Abstract
Asthma in the pediatric population remains a significant contributor to morbidity and increasing healthcare costs. Vitamin D3 insufficiency and deficiency have been associated with development of asthma. Recent studies in models of adult airway diseases suggest that the bioactive Vitamin D3 metabolite, calcitriol (1,25-dihydroxyvitamin D3; 1,25(OH)2D3), modulates responses to inflammation; however this concept has not been explored in developing airways in the context of pediatric asthma. We used human fetal airway smooth muscle (ASM) cells as a model of the early postnatal airway to explore how calcitriol modulates remodeling induced by pro-inflammatory cytokines. Cells were pre-treated with calcitriol and then exposed to TNFα or TGFβ for up to 72 h. Matrix metalloproteinase (MMP) activity, production of extracellular matrix (ECM), and cell proliferation were assessed. Calcitriol attenuated TNFα enhancement of MMP-9 expression and activity. Additionally, calcitriol attenuated TNFα and TGFβ-induced collagen III expression and deposition, and separately, inhibited proliferation of fetal ASM cells induced by either inflammatory mediator. Analysis of signaling pathways suggested that calcitriol effects in fetal ASM involve ERK signaling, but not other major inflammatory pathways. Overall, our data demonstrate that calcitriol can blunt multiple effects of TNFα and TGFβ in developing airway, and point to a potentially novel approach to alleviating structural changes in inflammatory airway diseases of childhood.
Keywords: Airway remodeling, Lung development, Prematurity, Pediatric asthma, Collagen
Introduction
Recurrent wheezing and asthma remain a significant clinical burden and are leading contributors to lung morbidity in children making this an important public health concern (Asher et al., 2006). Multiple factors contribute to the development of wheezing and asthma including genetics, in utero exposure to inflammation, environmental exposures, and maternal and neonatal nutritional status (Britt et al., 2013; Bush and Menzies-Gow, 2009). Akin to adult patient populations (Sutherland et al., 2010), deficiency of Vitamin D in children is strongly associated with development of asthmatic symptoms (Brehm et al., 2012; Freishtat et al., 2010). Recent studies show that lack of Vitamin D is linked to reduced lung function (Choi et al., 2013; Li et al., 2011) and airway remodeling (Gupta et al., 2011). Additionally, there is evidence that higher Vitamin D intake in pregnant women is associated with decreased rates of wheezing and asthma in their offspring (Camargo et al., 2007; Devereux et al., 2007; Zosky et al., 2014). While the topic of whether Vitamin D supplementation is of any benefit in healthy individuals has received much recent attention, these previous data suggest that in the setting of lung disease, reduction in Vitamin D levels enhances disease. Accordingly, it becomes important to understand the mechanisms by which Vitamin D influences airway structure or function in the context of neonatal and pediatric lung disease.
Children with asthma exhibit airway obstruction (Saglani et al., 2005) and increased airway hyperresponsiveness (Palmer et al., 2001). From a pathophysiologic perspective, these functional deficiencies are induced and maintained by inflammation. Inflammatory cytokines and growth factors promote airway remodeling through increased extracellular matrix (ECM) deposition, immune cell infiltration, and airway smooth muscle (ASM) cell proliferation (Malmstrom et al., 2013). Children with asthma have increased cytokine levels in bronchoalveolar lavage fluid, including tumor necrosis factor-α (TNFα) and transforming growth factor-β (TGFβ) (Barbato et al., 2003; Brown et al., 2012; Puthothu et al., 2009). Accordingly, understanding whether and how Vitamin D can influence the effects of such mediators in the inflamed airway becomes relevant to understanding the role of Vitamin D during childhood lung development, and in identification of novel strategies for alleviating inflammatory effects.
Vitamin D3 (cholecalciferol), is the inactive form of Vitamin D, while calcitriol (1,25(OH)2D3) is the hormonally active form of Vitamin D3. Calcitriol exerts its effects through binding the Vitamin D receptor (VDR), inducing genomic or non-genomic effects (Deeb et al., 2007). Upon calcitriol binding, VDR translocates from the plasma membrane to the nucleus where it transcriptionally activates genes via the VDR response element (VDRE), thereby affecting transcription of other genes (Deeb et al., 2007). VDR interacts with multiple proteins including the retinoid X receptor-α (RXRα) to mediate its transcriptional effects (Bettoun et al., 2003; Prufer and Barsony, 2002). The effects of VDR can also be through activities within the cytoplasm (Deeb et al., 2007). Thus far, these signaling mechanisms remain largely undefined in ASM.
Prior in vivo and in vitro studies suggest that calcitriol is a key regulator of inflammatory responses and airway remodeling in the adult lung. Early investigations in 50 day old rats born to mothers deprived of dietary Vitamin D3 showed reduced lung compliance in contrast to rats born to mothers whose diet was supplemented with Vitamin D (Gaultier et al., 1984). Additionally, VDR knockout mice prematurely develop pulmonary inflammation and an emphysematous phenotype by 4 months of age (Sundar et al., 2011). Vitamin D3 deficiency in mice challenged with ovalbumin show increased cytokine production by lymphocytes isolated from lung lymph nodes (Gorman et al., 2012). In adult human ASM, Vitamin D3 has been shown to reduce cytokine-induced chemokine secretion (Banerjee et al., 2008) and proliferation (Damera et al., 2009; Song et al., 2007). However, the effects of Vitamin D on remodeling beyond proliferation have not been examined, and not at all in the developing airway.
In the present study, using human fetal ASM cells as a model of developing airway, we tested the hypothesis that the VDR agonist calcitriol reduces TNFα and TGFβ-induced remodeling. We show that calcitriol attenuates the pro-inflammatory and pro-fibrotic effects of TNFα and TGFβ in terms of ECM formation and cell proliferation via specific signaling pathways. The relevance of this work lies in furthering our understanding of the role of calcitriol in perinatal lung inflammation and its consequences for pediatric airway diseases.
Materials and Methods
Materials
Cell culture reagents including fetal bovine serum (FBS), and Dulbecco’s Modified Eagle’s Medium F/12 (DMEM/F12) were purchased from Invitrogen (Carlsbad, CA). Chemicals and supplies were from Sigma (St. Louis, MO) unless otherwise specified. Human recombinant TNFα and TGFβ were from R&D Systems (Minneapolis, MN). Primary antibodies were from Cell Signaling Technology (Danvers, MA) unless otherwise noted.
Isolation of Human Fetal Airway Smooth Muscle Cells
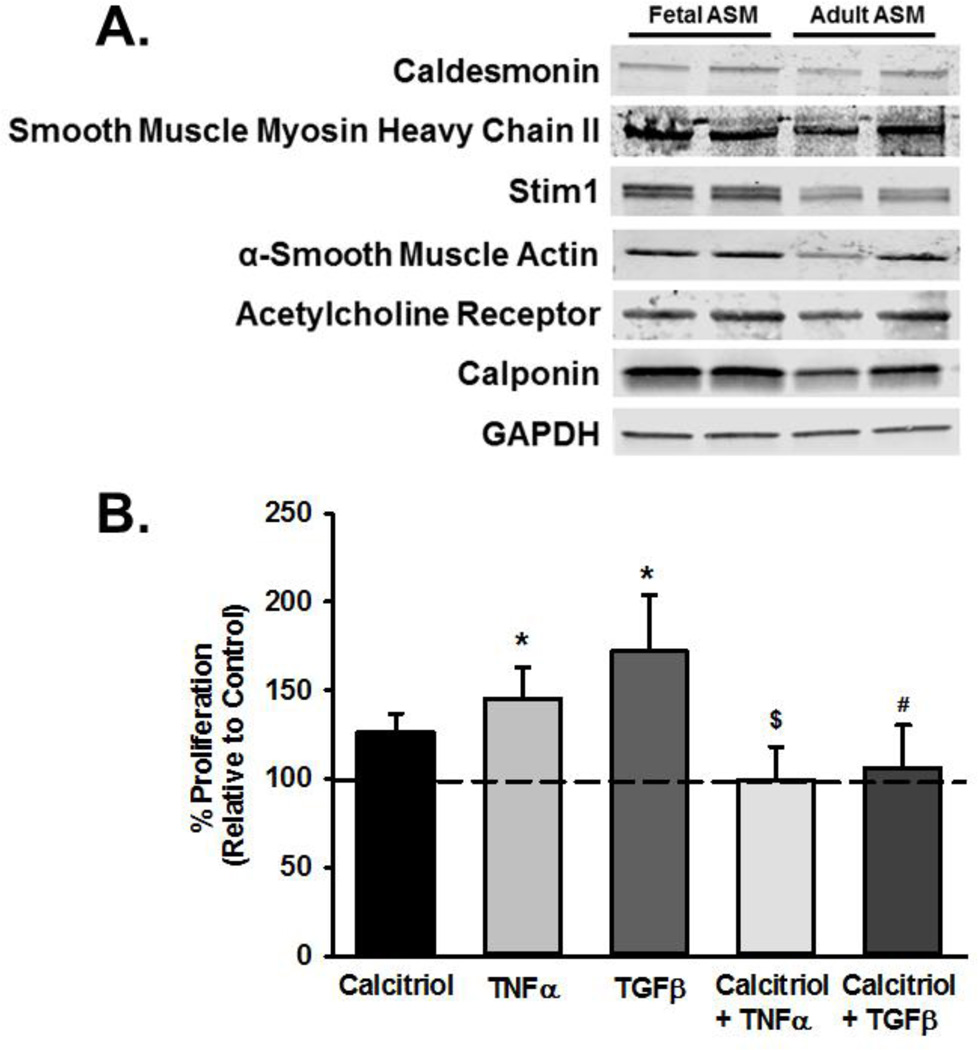
De-identified fetal human ASM cells from the canalicular stage (18–22 weeks gestation) were provided by Dr. Pandya from the University of Leicester, England or were purchased from Novogenix (Los Angeles, CA) under protocols approved and considered exempt by the Mayo Institutional Review Board and Ethics Committees in the UK. As previously described, cells were isolated from fetal tracheobronchial tissue through enzymatic dissociation (Hartman et al., 2012; Pandya et al., 2002). Cells were grown using standard techniques in a 95% air/5% CO2 humidified incubator in phenol red free DMEM/F12 with 10% FBS. All cells were serum deprived to arrest growth in 0.5% FBS media for at least 24 h prior to treatments. ASM phenotype in these samples was verified by expression of a number of smooth muscle markers such as smooth muscle myosin and actin, calponin, caldesmon, receptors for bronchoconstrictor agonists, and Ca2+ regulatory mechanisms (Figure 1). Expression of these markers were consistent with the pattern previously reported in other fetal ASM cells samples (Hartman et al., 2012), and justified the use of this in vitro cell model for the remainder of the study. Furthermore, the expression pattern was comparable to that adult human ASM cells (de-identifed samples from bronchi of normal lung areas from surgical samples of patients undergoing lung surgery for non-infectious, focal neoplasms; approved by Mayo IRB). In pilot studies, we determined that characteristics of fetal ASM cells in terms of [Ca2+]i responses to bronchoconstrictor agonist and cell proliferation were comparable for cells from the UK vs. those from Novogenix. Accordingly, data from these samples were pooled.
