Abstract
Objective
Focal junctional tourniquets (JTs) have been developed to control hemorrhage from proximal limb injuries. These devices may permit greater collateral perfusion than circumferential tourniquets. We hypothesized that JTs eliminate large-vessel pulse pressure yet allow a small amount of residual limb perfusion that could be useful for maintaining tissue viability.
Methods
Ten healthy control subjects were studied. Transthoracic echocardiography, Doppler ultrasound of the femoral artery (FA) and posterior tibial artery, and contrast-enhanced ultrasound (CEU) perfusion imaging of the anterior thigh extensor and calf plantar flexor muscles were performed at baseline and during application of a JT over the common FA. Intramuscular arterial pulsatility index was also measured from CEU intensity variation during the cardiac cycle.
Results
FA flow was eliminated by JTs in all subjects; posterior tibial flow was eliminated in all but one. Perfusion measured in the thigh and calf muscles was similar at baseline (0.33 ± 0.29 vs 0.29 ± 0.22 mL/min/g). Application of the JT resulted in a reduction of perfusion (P < .05) that was similar for the thigh and calf (0.08 ± 0.07 and 0.10 ± 0.03 mL/min/g). On CEU, microvascular flux rate was reduced by ≈55%, and functional microvascular blood volume was reduced by ≈35%. Arterial pulsatility index was reduced by ≈90% in the calf. JT inflation did not alter left ventricle dimensions, fractional shortening, cardiac output, or arterial elastance as a measure of total systolic load.
Conclusions
Application of a JT eliminates conduit arterial pulse and markedly reduces intramuscular pulse pressure, but thigh and calf skeletal muscle perfusion is maintained at 25% to 35% of basal levels. These data suggest that JTs that are used to control limb hemorrhage allow residual tissue perfusion even when pulse pressure is absent.
Application of tourniquets to occlude limb arterial inflow has been a mainstay of emergent therapy for life-threatening hemorrhage from trauma. A variety of tourniquets have been developed and applied both by civilian emergency first responders and by military personnel who treat battlefield injuries.1,2 The importance of continued advances in tourniquet technology is underscored by an analysis of U.S. military casualties who died after reaching a medical treatment facility, where it was estimated that more than half of the fatal wounds were potentially survivable, with hemorrhage being the overwhelming cause of death.3 In civilian trauma and in modern combat situations, unsuccessful hemorrhage control often involves bleeding from “junctional” wounds where anatomic constraints limit the effectiveness of standard hemostatic devices. This limitation has led to the development of focal tourniquets to control hemorrhage from junctional injuries.4,5 In contrast to circumferential tourniquets, focal tourniquets that occlude the common femoral artery (FA) may allow residual limb perfusion through collateral vessels originating from the internal iliac artery and pelvic vessels, which may be advantageous for maintaining tissue viability during prolonged tourniquet application. This notion is supported by long-term limb survival and favorable residual arterial flow in patients with infected pseudoaneurysms who undergo permanent ligation of the common FA without revascularization.6,7
In this study, we hypothesized that focal junctional tourniquets (JTs) allow residual skeletal muscle perfusion despite elimination of large and small arterial pulse pressure. To test this hypothesis, we used quantitative contrast-enhanced ultrasound (CEU) perfusion imaging, which has been used previously to evaluate limb muscle perfusion abnormalities in peripheral arterial disease (PAD) and diabetes.8-11 This technique has the advantage of providing information on functional microvascular blood volume (MBV) and intramuscular arterial/arteriolar pulse pressure.
METHODS
Study population
The study was approved by the human Institutional Review Board at Oregon Health & Science University. Ten consecutive healthy control subjects were studied after giving written informed consent. Participants between 19 and 50 years of age were recruited to reduce likelihood for pre-existing PAD. Exclusion criteria included pregnant or lactating women; history of cardiovascular disease or PAD; evidence of right-to-left shunt, ventricular dysfunction, or more than mild valve regurgitation or stenosis on screening echocardiography; ankle-brachial index (ABI) ≤0.9; and any unexplained symptoms of dyspnea, chest pain, or claudication.
