Abstract
Objective
We previously showed that cholesterol loading in vitro converts mouse aortic vascular smooth muscle cells (VSMC) from a contractile state to one resembling macrophages. In human and mouse atherosclerotic plaques it has become appreciated that ~40% of cells classified as macrophages by histological markers may be of VSMC origin. We therefore sought to gain insight into the molecular regulation of this clinically relevant process.
Approach and Results
VSMC of mouse (or human) origin were incubated with cyclodextrin-cholesterol complexes for 72 hours, at which time the expression at the protein and mRNA levels of contractile-related proteins were reduced and of macrophage markers increased. Concurrent was down regulation of miR-143/145, which positively regulate the master VSMC-differentiation transcription factor myocardin (MYOCD). Mechanisms were further probed in mouse VSMC. Maintaining the expression of MYOCD or miR-143/145 prevented and reversed phenotypic changes caused by cholesterol loading. Reversal was also seen when cholesterol efflux was stimulated after loading. Notably, despite expression of macrophage markers, bioinformatic analyses showed that cholesterol-loaded cells remained closer to the VSMC state, consistent with impairment in classical macrophage functions of phagocytosis and efferocytosis. In apoE-deficient atherosclerotic plaques, cells positive for VSMC and macrophage markers were found lining the cholesterol-rich necrotic core.
Conclusions
Cholesterol loading of VSMC converts them to a macrophage–appearing state by downregulating the miR-143/145-myocardin axis. Though these cells would be classified by immunohistochemistry as macrophages in human and mouse plaques, their transcriptome and functional properties imply that their contributions to atherogenesis would not be those of classical macrophages.
Keywords: atherosclerosis, cholesterol, plaque, smooth muscle cell differentiation, macrophage
INTRODUCTION
The hallmark of atherosclerotic plaque development is the accumulation in the arterial wall of cholesterol-engorged macrophage foam cells1–5. It has been generally accepted that the majority of plaque macrophages originate from circulating monocytes. Like intimal macrophages, vascular smooth muscle cells (VSMC) have also been shown to accumulate excess cholesterol, contributing to atherosclerosis development and progression (summarized in6). Formation of lipid-laden VSMC has been documented in both in vivo7–9 and in vitro studies10–14. In previous studies, we demonstrated that mouse aortic VSMC that were loaded with cholesterol assumed the appearance of macrophage foam cells, and we raised the intriguing possibility that foam cells in the plaque presumed to be macrophages may instead be of VSMC origin15. Following cholesterol loading, VSMC showed a marked decline in the expression of genes specifically related to the contractile phenotype (eg., smooth muscle alpha-actin), whereas expression of macrophage markers (eg., CD68) were increased at both the protein and mRNA levels.
That these phenomena are clinically relevant is supported by studies showing the presence of cells expressing both VSMC and macrophage markers in human atherosclerotic plaques8 and by the very recent demonstration that from 30–40% of cells positive for the macrophage marker CD68 in human plaques are likely to be of VSMC origin16. Despite these provocative findings, the molecular mechanisms underlying this transition remain poorly defined. Furthermore, whether these CD68-positive VSMC can support macrophage functions known to influence atherogenesis, such as innate immune signaling, phagocytosis and efferocytosis, is not known.
To address these outstanding questions, we have applied unbiased transcriptome and transcription factor binding site motif enrichment analyses to identify important molecular regulators of mouse VSMC phenotypic variation after cholesterol loading. In addition, using a global transcriptional data analysis algorithm (principal component analysis, or PCA) we determined the relationship between the cholesterol-induced CD68+ VSMC phenotype and that of authentic macrophages. These analyses pointed to multiple and independent pathways by which cholesterol loading suppresses the expression of the master regulator of the contractile state of VSMC, the transcription factor myocardin (MYOCD) and its co-activator, serum response factor (SRF)17. Notably, despite gaining a number of macrophage features, the molecular profile of the cells remained substantially that of VSMC, consistent with their poor activity relative to macrophages in functional assays. Furthermore, a number of the effects of cholesterol loading on the VSMC phenotype were largely reversible by the promotion of cholesterol efflux by HDL and other acceptors.
Similar to the plasticity of monocyte-derived macrophages in inflammatory sites18, then, VSMC in atherosclerotic plaques are also in dynamic phenotypic states, traditionally categorized as constitutive (or contractile) and synthetic19, altering their morphological and functional features depending on factors in their microenvironment. Collectively, our present data, taken with the findings in vivo by us and others16, 20 suggest that in atherosclerotic plaques, one such factor is cholesterol, which causes the development of a third phenotypic state that superficially resembles macrophages.
METHODS
Materials and Methods are available in the online-only Data Supplement
RESULTS
Cholesterol loading converts mouse aortic VSMC to a macrophage foam cell-like state
In contrast to mouse aortic VSMC incubated with 0.2% BSA, those incubated for 3 d with cyclodextrin (CD) cholesterol-complexes became foam cells, as evidenced by the appearance of Oil-Red-O stained lipid droplets (Fig. 1A). Cholesterol-loaded cells also showed a significant decrease in the protein expression of the VSMC marker, smooth muscle (SM) α-actin (ACTA2), while that of macrophage-related marker, CD68, increased (Fig. 1B). In contrast, BSA-incubated cells stained heavily with antibodies to ACTA2, but not to CD68 (Fig. 1B). To further characterize the phenotypic changes, we performed quantitative qRT-PCR assays. Cholesterol loading led to dramatic decreases in mRNA levels for VSMC-related genes Acta2, α-tropomyosin (Tpm1), smooth muscle myosin heavy chain (Myh11), and calponin (Cnn1), while those for the macrophage markers CD68 (Cd68) and Mac-2 (Lgals3), and the cholesterol efflux factor ABCA1 increased (Fig. 1C&D). Similar findings were found with human coronary artery smooth muscle cells (Supplemental Fig. I). These results are consistent with what we have previously reported15, and finding them in 2 species supports the general importance and clinical relevance of the findings as well as the undertaking of the series of experiments to be described.
Figure 1. Cholesterol loading of VSMC leads to foam-cell formation, loss of VSMC characteristics, and emergence of macrophage-like features.
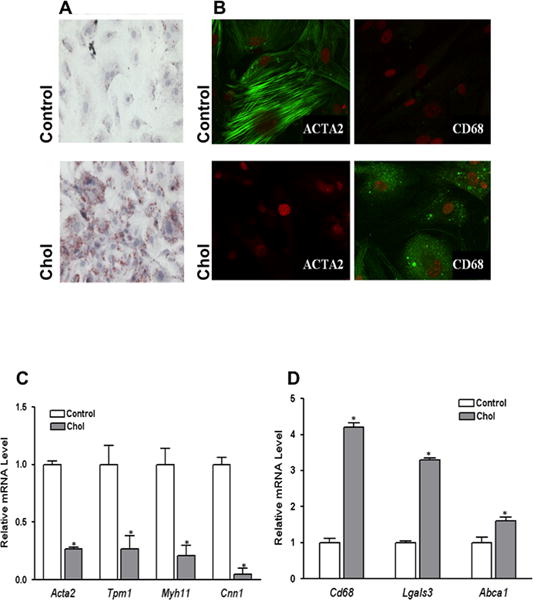
Subconfluent mouse aortic VSMC were treated with (Chol) or without (Control) cyclodextrin (CD)-cholesterol complexes in 0.2% BSA for 72 hours. After cholesterol loading cells assumed the appearance of foam cells with Oil Red O-stained lipid droplets (A). Immunostaining showed decreased protein levels of VSMC marker ACTA2, while macrophage marker CD68 was dramatically increased at the end of 72 hour cholesterol treatment period (B). Consistent changes to this phenotype shift are qRT-PCR analyses of VSMC (C) and macrophage marker (D) gene expression. Data shown are mean ± SD of triplicates of qRT-PCR reactions and are representative of two independent experiments.
