Abstract
Tardive dyskinesia (TD) is movement disorder commonly associated with chronic exposure to antidopaminergic medications which may be in some cases disfiguring and socially disabling. The consensus from a growing body of research on the incidence and prevalence of TD in the modern era of antipsychotics indicates that this disorder has not disappeared continues to challenge the effective management of psychotic symptoms in patients with schizophrenia. A fundamental component in an effective strategy for managing TD is its reliable and accurate assessment. In the present study, we examined the clinical utility of a brief handwriting dysfluency measure for quantifying TD. Digitized samples of handwritten circles and loops were obtained from 62 psychosis patients with or without TD and from 50 healthy subjects. Two measures of dysfluent pen movements were extracted from each vertical pen stroke, including normalized jerk and the number of acceleration peaks. TD patients exhibited significantly higher dysfluency scores than non-TD patients and controls. Severity of handwriting movement dysfluency was correlated with AIMS severity ratings for some tasks. The procedure yielded high degrees of test-retest reliability. These results suggest that measures of handwriting movement dysfluency may be particularly useful for objectively evaluating the efficacy of pharmacotherapeutic strategies for treating TD.
INTRODUCTION
Antipsychotic-induced tardive dyskinesia (TD) continues to challenge the effective management of psychotic symptoms in patients with schizophrenia. While individuals treated with second generation antipsychotics are at lower risk for developing TD, published reviews indicate a prevalence rate of 10-15% with an incidence of approximately 4% per year 1,2. Reports demonstrating lowered incidence and prevalence of TD with second generation antipsychotics3,4 are offset by the rapid growth in the number of antipsychotic prescriptions written over the past decade with an estimated number of patients exposed to dopamine blocking agents now in excess of five million1 with off-label indications accounting for a significant portion of uses5. Children and adolescents account for a large proportion of the increase in use of antipsychotic medications6. A 2009 report indicated that 60% of the prescriptions for antipsychotic medications in a veteran population were for off-label indications7.
As the number of patients exposed to antipsychotics continues to grow so does the need to monitor and manage risks associated with these medications. Current strategies for reducing risk of developing persistent TD are based on prevention by identifying patients at higher risk for TD and, where possible switching antipsychotic medications8,9. Effective medical treatment for existing TD, whether it involves the adjunctive use of potentially therapeutic medicines (e.g. tetrabenazine, amantadine, levetiracetam, or antioxidants) or switching antipsychotic mediation requires precise monitoring. The conventional approach to monitoring the efficacy of these putative treatments relies upon changes in the scores from the Abnormal Involuntary Movement Scale (AIMS)10. Placebo-controlled trials reporting clinical efficacy cite reductions in total AIMS score ranging from 15%-20%11,12 to as high as 44%13. While these reports appear encouraging, it is important to consider that the AIMS relies on a non-proportional scale that assigns a score (0-4) based on a qualitative impression of severity. For example, a reduction in observed severity of dyskinetic movements from “2” (mild) to “1” (minimal) represents a 50% improvement, despite the lack of objective criteria to conclude that a score of “2” is twice as severe as a score of “1”.
We and others have raised this issue in prior publications on instrumental assessments of TD14-16. While instrumental measures increase the objectivity and sensitivity when assessing severity of dyskinetic movements, they have not been widely accepted in the clinical setting for a number of reasons. Previously published instruments such as accelerometers, load cells, and high resolution motion sensing cameras require significant technical experience to properly calibrate, acquire, and analyze the movement data. These instruments are not always portable and the set-up time or training is not trivial. Given the limitations of both traditional severity rating scales and instrumental measures, there remains a need for a portable, objective, reliable, and sensitive procedure for assessing TD in the clinical setting. The need is particularly relevant for clinical trials designed to test pharmacotherapies for treating existing TD.
One potential measurement solution that overcomes the limitations of prior instrumental approaches involves handwriting movements. Examination of handwriting is a common practice in the clinical setting to assess severity of a movement disorder. For example, a standard test used by neurologists to assess tremor is to ask patients to draw an Archimedes circle. Similarly, parkinsonian micrographia can be detected by examining samples of handwriting. Research conducted in our laboratory over the past several years has led to the refinement of a quantitative approach for assessing parkinsonism by extracting stroke-by-stroke kinematic features associated with natural handwriting17-19. Among the many kinematic features this handwriting test provides, including duration, amplitude, velocity, acceleration, and applied pressure of each stroke of pen movement, the procedure also measures movement dysfluency.
The aim of the present study was to determine whether a brief handwriting test designed to measure degree of pen movement dysfluency can be used to assess the severity of TD. Based on prior experience with instrumental procedures for quantifying severity of TD, we can propose a main hypothesis for the present study; namely that patients with TD will exhibit greater kinematic dysfluency during simple handwriting movement compared with non-TD patients. However, it remains unknown which of the selected handwriting conditions or indices of movement dysfluency are sufficiently sensitive to detect TD. Robust differences between TD and non-TD patients would support the notion that handwriting kinematic assessment could enhance early detection or change in severity of TD.
