Abstract
Inflammation is a fundamental feature of several complex cardiometabolic diseases. Indeed, obesity, insulin resistance, metabolic dyslipidemia, and atherosclerosis are all closely linked inflammatory states. Increasing evidence suggests that the infectious, biome-related or endogenous activation of the innate immune system may contribute to the development of metabolic syndrome and cardiovascular disease. Here we describe the human experimental endotoxemia model for the specific study of innate immunity in understanding further the pathogenesis of cardiometabolic disease. In a controlled, experimental setting, administration of an intravenous bolus of purified Escherichia coli endotoxin activates innate immunity in healthy human volunteers. During endotoxemia, changes emerge in glucose metabolism, lipoprotein composition, and lipoprotein functions that closely resemble those observed chronically in inflammatory cardiovascular disease risk states. In this review we describe the transient systemic inflammation and specific metabolic consequences that develop during human endotoxemia. Such a model provides a controlled induction of systemic inflammation, eliminates confounding, undermines reverse causation, and possesses unique potential as a starting point for genomic screening and testing of novel therapeutics for treatment of the inflammatory underpinning of cardiometabolic disease.
Keywords: Inflammation, Immune System, Metabolic Syndrome, Cardiovascular Disease Prevention, Cytokine
INTRODUCTION: INNATE IMMUNITY AND CARDIOMETABOLIC DISEASE
Innate immunity, an ancient form of host defense, is the body's rapid, first-line response to environmental threats such as microbial infection.1 In contrast to the adaptive immune system — which is present only in higher-order vertebrates, and mediated primarily by somatically-generated receptors — the innate immune system relies inherently on basic detection machineries coded for and conserved within the germ-lines of higher and lower organisms, from plants and fruits flies to mammals.2, 3 For the specificity of innate immune receptors to be conferred genetically, innate immune recognition must be built upon small families of membrane receptors that recognize highly conserved pattern structures present in large groups of microorganisms.2 Perhaps the most prominent and widely-studied subgroup of these pattern recognition receptors (PRRs) is the Toll-like receptor (TLR) family, whose ten members are manifested in humans as cell surface receptors in a series of trouble-detecting sentinel cells.4 Individual Toll-like receptors are known to play important roles in the recognition of structures derived from pathogens such as fungi, protozoa, viruses, and bacteria. As such, the TLR family is now widely accepted as the major microbe sensing system in mammals.5, 6
A classic starting point for innate immunity is Toll-like receptor-4, which — through detection of bacterial lipopolysaccharide (LPS) — is crucial for the effective immune response to gram negative bacteria.7 The binding of LPS to TLR-4 leads to downstream activation of nuclear factor- κB (NF-κB), a nuclear transcription factor responsible for regulating gene products that initiate a generalized inflammatory response.8 Specifically, tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and other pro-inflammatory mediators are all synthesized and released into the systemic circulation, where they trigger the activation of the complement system, the coagulation cascade, and the acute phase response. TNF-α and IL-1β also play key roles in facilitating leukocyte migration by increasing the expression of adhesion molecules on endothelial cells. Lastly, the presence of circulating inflammatory cytokines enhances tissue perfusion, vascular permeability, and cell migration throughout the body. Altogether, these systemic changes work together to allow for the timely and efficient eradication of the invading microorganism.9, 10
Inappropriate or sustained triggering of innate immunity and inflammatory signaling may, however, contribute to various medical conditions and diseases. Excessive activation of inflammatory cytokines leads to septic shock, a leading cause of death in patients with bacterial infections.11 Several studies indicate that more moderate TLR-4 activation is also linked to immunodeficiency, asthma, obesity, diabetes, and atherosclerosis,12–15 all of which are known to possess substantial inflammatory components. An inflammatory insulin resistance (IR) and metabolic dyslipidemia emerges clinically during acute sepsis16 and chronic infections,17 possibly via activation of TLR-4 signaling. Furthermore, experimental studies of TLR-4 deficiency in mouse models demonstrate a reduction in both diet induced obesity18 and atherosclerosis.19 Lastly, genetic manipulation and therapeutic targeting of TLR-420 and NF-κB21, 22 have provided proof of concept that modulation of innate immune signaling attenuates IR and type 2 diabetes (T2DM) in dietary and obesity models. Taken together, therefore, several lines of evidence suggest that chronic TLR-4 activation by exogenous and/or host-derived molecules may lead to a pro-inflammatory state of increased cytokines, chemokines, and adhesion molecules, all of which can exacerbate the risk of cardiometabolic disease.23
HUMAN EXPERIMENTAL ENDOTOXEMIA: AN INTRODUCTION & HISTORY
Human experimental endotoxemia has emerged as a controlled model for the study of complex disease inflammatory responses and their modulation in vivo. Administration of an intravenous bolus of purified Escherichia coli endotoxin activates TLR-4 signaling and stimulates innate immunity in healthy human volunteers.24 Though administration of lower doses of E. coli LPS (0.2–2 ng/ kg body weight) is best acknowledged today as a transient model of moderate systemic inflammation, intravenous LPS for decades was used at higher doses (3–5 ng/kg body weight) to mimic the storm of inflammatory signaling seen in acute clinical inflammatory conditions such as bacteremia and sepsis.25–28 Indeed, sepsis-like changes in systemic hemodynamics, ventricular function, pulmonary gas exchange, and permeability have all been shown to emerge within hours of experimental administration of higher doses of LPS.29 These responses are driven by a sharp induction of pro-inflammatory cytokines (e.g. TNF-α, IL-1β, IL-6, IL-8), many of which circulate in the plasma at levels resembling those seen clinically in infection and early sepsis.29 Since the pathophysiological derangements observed in septic patients result from an acute, systemic inflammatory response to endotoxin derived from gram-negative bacteria, it was logical to first use experimental endotoxemia most directly, and proximally, as a controlled model for the study of sepsis.
As such a controlled, transient model of early sepsis, human experimental endotoxemia also offered a means to study multiple organ dysfunction in septic shock, a topic intrinsically difficult to investigate in critically-ill patients. Using thermodilution pulmonary-artery catheters and simultaneous radionuclide cineangiography, Suffredini et al. were able to monitor the initial cardiovascular effects of 4 ng/kg endotoxin in healthy volunteers.24 Experimental endotoxemia resulted in a hyperdynamic cardiovascular state involving an early increase in cardiac index (CI) with a concurrent reduction in systemic vascular resistance (SVR). An elevated heart rate and reduced mean arterial pressure were also manifested, altogether suggesting that experimental endotoxemia qualitatively mimicked the hyperdynamic circulatory pattern observed in septic shock. During endotoxemia, left ventricular ejection fraction (EF) was significantly depressed, while end-diastolic and end-systolic volume indexes both increased. Decreased myocardial contractility was further evidenced by a reduced ratio of peak systolic pressure to end-systolic volume index (SBP/ESVI), an observation consistent with clinical studies of septic shock.30–33 Indeed, the presence of diminishing left ventricular function in manners analogous to clinical septic shock demonstrated that endotoxin, its detection machineries, and its signaling mechanisms also possessed biological relevance to sepsis-related cardiac dysfunction in humans.
HUMAN EXPERIMENTAL ENDOTOXEMIA AS A MODEL FOR CARDIOMETABOLIC DISEASE
As a growing collection of recent literature has investigated the intricacies of the endotoxemia model, it has become widely acknowledged that human experimental endotoxemia may actually not be best-defined as a model of sepsis, but rather one of moderate systemic inflammation.9, 34, 35 Particularly, at lower doses of LPS, endotoxemia activates innate immunity at a level vastly more relevant to the low-grade, chronic inflammatory state observed in cardiometabolic disease.36, 37 In contrast to the near supraphysiologic hundred to thousand-fold increases in TNF-α following higher doses of LPS,38, 39 administration of lower doses leads to modest, several-fold increases in plasma-levels of cytokines40, 41 which more closely reflects, albeit in an acute manner, the subclinical inflammation which characterizes the metabolic syndrome and chronic cardiovascular diseases.42–44 Moreover, as a model for the inflammatory contributions to cardiometabolic disease, experimental endotoxemia has strong biological plausibility because it activates pathways known to be perturbed in obesity, diabetes, and atherosclerosis.18–20 Indeed in settings of risk for cardiometabolic disease, TLR-4 is activated intermittently, both locally and systemically, by host-derived antigens that are generated and circulate at more modest concentrations and thereby establish a dynamic low-grade inflammatory state even in sterile, non-infectious settings. Thus, at lower doses, E.coli endotoxemia is reasonably thought to have substantial relevance to diseases associated with subclinical activation of innate immunity.36, 45
A recent development that supports the legitimacy of the human endotoxemia model is an increasing awareness of the gut microbiome as a dynamic inflammatory and metabolic influence in human disease. In fact, systemic and recurrent episodes of low-grade inflammation may result from metabolic endotoxemia and metabolic bacteremia — two phenomena in which bacterial fragments or live bacteria cross the gut mucosal membrane and enter into the systemic circulation.46, 47 Mounting evidence suggests that high fat diets increase gut permeability, resulting in 2- to 3- fold post-prandial increases of bacterial LPS in the host circulation48–51 while also generating, via altered gut microbiome, systemically active metabolites that directly impact cardiometabolic diseases.52, 53 Though post-prandial circulating levels of LPS are notably 10–50 times lower than the levels observed in septicemia and infections,47 metabolic endotoxemia nonetheless activates TLR-4-dependent innate immunity and appears to serve as an important determinant in the pathogenesis of inflammatory induced obesity and type 2 diabetes.46 Distinct gut microbiota signatures (GMS) — likely conferred by long-term diet54 — have been linked with inflammatory, obese, and metabolic conditions,55–57 and modulation of gut microbiota signatures in animal models has proven to relieve metabolic dysfunction.58, 59 Additionally, atherosclerotic plaque contains microbes, likely oral and gut derived,60 while blood microbial load may be predictive of the development of diabetes.61 Our growing understanding that the immune response to bacteria is closely linked to cardiometabolic disease risk emphasizes further both the relevance and utility of human endotoxemia protocols.