Figure 1.
Effect of calcitriol on proliferation in fetal airway smooth muscle (ASM) treated with tumor necrosis factor (TNFα) and transforming growth factor (TGFβ). (A) ASM cells were from 18–22 week old gestational age fetal lung tissue. The ASM phenotype in serum-deprived samples was verified by Western analysis for expression of smooth muscle actin and myosin, calponin, caldesmon, ACh receptor and other calcium regulatory proteins (stromal interaction molecule STIM1 shown here). Such expression was largely comparable to that observed in adult ASM cells. (B) Fetal ASM were treated with vehicle or calcitriol, and then TNFα or TGFβ. Cellular proliferation was determined by Invitrogen Cyquant fluorescence assay. Data were normalized to vehicle treated cells. Both TNFα and TGFβ increased proliferation, and these effects were blunted by calcitriol. Data are presented as mean ± SEM, n=4 samples, p<0.05. * indicates significant difference from vehicle control; $ indicates significant difference from TNFα; # indicates significant difference from TGFβ.
Cell Treatments
Fetal ASM cells were pre-treated with vehicle (DMSO), 100 nM calcitriol or 100 nM of Vitamin D3 (Tocris, Bristol, United Kingdom) for 1 h and then exposed to control (media only), 10 ng/mL TNFα, or 0.5 ng/mL TGFβ for 24–72 h in the continued presence of calcitriol or Vitamin D3.
Proliferation
Human fetal ASM cells were treated with vehicle (DMSO) or 100 nM calcitriol, and then media only, 10 ng/mL TNFα, or 0.5 ng/mL TGFβ in media containing 1% fetal bovine serum for 48 h. Similar to previous studies (Hartman et al., 2012), cell proliferation was determined using a CyQuant fluorescence assay (Invitrogen) and following manufacturer’s protocol. Fluorescence was detected at 480 nm excitation using a Flexstation 3 plate reader (Molecular Devices, Sunnyvale, CA). For calculations, cell number was determined using a calibration curve and data was normalized to control treated cells (Hartman et al., 2012).
Western Blot Analysis
Cells were harvested using standard techniques (cell lysis buffer containing protease inhibitors). Using a Lowry protein assay (Bio-Rad, Hercules, CA) cell protein concentration was determined and ~30 µg total protein was loaded onto 10% or 4–15% gradient gels (Criterion Gel System; Bio-Rad, Hercules, CA). Proteins were transferred to nitrocellulose membranes using a Bio-Rad Trans-Blot Turbo rapid transfer system (Bio-Rad, Hercules, CA). Membranes were blocked with either Odyssey Blocking buffer (Li-Cor Biosciences, Lincoln, NE) or 5% milk in Tris-buffered saline with 0.1% Tween (TBST) for 1 h at room temperature. Membranes were incubated overnight in 1 µg/mL of primary antibody of interest at 4°C. Primary antibodies included: VDR (Santa Cruz Biotechnology, Santa Cruz, Dallas, TX), phosphorylated and total extracellular signal-regulated kinase 1/2 (ERK 1/2), phosphorylated and total c-Jun N-terminal kinase (JNK), phosphorylated and total p38, phosphorylated and total smad 2, p50 (Santa Cruz), p65 (Santa Cruz), and GAPDH. Following three washes with TBST, infrared dye-conjugated secondary antibody (LiCor Biosciences) was added. Membranes were imaged on a Li-Cor OdysseyXL system and densitometry was quantified with Image Studio software. Protein blots were normalized to GAPDH unless otherwise specified.
In-Cell Western
A previously described semi-quantitative immunofluorescence-based technique was modified for use with fetal ASM (Theiss et al., 2005). Upon reaching 70% confluence, human fetal ASM cells grown in black bottom 96-well plates (Corning Incorporated; Corning NY) were serum deprived for 24 h. The cells were then pre-treated with vehicle or 100 nM calcitriol and subsequently treated with vehicle, 10 ng/mL TNFα, or 0.5 ng/mL TGFβ for 72 h. Next, 0.016 N NH4OH was applied for 40 min in order to lyse and lift the cells from the plate. The plate was then washed three times with PBS and blocked with Li-Cor Odyssey Blocking Buffer for 60 min prior to overnight incubation in primary antibody with a concentration of 10 µg/mL at 4°C. Primary antibodies included: Collagen I (Abcam, Cambridge, MA), Collagen III (Abcam), and Fibronectin (Santa Cruz). Wells were then washed again and incubated for 60 min with infrared dye-conjugated secondary antibodies at 5 µg/mL. Plates were imaged using a Li-Cor Odyssey XL system with densitometry quantification. Data was normalized to cell count using CyQuant cell fluorescence assays performed on each plate prior to NH4OH cell lysis.
Gelatin Zymography
MMP-2 and MMP-9 activity in human fetal ASM was measured by gelatin zymography. Following cell treatments, media was collected from each group and concentrated using centrifugal filter units (Millipore, Beverly, MA). Concentrated media was combined with 4× SDS buffer (40% glycerol, 240 mM Tris-HCl pH 6.8, 8% SDS, 0.05% bromphenol) in a 4:1 ratio of concentrated media to SDS, respectively. Samples were then loaded onto a SDS-PAGE 10% gelatin gel (Bio-Rad Criterion). Following electrophoresis (100 V, 3 h), the gel was rinsed with deionized water, and then incubated at room temperature with gentle rocking in 1× zymogram renaturation buffer (Bio-Rad). The gel was then rinsed again with deionized water before being incubated for 36 h at 37°C in 1× zymogram developing buffer (Bio-Rad). The gels were stained with Coomassie blue for 1 h at room temperature, and subsequently de-stained (50% methanol, 40% deionized water, 10% acetic acid) for 30 min. Enzymatic activity was demonstrated by clear or unstained zones on the gel, representing the degrading action of the enzyme on the gelatin substrate. Enyzmatic activity of pro- and cleaved MMPs were quantified by imaging the gel on the Li-Cor OdysseyXL system, and standardizing to the cell lysate protein concentration. Data were normalized to vehicle control where appropriate.
Real-time PCR
Total RNA was extracted from cells using the RNeasy Mini Kit (Qiagen) following manufacturer’s protocol. Standard techniques were used to synthesize and amplify cDNA optimized for a Roche LightCycler 480. Real-time PCR was performed in triplicates per cDNA template. Primer sequences are summarized in Table 1 and product formation was confirmed by gel electrophoresis (Supplementary Figure S1). Expression of mRNA was calculated by the normalization of cycle threshold [C(t)] values of target gene to reference gene (GAPDH). The relative fold change was calculated using the ΔΔCt method.
Table 1.
Primers used for Quantitative real time PCR.
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Fibronectin | AGGAAGCCGAGGTTTTAACTG | AGGACGCTCATAAGTGTCACC |
| GAPDH | AAGGTGAAGGTCGGAGTCAACGGATT | CCATGGAATTTGCCATGGGAGGAATC |
| MMP-2 | GATACCCCTTTGACGGTAAGGA | CCTTCTCCCAAGGTCCATAGC |
| MMP-9 | TGTACCGCTATGGTTACACTCG | GGCAGGGACAGTTGCTTCT |
| Procollagen I | GAGGGCCAAGACGAAGACATC | CAGATCACGTCATCGCACAAC |
| Procollagen III | TTGAAGGAGGATGTTCCCATCT | ACAGACACATATTTGGCATGGTT |
| TIMP-1 | CTTCTGCAATTCCGACCTCGT | ACGCTGGTATAAGGTGGTCTG |
| TIMP-2 | GCTGCGAGTGCAAGATCAC | TGGTGCCCGTTGATGTTCTTC |
| VDR | TGCTATGACCTGTGAAGGCTGCAAAG | CCTCTGCACTTCCTCATCTGTCAGAA |
siRNA Transfection
Fetal ASM were transfected at 60–70% confluence using Lipofectamine as vehicle and scrambled or VDR siRNA (50 nM; Life Technologies, Grand Island, NY) under serum- and antibiotic-free conditions. After 6 h, 20% FBS was added to incubate cells in 10% FBS overnight. The following day, cells were treated with calcitriol and/or TNF-α for 24 h. Efficacy of siRNA knockdown was determined by assessing VDR expression 24 h post-transfection by Western blot.
Statistical Analysis
Experiments were performed using cells from at least 3 different fetal ASM samples, where each sample is from one individual. Multiple repetitions per sample were used for each experiment. Data were analyzed using unpaired t test. Values are expressed as mean ± standard error (SE) and statistical significance was established at p<0.05.
Results
Vitamin D reduces cytokine enhancement of ASM proliferation
Fetal ASM cells showed robust proliferation at baseline in minimal amounts of FBS, consistent with our previous report (Hartman et al., 2012). Exposure to TNFα or TGFβ for 48 h significantly increased cell proliferation (Figure 1; p<0.05). Pre-treatment with calcitriol blunted the effects of either cytokine on cell proliferation (Figure 1; p<0.05). The inactive form Vitamin D3 was without effect on proliferation under baseline conditions (not shown).