Study protocol
At the time of enrollment, a medical history and focused physical examination including blood pressure measurement, cardiopulmonary examination, and peripheral vascular examination were performed. In women of childbearing age, pregnancy was excluded by measurement of urine human chorionic gonadotropin concentration. Full echocardiography was performed. Vascular ultrasound of the FA at the site of device placement was performed to exclude presence of plaque, aneurysm, or any other pre-existing structural abnormalities.
Subjects were placed in the supine position and were fitted with a JT (SAM Medical Products, Wilsonville, Ore), which involves an adjustable belt and placement of a deflated compression bladder over the left common FA (Fig 1, A). Placement of the device generally required less than 30 seconds. ABI measurement and CEU perfusion imaging of the midanterior thigh extensor muscles and the midcalf plantar flexor muscles were performed. The targeted compression device was then inflated over the left common FA until complete absence of the posterior tibialis (PT) pulse measured by continuous-wave Doppler ultrasound with a single-element “pencil probe” (1058-C Vascular Mini-Lab; Parks Medical Electronics, Aloha, Ore). Complete absence of FA flow distal to the occluder was confirmed by color flow and pulsed-wave spectral Doppler ultrasound. CEU perfusion imaging was then repeated 1 minute after inflation of the occlusion device. The tourniquet was deflated, and return of pulse pressure was confirmed by PT Doppler signal. The total duration of occlusion was <7 minutes in all subjects.
Fig 1.
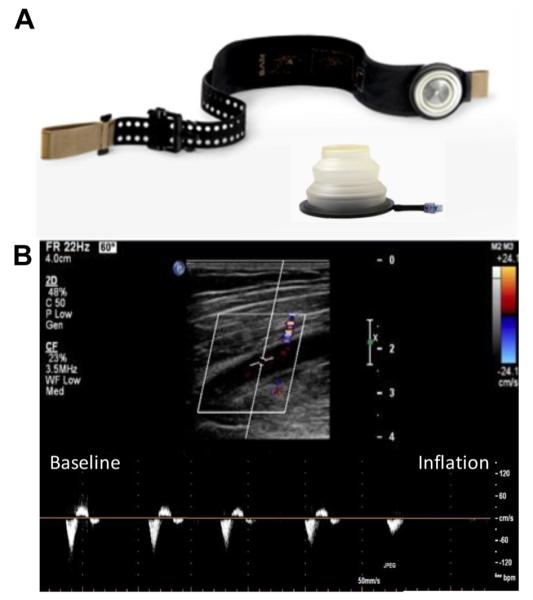
A, Image illustrating the inflatable junctional tourniquet (JT) device and the wrapping mechanism used to secure the device. B, Femoral artery (FA) ultrasound image showing B-mode long-axis ultrasound imaging of the FA (top) and pulsed-wave Doppler imaging of FA blood flow velocity (bottom) before and after tourniquet inflation, demonstrating complete elimination of pulsatile blood flow.
ABI
Before inflation of the JT, left leg dorsalis pedis and PT artery systolic blood pressures were measured with continuous-wave Doppler ultrasound by the single-element transducer. ABI was calculated by dividing the higher of the two pressures by brachial artery systolic blood pressure.
Echocardiography
Echocardiography was performed with a phased array transducer interfaced with a cardiac ultrasound imaging system (S5 transducer, iE33; Philips Ultrasound, Bothell, Wash). Two-dimensional echocardiography was performed at 1.7 MHz with tissue harmonic imaging receive filters. Pre-existing ventricular or valvular dysfunction, pulmonary hypertension, and intracardiac shunt were excluded before proceeding with the study protocol. All echocardiographic measurements were made according to guidelines published by the American Society of Echocardiography.12 Measurements made both at baseline and during inflation of the JT included left ventricular (LV) chamber dimension at end systole (LVIDs) and end diastole (LVIDd) and LV stroke volume (SV) calculated by the product of LV outflow tract area measured in the parasternal long-axis view and the time-velocity integral measured by pulsed-wave spectral Doppler in the apical five-chamber view. Arterial elastance (Ea) was calculated by (0.9 × systolic blood pressure)/(SV indexed to body surface area). Fractional shortening was calculated by (LVIDd – LVIDs)/LVIDd. Because Doppler values and chamber dimensions can be influenced by the presence of ultrasound contrast material,13 baseline and occlusion measurements were both made in the presence of contrast material.