Transcriptome profiling reveals multiple changes associated with cholesterol loading of VSMC
To identify key molecular mechanisms regulating VSMC phenotypic changes in vitro, RNA samples from control and cholesterol-treated VSMC were used for transcriptome profiling. The analysis revealed 613 genes for which differential probeset intensities were detected between the two sample groups (see on-line Methods and Materials and Supplemental Table 2). Of these, 270 genes were detected at higher mRNA levels in the cholesterol vs. control-treated VSMC and 343 genes were detected at lower levels. Heatmaps from the microarray analysis are shown in Fig. 2A. Consistent with the qRT-PCR data in Fig. 1, the transcriptome profiling detected (all p<0.01) cholesterol-loading up-regulation of macrophage markers Cd68 (2.9-fold) and Lgals3 (2.8-fold), and cholesterol transporters Abca1 (6.4-fold) and Abcg1 (8.3-fold), but downregulation of VSMC markers Cnn1 (−15-fold), Actg2 (−6.5-fold), and Tpm1 (−1.6-fold). Cholesterol-loading also up-regulated several macrophage-specific inflammatory cytokine mRNAs, including Ccl2 (15.8-fold) and Ccl7 (56-fold), as well as the Toll-like receptor Tlr4 (1.9-fold), which initiates pro-inflammatory responses to microbial and endogenous ligands.
Figure 2. Changes in VSMC transcriptome and function after cholesterol loading.
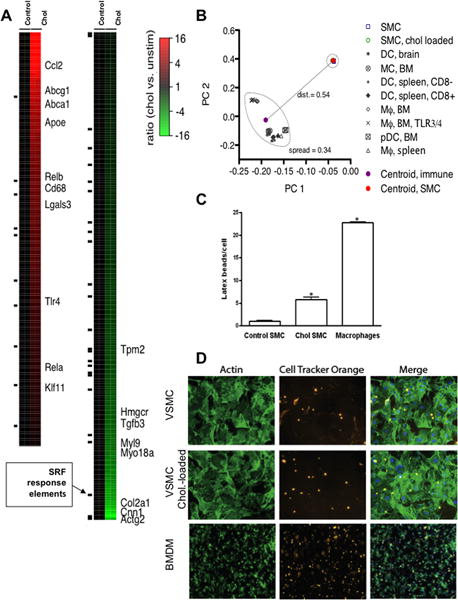
RNA samples from VSMC treated as in Fig. 1 were used for DNA microarray analyses (on-line Materials and Methods). (A) Heatmap colorations indicating differential mRNA expression of genes (rows) in each of the samples (columns; N=2 for each sample group) with respect to the average expression in the control sample group (red = higher expression than the control group average, green = lower). The heatmap shows upregulation of macrophage- and cholesterol efflux-related genes and downregulation of VSMC-related genes and Hmgcr (encoding the rate-limiting enzyme in cholesterol biosynthesis). (B) Comparison of cholesterol-loaded VSMC and macrophages using Principal Components Analysis (PCA; see on-line Materials and Methods for an explanation of PCA) of the microarray data, which shows that cholesterol-loaded VSMC are distinct from hematopoietic cells. Each data mark represents one of 31 microarray hybridizations, taken from five different studies. BM, bone marrow; pDC, plasmacytoid dendritic cell; PC1/2, principal components 1 and 2. (C) Control, cholesterol-loaded VSMC, and peritoneal macrophages were incubated with 1 μ latex beads (green). The beads inside the cells were counted to quantify phagocytotic activity. Data shown are mean ± SD and are representative of two independent experiments. (D) Efferocytosis activity was measured by exposing VSMC or BMDM for 2 h to dead Jurkat cells that had been pre-labeled with Cell Tracker Orange. After washing away non-internalized Jurkat cells, the internalized ones were visualized by fluorescent microscopy (center panels). After fixation, VSMC and BMDM were also stained for actin by phalloidin (left panels).
qRT-PCR results for selected genes identified on the microarray as significantly increased in expression are shown in Supplemental Fig. II. These results not only further validate the trends observed on the array, but by comparing them to qRT-PCR results with RNA isolated from bone marrow-derived macrophages (Supplemental Fig. III), they show that cholesterol-loading was a stronger relative influence on inflammation-related gene expression in VSMC. Additional qRT-PCR results showed that for some genes whose products have been associated with plaque rupture and thrombosis (Mmp2, Mmp9, Mmp13, F3 (Tissue Factor)), only that of Mmp13 was significantly increased in cholesterol-load VSMC (H. Nishi, E. Fisher, data not shown).
Principal Component Analysis of the transcriptome profiles characterizes the relationship between cholesterol-loaded VSMC and authentic macrophages
The microarray results, taken with those in Fig. 1, suggest that cholesterol-loaded VSMC assume some characteristics of macrophages, including inflammatory features. Thus, we wondered how globally similar they were to authentic macrophages and another monocyte-derived cell found in mouse and human plaques, dendritic cells (DC). For this, Principal Component Analysis (PCA) was performed on the microarray data and on published array results for macrophages (Mϕ), monocytes (MC), and dendritic cells (DC). PCA is a statistical technique for determining the key variables in a multidimensional data set that explain the differences in the observations, and can be used to simplify the analysis and visualization of multidimensional data sets. PCA is often applied to microarray expression data to summarize the ways in which gene responses vary under different conditions (for more detail see on-line Materials and Methods and21).
In the graph in Fig. 2B, each data mark represents one of the 31 microarray hybridizations. The scatter plot of the 2,969 most variable probesets across the combined dataset (Fig. 2B) shows the loading values for each microarray study for principal components 1 and 2. The two solid circle marks show the centroid of the loading values, for principal components 1 and 2 (i.e., PC1 and PC2; see on-line Materials and Methods for details) for the hematopoietic (magenta) and VSMC (red) hybridizations, respectively. The distance between the two centroids, 0.54, is greater than the average spread between the hematopoietic cell types (0.34), suggesting that cholesterol-loaded VSMC in vitro remain distinguishable from the hematopoietic cells, despite acquiring a foam cell appearance and some features of macrophages, while losing expression of commonly accepted markers of VSMC phenotype. Consistent with this are qRT-PCR results that show that the absolute mRNA levels of macrophage-associated factors Cd68 and Lgals3 in cholesterol-loaded VSMC are at most 20% of those in bone marrow-derived macrophages (H. Nishi, E. Fisher, data not shown).
