Abstract
We evaluated the impact of body mass index (BMI) and lifestyle risk factors on ethnic disparity in diabetes incidence among 89,198 Asian, Native Hawaiian, and white participants of the Multiethnic Cohort who completed multiple questionnaires. After 12 years of follow-up, 11,218 new cases were identified through self-report and health plan linkages. BMI was lowest in Chinese/Koreans, Japanese, and Filipinos (22.4, 23.5, 23.9 kg/m2). Using Cox regression, the unadjusted hazard ratios were 1.9 (Chinese/Korean), 2.1 (Japanese, Mixed-Asian), 2.2 (Filipino), 2.5 (Native Hawaiian), and 2.6 (Part-Asian) as compared to whites. With BMI added, the risk for Japanese, Filipinos, Chinese/Koreans, and Mixed-Asians increased (8–42%) but declined in Part-Asians and Native Hawaiians (17–31%). When lifestyle and dietary factors were also included, the risk was attenuated in all groups (6–14%). Despite their lower BMI, Asian Americans have a higher diabetes risk than whites, but dietary and lifestyle factors do not account for the excess risk.
Keywords: Type 2 diabetes mellitus, ethnicity, incidence, disparity, risk factors, Japanese, Filipinos, Native Hawaiians
Introduction
The number of people with type 2 diabetes is rising across the world, but the disease risk is considerably higher in Asians and Pacific Islanders than in whites.1;2 Recent estimates of diabetes prevalence in Japan, Philippines, China, and Korea were 5.1%, 6.9%, 9.0%, and 7.5%, respectively. In the United States, the National Health Interview Survey reported 20–40% higher rates of type 2 diabetes in Asian Americans than whites after adjustment for body mass index (BMI).3 Whereas high rates of obesity among Native Hawaiians and other Pacific Islanders appear to account for their elevated risk,4;5 a high prevalence of diabetes has been reported for Asian Americans at relatively low levels of BMI.6–9 Not only are incidence data on type 2 diabetes limited due to the lack of population-based registries, but the use of aggregated ethnicity categories has made it difficult to distinguish specific Asian groups.9;10 Given the serious complications due to diabetes in some ethnic groups, disaggregation is important to identify individuals at high risk for screening and/or treatment.11;12
While elevated incidence rates are well documented for Japanese and Native Hawaiians,6;7;13;14 only one cohort has examined risk prospectively in other Asian American groups.2 A cohort consisting of Kaiser Permanente Northern California members detected the highest diabetes incidence in Koreans, Pacific Islanders, and Filipinos (20.3, 19.9, and 14.7 cases per 1,000 person-years, respectively), while the rates for multiracial individuals, Japanese, Chinese, and whites were 9.7, 7.5, 6.5, and 6.3 per 1,000 person-years, respectively. Individuals with multiracial/ethnic admixture also appear to have a higher prevalence of obesity and overweight than those with one of the individual constituent ethnicities.15 To estimate annual incidence rates for type 2 diabetes in Filipinos, Chinese/Koreans, and Part-Asians, we used data from the Hawaii component of the Multiethnic Cohort (MEC)16 with close to 100,000 individuals whose diabetes status was established through follow-up questionnaires and linkages with health plans.7 In addition, we evaluated the impact of excess weight, as well as of other lifestyle and dietary factors previously linked to diabetes risk, on ethnic differences in diabetes risk.
Methods
Study Population and Data Collection
The MEC was established in 1993 through 1996 to study diet and cancer among different ethnic groups in Hawaii and California.16 Although whites, Japanese, and Native Hawaiians were targeted for recruitment in Hawaii, a substantial number of cohort members reported other Asian backgrounds. A comparison of white, Japanese, and Native Hawaiian cohort members with census data indicated that the MEC represents all levels of education although cohort members were somewhat better educated than the general population.16 Subjects entered the cohort by completing a 26-page, self-administered mailed survey that asked about demographic background, medical conditions, anthropometric measures, diet, and lifestyle factors. The participants marked all ethnic backgrounds (Black, Chinese, Filipino, Hawaiian, Japanese, Korean, Hispanic, white, and other) that applied to them. Dietary history was assessed using a food frequency questionnaire (FFQ) that has been described in detail elsewhere.17 In brief, the FFQ included 8 frequency categories for foods and 9 for beverages. Portion sizes were selected from 3 or 4 options. To define the specific performance of the FFQ in the 5 targeted ethnic groups, a calibration study using 24-hour dietary recalls was performed and showed acceptable values.17 Nutrient intake was determined using a customized ethnicity-specific food composition database based on the US Department of Agriculture and additional laboratory analyses performed in Hawaii.16
Identification of Diabetes Cases
Of the original 103,898 members within the Hawaii component of the MEC, 10,028 (9.7%) who reported a diagnosis of diabetes at baseline and 10 subjects with missing information were excluded. Since the MEC was established, annual linkages with state death certificate files have been performed to update vital status. Information on diabetes status was available at three time points, i.e., from two questionnaires and a linkage with health plans. Between February 1999 and September 2003, a short follow-up questionnaire completed by 84% of cohort members provided updated information on medical conditions, including diabetes. A medication questionnaire administered between 2003 and 2006 collected data on diabetes drugs for 38% of the 103,898 subjects.
