Bilkovski R, Schulte DM, Oberhauser F, Gomolka M, Udelhoven M, Hettich MM, et al. Role of WNT-5a in the determination of human mesenchymal stem cells into preadipocytes. Journal of Biological Chemistry. 2010;285(6170–6178) doi: 10.1074/jbc.M109.054338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fairbanks BD, Schwartz MP, Bowman CN, Anseth KS. Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: polymerization rate and cytocompatibility. Biomaterials. 2009;30(35):6702–6707. doi: 10.1016/j.biomaterials.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benoit DS, Tripodi MC, Blanchette JO, Langer SJ, Leinwand LA, Anseth KS. Integrin-linked kinase production prevents anoikis in human mesenchymal stem cells. Journal of biomedical materials research Part A. 2007;81(2):259–268. doi: 10.1002/jbm.a.31292. [DOI] [PubMed] [Google Scholar]
- 36.Lin C-C, Anseth KS. PEG hydrogels for the controlled release of biomolecules in regenerative medicine. Pharmaceutical research. 2009;26(3):631–643. doi: 10.1007/s11095-008-9801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue engineering. 1998;4(4):415–428. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 38.Biechele TL, Moon RT. Assaying beta-catenin/TCF transcription with beta-catenin/TCF transcription-based reporter constructs. Methods in molecular biology. 2008;468:99–110. doi: 10.1007/978-1-59745-249-6_8. [DOI] [PubMed] [Google Scholar]
- 39.Pfaffl M. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 2001;29(9) doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woods A, Wang G, Beier F. Regulation of chondrocyte differentiation by the actin cytoskeleton and adhesive interactions. Journal of cellular physiology. 2007;213(1):1–8. doi: 10.1002/jcp.21110. [DOI] [PubMed] [Google Scholar]
- 41.Bryant SJ, Anseth KS. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly (ethylene glycol) hydrogels. Journal of biomedical materials research. 2002;59(1):63–72. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- 42.Kim IL, Mauck RL, Burdick JA. Hydrogel design for cartilage tissue engineering: A case study with hyaluronic acid. Biomaterials. 2011;32:8771–8782. doi: 10.1016/j.biomaterials.2011.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park H, Temenoff JS, Tabata Y, Caplan AI, Mikos AG. Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials. 2007;28(21):3217–3227. doi: 10.1016/j.biomaterials.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li W-J, Tuli R, Okafor C, Derfoul A, Danielson KG, Hall DJ, et al. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials. 2005;26(6):599–609. doi: 10.1016/j.biomaterials.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 45.Cohn MJ, Tickle C. Limbs: a model for pattern formation within the vertebrate body plan. Trends in Genetics. 1996;12(7):253–257. doi: 10.1016/0168-9525(96)10030-5. [DOI] [PubMed] [Google Scholar]
- 46.Grogan SP, Olee T, Hiraoka K, Lotz MK. Repression of chondrogenesis through binding of notch signaling proteins HES-1 and HEY-1 to N-box domains in the COL2A1 enhancer site. Arthritis & Rheumatism. 2008;58(9):2754–2763. doi: 10.1002/art.23730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong Y, Jesse AM, Kohn A, Gunnell LM, Honjo T, Zuscik MJ, et al. RBPjκ-dependent Notch signaling regulates mesenchymal progenitor cell proliferation and differentiation during skeletal development. Development. 2010;137(9):1461–1471. doi: 10.1242/dev.042911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watanabe N, Tezuka Y, Matsuno K, Miyatani S, Morimura N, Yasuda M, et al. Suppression of differentiation and proliferation of early chondrogenic cells by Notch. J Bone Miner Metab. 2003;21(6):344–352. doi: 10.1007/s00774-003-0428-4. [DOI] [PubMed] [Google Scholar]
- 49.Oldershaw RA, Tew SR, Russell AM, Meade K, Hawkins R, McKay TR, et al. Notch signaling through jagged-1 is necessary to initiate chondrogenesis in human bone marrow stromal cells but must be switched off to complete chondrogenesis. Stem Cells. 2008;26(3):666–674. doi: 10.1634/stemcells.2007-0806. [DOI] [PubMed] [Google Scholar]