To further characterize cholesterol-loaded VSMC, we compared their gene expression profiles and transcriptional regulators to the core signature of mature tissue macrophages from various mouse organs identified by Gautier and colleagues22. Out of the 37 macrophage signature genes in the study, only 15 were up-regulated in cholesterol-loaded VSMC, as shown in Supplemental Fig. IV. Notably, two key marker genes, Mertk and Fcgr1 (CD64), universally associated with mature tissue macrophages, were not up-regulated by cholesterol loading.
Functional properties of cholesterol-loaded VSMC relative to those of macrophages
To test whether mouse VSMC converted to macrophage-like cells upon cholesterol-loading become competent macrophages we tested their ability to perform key macrophage functions. We incubated control VSMC, cholesterol-loaded VSMC, and mouse peritoneal macrophages with 1-μm latex beads for 24 hours and compared the phagocytic activities. As shown in Fig. 2C (representative images in Supplemental Fig. V), latex bead accumulation was significantly increased (6.3 ± 0.6 beads/cell) in cholesterol-loaded VSMC vs. control VSMC (1.1 ± 0.18 beads/cell). This was modest phagocytic activity, however, compared to that of peritoneal macrophages (25.5 ± 0.4/cell). Because the related activity of efferocytosis of apoptotic macrophages in plaques is crucial for limiting the dispersal of lipid and inflammatory substances from the deteriorating cells23, we wondered if the latex bead results presaged a similar deficiency of efferocytosis by cholesterol-loaded VSMC. As shown in Fig. 2D, indeed this was the case. Not only was the activity relative to BMDM only ~25%, it was not noticeably increased by cholesterol-loading in either cell type (Supplemental Fig. VI). These results suggest that in spite of their immunohistological appearance as macrophages in vitro (15 and Fig. 1) and in vivo8, 16, 20, cholesterol-loaded VSMC are relatively deficient in phagocytosis and efferocytosis.
Macrophage-marker positive cells of VSMC origin are located near necrotic cores in mouse atherosclerotic plaques
The functional assays suggested that in atherosclerotic plaques, cholesterol-loaded VSMC may contribute to the pathology because efferocytosis, as alluded to above, regulates the clearance of apoptotic cells and its deficiency expands the necrotic core and retards inflammation resolution in experimental atherosclerosis23. Using lineage-tracing techniques, the laboratories of Gary Owens and Robert Feil have found macrophage-like cells of VSMC origin in Apoe−/− mouse atheromas20, 24. To determine this using a non-lineage tracing technique, we first examined the microarray data to define VSMC markers that did not change after cholesterol loading. Caldesmon (CALD1), a calmodulin binding protein25, is barely detectable in monocyte-derived CD68+ cells in mouse atherosclerotic plaques26, but it is strongly expressed in VSMC independent of cholesterol loading.
We then performed double immunostaining with anti-CD68 and anti-CALD1 antibodies on plaque sections from Apoe−/− mice kept 16 weeks on Western diet. As shown in Supplemental Fig. VIIA, in the plaques there were (red) CALD1+ VSMC in the media or in the fibrous caps, and (green) CD68+ macrophages. Notably, we also identified a subset of double-stained (yellow) cells expressing both markers (Supplemental Fig. VIIB; arrows). We then used Oil Red O to enhance foam cell and necrotic lipid core features. These double-positive cells were lipid-rich and were mostly found close to the necrotic core (Supplemental Fig. VIIC). These histological and immunostaining data are consistent with the results in vitro, as VSMC cells that had migrated from the media to this location would be chronically exposed to cholesterol (which would allow uptake through multiple mechanisms over time14), likely inducing the expression of CD68. Furthermore, they are consistent with very recent data from human plaques that have also demonstrated cells doubly positive for ACTA2 and CD6816.
Collectively, the multiple lines of evidence in Figs. 1–2 and Supplemental Figs. I–VII demonstrate significant plasticity of VSMC in response to cholesterol-loading. The changes included down-regulation of classical VSMC markers and acquisition of macrophage features at the molecular and phenotypic levels in vitro and in vivo. Overall, however, the cholesterol-loaded VSMC were not as functionally active as authentic macrophages and in PCA, their global molecular signature remained predominately VSMC in nature.
Transcription factor binding site motif enrichment analysis
Despite the lack of the full assumption of the macrophage state with cholesterol loading, the microarray analysis still showed a remarkable number (613) of changes in the VSMC transcriptome. To identify molecular mechanisms that may underlie these broad changes we sought transcription factor binding site (TFBS) sequence patterns (“motifs”) that were statistically over-represented (on-line Materials and Methods). A comparison was made to TFBS sequence patterns from a literature-curated database of protein-DNA binding interactions (TRANSFAC27) that are likely to be expressed in VSMC (see on-line Materials and Methods). As shown in Fig. 3A, the analysis implicates serum response factor (SRF) and several other transcription factors, such as the SREBP family, PPARγ, NFKB, and KLFs, in the response of mouse aortic VSMC to cholesterol loading. Notably, LXR was not among the transcription factors implicated, despite its upregulation by cholesterol loading in other settings28.
Figure 3. Transcription factor binding site (TFBS) motif enrichment analysis and the effects of cholesterol-loading on VSMC expression of MYOCD, CNN1, and SRF.
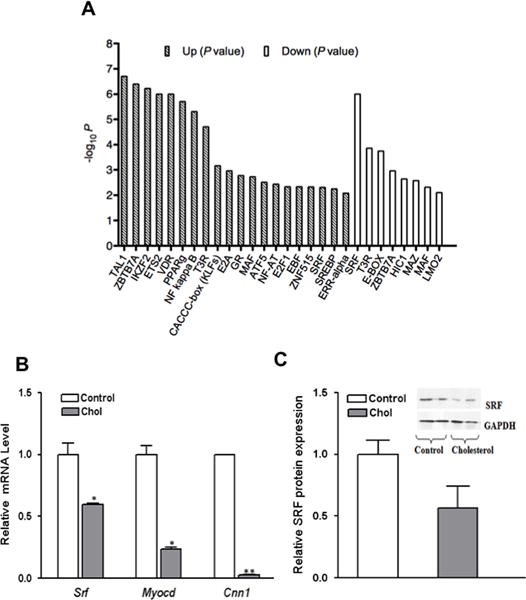
A) Bars show statistical significance (negative log10 P value) for over-representation of transcription factor binding sites within the promoters of the indicated gene set (genes that are cholesterol-upregulated or genes that are cholesterol-downregulated). Each bar corresponds to a TFBS motif whose occurrences were over- represented (P < 0.01) compared to the frequency expected by chance within promoters of genes with altered expression in response to cholesterol loading. Patterned bars indicate motifs whose occurrences are over-represented within the promoters of up-regulated genes; open bars correspond to down-regulated genes. Vertical scale is negative logarithmic, so increased bar height corresponds to greater statistical significance. SREBP indicates the sterol regulatory element (SRE), which can be bound by either SREBP-1 or SREBP-2. (B) VSMC were treated as in Fig. 1, and RNA was harvested for qRT- PCR analysis. In cholesterol loaded VSMC (gray bars), the mRNA abundances of Myocd, Srf, and calponin (Cnn1) decreased significantly compared to the control cells cultured in 0.2% BSA (C). Western blot analysis demonstrates that in cholesterol- loaded VSMC, there is ~50% reduction in SRF at the protein level compared to control cells (*p < 0.05; **p<0.001). Data shown are mean ± SD of triplicates of qRT-PCR assays (C) or Western blotting densitometric analysis (D) and are representative of two independent experiments.