Vascular ultrasound
Vascular ultrasound was performed with a linear array transducer (L9-3 transducer, IE33; Philips Ultrasound) to exclude pre-existing anatomic abnormalities of the left FA at the occluder inflation site. Long-axis color and spectral Doppler (3.5 MHz) imaging of the FA was performed immediately distal to the JT at baseline and during inflation.
Perfusion imaging
CEU perfusion imaging was performed with a multipulse decorrelation algorithm at 1.6 MHz and a mechanical index of 0.9. Lipid-shelled octafluoropropane microbubbles (Definity; Lantheus Medical Imaging, North Billerica, Mass) were diluted 1:15 in normal saline and infused intravenously at 0.5 mL/min for obtaining blood pool signal (IB) from the FA at a pulsing interval of once per cardiac cycle at end diastole. The infusion rate was then increased to 1.5 mL/min for perfusion imaging of the midanterior thigh extensor muscles and the midcalf plantar flexor muscles, which were imaged in the transaxial plane. Frames were acquired during continuous imaging at approximately 50 Hz and at end diastole during incremental prolongation of the pulsing interval from one to 15 cardiac cycles. Video intensity was measured from regions of interest placed over the thigh rectus femoris and calf soleus and gastrocnemius muscles after subtracting averaged frames obtained during continuous imaging to eliminate signal from tissue and large intramuscular vessels.14,15 Pulsing interval vs intensity data were fit to the function y = A (1 – e—β t), where y is intensity at time t, A is the plateau intensity reflecting MBV, and β is the rate constant of microbubble replenishment reflecting microvascular blood flux rate. Skeletal muscle MBV was quantified by the following: A/(1.06 × IB × F), where 1.06 is tissue density (g/cm3) and F is the scaling factor (3) that corrects for the different infusion rate for measuring IB to avoid dynamic range saturation. Microvascular blood flow was quantified by the product of MBV and β.16
Arterial pulsatility index
The pulse pressure within small intramuscular arteries and large arterioles was reflected by the CEU-determined arterial pulsatility index (API). Video intensity during continuous imaging was measured from a muscle region of interest drawn to exclude the presence of any large-conduit intramuscular arteries or veins. The API was calculated as the ratio of the peak systolic to diastolic intensity. Because high mechanical index imaging was used, this value reflects the ratio of mean systolic to diastolic velocity in the vascular bed. On the basis of the known beam elevation profile and the frame rate, signal was derived primarily from arterial vessels with a mean velocity of >4 cm/s, thereby excluding capillary and small to medium microvessels.17,18 According to Poiseuille’s law governing flow in vessels with Newtonian conditions, the API will directly reflect the ratio of systolic to diastolic pressure gradient, assuming minimal pulsatile change in vessel diameter.
Statistical methods
Data are expressed as mean (±standard deviation) unless otherwise stated. Comparisons of all continuous variables measured at baseline and during pneumatic tourniquet inflation were performed with the paired Student t-test (two-sided). Comparisons of API were made by a Wilcoxon signed rank test. Differences were considered significant at P < .05.
RESULTS
Subject data
All recruited subjects completed the study protocol without complication, and there were no echocardiographic reasons for exclusion. The study population consisted of five men and five women, aged 31 ± 7 years (range, 23-47 years). The mean body mass index of the population was 24.7 ± 3.3. Lower extremity examination findings were normal, and the mean baseline ABI was 1.12 ± 0.10. All subjects tolerated placement of the JT with only mild discomfort except for one individual, who subjectively reported moderate to severe discomfort.
Hemodynamic and echocardiographic data
Heart rate, blood pressure, and echocardiographic measurement of LV dimensions, SV, and SV index did not significantly change between baseline and inflation of the JT (Table). Fractional shortening, the end-systolic pressure–LV dimension relationship, and arterial elastance (Ea) also did not change, indicating that the JT did not produce any major change in LV systolic function or afterload.
Table.
