Abstract
Purpose
Older cancer survivors are a vulnerable population due to an increased risk for chronic diseases (e.g., cardiovascular disease) compounded with treatment late-effects and declines in physical functioning. Therefore, interventions that reduce chronic disease risk factors (i.e., blood pressure, chronic inflammation, & cortisol) are important in this population. Tai Chi Chih (TCC) is a mind-body exercise associated with reductions in chronic disease risk factors, but has not been examined with older cancer survivors. In a feasibility randomized controlled trial of TCC, we examined secondary outcomes of blood pressure, salivary cortisol, and inflammatory cytokines (interleukin (IL)-6, IL-12, tumor necrosis factor-α, IL-10, IL-4) due to their implications in chronic diseases.
Methods
Sixty-three senior female cancer survivors (Mage=67 years, SD=7.15) with physical functioning limitations (SF-12 physical functioning≤80 or role-physical≤72) were randomized to 12-weeks (60-minutes, three times a week) of TCC or Health Education control (HEC) classes. Resting blood pressure, 1-day salivary cortisol samples, and fasting plasma samples for cytokine multiplex assays were collected at baseline and 1-week post-intervention.
Results
Controlling for baseline values, the TCC group had significantly lower systolic blood pressure (SBP, p=0.002) and cortisol area-under-curve (AUC, p=0.02) at post-intervention than the HEC group. There was no intervention effect on inflammatory cytokines (p’s>0.05).
Conclusions
This TCC feasibility trial was associated with significant reductions in SBP and cortisol AUC in senior female cancer survivors. Larger, definitive trials are needed to confirm these findings.
Keywords: Senior Cancer Survivors, Tai Chi Chih, Randomized Controlled Trial, Blood Pressure, Cortisol, Inflammatory Cytokines
Introduction
An estimated 60% of cancer survivors are 65 years of age or older and the number of older survivors is projected to increase dramatically by the year 2020 [1]. Senior cancer survivors are a particularly vulnerable population because they have an increased risk for the development or progression of chronic diseases (e.g., cardiovascular, hypertension, stroke, Type 2 diabetes mellitus, arthritis, etc), alongside cancer and/or treatment-related late effects (e.g., pain, fatigue, lymphedema, etc.), risks for cancer recurrence and additional primaries, and declines in physical functioning [2–7]. Likewise, the coexistence of chronic diseases with late effects can accelerate declines in survivors’ health-related quality of life [8, 7] and worsen survival prognosis [9]. In senior cancer survivors, this is highly relevant as twice as many deaths occur as a result of chronic diseases other than cancer, with cardiovascular disease emerging as a leading cause of death[10]. Reported potential causes include lifestyle factors (decreased physical activity, increased weight gain), existence of comorbidities (e.g., hypertension , Type 2 diabetes mellitus), and treatment-related factors such as the adverse cardiac effects associated with chemotherapy and radiation treatment [3, 10–12, 7, 13]. This presents a need to intervene on senior cancer survivors’ modifiable risk factors (e.g., hypertension, chronic inflammation, lack of physical activity, etc.) that are associated with the most common chronic diseases (i.e., cardiovascular) in this population [14, 5]).
Exercise is associated with reductions in chronic disease risk factors [15] and reduced risk of cancer recurrence and all-cause mortality in cancer survivors [16, 17, 2, 18]. Unfortunately, a majority of older survivors do not meet physical activity recommendations, with female survivors older than 60 years of age spending 44% less time in physical activity than those younger than 50 years of age [19]. Tai Chi (TC) is a form of exercise that is also known as meditative movement because it combines physical activity with meditation [20]. There are various styles of TC, but generally the practice consists of focused, fluid physical movements (i.e., balance and shifting of body weight), which are performed in a specified order, coordinated with breathing and imagery to relax the mind, strengthen the body, and improve the flow of “qi” or life energy [21]. The metabolic equivalents (METs) of TC range from an estimated 3.3 METs (Yang-style Tai Chi Chuan) to 2.6 METs (Tai Chi Chih style); a moderate-intensity level similar to walking at 2 to 3 miles per hour [22, 23]. For the older cancer survivor, TC may be an appealing form of exercise because it consists of repetitive, steady movements that are considered safe (i.e., can be performed sitting or standing), easy to learn [21, 24], and can be performed in various types of locations (i.e., at home or other setting) with little to no cost.
Similar to traditional exercise, TC is associated with improvements in chronic disease risk factors such as cardiovascular factors (i.e., reductions in blood pressure (BP), sympathetic activity, lipid profiles, endothelial dysfunction) [21, 25–27], insulin markers (i.e., insulin-like growth factors, maintained insulin levels) [28], chronic inflammation (C-reactive protein (CRP) [29, 30], increased cell-mediated immunity to the Shingles virus [31, 32], functional capacity [33, 34], bone metabolism benefits [35] , and healthrelated quality of life (QOL) [33, 36, 29, 32, 37, 31, 34]. Although the mechanisms for TC influences on biological risk factors have not been established, proposed pathways include physical activity and stressreduction influences on the hypothalamic – pituitary – adrenal (HPA) axis and autonomic nervous system (i.e., decreased sympathetic activity, increased parasympathetic activity), which can interact with the immune system via different pathways [38–40]. Pro-inflammatory cytokines (such as Tumor necrosis factor (TNF)-α, IL-6) trigger downstream release of glucocorticiods (cortisol) via activation of the HPA axis, which in turn downregulates pro-inflammatory cytokines and mediates a shift to a T-helper2 (Th-2) anti-inflammatory cytokine balance [41]. However, chronically elevated cortisol levels may also lead to glucocorticoid receptor resistance, thus increasing susceptibility to inflammatory-related diseases [42]. This has relevance to senior cancer survivors because aging is associated with dysregulation of the immune system and chronic low-grade inflammation. Chronic inflammation is a risk factor for cardiovascular disease, linked to cancer growth and progression, and a predictor of physical decline and all-cause mortality in elderly adults [43, 44, 38, 45–48]. Therefore, reductions in chronic disease risk factors is important for reducing older survivor’s increased risk for cardiovascular disease and other chronic diseases with underlying chronic inflammation (e.g., arthritis, atherosclerosis, anemia, cancer, Alzheimer’s, etc.) [7, 49, 11, 10]. In this study, we examined blood pressure because hypertension is a major risk factor for cardiovascular disease [50, 51] and the prevalence of cardiovascular disease mortality/morbidity is high among senior cancer survivors [49, 10]. Additionally, we examined the pro-inflammatory cytokines IL-12, IL-6, TNF-α, and the anti-inflammatory cytokines IL-10 and IL-4 because these are reported by researchers to be major cytokines and/or have implications for chronic disease and/or cancer-related outcomes [41, 52]. Finally, salivary cortisol was examined due to its role in inflammatory responses and chronic disease risk [42, 41, 53].