As reported in detail previously,7 records for MEC members alive in 2007 were linked to the diabetes care registries of the two major insurers in Hawaii. Both health plans used specific algorithms based on multiple claims for diabetes-related treatment services to identify cases. Linkage of the MEC database was performed through probability matching of last and first name, middle initial, birth month and year, and sex for one health plan and by matching social security numbers, sex, and birthdates for the other plan. Individuals who indicated having diabetes at any point after baseline or who were classified as cases by one of the health plans were considered incident cases. Individuals who never reported diabetes and who were not identified as diabetic patients by the health plans were categorized as non-cases. The study was approved by the Institutional Review Boards of the University of Hawaii and Kaiser Permanente and conducted in accordance with the principles of the Declaration of Helsinki as revised in 2008.
For incident cases identified by one of the health plans, diagnosis dates were known starting in 2000; all those diagnosed before were assigned December 1, 1999. For cases from the other health plan, the date of diagnosis was assumed as July 1 of the diagnosis year and as July 1, 2007 for 120 cases with missing dates. Unless a date was provided by the health plan, we estimated diagnosis dates by computing the midpoint in time between the last report of not having diabetes and the first indication of diabetes. For incident cases, the follow-up time was calculated as the time between cohort entry and the estimated diagnosis date. For non-cases, the follow-up time was calculated as the time between the date of the baseline questionnaire and any of the following events if applicable: the date of death, the last date when data on diabetes status was available, i.e., the date of the questionnaire or health plan linkage.
Statistical analysis
In contrast to previous reports that used four ethnic categories,7 we created a more detailed ethnic classification for the current analysis. Individuals who only reported Chinese, Korean, Japanese, or Filipino ancestry were categorized as such. Chinese (N=1173) and Koreans (N=459) were combined due to the small numbers and the similar crude incidence rates of 12.8% and 11.1%. Persons who reported more than one Asian background were classified as Mixed-Asian and those with Chinese, Korean, Japanese, or Filipino background plus any non-Asian ancestry as Part-Asians. Next, anyone who reported Hawaiian ancestry was classified as such and then the same was done for whites. All remaining individuals, primarily African Americans and Latinos, were grouped as Other. Differences in baseline characteristics by ethnic group were tested by ANOVA for continuous and by χ2-tests for categorical variables.
The average annual incidence rates were computed by ethnic group as the sum of the number of newly diagnosed diabetes cases divided by the sum of person-years of follow-up and age-standardized to the total study population by the direct method. Cox proportional regression using PROC PHREG in the SAS software package, version 9.2 (SAS Institute, Inc., Cary, NC) was applied to estimate diabetes risk by ethnic group with whites as the reference group. To capture sex-specific associations, separate models for men and women were completed. First, we calculated hazard ratios (HR) and 95% confidence intervals (CI) using models stratified by age at cohort entry (continuous) and adjusted for sex and years of education (<12, 12, 13–15, and ≥16 years). The second model also included BMI (<22.5, 22.5–<25, 25–<30, and ≥30 kg/m2). In a third model, we further adjusted for all variables that were previously found to be associated with diabetes risk in this dataset, i.e., smoking status and physical activity, as well as intake of total energy, alcohol, red meat, dietary fiber, regular sodas, and coffee. Daily intakes of dietary fiber and red meat were converted to energy density values (per 4,184 kJ equivalent to 1,000 kcal);18 logarithmic transformations were applied to these dietary variables. For alcohol, coffee, and soda consumption, categorical variables were created (Table 1). To assess the importance of overweight/obesity and of all other covariates, the percent change in HR from model 1 to 2 and model 2 to 3 was computed and plotted by sex and ethnic group. As sensitivity analysis, the final model was repeated excluding cases with uncertain diagnosis date.
Table 1.