Because of their central importance in VSMC differentiation, we carried out a secondary computational analysis of SRF binding elements (CArG box elements) using a genome-wide database of CArG boxes that are phylogenetically conserved29. This more stringent bioinformatic analysis showed statistical over-representation of conserved CArG boxes within the promoters of cholesterol-downregulated genes (P<0.05, see on-line Materials and Methods), but not within the promoters of cholesterol-upregulated genes. Because of the strength of the SRF binding site over-representation in the unbiased and secondary analyses, and the general importance of the complex of SRF and Myocardin (MYOCD) in regulating smooth muscle gene transcription30, 31, we next focused on functional aspects of these two regulators of smooth muscle contractile phenotype in cholesterol-loaded VSMC.
Cholesterol loading downregulates the expression of SRF and MYOCD in VSMC
Because cholesterol loading in vitro results in a decrease of VSMC-related gene expression, we tested whether this resulted from reduced Myocd and Srf expression levels. qRT-PCR results shown in Fig. 3B demonstrate that, indeed, cholesterol-loaded VSMC have significantly lower levels of Myocd and Srf mRNA, with corresponding changes in the SRF/MYOCD-dependent marker calponin (Cnn1)30, 31. While cholesterol loading down-regulated SRF expression to ≈60% of that in control cells, Myocd mRNA was decreased even more, to ≈23% of control. Consistent with the qRT-PCR results, cholesterol-loaded VSMC were characterized by a lower level of SRF protein (Fig. 3C). We were unable to measure MYOCD protein due to the insensitivity of commercially available antibodies.
Overexpression of MYOCD prevents and reverses the loss of VSMC features after cholesterol-loading
Given the result that cholesterol loading suppresses SRF/MYOCD expression and the central importance of these factors to VSMC differentiation, we used Myocd adenoviral transduction (Ad-MYOCD) to test whether overexpression of Myocd would prevent VSMC from losing features of their phenotype and gaining those of macrophages. Prior to cholesterol loading, VSMC were transduced with Ad-MYOCD or Ad-Lacz (control) at a multiplicity of infection (moi) of 50, 100, and 300. Immunostaining (Fig. 4A) showed that Ad-MYOCD cells (300 moi) retained the VSMC phenotype with cholesterol loading (i.e., they remained ACTA2+ and CD68−), while control Ad-LacZ VSMC became ACTA2 poor and CD68+. Furthermore, the qRT-PCR data (Fig. 4B–C) demonstrate that Ad-MYOCD significantly increased Cnn1 and suppressed Cd68 mRNA expression in a dose-dependent manner. These results suggest that Ad-MYOCD transduction can completely (300 moi) or partially (100 moi) block the loss of VSMC features and the induction of CD68 upon cholesterol loading.
Figure 4. Maintaining MYOCD expression prevents and reverses phenotypic changes in VSMC associated with cholesterol loading.
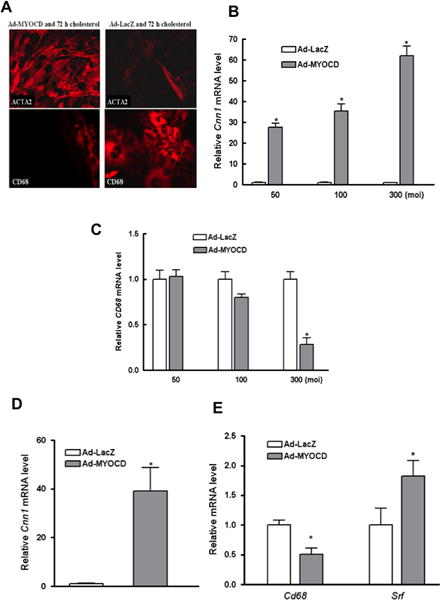
(A) Shown is immunostaining of mouse VSMC transduced with adenoviral vectors (MOI of 300) expressing either MYOCD (Ad-MYOCD) or LacZ (Ad-LacZ) prior to incubation with CD-cholesterol complexes for 72 hours (40×). Note the persistence of ACTA2 and the lack of CD68 in Ad-MYOCD vs. Ad-LacZ cells. (B–C) qRT-PCR analysis of mRNA from cells treated as in (A) showed an adenoviral vector-dose related maintenance and suppression, respectively, of VSMC marker Cnn1 and of macrophage marker Cd68. (D–E) Mouse VSMC were transduced with Ad-MYOCD or Ad-LacZ (MOI of 300) after 3 days of cholesterol loading. qRT-PCR results show increases of Cnn1 (D) and Srf mRNA (E), and suppression of Cd68 mRNA (E) in Ad-MYOCD cells (*p < 0.05; data shown are mean ± SD of triplicates of qRT-PCR reactions and are representative of two independent experiments).
Next, we tested whether MYOCD adenoviral transduction can restore the original VSMC phenotype after cholesterol loading. First, VSMC were loaded with cholesterol for 3 d (as before), then they were transduced with Ad-MYOCD or Ad-LacZ overnight. After virus was removed, the cells were kept in FBS for 3 d. qRT-PCR results (Fig. 4D and E) show that Ad-MYOCD increased Cnn1 and Srf mRNA and suppressed Cd68 expression. Thus, MYOCD over-expression not only prevents changes in VSMC after cholesterol-loading, but also can restore the original phenotype after these changes occur.
Role of Srebf2 in the phenotypic changes in cholesterol-loaded VSMC
SREBP-2 (encoded by Srebf2) controls the transcription of sterol-regulated genes32. The microarray results showed that, as expected, the Srebf2 mRNA level was suppressed in cholesterol-loaded cells (not shown). The finding of enrichment of the sterol regulatory element (SRE) within promoters of upregulated genes may reflect differential activation of SREBP isoforms in response to cholesterol loading (e.g., see33). It is possible, then, that in addition to SRF/MYOCD, SREBP-2 also contributes to the phenotypic changes in cholesterol-loaded VSMC because of the regulation of a critical sub-set of its target genes. If so, the loss of VSMC characteristics would be preventable by overexpressing SREBP-2.
To test this, VSMC were transduced overnight either with Ad-SREBP-2 or with control Ad-LacZ at moi of 300, then incubated with or without CD-cholesterol complexes. As shown in Supplemental Fig. VIII, cholesterol loading resulted in downregulation of VSMC marker ACTA2 and up-regulation of macrophage-related gene Mac-2 (Lgals3) to the same degree in all experimental groups. This was despite ~3× increases (p<0.01) in Srebf-2 mRNA with vector treatment (relative to the levels in unloaded control cells), making it unlikely that Srebf-2 regulated genes are key participants in the phenotypic changes when VSMC are cholesterol-loaded.