Vitamin D attenuates MMP-9 expression and activity
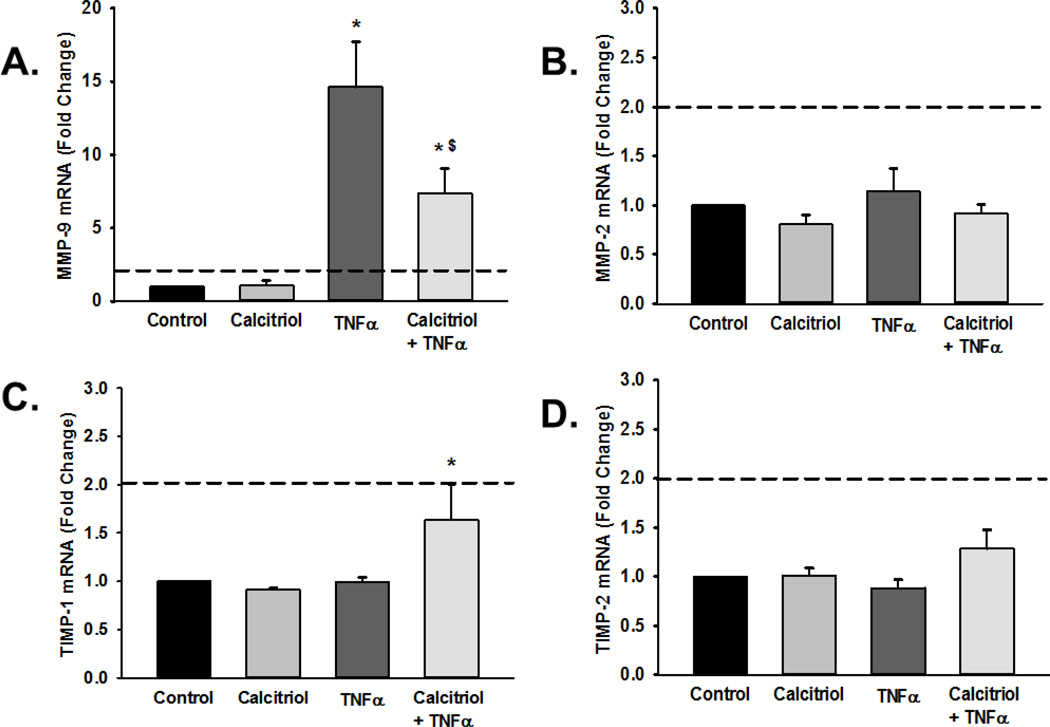
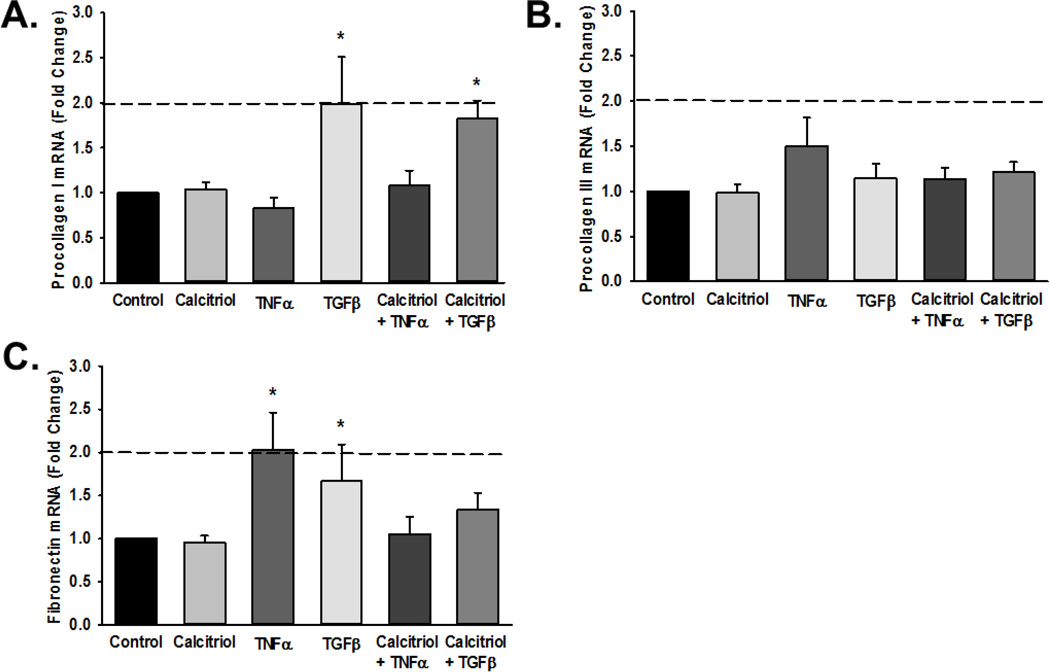
Pro-inflammatory cytokines such as TNFα modulate expression of a number of ECM regulatory proteins including the gelatinases MMP-2 and MMP-9 (Labrie and St-Pierre, 2013). Real-time PCR for mRNA expression of MMP-2 and MMP-9 showed that TNFα significantly increased MMP-9 mRNA levels (Figure 2A; p<0.05, functional threshold of >2-fold change represented by dotted line), while calcitriol blunted such effects. In contrast, calcitriol and TNFα did not affect MMP-2 mRNA levels (Figure 2B).
Figure 2.
Matrix metalloproteinase (MMP) and tissue inhibitor of metalloproteinase (TIMP) mRNA expression in fetal ASM. Cells were treated with vehicle or calcitriol, then TNF-α. (A) MMP-9, (B) MMP-2, (C) TIMP-1 and (D) TIMP-2 mRNA levels were measured by real time PCR. Calcitriol blunted cytokine-induced increase in MMP-9 mRNA but did not substantially influence MMP-2. Conversely, calcitriol increased TIMP-1 mRNA in the presence of TNFα. However, note that the dotted lines represent 2-fold change used to assess potential functional importance, and changes in TIMP-1 value were below this line. Data are presented as mean ± SEM, n=4 samples, p<0.05. * indicates significant difference from vehicle control; $ indicates significant difference from TNFα.
In addition to MMPs, tissue inhibitor of metalloproteinases (TIMPs) regulate ECM composition by inhibiting MMP activity (Visse and Nagase, 2003). We evaluated the effect of TNFα and calcitriol on TIMP-1 and TIMP-2 expression and found that TIMP-1 but not TIMP-2 mRNA levels were significantly increased in cells treated with both calcitriol and TNFα (Figure 2C–D; p<0.05), while exposure to either agent alone was without effect. However, it should also be noted that these changes were below the 2-fold threshold, suggesting that although statistically significant, may not represent functional importance.
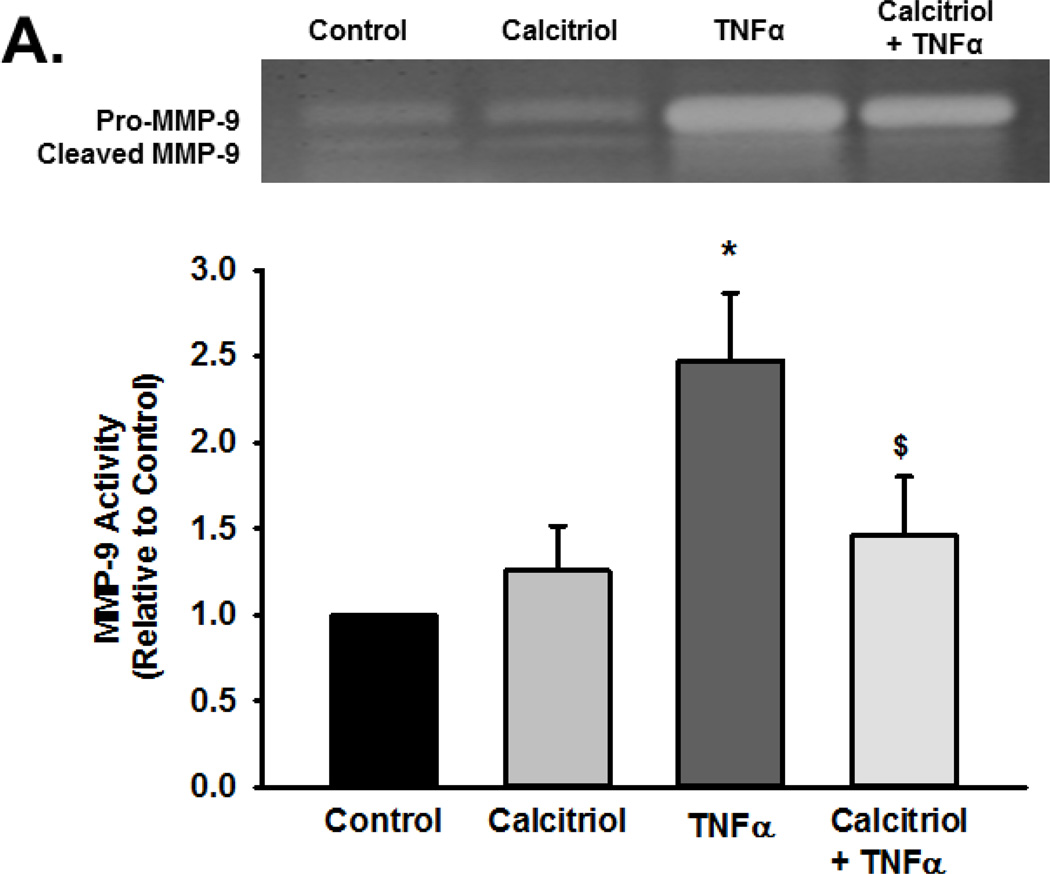
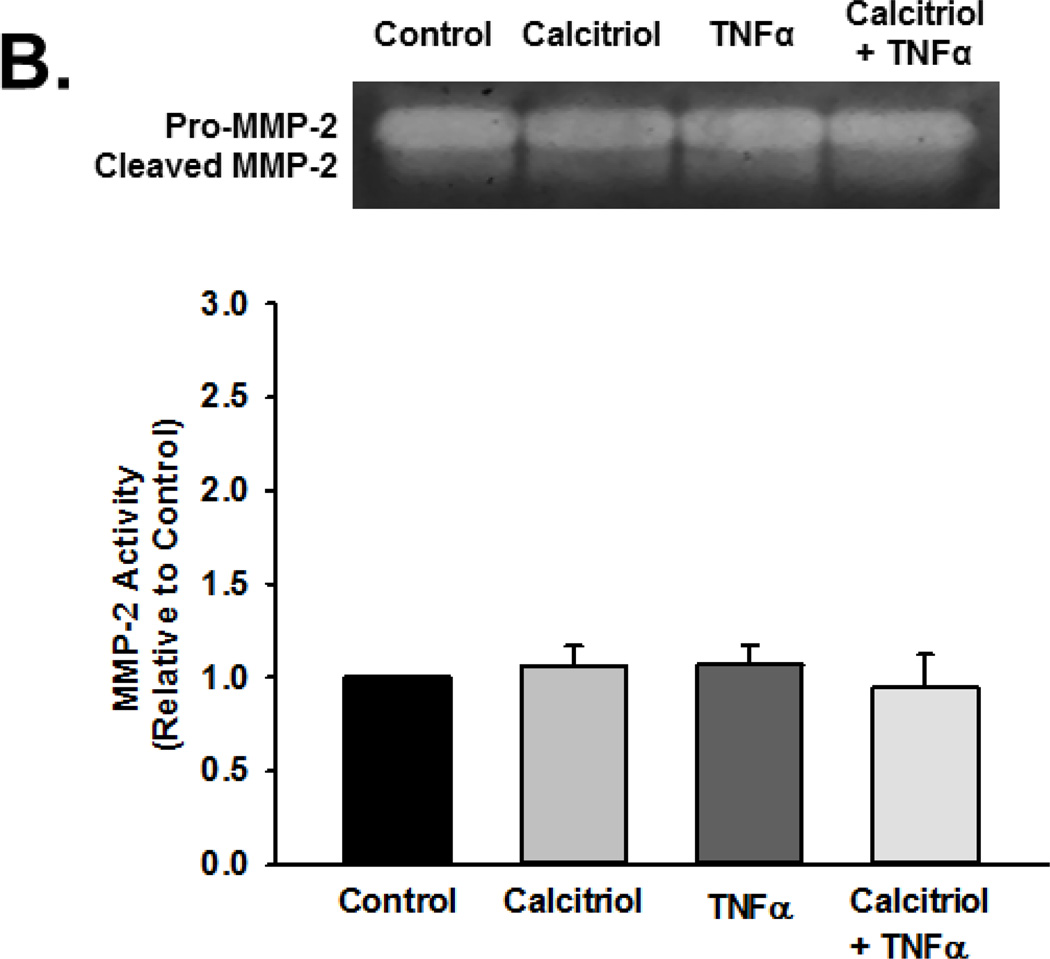
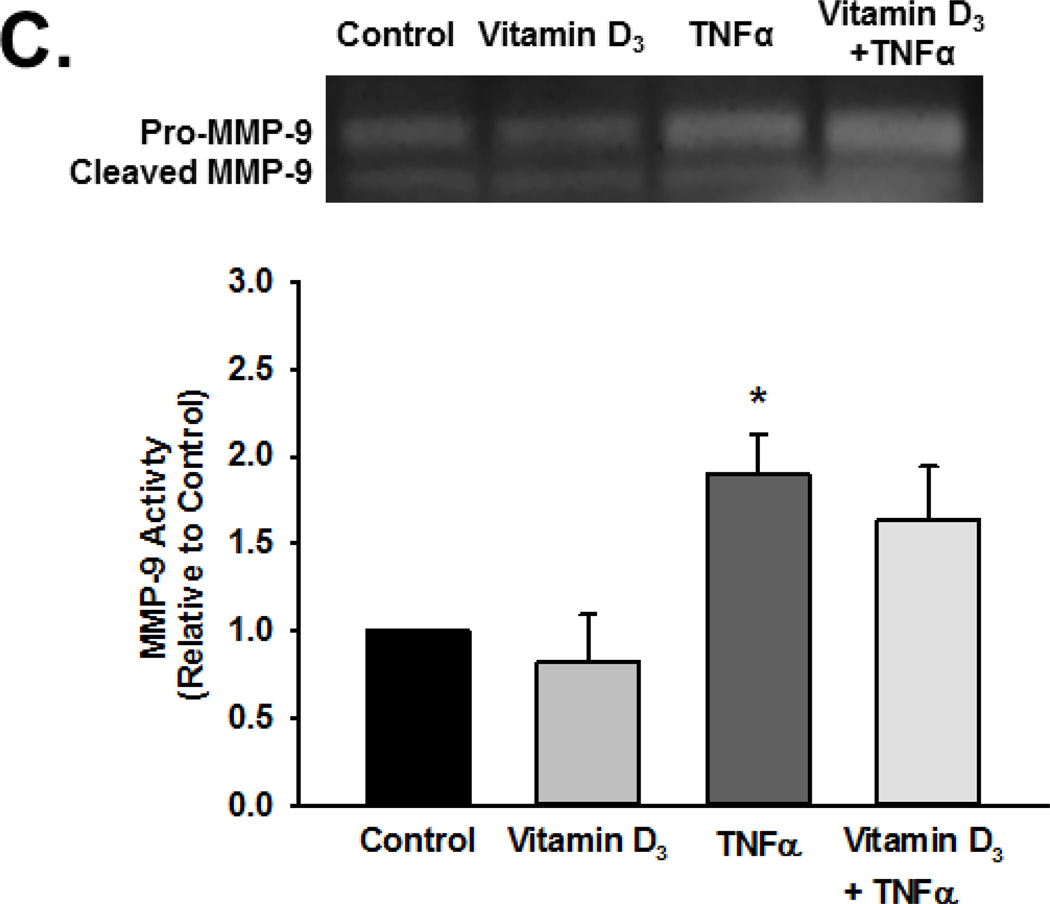
Based on the above findings, we explored MMP activity in the context of calcitriol and TNFα exposure using gelatin zymography. Pro-MMP-9 and cleaved MMP-9 exhibited gelatinase activity. Exposure to TNFα increased activity of total MMP-9 (pro and cleaved forms), although the extracellular pro-MMP-9 levels (and activity) were generally greater than that of cleaved MMP-9. Nonetheless, these data indicated increased MMP-9 secretion and activity (Figure 3A, summary bar graph shows cleaved MMP-9 p<0.05). Pre-treatment with calcitriol blunted TNFα-induced increase in both pro- and active MMP-9 in the extracellular medium (Figure 3A; p<0.05). Calcitriol by itself was without effect. In contrast to these observations, there was no effect of TNFα or calcitriol on MMP-2 activity (Figure 3B). Furthermore, the inactive Vitamin D3 did not attenuate TNFα induced enhancement of MMP-9 activity (Figure 3C).