- 50.Engin F, Yao Z, Yang T, Zhou G, Bertin T, Jiang MM, et al. Dimorphic effects of Notch signaling in bone homeostasis. Nat Med. 2008;14(3):299–305. doi: 10.1038/nm1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hilton MJ, Tu X, Wu X, Bai S, Zhao H, Kobayashi T, et al. Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat Med. 2008;14(3):306–314. doi: 10.1038/nm1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zanotti S, Smerdel-Ramoya A, Stadmeyer L, Durant D, Radtke F, Canalis E. Notch inhibits osteoblast differentiation and causes osteopenia. Endocrinology. 2008;149(8):3890–3899. doi: 10.1210/en.2008-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dong Y, Jesse AM, Kohn A, Gunnell LM, Honjo T, Zuscik MJ, et al. RBPjkappa-dependent Notch signaling regulates mesenchymal progenitor cell proliferation and differentiation during skeletal development. Development. 2010;137(9):1461–1471. doi: 10.1242/dev.042911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tao J, Chen S, Lee B. Alteration of Notch signaling in skeletal development and disease. Ann N Y Acad Sci. 2010;1192:257–268. doi: 10.1111/j.1749-6632.2009.05307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dishowitz MI, Mutyaba PL, Takacs JD, Barr AM, Engiles JB, Ahn J, et al. Systemic inhibition of canonical Notch signaling results in sustained callus inflammation and alters multiple phases of fracture healing. PLoS One. 2013;8(7):e68726. doi: 10.1371/journal.pone.0068726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fre S, Pallavi SK, Huyghe M, Lae M, Janssen KP, Robine S, et al. Notch and Wnt signals cooperatively control cell proliferation and tumorigenesis in the intestine. Proc Natl Acad Sci U S A. 2009;106(15):6309–6314. doi: 10.1073/pnas.0900427106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Im G-I, Lee J-M, Kim H-J. Wnt inhibitors enhance chondrogenesis of human mesenchymal stem cells in a long-term pellet culture. Biotechnology letters. 2011;33(5):1061–1068. doi: 10.1007/s10529-010-0514-3. [DOI] [PubMed] [Google Scholar]
- 58.Rudnicki JA, Brown AMC. Inhibition of Chondrogenesis by Wnt Gene Expression in Vivo and in Vitro. Developmental Biology. 1997;185(1):104–118. doi: 10.1006/dbio.1997.8536. [DOI] [PubMed] [Google Scholar]
- 59.Kuss P, Villavicencio-Lorini P, Witte F, Klose J, Albrecht AN, Seemann P, et al. Mutant Hoxd13 induces extra digits in a mouse model of synpolydactyly directly and by decreasing retinoic acid synthesis. The Journal of clinical investigation. 2009;119(1):146. doi: 10.1172/JCI36851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hidaka C, Goldring MB. Regulatory mechanisms of chondrogenesis and implications for understanding articular cartilage homeostasis. Current Rheumatology Reviews. 2008;4(3):136–147. [Google Scholar]
- 61.Ryu J-H, Kim S-J, Kim S-H, Oh C-D, Hwang S-G, Chun C-H, et al. Regulation of the chondrocyte phenotype by β-catenin. Development. 2002;129(23):5541–5550. doi: 10.1242/dev.129.23.5541. [DOI] [PubMed] [Google Scholar]
- 62.Hayward P, Brennan K, Sanders P, Balayo T, DasGupta R, Perrimon N, et al. Notch modulates Wnt signalling by associating with Armadillo/β-catenin and regulating its transcriptional activity. Development. 2005;132(8):1819–1830. doi: 10.1242/dev.01724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bian LM, Guvendiren M, Mauck RL, Burdick JA. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. P Natl Acad Sci USA. 2013;110(25):10117–10122. doi: 10.1073/pnas.1214100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goggs R, Carter SD, Schulze-Tanzil G, Shakibaei M, Mobasheri A. Apoptosis and the loss of chondrocyte survival signals contribute to articular cartilage degradation in osteoarthritis. The Veterinary Journal. 2003;166(2):140–158. doi: 10.1016/s1090-0233(02)00331-3. [DOI] [PubMed] [Google Scholar]
- 65.Byers BA, Mauck RL, Chiang IE, Tuan RS. Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissue-engineered cartilage. Tissue Engineering Part A. 2008;14(11):1821–1834. doi: 10.1089/ten.tea.2007.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Erickson IE, Kestle SR, Zellars KH, Farrell MJ, Kim M, Burdick JA, et al. High mesenchymal stem cell seeding densities in hyaluronic acid hydrogels produce engineered cartilage with native tissue properties. Acta biomaterialia. 2012;8(8):3027–3034. doi: 10.1016/j.actbio.2012.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
After 24 hours of treatment with BIO, there was no significant change in NICD1 activity. Thus, Wnt/β-catenin signaling activation does not have an effect on Notch signaling activity (n=3; average ± standard error; no significance detected between groups).
Primer Sequences for RT-PCR Gene Expression Analysis.
Continuous DAPT and BIO treatment of MSC pellet cultures did not have significant cytotoxic effects over a period of 3 weeks. After 21 days, MSC pellet cultures retain high cell viability (> 90%) as illustrated by confocal LIVE/DEAD imaging (green, calcein AM (live cells); red, ethidium homodimer (dead cells)).