In this manuscript, we report on secondary outcomes of BP, salivary cortisol, and inflammatory cytokines from a 12-week feasibility RCT of TCC in senior female cancer survivors. A prior publication has reported on the RCT’s primary outcomes of feasibility, acceptability, and health-related QOL outcomes [54]. In this RCT, we focused on female survivors to expand on prior TC trials conducted with breast cancer survivors [28, 36]. Additionally, we focused on senior survivors with some physical functioning limitations so that these findings would be generalizable to the real-world setting where many older survivors are experiencing declines in physical functioning [8]. Although prior RCTs of TCC and TC have been conducted with non-cancer senior adults and breast cancer survivors, to our knowledge no RCT has examined the effects of a TCC intervention on senior female cancer survivors’ chronic disease risk factors.
Methods
Study Design and Participants
The study design was a two-armed, parallel group, feasibility RCT that followed the guidelines of the Consolidated Standards of Reporting Trials (CONSORT) statement (Figure 1) [55]. It was approved by the University of Utah’s Institutional Review Board and registered at ClinicalTrials.gov (Identifier NCT01305044). Sample size power calculations were based on this feasibility trial’s primary outcomes of health-related QOL (SF-36), which have been reported previously [54]. The biomarker outcomes from this study would be used to determine the sample size necessary for a larger, definitive RCT.
Figure 1.
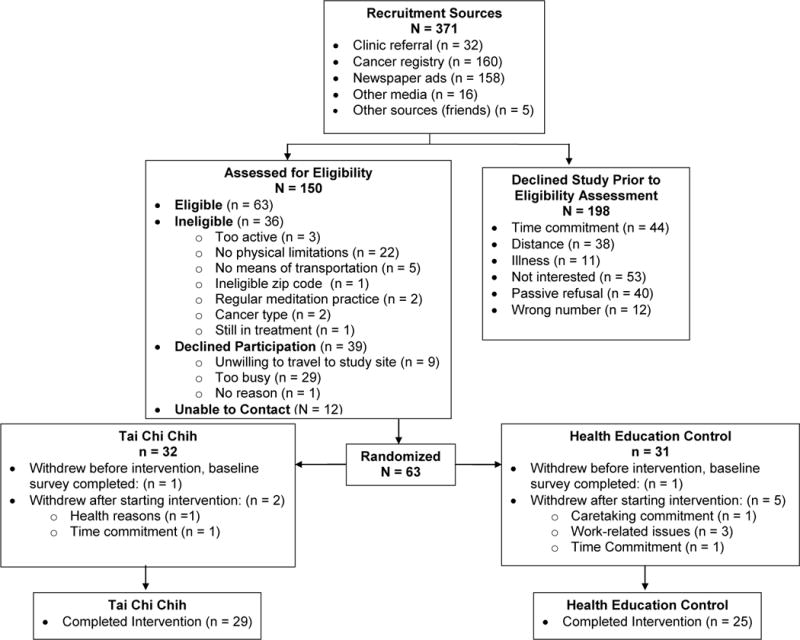
Consort diagram of the Tai Chi Chih Randomized Controlled Trial.
Senior female cancer survivors (age ≥ 55 years) with physical functioning limitations (SF-12 subscales: physical functioning ≤80, or role-physical ≤72) were recruited via Huntsman Cancer Institute (HCI) Tissue Resource & Applications Core registry (TRAC), Huntsman Cancer Hospital clinics, and community advertisements. The cut-off values for the SF-12 physical functioning and role-physical subscales were based on a TCC trial in which low functioning older adults (using similar SF-12 cut-off values) benefited the most from the intervention [32]. As mentioned prior, we focused on survivors with physical functioning limitations so that these findings would be generalizable to the real-world setting where many older survivors are experiencing declines in physical functioning [8]. Additional inclusion criteria were: 1) Diagnosis of solid tumor cancer, stages I-III, 2) ≥3 months since cancer treatment completion (exception of hormone therapy), with no detectable cancer, 3) not currently engaged in focused, intense physical activity for 30 minutes or more a day, for ≥ 3 days per week, and 4) no experience with Tai Chi, yoga, or similar types of mind-body exercises within the past six months. Further details of eligibility criteria are also reported in prior publication [54]. All participants provided written informed consent.
Measures
We examined one-week post-intervention outcomes in BP, salivary cortisol, and inflammatory cytokines because acute effects of a 12-week TCC intervention were of interest. This timeframe has also been used in other TCC interventions for older adults that examined biomarker outcomes [32, 30].
Blood Pressure
Resting systolic (SBP) and diastolic blood pressure (DBP) were assessed with an oscillating blood pressure cuff (Omron 5 Series model) at baseline (prior to randomization) and at one week post-intervention. BP was measured in the morning of participants’ physical assessment session1, after the participant had been seated for approximately five minutes.
Cortisol
Five saliva samples (awakening, 30 minutes after awakening, noon, 5pm, & 10pm) were collected on a weekend day at baseline and one-week post-intervention with Salivette® swabs (Sarstedt AG & Co.). Participants were asked to refrain from brushing teeth, eating, or drinking 30 minutes prior to collection. Salivary cortisol samples were assayed at the Kirschbaum Biopsychology Laboratory at Technical University of Dresden, Germany. The samples were prepared for biochemical analysis by centrifuging at 3000 rpm for 5 min, which resulted in a clear supernatant of low viscosity. Salivary free cortisol concentrations were determined employing a chemiluminescence immunoassay (CLIA) with high sensitivity of 0.16 ng/ml (IBL; Hamburg, Germany). Intra- and inter-assay coefficients of variation were below 8%.
Inflammatory Cytokines
Fasting blood samples were drawn on the morning of the study’s physical assessment sessions1 at baseline and one-week post-intervention. Prior to the blood draws, we ensured that participants did have illness or fever at the time of the blood draw. Plasma aliquots were assayed at the Associated Regional and University Pathologists (ARUP) Institute for Clinical and Experimental Pathology in Salt Lake City, Utah with a multiplexed cytokine assay developed at the Institute using a standard sandwich capture format [56]. This multiplex assay has a large dynamic range with good sensitivity, measuring from less than 10 to 10,000 pg/mL [57]. As mentioned prior, we analyzed the pro-inflammatory cytokines IL-12, IL-6, TNF-α, and the anti-inflammatory cytokines IL-10 and IL-4 because these are reported by researchers to be major cytokines and/or have implications for cancer-related outcomes [41, 52].