EVOKED INFLAMMATION INDUCES CARDIOMETABOLIC DISTURBANCES IN HUMANS
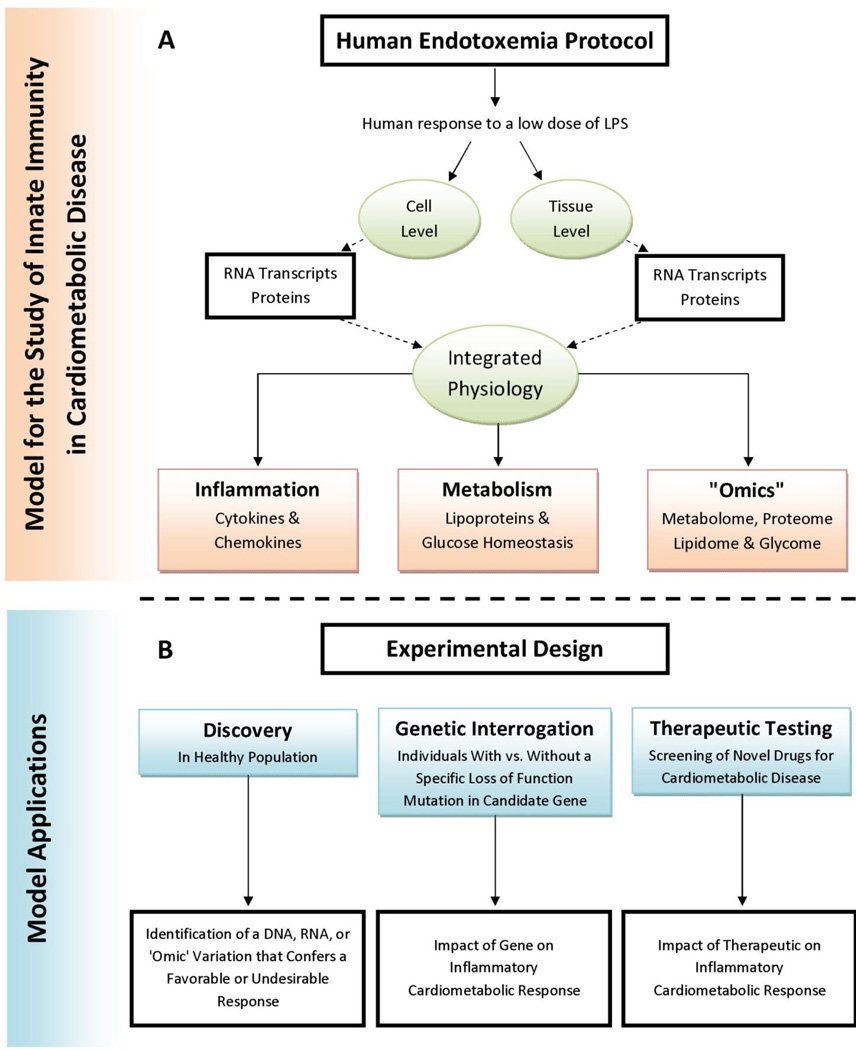
Following the administration of LPS in humans, several changes emerge that closely resemble those chronically observed in cardiovascular disease risk states (Figure A). To begin, experimental endotoxemia leads to significant system-wide alterations in glucose homeostasis. Agwunobi et al. were the first to document impaired insulin sensitivity following endotoxin administration in humans.62 More recently, our group further demonstrated that endotoxemia leads to the loss of both hepatic and peripheral insulin sensitivity.63 In a much larger sample, we have confirmed endotoxemia-induced insulin resistance in both European and African ancestry populations, and have revealed an apparent compensatory increase in pancreatic β-cell insulin secretion and function.64 Furthermore, we have shown that endotoxemia induces substantial adipose tissue inflammation — characterized by upregulation of chemokines, T-cell markers, macrophage markers, and many other genes — in a manner that parallels the abnormalities observed in adipose tissue in obesity and obesity-related insulin resistance.37, 63, 65, 66 Importantly, during experimental human endotoxemia, this adipose tissue inflammation has been shown to precede systemic insulin resistance.63 Last, our group has also shown that a subclinical, low-dose (0.6 ng/kg) endotoxemia produces a more subtle adipose tissue inflammation and a more modest insulin resistance more consistent with the extent of abnormality observed in metabolic syndrome and diabetes.36
Figure 1.
Panel A provides examples of the dynamic cardiometabolic responses to low-grade human endotoxemia. Panel B describes model applications for discovery, genetic and therapeutic purposes.
Inflammatory conditions are also characterized by widespread changes in plasma lipoproteins,67, 68 some of which may directly exacerbate the risk of cardiometabolic complications and atherosclerosis.69, 70 Hudgins et al. were the first to show that intravenous endotoxin in healthy human volunteers reproduces many of the lipid and lipoprotein changes observed in sepsis and atherogenic dyslipidemia — i.e., an increase in plasma triglycerides, an increase in small dense LDL particles, HDL remodeling, and a reduction in HDL particle size. Notably, these changes included a marked increase in HDL-serum amyloid-A (SAA) and a decline in HDL phospholipid, all while apolipoprotein A-I and HDL-C levels remained constant.71 More recently, our group examined the functional consequences of LPS-induced HDL remodeling and demonstrated that endotoxemia triggers HDL dysfunction — specifically by impairing HDL-macrophage cholesterol efflux function, the first step in reverse cholesterol transport (RCT)72 — independent of changes in plasma HDL-C and ApoA-I levels.73, 74 We observed that the activation of innate immunity modulates HDL composition in particular by inducing a substantial loss of HDL phospholipid and a specific decrease in small to medium sized HDL particles. Importantly, these changes followed a simultaneous induction of both HDL lipases and HDL enrichment with SAA,73–75 and coincided with an impaired capacity of the isolated HDL to efflux cholesterol from macrophages.73, 74 This loss of HDL RCT function is a pathologic hallmark of acute and chronic-recurrent clinical inflammatory syndromes that are associated with an increased risk of atherosclerosis and acute cardiovascular events. In fact, reduced HDL cholesterol-efflux function has been observed in insulin resistance,76 obesity,77 psoriasis,78 systemic lupus erythematosis,79 acute infections,80 and surgery-induced systemic inflammation,80 and has been shown to be an independent risk factor for coronary artery disease independent of HDL-C levels.81 Together, these human data underscore the clinical relevance of experimental endotoxemia in the study of the atherogenic dyslipidemia found in inflammatory cardiometabolic diseases.
ADVANTAGES OF THE MODEL
A distinct advantage of the experimental endotoxemia model is that it controls in a temporal manner the activation of innate immunity and its downstream responses in healthy human volunteers. As such, the model eliminates both confounding and reverse causation — features of observational studies in which inflammatory changes may result from other risk factors and the disease itself rather than being causal. Thus, the model provides a controlled framework for assessing the downstream impact of induced inflammation in vivo. Typically, endotoxemia studies are performed in healthy human volunteer samples. Though this certainly reduces direct translation to specific diseases, there are obvious advantages for experimental control of confounding parameters that may affect inflammatory outcomes. Individual studies may vary, but in general controllable parameters (often exclusions) include age range, obesity, pregnancy/lactating status, chronic or recurrent medical disorders including cardiovascular disease, diabetes mellitus, hypertension, malignancy, inflammatory and rheumatological disorders, HIV-1 infection, liver or kidney disease, tobacco use or use of any prescription medication or supplemental vitamins. Recent evidence suggests that race is a parameter which may affect response,64 while the influence of gender is still under debate.38, 82 Emerging data has demonstrated also that LPS responsiveness varies with circadian rhythm83 – consequently, most studies are performed at the same time of day, typically in the morning.