Figure 3.
Effect of calcitriol and Vitamin D3 on TNF-α-induced MMP-2 and MMP-9 activity. Fetal ASM were treated with vehicle, (A, B) calcitriol, or (C) Vitamin D3, followed by media only or TNFα. MMP-2 and MMP-9 activity in cell culture media was determined by zymography. Calcitriol blunted TNFα-induced increase in MMP-9 activity, but had no effect on MMP-2. Furthermore, the inactive Vitamin D3 was without effect on MMP-9 activity. Gels are representative results from independent experiments using at least 3 individual samples. For summary bar graphs, pro-and cleaved MMP data were quantified together and normalized to vehicle control. Data are presented as mean ± SEM, n=4 samples, p<0.05. * indicates significant difference from vehicle control; $ indicates significant difference from TNFα.
Vitamin D attenuates ECM protein expression and deposition
To determine if calcitriol modulates TNFα-induced ECM protein production at the transcriptional level, fetal ASM cells were pre-treated with either vehicle or calcitriol for 1 h, and then exposed to TNFα or TGFβ for 6 h. TGFβ significantly increased pro-collagen I and fibronectin, while TNFα increased fibronectin mRNA expression (Figure 4A–C). These effects were not significantly affected by calcitriol, although there was a trend towards decreased fibronectin by calcitriol in TNFα-treated cells. Pro-collagen III mRNA expression was not significantly altered by calcitriol, TNFα, or TGFβ (Figure 4B).
Figure 4.
Effect of calcitriol on mRNA expression of extracellular matrix (ECM) proteins. Fetal ASM were treated with vehicle or calcitriol, followed by TNF-α or TGF-β. (A) collagen I, (B) collagen III, and (C) fibronectin mRNA levels were measured by real time PCR. Data are normalized to vehicle control cells and presented as mean ± SEM, n=4 samples, p<0.05. * indicates significant difference from vehicle control
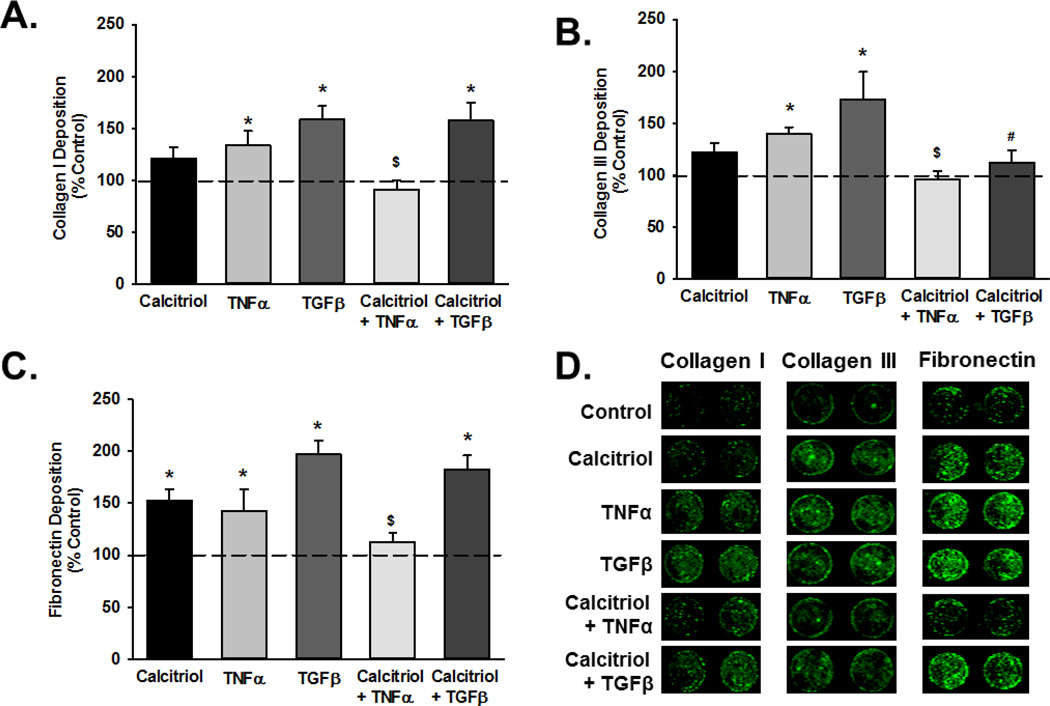
Although there were no early transcriptional changes, semi-quantitative In-Cell Western analysis of native ECM deposition showed that TNFα and TGFβ increased deposition of collagen I, collagen III and fibronectin (Figure 5A–C; p<0.05) while calcitriol attenuated these effects across all three ECM proteins, albeit not for both cytokines. For example, calcitriol reduced TGFβ-induced collagen III deposition, but did not affect increased deposition of collagen I and fibronectin (Figure 5).