MATERIALS AND METHODS
Subjects
Fifty right-handed healthy participants and 62 right-handed patients with psychosis were enrolled from two study sites (San Diego and Minneapolis). Of the 62 patients, 57 met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria for schizophrenia; four for schizoaffective disorder, and one for Type I bipolar disorder. Healthy participants represented a sample of convenience recruited from staff, students, and volunteers from the respective sites. Each participant read and signed institutionally approved informed consent prior to participating. Participation consisted of a brief medication and psychiatric history, administration of the Abnormal Involuntary Movement Scale (AIMS)10, a modified version of the Simpson-Angus Extrapyramidal Signs Scale (SAEPS)20, the Barnes Akathisia Scale (BAS)21 to assess the presence and severity of tardive dyskinesia, parkinsonism, and akathisia respectively, and the Positive and Negative Syndrome Scale (PANSS)22 to assess the nature and severity of psychosis. Upon completion of the clinical assessments, subjects completed the procedure for quantifying handwriting dysfluency. Table 1 shows the demographic and clinical characteristics of the study subjects.
Table 1.
Clinical and demographic characteristics of the study subjects1. Shown are the means (with standard deviations) unless otherwise indicated.
| Healthy Subjects | Non-TD Patients | Mild TD Patients | Moderate TD Patients | |
|---|---|---|---|---|
| N=50 | N=30 | N=20 | N=12 | |
| Age, years | 45.5 (9.5) | 50.4 (8.8) | 44.6 (11.4) | 50.2 (8.0) |
| Gender (% male) | 42% | 73% | 60% | 83% |
| PANSS-Total Score | 64.3 (16.4) | 72.5 (17.3) | 71.8 (15.9) | |
| APD | 4.0 (2.9) | 4.5 (4.1) | 5.3 (2.8) | |
| % on Anticholinergics | 13% | 10% | 27% | |
| SAEPS | 3.7 (3.7) | 5.2 (3.1) | 6.6 (3.1) | |
| BAS | 0.6 (0.8) | 1.3 (1.4) | 2.3 (0.9) | |
| AIMS | 1.1 (0.8) | 4.6 (1.1) | 8.0 (1.1) | |
Positive and Negative Syndrome Scale (PANNS); Antipsychotic Drugs (APD, in mg/day risperidone-equivalents), Simpson-Angus EPS scale (SAEPS), Barnes Akathisia Scale (BAS), Abnormal Involuntary Movement Scale (AIMS)
It is important to note that the clinical and handwriting data from the current study subjects have appeared in prior publications describing the antipsychotic effects on parkinsonism as measured by handwriting kinematics18,19. The present study; however describes new analyses focusing on handwriting dysfluency in TD patients.
Quantification of Handwriting Dysfluency
Apparatus
Handwriting movements were quantified using a non-inking pen with a Wacom UD 9”×12” digitizing tablet (with an active area of 22.9 cm × 30.5 cm) attached to a notebook computer. Pen movements were sampled at 100 Hz with an RMS accuracy of 0.01 cm. Data acquisition and analyses (see below) were performed using MovAlyzeR® software (Neuroscript, LLC http://www.neuroscriptsoftware.com/ Tempe, AZ, USA).
Procedures
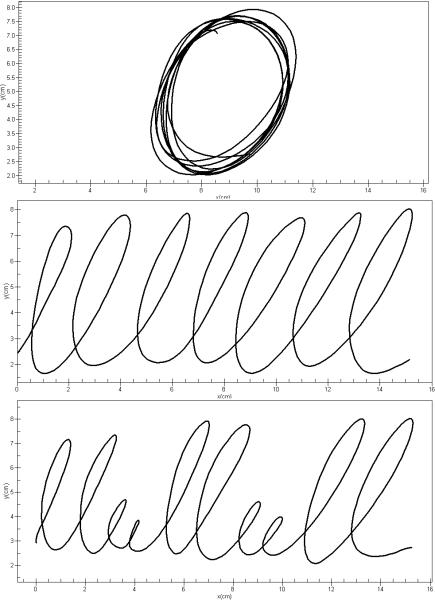
Subjects were comfortably seated at a table with the writing tablet positioned at an angle comfortable for writing. For the purpose of this study, we administered nine handwriting tests including repetitive overlaid circles, repetitive cursive l-loops written from left to right, and continuous complex “lleellee” cursive pattern each within 1, 2, and 4 cm vertical boundaries. Subjects were asked to perform the tasks at comfortable writing speeds. A frictionless template of horizontal lines was placed on the tablet to guide the subject to write the loops circles within the requisite 1, 2, or 4 cm vertical boundaries. Each trial was programmed to last 10 seconds. Figure 1 shows 4-cm exemplars of the three handwriting tasks.
Figure 1.
Handwriting samples of the three tasks executed under the 4-cm boundary condition. Shown are samples for the overlay circles (top), L-R loops (middle) and complex loops (bottom).