Further, when attempting to predict the biochemical and clinical consequences of activated innate immunity in disease, studying the evoked physiology may be of much greater value than measuring the resting levels of inflammatory markers, the strategy in epidemiological studies. Unlike more static blood risk factors (e.g., LDL-C), single time-point measurements of basal circulating levels of inflammatory markers (e.g., cytokines and acute phase proteins), that are putative biomarkers of cardiovascular disease,84, 85 may not necessarily reflect the physiology and pathophysiology of innate immune responses during dynamic disease processes in acute, sub-acute or even chronic disease. In fact, resting levels in non-stressed settings may have limited relevance to how the host responds during acute or recurrent pathophysiological stresses, as has been demonstrated, particularly in response to nutritional challenges.86, 87 Thus the evoked response might be more clinically informative than basal levels. In this context, in our own work (the largest human endotoxemia protocol published to date, n = 294),64 we have observed that (a) the LPS-induced cytokine responses had greater correlations with each other and with the subsequent increases in acute-phase proteins than the correlations observed for the pre-LPS cytokines with baseline biomarkers or with LPS-induced responses, (b) opposite trends in basal vs. endotoxemia-responses across race, with lower peak levels, but higher basal levels of inflammatory biomarkers in African Americans compared to European Americans, and (c) a genome wide-significant locus for evoked fever has no association with basal temperature.88–91 Thus, basal levels may not capture the dynamic pathophysiology, may have limited utility as markers of innate immune processes, and may also be relatively poor predictors of the evoked response and innate immune activity during inflammatory stress and in disease.
Experimental endotoxemia also provides a precise model for the study of the temporal patterns of innate immune responses in humans, from the early activation of systemic inflammation to the later resolution phase. This can offer a much more complete insight into the complex physiological, molecular, and genetic influences on the promotion and resolution of inflammation, insights that cannot be derived from single-time point estimates or repeated sampling of resting levels in traditional population studies and clinical trials. Furthermore, by making repeated measurements on the same individual over time, the model also can account for inter-individual variation. Coupled to the capacity to reveal biological differences in innate immune responses that are either enhanced by or only evident after the experimental perturbation, this allows more modest sample sizes than traditional static epidemiological designs.92–95
Though animal models of experimental endotoxemia have benefits over human models in terms of cost, feasibility and genetic manipulation, there are important differences between humans and model organisms that decrease the applicability of animal studies and highlight the advantages of the human experimental system. Many model organisms including mouse and zebrafish are LPS-tolerant relative to human,96, 97 and thus may not be ideal models of human disease. A noteworthy study directly compared gene expression changes in human severe blunt trauma, human burn injury, 2 ng/kg human endotoxemia, mouse trauma, mouse burn injury, and mouse endotoxemia at a mathematically scaled down dose.98 Although this study concluded that mice make poor models for inflammatory diseases, a subsequent publication using the same data came to a different conclusion.99 The results from these conflicting analyses revealed that though different etiologies of acute inflammatory stresses result in highly similar genomic responses in humans, the responses in corresponding mouse models may only partially overlap with the human conditions. While rodent models have specific utility, the ongoing controversy underscores the need for caution in extrapolating rodent models to study human inflammatory diseases, and emphasizes the value of human translational research models with direct relevance to human disease. Similarly, ex vivo endotoxemia models using human cells100, 101 allow for high-throughput profiling, however these models are not able to recapitulate the complexities of the multiple tissues and integration of cell-types involved in the whole-organism inflammatory response.102
Finally, controlled endotoxemia is a useful model for the evaluation of genetic influences on evoked clinical inflammatory phenotypes as well as the cytokine responses that drive clinical pathophysiologies. Genetic variation in TLR-4 is associated with differences in LPS responsiveness,103 while promoter polymorphisms in candidate genes such as TNF-α, IL-10, and IL-6 have all been studied with the intent of demonstrating the importance of specific genes and pathways on the induced inflammatory response.104, 105 Recent studies have probed the cell-specific transcriptomic106 underpinning of the evoked response to endotoxemia, with novel data revealing the potential role of tissue-specific inflammatory modulation of non-coding RNA in inflammatory cardiometabolic disease.107 As a controlled model of proven relevance to inflammatory diseases, metabolic syndrome and cardiovascular disease, human experimental endotoxemia provides a probe for the study of therapeutic influences on inflammatory atherogenic stress, with important clinical and translational implications, as discussed in the next section. Altogether, experimental endotoxemia provides a well-characterized, reproducible, and tractable model of inflammation in which novel therapies and genomic influences can be tested for their ability to modulate evoked inflammation and its specific metabolic consequences. Overall, such natural genomic variations and experimental interventions offer a starting point and screening strategy for development of novel therapies for treatment of acute and chronic human inflammatory and cardiometabolic diseases.
EVIDENCE FOR TRANSLATION AND CLINICAL RELEVANCE
Although the model is unable to capture the chronicity of inflammation, findings from human endotoxemia have proven to be relevant to the clinical course of both acute inflammatory and chronic inflammatory disease states. TNFα blockers and IL-1 pathway antagonists that showed partial suppression of the inflammatory response in human endotoxemia models may have failed in trials of sepsis, but now are mainstays in the treatment of rheumatoid arthritis, inflammatory bowel disease, ankylosing spondylitis, and gout.108–111 Indeed, endotoxemia protocols have been used safely in humans for decades to test the efficacy of LPS antagonists,112, 113 IL-1 receptor antagonists,114, 115 IL-10 infusions,116, 117 and TNFα blockers118, 119 in mitigating the system-wide dysfunction which results from excessive innate immune signaling. Evoked endotoxemia can be used to inform mechanism of action of therapeutics, potentially identifying novel applications, or contraindicating utility. Thalidomide was thought to offer therapeutic benefit through modulation of TNFα, however results in clinical trials on TNFα modulation were conflicting. In an evoked endotoxemia protocol, thalidomide was found to have no significant effect on the TNFα response to LPS, but significantly decreased the IL-6 response, suggesting IL-6 rather than TNFα as a potential target.120 Similarly, evoked endotoxemia has been used to understand the specific in vivo effects of different doses of prednisolone, revealing target effects on fibrinolytic pathways and chemokine responses.121, 122 Dobutamine, a catecholamine used to treat septic myocardial dysfunction, has no effect on inflammatory responses to evoked endotoxemia in vivo, despite effects in vitro, highlighting the importance of the human model.123 In our own work, we found no effect of fenofibrate on response to evoked endotoxemia,124 contrasting with a modulating effect of high-dose n-3 PUFA supplementation in the same trial.125 In addition to modeling pharmacologic interventions, experimental endotoxemia has also been applied to study the capacity of nutrients to modify the systemic inflammatory response. Notably, the anti-inflammatory properties of omega-3 polyunsaturated fatty acids have been assessed by administering fish oil either parenterally126 or through dietary supplementation125, 127 prior to the endotoxin challenge, while habitual dietary intake of soy-derived foods may also modify the response to endotoxemia.128
Differences in the evoked responses, and the genetic determinants of these differences, may indeed relate to the clinical course of future disease. For example, our group has shown recently that genetic variation associated with the evoked IL1RA response during experimental endotoxemia is also predictive of patient survival in septic shock.129 Further, as noted above, evoked endotoxemia revealed a novel genomic locus for the febrile response to LPS (but not resting body temperature) and this locus also associates with outcomes following severe trauma and sepsis.91 Other common genetic variants influencing both response to evoked endotoxemia and disease risk have been described. A SNP in MMP-8, previously shown to associate with mortality in pneumonia, was found to modulate the inflammatory response to evoked endotoxemia.130 Similarly, genetic variation in fibrinogen and CRP relate to the endotoxemia response.131, 132 Finally, experimental endotoxemia revealed polymorphism-specific effects in TNF, with the Asp299Gly and Thr399Ile, but not the -308 G/A polymorphisms associating with inflammatory response,133, 134 while variation in IL-6 was not associated with alterations in the IL-6 response to endotoxemia.105 These studies thus highlight common genetic underpinnings of evoked endotoxemia and inflammatory responses, which then direct functional studies and clinical translation.
LIMITATIONS AND CHALLENGES OF THE MODEL
The human experimental endotoxemia model generates a low-grade acute systemic inflammatory state and admittedly does not fully capture chronic subclinical inflammation as is present in cardiometabolic disease. Additionally, due to Food and Drug Administration restrictions on the use of experimental endotoxemia in humans, most protocols are now restricted to relatively young (< age 45), healthy, non-obese (BMI <30) non-smoking individuals. Because of the small number of subjects who have undergone endotoxemia studies and their relatively young age, it has not been possible in this field to date to perform cardiovascular disease outcome studies and evaluate the relationship between LPS phenotype and future cardiovascular events. Additionally, there is also no currently established association between the LPS response and established inflammatory biomarkers of CVD. However, as noted, relative to resting inflammatory biomarker levels (which in fact are very modest predictors of CVD), the evoked inflammatory biomarker response to endotoxemia may better reflect the pathophysiology, the genetic underpinnings and the therapeutic modulation of innate immunity during inflammatory stress. A pragmatic approach to overcome limitations on predictive capacity of the model is to use the endotoxemia model as a tool to focus on specific responses or characteristics of interest and then to assess the relation of those characteristics to incident clinical disease in independent epidemiological studies.129
Moreover, the single-exposure human endotoxemia model, as approved currently for use within the US by the FDA, is unable to capture sustained activation of innate immunity which may occur with chronic or repeated exposures to innate immune ligands (e.g. chronic infections, inflammatory bowel disease, or chronic obstructive pulmonary disease). However, repeated-exposure models have been applied by researchers in Europe, further illuminating the biology of chronic innate immune stimulation in clinical disease. Five consecutive days of 2 ng/kg endotoxin administration leads to endotoxin tolerance,135 with evidence of attenuated release of both pro-inflammatory and anti-inflammatory cytokines over time, leading to less leukocyte and endothelial activation, an effect which may last several weeks.102 In the same model, endothelial dysfunction gradually declines as endotoxin tolerance emerges,136 whereas LPS tolerance does not seem to protect against ischemia-reperfusion injury.137 The repeated-exposure model has also been used for the study of sepsis-induced immunoparalysis, where IFN-γ treatment has partially reversed immune suppression and furthered pharmacologic interest for immunostimulation in sepsis.138 Though these findings provide key initial insights into the elements of endotoxin tolerance, much research is still needed to elucidate the role of repeated LPS exposure on cardiometabolic physiology.