Figure 5.
Effect of calcitriol on ECM deposition. (A) collagen I, (B) collagen III, and (C) fibronectin deposition by fetal ASM was evaluated by In-Cell Western (representative examples of wells are shown in panel D), with normalization to control. Both TNFα and TGFβ increased ECM deposition, and calcitriol blunted these effects, albeit not for every ECM protein and cytokine. Wells are representative results from independent experiments using at least 3 individual samples. Data are presented as mean ± SEM, n=4 samples, p<0.05. * indicates significant difference from vehicle control; $ indicates significant difference from TNFα; # indicates significant difference from TGFβ.
Mechanisms of calcitriol effect in fetal ASM
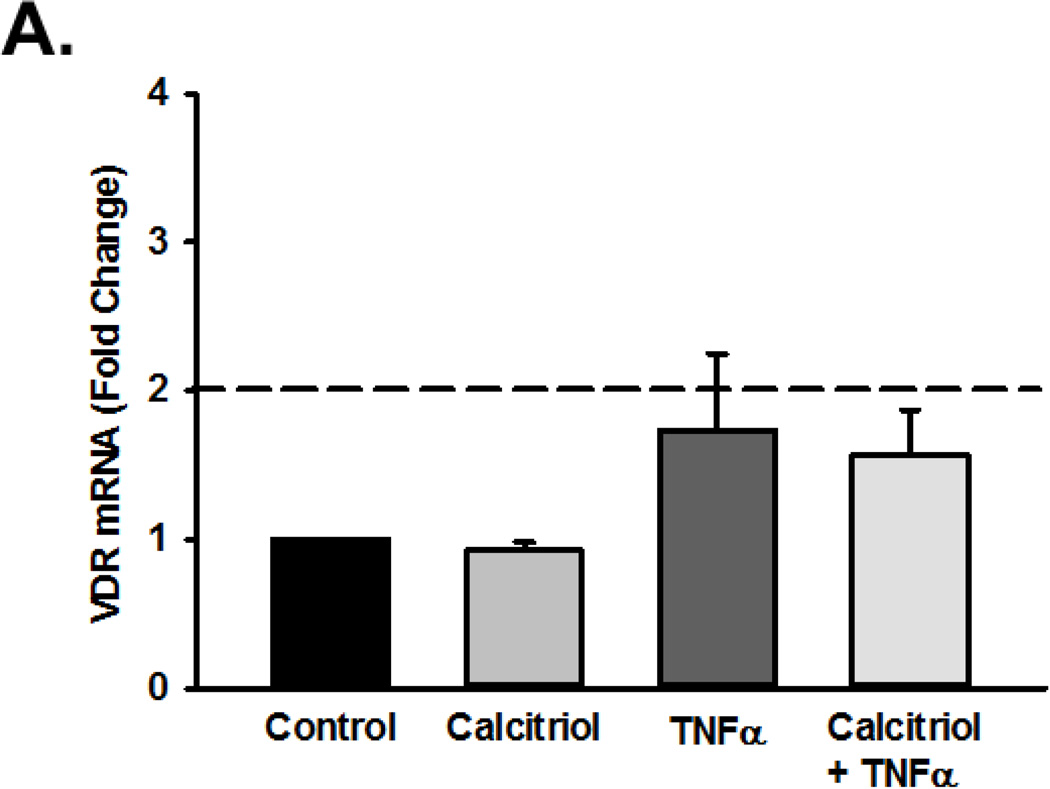
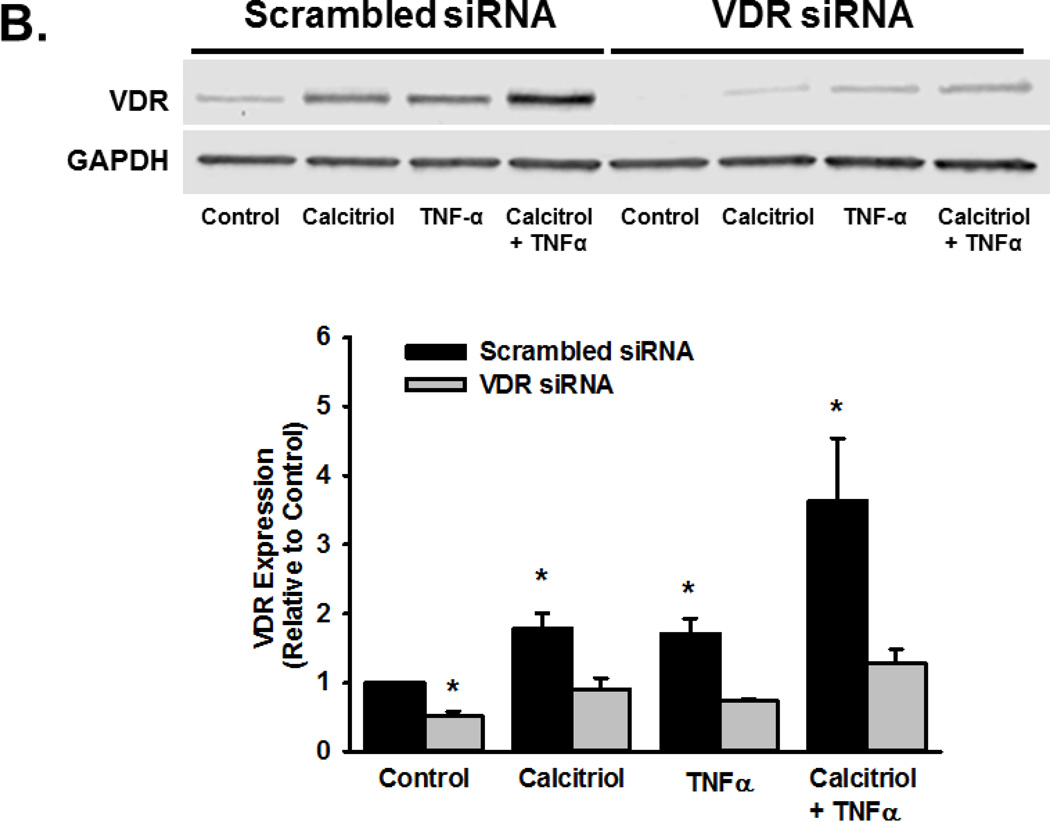
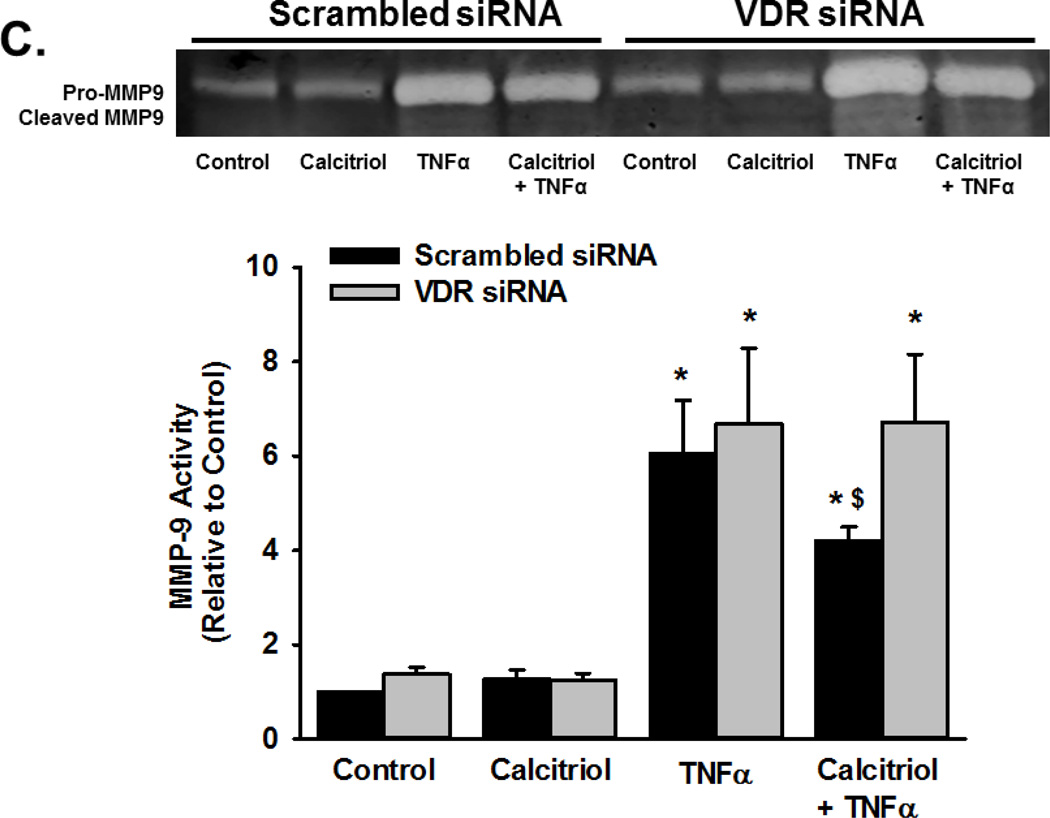
To determine if the effects of calcitriol were mediated through VDR, we first examined VDR mRNA and protein expression in fetal ASM cells, both of which were detectable. Calcitriol and TNFα did not significantly affect VDR mRNA levels (Figure 6A), but did increase VDR protein levels after 24 h (Figure 6B; p<0.05). VDR knockdown, via siRNA, blunted the ability of calcitriol and TNFα to increase VDR protein levels (Figure 6B; p<0.05). In cells treated with VDR siRNA, the ability of calcitriol to attenuate TNFα-induced MMP-9 activity was diminished (Figure 6C; p<0.05).
