Abstract
Stem and progenitor cells play important roles in organogenesis during development and in tissue homeostasis and response to injury postnatally. As the regenerative capacity of many human tissues is limited, cell replacement therapies hold great promise for human disease management. Pluripotent stem cells such as embryonic stem (ES) cells and induced pluripotent stem (iPS) cells are prime candidates for the derivation of unlimited quantities of clinically relevant cell types through development of directed differentiation protocols, i.e. the recapitulation of developmental milestones in in vitro cell culture. Tissue-specific progenitors, including progenitors of endodermal origin, are important intermediates in such protocols since they give rise to all mature parenchymal cells. In this review, we focus on the in vivo biology of embryonic endodermal progenitors in terms of key transcription factors and signaling pathways. We critically review the emerging literature aiming to apply this basic knowledge to achieve the efficient and reproducible in vitro derivation of endodermal progenitors such as pancreas, liver and lung precursor cells.
Introduction
Gastrulation, the step that follows blastula formation during development, consists of the separation of the three embryonic germ layers, namely ectoderm, endoderm and mesoderm. Vertebrate animal models have revealed highly conserved mechanisms of endoderm morphogenesis and determination [Grapin-Botton and Constam, 2007; Kimelman and Griffin, 2000]. Following establishment of the endoderm, complex morphogenetic movements and crosstalk with mesodermal tissues lead to an endodermal gut tube with an established anterior-posterior (AP) axis and several progenitor domains that will give rise to the parenchyma of endodermal organs (thyroid, lung, pancreas, liver, gastrointestinal (GI) tract). Although our understanding of endodermal differentiation has greatly advanced in recent years [Zorn and Wells, 2009], several gaps in our knowledge remain concerning the specification of endodermal progenitors.
A particularly attractive system to study developmental cell fate decisions in vitro is the use of pluripotent stem cells (PSCs), such embryonic stem (ES) cells or their engineered equivalent, induced pluripotent stem (iPS) cells. ES cells are pluripotent cells derived from the inner cell mass at the blastocyst stage of vertebrate development [Smith, 2001]. Mouse ES cells were first derived in 1981 [Evans and Kaufman, 1981; Martin, 1981] and have been routinely used for gene targeting in mice [Manis, 2007]. The derivation of human ES (hES) cells in 1998 [Thomson et al., 1998] was a major breakthrough since the derivation of clinically relevant populations of any embryonic germ layer origin was possible for the first time [Murry and Keller, 2008]. Several approaches have been developed in attempts to differentiate ES cells into desired functional lineages. Of these, the method of “directed differentiation”, i.e. the multi-stage recapitulation in vitro of developmental milestones that are known to occur during embryonic development in vivo, has been proven to be particularly successful [Gadue et al., 2005]. Optimization of directed differentiation has led to the establishment of efficient protocols for the derivation of various cell types from mouse and human ES cells [Gouon-Evans et al., 2006; Kattman et al., 2006; Wichterle et al., 2002].
Although hES cells have been a successful tool in stem cell research, several issues such as difficulty of derivation, ethical concerns and immunogenicity may limit their clinical use in cell therapies. The groundbreaking paper by [Takahashi and Yamanaka, 2006] described the reprogramming of mouse fibroblasts to iPS cells by the transfer of four transcription factors (TFs), Oct3/4, Sox2, c-Myc and Klf4. This discovery opened a new, exciting chapter in the history of stem cell biology. Soon after, the derivation of human iPS cells was reported [Takahashi et al., 2007; Yu et al., 2007] and in vitro human disease modeling became a possibility. Currently, there are several human iPS cell disease models (reviewed in [Wu and Hochedlinger, 2011]) and efforts to study complex diseases in vitro or develop drug screening platforms are underway [Ikonomou et al., 2011; Inoue and Yamanaka, 2011].
The derivation of functional differentiated progeny from PSCs is a sine qua non for the success of disease modeling or cell-based therapies. Despite the fact that several serious ailments affect tissues of endodermal origin, protocols to differentiate PSCs to endodermal lineages are still underdeveloped. Understanding the inductive signals and epigenetic and genetic networks that govern endodermal progenitor formation in vivo will be instrumental in deriving such progenitors in vitro at high fidelity and purity.