Blocks of three trials per condition were performed in random order. Subjects began the trial when prompted by the examiner and data collection began at the moment the pen came in contact with the tablet. Trials with an initiation delay of more than 5 seconds or interruptions or pen lifts of more than 3 seconds were re-administered. The total duration of the handwriting test was less than 10 minutes.
Data Reduction
Data in both X and Y coordinates were low pass filtered at 8 Hz using a sinusoidal transition band from 3.5 to 12.5 Hz23. Subsequently, the first, second, and third time derivatives (i.e., velocity, acceleration, and jerk, respectively) were calculated. Movements were then segmented into successive up and down strokes. Each trial contained at least 16 up and down pen strokes. Two kinematic variables reflecting smoothness of pen movements were extracted from the processed data. These included (1) normalized jerk averaged across a trial (ANJ) and (2) the number of acceleration peaks of the vertical movement component (APK). Normalized jerk is unitless as it is normalized for stroke duration and length. ANJ was calculated using the following formula: . APK was determined for both ascending and descending strokes. Higher ANJ scores and increased APK per stroke are indicative of dysfluent writing movements.
Statistical Analyses
Two-way analyses of variance (ANOVAs) with participant group and handwriting task condition as main factors were used to test our primary hypothesis that patients with TD will exhibit greater kinematic dysfluency during simple handwriting movement compared with non-TD patients. Separate ANOVAs were used for each of the two independent variables (ANJ and APK). Because variances were proportional to the mean scores for ANJ, ANJ scores were transformed using the natural logarithm to remove inequality of variance. Test-retest reliability was examined using Cronbach's alpha25 procedures derived from the results of two-way ANOVAs. We also examined the relationship between ANJ and APK scores and demographic and clinical variables such as age, severity of psychosis, clinical ratings for extrapyramidal signs, and antipsychotic dose. For the ANOVA main effects and post-hoc comparisons we chose α<0.05 to reflect statistical significance. Where appropriate (e.g. correlational tests for each of the 9 handwriting tasks), Bonferroni correction was used to adjust α for multiple comparisons by dividing α by the number of handwriting tasks (n=9). We used Statistica® version 10 (StatSoft, Inc. www.statsoft.com Tulsa, OK) for all statistical analyses.
RESULTS
Instrument Reliability
Nine healthy subjects and 15 psychosis patients on stable medications were tested twice separated by approximately one month. Table 2 shows the results of the instrument reliability testing. Cronbach's α coefficients ranged from 0.84 to 0.92 for healthy subjects and 0.67 to 0.92 for stable psychosis patients. Reliability coefficients for log(ANJ) were above 0.80 for all subjects indicating good test-retest reliability for this measure. The relatively low coefficient for APK (0.67) for overlay circle task indicates that this variable may be measuring a multidimensional rather than a unidimensional latent construct.
Table 2.
Results from test-retest reliability analysis. Shown are Cronbach's α coefficients derived from repeated measures ANOVA for two handwriting dysfluency measures, log (ANJ) and APK for 3 categories of handwriting tasks for 9 healthy subjects and 15 medically stable psychosis patients.
| Group | Log(ANJ) | APK | ||||
|---|---|---|---|---|---|---|
| Overlay Circles | L-R Loops | Complex Loops | Overlay Circles | L-R Loops | Complex Loops | |
| Healthy Participants | 0.91 | 0.92 | 0.84 | 0.87 | 0.90 | 0.89 |
| Psychosis Patients | 0.80 | 0.90 | 0.92 | 0.67 | 0.90 | 0.92 |
Tardive Dyskinesia (TD) Effects
For the first set of analyses, patients were placed into groups based on the severity of overall TD. Group 1 included 30 patients with total AIMS score (items 1-7) less than 3 (non-TD). Group 2 included 20 patients with total AIMS score (items 1-7) between 3 and 6 (mild TD), Group 3 included 12 patients with total AIMS score greater than 6 (moderate TD). The initial analysis involved a 2-way ANOVA with 4 levels for the Group factor (healthy subjects, non-TD, mild TD, and moderate TD patients) and nine levels for the handwriting Task factor (overlay circles, L-R loops, and complex loops performed at 1, 2, and 4 cm). Results for log(ANJ) revealed a significant main effect for Group (F3,968 = 81.75; p<0.0001) and a significant main effect for Task (F8,968 = 3.56; p<0.001). The Group by Task interaction was not statistically significant (F24, 968 = 0.46; p>0.10).
Univariate ANOVAs were used to evaluate significant group effects for patients only for each of the nine tasks separately. These results, along with the means and standard deviations for each group are listed in Table 3. Post-hoc analyses (Scheffé tests) revealed that the significant effects for ANJ were driven by significant differences (p<0.05) between the non-TD group and the group with moderate TD. Additionally, the results show consistently greater dysfluency between patients with moderate TD and non-TD patients for the 4-cm boundary conditions.
Table 3.