Figure 6.
Role of Vitamin D receptor (VDR) in calcitriol effects in fetal ASM. (A) Fetal ASM cells expressed VDR mRNA, which was not substantially altered by exposure to calcitriol or TNF-α. (B) Western analysis showed that VDR protein levels were maintained following calcitriol or TNFα treatment. (C) siRNA knockdown of VDR (verified by Western analysis (B)) blunted calcitriol effects on MMP-9 activity in the presence of TNFα, as assessed by zymography. Western blots are representative results from independent experiments using at least 3 individual samples. Data are presented as mean ± SEM, n=at least 3 samples, p<0.05. * indicates significant difference from vehicle control; $ indicates significant difference from TNFα.
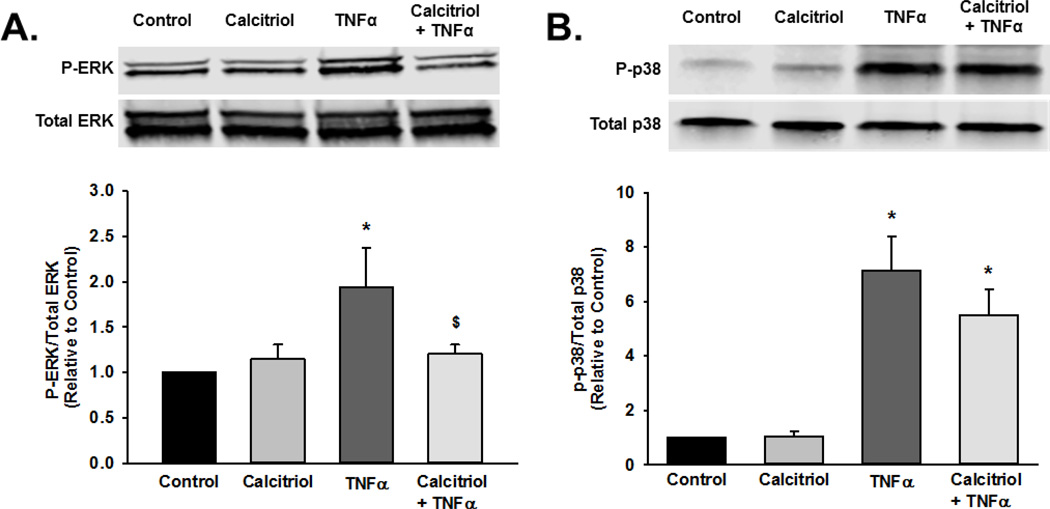
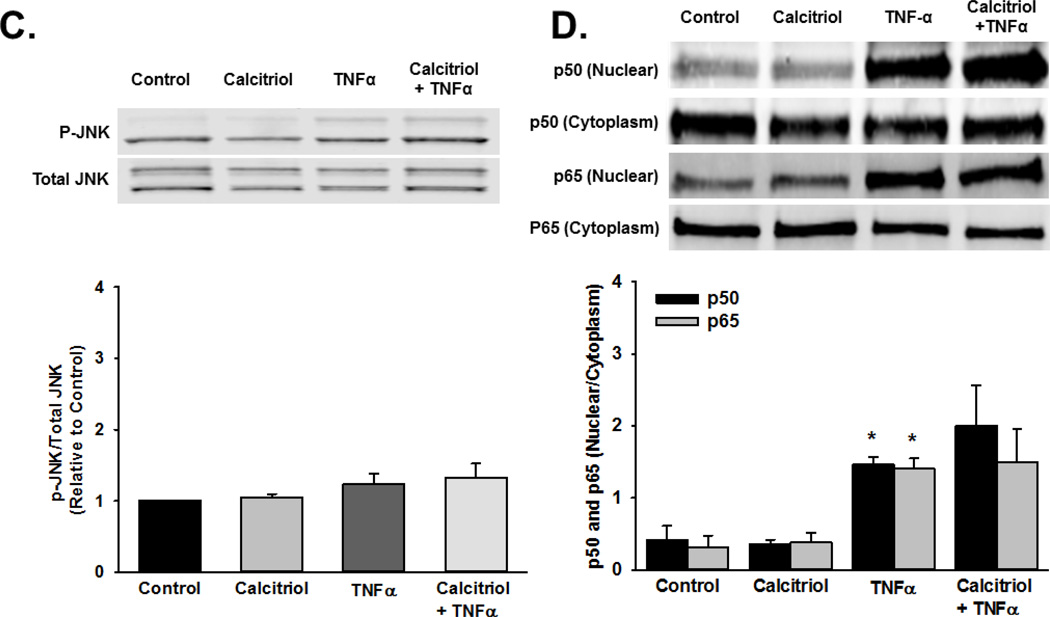
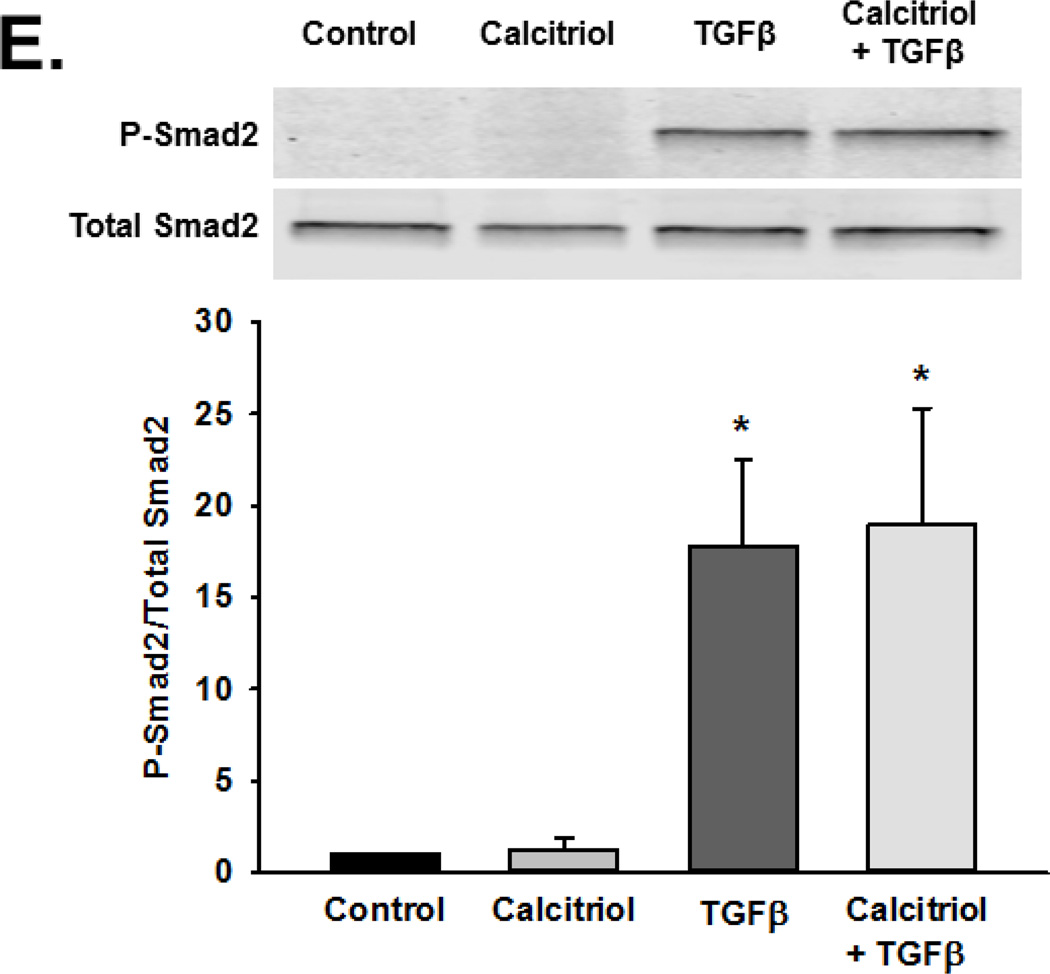
To explore potential effects of calcitriol on signaling mechanisms mediated by TNFα and TGFβ, we examined MAP kinase pathway phosphorylation, p50 and p65 nuclear translocation, and Smad2 phosphorylation. Human fetal ASM were pre-treated with vehicle or calcitriol for 1 h prior to treatment with vehicle, TNFα, or TGFβ for 30 min. Cells were harvested and phosphorylation of p38, ERK, and JNK were assessed by Western blot. TNFα stimulated phosphorylation of p38, ERK, and JNK, while calcitriol pre-treatment inhibited ERK phosphorylation but did not affect p38 and JNK (Figure 7A–C). We also assessed the effect of calcitriol on TNFα-induced nuclear translocation of the NFκB subunit p50 and p65. Calcitriol pretreatment did not prevent TNFα-induced p50 and p65 nuclear translocation (Figure 7D). Additionally, calcitriol did not inhibit TGFβ-induced Smad2 phosphorylation (Figure 7E).
Figure 7.
Mechanisms of calcitriol action in fetal ASM. Calcitriol blunted TNFα-induced phosphorylation of (A) ERK but not (B) p38, (C) JNK phosphorylation, (D) nuclear translocation of the p50 and p65 NFκB subunits, and TGFβ-induced (E) Smad2 phosphorylation. Western blots are representative results from independent experiments using at least 3 individual samples. Data are presented as mean ± SEM, n=3 samples, p<0.05. * indicates significant difference from control.