Indeed, many compounds including cytokine pathway modulators, endotoxin antagonists, nutritional supplements, hormones, and novel therapeutics, among others, have all to varying degrees been shown to influence the systemic inflammatory response in human experimental endotoxemia.35 However, due to the complexity of the inflammatory response, no single intervention has been shown to blunt the entire inflammatory spectrum during endotoxemia. In fact, TNFα blockers118, 119 and IL-1 antagonists114, 139 only partially mitigated the systemic inflammatory response in human endotoxemia, and were brought to clinical trials of sepsis in part because of promising results indicating decreased mortality with such compounds in rodent models of endotoxemia. Recent data suggesting that rodent models of endotoxemia correlate poorly with human conditions may partly explain why these compounds failed in clinical trials of human sepsis.98 Our recent work, however, show that genetic variation associated with the evoked IL1RA response to experimental endotoxemia is predictive of patient survival in clinical cohorts with septic shock.129 These data have re-ignited a discussion on whether IL-1 pathway modulation might provide clinical benefit in sepsis if targeted to subsets of patients with specific genetic or biomarker features i.e., a “precision medicine” approach. As noted also, TNF pathway blockers and IL-1 antagonists ultimately succeeded in clinical translation and are now mainstays in the treatment of several rheumatological and inflammatory disorders.108–111
FUTURE AND CONCLUSION
Human experimental endotoxemia has established utility as a controlled model of systemic inflammation. Coupled to contemporary genomics, transcriptomics, and strategies for development of novel therapeutics, the model provides a unique platform for clinical, genetic, and pharmacological research applications in inflammatory and cardiometabolic diseases (Figure B). Controlled sampling within the structure of the experimental model permits cell- and tissue-specific interrogation of genomic, epigenetic, and transcriptomic responses to the activation of innate immunity, and may help identify unique molecules or pathways for future treatments that target cell-specific components of innate immunity. Admittedly, human experimental endotoxemia is just one of several complementary approaches which all possess the advantage of studying genomic and transcriptomic regulation in context.100, 101 In all human experimental models, increasing sophistication in multiple “omics” profiling and integrative genomics combined with the evoked phenotypic responses allows for enhanced discovery and profiling of novel pathways and therapeutics even with limited trial sample sizes.91, 92, 95, 129 Alongside discovery, the human endotoxemia model allows for the assessment of specific genetic, pharmacologic, and lifestyle exposures on cell and organ level responses, as well as the effect of these exposures on the dynamic integrated human host physiology. Lastly, in light of recent evidence demonstrating the challenges in extrapolating rodent endotoxemia models to human inflammatory disease,98 greater emphasis on human translational experimental models is warranted.
With the advent of whole exome and genome sequencing, we now have the unique opportunity with the human endotoxemia model to examine the impact of specific loss of function alleles in the human genome on innate immune physiologies as well as the cell-specific mechanisms underlying the host response in these “knock-out” in vivo. Certainly, an exciting future exists in the controlled, clinically-relevant, human endotoxemia model where precision and personalized initiatives may be discovered, evaluated, and expanded into diagnostic, prognostic, and therapeutic applications.
SIGNIFICANCE.
Inflammation is a key feature of several complex cardiometabolic diseases, and evidence suggests the activation of the innate immune system may be a contributing factor in the pathophysiology of obesity, insulin resistance, metabolic dyslipidemia, and atherosclerosis. Here we describe the human experimental endotoxemia model for the specific study of innate immunity in understanding further the pathogenesis of cardiometabolic diseases in humans. In a controlled, experimental setting, administration of an intravenous bolus of purified Escherichia coli endotoxin activates innate immunity in healthy human volunteers, and elicits specific metabolic consequences that closely resemble those observed chronically in inflammatory cardiovascular disease risk states. Such a model allows for the controlled study of innate immune influences on cardiometabolic physiology, but perhaps more importantly is uniquely positioned with contemporary technology as a starting point for genomic screening and testing of novel therapeutics for treatment of the inflammatory underpinning of cardiometabolic disease.
ACKNOWLEDGEMENTS
None
SOURCES OF FUNDING:
This work and related research was supported by NIH-NHLBI SCCOR Project grant (P50-HL-083799), R01-HL113147 and K24-HL107643 to MPR. JFF is supported by a postdoctoral fellowship grant from the American Heart Association (12POST11840017). MPR is also supported by H111694, DK-090505 and HL-108636.
ABBREVIATIONS
- TLR
Toll-like Receptor
- LPS
Lipopolysaccharide
- TNF-α
Tumor Necrosis Factor-α
- IL
Interleukin
- NF-κB
Nuclear Factor-κB
- RCT
Reverse Cholesterol Transport
- SAA
Serum Amyloid-A
- CVD
Cardiovascular disease
Footnotes
DISCLOSURES:
None
REFERENCES
- 1.Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RAB. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R, Janeway C. Innate immunity. New England Journal of Medicine. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 3.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes care. 2004;27:813–823. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 5.Takeda K, Akira S. Toll-like receptors in innate immunity. International immunology. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 6.Moresco EMY, LaVine D, Beutler B. Toll-like receptors. Current biology : CB. 2011;21:R488–R493. doi: 10.1016/j.cub.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 7.Parker LC, Prince LR, Sabroe I. Translational mini-review series on toll-like receptors: Networks regulated by toll-like receptors mediate innate and adaptive immunity. Clinical & Experimental Immunology. 2007;147:199–207. doi: 10.1111/j.1365-2249.2006.03203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong ET, Tergaonkar V. Roles of nf-kappab in health and disease: Mechanisms and therapeutic potential. Clinical science. 2009;116:451–465. doi: 10.1042/CS20080502. [DOI] [PubMed] [Google Scholar]
- 9.Andreasen AS, Krabbe KS, Krogh-Madsen R, Taudorf S, Pedersen BK, Moller K. Human endotoxemia as a model of systemic inflammation. Current medicinal chemistry. 2008;15:1697–1705. doi: 10.2174/092986708784872393. [DOI] [PubMed] [Google Scholar]
- 10.Sherwood ER, Toliver-Kinsky T. Mechanisms of the inflammatory response. Best Practice & Research Clinical Anaesthesiology. 2004;18:385–405. doi: 10.1016/j.bpa.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Ulevitch RJ, Tobias PS. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annual review of immunology. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 12.Ferwerda B, McCall MB, Verheijen K, Kullberg BJ, van der Ven AJ, Van der Meer JW, Netea MG. Functional consequences of toll-like receptor 4 polymorphisms. Molecular medicine. 2008;14:346–352. doi: 10.2119/2007-00135.Ferwerda. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Neill LAJ, Bryant CE, Doyle SL. Therapeutic targeting of toll-like receptors for infectious and inflammatory diseases and cancer. Pharmacological Reviews. 2009;61:177–197. doi: 10.1124/pr.109.001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Werner M, Topp R, Wimmer K, Richter K, Bischof W, Wjst M, Heinrich J. Tlr4 gene variants modify endotoxin effects on asthma. The Journal of allergy and clinical immunology. 2003;112:323–330. doi: 10.1067/mai.2003.1648. [DOI] [PubMed] [Google Scholar]
- 15.Cook DN, Pisetsky DS, Schwartz DA. Toll-like receptors in the pathogenesis of human disease. Nature immunology. 2004;5:975–979. doi: 10.1038/ni1116. [DOI] [PubMed] [Google Scholar]