Mean (with standard deviation) log(ANJ) for the nine handwriting tasks. The univariate analyses of variance describe main effects of Group with 3 patient groups.
| Handwriting Task | Healthy Participants | Non-TD Patients | Mild TD Patients | Moderate TD Patients | F Statistic | p-value |
|---|---|---|---|---|---|---|
| Overlay Circles; 1 cm | 2.36 (0.37) | 2.62 (0.54) | 2.88 (0.85) | 3.17 (1.26) | F2,59 = 2.01 | p<0.10 |
| Overlay Circles; 2 cm | 2.54 (0.53) | 2.71 (0.56) | 2.94 (0.93) | 3.63 (1.27) | F2,59 = 4.94 | p≤0.01 |
| Overlay Circles; 4 cm | 2.65 (0.58) | 2.93 (0.83) | 3.16 (1.06) | 3.79 (1.05) | F2,59 = 3.53 | p<0.05 |
| L-R Loops; 1 cm | 2.45 (0.46) | 3.01 (0.84) | 3.17 (0.79) | 3.82 (1.01) | F2,57 = 3.60 | p<0.05 |
| L-R Loops; 2 cm | 2.59 (0.56) | 2.94 (0.74) | 3.34 (0.93) | 3.80 (1.11) | F2,57 = 4.20 | p<0.05 |
| L-R Loops; 4 cm | 2.64 (0.62) | 3.11 (0.80) | 3.34 (0.97) | 3.95 (1.17) | F2,58 = 3.35 | p<0.05 |
| Complex Loops 1 cm | 2.48 (0.58) | 3.07 (1.02) | 3.24 (0.98) | 3.71 (1.00) | F2,57 = 1.62 | p>0.10 |
| Complex Loops 2 cm | 2.52 (0.50) | 2.89 (0.75) | 3.12 (0.79) | 3.52 (1.05) | F2,57 = 2.46 | p<0.10 |
| Complex Loops 4 cm | 2.58 (0.73) | 2.99 (0.58) | 3.49 (1.05) | 3.84 (1.21) | F2,58 = 4.22 | p<0.05 |
Results for APK revealed a significant main effect for group (F3,968 = 46.86; p<0.0001) and a significant main effect for task (F8,968 = 5.82; p<0.0001). The group by task interaction was not statistically significant (F24, 968 = 0.42; p>0.10). Univariate ANOVAs were used to evaluate group effects for patients only for each of the nine tasks separately. These results, along with the means and standard deviations for each groups appear in Table 4. As with ANJ, post-hoc tests of APK revealed differences only between non-TD and moderate TD patients and only for two handwriting tasks.
Table 4.
Mean (with standard deviation) APK for the nine handwriting tasks. The univariate analyses of variance describe main effects of Group with 3 patient groups.
| Handwriting Task | Healthy Participants | Non-TD Patients | Mild TD Patients | Moderate TD Patients | F-Statistic | P-value |
|---|---|---|---|---|---|---|
| Overlay Circles; 1 cm | 2.23 (0.42) | 2.59 (0.94) | 2.68 (1.28) | 3.29 (2.23) | F2,59 = 1.88 | p>0.10 |
| Overlay Circles; 2 cm | 2.53 (0.88) | 2.69 (0.87) | 2.94 (1.56) | 4.29 (3.36) | F2,59 = 3.27 | p=0.02 |
| Overlay Circles; 4 cm | 2.75 (1.22) | 3.27 (1.88) | 3.69 (2.60) | 4.65 (3.10) | F2,59 = 1.44 | p>0.10 |
| L-R Loops; 1 cm | 2.44 (0.47) | 3.05 (1.03) | 3.20 (1.28) | 3.93 (1.90) | F2,57 = 1.83 | p>0.10 |
| L-R Loops; 2 cm | 2.65 (0.77) | 3.19 (1.35) | 3.59 (1.48) | 4.63 (2.90) | F2,57 = 2.71 | p>0.10 |
| L-R Loops; 4 cm | 2.95 (1.16) | 3.63 (1.87) | 3.83 (1.85) | 4.91 (3.01) | F2,58 = 1.58 | p>0.10 |
| Complex Loops 1 cm | 2.41 (0.54) | 2.90 (0.95) | 3.21 (1.41) | 3.70 (1.94) | F2,57 = 1.48 | p>0.10 |
| Complex Loops 2 cm | 2.43 (0.51) | 2.72 (0.60) | 3.11 (1.19) | 3.74 (1.93) | F2,57 = 3.31 | p<0.05 |
| Complex Loops 4 cm | 2.59 (0.70) | 3.20 (1.04) | 3.83 (1.72) | 4.55 (2.76) | F2,58 = 2.75 | p<0.10 |
For the second set of analyses, patients were divided into two groups based on the severity of upper extremity TD (UETD). Group 1 included 27 patients with upper extremity AIMS score of zero. Group 2 included 34 patients with an upper extremity AIMS score of at least 1 (minimal TD). This analysis involved a 2-way ANOVA with 3 levels for the Group factor (healthy subjects, no UETD, and at least minimal UETD) and nine levels for the handwriting Task factor (see above). Results for log(ANJ) revealed a significant main effect for Group (F2,969 = 100.46; p<0.0001) and a significant main effect for Task (F8,969 = 3.42; p<0.0001). The group by task interaction was not statistically significant (F16, 969 = 0.64; p>0.10). Results for APK revealed a significant main effect for group (F2,969 = 58.34; p<0.0001) and a significant main effect for task (F8,969 = 5.71; p<0.0001). The group by task interaction was not statistically significant (F16, 969 = 0.44; p>0.10).