Hemodynamic and echocardiographic data
| Baseline | Occlusion | |
|---|---|---|
| Heart rate, beats/min | 60 ± 11 | 63 ± 12 |
| Systolic blood pressure, mm Hg | 115 ± 17 | 111 ± 16 |
| Diastolic blood pressure, mm Hg | 69 ± 11 | 68 ± 8 |
| LVIDd, cm | 4.9 ± 0.4 | 4.8 ± 0.7 |
| LVIDs, cm | 3.2 ± 0.3 | 3.1 ± 0.2 |
| Fractional shortening, % | 35 ± 5 | 38 ± 7 |
| SV, mL | 76 ± 25 | 80 ± 23 |
| SV index, mL/m2 | 39 ± 11 | 41 ± 11 |
| Cardiac output, mL/min | 4.4 ± 1.0 | 4.9 ± 1.3 |
| Cardiac index, mL/min/m2 | 2.3 ± 0.4 | 2.5 ± 0.6 |
| Ea, mm Hg/mL | 1.45 ± 0.36 | 1.37 ± 0.57 |
Ea, Arterial elastance; LVIDd, diastolic left ventricular internal diameter; LVIDs, systolic left ventricular internal diameter; SV, stroke volume.
Conduit vessel and skeletal muscle blood flow
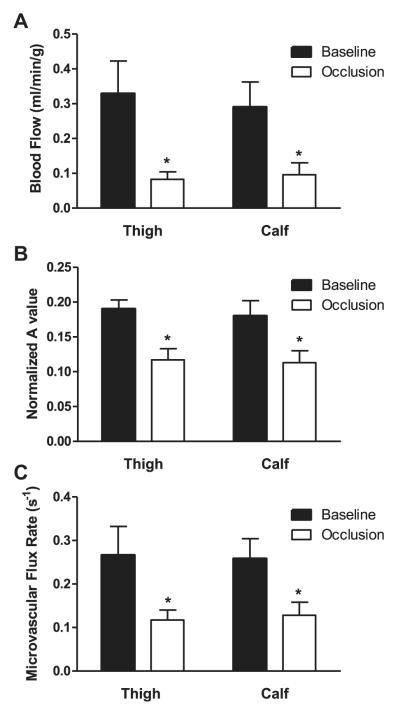
During pneumatic JT inflation, there was immediate elimination of the triphasic FA Doppler signal in all subjects (Fig 1). Further inflation of the cuff was needed in several subjects because of reappearance of a weak PT Doppler signal within 1 minute of initial inflation. However, in the one patient with moderate to severe discomfort, the PT Doppler signal was not entirely eliminated for the full duration because of discomfort during further pneumatic inflation. CEU perfusion imaging was successfully performed for both the thigh and calf in all patients (Figs 2 and 3). Baseline blood flow in the anterior thigh extensors and calf plantar flexor muscle groups was similar (Fig 3). During JT occlusion, skeletal muscle blood flow was significantly reduced by 65% to 75% for both the thigh and calf muscle groups (Fig 3). In the patient in whom the PT pulse was reduced but not entirely eliminated, the degree to which perfusion was reduced in the calf was less than in the group as a whole (18% reduction). Parametric analysis demonstrated that JT inflation produced significant reductions in both functional MBV and microvascular flux rate, with slightly greater reductions in the latter (Fig 3). Cyclical video intensity was sufficiently robust to measure API in the thigh in six subjects and in the calf in seven subjects. The API was significantly lower during JT occlusion compared with baseline (P < .05), and the reduction in API tended to be greater in the calf (8% ± 13% of baseline) than in the thigh (39% ± 43% of baseline).
Fig 2.