Definitive endoderm
There are excellent reviews on vertebrate endoderm development [Grapin-Botton, 2008; Zorn and Wells, 2009]; our review will focus mostly on signaling pathways and TFs that have been important in definitive endoderm derivation from human and mouse PSCs.
Transcription Factors for Marking Endodermal Development
The formation of the endodermal germ layer also called definitive endoderm (DE) as opposed to primitive endoderm (an extraembryonic layer with negligible contribution to gut tube), starts at gastrulation and is preceded by the formation of the primitive streak (PS), the region from which definitive endodermal precursor cells will emerge. Anatomically, the PS is a zone of cells at the interface of the epiblast and visceral endoderm (VE) at the posterior end of the embryo [Gadue et al., 2005]. In the mouse embryo, precise spatiotemporal PS formation depends on a combination of pathways such as Wnt and Nodal in the posterior epiblast and inhibitors thereof, such as Lefty and Cerberus, in the anterior VE [Conlon et al., 1994; Liu et al., 1999; Perea-Gomez et al., 2002]. Nodal belongs to the TGF-β superfamily of growth factors and Nodal signaling is involved both in PS induction and left-right asymmetry [Schier, 2003]. During late PS formation the expression of Nodal transcripts in the embryo becomes restricted predominantly to the developing node in the vicinity of the anterior primitive streak, and high levels of Nodal signaling are associated with further spatiotemporal patterning of the late anterior primitive streak marked by first induction of TFs required for endoderm formation. It is currently believed that PS contains mesendodermal progenitors characterized by the expression of the transcription factor Brachyury [Conlon et al., 1994; Kimelman and Griffin, 2000; Rodaway and Patient, 2001].
TFs of the forkhead, Gata and Sox families are implicated in the formation of DE with conserved roles in vertebrates and invertebrates [Grapin-Botton and Constam, 2007]. Furthermore, Foxa and Gata factors have been called “pioneer” factors [Zaret et al., 2008] because they bind to many endodermal-associated gene loci, even in regions of compact chromatin, thus rendering these loci accessible to other transcription factors and bestowing lineage-specific competence to multipotent endodermal progenitors. For example, Foxa2 (HNF3β) is a member of the forkhead family of TFs and has important roles in the derivation of the node, notochord and endoderm [Ang et al., 1993; Monaghan et al., 1993; Weinstein et al., 1994{Ang, 1994 #233]. Within the anterior primitive streak Foxa2 expression marks the emergence of the earliest endodermal progenitors and along with Foxa1 (HNF3a) is involved in the maintenance of DE and the formation of several endodermal tissues such as lung and liver, as indicated by fate mapping and double knock-out studies [Ang et al., 1993; Lee et al., 2005; Wan et al., 2005]. The expression of another forkhead factor, Foxa3 (HNF3?) starts later in endodermal development and is initially restricted to more posterior structures such as liver, pancreas, midgut and hindgut [Monaghan et al., 1993]. Although Gata4/6 factors in the mouse are initially important for the determination of VE [Morrisey et al., 1998; Patient and McGhee, 2002], they have important roles in the development of DE [Carrasco et al., 2012; Morrisey et al., 1998; Watt et al., 2007; Xuan et al., 2012]. For example, Gata4 and Gata6 are expressed early in mouse developing DE and are crucial for the survival, differentiation and morphogenesis of the foregut [Kuo et al., 1997; Morrisey et al., 1998]. Deletion of either of these transcription factors is lethal prior to the completion of gastrulation. Once the lung forms, Gata6, but not Gata4 continues to be expressed in the lung epithelium and is required for appropriate lung epithelial differentiation, as evidenced by the observation that GATA6-null ES cells fail to contribute to developing respiratory epithelium in chimeric mouse embryos [Morrisey et al., 1998]. In addition, combined deletion of Gata4/Gata6 in the developing pancreas of conditional double knock-out mice results in a reduced Pdx1+ pancreatic progenitor pool and severe defects in pancreatic organogenesis [Carrasco et al., 2012; Xuan et al., 2012]. Gata4 and Foxa1 also facilitate hepatic differentiation as they bind the albumin enhancer (early marker of the liver endoderm) in early endoderm [Bossard and Zaret, 1998]. Regarding Sox factors, Sox17 is indispensable for the derivation of DE [Kanai-Azuma et al., 2002]. The absence of DE in a Sox17-null mouse reveals important cell-autonomous roles for this factor both in the foregut where its absence leads to increased apoptosis and in the mid and hindgut regions where DE cells fail to displace extraembryonic endoderm [Kanai-Azuma et al., 2002]. Another Sox factor, Sox2, has been found to play important functional roles in both early endodermal gut tube patterning [Ishii et al., 1998; Matsushita et al., 2008] as well as the differentiation of DE-derived epithelia, such as the proximal lung epithelium [Domyan et al., 2011; Que et al., 2009; Wang et al., 2013].