Intervention
After completion of the baseline survey, HCI’s Research Informatics Shared Resource randomized participants in blocks of 2 to 4 to TCC or Health Education Classes (HEC). A single-blinded design was not followed because informed consent procedures required that participants be informed that they would be randomly assigned to either TCC or HEC. Statisticians were blinded to study group allocation. The TCC and HEC each comprised 60 minute sessions, three days per week, over a 12-week period (i.e., total of 36 sessions). The class frequency and intervention duration was based on an amount found to be efficacious for physical function outcomes in TCC and other TC forms involving 20–25 movements [21, 34, 58]. This was also viewed as an acceptable length in this senior sample for whom time commitment (i.e., time required to attend classes and for traveling) is a barrier to engaging in physical activity [59]. Our intervention duration is consistent with exercise interventions for cancer survivors [60] and TC and Qigong RCTs with cancer survivors that have ranged from 45 minutes to 2 hours per session, from 2 to 5 times a week, for 5 to 12 weeks [58]. Due to space limitations, the HEC classes were held at HCI, whereas the TCC sessions were held at a community senior center within two miles of HCI (i.e., held at similar times on the same three weekdays).
The TCC intervention was led by an experienced instructor who was accredited through teacher training provided by the TCC community. The TCC movements consist of 19 non-strenuous movements and one standing pose: Rocking Motion, Bird Flaps its Wings, Around the Platter, Around the Platter variation, Bass Drum, Daughter on the Mountaintop, Carry the Ball, Push Pull, Pulling in Energy, Pulling Taffy, Pulling Taffy –Anchor, Pulling Taffy-Wrist Circles, Pulling Taffy-Perpetual Motion, Working the Pulley, Light at the Top of the Head, Joyous Breath, Passing Clouds, Six Healing Sounds, and Cosmic Consciousness Pose. Sessions began with a 20-minute warm-up that included seated meditation, self-massage of acupressure points, and light stretching, followed by 30 minutes of TCC movements and 10 minutes of closing movements. Participants were informed that all movements could be performed seated if needed. HEC served as an attention control group and focused on topics relevant to aging (e.g., successful aging, pain, sleep changes, & social roles), with the majority of topics spanning two classes each. These classes were led by a variety of health specialists (i.e., gerontological oncologist, nutritionist, physical therapist, social worker, & health promotion specialists). Make-up classes were not offered to participants; however, the TCC participants received a DVD of the instructor performing the TCC movements (data on home practice was not collected). Class attendance was encouraged by the research coordinator (i.e., missed classes were followed up with a phone call to the participant) and attendance was recorded by study staff. Participants were asked not to begin new physical activity during the study.
Data Analysis
Pearson chi-square tests for categorical data and Wilcoxon tests for continuous data (due to data skewness) were used to compare study groups on baseline sociodemographics. The cortisol and inflammatory cytokine data were log-transformed due to appreciable skewness. Analysis of covariance (ANCOVA), controlling for baseline, compared the TCC and HEC groups at post-intervention on BP, salivary cortisol, and inflammatory cytokines. Post-hoc analyses were conducted with Pearson bivariate correlations and Fishers Exact tests. Analyses were conducted on participants with complete data at baseline and post-intervention. In this feasibility trial, intent-to-treat analyses were not conducted because we did not have post-intervention data on participants who withdrew from the study to conduct complete case analysis and our sample size was too small to conduct multiple imputation techniques. Analyses were conducted with SAS (version 9.2) and statistical significance was based on p<0.05.
Results
Feasibility
As shown in the CONSORT diagram (Figure 1), we randomized 63 survivors to a study group and 54 completed the intervention. The overall retention rate for the intervention (i.e., the proportion who remained enrolled) was 86% and did not significantly differ between study groups [TCC = 91% (3 out of 32 withdrew), HEC = 81% (6 out of 31 withdrew); p=0.44]. The most common reasons for withdrawing after randomization were work-related reasons and time commitment. The overall class attendance was 81% and did not differ between study groups (TCC=79%, HEC=83%; p=0.36). There were no adverse events to report. We have also reported details of the trial’s feasibility and acceptability in a prior manuscript [54].
Sociodemographics and Medical Characteristics
The study groups did not significantly differ in baseline sociodemographics and medical characteristics (Table 1). The median age was 66.54 years (55 – 84 years) and the majority had a history of breast cancer (80% of the N=54 included in data analysis); other types included colorectal, cervical, uterine, thyroid, bladder, and nasopharyngeal. The number of years since cancer diagnosis or treatment did not differ between study groups (Table 1, p’s>.05). Importantly, the study groups were balanced on cancer treatments (including hormone treatment) and self-reported use of medications (i.e., cardiovascular, diabetic, antidepressants, & corticosteroids; p’s>.05). Participants reported an average of 1.90 (SD=1.22) comorbidities (i.e., depression, hypertension, heart attack, diabetes, arthritis, or chronic obstructive pulmonary disease), and the total number of comorbidities did not differ between groups (p=0.85). We did not include cancer treatments, medication use, or comorbidities as covariates in the model because these were balanced between study groups and the study was underpowered to include these as covariates. In our main analysis, we controlled for baseline assessments of the variable of interest (i.e., blood pressure, cortisol, & cytokines) to control experimental error and increase the precision in which the intervention effect could be measured.
Table 1.
Baseline Sociodemographic and Medical Characteristics of Participants by Study Arm
| TCC n=29 |
HEC n=25 |
p-value | |
|---|---|---|---|
| Age, Mdn years (range) | 65.9 (55 – 82) | 66.7 (59 – 84) | 0.74 |
| Non-Latino | 27 (93%) | 24 (96%) | 0.64 |
| White Race | 28 (97%) | 25 (100%) | 0.54 |
| Marital Status | 0.35 | ||
| Married/living as married | 21 (72%) | 21 (84%) | |
| Not Marrieda | 8 (23%) | 4 (16%) | |
| Educationb | 0.06 | ||
| High School, some vocational school or college | 20 (69%) | 10 (42%) | |
| College degree/Post-grad | 9 (31%) | 14 (58%) | |
| Religion | 0.53 | ||
| Latter-Day-Saints/Mormon | 12 (41%) | 11 (44%) | |
| Other | 17 (59%) | 14 (56%) | |
| Employment | 0.37 | ||
| Employed | 6 (21%) | 8 (32%) | |
| Not employedc | 23 (79%) | 17 (68%) | |
| Income ≥$50,000 | 16 (55%) | 9 (33%) | 0.30 |
| Breast Cancer Diagnosis | 22 (76%) | 21 (84%) | 0.38 |
| Cancer Stage | 0.11 | ||
| Stage I | 9 (31%) | 7 (29%) | |
| Stage II | 7 (24%) | 12 (50%) | |
| Stage III | 13 (45%) | 5 (21%) | |
| Cancer Treatment | |||
| Surgery | 24 (83%) | 22 (88%) | 0.44 |
| Radiation | 18 (62%) | 15 (60%) | 0.55 |
| Chemotherapy | 16 (55%) | 16 (64%) | 0.58 |
| Current Hormone Treatment | 7 (24%) | 4 (16%) | 0.35 |
| Years Since Diagnosis Mdn (range) | 9.0 (1 – 31) | 8.0 (1 – 40) | 0.50 |
| Years Since Treatment Mdn (range) | 8.5 (0 – 31) | 6.0 (1 – 28) | 0.28 |
| Comorbiditiesd | |||
| Heart Attack | 5 (17%) | 0 (0%) | 0.05 |
| Arthritis | 17 (59%) | 16 (64%) | 0.78 |
| Hypertension | 16 (55%) | 13 (52%) | 1.00 |
| Depression | 12 (41%) | 11 (44%) | 1.00 |
| Type 2 Diabetes Mellitus | 2 (7%) | 3 (12%) | 0.65 |
| COPD | 2 (7%) | 4 (16%) | 0.39 |
Note. Unless specified, values represent % (n). Wilcoxon tests were used for continuous data and Pearson Chi-square tests were used for categorical data. Fishers Exact test was used for categorical data with <5 per cell. Mdn =Median, TCC=Tai Chi Chih, HEC=Health Education Class, COPD=chronic obstructive pulmonary disease.