- 16.Van Cromphaut SJ, Vanhorebeek I, Van den Berghe G. Glucose metabolism and insulin resistance in sepsis. Current pharmaceutical design. 2008;14:1887–1899. doi: 10.2174/138161208784980563. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Real JM, Lopez-Bermejo A, Vendrell J, Ferri MJ, Recasens M, Ricart W. Burden of infection and insulin resistance in healthy middle-aged men. Diabetes care. 2006;29:1058–1064. doi: 10.2337/diacare.2951058. [DOI] [PubMed] [Google Scholar]
- 18.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. Tlr4 links innate immunity and fatty acid–induced insulin resistance. The Journal of Clinical Investigation. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein e. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsukumo DML, Carvalho-Filho MA, Carvalheira JBC, Prada PO, Hirabara SM, Schenka AA, Araújo EP, Vassallo J, Curi R, Velloso LA, Saad MJA. Loss-of-function mutation in toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 21.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shoelson SE, Lee J, Yuan M. Inflammation and the ikk beta/i kappa b/nf-kappa b axis in obesity- and diet-induced insulin resistance. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2003;27(Suppl 3):S49–S52. doi: 10.1038/sj.ijo.0802501. [DOI] [PubMed] [Google Scholar]
- 23.Frantz S, Ertl G, Bauersachs J. Mechanisms of disease: Toll-like receptors in cardiovascular disease. Nat Clin Pract Cardiovasc Med. 2007;4:444–454. doi: 10.1038/ncpcardio0938. [DOI] [PubMed] [Google Scholar]
- 24.Suffredini AF, Fromm RE, Parker MM, Brenner M, Kovacs JA, Wesley RA, Parrillo JE. The cardiovascular response of normal humans to the administration of endotoxin. N Engl J Med. 1989;321:280–287. doi: 10.1056/NEJM198908033210503. [DOI] [PubMed] [Google Scholar]
- 25.Elin RJ, Wolff SM. Biology of endotoxin. Annu Rev Med. 1976;27:127–141. doi: 10.1146/annurev.me.27.020176.001015. [DOI] [PubMed] [Google Scholar]
- 26.Michie HR, Spriggs DR, Manogue KR, Sherman ML, Revhaug A, O'Dwyer ST, Arthur K, Dinarello CA, Cerami A, Wolff SM, et al. Tumor necrosis factor and endotoxin induce similar metabolic responses in human beings. Surgery. 1988;104:280–286. [PubMed] [Google Scholar]
- 27.Deitch EA. Animal models of sepsis and shock: A review and lessons learned. Shock. 1998;9:1–11. doi: 10.1097/00024382-199801000-00001. [DOI] [PubMed] [Google Scholar]
- 28.O'Reilly M, Newcomb DE, Remick D. Endotoxin, sepsis, and the primrose path. Shock. 1999;12:411–420. doi: 10.1097/00024382-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Martich GD, Boujoukos AJ, Suffredini AF. Response of man to endotoxin. Immunobiology. 1993;187:403–416. doi: 10.1016/S0171-2985(11)80353-0. [DOI] [PubMed] [Google Scholar]
- 30.Ellrodt AG, Riedinger MS, Kimchi A, Berman DS, Maddahi J, Swan HJC, Murata GH. Left ventricular performance in septic shock: Reversible segmental and global abnormalities. American heart journal. 1985;110:402–409. doi: 10.1016/0002-8703(85)90163-2. [DOI] [PubMed] [Google Scholar]
- 31.Parker MM, Suffredini AF, Natanson C, Ognibene FP, Shelhamer JH, Parrillo JE. Responses of left ventricular function in survivors and nonsurvivors of septic shock. Journal of Critical Care. 1989;4:19–25. [Google Scholar]
- 32.Parker MM, Shelhamer JH, Bacharach SL, Green MV, Natanson C, Frederick TM, Damske BA, Parrillo JE. Profound but reversible myocardial depression in patients with septic shock. Annals of internal medicine. 1984;100:483–490. doi: 10.7326/0003-4819-100-4-483. [DOI] [PubMed] [Google Scholar]
- 33.Landesberg G, Gilon D, Meroz Y, Georgieva M, Levin PD, Goodman S, Avidan A, Beeri R, Weissman C, Jaffe AS, Sprung CL. Diastolic dysfunction and mortality in severe sepsis and septic shock. European heart journal. 2012;33:895–903. doi: 10.1093/eurheartj/ehr351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calvano SE, Coyle SM. Experimental human endotoxemia: A model of the systemic inflammatory response syndrome? Surg Infect (Larchmt) 2012;13:293–299. doi: 10.1089/sur.2012.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Remick DG, Ward PA. Evaluation of endotoxin models for the study of sepsis. Shock. 2005;24(Suppl 1):7–11. doi: 10.1097/01.shk.0000191384.34066.85. [DOI] [PubMed] [Google Scholar]
- 36.Mehta NN, Heffron SP, Patel PN, Ferguson J, Shah RD, Hinkle CC, Krishnamoorthy P, Shah R, Tabita-Martinez J, Terembula K, Master SR, Rickels MR, Reilly MP. A human model of inflammatory cardio-metabolic dysfunction; a double blind placebo-controlled crossover trial. Journal of translational medicine. 2012;10:124. doi: 10.1186/1479-5876-10-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah R, Lu Y, Hinkle CC, McGillicuddy FC, Kim R, Hannenhalli S, Cappola TP, Heffron S, Wang X, Mehta NN, Putt M, Reilly MP. Gene profiling of human adipose tissue during evoked inflammation in vivo. Diabetes. 2009;58:2211–2219. doi: 10.2337/db09-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coyle SM, Calvano SE, Lowry SF. Gender influences in vivo human responses to endotoxin. Shock. 2006;26:538–543. doi: 10.1097/01.shk.0000232589.39001.4d. [DOI] [PubMed] [Google Scholar]
- 39.Elin RJ, Wolff SM, McAdam KPWJ, Chedid L, Audibert F, Bernard C, Oberling F. Properties of reference escherichia coli endotoxin and its phthalylated derivative in humans. Journal of Infectious Diseases. 1981;144:329–336. doi: 10.1093/infdis/144.4.329. [DOI] [PubMed] [Google Scholar]
- 40.Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and il-6 infusion inhibit endotoxin-induced tnf-alpha production in humans. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17:884–886. doi: 10.1096/fj.02-0670fje. [DOI] [PubMed] [Google Scholar]
- 41.Taudorf S, Krabbe KS, Berg RM, Pedersen BK, Moller K. Human models of low-grade inflammation: Bolus versus continuous infusion of endotoxin. Clinical and vaccine immunology : CVI. 2007;14:250–255. doi: 10.1128/CVI.00380-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winkler G, Salamon F, Harmos G, Salamon D, Speer G, Szekeres O, Hajos P, Kovacs M, Simon K, Cseh K. Elevated serum tumor necrosis factor-alpha concentrations and bioactivity in type 2 diabetics and patients with android type obesity. Diabetes research and clinical practice. 1998;42:169–174. doi: 10.1016/s0168-8227(98)00109-0. [DOI] [PubMed] [Google Scholar]
- 44.Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitamins and hormones. 2006;74:443–477. doi: 10.1016/S0083-6729(06)74018-3. [DOI] [PubMed] [Google Scholar]
- 45.Mullington J, Korth C, Hermann DM, Orth A, Galanos C, Holsboer F, Pollmächer T. Dose-dependent effects of endotoxin on human sleep. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2000;278:R947–R955. doi: 10.1152/ajpregu.2000.278.4.R947. [DOI] [PubMed] [Google Scholar]
- 46.Piya MK, Harte AL, McTernan PG. Metabolic endotoxaemia: Is it more than just a gut feeling? Curr Opin Lipidol. 2013;24:78–85. doi: 10.1097/MOL.0b013e32835b4431. [DOI] [PubMed] [Google Scholar]
- 47.Shen J, Obin MS, Zhao L. The gut microbiota, obesity and insulin resistance. Molecular Aspects of Medicine. 2013;34:39–58. doi: 10.1016/j.mam.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Harte AL, Varma MC, Tripathi G, McGee KC, Al-Daghri NM, Al-Attas OS, Sabico S, O’Hare JP, Ceriello A, Saravanan P, Kumar S, McTernan PG. High fat intake leads to acute postprandial exposure to circulating endotoxin in type 2 diabetic subjects. Diabetes care. 2012;35:375–382. doi: 10.2337/dc11-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, Alessi MC, Chamontin B, Ferrieres J. Energy intake is associated with endotoxemia in apparently healthy men. The American journal of clinical nutrition. 2008;87:1219–1223. doi: 10.1093/ajcn/87.5.1219. [DOI] [PubMed] [Google Scholar]
- 50.Brun P, Castagliuolo I, Di Leo V, Buda A, Pinzani M, Palu G, Martines D. Increased intestinal permeability in obese mice: New evidence in the pathogenesis of nonalcoholic steatohepatitis. American journal of physiology. Gastrointestinal and liver physiology. 2007;292:G518–G525. doi: 10.1152/ajpgi.00024.2006. [DOI] [PubMed] [Google Scholar]