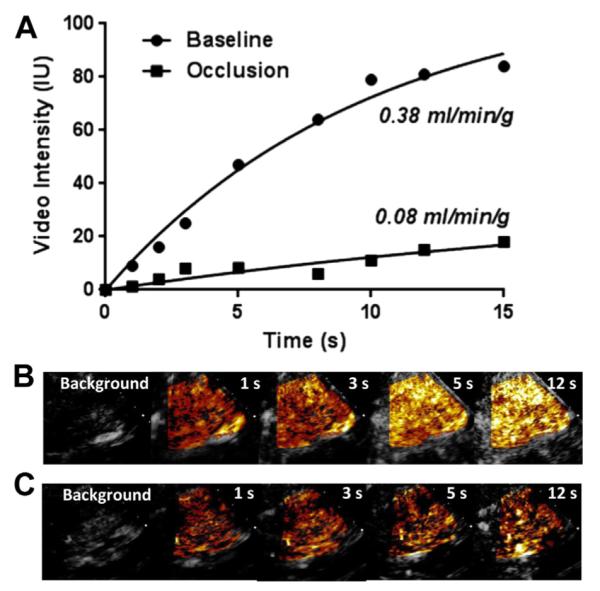
Example of time vs video intensity data (A) and images at increasing time after the destructive pulse before (B) and after (C) tourniquet inflation with contrast-enhanced ultrasound (CEU). Contrast signal is background subtracted and color coded (orange to yellow to white). Time-intensity data illustrate a reduction in both the microvascular flux rate (curve rate constant) and functional blood volume (plateau intensity) with tourniquet occlusion.
Fig 3.
Mean (±standard error of the mean) (A) microvascular blood flow, (B) microvascular blood volume (MBV; a value normalized to blood pool), and (C) microvascular flux rate (β) in thigh and calf skeletal muscle at baseline and after tourniquet occlusion. *P < .05 vs baseline.
DISCUSSION
The development of focal JTs has been driven primarily by the increasing proportion of battlefield injuries in recent military conflicts that involve proximal limb trauma. Traditional tourniquets are limited in their ability to control junctional hemorrhage stemming from injuries proximal to where a circumferential device can be placed. JTs have also been used to treat nonmilitary trauma and to control bleeding in limb surgical procedures. These devices appear to be effective in reducing hemorrhage.4,5,19 Moreover, studies performed in a porcine model have demonstrated complete elimination of distal pulsatile blood flow by Doppler ultrasound and loss of entry of radiographic contrast material into large distal vessels on computed tomography.4
A consideration with all forms of tourniquets is whether it is possible to achieve a balance between complete hemostasis and maintenance of tissue viability with prolonged application. Studies performed in nonhuman primates and humans by a variety of techniques, such as radiolabeled microspheres, thermodiffusion, and impedance plethysmography, have demonstrated that brief application of circumferential tourniquets reduces muscle flow by 80% to 99%.20-22 Little is known about tissue blood flow in the proximal and distal limb after application of a JT. On the basis of vascular anatomic considerations, focal occlusion of the common FA would allow some degree of residual perfusion from pre-existing pelvic and other collaterals arising from the internal iliac and circumflex arterial systems. In other words, hemostasis may be achieved by femoral JTs through reduction in pulsatile pressure in large to medium vessels, yet a small amount of residual flow may prevent necrosis, which, although it is unproved, may lead to better long-term function and wound healing. This notion is supported by the continued viability and even functionality of limbs in patients with infected pseudoaneurysms who undergo complete and permanent ligation of the FA.6 On angiographic evaluation, residual perfusion is likely to occur through collateral pathways originating from the internal iliac artery (inferior gluteal, obturator, inferior epigastric) that may then anastomose with the profunda femoris artery or the circumflex branches.7
In the present study, we used quantitative CEU perfusion imaging to examine tissue blood flow during application of a pneumatic tourniquet designed to control junctional hemorrhage. Advantages of using CEU for assessment of limb perfusion are that it is rapid and safe, provides spatial assessment of perfusion, and has sufficient sensitivity to detect flow even when it is severely reduced.15,23 Limb skeletal muscle perfusion imaging with CEU at rest and hyperemic flow during exercise stress has been demonstrated previously to provide information on the severity of PAD and correlates better with claudication symptoms than ABI or exercise ABI does.8 It has also been used to evaluate the presence of a collateral arterial network in severe PAD.10 In the current study, we found that a JT can completely eliminate large-vessel pulse pressure, yet thigh and calf muscle perfusion are reduced by only 65% to 75%. Although the minimal perfusion necessary to maintain tissue viability in various limb tissues and muscle fiber types is unclear, microsphere studies have suggested that somewhere between 7% and 40% of normal resting flow is sufficient to prevent histologic muscle necrosis irrespective of the duration of ischemia.24 This rather wide range produces uncertainty with regard to the amount of flow needed to maintain viability, which can be defined either histologically (prevention of necrosis in different tissues) or in terms of outcomes (need for amputation). Hence, we cannot say with certainty that the degree of hypoperfusion during JT inflation in our study would not result in necrosis in different tissue beds or that duration would not have an effect on different tissues.