Includes divorced, separated, never married, widowed.
One person’s data not reported in HEC.
Includes retired, unable to work, or unemployed.
Self-reported data from the question: “Have you ever been treated or told by a doctor that you have had any of the following…”.
Blood Pressure
ANCOVAs, controlling for baseline, examined if the study groups’ SBP and DBP differed at post-intervention. Complete data for analysis was available for 29 TCC participants and 24 HEC participants. The TCC group had significantly lower SBP at post-intervention than the HEC group (TCC adjM=119.00, SE=2.81; HEC adjM =132.57, SE=3.09, p=0.002; Figure 2).2 The study groups did not significantly differ for DBP (TCC adjM =79.62, SE=2.18; HEC adjM =79.69, SE=2.40, p=0.98).
Figure 2.

Systolic (top) and diastolic (middle) blood pressure and cortisol AUC (bottom) adjusted means for study groups (N=54) at baseline and post-intervention. Bars represent standard errors. *p<.05.
Cortisol
The analyses included participants with complete data for all five collection samples (TCC n=20, HEC n=19). Nine participants (TCC n=6, HEC n=3) were missing at least one of the five samples due to insufficient saliva production. Then, four participants (TCC n=2, HEC n=2) with high cortisol profiles were excluded because of values greater than 75 nmol/L, which research has suggested removal of as the high levels may be due to altered pH-values or suspected blood contamination [61, 62]. Two participants (one each in TCC and HEC) whose first (awakening) and second (30-minutes after awakening) sample collection times were at least 3 hours apart were excluded from analysis.
ANCOVA results for the log-transformed cortisol awakening response (CAR; difference between awakening and 30 minutes after awakening), controlling for CAR baseline, revealed that the study groups did not significantly differ at post-intervention (TCC adjM =0.37, SE=0.13; HEC adjM =0.46, SE=0.13; p=0.63). Similar results were found when the analysis included participants whose first and second samples were at least 3 hours apart (p=0.19). Next, we examined whether the study groups differed in their diurnal slopes (i.e., the 30 minutes after awakening sample to the 10 pm sample) and did not find significant group differences (TCC b = −0.69, HEC b = −0.59; p=0.28). Finally, we compared the groups at post-intervention on their log-transformed cortisol area-under-the-curve values (AUC, calculated with the Trapezoid rule), controlling for baseline cortisol AUC.3 Results indicated that the TCC group had significantly lower post-intervention cortisol AUC than the HEC group (TCC adjM =24.65, SE=1.52; HEC adjM =29.95, SE=1.56, p=0.02; Figure 2).
Inflammatory Cytokines
Complete data for analysis of the inflammatory cytokines was available for 28 TCC participants and 24 HEC participants because two participants (one each in TCC and HEC) did not provide blood samples. ANCOVAs, controlling for baseline, were conducted on log-transformed pro- and anti-inflammatory cytokines (IL-12, IL-6, TNF-α, IL-10, & IL-4) at post-intervention. Results indicated that the study groups did not significantly differ among the pro- and anti-inflammatory cytokines at post-intervention (all p’s>.05). Summary data of the log-transformed values at baseline and post-intervention are presented in Table 2.
Table 2.
Summary Data of Log-transformed Inflammatory Cytokines at Baseline and Post-Intervention
| TCC Mdn (Q1, Q3) n=28 |
HEC Mdn (Q1, Q3) n=24 |
|
|---|---|---|
| IL-12 pg/ml | ||
|
| ||
| Baseline | 3.5 (2.0, 8.3) | 2.5 (1.6, 10.0) |
| Post-Intervention | 4.7 (3.3, 12.5) | 3.7 (1.3, 7.4) |
|
| ||
| IL-6 pg/ml | ||
|
| ||
| Baseline | 6.8 (4.1, 24.2) | 6.8 (3.9, 11.2) |
| Post-Intervention | 9.1 (4.3, 29.0) | 5.3 (3.4, 8.2) |
|
| ||
| TNF-α pg/ml | ||
|
| ||
| Baseline | 19.7 (0, 101.5) | 34.4 (0, 85.2) |
| Post-Intervention | 37.8 (0, 77.9) | 45.1 (0, 109.1) |
|
| ||
| IL-4 pg/ml | ||
|
| ||
| Baseline | 1.0 (0.5, 3.1) | 0.9 (0.6, 2.3) |
| Post-Intervention | 1.5 (0.6, 3.6) | 1.1 (0.5, 2.2) |
|
| ||
| IL-10 pg/ml | ||
|
| ||
| Baseline | 5.9 (4.2, 19.8) | 4.5 (3.4, 7.7) |
| Post-Intervention | 5.8 (4.3, 16.3) | 5.0 (2.9, 7.7) |
Note. Table includes participants with complete post-intervention data. TCC=Tai Chi Chih, HEC=Health Education Control, Mdn=Median, Q1=Lower Quartile, Q3= Upper Quartile, IL=Interleukin, TNF=Tumor Necrosis Factor.
Post-hoc Analyses
In our prior publication, we reported on SF-36 QOL outcomes [54]. For exploratory purposes, we examined associations of changes in the biomarker outcomes (blood pressure, inflammatory cytokines, AUC cortisol) with changes in the mental (MCS) and physical component summary (PCS) scores for each group using Pearson bivariate correlations. These results did not reveal significant associations of the biomarkers with PCS or MCS for either the TCC or HEC group (all p ’s >0.05).