- 51.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 52.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WHW, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. The New England journal of medicine. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y-Y, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 56.De Bandt JP, Waligora-Dupriet AJ, Butel MJ. Intestinal microbiota in inflammation and insulin resistance: Relevance to humans. Current opinion in clinical nutrition and metabolic care. 2011;14:334–340. doi: 10.1097/MCO.0b013e328347924a. [DOI] [PubMed] [Google Scholar]
- 57.Cani PD, Osto M, Geurts L, Everard A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut microbes. 2012;3:279–288. doi: 10.4161/gmic.19625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carvalho BM, Guadagnini D, Tsukumo DM, Schenka AA, Latuf-Filho P, Vassallo J, Dias JC, Kubota LT, Carvalheira JB, Saad MJ. Modulation of gut microbiota by antibiotics improves insulin signalling in high-fat fed mice. Diabetologia. 2012;55:2823–2834. doi: 10.1007/s00125-012-2648-4. [DOI] [PubMed] [Google Scholar]
- 59.Murphy EF, Cotter PD, Hogan A, O'Sullivan O, Joyce A, Fouhy F, Clarke SF, Marques TM, O'Toole PW, Stanton C, Quigley EM, Daly C, Ross PR, O'Doherty RM, Shanahan F. Divergent metabolic outcomes arising from targeted manipulation of the gut microbiota in diet-induced obesity. Gut. 2013;62:220–226. doi: 10.1136/gutjnl-2011-300705. [DOI] [PubMed] [Google Scholar]
- 60.Koren O, Spor A, Felin J, Fak F, Stombaugh J, Tremaroli V, Behre CJ, Knight R, Fagerberg B, Ley RE, Backhed F. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4592–4598. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amar J, Serino M, Lange C, Chabo C, Iacovoni J, Mondot S, Lepage P, Klopp C, Mariette J, Bouchez O, Perez L, Courtney M, Marre M, Klopp P, Lantieri O, Dore J, Charles M, Balkau B, Burcelin R Group DESIRS. Involvement of tissue bacteria in the onset of diabetes in humans: Evidence for a concept. Diabetologia. 2011;54:3055–3061. doi: 10.1007/s00125-011-2329-8. [DOI] [PubMed] [Google Scholar]
- 62.Agwunobi AO, Reid C, Maycock P, Little RA, Carlson GL. Insulin resistance and substrate utilization in human endotoxemia. The Journal of clinical endocrinology and metabolism. 2000;85:3770–3778. doi: 10.1210/jcem.85.10.6914. [DOI] [PubMed] [Google Scholar]
- 63.Mehta NN, McGillicuddy FC, Anderson PD, Hinkle CC, Shah R, Pruscino L, Tabita-Martinez J, Sellers KF, Rickels MR, Reilly MP. Experimental endotoxemia induces adipose inflammation and insulin resistance in humans. Diabetes. 2010;59:172–181. doi: 10.2337/db09-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferguson J, Patel P, Shah R, Mulvey C, Gadi R, Nijjar P, Usman H, Mehta N, Shah R, Master S, Propert K, Reilly M. Race and gender variation in response to evoked inflammation. Journal of translational medicine. 2013;11:63. doi: 10.1186/1479-5876-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Jr, AWF Ccr2 modulates inflammatory and metabolic effects of high-fat feeding. The Journal of Clinical Investigation. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anderson PD, Mehta NN, Wolfe ML, Hinkle CC, Pruscino L, Comiskey LL, Tabita-Martinez J, Sellers KF, Rickels MR, Ahima RS, Reilly MP. Innate immunity modulates adipokines in humans. The Journal of clinical endocrinology and metabolism. 2007;92:2272–2279. doi: 10.1210/jc.2006-2545. [DOI] [PubMed] [Google Scholar]
- 67.Levels JHM, Pajkrt D, Schultz M, Hoek FJ, van Tol A, Meijers JCM, van Deventer SJH. Alterations in lipoprotein homeostasis during human experimental endotoxemia and clinical sepsis. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2007;1771:1429–1438. doi: 10.1016/j.bbalip.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 68.Grunfeld C, Pang M, Doerrler W, Shigenaga JK, Jensen P, Feingold KR. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. Journal of Clinical Endocrinology & Metabolism. 1992;74:1045–1052. doi: 10.1210/jcem.74.5.1373735. [DOI] [PubMed] [Google Scholar]
- 69.van der Westhuyzen DR, de Beer FC, Webb NR. Hdl cholesterol transport during inflammation. Current opinion in lipidology. 2007;18:147–151. doi: 10.1097/MOL.0b013e328051b4fe. 110.1097/MOL.1090b1013e328051b328054fe. [DOI] [PubMed] [Google Scholar]
- 70.Khovidhunkit W, Kim M-S, Memon RA, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C. Thematic review series: The pathogenesis of atherosclerosis. Effects of infection and inflammation on lipid and lipoprotein metabolism mechanisms and consequences to the host. Journal of lipid research. 2004;45:1169–1196. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- 71.Hudgins LC, Parker TS, Levine DM, Gordon BR, Saal SD, Jiang XC, Seidman CE, Tremaroli JD, Lai J, Rubin AL. A single intravenous dose of endotoxin rapidly alters serum lipoproteins and lipid transfer proteins in normal volunteers. Journal of lipid research. 2003;44:1489–1498. doi: 10.1194/jlr.M200440-JLR200. [DOI] [PubMed] [Google Scholar]
- 72.Wang X, Rader DJ. Molecular regulation of macrophage reverse cholesterol transport. Current opinion in cardiology. 2007;22:368–372. doi: 10.1097/HCO.0b013e3281ec5113. 310.1097/HCO.1090b1013e3281ec5113. [DOI] [PubMed] [Google Scholar]
- 73.McGillicuddy FC, de la Llera Moya M, Hinkle CC, Joshi MR, Chiquoine EH, Billheimer JT, Rothblat GH, Reilly MP. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119:1135–1145. doi: 10.1161/CIRCULATIONAHA.108.810721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de la Llera Moya M, McGillicuddy FC, Hinkle CC, Byrne M, Joshi MR, Nguyen V, Tabita-Martinez J, Wolfe ML, Badellino K, Pruscino L, Mehta NN, Asztalos BF, Reilly MP. Inflammation modulates human hdl composition and function in vivo. Atherosclerosis. 2012;222:390–394. doi: 10.1016/j.atherosclerosis.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Badellino KO, Wolfe ML, Reilly MP, Rader DJ. Endothelial lipase is increased in vivo by inflammation in humans. Circulation. 2008;117:678–685. doi: 10.1161/CIRCULATIONAHA.107.707349. [DOI] [PubMed] [Google Scholar]
- 76.Cavallero E, Brites F, Delfly B, Nicolaiew N, Decossin C, De Geitere C, Fruchart JC, Wikinski R, Jacotot B, Castro G. Abnormal reverse cholesterol transport in controlled type ii diabetic patients. Studies on fasting and postprandial lpa-i particles. Arterioscler Thromb Vasc Biol. 1995;15:2130–2135. doi: 10.1161/01.atv.15.12.2130. [DOI] [PubMed] [Google Scholar]
- 77.Mooradian AD, Haas MJ, Wehmeier KR, Wong NCW. Obesity-related changes in high-density lipoprotein metabolism. Obesity. 2008;16:1152–1160. doi: 10.1038/oby.2008.202. [DOI] [PubMed] [Google Scholar]
- 78.Holzer M, Wolf P, Curcic S, Birner-Gruenberger R, Weger W, Inzinger M, El-Gamal D, Wadsack C, Heinemann A, Marsche G. Psoriasis alters hdl composition and cholesterol efflux capacity. Journal of lipid research. 2012;53:1618–1624. doi: 10.1194/jlr.M027367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McMahon M, Grossman J, FitzGerald J, Dahlin-Lee E, Wallace DJ, Thong BY, Badsha H, Kalunian K, Charles C, Navab M, Fogelman AM, Hahn BH. Proinflammatory high-density lipoprotein as a biomarker for atherosclerosis in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 2006;54:2541–2549. doi: 10.1002/art.21976. [DOI] [PubMed] [Google Scholar]
- 80.Van Lenten BJ, Hama SY, de Beer FC, Stafforini DM, McIntyre TM, Prescott SM, La Du BN, Fogelman AM, Navab M. Anti-inflammatory hdl becomes pro-inflammatory during the acute phase response. Loss of protective effect of hdl against ldl oxidation in aortic wall cell cocultures. J Clin Invest. 1995;96:2758–2767. doi: 10.1172/JCI118345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. New England Journal of Medicine. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Eijk LT, Dorresteijn MJ, Smits P, van der Hoeven JG, Netea MG, Pickkers P. Gender differences in the innate immune response and vascular reactivity following the administration of endotoxin to human volunteers. Crit Care Med. 2007;35:1464–1469. doi: 10.1097/01.CCM.0000266534.14262.E8. [DOI] [PubMed] [Google Scholar]
- 83.Alamili M, Bendtzen K, Lykkesfeldt J, Rosenberg J, Gogenur I. Pronounced inflammatory response to endotoxaemia during nighttime: A randomised cross-over trial. PLoS One. 2014;9:e87413. doi: 10.1371/journal.pone.0087413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, Gallimore JR, Pepys MB. Low grade inflammation and coronary heart disease: Prospective study and updated meta-analyses. BMJ. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pradhan AD, Manson JE, Rossouw JE, Siscovick DS, Mouton CP, Rifai N, Wallace RB, Jackson RD, Pettinger MB, Ridker PM. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease: Prospective analysis from the women's health initiative observational study. JAMA. 2002;288:980–987. doi: 10.1001/jama.288.8.980. [DOI] [PubMed] [Google Scholar]