Post-hoc t-tests were used to evaluate differences between patients with (n=34) and without UETD (n=27) for each of the nine tasks separately. For log(ANJ), results revealed significantly greater handwriting dysfluency for the TD compared to non-TD patients for three conditions: 2-cm overlay circles (t=2.75; df=58; p<0.01); 1-cm L-R loops (t=2.04; df=58; p<0.05); and 2-cm L-R loops (t=2.40; df=58; p<0.02). For APK, results revealed significantly greater handwriting dysfluency for the TD compared to non-TD patients for only two conditions: 2-cm overlay circles (t=2.10; df=58; p<0.05) and the 1-cm L-R loops (t=2.03; df=58; p<0.05).
Correlational analyses were used to examine the relationships between log(ANJ) and APK scores and demographic and clinical characteristics. We found no statistically significant correlations between handwriting dysfluency and age (for controls or patients), severity of psychopathology (based on PANSS total score), daily dose of antipsychotic medication (in mg risperidone equivalents/day), severity of parkinsonism (based on SAEPS total score) or severity of akathisia (based on BAS global score). Statistically significant correlation coefficients were found for associations between each of the three pairs of log(ANJ) and APK and TD severity (based on total AIMS score for items 1-7) after Bonferroni correction for multiple comparisons for four of the nine handwriting task. These included: 2-cm overlay circles (r=0.41; p=0.001 for ANJ and r=0.37; p=0.003 for APK); 2-cm L-R loops (r=0.37; p=0.004 for ANJ); 2-cm complex loops (r=0.36; p=0.004 for APK); and 4-cm complex loops (r=0.35; p=0.006 for ANJ).
To further characterize the sensitivity and specificity of our handwriting dysfluency measures in TD we performed discriminant function analyses. Grouping patients into TD and non-TD based on UETD AIMS scores (see above), we were able to accurately classify only 67% of the patients based on the log(ANJ) score alone for the 2-cm overlay circles task. Grouping based on total AIMS score yielded 100% specificity (with 20 non-TD psychosis patients); however, only 33% sensitivity (with 12 TD psychosis patients) for ANJ only for the 4-cm complex loop task. Classification models employing different handwriting tasks and combinations of ANJ or APK did not improve these results. Overall, using AIMS-based methods for diagnosing patients as having TD, our handwriting dysfluency measures did not appear to exhibit adequate sensitivity as a diagnostic measure for TD.
We found that log(ANJ) and APK scores were strongly associated. Figure 3 shows an example of this relationship for 4-cm complex loops in patients with or without TD. This is not surprising given that the APK measures the number of changes in acceleration (second derivative of displacement) while jerk (third derivative of displacement) measures the rate of change in acceleration with respect to time. Both measure dysfluent movements. The difference between these two measures rests only in the calculation of normalized jerk which removes the effects of stroke duration and length which could systematically increase scores for slower or bradykinetic writers. Given their overlapping constructs, one would anticipate that these measures would yield similar results on difference tests involving TD and non-TD patients. However, this was supported for only one handwriting condition (2-cm overlay circles), a disparity that could be explained by increased relative variability for the APK measure.
Figure 3.
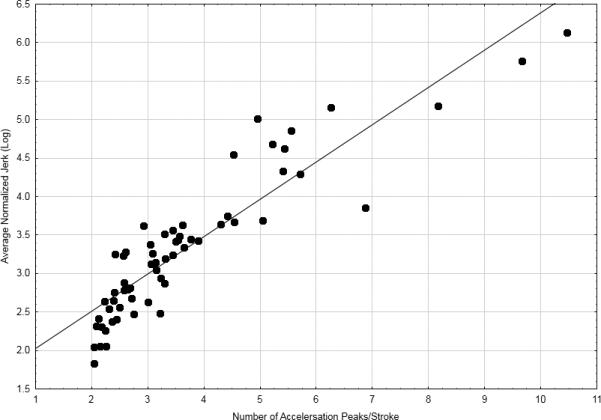
Relationship between log(ANJ) and APK during production of 4-cm complex loops in patients with or without TD (n=62; r=0.91;p<0.0001)
DISCUSSION
The findings from the present study supported our primary hypothesis that patients with TD exhibit significantly greater kinematic dysfluency during simple handwriting movement compared with non-TD patients. Dysfluent handwriting movements were more evident when producing larger (4-cm) loops and circles than smaller (1-cm) loops and circles. While we found that our two measures of handwriting dysfluency were strongly co-linear, the measure of normalized jerk averaged across pen strokes (ANJ) detected greater separation of TD from non-TD patients compared with the measure based on the number of acceleration peaks within pen strokes (APK).
