Abstract
Objective
This study was conducted to resolve the striking controversy between our previous report that high density lipoprotein (HDL) enhances activated protein C (APC)/Protein S anticoagulant actions and a subsequent, contradicting report that HDL lacks this activity.
Approach and Results
When fresh HDL preparations from two labs were subjected to Superose 6 column chromatography, fractions containing HDL enhanced APC:Protein S anticoagulant actions in clotting assays, thereby validating our previous report. Moreover, the ability of HDL to enhance the anticoagulant actions of APC:protein S was neutralized by anti-apoA-I antibodies, further indicating that the activity is due to HDL particles and not due to contaminating phospholipid vesicles. Density gradient subfractionation studies of HDL showed that large HDL subfractions (densities between 1.063 and 1.125 g/mL) contained the APC:protein S enhancing activity. Fresh HDL stored at 4° C gradually lost its anticoagulant enhancing activity over 14 days, indicating moderate instability in this activity of purified HDL.
Conclusions
These studies conclusively demonstrate that freshly prepared HDL fractions possess anticoagulant activity. Fractions from Superose 6 columns that contain HDL reproducibly enhance APC:Protein S anticoagulant activity, consistent with the hypothesis that HDL has antithrombotic activity and with the observation that low HDL levels are found in male venous thrombosis patients. Understanding the basis for this activity could lead to novel therapeutic approaches to regulate venous thrombosis.
Keywords: HDL, activated protein C, thrombosis, protein S, prothrombinase
Introduction
High Density Lipoprotein (HDL) particles, defined by density of 1.063-1.21 g/mL, are heterogeneous in size and composition 1, 2. Although there is some controversy, extensive epidemiological data show that endogenous HDL is positively associated with cardioprotection 2–4 but much remains unknown about mechanism(s) for HDL’s benefits. HDL deficiency is associated with venous thrombosis (VTE) 5–8. The multiple antithrombotic properties of HDL 9 may help explain why the deficiency of HDL is associated with thrombotic diseases. In 1999, we discovered that purified HDL enhances activated protein C (APC):Protein S anticoagulant action in plasma clotting and factor Va inactivation assays, giving rise to our hypothesis that HDL helps protect against venous thrombosis10. However, in 2010, Oslakovic et al 11 challenged our discovery in reporting that APC:Protein S enhancement was not an intrinsic property of HDL and that this APC:Protein S enhancement activity was due to phospholipid contaminants in their HDL preparation based on fractionation of HDL on Superose 6. To resolve this striking conflict, here we also used Superose 6 chromatographic analyses and characterized two new sources for fresh HDL. Here we provide new data that validate that our initial report 10 and extend the characterization of HDL’s ability to enhance APC:Protein S activity.
Materials and Methods
are available in the online-only Data Supplement.
Results
Our original report demonstrating anticoagulant activity of HDL utilized fresh HDL prepared at the Scripps lab, as described 10. In these new studies, we again utilized freshly prepared, never-frozen, purified human HDL, but obtained HDL from two outside sources, the laboratory of Joseph L Witztum at the University of California, San Diego, and a commercial product of Intracel Resources. When each HDL prep was subjected to Superose 6 chromatography as described 11 and column fractions were assayed, each HDL prep gave similar results. For example, lipid, apoA-I, and protein profiles of Superose 6 column fractions for one representative HDL prep are seen in Fig.1A. ApoA-I eluted in a typical pattern consistent with being on intact HDL particles, with the major retained peak of apoA-1 co-eluting with the lipid fractions at elution volumes between 15 and 17 mL, which were well separated from the void volume (Vo) fractions (8 to 9 mL). Importantly, all the apoA-I was associated with HDL particles.
Figure 1.