Composition of the Hawaii component of the Multiethnic Cohort study at baselinea
| White | Japanese | Filipino | Chinese/Korean | Mixed-Asian | Part-Asian | Native Hawaiian | Other | All | |
|---|---|---|---|---|---|---|---|---|---|
| Number | 31564 | 36712 | 3871 | 1632 | 694 | 8480 | 4772 | 1473 | 89198 |
| Male (%) | 50.6 | 46.9 | 48.5 | 11.3 | 38.9 | 41.5 | 44.3 | 53.5 | 47.0 |
| Incidence (cases/1,000 pyb) | 9.9 | 21.1 | 21.3 | 17.9 | 16.3 | 24.9 | 22.8 | 19.2 | 10.6 |
| Age at cohort entry (years) | 56 (16) | 62 (16) | 57 (14) | 57 (17) | 51 (9) | 53 (13) | 57 (15) | 54 (13) | 58 (17) |
| Physical activity (METc) | 1.61 (0.36) | 1.62 (0.34) | 1.73 (0.35) | 1.59 (0.35) | 1.59 (0.39) | 1.61 (0.40) | 1.64 (0.38) | 1.62 (0.40) | 1.62 (0.36) |
| Body mass index (kg/m2) | 24.6 (5.28) | 23.5 (4.61) | 23.9 (4.25) | 22.4 (4.27) | 24.4 (5.52) | 26.1 (6.24 | 27.7 (7.34) | 26.0 (6.02) | 24.3 (5.05) |
| Education (years) | |||||||||
| <12 | 4.5 | 10.1 | 26.6 | 9.4 | 5.8 | 8.5 | 14.0 | 18.3 | 9.0 |
| 12 | 17.4 | 32.2 | 25.2 | 29.4 | 28.1 | 38.6 | 42.0 | 26.3 | 27.6 |
| 13–15 | 31.4 | 27.5 | 24.6 | 23.3 | 34.3 | 31.7 | 28.2 | 28.5 | 29.2 |
| ≥16 | 46.7 | 30.2 | 23.6 | 37.9 | 31.8 | 21.2 | 15.8 | 26.9 | 34.2 |
| Smoking status | |||||||||
| Never | 38.2 | 51.7 | 51.5 | 62.7 | 47.1 | 42.1 | 37.1 | 46.2 | 45.3 |
| Past | 45.4 | 36.5 | 33.5 | 24.3 | 34.2 | 36.1 | 38.6 | 34.8 | 39.3 |
| Current | 16.4 | 11.8 | 15.0 | 13.0 | 18.7 | 21.8 | 24.3 | 19.0 | 15.4 |
| Total energy (kJ) | 8222 (4435) | 8071 (4255) | 9347 (6100) | 7176 (4134) | 8724 (4996) | 9230 (6100) | 9678 (6636) | 8678 (6100) | 8322 (4665) |
| Red meat (g/4184 kJ/day) | 20.9 (20.8) | 23.7 (19.6) | 24.3 (19.99) | 24.4 (21.5) | 28.7 (20.4) | 28.6 (20.8) | 27.1 (21.2) | 25.2 (23.8) | 23.5 (20.7) |
| Dietary fiber (g/4184 kJ/day) | 11.2 (5.6) | 10.16 (5.5) | 9.75 (5.3) | 10.9 (5.42) | 8.88 (5.0) | 9.15 (5.1) | 9.55 (5.4) | 10.57 (5.7) | 10.4 (5.5) |
| Alcohol (drinks) | |||||||||
| <1/month | 32.8 | 64.5 | 62.8 | 73.0 | 62.0 | 55.4 | 49.7 | 50.0 | 51.4 |
| 1/month –<1/day | 34.9 | 22.1 | 26.6 | 21.3 | 25.6 | 28.9 | 30.4 | 31.7 | 28.1 |
| >1/day | 32.3 | 13.4 | 10.6 | 5.7 | 12.4 | 15.7 | 19.9 | 18.3 | 20.5 |
| Coffee (cups/day) | |||||||||
| 0–<1 | 16.0 | 18.8 | 21.8 | 22.8 | 20.2 | 22.5 | 20.8 | 20.8 | 18.5 |
| 1–2 | 28.0 | 35.3 | 33.8 | 32.7 | 29.4 | 28.7 | 29.8 | 28.3 | 31.5 |
| 2–3 | 27.3 | 24.0 | 20.9 | 19.0 | 21.3 | 20.6 | 20.0 | 21.0 | 24.3 |
| ≥3 | 11.8 | 7.46 | 7.8 | 4.7 | 7.6 | 7.7 | 7.67 | 8.3 | 9.0 |
| Regular sodas | |||||||||
| None | 53.2 | 37.1 | 23.7 | 39.6 | 30.4 | 32.5 | 35.2 | 37.7 | 41.7 |
| <1/week | 23.1 | 36.7 | 35.3 | 38.9 | 35.2 | 31.6 | 29.3 | 29.6 | 30.9 |
| >1/week | 23.7 | 26.2 | 41.0 | 21.5 | 34.4 | 35.9 | 35.5 | 32.7 | 27.4 |
For all categorical variables, percentages are shown; for continuous variables, medians with interquartile ranges are presented
Person-years
Metabolic equivalent of task
Results
After excluding subjects with prevalent diabetes or missing data at baseline, 89,198 cohort members were part of this analysis (Table 1). After 12 years of follow-up, 11,218 incident cases were identified. Whites and Japanese constituted the largest groups with >30,000 persons each. The respective numbers for Filipinos and Chinese/Koreans were 3,871 and 1,632. In addition, 694 Mixed-Asians, 8,480 Part-Asians, and 4,772 Native Hawaiians were included. The median age at cohort entry was close to 60 years. The ethnic groups within the Hawaii component of the MEC differed in several ways (p<0.0001 for all baseline characteristics except physical activity). Mean BMI was lowest in Chinese/Koreans, Japanese, and Filipinos (22.4, 23.5, 23.9 kg/m2) and higher in Mixed-Asians, whites, Part-Asians, and Native Hawaiians (24.4, 24.6, 26.1, 27.7 kg/m2). In comparison to other ethnic groups, whites reported higher alcohol and coffee and lower soda intake. The overall diabetes incidence was 10.6 per 1,000 person-years with a higher rate for men than women (11.9 vs. 9.4). Part-Asians had the highest age-adjusted incidence rate at 24.9, followed by Native Hawaiians at 22.8, Filipinos at 21.3, Japanese at 21.1, Mixed-Asians at 16.3, while whites were lowest at 9.9 cases per 1,000 person-years.