Discussion
Recent clinical studies suggest that Vitamin D3 may be important for maintaining the structural integrity and caliber of the airway in children with asthma (Choi et al., 2013; Gupta et al., 2011; Sutherland et al., 2010). However, the role of bioactive Vitamin D3 metabolites in the developing airway, particularly in ASM, remains undefined. Using human fetal ASM cells as a model, we evaluated the role of calcitriol and its receptor, VDR, in cell proliferation, MMP activity, and ECM deposition following stimulation with TNFα and TGFβ: two inflammatory mediators that can contribute to airway remodeling in the context of perinatal inflammation that may occur in the setting of prenatal maternal infection, and postnatal infection and inflammation, predisposing the growing lung to diseases such as asthma (Britt et al., 2013). Our findings that calcitriol may have a suppressive effect on features of inflammation-induced airway remodeling suggest the importance of calcitriol in preventing detrimental structural changes in the growing lung, and thus to the need for better understanding of Vitamin D levels in infants and children at risk for asthma.
In adults, phenotypic changes to ASM, mediated by pro-inflammatory cytokines, are important for the airway remodeling process (Doherty and Broide, 2007). While the cytokines and other factors involved in the remodeling process may differ in infants and children, it is likely that features such as increased cell proliferation and altered or increased ECM (and fibrosis) does occur. With the hypothesis that such changes would be blunted by calcitriol, we evaluated the effects of calcitriol on fetal ASM proliferation and ECM production induced by TNFα and TGFβ. Our findings of blunting effects of calcitriol on the increased fetal ASM proliferation and ECM by TNFα and TGFβ are consistent with previous studies in adult human ASM demonstrating that calcitriol reduces serum and platelet derived growth factor-induced proliferation (Damera et al., 2009; Song et al., 2007). Damera et al. reported that calcitriol inhibits cell cycle progression but did not induce apoptosis in human ASM (Damera et al., 2009).
Altered and/or enhanced ECM composition is a key aspect of structural remodeling in the asthmatic airway. While resident fibroblasts are well-known to produce a number of ECM proteins, there is now increasing recognition in adults that the ASM is also a major source of ECM proteins (Black et al., 2003; Burgess et al., 2009). In this regard, there is currently no information on whether human fetal ASM produces ECM, and the data presented in this study are novel in themselves. However, given the rapid postnatal growth of the lung, a proliferative and ECM-producing phenotype of the developing ASM is to be expected. Accordingly, we expected that calcitriol would blunt ECM production by fetal ASM. TNFα significantly increased fibronectin mRNA expression, in contrast, TGFβ increased pro-collagen I mRNA expression which was not altered by calcitriol. Interestingly, pro-collagen III mRNA expression levels were not significantly altered by calcitriol, TNFα, or TGFβ, at least 6 h after treatment. However, TNFα and TGFβ did result in increased extracellular deposition of all three ECM proteins. We suspect that mRNA levels of ECM proteins are upregulated by TNFα and TGFβ at a time point later than 6 h. Nonetheless, calcitriol reduced deposition of collagen I, collagen III, and fibronectin induced by TNFα. Conversely, calcitriol reduced TGFβ-induced collagen III deposition, but not collagen I and fibronectin.
In addition to collagens and fibronectin, MMPs are key mediators of airway inflammation and contributors to ECM composition and immune cell infiltration in airway diseases (McKleroy et al., 2013). MMP-2 and MMP-9 are gelatinases that target collagens and gelatins for degradation to regulate ECM composition. Mouse models of VDR deletion and Vitamin D3 deficiency suggest that Vitamin D3 regulates MMP-9 expression and activity in the lung (Sundar et al., 2011). We observed that TNFα increased MMP-9 but not MMP-2 mRNA levels and activity in human fetal ASM. These effects were reduced by calcitriol but not by the biologically inactive Vitamin D3. In contrast, TGFβ did not affect MMP-2 and MMP-9 activity (data not shown). These findings are similar to previous studies in adult human ASM demonstrating that calcitriol reduces asthmatic serum-induced enhancement of MMP-9 protein and mRNA expression (Song et al., 2007). Using siRNA against VDR, we demonstrated that the effect of calcitriol on TNFα-induced MMP-9 activity is mediated by this receptor. These differential effects on TNFα- vs. TGFβ-induced ECM deposition and MMPs suggest that calcitriol may interfere with upstream signaling pathways specific to these cytokines, or differentially affect transcription of specific ECM proteins via co-activators and more nuanced mechanisms that were not explored here.
Tissue inhibitors of matrix metalloproteinases (TIMPs) inhibit MMP activity and have notable anti-fibrotic effects in the lung (Visse and Nagase, 2003). Based on the effects of calcitriol on MMP-9 activity, we assessed the effects of calcitriol on mRNA expression of TIMP-1 and TIMP-2 which negatively regulate MMP-9 activity. Our data show that decreased MMP-9 activity was associated with increased mRNA levels of TIMP-1 in cells treated with both TNFα and calcitriol. Although calcitriol treatment independently did not increase TIMP-1, we speculate that calcitriol could increase its transcription in the presence of TNFα. Calcitriol increased TIMP-1 expression in stimulated peripheral blood mononuclear cells from patients with tuberculosis (Anand and Selvaraj, 2009). Vitamin D3 deficiency in mice increased MMP-9/TIMP-1 ratio in the lung following cigarette exposure (Crane-Godreau et al., 2013). Although these data suggest that calcitriol could regulate TIMP-1 expression, their effects in ASM per se have not been established, and further studies are needed to determine underlying mechanisms by which calcitriol regulates TIMP-1 expression vs. MMP-9 activity.
To determine potential mechanisms by which calcitriol can modulate pro-inflammatory responses in fetal ASM, we evaluated expression of VDR and found it expressed to substantial levels at baseline. Expression of VDR mRNA per se was not significantly altered by calcitriol and TNFα. However, and interestingly, both calcitriol and TNFα did increase VDR protein levels, suggesting a crosstalk between the VDR and TNFα-mediated pathways that help maintain VDR levels even in the presence of inflammation. This further supports the idea that calcitriol may be used to benefit under these conditions.
MAP kinase, NFκB, and Smad signaling pathways regulate airway remodeling including proliferation, ECM deposition, and MMP-9 activity (Doherty and Broide, 2007; Pera et al., 2010). While the mechanisms by which cytokines influence developing ASM are not well-known, we found that TNFα increased phosphorylation of ERK, JNK, and p38. Nuclear translocation of NFκB subunit, p50 and p65, was induced by TNFα, while TGFβ stimulated Smad2 phosphorylation, suggesting that the same mechanisms may play a role in developing ASM. Interestingly, calcitriol did reduce ERK phosphorylation stimulated by TNFα, but did not affect p38 and Smad2 phosphorylation, or p50 and p65 nuclear translocation. Previous studies using adult human ASM have shown that calcitriol can disrupt p65 nuclear translocation through enhancing p65 export from the nucleus (Agrawal et al., 2012). Surprisingly, we were unable to detect such an effect in fetal ASM. These contrasting observations may be due to variations in treatment protocols or could represent intrinsic differences between fetal and adult ASM. Nonetheless, it appears that calcitriol interactions with pro-inflammatory signaling pathways in developing ASM may differ from the adult.
Modulation of pro-inflammatory signaling mechanisms by VDR remains undefined in ASM. It has been postulated that VDR can induce its anti-proliferative effects via non-genomic effects in the cytoplasm and transcriptional activation and/or repression (Deeb et al., 2007). These effects include inhibition of the ERK and Akt pathways, which are key regulators of cell proliferation (Deeb et al., 2007). The anti-proliferative effects of calcitriol in ASM have been attributed to inhibition of the ERK signaling pathway (Damera et al., 2009). Our data demonstrate that unlike in adult ASM, calcitriol may have inhibitory effects on only some pathways, but the ERK inhibition at least suggests an effect on cellular proliferation and potentially the transcription factors, AP-1 and cFos. Future studies will be needed to determine how VDR regulates the ERK pathway via genomic and non-genomic mechanisms.
Remodeling of the airway is a central feature of asthma and influences disease severity. Children with moderate to severe asthma demonstrate airway obstruction and hyperresponsiveness, corresponding to thickening of airway smooth muscle and reticular basement membrane (Barbato et al., 2003; Saglani et al., 2005). The present studies demonstrate that calcitriol can regulate multiple aspects of airway remodeling in a model of developing ASM. Future studies are needed to examine the mechanistic actions of calcitriol and its therapeutic potential for the developing lung. Such strategies could lead to clinical advances in the treatment of young children with asthmatic symptoms.
Supplementary Material
Acknowledgements
The authors acknowledge funding from the National Institutes of Health (T32 HL 105355 (Britt, Vogel), F32 HL123075 (Britt), R01 HL 056470 (Prakash, Martin), R01 HL 088029 (Prakash); R01 HL 090595 (Pabelick)), the Mayo Clinic Departments of Anesthesiology (Pabelick, Prakash) and Obstetrics/Gynecology (Faksh), and the Mayo Graduate School (Chu).
Footnotes
This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: [10.1002/jcp.24814]
Additional Supporting Information may be found in the online version of this article.