The statistical associations between TD severity and handwriting dysfluency were generally weak, accounting for less than 17% of the variance in TD severity. Similarly, we were unable to attain better than 67% sensitivity or specificity from our handwriting dysfluency measures for distinguishing TD from non-TD patients. Because both analyses rely on the accuracy of a non-proportional ordinal scale for rating severity of observable dyskinetic movements, these negative findings likely stem from a common procedural limitation. While the reliability and validity of the AIMS is well established, the procedure forces the rater to fit an observation into one of 4 categories. One such category (a score of “1’) is used for questionable movements. Patients scoring “1” on any or multiple items are generally not considered to have TD; however, these same patients could perform outside the normal range on tasks of fine motor control or steadiness as previously shown26. The relatively low sensitivity and specificity could be attributed to how patients were classified into TD and non-TD groups and that some non-TD patients failing to meet criteria for TD could nonetheless manifest subclinical dyskinesia.
Prior research has shown that measures of isometric force steadiness can distinguish dyskinetic from non-dyskinetic patients14,26. The present findings of abnormal acceleration control among dyskinetic patients are consistent with studies of hand force steadiness. This is not unexpected. From the principles of basic physics, we know that acceleration and force are two measures of the same mechanical process. To the extent that force is a product of an object's mass and it's acceleration (Newton's second law), it stands to reason that changes in acceleration over time (operationalized here as the duration of a pen stroke) will lead to changes in force over that same time period. The convergence of findings from research employing different methods that dyskinesia manifests as a disruption in the control of muscle force may inform a general principle governing the role of dopamine in motor control.
Clinicians and researchers have searched for means to detect subtle changes in the neuromotor system to monitor the emergence of movement side effects in patients treated with psychotropic medications. Interestingly, in the late 1950s and early 1960s handwriting was considered an ideal candidate for such a monitoring system. Haase27 was the first to demonstrate a relationship between clinical effectiveness of neuroleptic medication for treating psychosis and their side effects by observing handwriting. Haase noted that as neuroleptic dosage increases, patients’ handwriting exhibited classic features of parkinsonism such as slowing (bradykinesia), tremor, and micrographia. Haase developed the notion of the “neuroleptic threshold” defined as the minimum dose a patient needs to obtain clinical efficacy while minimizing any of these extrapyramidal side effects. Modern investigators28 using digitizing tablets and computer software and positron emission tomography have confirmed a direct link between dopamine receptor occupancy and reduction in handwriting size and other parkinsonian features. The present study extends prior handwriting research supporting a role in the assessment of antipsychotic-induced parkinsonism to the quantitative assessment of tardive dyskinesia.
Dyskinetic hand movements need not develop solely as a consequence of long term exposure to dopamine receptor blocking drugs. While spontaneous dyskinesia is rare in the general population, it has been observed with greater frequency in diabetes patients29 and drug naïve schizophrenia30. With prevalence estimates of spontaneous dyskinesia among patients with psychosis of approximately 17%30 , distinguishing spontaneous from iatrogenic cause of the dyskinesia at presentation is difficult at best and will remain a confound in epidemiological studies of dyskinesia associated with second generation antipsychotics. Exceptions are studies of the schizophrenia prodrome. Because of the putative neurobiological mechanisms linking spontaneous dyskinesia with schizophrenia31, 32, some have explored the sensitivity of quantifying handwriting movement dysfluencies as an alternative. In one such study of adolescents at high risk for schizophrenia33, investigators found that those identified has having a prodromal or ultra-high risk syndrome had significantly higher mean scores on the ANJ measure for complex loops. Their findings suggest that dysfluent handwriting movements may also serve as an early behavioral biomarker of schizophrenia in high risk adolescents.
The study has some limitations. First our grouping of patients into TD and non-TD subgroups based on AIMS cut point scores was not based on consensus from the published literature as such criteria have not been established. To our knowledge there is no established AIMS-based definition of mild or moderate TD when interpreting the total AIMS score. However, the research diagnostic criteria for TD34 defines TD as at least two “2”s or one “3” on any of the 7 items from the AIMS. Our criteria for non-TD (total AIMS score of less than 3) is consistent with that definition. Second, we intentionally excluded left handed and enrolled only right handed subjects to remove potential handedness confound. We could find no support for the notion that left- handed TD patients exhibit different levels of handwriting dysfluency (when writing with their dominant hand) than right-handed patients. Third, there was a discrepancy in the gender distribution between patients and controls in our study. While our male-female ratio is representative of the schizophrenia population in general, matching gender with our healthy control sample in a case control design would not have altered the significant effects of TD severity on handwriting dysfluency. In our prior study19 of the antipsychotic effects on handwriting kinematics we observed a significant effect of gender on ANJ with males showing greater dysfluency than females; however, there was no group-by-gender interaction indicating that the gender difference was the same for antipsychotic treated patients as well as healthy controls and not likely related to antipsychotic dose or presence or severity of movement disorder.