The basic Cox regression models stratified by age at cohort entry and adjusted for sex and years of education showed a similar trend as the incidence rates (Table 2). In model 1, the relative risk for Part-Asians and Native Hawaiians were 2.64 and 2.47 in comparison to whites. The respective HRs for Filipinos, Japanese, Chinese/Koreans, and Mixed-Asians were 2.24, 2.11, 1.94, and 2.07. When BMI was included in the model, the risk estimates for Japanese, Filipinos, Chinese/Koreans and Mixed-Asians increased by 28, 23, 42 and 8% but declined in Part-Asians and Native Hawaiians by 17 and 31%. Taking into account all dietary and lifestyle factors attenuated the risk across ethnic groups between 6–14%. In model 3, the HRs for Japanese, Filipinos, and Chinese/Koreans were 2.42, 2.48, and 2.40, whereas Mixed and Part-Asians had HRs of 1.94. Native Hawaiians had the lowest HR (1.56) as compared to whites. Excluding cases with uncertain diagnosis date did not substantially change the relative comparison of all ethnic groups with whites despite a slight attenuation of the risk estimates.
Table 2.
Risk of developing type 2 diabetes by ethnic group and sex, Hawaii component of the Multiethnic Cohort
| Sex | Ethnicity | Number | Model 1a | Model 2b | Model 3c | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Cases | Non-cases | HR | 95% CI | HR | 95% CI | HR | 95% CI | |||||
| Both | White | 2231 | 29333 | Ref | Ref | Ref | ||||||
| Japanese | 5522 | 31190 | 2.11 | 2.01 | 2.22 | 2.71 | 2.57 | 2.85 | 2.42 | 2.29 | 2.55 | |
| Filipino | 634 | 3237 | 2.24 | 2.04 | 2.45 | 2.76 | 2.52 | 3.02 | 2.48 | 2.26 | 2.72 | |
| Chinese/Korean | 201 | 1431 | 1.94 | 1.68 | 2.24 | 2.76 | 2.39 | 3.20 | 2.40 | 2.07 | 2.78 | |
| Mixed-Asian | 103 | 591 | 2.07 | 1.70 | 2.52 | 2.23 | 1.83 | 2.72 | 1.94 | 1.59 | 2.36 | |
| Part-Asian | 1529 | 6951 | 2.64 | 2.46 | 2.82 | 2.18 | 2.04 | 2.33 | 1.94 | 1.81 | 2.08 | |
| Native Hawaiian | 799 | 3973 | 2.47 | 2.27 | 2.68 | 1.72 | 1.58 | 1.87 | 1.58 | 1.45 | 1.72 | |
| Other | 199 | 1274 | 1.85 | 1.60 | 2.14 | 1.60 | 1.38 | 1.85 | 1.50 | 1.30 | 1.74 | |
|
| ||||||||||||
| Men | White | 1316 | 14647 | Ref | Ref | Ref | ||||||
| Japanese | 2913 | 14296 | 2.04 | 1.91 | 2.18 | 2.48 | 2.32 | 2.65 | 2.28 | 2.13 | 2.44 | |
| Filipino | 319 | 1560 | 1.93 | 1.70 | 2.19 | 2.42 | 2.14 | 2.75 | 2.26 | 1.99 | 2.57 | |
| Chinese/Korean | 25 | 159 | 1.76 | 1.19 | 2.62 | 2.48 | 1.67 | 3.68 | 2.19 | 1.47 | 3.25 | |
| Mixed-Asian | 49 | 221 | 2.06 | 1.55 | 2.74 | 2.24 | 1.68 | 2.98 | 2.00 | 1.50 | 2.67 | |
| Part-Asian | 692 | 2829 | 2.36 | 2.15 | 2.59 | 1.97 | 1.79 | 2.17 | 1.83 | 1.66 | 2.02 | |
| Native Hawaiian | 357 | 1757 | 2.08 | 1.85 | 2.35 | 1.50 | 1.33 | 1.69 | 1.44 | 1.27 | 1.62 | |
| Other | 120 | 668 | 1.78 | 1.48 | 2.15 | 1.57 | 1.30 | 1.90 | 1.52 | 1.26 | 1.83 | |
|
| ||||||||||||
| Women | White | 915 | 14686 | Ref | Ref | Ref | ||||||
| Japanese | 2609 | 16894 | 2.22 | 2.05 | 2.39 | 3.03 | 2.80 | 3.27 | 2.53 | 2.33 | 2.76 | |
| Filipino | 315 | 1677 | 2.68 | 2.35 | 3.05 | 3.21 | 2.82 | 3.66 | 2.67 | 2.34 | 3.06 | |
| Chinese/Korean | 176 | 1272 | 2.10 | 1.78 | 2.47 | 3.04 | 2.58 | 3.58 | 2.50 | 2.12 | 2.95 | |