In vitro derivation of definitive endoderm
Given the detailed in vivo characterization of the temporal kinetics of expression of endodermally-enriched TFs in developing embryos, investigators have utilized these TFs to track the sequence of endodermal formation from PSCs undergoing differentiation in vitro. ES cell lines engineered to carry reporter genes (e.g. green fluorescence protein; GFP) targeted to the loci encoding several primitive streak or DE-associated TFs have proven to be important tools in the elucidation of the developmental processes and signals that lead to DE derivation [Gadue et al., 2005]. For instance, by using a mouse ES cell line with GFP knocked in to the brachyury locus (Bry-GFP), Gadue et al. [Gadue et al., 2006] demonstrated that DE, unlike VE, is the progeny of Bry-GFP+ cells and can be derived in vitro from mouse ES cells by inducing Nodal signaling via the addition of high levels (30–100 ng/ml) of activin A (hereafter referred to as activin) [Kubo et al., 2004]. In a similar approach, [Tada et al., 2005] engineered a Gsc-GFP knock-in mouse ES cell line and demonstrated that the mesendodermal stage of mouse development can be captured in culture as Gsc-GFP+E-cadherin(ECD)+PDGFRa(aR)+ cells which can then give rise to DE and mesodermal lineages through Gsc+ECD+aR− and Gsc+ECD+aR+ intermediates, respectively. Further work from the same laboratory using a double knock-in line, Gsc-GFP/Sox17-hCD25, identified Gsc+Sox17+ cells as bona fide DE cells and Cxcr4 as a DE-specific cell surface marker following activin stimulation of ES cells [Yasunaga et al., 2005]. To gain additional insights in the mechanisms of endodermal differentiation, the Keller laboratory developed an elaborate model of PS by the generation of the double knock-in line Foxa2-hCD4/Bry-GFP. By using this line, Gadue and coworkers observed that although the PS population was Foxa2+Bry+, the expression levels of Foxa2 within this population correlated with distinct differentiation outcomes [Gadue et al., 2006]. Foxa2hi cells exhibited DE and cardiac potential whereas Foxa2lo cells exhibited hematopoietic potential. Furthermore, this model recapitulated the signaling events that take place in vivo since high activin levels induced DE whereas Wnt or low levels of activin or both gave rise to paraxial mesoderm.
The identification of activin/nodal as the single most important signal for the derivation of mouse and human DE, has been a significant discovery in many aspects. First, it led to the routine, high-efficiency derivation from PSCs of DE endowed with the potential to differentiate to downstream endodermal lineages such as pancreatic, hepatic and intestinal cells [Gouon-Evans et al., 2006; Kubo et al., 2004]. Also, the identification of cell surface marker combinations such as C-KIT/CXCR4 [Gouon-Evans et al., 2006], or CXCR4/EpCAM [Yasunaga et al., 2005], that can distinguish DE from VE made possible the high-purity isolation of endodermal progeny of genetically un-manipulated ES or iPS cells. Most importantly, activin (alone or in conjunction with low levels of Wnt and serum) efficiently induces DE in hES cells [D’Amour et al., 2005; Green et al., 2011; Wang et al., 2011]. Although in the undifferentiated state Activin and FGF signaling cooperatively control hES cell pluripotency through direct NANOG targeting [Vallier et al., 2005; Xu et al., 2008a], Smad2/3 signaling appears to activate disparate gene networks in undifferentiated hES cells vs. hES cell-derived DE [Brown et al., 2011].