CEU perfusion imaging is based on the destruction of microbubble contrast agents within the volume of ultrasound field and kinetic modeling of the replenishment of microbubbles into the sector volume.16 Data on the rate and extent of signal replenishment when normalized to blood pool can provide parametric assessment of MBV and microvascular flux rate.16 Although CEU has been used to study limb perfusion in models of PAD,15,23 this study represents its first application to study hemostatic devices. Our results suggest that flux rate is influenced more than MBV, arguing against large territories of muscle that are completely devoid of perfusion.
During CEU imaging, frames obtained with rapid pulsing intervals (continuous imaging) at a high mechanical index are used as background to subtract signal from all but the capillary and other small noncapillary microvessels that are characterized by slow blood velocity.14,17 Analysis of the cyclic intensity of frames obtained during continuous imaging provides a measure of the degree to which intramuscular arterial velocity changes from systole to diastole. As predicted by hydrodynamic principles, cyclical intensity will then be linearly related to pulse pressure. Although the API measured from peak systolic and diastolic intensity during continuous high-power imaging could not be measured in all subjects, there was a clear reduction in pulse pressure during JT inflation that was greater for the more distal calf muscle groups.
There are several limitations of this study. The number of subjects was small. The duration of tourniquet occlusion was brief because of our concerns about discomfort and the lack of a priori knowledge that residual perfusion would be present. Accordingly, we are not able to assess whether flow reduction changes over time. Further studies using segmental limb CEU will likely provide insight into spatial-temporal patterns of flow for proximal and distal limb muscle groups. We also used the minimum occlusion pressure necessary to eliminate the PT pulse signal on Doppler ultrasound. Although it is possible that higher inflation pressures during field use, where recommendations are to “inflate to desired effect,” could further reduce blood flow, we do not believe this is the case because our pulsed-wave Doppler data showed complete obliteration of FA flow at the inflation pressures used. We did not compare results of the JT with other forms of tourniquet occlusion during which there are other potential sources for residual flow, such as from intraosseal sources.25 This study was performed in a controlled clinical environment and did not study a hemorrhage control model, which may limit extrapolation to field applications. Although we can verify the presence of sufficient flow to maintain viability, there is no accurate way of extrapolating our CEU data to determine the degree of blood loss that would occur with transection of different thigh or calf muscle groups. Finally, the results of our study were not performed in a situation of hemorrhagic shock, in which peripheral vasoconstriction could influence the degree of muscle perfusion.
CONCLUSIONS
Using quantitative CEU perfusion imaging, we have demonstrated that application of a focal JT device to the common FA resulted in a significant reduction in but not elimination of distal skeletal muscle blood flow despite elimination of pulsatile flow by Doppler ultrasound in both proximal and distal vessels and severe reduction in intramuscular pulse pressure. Application of this tourniquet to occlude FA blood flow did not result in changes in cardiac function or afterload compared with baseline. The results of the study may serve as a foundation for the use of CEU as a rapid noninvasive method to evaluate different types of tourniquet devices and regional heterogeneity of flow during tourniquet occlusion.
Clinical Relevance.
The application of tourniquets to the lower extremity is intended to stem bleeding either from trauma or during surgery. The potential for collateral inflow remains with focal femoral occlusion. Using quantitative contrast-enhanced ultrasound perfusion imaging, we have demonstrated that despite elimination of femoral flow and distal pulses, a small but substantial amount of perfusion remains in skeletal muscle that may be sufficient for maintaining tissue viability. This study provides the first measurements of perfusion during junctional tourniquet application and also introduces the use of contrast-enhanced ultrasound perfusion imaging as a technique to assess regional perfusion in the investigation of mechanical hemostasis.
Acknowledgments
The study was supported by an investigator-initiated grant from SAM Medical Products Inc, Wilsonville, Ore.
