Guanfacine is used clinically as either monotherapy or adjunct therapy (along with psychostimulants) for the treatment of attention deficit hyperactivity disorder (ADHD) due to its hypothesized action of increasing network connections in the prefrontal cortex (1). Guanfacine is a selective α2A-adrenoreceptor (α2A-receptor) agonist that activates central nervous system (CNS) norepinephrine receptors in the locus coeruleus. This action results in reduced peripheral sympathetic tone of both systolic (SBP) and diastolic blood pressure (DBP), which was its original indication as an antihypertensive (2). Additionally, guanfacine has been investigated for the treatment of substance abuse disorders (SUD) due to its ability to decrease both stress and cue induced craving (3). Dextroamphetamine (AMP) is a monoamine agonist that blocks neurotransmitter reuptake at the presynaptic transporter and is also taken up by the vesicular monoamine transporter 2, resulting in catecholamine release and CNS stimulation (4). AMP is a well-established therapy for ADHD; however, it also has a propensity to be abused due to its similarity to drugs of abuse such as cocaine and methamphetamine (5) and its cognitive enhancing properties.
As part of a larger study, nine otherwise healthy nicotine dependent volunteers were recruited to take part in a positron emission tomography (PET) protocol examining the effects of oral guanfacine on dopamine release. All subjects were required to stop smoking by midnight the night before all scans, which was verified by carbon monoxide levels less than 10 ppm. Subjects were imaged at baseline (under no pharmacological intervention) and after an oral AMP challenge to assess pre-guanfacine treatment dopamine release the following day. As part of the PET scanning procedure, vital sign measurements were taken twice at baseline and then every 15 minutes after being administered AMP for the duration of the 180-minute scans, which were averaged to reduce potential variability for the purpose of this case series. After pre-treatment scans were complete, subjects took part in a guanfacine escalation paradigm to 3mg daily over fifteen days then remained on a steady state of 3mg guanfacine for eight additional days. To confirm medication compliance, a riboflavin marker detectable in urine by ultraviolet light was added to each dose. After the three weeks of guanfacine monotherapy, subjects took part in a post-guanfacine treatment baseline scan accompanied by a fourth and final scan using the same AMP challenge to examine the effects of chronic guanfacine treatment on dopamine release.
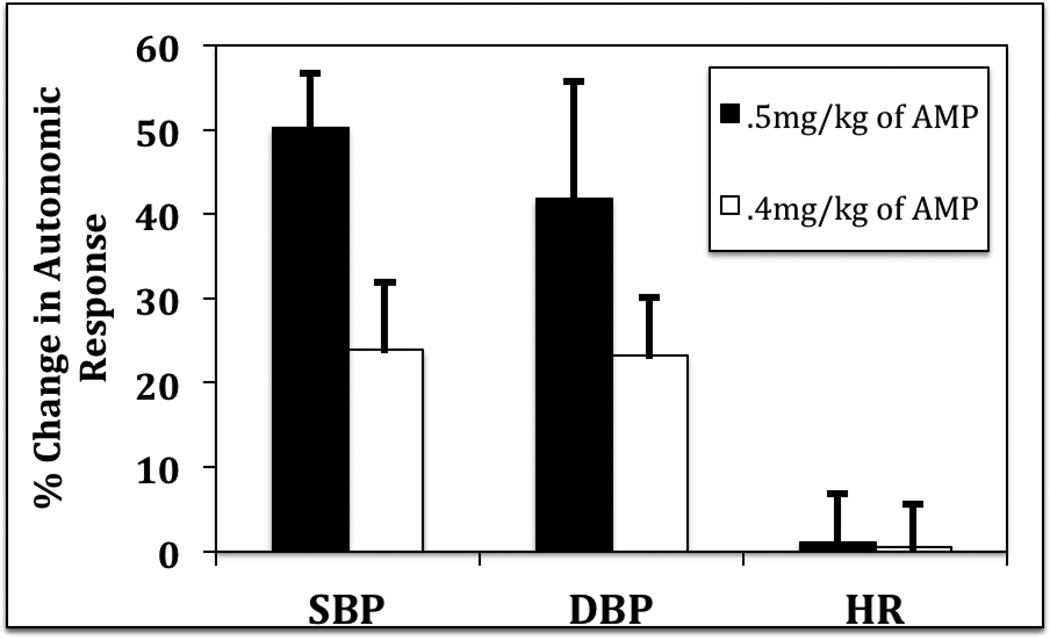
All nine subjects completed the pre-AMP challenge baseline scans without incident and remained normotensive throughout scanning sessions evidenced by an average SBP of 129 mmHg, an average DBP of 75 mmHg, and an average heart rate (HR) of 73 bpm. The first two subjects in the study received 0.5mg/kg (mean of 40mg per dose) of AMP for their pre-guanfacine treatment dopamine release scans without incident and experienced expected autonomic nervous system (ANS) changes as SBP increased by 14% (mean of 151 mmHg), DBP increased by 12% (mean of 76 mmHg), and HR increased by 10% (mean of 80 bpm) over the session as compared to pre-AMP baseline. After the three weeks of guanfacine montherapy, the same two subjects were scanned before a second AMP challenge and, as expected, decreases in ANS measures were observed as compared to their pre-guanfacine baseline scans. Specifically, there was a 13% decrease in SBP (mean of 114 mmHg), a 1% increase in DBP (mean of 69 mmHg), and a 19% decrease in HR (mean of 59 bpm), respectively. Surprisingly however, after receiving 0.5mg/kg of AMP after three weeks of guanfacine monotherapy, those two subjects became acutely stage II hypertensive as reflected by a 50% increase in SBP (mean of 170 mmHg), a 42% increase in DBP (mean of 97 mmHg), and a 1% decrease in HR (mean of 59 bpm). Subjects were asymptomatic with the exception of a mild to moderate headache that subsided without intervention in one of the subjects (See Figure 1).