- 86.Krug S, Kastenmuller G, Stuckler F, Rist MJ, Skurk T, Sailer M, Raffler J, Romisch-Margl W, Adamski J, Prehn C, Frank T, Engel KH, Hofmann T, Luy B, Zimmermann R, Moritz F, Schmitt-Kopplin P, Krumsiek J, Kremer W, Huber F, Oeh U, Theis FJ, Szymczak W, Hauner H, Suhre K, Daniel H. The dynamic range of the human metabolome revealed by challenges. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:2607–2619. doi: 10.1096/fj.11-198093. [DOI] [PubMed] [Google Scholar]
- 87.van Ommen B, van der Greef J, Ordovas JM, Daniel H. Phenotypic flexibility as key factor in the human nutrition and health relationship. Genes Nutr. 2014;9:423. doi: 10.1007/s12263-014-0423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paalani M, Lee JW, Haddad E, Tonstad S. Determinants of inflammatory markers in a bi-ethnic population. Ethn Dis. 2011;21:142–149. [PMC free article] [PubMed] [Google Scholar]
- 89.Steffen BT, Steffen LM, Tracy R, Siscovick D, Jacobs D, Liu K, He K, Hanson NQ, Nettleton JA, Tsai MY. Ethnicity, plasma phospholipid fatty acid composition and inflammatory/endothelial activation biomarkers in the multi-ethnic study of atherosclerosis (mesa) Eur J Clin Nutr. 2012;66:600–605. doi: 10.1038/ejcn.2011.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, Wians FH, Jr, Grundy SM, de Lemos JA. Race and gender differences in c-reactive protein levels. J Am Coll Cardiol. 2005;46:464–469. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 91.Ferguson JF, Meyer NJ, Qu L, Xue C, Liu Y, DerOhannessian SL, Rushefski M, Paschos GK, Tang S, Schadt EE, Li M, Christie JD, Reilly MP. Integrative genomics identifies 7p11.2 as a novel locus for fever and clinical stress response in humans. Human Molecular Genetics. 2014 doi: 10.1093/hmg/ddu589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Franco LM, Bucasas KL, Wells JM, Nino D, Wang X, Zapata GE, Arden N, Renwick A, Yu P, Quarles JM, Bray MS, Couch RB, Belmont JW, Shaw CA. Integrative genomic analysis of the human immune response to influenza vaccination. Elife. 2013;2:e00299. doi: 10.7554/eLife.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Daly AK. Genome-wide association studies in pharmacogenomics. Nat Rev Genet. 2010;11:241–246. doi: 10.1038/nrg2751. [DOI] [PubMed] [Google Scholar]
- 94.Dillon MA, Harris B, Hernandez ML, Zou B, Reed W, Bromberg PA, Devlin RB, Diaz-Sanchez D, Kleeberger S, Zhou H, Lay JC, Alexis NE, Peden DB. Enhancement of systemic and sputum granulocyte response to inhaled endotoxin in people with the gstm1 null genotype. Occup Environ Med. 2011;68:783–785. doi: 10.1136/oem.2010.061747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen R, Mias GI, Li-Pook-Than J, Jiang L, Lam HY, Miriami E, Karczewski KJ, Hariharan M, Dewey FE, Cheng Y, Clark MJ, Im H, Habegger L, Balasubramanian S, O'Huallachain M, Dudley JT, Hillenmeyer S, Haraksingh R, Sharon D, Euskirchen G, Lacroute P, Bettinger K, Boyle AP, Kasowski M, Grubert F, Seki S, Garcia M, Whirl-Carrillo M, Gallardo M, Blasco MA, Greenberg PL, Snyder P, Klein TE, Altman RB, Butte AJ, Ashley EA, Gerstein M, Nadeau KC, Tang H, Snyder M. Personal omics profiling reveals dynamic molecular and medical phenotypes. Cell. 2012;148:1293–1307. doi: 10.1016/j.cell.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Munford RS. Murine responses to endotoxin: Another dirty little secret? J Infect Dis. 2010;201:175–177. doi: 10.1086/649558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Novoa B, Bowman TV, Zon L, Figueras A. Lps response and tolerance in the zebrafish (danio rerio) Fish Shellfish Immunol. 2009;26:326–331. doi: 10.1016/j.fsi.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, López CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rahme LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG Inflammation t, Host Response to Injury LSCRP. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proceedings of the National Academy of Sciences. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Takao K, Miyakawa T. Genomic responses in mouse models greatly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1401965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fairfax BP, Humburg P, Makino S, Naranbhai V, Wong D, Lau E, Jostins L, Plant K, Andrews R, McGee C, Knight JC. Innate immune activity conditions the effect of regulatory variants upon monocyte gene expression. Science. 2014;343 doi: 10.1126/science.1246949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee MN, Ye C, Villani A-C, Raj T, Li W, Eisenhaure TM, Imboywa SH, Chipendo PI, Ran FA, Slowikowski K, Ward LD, Raddassi K, McCabe C, Lee MH, Frohlich IY, Hafler DA, Kellis M, Raychaudhuri S, Zhang F, Stranger BE, Benoist CO, De Jager PL, Regev A, Hacohen N. Common genetic variants modulate pathogen-sensing responses in human dendritic cells. Science. 2014;343 doi: 10.1126/science.1246980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kox M, de Kleijn S, Pompe JC, Ramakers BP, Netea MG, van der Hoeven JG, Hoedemaekers CW, Pickkers P. Differential ex vivo and in vivo endotoxin tolerance kinetics following human endotoxemia. Crit Care Med. 2011;39:1866–1870. doi: 10.1097/CCM.0b013e3182190d5d. [DOI] [PubMed] [Google Scholar]
- 103.Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, Frees K, Watt JL, Schwartz DA. Tlr4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–191. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- 104.Fijen JW, Tulleken JE, Hepkema BG, van der Werf TS, Ligtenberg JJM, Zijlstra JG. The influence of tumor necrosis factor–α and interleukin-10 gene promoter polymorphism on the inflammatory response in experimental human endotoxemia. Clinical Infectious Diseases. 2001;33:1601–1603. doi: 10.1086/323197. [DOI] [PubMed] [Google Scholar]
- 105.Endler G, Marsik C, Joukhadar C, Marculescu R, Mayr F, Mannhalter C, Wagner OF, Jilma B. The interleukin-6 g(−174)c promoter polymorphism does not determine plasma interleukin-6 concentrations in experimental endotoxemia in humans. Clinical Chemistry. 2004;50:195–200. doi: 10.1373/clinchem.2003.022459. [DOI] [PubMed] [Google Scholar]
- 106.Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, Chen RO, Brownstein BH, Cobb JP, Tschoeke SK, Miller-Graziano C, Moldawer LL, Mindrinos MN, Davis RW, Tompkins RG, Lowry SF. A network-based analysis of systemic inflammation in humans. Nature. 2005;437:1032–1037. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- 107.Liu Y, Ferguson JF, Xue C, Ballantyne RL, Silverman IM, Gosai SJ, Serfecz J, Morley MP, Gregory BD, Li M, Reilly MP. Tissue-specific rna-seq in human evoked inflammation identifies blood and adipose lincrna signatures of cardiometabolic diseases. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:902–912. doi: 10.1161/ATVBAHA.113.303123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Elliott MJ, Maini RN, Feldmann M, Kalden JR, Antoni C, Smolen JS, Leeb B, Breedveld FC, Macfarlane JD, Bijl H, et al. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (ca2) versus placebo in rheumatoid arthritis. Lancet. 1994;344:1105–1110. doi: 10.1016/s0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- 109.van Dullemen HM, van Deventer SJ, Hommes DW, Bijl HA, Jansen J, Tytgat GN, Woody J. Treatment of crohn's disease with anti-tumor necrosis factor chimeric monoclonal antibody (ca2) Gastroenterology. 1995;109:129–135. doi: 10.1016/0016-5085(95)90277-5. [DOI] [PubMed] [Google Scholar]
- 110.Gorman JD, Sack KE, Davis JC., Jr Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. The New England journal of medicine. 2002;346:1349–1356. doi: 10.1056/NEJMoa012664. [DOI] [PubMed] [Google Scholar]
- 111.Burns CM, Wortmann RL. Gout therapeutics: New drugs for an old disease. The Lancet. 2011;377:165–177. doi: 10.1016/S0140-6736(10)60665-4. [DOI] [PubMed] [Google Scholar]