References Cited
- Agrawal T, Gupta GK, Agrawal DK. Calcitriol decreases expression of importin alpha3 and attenuates RelA translocation in human bronchial smooth muscle cells. J Clin Immunol. 2012;32(5):1093–1103. doi: 10.1007/s10875-012-9696-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand SP, Selvaraj P. Effect of 1, 25 dihydroxyvitamin D(3) on matrix metalloproteinases MMP-7, MMP-9 and the inhibitor TIMP-1 in pulmonary tuberculosis. Clin Immunol. 2009;133(1):126–131. doi: 10.1016/j.clim.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK, Williams H. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368(9537):733–743. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Damera G, Bhandare R, Gu S, Lopez-Boado Y, Panettieri R, Jr, Tliba O. Vitamin D and glucocorticoids differentially modulate chemokine expression in human airway smooth muscle cells. Br J Pharmacol. 2008;155(1):84–92. doi: 10.1038/bjp.2008.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbato A, Turato G, Baraldo S, Bazzan E, Calabrese F, Tura M, Zuin R, Beghe B, Maestrelli P, Fabbri LM, Saetta M. Airway inflammation in childhood asthma. Am J Respir Crit Care Med. 2003;168(7):798–803. doi: 10.1164/rccm.200305-650OC. [DOI] [PubMed] [Google Scholar]
- Bettoun DJ, Burris TP, Houck KA, Buck DW, 2nd, Stayrook KR, Khalifa B, Lu J, Chin WW, Nagpal S. Retinoid X receptor is a nonsilent major contributor to vitamin D receptor-mediated transcriptional activation. Mol Endocrinol. 2003;17(11):2320–2328. doi: 10.1210/me.2003-0148. [DOI] [PubMed] [Google Scholar]
- Black JL, Burgess JK, Johnson PR. Airway smooth muscle--its relationship to the extracellular matrix. Respir Physiol Neurobiol. 2003;137(2–3):339–346. doi: 10.1016/s1569-9048(03)00157-5. [DOI] [PubMed] [Google Scholar]
- Brehm JM, Acosta-Perez E, Klei L, Roeder K, Barmada M, Boutaoui N, Forno E, Kelly R, Paul K, Sylvia J, Litonjua AA, Cabana M, Alvarez M, Colon-Semidey A, Canino G, Celedon JC. Vitamin D insufficiency and severe asthma exacerbations in Puerto Rican children. Am J Respir Crit Care Med. 2012;186(2):140–146. doi: 10.1164/rccm.201203-0431OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt RD, Jr, Faksh A, Vogel E, Martin RJ, Pabelick CM, Prakash YS. Perinatal factors in neonatal and pediatric lung diseases. Expert Rev Respir Med. 2013;7(5):515–531. doi: 10.1586/17476348.2013.838020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SD, Baxter KM, Stephenson ST, Esper AM, Brown LA, Fitzpatrick AM. Airway TGF-beta1 and oxidant stress in children with severe asthma: association with airflow limitation. J Allergy Clin Immunol. 2012;129(2):388–396. doi: 10.1016/j.jaci.2011.11.037. 396 e381-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess JK, Ceresa C, Johnson SR, Kanabar V, Moir LM, Nguyen TT, Oliver BG, Schuliga M, Ward J. Tissue and matrix influences on airway smooth muscle function. Pulm Pharmacol Ther. 2009;22(5):379–387. doi: 10.1016/j.pupt.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Bush A, Menzies-Gow A. Phenotypic differences between pediatric and adult asthma. Proc Am Thorac Soc. 2009;6(8):712–719. doi: 10.1513/pats.200906-046DP. [DOI] [PubMed] [Google Scholar]
- Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, Kleinman K, Gillman MW. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. 2007;85(3):788–795. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CJ, Seo M, Choi WS, Kim KS, Youn SA, Lindsey T, Choi YJ, Kim CM. Relationship between serum 25-hydroxyvitamin D and lung function among Korean adults in Korea National Health and Nutrition Examination Survey (KNHANES), 2008–2010. J Clin Endocrinol Metab. 2013;98(4):1703–1710. doi: 10.1210/jc.2012-3901. [DOI] [PubMed] [Google Scholar]
- Crane-Godreau MA, Black CC, Giustini AJ, Dechen T, Ryu J, Jukosky JA, Lee HK, Bessette K, Ratcliffe NR, Hoopes PJ, Fiering S, Kelly JA, Leiter JC. Modeling the influence of vitamin D deficiency on cigarette smoke-induced emphysema. Front Physiol. 2013;4:132. doi: 10.3389/fphys.2013.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damera G, Fogle HW, Lim P, Goncharova EA, Zhao H, Banerjee A, Tliba O, Krymskaya VP, Panettieri RA., Jr Vitamin D inhibits growth of human airway smooth muscle cells through growth factor-induced phosphorylation of retinoblastoma protein and checkpoint kinase 1. Br J Pharmacol. 2009;158(6):1429–1441. doi: 10.1111/j.1476-5381.2009.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7(9):684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- Devereux G, Litonjua AA, Turner SW, Craig LC, McNeill G, Martindale S, Helms PJ, Seaton A, Weiss ST. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr. 2007;85(3):853–859. doi: 10.1093/ajcn/85.3.853. [DOI] [PubMed] [Google Scholar]
- Doherty T, Broide D. Cytokines and growth factors in airway remodeling in asthma. Curr Opin Immunol. 2007;19(6):676–680. doi: 10.1016/j.coi.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Freishtat RJ, Iqbal SF, Pillai DK, Klein CJ, Ryan LM, Benton AS, Teach SJ. High prevalence of vitamin D deficiency among inner-city African American youth with asthma in Washington, DC. J Pediatr. 2010;156(6):948–952. doi: 10.1016/j.jpeds.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaultier C, Harf A, Balmain N, Cuisinier-Gleizes P, Mathieu H. Lung mechanics in rachitic rats. Am Rev Respir Dis. 1984;130(6):1108–1110. doi: 10.1164/arrd.1984.130.6.1108. [DOI] [PubMed] [Google Scholar]
- Gorman S, Tan DH, Lambert MJ, Scott NM, Judge MA, Hart PH. Vitamin D(3) deficiency enhances allergen-induced lymphocyte responses in a mouse model of allergic airway disease. Pediatr Allergy Immunol. 2012;23(1):83–87. doi: 10.1111/j.1399-3038.2011.01146.x. [DOI] [PubMed] [Google Scholar]
- Gupta A, Sjoukes A, Richards D, Banya W, Hawrylowicz C, Bush A, Saglani S. Relationship between serum vitamin D, disease severity, and airway remodeling in children with asthma. Am J Respir Crit Care Med. 2011;184(12):1342–1349. doi: 10.1164/rccm.201107-1239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman WR, Smelter DF, Sathish V, Karass M, Kim S, Aravamudan B, Thompson MA, Amrani Y, Pandya HC, Martin RJ, Prakash YS, Pabelick CM. Oxygen dose responsiveness of human fetal airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2012;303(8):L711–L719. doi: 10.1152/ajplung.00037.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie M, St-Pierre Y. Epigenetic regulation of mmp-9 gene expression. Cell Mol Life Sci. 2013;70(17):3109–3124. doi: 10.1007/s00018-012-1214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Peng M, Jiang L, Sun Q, Zhang K, Lian F, Litonjua AA, Gao J, Gao X. Vitamin D deficiency is associated with decreased lung function in Chinese adults with asthma. Respiration. 2011;81(6):469–475. doi: 10.1159/000322008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmstrom K, Pelkonen AS, Makela MJ. Remodeling, inflammation and airway responsiveness in early childhood asthma. Curr Opin Allergy Clin Immunol. 2013;13(2):203–210. doi: 10.1097/ACI.0b013e32835e122c. [DOI] [PubMed] [Google Scholar]
- McKleroy W, Lee TH, Atabai K. Always cleave up your mess: targeting collagen degradation to treat tissue fibrosis. Am J Physiol Lung Cell Mol Physiol. 2013;304(11):L709–L721. doi: 10.1152/ajplung.00418.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LJ, Rye PJ, Gibson NA, Burton PR, Landau LI, Lesouef PN. Airway responsiveness in early infancy predicts asthma, lung function, and respiratory symptoms by school age. Am J Respir Crit Care Med. 2001;163(1):37–42. doi: 10.1164/ajrccm.163.1.2005013. [DOI] [PubMed] [Google Scholar]
- Pandya HC, Snetkov VA, Twort CH, Ward JP, Hirst SJ. Oxygen regulates mitogen-stimulated proliferation of fetal human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2002;283(6):L1220–L1230. doi: 10.1152/ajplung.00268.2001. [DOI] [PubMed] [Google Scholar]
- Pera T, Gosens R, Lesterhuis AH, Sami R, van der Toorn M, Zaagsma J, Meurs H. Cigarette smoke and lipopolysaccharide induce a proliferative airway smooth muscle phenotype. Respir Res. 2010;11:48. doi: 10.1186/1465-9921-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prufer K, Barsony J. Retinoid X receptor dominates the nuclear import and export of the unliganded vitamin D receptor. Mol Endocrinol. 2002;16(8):1738–1751. doi: 10.1210/me.2001-0345. [DOI] [PubMed] [Google Scholar]
- Puthothu B, Bierbaum S, Kopp MV, Forster J, Heinze J, Weckmann M, Krueger M, Heinzmann A. Association of TNF-alpha with severe respiratory syncytial virus infection and bronchial asthma. Pediatr Allergy Immunol. 2009;20(2):157–163. doi: 10.1111/j.1399-3038.2008.00751.x. [DOI] [PubMed] [Google Scholar]
- Saglani S, Malmstrom K, Pelkonen AS, Malmberg LP, Lindahl H, Kajosaari M, Turpeinen M, Rogers AV, Payne DN, Bush A, Haahtela T, Makela MJ, Jeffery PK. Airway remodeling and inflammation in symptomatic infants with reversible airflow obstruction. Am J Respir Crit Care Med. 2005;171(7):722–727. doi: 10.1164/rccm.200410-1404OC. [DOI] [PubMed] [Google Scholar]
- Song Y, Qi H, Wu C. Effect of 1,25-(OH)2D3 (a vitamin D analogue) on passively sensitized human airway smooth muscle cells. Respirology. 2007;12(4):486–494. doi: 10.1111/j.1440-1843.2007.01099.x. [DOI] [PubMed] [Google Scholar]
- Sundar IK, Hwang JW, Wu S, Sun J, Rahman I. Deletion of vitamin D receptor leads to premature emphysema/COPD by increased matrix metalloproteinases and lymphoid aggregates formation. Biochem Biophys Res Commun. 2011;406(1):127–133. doi: 10.1016/j.bbrc.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland ER, Goleva E, Jackson LP, Stevens AD, Leung DY. Vitamin D levels, lung function, and steroid response in adult asthma. Am J Respir Crit Care Med. 2010;181(7):699–704. doi: 10.1164/rccm.200911-1710OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiss AL, Simmons JG, Jobin C, Lund PK. Tumor necrosis factor (TNF) alpha increases collagen accumulation and proliferation in intestinal myofibroblasts via TNF receptor 2. J Biol Chem. 2005;280(43):36099–36109. doi: 10.1074/jbc.M505291200. [DOI] [PubMed] [Google Scholar]
- Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92(8):827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- Zosky GR, Hart PH, Whitehouse AJ, Kusel MM, Ang W, Foong RE, Chen L, Holt PG, Sly PD, Hall GL. Vitamin D Deficiency at 16–20 Weeks Gestation Is Associated with Impaired Lung Function and Asthma at 6 Years of Age. Ann Am Thorac Soc. 2014 doi: 10.1513/AnnalsATS.201312-423OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.