Finally, older age is associated with a high-risk biological profile (e.g., higher BP & chronic lowgrade inflammation) due to more comorbidities, which could have influenced our biomarker outcomes. To examine this, we divided the sample into a younger (55 to 64 years of age, n=21) and an older age group (> 65 years of age, n=33), and compared these age groups on reported baseline comorbidities (i.e., hypertension, heart attack history, diabetes, arthritis, depression, & COPD) with Fishers Exact tests. These results did not reveal any age differences for proportion of comorbidities among the younger and older age groups (p’s> 0.05). We also conducted Pearson bivariate correlations to examine the associations of age with baseline blood pressure, cortisol (i.e., CAR, AUC), and the inflammatory cytokines. These results were also nonsignificant (p’s> 0.05), suggesting that older age was not associated with our biomarker outcomes.
Discussion
Our 12-week TCC intervention for senior female cancer survivors was associated with lower levels of SBP and cortisol AUC at post-intervention in the TCC group compared to the HEC group. These SBP findings are consistent with other TC interventions of similar length that have found significant BP reductions in different populations (i.e., general and older adults, cardiovascular disease patients) [26, 21, 27, 63]. Although we did not find reductions in DBP, we view the reductions in SBP as noteworthy because isolated SBP hypertension is the most common type of uncontrolled hypertension in older adults and it is associated with an increased risk for coronary heart disease, stroke, and end-stage renal disease [50]. Reduced BP is also highly relevant for senior cancer survivors considering that cardiovascular disease is a leading cause of morbidity and mortality in long-term survivors [49, 10]. Thus, it is essential that senior cancer survivors maintain a normotensive status in order to manage their cardiovascular risk and TC exercise may be offered as a non-pharmacological intervention for managing this risk.
In regards to the cortisol outcomes, there are a limited number of TC interventions that have examined cortisol in cancer survivors and these studies did not find significant differences compared to a control group [64, 36]. Similar to Chen and colleagues (2013), we did not find that our study groups differed on their slopes or CAR; however, we found that AUC cortisol was lower for the TCC group than the HEC group. This may be an important outcome considering that elevated AUC cortisol has been found in advanced-stage depressed cancer patients (ovarian cancer) [65] and elevated cortisol levels are an indicator of early mortality for metastatic breast cancer patients [66].
We did not find an intervention effect for the inflammatory cytokine levels, which is consistent with cytokine outcomes reported in prior TC studies conducted with healthy older adults and breast cancer survivors [30, 36, 28]. One speculation for our null finding is that TC has a limited influence on senior cancer survivors’ inflammatory cytokines due to a complex interaction of factors associated with aging (chronic low-grade inflammation), existence of comorbidities, and cancer treatment late effects that can result in immune dysregulation [39, 48, 43, 44, 38]. However, exercise research with older adults suggests there is a dose-response relationship between regular exercise and reductions in age-related inflammation [67]. Particularly, C-reactive protein (CRP, downstream inflammatory marker) tends to be the most consistently responsive to exercise, whereas, the evidence for inflammatory cytokines, such as IL-6 and TNF-α, has been less consistent [67]. Likewise, TC studies with older adults and cancer survivors have reported reductions in CRP [29, 68], but normalized IL-6 levels have only been found among older adults who had high baseline levels, with no effects for other inflammatory cytokines [30]. Another speculation is that the intervention did not result in increased aerobic fitness or fat loss (i.e., fat mass & adipose tissue), which could have led to reductions in inflammatory cytokines [67]. A TC intervention with breast cancer survivors did find associations between decreased fat mass and increased fat-free mass with increased IL-6 (muscle-derived IL-6 has anti-inflammatory effects) and decreased IL-2 levels [28]. Future TC research with senior cancer survivors would benefit from including downstream markers of the inflammatory process, such as CRP, and assessments of aerobic fitness and fat loss (i.e., fat mass & adipose tissue) to allow the examination of potential inflammatory mechanisms.
Our study has a few limitations to be noted. First, the mechanisms that may have driven the SBP and cortisol AUC outcomes are unknown. We can speculate that our findings may have been a result of psychosocial processes (e.g., stress reductions), increases in physical activity, or the combined effects of these. All of these are associated with improved chronic disease risk factors [69, 15, 18]. However, we did not find any associations of changes in mental-health or physical-health QOL with changes in the biomarker outcomes. A review on TC RCTs for patients with cardiovascular conditions indicated that TC was associated with greater reductions in BP when compared to a health education control or to no treatment, but outcomes were equivalent to physical exercise [63]. This suggests that TC may be associated with reductions in BP due to physical activity. Future assessments of psychosocial factors (i.e., stress) and objective fitness outcomes (i.e., aerobic fitness & fat loss) may help clarify the underlying mechanisms behind TCC’s effects.
Another limitation is that the outcomes were limited to one-week post-intervention. In this feasibility trial, we were interested in the acute outcomes of TCC on senior female survivors’ chronic disease risk factors. However, a 2-month or longer follow-up may reveal whether TC practice and these BP and cortisol outcomes can be maintained or additional biomarker improvements observed (i.e., inflammatory cytokines). Very few studies have examined the long-term effects of mind-body activities on biomarker levels, with the exception of an 8-week stress-reduction intervention with yoga that found continued reductions in cortisol and inflammatory cytokines over a one year follow-up period [70]. As noted, future studies should assess whether similar ongoing physiological benefits would be observed in senior cancer survivors who are more long-term TC practitioners, as this may have implications for the management of chronic diseases.
Additionally, these findings are limited to older female, mainly Caucasian, cancer survivors, thus may not be generalizable to older male cancer survivors or older racial/ethnic minority survivors. Future TCC studies should examine whether similar BP and cortisol outcomes are observed with prostate cancer survivors, a population that is at an increased risk for diabetes and heart disease due to the side effects of androgen deprivation therapy [71]. These findings should also be examined in more racially/ethnically diverse survivor populations. We are not aware of any Tai Chi trials that have examined the uptake or efficacy of Tai Chi for improving biomarker outcomes in ethnic minority survivors. Additionally, these findings are limited to senior female survivors with limitations in physical functioning. Other TC studies with breast cancer survivors that did not limit by physical functioning have found beneficial outcomes in functional capacity [33, 34], insulin levels [28], QOL [33, 36, 34] and bone metabolism [35].
A final limitation is that the reliability of our cortisol results is limited by a single-day collection, rather than a multiple-day collection. Given the day-to-day variability in cortisol [72], future studies should aim to replicate these findings by including two to three days of salivary cortisol collections. Despite these issues, we view our findings for lower cortisol AUC in the TCC group as promising in light of prior studies that found elevated cortisol levels predicted poor survival outcomes for cancer survivors [73, 66].