| Mixed-Asian | 54 | 370 | 2.14 | 1.62 | 2.82 | 2.31 | 1.75 | 3.04 | 1.88 | 1.42 | 2.48 | |
| Part-Asian | 837 | 4122 | 2.98 | 2.71 | 3.28 | 2.45 | 2.22 | 2.70 | 2.06 | 1.86 | 2.28 | |
| Native Hawaiian | 442 | 2216 | 2.93 | 2.61 | 3.29 | 1.99 | 1.77 | 2.23 | 1.73 | 1.53 | 1.95 | |
| Other | 79 | 606 | 1.93 | 1.53 | 2.43 | 1.63 | 1.29 | 2.05 | 1.48 | 1.18 | 1.87 | |
Hazard ratios (HR) and 95% confidence intervals (95% CI) obtained by Cox regression stratified by age and adjusted for sex and education
Model 1 plus adjustment for BMI
Model 2 plus adjustment for smoking status, physical activity, intake of total energy, alcohol, red meat, dietary fiber, regular sodas, and coffee
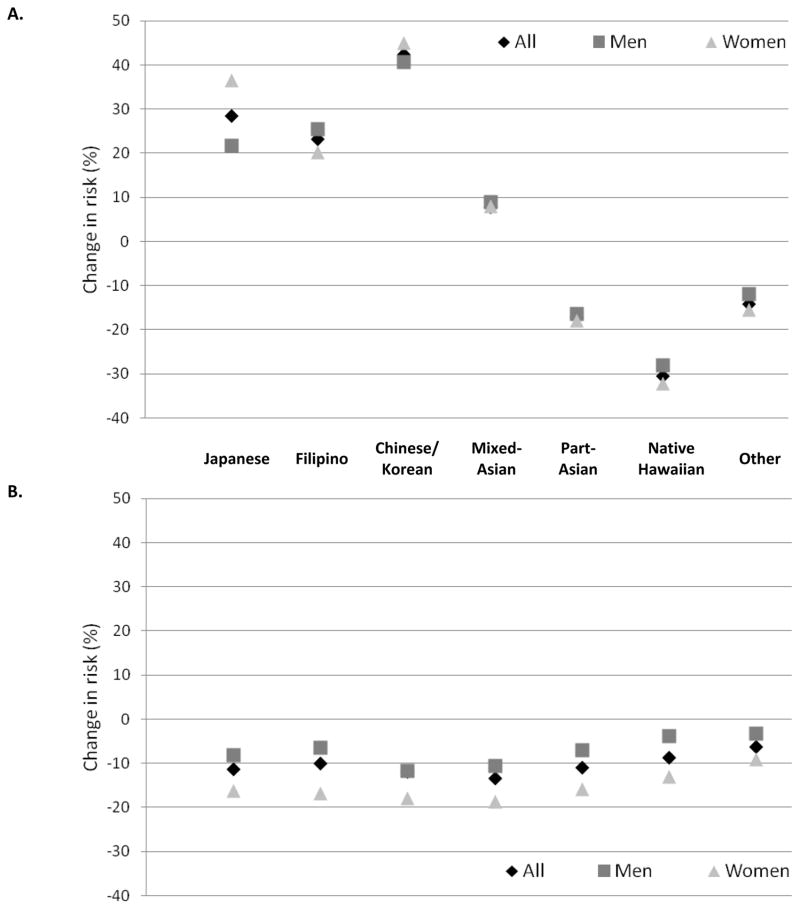
With very few exceptions, the risk estimates in the sex-specific models 1–3 (Table 2) were higher in women than men despite their 14% lower overall risk in the fully adjusted model (HR=0.86; 95% CI: 0.83–0.90). For example in model 3, the respective HRs for Japanese, Filipino, Chinese/Korean, and Native Hawaiian women were 2.53, 2.67, 2.50, and 1.73, whereas the values for men were 2.28, 2.26, 2.19, and 1.44. The change in risk estimates from model 1 to 2 was similar in men and women (Figure 1A). However, women experienced a stronger reduction of relative risk than men (9–19% vs. 3–12%) when model 3 was compared to model 2 (Figure 1B).
Figure 1.
Change in diabetes risk (% of hazard ratio) by ethnic group after adjustment for BMI (A) and dietary and lifestyle risk factors including smoking status, physical activity, intake of total energy, red meat, alcohol, dietary fiber, regular sodas, and coffee (B). All models are adjusted for age and education.
Discussion
This prospective analysis showed that, for individuals who reported a single Asian ancestry, diabetes incidence was similar for Japanese and Filipinos and only slightly lower in Chinese/Koreans. After the inclusion of all available predictors, the risk was approximately 2.4 in these single ancestry groups compared to whites, while for Part- and Mixed-Asians it was 1.9. Interestingly, the unadjusted risk estimates increased substantially for all Asians when BMI was added to the models but decreased for Part-Asians and Native Hawaiians, the two groups with the highest mean BMI. The finding of a higher type 2 diabetes risk independent of BMI across Asian groups agrees with numerous reports.2;9;19;20 Compared to BMI, further adjustment for dietary and lifestyle covariates affected the relative risks only to a small degree (6.4–13.5%) and in the same direction across ethnic groups. In addition to providing incidence data across ethnic groups using the same data sources, the novelty of the current analysis is the finding that the many available dietary lifestyle factors did not account for the ethnic disparity in risk as indicated by the elevated risk estimates for all ethnic groups in the fully-adjusted models. The relatively weak contribution of dietary and lifestyle factors in comparison to BMI agrees with a previous analysis of attributable risk in this cohort.21 Although women experienced a 14% lower diabetes risk, a higher proportion of the ethnic difference was explained by dietary and lifestyle factors in women.