Roles of miR-143/145 in VSMC phenotypic changes in response to cholesterol-loading
Because several recent studies have demonstrated miR-143 and miR-145 work together to regulate MYOCD expression and VSMC phenotypic switching34, we next focused on the roles of these micro RNAs in the response to cholesterol loading. First, we established that both miR-143 and miR-145 are downregulated in cholesterol-loaded mouse aortic VSMC (Fig. 5A), consistent with prior reports that their expression is low in the cholesterol-laden aorta of atherosclerotic Apoe−/− mice, especially when plasma hyperlipidemia is even further elevated by a high fat diet35, 36; also contributing to this could be the infiltration of authentic macrophages, which have comparatively low levels of miR-143/miR-145 expression (~15% of that in control VSMC; H. Nishi, E. Fisher, unpublished data).
Figure 5. Relationships among cholesterol loading, miR-143/145 expression, and VSMC phenotypic state.
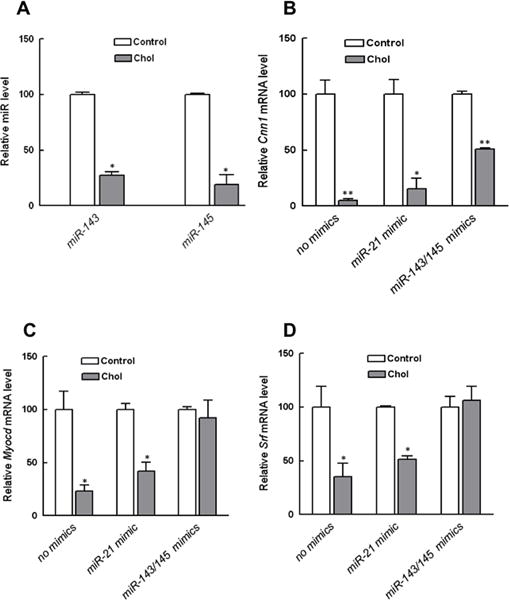
(A). Mouse VSMC were treated as in Fig. 1. qRT-PCR analysis showed that cholesterol-loading suppressed the expression of both miR-143 and miR-145. (B) Mouse VSMC were pretreated with miR-143 and miR-145 before cholesterol loading, which significantly antagonized the down-regulation of VSMC marker Cnn1 and maintained expression of the regulatory factors Myocd (C) and Srf (D). *p<0.05, *p < 0.001. Data shown are mean ± SD of triplicates of qRT-PCR reactions and are representative of two independent experiments.
Next, we used miRNA mimics to test whether restoring their levels ameliorates the effects of cholesterol loading. As shown in Fig. 5B, the mRNA level of the VSMC marker CNN1 was significantly higher in cells treated with miR-143/miR-145 mimics prior to cholesterol loading compared to the two control groups. The protective effects of the miR-143/miR-145 pre-treatment were likely due to their maintenance of MYOCD and SRF expression (Fig. 5C and D) in the face of cholesterol-loading.
ApoA1 and HDL reverse phenotypic changes induced by cholesterol-loading of VSMC
A question of both fundamental and clinical interest is whether the phenotypic changes in VSMC upon cholesterol-loading can be reversed by unloading, particularly by natural acceptors of cholesterol. To address this, VSMC were treated as before for 72 h with CD-cholesterol complexes, and then incubated for an additional 72 h with either apoA1 or the control protein, BSA. As shown in Fig. 6, as expected, cholesterol-loading reduced and increased the expression of ACTA2 and CD68, respectively, at both the protein and mRNA levels (panels A and C). After 72 h of cholesterol efflux mediated by apoA1, however, the typical VSMC appearance and marker expression were restored, and lost was the expression of macrophage markers (panels B and C). Similar results were seen with cholesterol acceptors HDL and CD (data not shown), indicating that the effects were due to cholesterol efflux in general, and not a specific pathway.
Figure 6. Cholesterol efflux promoted by apoA1 or HDL reverses phenotypic changes induced by cholesterol-loading of VSMC.
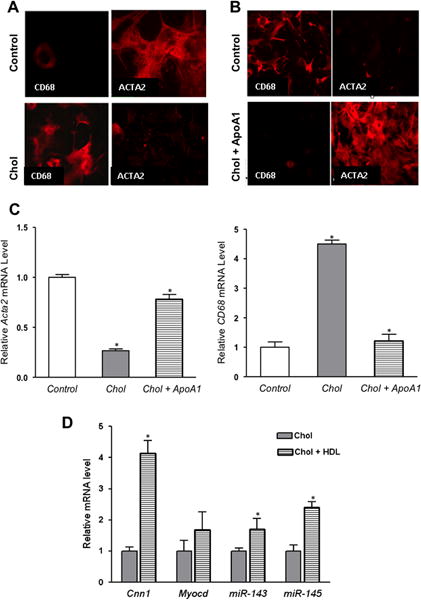
(A) Mouse VSMC were treated and immunostained as in Fig. 1. Images shown are (40×). As expected, the protein level of ACTA2 decreased, while that of CD68 increased. (B) Next, cholesterol-loaded VSMC were incubated with apoA1 for 72 h, which restored the typical VSMC appearance and ACTA2 expression, with loss of CD68 expression. (C) Consistent with immunostaining, cholesterol-loaded cells had reduced and increased, respectively, Acta2 and Cd68 mRNA levels. (D) Cholesterol-loaded VSMC were treated as in panel (C), but HDL was used instead of apoA1. By qRT-PCR analysis, there were recoveries of the expression of the VSMC marker Cnn1 as well as of the regulatory factors Myocd and miR-143/145. *p < 0.5 vs. control). Data shown are mean ± SD of triplicates of qRT-PCR reactions and are representative of two independent experiments.
Because of the results implicating the miR-143/145 regulatory network (Fig. 5) in the molecular response of VSMC to cholesterol loading, we also measured efflux-associated changes in the levels of these micro RNAs and their key target Myocd. As shown in panel D, cholesterol unloading of VSMC by HDL increased expression of Myocd, miR-143, and miR-145, suggesting that these changes were the mechanistic basis of the phenotypic reversibility (panels B and C).
DISCUSSION
Though it is commonly assumed that the majority of atherosclerotic plaque foam cells originate from the circulating monocytes recruited into the vessel wall, VSMC can also become lipid-laden in vitro and in vivo (e.g.,14, 16). It is also well-established that VSMC that migrate from the medial layer into the plaque intima lose expression of many factors related to the contractile phenotype, such as myosin heavy chain and smooth muscle cell actin. Recent data from mouse20, 24 and human plaques16 confirm that a subset of foam cells originate from VSMC, and extend these findings by showing that many of these cells display macrophage markers. Indeed, it is estimated that ~30–40% of foam cells in mouse or human plaques positive for standard macrophage markers, such as CD68 or Mac-2, are of VSMC origin16, 20, 24. This would be consistent with our previous report that cholesterol loading of mouse aortic SMC in vitro caused them to rapidly become foam cells, to lose the expression of several contractile VSMC-related markers, and to gain the expression of several macrophage-related markers15. That similar observations are now made with human coronary artery smooth muscle cells (Supplemental Fig. I) supports the general importance and clinical relevance of the mechanistic findings in the present study.