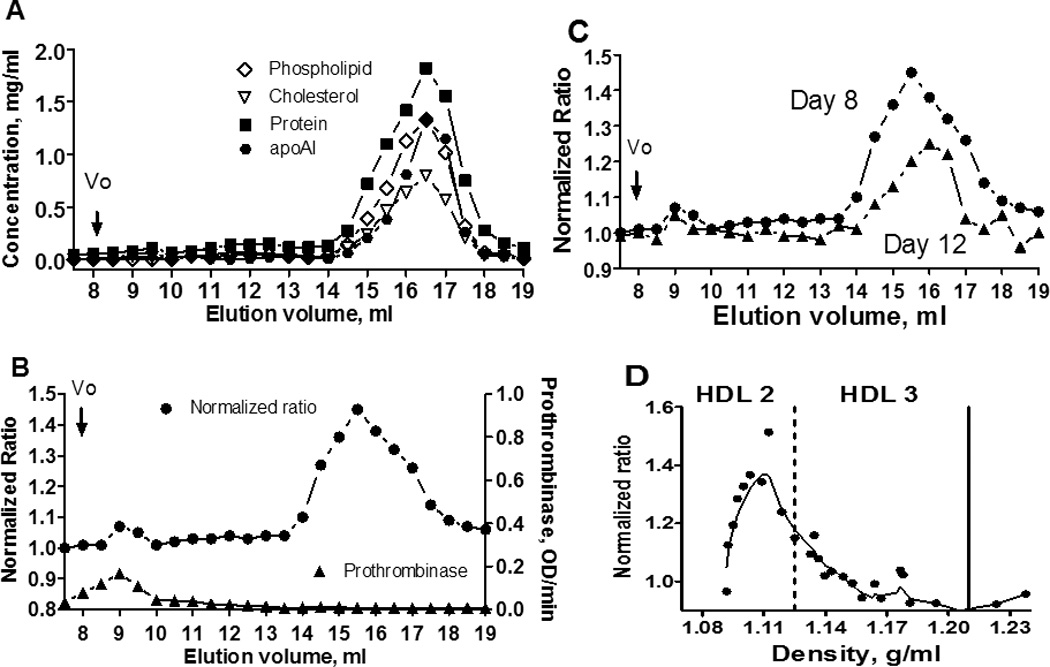
Characterization of HDL using Superose 6 chromatography and density gradient subfractionation. (A) Protein and lipid contents of the Superose 6 column fractions. (B) Anticoagulant and procoagulant profiles for Superose 6 column fractions. (C) Loss of anticoagulant activity over time for the HDL- containing fractions. (D) Anticoagulant activity of HDL subfractions following density gradient subfractionation. Dotted vertical line indicates density of 1.125 g/mL and solid vertical line a density of 1.21 g/mL. Enhancement of APC:Protein S anticoagulant activity is reflected in the “Normalized Ratio” which was calculated as the ratio of prothrombin times plus and minus APC:Protein S normalized to 1.0 for samples lacking Superose 6 fractions.
To study the anticoagulant APC:Protein S enhancing activity of Superose 6 column fractions, a modified prothrombin time clotting assay was used (See Methods). As seen in Fig. 1B, addition of Superose 6 fractions containing purified HDL that eluted between 15 and 17 mL caused a marked increase in the normalized APC/Protein S ratio, reflecting enhancement of APC:Protein S anticoagulant activity. In contrast, these HDL-containing fractions had no detectable prothrombinase activity. As reported by Oslakovic 11, void volume fractions slightly stimulated prothrombinase activity in reaction mixtures containing purified factors Xa and Va with prothrombin, and void volume fractions also had positive but minimal effects on APC:Protein S anticoagulant activity. Thus, chromatography of fresh, never-frozen HDL on Superose 6 showed that fractions containing HDL contained > 90 % of the APC:Protein S enhancing activity of the HDL preparation. This pattern was similarly observed for three different HDL preparations analyzed six times on the Superose 6 column.