The high diabetes risk in Japanese had been shown previously in the Honolulu Heart Program,6 Japanese migrants living in Hawaii and Los Angeles14 and in Washington state,14 and in the MEC.7 The prevalence in the Philippines is 10% at this time1 but appears to be increasing fast.22;23 The high incidence in Filipinos agrees with the only previous prospective study that reported a 3-fold higher risk as compared to whites.2 Prevalences between 23, 524, 68, 1625, and 2126 were reported for Filipino Americans with moderate BMI levels; the variations may be due to time of immigration and acculturation status.9 Whereas China is now the country with the highest number of diabetes patients in the world,1 reports on Chinese Americans vary.9 In the California incidence study Chinese had a 1.8-fold higher risk than whites,2 several prevalence studies showed similar risks,3;24 and a Canadian report found no elevated prevalence.27 Incidence2 and prevalence28 rates in Korean Americans was comparable to those for Chinese Americans although the prevalence in Korea still appears to be lower than in China.1
An investigation into percentage of Hawaiian ancestry and ethnic admixture reported that Part-Hawaiians of predominantly Asian ancestry had higher 2-hour glucose concentrations than full-Hawaiians, whereas part-Hawaiians with non-Asian admixtures had lower levels.29 This agrees with the current relative risk of only 1.58 for Native Hawaiians after re-classifying those with any Asian ancestry as Part-Asian, whereas anyone with any Native Hawaiian ancestry had a risk of 1.93.7 Asian admixture may augment diabetes risk in Native Hawaiians beyond the contribution of obesity. This important but complex factor may have obscured the relation between ancestry and diabetes susceptibility in previous reports.29
The limitations of the current analysis include the fact that only Japanese and Native Hawaiians were targeted for recruitment into the MEC.16 Therefore, participation rates could not be computed for Filipinos and Chinese/Koreans. More importantly, the small sample may not represent the population of these groups in Hawaii and California. The same is true for the FFQ, which was developed for the 5 targeted ethnic groups in Hawaii and Los Angeles, i.e., whites, Japanese, Native Hawaiians, African Americans, and Latinos, and may be missing foods commonly consumed by other Asian groups.17 Although we classified all participants who reported Native Hawaiian and at least one Asian ancestry as Part-Asian, we cannot be certain about all admixtures for persons in the Native Hawaiian category. For historic and political reasons, such individuals may not be able or willing to report all other ethnic contributions in their ancestors. The validity of the diabetes diagnosis obtained through different methods varied and the exact date of diagnosis was not known for all cases.7 Although no data documenting the quality of the diabetes care registries for Hawaii are available, validation studies of similar registries in other locations have shown that care registries are highly specific and adequately sensitive.30 Self-reported diabetes diagnoses were found to be valid in other studies.31 Although of great interest because of the rising diabetes burden, data for other Asian and Pacific Islander groups were not available in our dataset.1
The population-based, large multiethnic cohort and a large variation in diabetes risk across groups are strengths of this report. During a follow-up period of nearly 12 years, we identified a large number of new diabetes cases across ethnic groups including more than 600 cases in Filipinos. This group has largely been ignored despite being one of the fastest growing immigrant groups in the United States and there is an urgent need to examine health issues among this population. Despite some uncertainties in ethnicity reporting and diagnosis status, the validity of the incidence rates should be high since they are based on numerators and denominators from one cohort.
The proposed biologic mechanisms for the high susceptibility of Asians to type 2 diabetes are complex.32;33 A central role of visceral adipose tissue in lipid and glucose metabolism due to the production of various hormones and cytokines appears likely.34 Since visceral fat has a stronger association with insulin resistance and type 2 diabetes mellitus than subcutaneous fat, the higher proportion of abdominal visceral fat relative to subcutaneous adipose tissue among Asian Americans than whites may account for the adverse effects of excess body weight32;34 although in our cohort measures of abdominal adiposity did not show a stronger association with diabetes than BMI.35 In addition, a recent summary of published report concluded that insulin sensitivity in East Asians was higher and insulin response was lower than in whites.33 Because the stabilization point in the hyperbolic relation to maintain the normal blood glucose levels appears to be located around an unstable extreme point for East Asians, i.e. high insulin sensitivity and relatively low insulin response, even a small change in insulin secretory function may lead to a rapid nonlinear decrease in the threshold level of insulin resistance above which diabetes occurs. As a result of the high insulin sensitivity in Asians, a limited capacity for insulin secretion may have evolved over time and this low β-cell function may contribute to the high diabetes risk in persons of Asian ancestry.32;33
The current analysis revealed that Filipinos and Chinese/Koreans experience an equally high risk for type 2 diabetes as Japanese despite their low rates of overweight and obesity. Multiracial individuals with any Asian ancestry also have elevated diabetes incidence rates, whereas a large proportion of the excess diabetes risk in Native Hawaiians is the result of overweight and obesity. BMI status, dietary and lifestyle factors did not account for the ethnic disparity in diabetes risk. Therefore, the role of biologic differences in body fat distribution and insulin secretion needs to be elucidated in more detail to find out how to protect Asians against this excessive risk to develop diabetes while promoting public health measures that prevent further increases in obesity with all its health consequences at the same time.36
Acknowledgments
Funding The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health [R37 CA54281 to L.N.K. and R21 DK073816 to G.M.].