Author conflict of interest: B.P.D. is supported by the Clinician Research Program (12CRP11890055) from the American Heart Association. J.R.L. is supported by grants R01-HL-078610 and R01-HL111969 from the National Institutes of Health, Bethesda, Md.
Footnotes
Clinical trial registration: NCT02092415.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
AUTHOR CONTRIBUTIONS Conception and design: BD, JB, GL, JL
Analysis and interpretation: BD, JB, BM, GL, JL
Data collection: BD, JB, BM, JL
Writing the article: BD, JB, BM, GL, JL
Critical revision of the article: BD, JB, BM, GL, JL
Final approval of the article: BD, JB, BM, GL, JL
Statistical analysis: BD, JL
Obtained funding: JL
Overall responsibility: JL
REFERENCES
- 1.Kragh JF, Jr, Walters TJ, Baer DG, Fox CJ, Wade CE, Salinas J, et al. Practical use of emergency tourniquets to stop bleeding in major limb trauma. J Trauma. 2008;64:S38–49. doi: 10.1097/TA.0b013e31816086b1. discussion: S49-50. [DOI] [PubMed] [Google Scholar]
- 2.Welling DR, Burris DG, Hutton JE, Minken SL, Rich NM. A balanced approach to tourniquet use: lessons learned and relearned. J Am Coll Surg. 2006;203:106–15. doi: 10.1016/j.jamcollsurg.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 3.Eastridge BJ, Hardin M, Cantrell J, Oetjen-Gerdes L, Zubko T, Mallak C, et al. Died of wounds on the battlefield: causation and implications for improving combat casualty care. J Trauma. 2011;71:S4–8. doi: 10.1097/TA.0b013e318221147b. [DOI] [PubMed] [Google Scholar]
- 4.Kheirabadi BS, Terrazas IB, Hanson MA, Kragh JF, Jr, Dubick MA, Blackbourne LH. In vivo assessment of the combat ready clamp to control junctional hemorrhage in swine. J Trauma Acute Care Surg. 2013;74:1260–5. doi: 10.1097/TA.0b013e31828cc983. [DOI] [PubMed] [Google Scholar]
- 5.Kragh JF, Jr, Murphy C, Dubick MA, Baer DG, Johnson J, Blackbourne LH. New tourniquet device concepts for battlefield hemorrhage control. US Army Med Dep J. 2011:38–48. [PubMed] [Google Scholar]
- 6.Arora S, Weber MA, Fox CJ, Neville R, Lidor A, Sidawy AN. Common femoral artery ligation and local debridement: a safe treatment for infected femoral artery pseudoaneurysms. J Vasc Surg. 2001;33:990–3. doi: 10.1067/mva.2001.114212. [DOI] [PubMed] [Google Scholar]
- 7.Hu ZJ, Wang SM, Li XX, Li SQ, Huang XL. Tolerable hemodynamic changes after femoral artery ligation for the treatment of infected femoral artery pseudoaneurysm. Ann Vasc Surg. 2010;24:212–8. doi: 10.1016/j.avsg.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Lindner JR, Womack L, Barrett EJ, Weltman J, Price W, Harthun NL, et al. Limb stress-rest perfusion imaging with contrast ultrasound for the assessment of peripheral arterial disease severity. JACC Cardiovasc Imaging. 2008;1:343–50. doi: 10.1016/j.jcmg.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Womack L, Peters D, Barrett EJ, Kaul S, Price W, Lindner JR. Abnormal skeletal muscle capillary recruitment during exercise in patients with type 2 diabetes mellitus and microvascular complications. J Am Coll Cardiol. 2009;53:2175–83. doi: 10.1016/j.jacc.2009.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duerschmied D, Zhou Q, Rink E, Harder D, Freund G, Olschewski M, et al. Simplified contrast ultrasound accurately reveals muscle perfusion deficits and reflects collateralization in PAD. Atherosclerosis. 2009;202:505–12. doi: 10.1016/j.atherosclerosis.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 11.Coggins M, Lindner J, Rattigan S, Jahn L, Fasy E, Kaul S, et al. Physiologic hyperinsulinemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes. 2001;50:2682–90. doi: 10.2337/diabetes.50.12.2682. [DOI] [PubMed] [Google Scholar]