DE patterning and endodermal primordia formation
The morphogenetic processes that result in the formation of the endodermal sheet during gastrulation, the closure of the primitive gut tube and the subsequent emergence of endodermal organ primordia coincide with signals that pattern the DE along the AP and dorso-ventral (DV) axes and gradually restrict the lineage competence of the various endodermal progenitor populations [Zorn and Wells, 2009]. Precise spatial arrangement of endodermal cells appears to take place as early as the gastrulation stage since cells that leave the PS first contribute to the foregut whereas cells that exit later contribute to posterior endoderm [Lawson and Pedersen, 1987]. Experimental models based on the co-culture of developing mouse germ layers have suggested that cells in the early endoderm are still plastic since instructive signals from the posterior mesoderm/ectoderm can alter the fate of anterior endoderm and vice versa [Wells and Melton, 2000]. Other experimental models have compared the relative plasticity of anterior vs posterior DE in developing chick embryos, suggesting the concept of ‘posterior dominance’ [Kimura et al., 2007; Kimura et al., 2006; Kumar et al., 2003a] where anterior DE may be instructed to change to a posterior fate whereas after posterior patterning, DE appears to lose competence to respond to anteriorization signals.
After the formation of the anterior and caudal intestinal portals, the regionalization of the emerging gut tube is marked by an anterior Sox2+ domain moving posteriorly and a posterior Cdx2+ domain moving anteriorly which finally form a clear boundary at midgut level [Sherwood et al., 2009]. This broad A-P patterning of the endodermal gut tube is also characterized by spatially-enriched expression of additional TFs, such as Tbx1 and Hoxa1,2 in anterior foregut [Longmire et al., 2012; Sherwood et al., 2009] and Foxa3 in posterior foregut endoderm and mid- and hindgut [Monaghan et al., 1993; Sherwood et al., 2009]. In mouse, the appearance of endodermal primordia that will give rise to the epithelial of organs derived from the gut tube is complete by embryonic day 9.5 (E9.5) and is characterized by discreet domains of transcription factor expression, such as Nkx2-1 in lung and thyroid primordia [Kimura et al., 1996], Pax8 in thyroid [Mansouri et al., 1998] Hhex in liver and thyroid [Martinez-Barbera et al., 2000], Ptf1a in pancreas [Kawaguchi et al., 2002] and Cdx2 in the small intestine and cecum/colon primordia [Gao et al., 2009; Sherwood et al., 2009].
DE patterning and progenitor domain specification depend on reciprocal interactions between the endoderm and surrounding non-endodermal tissues. Sonic hedgehog (Shh) signaling is necessary for anterior foregut development and is a typical example of epithelial-mesenchymal interactions since endodermal SHH acts on the surrounding splanchnic mesenchyme which in turn proliferates and signals to the developing lungs, trachea and esophagus [Litingtung et al., 1998; Motoyama et al., 1998]. Retinoic acid (RA), FGF4, and Wnt signaling have been described as posteriorizing signals in endodermal development in several model organisms [Bayha et al., 2009; Dessimoz et al., 2006; McLin et al., 2007; Stafford and Prince, 2002]. RA signaling inhibition during early somitogenesis in chick embryos abolishes the expression of posterior foregut endoderm markers such as Pdx1 and intestinal markers such as CdxA (the homologue of mammalian Cdx2) [Bayha et al., 2009] whereas RA overexpression during late gastrulation in zebrafish shifts the pancreatic and liver domains anteriorly [Stafford and Prince, 2002]. High levels of mesodermal FGF4 establish the posterior domain of the folding gut tube in chick embryos as well as in mouse endodermal explant cultures [Wells and Melton, 2000]. For example, FGF4 overexpression during early somitogenesis expands the posterior domain (loss of Hhex (Hex1) and Nkx2-1), while FGF4 inhibition results in expansion of Hhex posteriorly [Dessimoz et al., 2006]. In the ventral foregut, specification of the liver and lung domains have been suggested in mouse models to be regulated by different levels of FGFs secreted from adjacent mesodermal structures such as the cardiac mesenchyme [Serls et al., 2005]. Finally, foregut development is repressed posteriorly through mesodermal Wnt signaling, whereas the latter is suppressed anteriorly by endoderm-secreted Wnt inhibitors [McLin et al., 2007].