In the present study, we have focused on the underlying molecular bases for these effects of cholesterol loading on VSMC. Based on a variety of bioinformatic and functional approaches applied to primary mouse cells, the major findings are: 1) The master transcription factors that control the VSMC contractile phenotype, SRF and MYOCD, as well as two key microRNAs that, in turn, regulate them, miR-143/145, decrease in expression upon cholesterol-loading. That these changes are causal is supported by experiments in which maintaining MYOCD or miR-143/145 levels either prevents or reverses the effects of cholesterol loading; 2) Despite the induced expression of some macrophage markers in cholesterol-loaded VSMC, by Principal Components Analysis (PCA) the overall transcriptome remains closer to that of VSMC than that of authentic macrophages; 3) Consistent with the PCA analysis, cholesterol-loaded VSMC are relatively poor in the macrophage activities of phagocytosis and efferocytosis; and, 4) Phenotypic changes caused by cholesterol-loading can be prevented by promotion of cholesterol efflux by artificial and natural acceptors.
There are a number of clinically relevant implications of our results, especially in light of the recent evidence noted above that the phenotypic changes corresponding to what we find in mouse VSMC loaded with cholesterol in vitro occur with frequency in the atheroma of mice and humans16, 20, 24. First, the molecular mechanisms in vivo are likely to be similar to what we discovered in vitro, as suggested by the down-regulation of miR-143/145 in the aortae of apoE-deficient mice, which becomes even more profound when they are fed a western-type diet to exacerbate their hypercholesterolemia35, 36.
Second, the functional consequences of the changes observed in vitro, if they were to occur in vivo, may be adverse. For example, while the overall transcriptome remained VSMC-centric, there was up-regulation of several pro-atherogenic factors, such as macrophage-specific inflammatory cytokine mRNAs, including Ccl2 and Ccl7, as well as the Toll-like receptor Tlr4, consistent with the transcription factor binding factor analysis that identified over-representation of mRNAs encoded by genes with NF-kβ sites. Furthermore, our finding of cells of VSMC origin with macrophage features that border the necrotic core (Supplemental Fig. VII) and likely deficient in efferocytosis (Fig. 2D and Supplemental Fig. VI), suggests that they would inefficiently clear apoptotic cells and consequently contribute to core expansion23. That cells in this location would be particularly susceptible to cholesterol loading and its induction of phenotypic/functional changes is entirely plausible, given the high ambient extracellular cholesterol they are exposed to, some of it in the form of cholesteryl ester-rich inclusions derived from necrotic foam cells, which are avidly taken up by VSMC14, and the impairment in ABCA1 expression and function in intima-type arterial smooth muscle cells7. Another possible consequence of clinical significance could be that the cholesterol loading of VSMC attenuates their ability to elaborate extracellular matrix components that serve to stabilize plaques, as implied by the down regulation of a number of relevant genes on the microarray (see Supplemental Table 3 for examples).
Third, the reversibility of the effects of cholesterol-loading by efflux may contribute to the formation of SMC-containing fibrous caps that we previously observed when mouse plaques were placed into an atherosclerosis regression environment in which HDL was increased37, 38. Notably, after the change in the plasma lipoprotein profile, the number of sub-endothelial cells that were CD68+ decreased and those ACTA2+ increased. Furthermore, the transcriptome profile of CD68+ cells that were laser captured from regressing plaques showed enrichment in the expression of genes associated with the contractile apparatus26. If these phenomena represented the restoration in CD68+ VSMC aspects of the ACTA2+ phenotypic state, and similar effects occur in foam cells of VSMC origin in human plaques, then emerging therapies to clinically increase cholesterol efflux by apoA1 or HDL infusions or by anti-miR33 treatment (reviewed in39) may also promote fibrous cap formation in patients, resulting in stabilization of vulnerable plaques.
While we have provided data to implicate down-regulation of the miR-143/145/SRF/MYOCD axis as the molecular mechanism of the loss of the contractile VSMC phenotype, of interest are two questions, namely, how cholesterol “regulates the regulators” of the contractile phenotype and what turns on the macrophage-specific factors. An obvious candidate because of its role in cholesterol regulation of gene expression32- SREBP2- turned out not to address either question. Instead, the answers to both questions may involve the transcription factor KLF4. KLF4 is a repressor of SRF/MYOCD40, and itself is repressed by the miR-143/145/SRF/MYOCD axis in a complicated feedback loop35. Thus, it is expected that KLF4 will increase after cholesterol loading because miR-143/145/SRF/MYOCD all become down-regulated. In preliminary studies, this is indeed the case; moreover, when MYOCD levels are maintained in cholesterol-loaded VSMC, KLF4 expression was suppressed (Supplemental Fig. IXA). The connection between KLF4 and the macrophage-marker expression (question 2) comes from studies showing that it is a required factor in monocyte differentiation41. Thus, it is tempting to speculate that the increased expression of KLF4 after cholesterol loading participates not only in the loss of the contractile phenotype, but also in the assumption of some of the monocyte-macrophage features by cholesterol-loaded VSMC.
Another likely factor in the loss of key phenotypic features of contractile VSMC upon cholesterol-loading is TGFβ. TGFβ is a long-established promoter of this phenotype of VSMC, at least in part by inducing Myocd expression42, which we have recently reported is an effect upstream of miR-143/14543. Most intriguing, then, is that cholesterol-enrichment of cells has been reported to dampen TGFβ signaling44. The mechanism is likely related to changes in membrane fluidity or lipid raft characteristics regulated by cholesterol content, which affects a host of receptors, channels, and other membrane-associated proteins (e.g.,45). These considerations and our exciting finding in preliminary studies that cholesterol-loaded mouse VSMC exhibit attenuation of TGFβ signaling (Supplemental Fig. IXB) support it being a plausible player in the loss of the contractile VSMC phenotype.
It should be noted that in addition to miR-143/145, there is another factor that is a molecular link among SRF, MYOCD, and KLF4. TET2 (ten-eleven-translocation 2) is a DNA de-methylase recently shown to be enriched in contractile VSMC, and its knockdown, similar to cholesterol-loading, inhibited expression of SRF and MYOCD with concomitant up-regulation of KLF446. That TET2 is potentially relevant to the present results is further supported by the transcription factor binding site (TFBS) sequence patterns analysis. As shown in Fig. 3, the binding site sequence pattern for EBF1, an interaction partner for TET247, was over-represented among the 5′ regulatory regions of VSMC genes that went up in response to cholesterol loading.
Though our results have been discussed mainly with regard to atherosclerosis, it is interesting to consider them in the general context of VSMC differentiation. Unlike terminally differentiated skeletal or cardiac muscle cells, VSMC remain remarkably plastic19, with changes in their phenotype occurring in a variety of vascular disorders besides atherosclerosis48. A signature feature of such modifications is reduced expression of VSMC marker genes, such as Myh11, Cnn1, and the master regulator of these contractile genes, Myocd. The novelty of the present findings is that these changes in VSMC can be provoked simply by cholesterol-loading. New methods, such as the elegant approach of tracing VSMC lineage in situ using epigenetic marks49, will improve the ability to detect phenotypically modified VSMC and better define the circumstances in vivo in which they occur.