In summary, a major strength of our study is that we have demonstrated that TCC interventions have the potential to improve chronic disease risk factors (i.e., SBP, cortisol) in senior cancer survivors with physical functioning limitations. This has important implications for older cancer survivors who have increased morbidity and mortality risks due to common chronic diseases, particularly cardiovascular disease [9, 3, 10]. However, our results should also be viewed as preliminary due to the small and heterogeneous sample of senior survivors in this feasibility trial. Future large-scale efficacy trials are needed to replicate our findings across a wider range of older cancer survivors (i.e., both men & women, more ethnically diverse populations), examine biomarker outcomes at longer follow-up periods, and to help determine the mechanisms (i.e., psychosocial factors and/or improved physical fitness and fat loss) responsible for these outcomes. These findings can help determine effective behavioral interventions for the prevention and management of chronic diseases in older cancer survivors.
Implications.
Senior survivors’ have an increased risk for chronic diseases; however, TCC interventions may help reduce associated risk factors.
Acknowledgments
Preparation of this manuscript was funded by a National Institutes of Health National Center for Complementary and Alternative Medicine Research Fellowship in Complementary and Alternative Medicine (T-32 AT00378) at the University of North Carolina for the first author (R.A. Campo). The Health Education & Active Living in Surviving Seniors (HEALS) Project was funded by a grant from the National Cancer Institute (R21CA135250) awarded to Dr. Anita Kinney and by the Huntsman Cancer Foundation. Additional support was provided by the Shared Resources (P30 CA042014) for use of the Research Informatics Shared Resource and the Study Design and Biostatistics Center. Additionally, the Linda B. and Robert B. Wiggins Wellness-Survivorship Center at Huntsman Cancer Institute provided support for the study physical assessments. This content is solely the responsibility of the authors and does not necessarily represent the official views of the funding and supporting agencies. The authors have no conflicts of interest to report. We thank the Associated Regional and University Pathologists’ Institute for Clinical and Experimental Pathology at the University of Utah and the Kirschbaum Biopsychology Laboratory at Technical University of Dresden, Germany for assays of the inflammatory cytokines and cortisol samples, respectively.
Footnotes
The study’s physical assessments (i.e., blood draw for cytokines, blood pressure assessed) were held during a morning session one week before the classes began, before participants’ randomization to study group, and during a morning session one week following the last study class. The sessions were held in a group format in which all the participants attended.
We also conducted an ANCOVA subanalysis for SBP that excluded the five TCC participants who reported a history of a heart attack and found similar results (p=0.004).
The sample times for the cortisol AUC analysis were fixed at 7:00 am, 7:30 am, 12:00 noon, 5:00 pm, and 10:00 pm. We recognize that this is a study limitation and that these results should be viewed as preliminary.
Conflict of Interest
All authors declare that they have no conflict of interest.
References
- 1.Parry C, Kent EE, Mariotto AB, Alfano CM, Rowland JH. Cancer survivors: a booming population. Cancer Epidemiol Biomarkers Prev. 2011;20(10):1996–2005. doi: 10.1158/1055-9965.EPI-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ACS. Cancer Treatment and Survivorship Facts & Figures 2012–2013. Atlanta: American Cancer Society; 2012. [Google Scholar]
- 3.Byers T, Patnaik JL. Missed opportunities for chronic disease prevention after breast cancer. Womens Health (Lond Engl) 2011;7(6):619–21. doi: 10.2217/whe.11.66. [DOI] [PubMed] [Google Scholar]
- 4.Demark-Wahnefried W, Pinto BM, Gritz ER. Promoting health and physical function among cancer survivors: potential for prevention and questions that remain. JClinOncol. 2006;24(32):5125–31. doi: 10.1200/JCO.2006.06.6175. [DOI] [PubMed] [Google Scholar]
- 5.Rao AV, Demark-Wahnefried W. The older cancer survivor. Crit RevOncolHematol. 2006;60(2):131–43. doi: 10.1016/j.critrevonc.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Rowland JH, Yancik R. Cancer survivorship: the interface of aging, comorbidity, and quality care. J NatlCancer Inst. 2006;98(8):504–5. doi: 10.1093/jnci/djj154. [DOI] [PubMed] [Google Scholar]
- 7.Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: age, health, and disability. J GerontolA BiolSciMed Sci. 2003;58(1):82–91. doi: 10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- 8.Yancik R, Ganz PA, Varricchio CG, Conley B. Perspectives on comorbidity and cancer in older patients: approaches to expand the knowledge base. J Clin Oncol. 2001;19(4):1147–51. doi: 10.1200/JCO.2001.19.4.1147. [DOI] [PubMed] [Google Scholar]
- 9.Patnaik JL, Byers T, Diguiseppi C, Denberg TD, Dabelea D. The influence of comorbidities on overall survival among older women diagnosed with breast cancer. J Natl Cancer Inst. 2011;103(14):1101–11. doi: 10.1093/jnci/djr188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13(3):R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yusuf SW, Sami S, Daher IN. Radiation-induced heart disease: a clinical update. Cardiology research and practice. 2011;2011:317659. doi: 10.4061/2011/317659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weaver KE, Foraker RE, Alfano CM, Rowland JH, Arora NK, Bellizzi KM, et al. Cardiovascular risk factors among long-term survivors of breast, prostate, colorectal, and gynecologic cancers: a gap in survivorship care? J Cancer Surviv. 2013;7(2):253–61. doi: 10.1007/s11764-013-0267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haque R, Prout M, Geiger AM, Kamineni A, Thwin SS, Avila C, et al. Comorbidities and cardiovascular disease risk in older breast cancer survivors. Am J Manag Care. 2014;20(1):86–92. [PMC free article] [PubMed] [Google Scholar]
- 14.Keating NL, Norredam M, Landrum MB, Huskamp HA, Meara E. Physical and mental health status of older long-term cancer survivors. J Am GeriatrSoc. 2005;53(12):2145–52. doi: 10.1111/j.1532-5415.2005.00507.x. [DOI] [PubMed] [Google Scholar]