In conclusion, the findings of the present study offer empirical support that TD can be detected and objectively quantified with a very brief and easily administered battery of handwriting movement tasks. Measures of handwriting acceleration overcome many of the limitations inherent in prior instrumental approaches. Handwriting movement analyses are naturalistic, require minimal training, involve a low-cost commercially available digitizing tablet, notebook computer, and software, require no analytic decisions such as trial segmentation, and can be performed in any clinical setting in less than ten minutes. Lastly, the procedure can be standardized for use across multiple sites with no known site-related variation.
Figure 2.
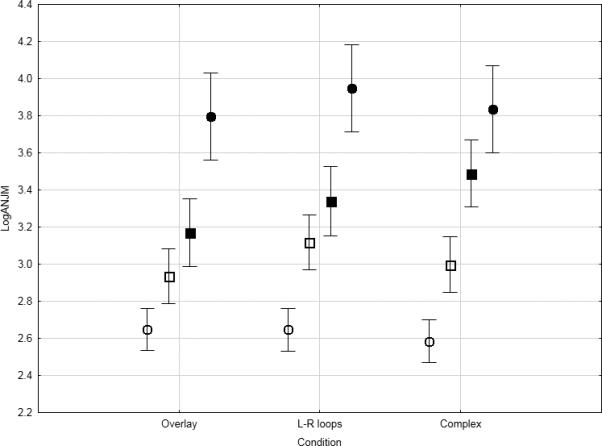
Means (with standard error bars) for log(ANJ) scores for healthy participants (open circles), non-TD patients (open squares), and patients with mild (filled squares) and moderate (filled circles) TD for three 4-cm handwriting tasks.
Acknowledgments
This research was supported by NIH grant R44 MH073192.
Footnotes
Author Disclosure Information
Drs Caligiuri, Dean and Lohr disclose no financial conflicts of interest with commercial entities involved in this research. Dr. Teulings is founder and owner of Neuroscript, LLC, a privately held company that produces and markets MovalyZeR® software used in this research.
REFERENCES
- 1.Cloud LJ, Zutshi D, Factor SA. Tardive dyskinesia: Therapeutic options for an increasingly common disorder. Neurotherapeutics. 2014;11:166–176. doi: 10.1007/s13311-013-0222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Correll CU, Schenk EM. Tardive dyskinesia and new antipsychotics. Curr Op Psychiatry. 2008;21:151–156. doi: 10.1097/YCO.0b013e3282f53132. [DOI] [PubMed] [Google Scholar]
- 3.Woods SW, Morgenstern H, Saksa JR, et al. Incidence of tardive dyskinesia with atypical versus conventional antipsychotic medications: a prospective cohort study. J Clin Psychiatry. 2010;71:463–474. doi: 10.4088/JCP.07m03890yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahn RS, Fleischhacker WW, Boter H, et al. the EUFEST study group. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomized clinical trial. Lancet. 2008;371:1085–1097. doi: 10.1016/S0140-6736(08)60486-9. [DOI] [PubMed] [Google Scholar]
- 5.Dean CE. The death of specificity in psychiatry: cheers or tears? Perspect Biol Med. 2012;55:443–460. doi: 10.1353/pbm.2012.0028. [DOI] [PubMed] [Google Scholar]
- 6.Olfson M, Blanco C, Liu S- M, et al. National trends in the office-based treatment of children, adolescents, and adults with antipsychotics. Arch Gen Psychiatr. 2012;69:1247–1256. doi: 10.1001/archgenpsychiatry.2012.647. [DOI] [PubMed] [Google Scholar]
- 7.Leslie D, Mohamed S, Rosenheck RA. Off-label use of antipsychotic medications in the Department of Veterans Affairs health care system. Psychiatr Serv. 2009;60:1175–1181. doi: 10.1176/ps.2009.60.9.1175. [DOI] [PubMed] [Google Scholar]
- 8.Jankelowitz SK. Treatment of neuroleptic-induced tardive dyskinesia. Neuropsychiatric Disease and Treatment. 2013;9:1371–1380. doi: 10.2147/NDT.S30767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Harten PN, Tenback DE. Tardive dyskinesia: clinical presentation and treatment. Int Rev Neurobiol. 2011;98:187–210. doi: 10.1016/B978-0-12-381328-2.00008-0. [DOI] [PubMed] [Google Scholar]
- 10.Guy W. ECDEU Assessment Manual for Psychopharmacology, rev. DHEW Pub. No. (ADM. 76-338) National Institute of Mental Health; Rockville, MD: 1976. National Institute of Mental Health. Abnormal Involuntary Movement Scale (AIMS). pp. 534–537. [Google Scholar]
- 11.Angus S, Sugars J, Boltezar R, et al. A controlled trial of amantadine hydrochloride and neuroleptics in the treatment of tardive dyskinesia. J Clin Psychopharmacol. 1997;17:88–91. doi: 10.1097/00004714-199704000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Pappa S, Isouli S, Apostolou G, et al. Effects of amantadine on tardive dyskinesia: a randomized, double-blind, placebo-controlled study. Clin Neuropharmacol. 2010;33:271–275. doi: 10.1097/wnf.0b013e3181ffde32. [DOI] [PubMed] [Google Scholar]
- 13.Woods SW, Saksa JR, Baker CB, et al. Effects of levetiracetam on tardive dyskinesia: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2008;69:546–554. doi: 10.4088/jcp.v69n0405. [DOI] [PubMed] [Google Scholar]