- 12.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Hundley WG, Kizilbash AM, Afridi I, Franco F, Peshock RM, Grayburn PA. Administration of an intravenous perfluorocarbon contrast agent improves echocardiographic determination of left ventricular volumes and ejection fraction: comparison with cine magnetic resonance imaging. J Am Coll Cardiol. 1998;32:1426–32. doi: 10.1016/s0735-1097(98)00409-4. [DOI] [PubMed] [Google Scholar]
- 14.Dawson D, Vincent MA, Barrett EJ, Kaul S, Clark A, Leong-Poi H, et al. Vascular recruitment in skeletal muscle during exercise and hyperinsulinemia assessed by contrast ultrasound. Am J Physiol Endocrinol Metab. 2002;282:E714–20. doi: 10.1152/ajpendo.00373.2001. [DOI] [PubMed] [Google Scholar]
- 15.Ryu JC, Davidson BP, Xie A, Qi Y, Zha D, Belcik JT, et al. Molecular imaging of the paracrine proangiogenic effects of progenitor cell therapy in limb ischemia. Circulation. 2013;127:710–9. doi: 10.1161/CIRCULATIONAHA.112.116103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation. 1998;97:473–83. doi: 10.1161/01.cir.97.5.473. [DOI] [PubMed] [Google Scholar]
- 17.Pascotto M, Leong-Poi H, Kaufmann B, Allrogen A, Charalampidis D, Kerut EK, et al. Assessment of ischemia-induced microvascular remodeling using contrast-enhanced ultrasound vascular anatomic mapping. J Am Soc Echocardiogr. 2007;20:1100–8. doi: 10.1016/j.echo.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Pascotto M, Wei K, Micari A, Bragadeesh T, Goodman NC, Kaul S. Phasic changes in arterial blood volume is influenced by collateral blood flow: implications for the quantification of coronary stenosis at rest. Heart. 2007;93:438–43. doi: 10.1136/hrt.2006.089631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mann-Salinas EA, Kragh JF, Jr, Dubick MA, Baer DG, Blackbourne LH. Assessment of users to control simulated junctional hemorrhage with the combat ready clamp (CRoC) Int J Burns Trauma. 2013;3:49–54. [PMC free article] [PubMed] [Google Scholar]
- 20.Zapletal C, Herzog L, Martin G, Klar E, Meeder PJ, Buchholz J. Thermodiffusion for the quantification of tissue perfusion in skeletal muscle—clinical evaluation in standardized traumatological procedures with tourniquet and potential application in the diagnosis of compartment syndrome. Microvasc Res. 2003;66:164–72. doi: 10.1016/s0026-2862(03)00043-8. [DOI] [PubMed] [Google Scholar]
- 21.Klenerman L, Crawley J. Limb blood flow in the presence of a tour-niquet. Acta Orthop Scand. 1977;48:291–5. doi: 10.3109/17453677708988771. [DOI] [PubMed] [Google Scholar]
- 22.Wenke JC, Walters TJ, Greydanus DJ, Pusateri AE, Convertino VA. Physiological evaluation of the U.S. Army one-handed tourniquet. Mil Med. 2005;170:776–81. doi: 10.7205/milmed.170.9.776. [DOI] [PubMed] [Google Scholar]
- 23.Carr CL, Qi Y, Davidson B, Chadderdon S, Jayaweera AR, Belcik JT, et al. Dysregulated selectin expression and monocyte recruitment during ischemia-related vascular remodeling in diabetes mellitus. Arterioscler Thromb Vasc Biol. 2011;31:2526–33. doi: 10.1161/ATVBAHA.111.230177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrasek PF, Homer-Vanniasinkam S, Walker PM. Determinants of ischemic injury to skeletal muscle. J Vasc Surg. 1994;19:623–31. doi: 10.1016/s0741-5214(94)70035-4. [DOI] [PubMed] [Google Scholar]
- 25.Blond L, Madsen JL. Bone marrow perfusion in healthy subjects assessed by scintigraphy after application of a tourniquet. Acta Orthop Scand. 2003;74:460–4. doi: 10.1080/00016470310017794. [DOI] [PubMed] [Google Scholar]