Dorsoventral patterning of the gut tube is more evident in the anterior foregut where BMP4 expression ventrally and its inhibitor, noggin, dorsally appear to set the boundaries for the prospective trachea and esophagus domains, respectively [Domyan et al., 2011; Que et al., 2006]. Both Shh and BMP4 signaling also have roles in the separation of the developing trachea and esophagus as their disruption leads to morphogenetic defects such esophageal atresia and tracheoesophageal fistula. In the posterior foregut endoderm, the TF Hlxb9 is necessary for the emergence of the dorsal pancreatic bud [Harrison et al., 1999; Li et al., 1999] and it is the only TF with a dorsoventral expression gradient in the posterior endoderm [Sherwood et al., 2009].
Several publications demonstrate that AP patterning information is relevant in directed differentiation of ES cells where the spatial aspects of development are lost. RA is an example of a successful use of an endodermal posteriorizing signal for the derivation of pancreatic progenitors. In hES cell pancreatic differentiation, RA has been used to direct hES cell-derived DE to a posterior foregut endodermal fate [D’Amour et al., 2006], or induce PDX1 expression in sorted CXCR4+ DE [Cai et al., 2010]. Similarly, RA promoted the derivation of Pdx1-GFP+ cells from mouse ES cells [Micallef et al., 2005]. Promotion of anterior foregut fate was a key step in mouse and human PSC protocols aiming to render DE progenitors lung-, thyroid- and thymus-competent [Green et al., 2011; Longmire et al., 2012; Mou et al., 2012]. This was achieved by brief inhibition of BMP4 and TGF-β signaling followed by re-activation of BMP4 signaling and addition of other ventralizing factors such as FGF2 and WNT. In our work, ES cell-derived endoderm contained approximately 40% of Foxa3+ cells in the absence of “anteriorization”, indicating significant AP patterning of the cells in response to activin, analogous to the posterior-dominant fashion of DE patterning that has been described in vivo [Kumar et al., 2003b].
Characterization and expansion of in vitro DE progenitors
Directed differentiation protocols are characterized by the semiautonomous nature of intermediate developmental stages and by modularity (e.g. the DE progenitor “module” can be combined with different downstream differentiation “modules” for diverse outcomes such as mature liver, pancreas or intestinal cell types) [Lenas et al., 2009]. Since embryonic progenitors normally exist as transient, short-lived cell populations in vivo, a major goal of PSC-based regenerative medicine is the in vitro derivation and expansion of stable, self-renewing populations of their in vitro counterparts. Concerning DE progenitors, there have been several strategies to establish and study such populations in vitro [Semb, 2008]. Constitutive overexpression of SOX17 and SOX7 in hES cells led to the establishment of cell lines with characteristics of DE and extraembryonic endoderm, respectively [Seguin et al., 2008]. The SOX17-overexpression line demonstrated high levels of C-KIT/CXCR4 co-expression, and efficient pancreatic and hepatic differentiation even in the absence of activin treatment. Interestingly, this population also expressed OCT4 and NANOG, known markers of PSCs. A cell population with anterior DE potential was isolated by using a Hex reporter mouse ES cell line and sorting Hex+Cxcr4+ cells post activin stimulation [Morrison et al., 2008]. These cells expressed markers of anterior DE such as Cer1 and Otx2 but not of posterior DE such as Cdx2. They were capable of limited self-renewal (2000-fold expansion) in a medium containing FGF2, BMP4 and VEGF and of differentiation towards pancreatic and liver lineages.
A major breakthrough in the in vitro derivation of expandable DE progenitors [Cheng et al., 2012], was reported with the serendipitous finding of morphologically distinct colonies appearing during the course of hES cell hepatic differentiation. Isolation of these colonies led to the propagation and characterization of a self-renewing endodermal progenitor (EP) cell population from both human ES and iPS cell cultures. EP cells are CXCR4+CD117+FOXA1+, can be cultured under serum-free conditions in the presence of BMP4, FGF2, EGF and VEGF, and have an expansion capacity of >1016 fold. Notably, EP cells can undergo efficient pancreatic differentiation in vitro and hepatic and intestinal differentiation in vitro and in vivo. A self-renewing population of SOX2+ human foregut endodermal progenitor cells (hFSCs) derived from PSCs in vitro was also reported recently [Hannan et al., 2013]. hFSCs were derived under serum-free conditions in media containing activin, BMP4, FGF2, EGF and HGF and maintained expression of SOX2 and pan-endodermal markers after serial passaging. Similar to EP cells, hFSCs were able to give rise to pancreatic and hepatic progeny in vitro, but they also expressed lung/thyroid markers such as NKX2-1 upon transplantation in NOD-SCID mice. Whether EP cells and hFSCs represent distinct progenitor populations or interconvertible stable states of the same endodermal progenitor will require further studies.