- 14.Lohr JB, Caligiuri MP. Quantitative instrumental assessment of tardive dyskinesia: A review. Neuropsychopharmacology. 1992;6:231–239. [PubMed] [Google Scholar]
- 15.Caligiuri MP. Instrumental measurement of tardive dyskinesia. In: Yassa R, Nair NPV, Jeste DV, editors. Neuroleptic-induced Movement Disorders: A Comprehensive Survey. Cambridge University Press; 1997. pp. 241–258. [Google Scholar]
- 16.Dean CE, Russell J, Kukowski M, et al. Clinical ratings scales and instruments: how do they compare in assessing abnormal involuntary movements? J Clin Psychopharmacol. 2004;24:298–304. doi: 10.1097/01.jcp.0000125681.97466.e7. [DOI] [PubMed] [Google Scholar]
- 17.Caligiuri MP, Teulings HL, Filoteo, et al. Quantitative measurement of handwriting in the assessment of drug-induced parkinsonism. Hum Mov Sci. 2006;4-5:510–522. doi: 10.1016/j.humov.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Caligiuri MP, Teulings HL, Dean CE, et al. Handwriting movement analyses for monitoring drug-induced motor side effects in schizophrenia patients treated with risperidone. Hum Mov Sci. 2009;5:633–642. doi: 10.1016/j.humov.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caligiuri MP, Teulings HL, Dean CE, et al. Handwriting movement kinematics for quantifying extrapyramidal side effects in patients treated with atypical antipsychotics. Psychiatr Res. 2010;177:77–83. doi: 10.1016/j.psychres.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scan. 1970;212(Suppl 44):11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 21.Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatr. 1989;154:672–676. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- 22.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schiz Bull. 1987;32:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 23.Teulings HL, Maarse FJ. Digital recording and processing of handwriting movements. Hum Mov Sci. 1984;3:193–217. [Google Scholar]
- 24.Teulings HL, Contreras-Vidal JL, Stelmach GE, et al. Coordination of fingers, wrist, and arm in Parkinsonian handwriting. Exp Neurol. 1997;146:159–170. doi: 10.1006/exnr.1997.6507. [DOI] [PubMed] [Google Scholar]
- 25.Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;6:297–334. [Google Scholar]
- 26.Caligiuri MP, Lohr JB. A potential mechanism underlying the voluntary suppression of tardive dyskinesia. J Psych Res. 1989;23:257–266. doi: 10.1016/0022-3956(89)90031-9. [DOI] [PubMed] [Google Scholar]
- 27.Haase HJ. Extrapyramidal modification of fine movements: a “conditio sine qua non” of the fundamental therapeutic action of neuroleptic drugs. Rev Can Biol. 1961;20:425–449. [PubMed] [Google Scholar]
- 28.Künstler U, Juhnhold U, Knapp WH, et al. Positive correlation between reduction in handwriting area and D2 dopamine receptor occupancy during treatment with neuroleptic drugs. Psychiatr Res. 1999;90:31–39. doi: 10.1016/s0925-4927(98)00054-7. [DOI] [PubMed] [Google Scholar]
- 29.Merrill M, Lyon JL, Matiaco PM. Tardive and spontaneous dyskinesia incidence in the general population. BMC Psychiatry. 2013;13(152):1–9. doi: 10.1186/1471-244X-13-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pappa S, Dazzan P. Spontaneous movement disorders in antipsychotic-naïve patients with first-episode psychosis: a systematic review. Psychol Med. 2009;39:1065–1076. doi: 10.1017/S0033291708004716. [DOI] [PubMed] [Google Scholar]
- 31.Graybiel AM. The basal ganglia and cognitive pattern generators. Schiz Bull. 1997;23:459–469. doi: 10.1093/schbul/23.3.459. [DOI] [PubMed] [Google Scholar]
- 32.Andreasen NC, Paradiso S, O'Learey DS. ‘Cognitive dysmetria’ as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schiz Bull. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- 33.Dean DJ, Teulings H-L, Caligiuri MP, et al. Handwriting analysis indicates spontaneous dyskinesias in neuroleptic-naïve adolescents at high-risk for psychosis. J Vis Exp. 2013 Nov 21;(81):e50852, 1–12. doi: 10.3791/50852. doi: 10.3791/50852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schooler N, Kane J. Research diagnoses for tardive dyskinesia. Arch Gen Psychiatr. 1982;39:486–487. doi: 10.1001/archpsyc.1982.04290040080014. [DOI] [PubMed] [Google Scholar]