In the course of our multiple analyses of different HDL preparations using Superose 6 chromatography, we observed that very fresh HDL showed the highest amount of anticoagulant enhancing activity in HDL fractions. Upon storage of never-frozen HDL at 3 – 5 °C, HDL gradually lost its activity over days. For example, when aliquots of the same HDL preparation were analyzed on day 8 and day 12 after purification, approximately 40 % of the activity was lost over the four day period (Figure 1C). After 3 weeks storage in the cold at 4°C, but not frozen, almost no APC:Protein S enhancing activity (< 10 % of original activity) was measureable. When the HDL preparation was frozen for 24 h at −20°C and later compared with the same non frozen HDL preparation undergoing identical chromatography analyses, the pool of Superose 6 apo-AI containing fractions from the frozen HDL had less anticoagulant activity than the pool of apo-AI fractions from fresh HDL (Suppl. Fig. IA). However, the pool fractions from the void volume (Vo) of the column showed an opposite effect. The Vo pool from the frozen-thawed HDL had more anticoagulant activity than the Vo pool from the fresh, never frozen HDL (Suppl. Fig. IA). When the procoagulant activity of the fractions was tested with a prothrombinase assay, the Vo pool from the frozen HDL had an enhanced procoagulant activity compared to the Vo pool from the fresh HDL fractions (Suppl. Fig. IB). No significant procoagulant activity was detected in fractions containing apoA-I from either fresh or frozen-thawed HDL preparations (Suppl. Fig. IB).
To confirm that the anticoagulant enhancing activity of HDL preparations was due to HDL particles, immobilized antibodies against apoA-I, were tested for their ability to absorb the anticoagulant activity of Superpose 6 fractions. Immobilized anti–apoA-I IgG adsorbed 86% of the anticoagulant activity, whereas no significant (< 5%) adsorption of the activity was observed for control Sepharose-IgG beads (Suppl. Fig. II). Thus, HDL fractions containing apoA-I provided the anticoagulant activity observed, and anticoagulant activity was not due to contaminants in the HDL preparations.
To characterize further HDL’s anticoagulant property, we prepared HDL subfractions by sequential density gradient ultracentrifugation over the 1.063-1.21 g/mL density range1. The density of each subfraction was measured by a densitometer at room temperature and ranged from 1.091 to 1.296 g/mL. Each fraction was dialyzed against TBS buffer containing 0.1% BSA and 0.2 mM EDTA and then assayed for their anticoagulant enhancing activity, as shown in Figure 1D. HDL subfractions (HDL2) ranging from 1.09 to 1.14 g/mL contained the predominant anticoagulant enhancing activity.
Discussion
Our studies show that when fresh, never-frozen HDL preparations, sourced from two independent laboratories outside of Scripps Research Institute, were analyzed using Superose 6 chromatography, the column fractions containing HDL but not the void volume fractions contained > 90 % of the preparation’s ability to enhance APC/protein S anticoagulant activity in plasma clotting assays. Moreover, anti-apoA-I antibodies removed most of the HDL preparation’s ability to enhance APC:Protein S activity, and specifically, HDL2 subfractions were the major source of this anticoagulant activity. We further show that the ability of fresh HDL to mediate this activity is lost as the HDL ages and appears to be completely lost after storage in the cold for 3 weeks, even without freezing. Thus, the data in these new studies validate and extend our previous report 10 about HDL’s ability to enhance APC: Protein S activity. We speculate that the conflicting report from Oslakovic and Dahlback11 was due to differences in HDL preparation, notably, their inferior quality HDL preparations, i.e., HDL which was stored frozen at −20 °C prior to bioassays and which had been prepared from “lipidmic” frozen blood bank plasma 12 as we confirmed in our chromatography experiments using fresh, never frozen vs frozen-thawed HDL. Functional activity studies using lipoproteins usually employ fresh, never-frozen preparations, as it is well-known that freeze-thawing disrupts lipoprotein particle structure rendering such preparations unsuitable for biological studies unless they are specially cryopreserved 13. Thus, it is likely that freeze-thaw cycles promoted development of phospholipid vesicles eluting in the void volume of Superose 6 columns in their HDL preparation and/or that unknown factors related to the long-term, frozen storage of HDL, led to the loss of HDL’s ability to enhance APC:Protein S.
In summary, our studies confirm that fresh HDL possesses anticoagulant cofactor activity, as we previously reported. 10 Understanding the components of HDL and the mechanisms by which this beneficial property occur could lead to novel therapeutic approaches to the prevention of venous thrombosis.