Figure 1.
Relative change between guanfacine and guanfacine + dextroamphetamine (AMP) (along with S.E.M) in systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate (HR). AMP was given as two different single doses.
Doses of AMP were then adjusted to 0.4mg/kg in order to safely complete the study on the seven additional subjects (mean of 31mg per dose) and, again, an expected autonomic response was observed during their pre-guanfacine treatment dopamine release scan as AMP increased SBP by 16% (mean of 148 mmHg) and DBP by 9% (mean of 83 mmHg), and decreased HR by 5% (mean of 69 bpm) as compared to their pre-AMP baseline scans. After the three weeks of guanfacine monotherapy, the seven subjects were scanned before their second AMP challenge and deceases in SBP of .1% (mean of 127 mmHg), DBP of 9% (mean of 71 mmHg), and HR of 8% (mean of 65 bpm) were observed as compared to their pre-AMP baseline. Those same subjects experienced asymptomatic acute stage I hypertension (rather than stage II hypertension previously observed with 0.5mg/kg in the prior two subjects) after receiving 0.4mg/kg of AMP. Although autonomic changes were still observed, they were of more benign nature evidenced by a 24% increase in SBP (mean of 156 mmHg), a 23% increase in DBP (mean of 87 mmHg), and a 1% increase in HR (mean of 65 bpm) as compared the post-guanfacine baseline scan (See Figure 1).
Discussion
The observed autonomic response post-guanfacine along with single-dose AMP was unexpected and not consistent with a previous report that showed combined AMP and guanfacine did not produce significant cardiovascular changes (6). However, subjects from the aforementioned multisite study were on AMP monotherapy for at least one month before being started on guanfacine (6). That stands in contrast to subjects from this case series who were initially on guanfacine for three weeks then given an acute dose of AMP as part of a research protocol. Thus, to the best of our knowledge, this is the first evidence of a potential novel guanfacine-AMP interaction. Furthermore, lowering the dose of AMP in order to safely complete the study resulted in a similar, albeit less severe, autonomic response, thus, potentially demonstrating an AMP dose response phenomenon within this novel drug-drug interaction.
One potential mechanism to explain this unanticipated response is an adrenergic surge after AMP administration. Guanfacine activates α2A norepinephrine autoreceptors, which in turn, results in an increase in norepinephrine feedback on the presynaptic nerve terminal, thus, resulting in reductions in sympathetic tone (2). We propose that these actions of guanfacine may have resulted in increases of intracellular concentrations of norepinephrine and once the acute dose of AMP was administered, those increased intracellular concentrations of norepinephrine flooded the synapse resulting in a heightened autonomic response.
There are several important clinical implications to these findings. First, these results suggest that an adrenergic surge is possible when giving AMP to patients who are concurrently taking guanfacine, thus, a careful cardiac family history should be taken when contemplating switching patients from guanfacine to AMP. Additionally, based on the potential dose dependent AMP phenomenon observed within this novel guanfacine-AMP interaction, if patients are decided to switch to AMP, starting doses should be minimal or a total washout period could be justified. Lastly, AMP, due its similar mechanism to that of drugs of abuse (i.e., cocaine and methamphetamine) (5) and its cognitive enhancement capabilities (i.e., study drug), has a high propensity to be misused. Consequently, patients already on guanfacine for ADHD or SUD treatment might be at higher risk for cardiac events if AMP is misused or abused. Thus, this drug-drug interaction could be especially relevant to patients with pre-existing cardiac conditions and in emergency departments.
Although novel, these findings are in a small number of nicotine-dependent subjects and should be interpreted cautiously. Studies consisting of larger cohorts, including healthy subjects, and further examination of other α2A-receptor agonists would be beneficial to generalize these findings.
Acknowledgments
The authors disclose no conflicts of interest and would like to acknowledge the Yale PET Center for all its wonder staff members. This work was made possible through funding provided by NIDA, ORWH, and the FDA Office of Women’s Health (K02DA031750 and P50DA033945).
References
- 1.Arnsten AF, Pliszka SR. Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacology, biochemistry, and behavior. 2011;99:211–216. doi: 10.1016/j.pbb.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Zwieten P, Thoolen M, & Timmermans P. The pharmacology of centrally acting antihypertensive drugs. British Journal of Clinical Pharmacology. 1983;15:455S–462S. [Google Scholar]
- 3.Fox HC, Seo D, Tuit K, et al. Guanfacine effects on stress, drug craving and prefrontal activation in cocaine dependent individuals: preliminary findings. Journal of psychopharmacology. 2012;26:958–972. doi: 10.1177/0269881111430746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eiden LE, Weihe E. VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse. Annals of the New York Academy of Sciences. 2011;1216:86–98. doi: 10.1111/j.1749-6632.2010.05906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spencer TJ, Greenbaum M, Ginsberg LD, et al. Safety and effectiveness of coadministration of guanfacine extended release and psychostimulants in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19:501–510. doi: 10.1089/cap.2008.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]