- 112.Bunnell E, Lynn M, Habet K, Neumann A, Perdomo CA, Friedhoff LT, Rogers SL, Parrillo JE. A lipid a analog, e5531, blocks the endotoxin response in human volunteers with experimental endotoxemia. Critical Care Medicine. 2000;28:2713–2720. doi: 10.1097/00003246-200008000-00005. [DOI] [PubMed] [Google Scholar]
- 113.Kumar A, Bunnell E, Lynn M, Anel R, Habet K, Neumann A, Parrillo JE. Experimental human endotoxemia is associated with depression of load-independent contractility indices*: Prevention by the lipid a analogue e5531. CHEST Journal. 2004;126:860–867. doi: 10.1378/chest.126.3.860. [DOI] [PubMed] [Google Scholar]
- 114.Van Zee KJ, Coyle SM, Calvano SE, Oldenburg HS, Stiles DM, Pribble J, Catalano M, Moldawer LL, Lowry SF. Influence of il-1 receptor blockade on the human response to endotoxemia. The Journal of Immunology. 1995;154:1499–1507. [PubMed] [Google Scholar]
- 115.Granowitz E, Porat R, Mier J, Orencole S, Callahan M, Cannon J, Lynch E, Ye K, Poutsiaka D, Vannier E. Hematologic and immunomodulatory effects of an interleukin-1 receptor antagonist coinfusion during low-dose endotoxemia in healthy humans. Blood. 1993;82:2985–2990. [PubMed] [Google Scholar]
- 116.Pajkrt D, Camoglio L, Tiel-van Buul MC, de Bruin K, Cutler DL, Affrime MB, Rikken G, van der Poll T, ten Cate JW, van Deventer SJ. Attenuation of proinflammatory response by recombinant human il-10 in human endotoxemia: Effect of timing of recombinant human il-10 administration. The Journal of Immunology. 1997;158:3971–3977. [PubMed] [Google Scholar]
- 117.Olszyna DP, Pajkrt D, Lauw FN, van Deventer SJH, van der Poll T. Interleukin 10 inhibits the release of cc chemokines during human endotoxemia. Journal of Infectious Diseases. 2000;181:613–620. doi: 10.1086/315275. [DOI] [PubMed] [Google Scholar]
- 118.Suffredini AF, Reda D, Banks SM, Tropea M, Agosti JM, Miller R. Effects of recombinant dimeric tnf receptor on human inflammatory responses following intravenous endotoxin administration. The Journal of Immunology. 1995;155:5038–5045. [PubMed] [Google Scholar]
- 119.van der Poll T, Coyle SM, Levi M, Jansen PM, Dentener M, Barbosa K, Buurman WA, Hack CE, ten Cate JW, Agosti JM, Lowry SF. Effect of a recombinant dimeric tumor necrosis factor receptor on inflammatory responses to intravenous endotoxin in normal humans. Blood. 1997;89:3727–3734. [PubMed] [Google Scholar]
- 120.Shannon E, Noveck R, Sandoval F, Kamath B, Kearney M. Thalidomide suppressed interleukin-6 but not tumor necrosis factor-alpha in volunteers with experimental endotoxemia. Transl Res. 2007;150:275–280. doi: 10.1016/j.trsl.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 121.de Kruif MD, Lemaire LC, Giebelen IA, Groot AP, Pater JM, van den Pangaart PS, Elliott PJ, van der Poll T. Effects of prednisolone on the systemic release of mediators of cell-mediated cytotoxicity during human endotoxemia. Shock. 2008;29:458–461. doi: 10.1097/shk.0b013e3181598a6a. [DOI] [PubMed] [Google Scholar]
- 122.de Kruif MD, Lemaire LC, Giebelen IA, van Zoelen MA, Pater JM, van den Pangaart PS, Groot AP, de Vos AF, Elliott PJ, Meijers JC, Levi M, van der Poll T. Prednisolone dose-dependently influences inflammation and coagulation during human endotoxemia. Journal of immunology. 2007;178:1845–1851. doi: 10.4049/jimmunol.178.3.1845. [DOI] [PubMed] [Google Scholar]
- 123.Lemaire LC, de Kruif MD, Giebelen IA, Levi M, van der Poll T, Heesen M. Dobutamine does not influence inflammatory pathways during human endotoxemia. Crit Care Med. 2006;34:1365–1371. doi: 10.1097/01.CCM.0000215514.96888.E3. [DOI] [PubMed] [Google Scholar]
- 124.Mulvey CK, Ferguson JF, Tabita-Martinez J, Kong S, Shah RY, Patel PN, Master SR, Usman MH, Propert KJ, Shah R, Mehta NN, Reilly MP. Peroxisome proliferator-activated receptor-alpha agonism with fenofibrate does not suppress inflammatory responses to evoked endotoxemia. Journal of the American Heart Association. 2012;1:e002923. doi: 10.1161/JAHA.112.002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ferguson JF, Mulvey CK, Patel PN, Shah RY, Doveikis J, Zhang W, Tabita-Martinez J, Terembula K, Eiden M, Koulman A, Griffin JL, Mehta NN, Shah R, Propert KJ, Song WL, Reilly MP. Omega-3 pufa supplementation and the response to evoked endotoxemia in healthy volunteers. Mol Nutr Food Res. 2014;58:601–613. doi: 10.1002/mnfr.201300368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pluess TT, Hayoz D, Berger MM, Tappy L, Revelly JP, Michaeli B, Carpentier YA, Chiolero RL. Intravenous fish oil blunts the physiological response to endotoxin in healthy subjects. Intensive care medicine. 2007;33:789–797. doi: 10.1007/s00134-007-0591-5. [DOI] [PubMed] [Google Scholar]
- 127.Michaeli B, M. Berger M, Revelly J-P, Tappy L, Chioléro R. Effects of fish oil on the neuro-endocrine responses to an endotoxin challenge in healthy volunteers. Clinical Nutrition. 2007;26:70–77. doi: 10.1016/j.clnu.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 128.Ferguson JF, Ryan MF, Gibney ER, Brennan L, Roche HM, Reilly MP. Dietary isoflavone intake is associated with evoked responses to inflammatory cardiometabolic stimuli and improved glucose homeostasis in healthy volunteers. Nutr Metab Cardiovasc Dis. 2014 doi: 10.1016/j.numecd.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Meyer NJ, Ferguson JF, Feng R, Wang F, Patel PN, Li M, Xue C, Qu L, Liu Y, Boyd JH, Russell JA, Christie JD, Walley KR, Reilly MP. A functional synonymous coding variant in the il1rn gene is associated with survival in septic shock. Am J Respir Crit Care Med. 2014;190:656–664. doi: 10.1164/rccm.201403-0586OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rella JM, Jilma B, Fabry A, Kaynar AM, Mayr FB. Mmp-8 genotypes influence the inflammatory response in human endotoxemia. Inflammation. 2014;37:451–456. doi: 10.1007/s10753-013-9758-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kovar FM, Marsik C, Jilma B, Mannhalter C, Quehenberger P, Handler S, Wagner OF, Endler G. The fibrinogen-148 c/t polymorphism influences inflammatory response in experimental endotoxemia in vivo. Thromb Res. 2007;120:727–731. doi: 10.1016/j.thromres.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 132.Marsik C, Sunder-Plassmann R, Jilma B, Kovar FM, Mannhalter C, Wagner O, Rumpold H, Endler G. The c-reactive protein (+)1444c/t alteration modulates the inflammation and coagulation response in human endotoxemia. Clin Chem. 2006;52:1952–1957. doi: 10.1373/clinchem.2006.069823. [DOI] [PubMed] [Google Scholar]
- 133.Marsik C, Jilma B, Joukhadar C, Mannhalter C, Wagner O, Endler G. The toll-like receptor 4 asp299gly and thr399ile polymorphisms influence the late inflammatory response in human endotoxemia. Clin Chem. 2005;51:2178–2180. doi: 10.1373/clinchem.2005.051649. [DOI] [PubMed] [Google Scholar]
- 134.Kovar FM, Marsik C, Cvitko T, Wagner OF, Jilma B, Endler G. The tumor necrosis factor alpha-308 g/a polymorphism does not influence inflammation and coagulation response in human endotoxemia. Shock. 2007;27:238–241. doi: 10.1097/01.shk.0000239768.64786.3a. [DOI] [PubMed] [Google Scholar]
- 135.Draisma A, Pickkers P, Bouw MP, van der Hoeven JG. Development of endotoxin tolerance in humans in vivo. Crit Care Med. 2009;37:1261–1267. doi: 10.1097/CCM.0b013e31819c3c67. [DOI] [PubMed] [Google Scholar]
- 136.Draisma A, Bemelmans R, van der Hoeven JG, Spronk P, Pickkers P. Microcirculation and vascular reactivity during endotoxemia and endotoxin tolerance in humans. Shock. 2009;31:581–585. doi: 10.1097/SHK.0b013e318193e187. [DOI] [PubMed] [Google Scholar]
- 137.Draisma A, de Goeij M, Wouters CW, Riksen NP, Oyen WJ, Rongen GA, Boerman OC, van Deuren M, van der Hoeven JG, Pickkers P. Endotoxin tolerance does not limit mild ischemia-reperfusion injury in humans in vivo. Innate Immun. 2009;15:360–367. doi: 10.1177/1753425909105548. [DOI] [PubMed] [Google Scholar]
- 138.Leentjens J, Kox M, Koch RM, Preijers F, Joosten LA, van der Hoeven JG, Netea MG, Pickkers P. Reversal of immunoparalysis in humans in vivo: A double-blind, placebo-controlled, randomized pilot study. Am J Respir Crit Care Med. 2012;186:838–845. doi: 10.1164/rccm.201204-0645OC. [DOI] [PubMed] [Google Scholar]
- 139.Preas HL, 2nd, Reda D, Tropea M, Vandivier RW, Banks SM, Agosti JM, Suffredini AF. Effects of recombinant soluble type i interleukin-1 receptor on human inflammatory responses to endotoxin. Blood. 1996;88:2465–2472. [PubMed] [Google Scholar]