- 15.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006;174(6):801–9. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293(20):2479–86. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 17.Irwin ML, Smith AW, McTiernan A, Ballard-Barbash R, Cronin K, Gilliland FD, et al. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. J Clin Oncol. 2008;26(24):3958–64. doi: 10.1200/JCO.2007.15.9822. doi:26/24/3958 [pii] 10.1200/JCO.2007.15.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Courneya KS, Vallance JK, McNeely ML, Karvinen KH, Peddle CJ, Mackey JR. Exercise issues in older cancer survivors. Crit Rev Oncol Hematol. 2004;51(3):249–61. doi: 10.1016/j.critrevonc.2004.05.001. doi:10.1016/j.critrevonc.2004.05.001 S1040842804000848[pii] [DOI] [PubMed] [Google Scholar]
- 19.Irwin ML, McTiernan A, Bernstein L, Gilliland FD, Baumgartner R, Baumgartner K, et al. Physical activity levels among breast cancer survivors. Med SciSports Exerc. 2004;36(9):1484–91. [PMC free article] [PubMed] [Google Scholar]
- 20.Larkey L, Jahnke R, Etnier J, Gonzalez J. Meditative movement as a category of exercise: implications for research. J Phys Act Health. 2009;6(2):230–8. doi: 10.1123/jpah.6.2.230. [DOI] [PubMed] [Google Scholar]
- 21.Rogers CE, Larkey LK, Keller C. A review of clinical trials of tai chi and qigong in older adults. West J Nurs Res. 2009;31(2):245–79. doi: 10.1177/0193945908327529. doi:31/2/245 [pii] 10.1177/0193945908327529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hui SS, Woo J, Kwok T. Evaluation of energy expenditure and cardiovascular health effects from Tai Chi and walking exercise. Hong Kong medical journal = Xianggang yi xue za zhi / Hong Kong Academy of Medicine. 2009;15(Suppl 2):4–7. [PubMed] [Google Scholar]
- 23.Fontana JA, Colella C, Wilson BR, Baas L. The energy costs of a modified form of T’ai Chi exercise. Nurs Res. 2000;49(2):91–6. doi: 10.1097/00006199-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Jahnke R, Larkey L, Rogers C, Etnier J, Lin F. A comprehensive review of health benefits of qigong and tai chi. American journal of health promotion : AJHP. 2010;24(6):e1–e25. doi: 10.4278/ajhp.081013-LIT-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motivala SJ, Sollers J, Thayer J, Irwin MR. Tai Chi Chih acutely decreases sympathetic nervous system activity in older adults. J Gerontol A Biol Sci Med Sci. 2006;61(11):1177–80. doi: 10.1093/gerona/61.11.1177. [DOI] [PubMed] [Google Scholar]
- 26.Yeh GY, Wang C, Wayne PM, Phillips RS. The effect of tai chi exercise on blood pressure: a systematic review. Prev Cardiol. 2008;11(2):82–9. doi: 10.1111/j.1751-7141.2008.07565.x. [DOI] [PubMed] [Google Scholar]
- 27.Lan C, Chen SY, Wong MK, Lai JS. Tai Chi Chuan Exercise for Patients with Cardiovascular Disease. Evid Based Complement Alternat Med. 2013;2013:983208. doi: 10.1155/2013/983208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janelsins MC, Davis PG, Wideman L, Katula JA, Sprod LK, Peppone LJ, et al. Effects of Tai Chi Chuan on insulin and cytokine levels in a randomized controlled pilot study on breast cancer survivors. Clin Breast Cancer. 2011;11(3):161–70. doi: 10.1016/j.clbc.2011.03.013. doi:S1526-8209(11)00014-0 [pii] 10.1016/j.clbc.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lavretsky H, Alstein LL, Olmstead RE, Ercoli LM, Riparetti-Brown M, Cyr NS, et al. Complementary use of tai chi chih augments escitalopram treatment of geriatric depression: a randomized controlled trial. Am J Geriatr Psychiatry. 2011;19(10):839–50. doi: 10.1097/JGP.0b013e31820ee9ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irwin MR, Olmstead R. Mitigating cellular inflammation in older adults: a randomized controlled trial of Tai Chi Chih. Am J Geriatr Psychiatry. 2012;20(9):764–72. doi: 10.1097/JGP.0b013e3182330fd3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irwin MR, Olmstead R, Oxman MN. Augmenting immune responses to varicella zoster virus in older adults: a randomized, controlled trial of Tai Chi. J Am GeriatrSoc. 2007;55(4):511–7. doi: 10.1111/j.1532-5415.2007.01109.x. [DOI] [PubMed] [Google Scholar]
- 32.Irwin MR, Pike JL, Cole JC, Oxman MN. Effects of a behavioral intervention, Tai Chi Chih, on varicella-zoster virus specific immunity and health functioning in older adults. Psychosom Med. 2003;65(5):824–30. doi: 10.1097/01.psy.0000088591.86103.8f. [DOI] [PubMed] [Google Scholar]
- 33.Mustian KM, Palesh OG, Flecksteiner SA. Tai Chi Chuan for breast cancer survivors. Med Sport Sci. 2008;52:209–17. doi: 10.1159/000134301. doi:10.1159/000134301 [pii] 10.1159/000134301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mustian KM, Katula JA, Zhao H. A pilot study to assess the influence of tai chi chuan on functional capacity among breast cancer survivors. J SupportOncol. 2006;4(3):139–45. [PubMed] [Google Scholar]
- 35.Peppone LJ, Mustian KM, Janelsins MC, Palesh OG, Rosier RN, Piazza KM, et al. Effects of a structured weight-bearing exercise program on bone metabolism among breast cancer survivors: a feasibility trial. Clin Breast Cancer. 2010;10(3):224–9. doi: 10.3816/CBC.2010.n.030. doi:S1526-8209(11)70032-5 [pii] 10.3816/CBC.2010.n.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sprod LK, Janelsins MC, Palesh OG, Carroll JK, Heckler CE, Peppone LJ, et al. Health-related quality of life and biomarkers in breast cancer survivors participating in tai chi chuan. J Cancer Surviv. 2012;6(2):146–54. doi: 10.1007/s11764-011-0205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mustian KM, Katula JA, Gill DL, Roscoe JA, Lang D, Murphy K. Tai Chi Chuan, health-related quality of life and self-esteem: a randomized trial with breast cancer survivors. Support Care Cancer. 2004;12(12):871–6. doi: 10.1007/s00520-004-0682-6. [DOI] [PubMed] [Google Scholar]
- 38.Irwin M, Pike J, Oxman M. Shingles Immunity and Health Functioning in the Elderly: Tai Chi Chih as a Behavioral Treatment. EvidBasedComplement AlternatMed. 2004;1(3):223–32. doi: 10.1093/ecam/neh048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lutgendorf SK, Costanzo ES. Psychoneuroimmunology and health psychology: an integrative model. Brain BehavImmun. 2003;17(4):225–32. doi: 10.1016/s0889-1591(03)00033-3. [DOI] [PubMed] [Google Scholar]
- 40.Friedman EM, Irwin MR. Modulation of immune cell function by the autonomic nervous system. Pharmacol Ther. 1997;74(1):27–38. doi: 10.1016/s0163-7258(96)00200-8. [DOI] [PubMed] [Google Scholar]
- 41.Elenkov IJ. Glucocorticoids and the Th1/Th2 balance. Ann N Y Acad Sci. 2004;1024:138–46. doi: 10.1196/annals.1321.010. [DOI] [PubMed] [Google Scholar]