Footnotes
Declaration of Conflicting Interests The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.International Diabetes Federation. [Accessed February 6, 2014];IDF Diabetes Atlas. 2013 www.idf.org/diabetesatlas[serial online]
- 2.Karter AJ, Schillinger D, Adams AS, et al. Elevated rates of diabetes in Pacific Islanders and Asian subgroups: The Diabetes Study of Northern California (DISTANCE) Diabetes Care. 2013;36:574–579. doi: 10.2337/dc12-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JW, Brancati FL, Yeh HC. Trends in the prevalence of type 2 diabetes in Asians versus whites: results from the United States National Health Interview Survey, 1997–2008. Diabetes Care. 2011;34:353–357. doi: 10.2337/dc10-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grandinetti A, Kaholokula JK, Theriault AG, Mor JM, Chang HK, Waslien C. Prevalence of diabetes and glucose intolerance in an ethnically diverse rural community of Hawaii. Ethn Dis. 2007;17:250–255. [PubMed] [Google Scholar]
- 5.Davis J, Busch J, Hammatt Z, et al. The relationship between ethnicity and obesity in Asian and Pacific Islander populations: a literature review. Ethn Dis. 2004;14:111–118. [PubMed] [Google Scholar]
- 6.Burchfiel CM, Curb JD, Rodriguez BL, et al. Incidence and predictors of diabetes in Japanese-American men. The Honolulu Heart Program. Ann Epidemiol. 1995;5:33–43. doi: 10.1016/1047-2797(94)00038-u. [DOI] [PubMed] [Google Scholar]
- 7.Maskarinec G, Erber E, Grandinetti A, et al. Diabetes incidence based on linkages with health plans: the multiethnic cohort. Diabetes. 2009;58:1732–1738. doi: 10.2337/db08-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Araneta MR, Wingard DL, Barrett-Connor E. Type 2 diabetes and metabolic syndrome in Filipina-American women: a high-risk nonobese population. Diabetes Care. 2002;25:494–499. doi: 10.2337/diacare.25.3.494. [DOI] [PubMed] [Google Scholar]
- 9.Staimez LR, Weber MB, Narayan KM, Oza-Frank R. A systematic review of overweight, obesity, and type 2 diabetes among Asian American subgroups. Curr Diabetes Rev. 2013;9:312–331. doi: 10.2174/15733998113099990061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNeely MJ, Boyko EJ. Type 2 diabetes prevalence in Asian Americans: results of a national health survey. Diabetes Care. 2004;27:66–69. doi: 10.2337/diacare.27.1.66. [DOI] [PubMed] [Google Scholar]
- 11.King GL, McNeely MJ, Thorpe LE, et al. Understanding and addressing unique needs of diabetes in Asian Americans, native Hawaiians, and Pacific Islanders. Diabetes Care. 2012;35:1181–1188. doi: 10.2337/dc12-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanaya AM, Adler N, Moffet HH, et al. Heterogeneity of diabetes outcomes among asians and pacific islanders in the US: the diabetes study of northern california (DISTANCE) Diabetes Care. 2011;34:930–937. doi: 10.2337/dc10-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grandinetti A, Chang HK, Mau MK, et al. Prevalence of glucose intolerance among Native Hawaiians in two rural communities. Native Hawaiian Health Research (NHHR) Project. Diabetes Care. 1998;21:549–554. doi: 10.2337/diacare.21.4.549. [DOI] [PubMed] [Google Scholar]
- 14.Fujimoto WY, Boyko EJ, Hayashi T, et al. Risk Factors for Type 2 Diabetes: Lessons Learned from Japanese Americans in Seattle. J Diabetes Investig. 2012;3:212–224. doi: 10.1111/j.2040-1124.2012.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albright CL, Steffen AD, Wilkens LR, Henderson BE, Kolonel LN. The prevalence of obesity in ethnic admixture adults. Obesity (Silver Spring) 2008;16:1138–1143. doi: 10.1038/oby.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolonel LN, Henderson BE, Hankin JH, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;151:346–357. doi: 10.1093/oxfordjournals.aje.a010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stram DO, Hankin JH, Wilkens LR, Henderson B, Kolonel LN. Calibration of the dietary questionnaire for a multiethnic cohort in Hawaii and Los Angeles. Am J Epidemiol. 2000;151:358–370. doi: 10.1093/oxfordjournals.aje.a010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willett W. Nutritional epidemiology. New York: Oxford University Press; 1998. [Google Scholar]
- 19.Shai I, Jiang R, Manson JE, et al. Ethnicity, obesity, and risk of type 2 diabetes in women: a 20-year follow-up study. Diabetes Care. 2006;29:1585–1590. doi: 10.2337/dc06-0057. [DOI] [PubMed] [Google Scholar]