Supplementary Material
Significance.
This study confirms and extends previous data showing that the activity profile of large HDL subfractions includes an ability to enhance the anticoagulant actions of APC:protein S. This activity may contribute to the multi-faceted antithrombotic properties of HDL. Mechanisms for the anticoagulant enhancing activity of HDL remain unidentified, and the moderate instability of this activity of fresh, never-frozen HDL over several weeks makes applications of lipidomics and proteomics methodologies to identify HDL’s active components challenging.
Acknowledgements
We thank Ms. Lacthu Tonnu for excellent technical assistance.
Sources of Funding: This work was supported in part by NIH grants HL021544 and HL052246 (JHG) and HL 088093 (CLB and JLW).
Non-standard Abbreviations
- HDL
High Density Lipoprotein
- APC
Activated Protein C
- VTE
venous thrombosis
- FVa
Factor Va
- apoAI
apolipoprotein A1
Footnotes
Disclosures: None.
References
- 1.Havel RJ, Eder HA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rye KA, Barter PJ. Cardioprotective functions of HDLs. J Lipid Res. 2014;55:168–179. doi: 10.1194/jlr.R039297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rader DJ, Hovingh GK. HDL and cardiovascular disease. Lancet. 2014;384:618–625. doi: 10.1016/S0140-6736(14)61217-4. [DOI] [PubMed] [Google Scholar]
- 4.Durrington P. Dyslipidaemia. Lancet. 2003;362:717–731. doi: 10.1016/S0140-6736(03)14234-1. [DOI] [PubMed] [Google Scholar]
- 5.Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117:93–102. doi: 10.1161/CIRCULATIONAHA.107.709204. [DOI] [PubMed] [Google Scholar]
- 6.Deguchi H, Pecheniuk NM, Elias DJ, Averell PM, Griffin JH. High-density lipoprotein deficiency and dyslipoproteinemia associated with venous thrombosis in men. Circulation. 2005;112:893–899. doi: 10.1161/CIRCULATIONAHA.104.521344. [DOI] [PubMed] [Google Scholar]
- 7.Eichinger S, Pecheniuk NM, Hron G, Deguchi H, Schemper M, Kyrle PA, Griffin JH. High-density lipoprotein and the risk of recurrent venous thromboembolism. Circulation. 2007;115:1609–1614. doi: 10.1161/CIRCULATIONAHA.106.649954. [DOI] [PubMed] [Google Scholar]
- 8.Vaya A, Falco C, Simo M, Ferrando F, Mira Y, Todoli J, Espana F, Corella D. Influence of lipids and obesity on haemorheological parameters in patients with deep vein thrombosis. Thromb Haemost. 2007;98:621–626. [PubMed] [Google Scholar]
- 9.Mineo C, Deguchi H, Griffin JH, Shaul PW. Endothelial and antithrombotic actions of HDL. Circ Res. 2006;98:1352–1364. doi: 10.1161/01.RES.0000225982.01988.93. [DOI] [PubMed] [Google Scholar]
- 10.Griffin JH, Kojima K, Banka CL, Curtiss LK, Fernandez JA. High-density lipoprotein enhancement of anticoagulant activities of plasma protein S and activated protein C. J Clin Invest. 1999;103:219–227. doi: 10.1172/JCI5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oslakovic C, Norstrom E, Dahlback B. Reevaluation of the role of HDL in the anticoagulant activated protein C system in humans. J Clin Invest. 2010;120:1396–1399. doi: 10.1172/JCI42260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oslakovic C, Krisinger MJ, Andersson A, Jauhiainen M, Ehnholm C, Dahlback B. Anionic phospholipids lose their procoagulant properties when incorporated into high density lipoproteins. J Biol Chem. 2009;284:5896–5904. doi: 10.1074/jbc.M807286200. [DOI] [PubMed] [Google Scholar]
- 13.Rumsey SC, Galeano NF, Arad Y, Deckelbaum RJ. Cryopreservation with sucrose maintains normal physical and biological properties of human plasma low density lipoproteins. J Lipid Res. 1992;33:1551–1561. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