- 42.Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci U S A. 2012;109(16):5995–9. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Licastro F, Candore G, Lio D, Porcellini E, Colonna-Romano G, Franceschi C, et al. Innate immunity and inflammation in ageing: a key for understanding age-related diseases. Immunity & ageing : I & A. 2005;2:8. doi: 10.1186/1742-4933-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120(4):435–46. doi: 10.1111/j.1365-2567.2007.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 46.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cesari M, Penninx BW, Pahor M, Lauretani F, Corsi AM, Rhys Williams G, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59(3):242–8. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- 48.Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39(5):687–99. doi: 10.1016/j.exger.2004.01.009. doi:10.1016/j.exger.2004.01.009 S0531556504000531 [pii] [DOI] [PubMed] [Google Scholar]
- 49.Daher IN, Daigle TR, Bhatia N, Durand JB. The prevention of cardiovascular disease in cancer survivors. Texas Heart Institute journal / from the Texas Heart Institute of St Luke’s Episcopal Hospital, Texas Children’s Hospital. 2012;39(2):190–8. [PMC free article] [PubMed] [Google Scholar]
- 50.He J, Whelton PK. Elevated systolic blood pressure and risk of cardiovascular and renal disease: overview of evidence from observational epidemiologic studies and randomized controlled trials. Am Heart J. 1999;138(3 Pt 2):211–9. doi: 10.1016/s0002-8703(99)70312-1. [DOI] [PubMed] [Google Scholar]
- 51.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303(20):2043–50. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 52.Lutgendorf SK, Sood AK. Biobehavioral factors and cancer progression: physiological pathways and mechanisms. Psychosom Med. 2011;73(9):724–30. doi: 10.1097/PSY.0b013e318235be76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elenkov IJ, Chrousos GP. Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann N Y Acad Sci. 2002;966:290–303. doi: 10.1111/j.1749-6632.2002.tb04229.x. [DOI] [PubMed] [Google Scholar]
- 54.Campo RA, O’Connor K, Light KC, Nakamura Y, Lipschitz DL, LaStayo PC, et al. Feasibility and acceptability of a Tai Chi Chih randomized controlled trial in senior female cancer survivors. Integrative cancer therapies. 2013;12(6):464–74. doi: 10.1177/1534735413485418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2011;9(8):672–7. doi: 10.1016/j.ijsu.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 56.Martins TB, Pasi BM, Pickering JW, Jaskowski TD, Litwin CM, Hill HR. Determination of cytokine responses using a multiplexed fluorescent microsphere immunoassay. Am J Clin Pathol. 2002;118(3):346–53. doi: 10.1309/N0T6-C56B-GXB2-NVFB. [DOI] [PubMed] [Google Scholar]
- 57.Hill HR, Martins TB. The flow cytometric analysis of cytokines using multi-analyte fluorescence microarray technology. Methods. 2006;38(4):312–6. doi: 10.1016/j.ymeth.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 58.Zeng Y, Luo T, Xie H, Huang M, Cheng AS. Health benefits of qigong or tai chi for cancer patients: a systematic review and meta-analyses. Complementary therapies in medicine. 2014;22(1):173–86. doi: 10.1016/j.ctim.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 59.Schutzer KA, Graves BS. Barriers and motivations to exercise in older adults. Prev Med. 2004;39(5):1056–61. doi: 10.1016/j.ypmed.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 60.Galvao DA, Newton RU. Review of exercise intervention studies in cancer patients. J Clin Oncol. 2005;23(4):899–909. doi: 10.1200/JCO.2005.06.085. [DOI] [PubMed] [Google Scholar]
- 61.Kunz-Ebrecht SR, Kirschbaum C, Marmot M, Steptoe A. Differences in cortisol awakening response on work days and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology. 2004;29(4):516–28. doi: 10.1016/s0306-4530(03)00072-6. doi:S0306453003000726 [pii] [DOI] [PubMed] [Google Scholar]
- 62.Aardal E, Holm AC. Cortisol in saliva–reference ranges and relation to cortisol in serum. European journal of clinical chemistry and clinical biochemistry : journal of the Forum of European Clinical Chemistry Societies. 1995;33(12):927–32. doi: 10.1515/cclm.1995.33.12.927. [DOI] [PubMed] [Google Scholar]
- 63.Yeh GY, Wang C, Wayne PM, Phillips R. Tai chi exercise for patients with cardiovascular conditions and risk factors: A systematic review. J Cardiopulm Rehabil Prev. 2009;29(3):152–60. doi: 10.1097/HCR.0b013e3181a33379. doi:10.1097/HCR.0b013e3181a33379 01273116-200905000-00002 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Z, Meng Z, Milbury K, Bei W, Zhang Y, Thornton B, et al. Qigong improves quality of life in women undergoing radiotherapy for breast cancer: Results of a randomized controlled trial. Cancer. 2013 doi: 10.1002/cncr.27904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lutgendorf SK, Weinrib AZ, Penedo F, Russell D, DeGeest K, Costanzo ES, et al. Interleukin-6, cortisol, and depressive symptoms in ovarian cancer patients. J Clin Oncol. 2008;26(29):4820–7. doi: 10.1200/JCO.2007.14.1978. doi:JCO.2007.14.1978 [pii] 10.1200/JCO.2007.14.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92(12):994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- 67.Woods JA, Wilund KR, Martin SA, Kistler BM. Exercise, inflammation and aging. Aging and disease. 2012;3(1):130–40. [PMC free article] [PubMed] [Google Scholar]
- 68.Oh B, Butow PN, Mullan BA, Clarke SJ, Beale PJ, Pavlakis N, et al. Effect of medical Qigong on cognitive function, quality of life, and a biomarker of inflammation in cancer patients: a randomized controlled trial. Support Care Cancer. 2011 doi: 10.1007/s00520-011-1209-6. [DOI] [PubMed] [Google Scholar]
- 69.Christian LM, Deichert NT, Gouin J-P, Graham JE, Kiecolt-Glaser JK. Psychological influences on neuroendocrine and immune outcomes. In: Berntson JTCGG, editor. Handbook of Neuroscience for the Behavioral Sciences Hoboken. New Jersey: John Wiley and Sons; 2009. pp. 1260–79. [Google Scholar]
- 70.Carlson LE, Speca M, Faris P, Patel KD. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain Behav Immun. 2007;21(8):1038–49. doi: 10.1016/j.bbi.2007.04.002. doi:S0889-1591(07)00085-2 [pii] 10.1016/j.bbi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 71.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24(27):4448–56. doi: 10.1200/JCO.2006.06.2497. doi:24/27/4448 [pii] 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 72.Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology. 1994;19(4):313–33. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 73.Abercrombie HC, Giese-Davis J, Sephton S, Epel ES, Turner-Cobb JM, Spiegel D. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology. 2004;29(8):1082–92. doi: 10.1016/j.psyneuen.2003.11.003. [DOI] [PubMed] [Google Scholar]