- 20.Nyamdorj R, Pitkaniemi J, Tuomilehto J, et al. Ethnic comparison of the association of undiagnosed diabetes with obesity. Int J Obes (Lond) 2010;34:332–339. doi: 10.1038/ijo.2009.225. [DOI] [PubMed] [Google Scholar]
- 21.Steinbrecher A, Morimoto Y, Heak S, et al. The preventable proportion of type 2 diabetes by ethnicity: the multiethnic cohort. Ann Epidemiol. 2011;21:526–535. doi: 10.1016/j.annepidem.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramachandran A, Snehalatha C, Shetty AS, Nanditha A. Trends in prevalence of diabetes in Asian countries. World J Diabetes. 2012;3:110–117. doi: 10.4239/wjd.v3.i6.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ku GM, Kegels G. The performance of the Finnish Diabetes Risk Score, a modified Finnish Diabetes Risk Score and a simplified Finnish Diabetes Risk Score in community-based cross-sectional screening of undiagnosed type 2 diabetes in the Philippines. Prim Care Diabetes. 2013;7:249–259. doi: 10.1016/j.pcd.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Wang EJ, Wong EC, Dixit AA, Fortmann SP, Linde RB, Palaniappan LP. Type 2 diabetes: identifying high risk Asian American subgroups in a clinical population. Diabetes Res Clin Pract. 2011;93:248–254. doi: 10.1016/j.diabres.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuasay LC, Lee ES, Orlander PP, Steffen-Batey L, Hanis CL. Prevalence and determinants of type 2 diabetes among Filipino-Americans in the Houston, Texas metropolitan statistical area. Diabetes Care. 2001;24:2054–2058. doi: 10.2337/diacare.24.12.2054. [DOI] [PubMed] [Google Scholar]
- 26.Gomez SL, Kelsey JL, Glaser SL, Lee MM, Sidney S. Immigration and acculturation in relation to health and health-related risk factors among specific Asian subgroups in a health maintenance organization. Am J Public Health. 2004;94:1977–1984. doi: 10.2105/ajph.94.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan NA, Wang H, Anand S, et al. Ethnicity and sex affect diabetes incidence and outcomes. Diabetes Care. 2011;34:96–101. doi: 10.2337/dc10-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim MT, Juon HS, Hill MN, Post W, Kim KB. Cardiovascular disease risk factors in Korean American elderly. West J Nurs Res. 2001;23:269–282. doi: 10.1177/01939450122045140. [DOI] [PubMed] [Google Scholar]
- 29.Grandinetti A, Keawe’aimoku KJ, Chang HK, et al. Relationship between plasma glucose concentrations and Native Hawaiian Ancestry: The Native Hawaiian Health Research Project. Int J Obes Relat Metab Disord. 2002;26:778–782. doi: 10.1038/sj.ijo.0802000. [DOI] [PubMed] [Google Scholar]
- 30.Saydah SH, Geiss LS, Tierney E, Benjamin SM, Engelgau M, Brancati F. Review of the performance of methods to identify diabetes cases among vital statistics, administrative, and survey data. Ann Epidemiol. 2004;14:507–516. doi: 10.1016/j.annepidem.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Molenaar EA, Van Ameijden EJ, Grobbee DE, Numans ME. Comparison of routine care self-reported and biometrical data on hypertension and diabetes: results of the Utrecht Health Project. Eur J Public Health. 2007;17:199–205. doi: 10.1093/eurpub/ckl113. [DOI] [PubMed] [Google Scholar]
- 32.Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281:64–91. doi: 10.1111/nyas.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care. 2013;36:1789–1796. doi: 10.2337/dc12-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayashi T, Boyko EJ, McNeely MJ, Leonetti DL, Kahn SE, Fujimoto WY. Visceral adiposity, not abdominal subcutaneous fat area, is associated with an increase in future insulin resistance in Japanese Americans. Diabetes. 2008;57:1269–1275. doi: 10.2337/db07-1378. [DOI] [PubMed] [Google Scholar]
- 35.Steinbrecher A, Heak S, Morimoto Y, Grandinetti A, Kolonel LN, Maskarinec G. Various Adiposity Measures Show Similar Positive Associations With Type 2Diabetes in Caucasians, Native Hawaiians, and Japanese Americans: The Multiethnic Cohort. Asia Pac J Public Health. 2012 Apr 11; doi: 10.1177/1010539512440819. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Binns C, Low WY. Obesity: upsetting the public health balance. Asia Pac J Public Health. 2013;25:121–123. doi: 10.1177/1010539513479003. [DOI] [PubMed] [Google Scholar]