In summary, the present results provide strong experimental evidence that cholesterol-loading causes changes in VSMC in vitro that result not only in their loss of the contractile phenotype, but also in their being mistaken for macrophages. The recent demonstration that up to 40% of human plaque “macrophage” foam cells are of VSMC origin16 is entirely consistent with these findings, making it very plausible that the mechanism we uncovered in vitro- the down-regulation by cholesterol of miR-143 and miR-145, which, in turn, down-regulates the MYOCD/SRF axis in VSMC of both murine and human origin- is similarly operative in vivo. Furthermore, the reversibility of the changes has implications not only for the understanding of the fundamental biology of plaques, but also for the therapeutic approach to their stabilization in patients experiencing, or at risk of, acute coronary syndromes.
Supplementary Material
SIGNIFICANCE.
We previously showed that cholesterol loading in vitro converts mouse aortic vascular smooth muscle cells (VSMC) from a contractile state to one resembling macrophages. In human and mouse atherosclerotic plaques it has become appreciated that ~40% of cells classified as macrophages by histological markers may be of VSMC origin. The present studies show that cholesterol loading of VSMC converts them to a macrophage–appearing state by downregulating the miR-143/145-myocardin/SRF axis. Though these cells would be classified by immunohistochemistry as macrophages in human and mouse plaques, their transcriptome and functional properties imply that their contributions to atherogenesis would not be those of classical macrophages, yet they would be considered as such by conventional criteria.
Acknowledgments
None
FUNDING SOURCES
This work was supported by funding from the National Institutes of Health to EAF (HL084312, HL098055), JMM (HL117907), KJM (HL117334, HL108182), and SAR (HL098807). YV was supported by NIH training grant T32 HL098129.
EAF reports being a member of the Speaker’s Bureau of Merck and receiving an investigator-initiated research grant from the same company.
ABBREVIATIONS
- ACTA2
Smooth muscle cell alpha actin
- Ad
Adenovirus
- Apoe
Apolipoprotein E
- CD
Cyclodextrin
- Mϕ
Macrophage or macrophages
- MYOCD
Myocardin
- PCA
Principal component analysis
- SM
Smooth muscle
- SRF
Serum response factor
- VSMC
Vascular smooth muscle cell or cells
Footnotes
DISCLOSURES
The other authors have no relevant disclosures.
References
- 1.Stary HC, Chandler AB, Glagov S, Guyton JR, Insull W, Jr, Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner WD, Wissler RW. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the committee on vascular lesions of the council on arteriosclerosis, american heart association. Circulation. 1994;89:2462–2478. doi: 10.1161/01.cir.89.5.2462. [DOI] [PubMed] [Google Scholar]
- 2.Brewer HB., Jr The lipid-laden foam cell: An elusive target for therapeutic intervention. The Journal of clinical investigation. 2000;105:703–705. doi: 10.1172/JCI9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glass CK, Witztum JL. Atherosclerosis. The road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 5.Tabas I. Consequences of cellular cholesterol accumulation: Basic concepts and physiological implications. The Journal of clinical investigation. 2002;110:905–911. doi: 10.1172/JCI16452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allahverdian S, Pannu PS, Francis GA. Contribution of monocyte-derived macrophages and smooth muscle cells to arterial foam cell formation. Cardiovascular research. 2012;95:165–172. doi: 10.1093/cvr/cvs094. [DOI] [PubMed] [Google Scholar]
- 7.Choi HY, Rahmani M, Wong BW, Allahverdian S, McManus BM, Pickering JG, Chan T, Francis GA. Atp-binding cassette transporter a1 expression and apolipoprotein a-i binding are impaired in intima-type arterial smooth muscle cells. Circulation. 2009;119:3223–3231. doi: 10.1161/CIRCULATIONAHA.108.841130. [DOI] [PubMed] [Google Scholar]
- 8.Andreeva ER, Pugach IM, Orekhov AN. Subendothelial smooth muscle cells of human aorta express macrophage antigen in situ and in vitro. Atherosclerosis. 1997;135:19–27. doi: 10.1016/s0021-9150(97)00136-6. [DOI] [PubMed] [Google Scholar]
- 9.Faggiotto A, Ross R, Harker L. Studies of hypercholesterolemia in the nonhuman primate. I. Changes that lead to fatty streak formation. Arteriosclerosis. 1984;4:323–340. doi: 10.1161/01.atv.4.4.323. [DOI] [PubMed] [Google Scholar]
- 10.Mietus-Snyder M, Gowri MS, Pitas RE. Class a scavenger receptor up-regulation in smooth muscle cells by oxidized low density lipoprotein. Enhancement by calcium flux and concurrent cyclooxygenase-2 up-regulation. The Journal of biological chemistry. 2000;275:17661–17670. doi: 10.1074/jbc.275.23.17661. [DOI] [PubMed] [Google Scholar]
- 11.Frontini MJ, O’Neil C, Sawyez C, Chan BM, Huff MW, Pickering JG. Lipid incorporation inhibits src-dependent assembly of fibronectin and type i collagen by vascular smooth muscle cells. Circulation research. 2009;104:832–841. doi: 10.1161/CIRCRESAHA.108.187302. [DOI] [PubMed] [Google Scholar]
- 12.Klouche M, Rose-John S, Schmiedt W, Bhakdi S. Enzymatically degraded, nonoxidized ldl induces human vascular smooth muscle cell activation, foam cell transformation, and proliferation. Circulation. 2000;101:1799–1805. doi: 10.1161/01.cir.101.15.1799. [DOI] [PubMed] [Google Scholar]
- 13.Pomerantz KB, Hajjar DP. Eicosanoid metabolism in cholesterol-enriched arterial smooth muscle cells: Reduced arachidonate release with concomitant decrease in cyclooxygenase products. Journal of lipid research. 1989;30:1219–1231. [PubMed] [Google Scholar]
- 14.Wolfbauer G, Glick JM, Minor LK, Rothblat GH. Development of the smooth muscle foam cell: Uptake of macrophage lipid inclusions. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:7760–7764. doi: 10.1073/pnas.83.20.7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rong JX, Shapiro M, Trogan E, Fisher EA. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13531–13536. doi: 10.1073/pnas.1735526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allahverdian S, Chehroudi AC, McManus BM, Abraham T, Francis GA. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation. 2014 doi: 10.1161/CIRCULATIONAHA.113.005015. [DOI] [PubMed] [Google Scholar]
- 17.Wang DZ, Olson EN. Control of smooth muscle development by the myocardin family of transcriptional coactivators. Current opinion in genetics & development. 2004;14:558–566. doi: 10.1016/j.gde.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Gordon S, Martinez FO. Alternative activation of macrophages: Mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiological reviews. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 20.Shankman LS, A M, Owens G. Smooth muscle cells modulate to a macrophage-like state in vivo. ATVB, 2011, Chicago, Ill. 2011:205. [Google Scholar]