Often overlooked in directed differentiation protocols, the culture substratum can be a substantial component in terms of paracrine or adhesion signaling. The judicious substratum selection for the in vitro derivation and expansion of DE progenitors has been stressed in several recent papers. Although mouse DE progenitors can be cultured on mouse embryonic fibroblast feeders [Morrison et al., 2008; Seguin et al., 2008], their self-renewal potential was considerably increased (up to 6-million-fold expansion) when cultured on pancreas-derived mesenchymal primary lines. Likewise, human SOX17+/FOXA2+ endodermal cells were capable of 65-million-fold expansion when cultured on the same mesenchyme while retaining their potential for endocrine pancreatic differentiation [Sneddon et al., 2012]. Expansion of human endodermal progenitors has also been attempted on more defined substrata as demonstrated by the absolute requirement of Matrigel for the long-term EP cell culture [Cheng et al., 2012]. In an attempt to dissect the integrin signaling involved in the derivation of DE from hES cells, the Willert group employed cellular combinatorial arrays of known extracellular matrix (ECM) proteins [Brafman et al., 2013b]. Fibronectin and vitronectin were identified as the key ECM components that promote DE derivation. ECM signaling was mediated by the integrins ITGA5 and ITGAV, and their co-expression was used for the purification by FACS of a population with high levels of DE and primitive gut tube markers.
Endodermal tissue-specific progenitors
DE is the precursor stage to all endodermal lineages. As discussed below, the unambiguous and efficient derivation of embryonic tissue-specific progenitors such as hepatic, pancreatic, intestinal and lung progenitors downstream of DE are vital for the successful derivation of mature cell types such as hepatocytes, β-cells, enterocytes and Type II alveolar epithelial cells. In the following section, the various endoderm domain progenitors are reviewed with emphasis on recent significant advances in derivation of anterior foregut progenitors.
Anterior foregut progenitors
In contrast to the more mature field of ES cell directed differentiation to pancreatic/liver lineages, the derivation of anterior lineages such as lung, thyroid and thymus has only recently been reported. Several factors such as the paucity of developmental studies on progenitor specification, the lack of appropriate ES cell reporter lines and the incomplete understanding of anterior foregut patterning can in part explain this discrepancy.
Thyroid and lung primordia arise within the anterior foregut at E8.5 and E9.0, respectively and are characterized by the expression of the homeodomain TF Nkx2-1 (also known as thyroid transcription factor-1; Ttf1 or Titf1) [Desai et al., 2004; Hösgör et al., 2002; Kimura et al., 1996; Lazzaro et al., 1991]. Although Nkx2-1 is not necessary for specification of either organ, its absence results in elimination of the thyroid rudiment through apoptosis and in profound defects in lung branching morphogenesis and differentiation [Kimura et al., 1996; Kimura et al., 1999]. The major epithelial compartment of the thyroid gland also requires the function of Pax8, a paired box TF [Mansouri et al., 1998]. In the absence of Pax8, the thyroid anlage evagivates from the foregut but it fails to develop and the final organ is completely devoid of follicular cells.
There are few reports on the putative signals that specify lung and thyroid primordia. FGF signaling through the FGFR4 was found to be sufficient for the induction of Nkx2-1 and its target genes SPC and CC10 (lung) and Tg (thyroid) in in vitro culture of ventral foregut explants [Serls et al., 2005]. The activation of Nkx2-1 expression required high doses of FGF2 (500 ng/ml) and was interpreted as reflecting the in vivo FGF levels originating from the cardiac mesoderm in close proximity to the lung and thyroid primordia. Wnt signaling has also been shown to be important for lung specification [Goss et al., 2009; Harris-Johnson et al., 2009]. In a knockout mouse lacking Wnt2/2b, lung agenesis was observed while the thyroid specified and developed normally. Conversely, expression of stabilized β-catenin in the foregut resulted in posterior expansion of the Nkx2-1 expressing domain, indicating that Wnt signaling was necessary and sufficient for the specification of lung progenitors within the foregut [Goss et al., 2009]. Using a conditional foregut β-catenin knockout, Harris-Johnson et al. reached similar conclusions although they also detected the transient expression of Nkx2-1 in the prospective lung domain, indicating that canonical Wnt signaling may be required for maintenance of early primordial lung progenitors rather than for their initial lineage specification from endoderm [Harris-Johnson et al., 2009]. The same group demonstrated that BMP4 signaling is required for trachea formation and lung bud initiation through repression of Sox2 [Domyan et al., 2011]. Sox2 is initially involved in the dorsoventral patterning of anterior foregut (trachea-esophagus separation) and subsequently in the differentiation of proximal lineages in the developing lung [Que et al., 2007; Que et al., 2009].