- 21.Raychaudhuri S, Stuart JM, Altman RB. Principal components analysis to summarize microarray experiments: Application to sporulation time series. Pacific Symposium on Biocomputing Pacific Symposium on Biocomputing. 2000:455–466. doi: 10.1142/9789814447331_0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma’ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, Randolph GJ, Immunological Genome C. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nature immunology. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feil S, Fehrenbacher B, Lukowski R, Essmann F, Schulze-Osthoff K, Schaller M, Feil R. Transdifferentiation of vascular smooth muscle cells to macrophage-like cells during atherogenesis. Circulation research. 2014;115:662–667. doi: 10.1161/CIRCRESAHA.115.304634. [DOI] [PubMed] [Google Scholar]
- 25.Huber PA. Caldesmon. The international journal of biochemistry & cell biology. 1997;29:1047–1051. doi: 10.1016/s1357-2725(97)00004-6. [DOI] [PubMed] [Google Scholar]
- 26.Feig JE, Vengrenyuk Y, Reiser V, Wu C, Statnikov A, Aliferis CF, Garabedian MJ, Fisher EA, Puig O. Regression of atherosclerosis is characterized by broad changes in the plaque macrophage transcriptome. PloS one. 2012;7:e39790. doi: 10.1371/journal.pone.0039790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wingender E, Chen X, Hehl R, Karas H, Liebich I, Matys V, Meinhardt T, Pruss M, Reuter I, Schacherer F. Transfac: An integrated system for gene expression regulation. Nucleic acids research. 2000;28:316–319. doi: 10.1093/nar/28.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calkin AC, Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors lxr and fxr. Nature reviews. Molecular cell biology. 2012;13:213–224. doi: 10.1038/nrm3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benson CC, Zhou Q, Long X, Miano JM. Identifying functional single nucleotide polymorphisms in the human cargome. Physiological genomics. 2011;43:1038–1048. doi: 10.1152/physiolgenomics.00098.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J, Kitchen CM, Streb JW, Miano JM. Myocardin: A component of a molecular switch for smooth muscle differentiation. Journal of molecular and cellular cardiology. 2002;34:1345–1356. doi: 10.1006/jmcc.2002.2086. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z, Wang DZ, Pipes GC, Olson EN. Myocardin is a master regulator of smooth muscle gene expression. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:7129–7134. doi: 10.1073/pnas.1232341100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown MS, Goldstein JL. The srebp pathway: Regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 33.Bommer GT, MacDougald OA. Regulation of lipid homeostasis by the bifunctional srebf2-mir33a locus. Cell metabolism. 2011;13:241–247. doi: 10.1016/j.cmet.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rangrez AY, Massy ZA, Metzinger-Le Meuth V, Metzinger L. Mir-143 and mir-145: Molecular keys to switch the phenotype of vascular smooth muscle cells. Circulation. Cardiovascular genetics. 2011;4:197–205. doi: 10.1161/CIRCGENETICS.110.958702. [DOI] [PubMed] [Google Scholar]
- 35.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D. Mir-145 and mir-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elia L, Quintavalle M, Zhang J, Contu R, Cossu L, Latronico MV, Peterson KL, Indolfi C, Catalucci D, Chen J, Courtneidge SA, Condorelli G. The knockout of mir-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: Correlates with human disease. Cell death and differentiation. 2009;16:1590–1598. doi: 10.1038/cdd.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rong JX, Li J, Reis ED, Choudhury RP, Dansky HM, Elmalem VI, Fallon JT, Breslow JL, Fisher EA. Elevating high-density lipoprotein cholesterol in apolipoprotein e-deficient mice remodels advanced atherosclerotic lesions by decreasing macrophage and increasing smooth muscle cell content. Circulation. 2001;104:2447–2452. doi: 10.1161/hc4501.098952. [DOI] [PubMed] [Google Scholar]
- 38.Trogan E, Fayad ZA, Itskovich VV, Aguinaldo JG, Mani V, Fallon JT, Chereshnev I, Fisher EA. Serial studies of mouse atherosclerosis by in vivo magnetic resonance imaging detect lesion regression after correction of dyslipidemia. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:1714–1719. doi: 10.1161/01.ATV.0000139313.69015.1c. [DOI] [PubMed] [Google Scholar]
- 39.Feig JE, Hewing B, Smith JD, Hazen SL, Fisher EA. High-density lipoprotein and atherosclerosis regression: Evidence from preclinical and clinical studies. Circulation research. 2014;114:205–213. doi: 10.1161/CIRCRESAHA.114.300760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y, Sinha S, McDonald OG, Shang Y, Hoofnagle MH, Owens GK. Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. The Journal of biological chemistry. 2005;280:9719–9727. doi: 10.1074/jbc.M412862200. [DOI] [PubMed] [Google Scholar]
- 41.Feinberg MW, Wara AK, Cao Z, Lebedeva MA, Rosenbauer F, Iwasaki H, Hirai H, Katz JP, Haspel RL, Gray S, Akashi K, Segre J, Kaestner KH, Tenen DG, Jain MK. The kruppel-like factor klf4 is a critical regulator of monocyte differentiation. The EMBO journal. 2007;26:4138–4148. doi: 10.1038/sj.emboj.7601824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurpinski K, Lam H, Chu J, Wang A, Kim A, Tsay E, Agrawal S, Schaffer DV, Li S. Transforming growth factor-beta and notch signaling mediate stem cell differentiation into smooth muscle cells. Stem cells. 2010;28:734–742. doi: 10.1002/stem.319. [DOI] [PubMed] [Google Scholar]
- 43.Long X, Miano JM. Transforming growth factor-beta1 (tgf-beta1) utilizes distinct pathways for the transcriptional activation of microrna 143/145 in human coronary artery smooth muscle cells. The Journal of biological chemistry. 2011;286:30119–30129. doi: 10.1074/jbc.M111.258814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen CL, Liu IH, Fliesler SJ, Han X, Huang SS, Huang JS. Cholesterol suppresses cellular tgf-beta responsiveness: Implications in atherogenesis. Journal of cell science. 2007;120:3509–3521. doi: 10.1242/jcs.006916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. Atp-binding cassette transporters and hdl suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu R, Jin Y, Tang WH, Qin L, Zhang X, Tellides G, Hwa J, Yu J, Martin KA. Ten-eleven translocation-2 (tet2) is a master regulator of smooth muscle cell plasticity. Circulation. 2013;128:2047–2057. doi: 10.1161/CIRCULATIONAHA.113.002887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guilhamon P, Eskandarpour M, Halai D, Wilson GA, Feber A, Teschendorff AE, Gomez V, Hergovich A, Tirabosco R, Fernanda Amary M, Baumhoer D, Jundt G, Ross MT, Flanagan AM, Beck S. Meta-analysis of idh-mutant cancers identifies ebf1 as an interaction partner for tet2. Nature communications. 2013;4:2166. doi: 10.1038/ncomms3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen AT, Gomez D, Bell RD, Campbell JH, Clowes AW, Gabbiani G, Giachelli CM, Parmacek MS, Raines EW, Rusch NJ, Speer MY, Sturek M, Thyberg J, Towler DA, Weiser-Evans MC, Yan C, Miano JM, Owens GK. Smooth muscle cell plasticity: Fact or fiction? Circulation research. 2012 doi: 10.1161/CIRCRESAHA.112.281048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gomez D, Shankman LS, Nguyen AT, Owens GK. Detection of histone modifications at specific gene loci in single cells in histological sections. Nature methods. 2013;10:171–177. doi: 10.1038/nmeth.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