More anterior endodermal tissues, such as the thymic epithelial compartment and the parathyroid glands, are derived from the third pharyngeal pouches [Gordon and Manley, 2011]. Both organs arise from a common primordium that is patterned by E11.5 to thymus and parathyroid domains. Ensuing morphogenetic movements result in separation of the thymus lobes that move caudally from the parathyroid glands that move laterally to the developing thyroid. Early expression of the TFs Foxn1 and Gcm2 defines the thymus and parathyroid domains respectively; nevertheless, neither factor is required for specification of the respective tissue [Gordon and Manley, 2011]. The thymus epithelial cells (TECs) are organized into two compartments in the adult organ, the cortical (inner) and the medullar (outer) with distinct functional roles in thymocyte differentiation. Although a dual germ layer origin of cTECs and mTECs (neuroectodermal and endodermal) was proposed in early theories of thymus development (reviewed in [Rodewald, 2008]), it is now established that TECs have a common endodermal origin [Rossi et al., 2006]. Regarding the pathways that are involved in thymus/parathyroid specification, little is known; BMP4 and Wnt are presumptive thymus specification signals whereas Shh appears to be required for the specification of the parathyroid glands [Gordon and Manley, 2011].
There have been sporadic attempts to derive the most anterior foregut endoderm (AFE) lineages such as parathyroid epithelium [Bingham et al., 2009] and thymic epithelial progenitors [Hidaka et al., 2010; Lai and Jin, 2009] from ES cells. These data are hard to interpret because of the non-developmental nature of the protocols (e.g. absence of activin stimulation) and the seemingly stochastic expression of parathyroid- and thymus-related genes. Similarly, early attempts to derive lung epithelium from ES cells relied heavily on the inefficient expression of late lung markers or the use of surfactant protein C as an indicator of mature alveolar cell differentiation when it is expressed in both developing lung epithelium and Type II alveolar epithelial cells (reviewed in [Wetsel et al., 2011]). Thyroid differentiation of ES cells has been also reported based on the expression of late thyroid markers such as thyroglobulin or the use of a TSHR reporter, a gene that is dispensable for normal thyroid gland development [Arufe et al., 2006; Ma et al., 2009].
Three recent studies have reported efficient derivation of AFE progenitors from PSCs in vitro [Brafman et al., 2013a; Green et al., 2011; Kearns et al., 2013]. Snoeck and colleagues demonstrated that inhibition of BMP4 and TGF-β signaling at the DE stage of hES or iPS cell directed differentiation leads to AFE derivation as witnessed by the reduced expression of hepatic and intestinal markers and the up-regulation of anterior markers such as SOX2, TBX1, NKX2-5 and PAX9 [Green et al., 2011]. The “anteriorized” endoderm was then differentiated to a ventral foregut fate with treatment with WNT3a, KGF (FGF7), FGF10, BMP4 and EGF and could give rise to lung and parathyroid lineages in response to RA and SHH signaling, respectively. Using a similar approach and a Pax9-Venus knock-in mouse ES cell line, a subset of AFE cells (PAX9+ cells) were sorted to purity and were found to possess a gene signature reminiscent of PAX9+ cells from E9.5 anterior mouse foregut [Kearns et al., 2013]. Since all known markers of AFE are either TFs (SOX2, PAX9, TBX1) or secreted molecules (CER) the recent discovery of human AFE putative cell surface markers is noteworthy [Brafman et al., 2013a]. A SOX2-GFP hES cell reporter line was used to derive and sort SOX2+ AFE-like cells by BMP4/TGF-β signaling inhibition at the DE stage. These cells had expression of AFE markers such as TBX1 and PAX9 and exhibited robust differentiation to NKX2-1+ progenitors upon lung/thyroid directed differentiation based on published protocols (see below). Most importantly, the enriched expression of the cell surface markers CD56+ (NCAM) and CD271+ (NGFR) appeared to distinguish SOX2+ from SOX2− cells at the AFE stage. Whether these markers can reliably be used with any human PSC line to purify AFE and whether CD56+CD271+ co-expression delineates AFE from other mammalian species in vitro and in vivo are still open questions.
Blocking of DE posterior patterning as a prerequisite for the efficient derivation of lung progenitors was a prominent feature in work focused on the derivation of lung epithelium from PSCs [Longmire et al., 2012; Mou et al., 2012]. In our published work, treatment of mouse DE cells with noggin and SB43142 to inhibit BMP4 and activin/TGF-β signaling followed by combined BMP4 and FGF2 signaling were necessary and sufficient conditions to specify Nkx2-1-expressing lung/thyroid progenitors in vitro from mouse ES cells [Longmire et al., 2012]. FACS-purified progenitors using a Nkx-2-1-GFP mouse ES cell reporter line expressed markers of mature proximal (CC10+, Foxj1+) and distal (SPC+, T1a+) lung as well as thyroid (Pax8+, Tg+) lineages and were able to repopulate 3D decellularized lung scaffolds. Rajagopal and colleagues developed a similar protocol to derive Nkx2-1+ lung progenitors by TGF-β signaling antagonism in DE cells followed by stimulation of BMP4, FGF2 and Wnt signaling. These progenitors adopted an airway progenitor identity upon treatment with BMP7 and FGF7. PSCs exposed to this differentiation protocol followed by bulk subcutaneous injection into immunodeficient mice gave rise to organized epithelia surrounding lumenal structures. Cells within these grafted epithelia growing subcutaneously expressed Nkx2-1 as well as markers of airway basal (p63), club (Clara) (CC10), and goblet (Muc5AC) cells. Additional cells formed cilia and expressed Foxj1. As a proof of principle that this protocol can be applied to human disease-specific iPS cells generated from patients, Nkx2-1+ progenitors were derived from iPS cells from cystic fibrosis (CF) patients following the same temporal addition of growth factors and inhibitors identified in the mouse protocol. Derivation of human CFTR-expressing human epithelia was the focus of another study using human cystic fibrosis disease-specific iPS cells [Wong et al., 2012]. Although this work follows the method of directed differentiation, there are notable differences, compared to previously published protocols in the cytokines used to induce each developmental stage. The authors used high doses of FGF2 and SHH to derive anterior foregut progenitors followed by treatment with FGF7, FGF10 and BMP4. FGF18 addition and culture in an air-liquid interface was then employed to produce a monolayered epithelium expressing CFTR. As a proof of concept, translocation of deltaF508 mutant CFTR, the ion channel misfolded in CF, to the plasma membrane was achieved by treating CF iPS cell-derived CFTR-expressing cells with a CF “corrector” investigational drug [Wong et al., 2012].
Since Type II alveolar epithelial cells are indispensable for distal lung epithelium function and maintenance, they are prime targets for lung cell replacement therapy. Two recent papers have focused on the derivation of distal alveolar epithelium via directed differentiation of human PSCs. In both publications the stated goals were the sequential differentiation of cells into DE followed by AFE and the induction of NKX2-1 lung progenitors competent to subsequently differentiate and mature into distal alveolar epithelial lineages [Ghaedi et al., 2013; Huang et al., 2014]. [Huang et al., 2014] optimized conditions for derivation of ventralized AFE previously reported by the same group [Green et al., 2011] resulting in cultures containing about 85% FOXA2+NKX2-1+ cells. These cells were competent for multi-lineage lung differentiation since further culturing of the bulk population or kidney capsule transplantation into immunodeficient mice gave rise to cells expressing markers of ciliated, club, basal, type I and type II lung alveolar epithelial cells in vitro or in vivo. In vitro the cells were able to differentiate mostly to SPB+ progeny, by combined WNT, FGF10 and KGF exposure followed by dexamethasone and cAMP. Using a similar approach, [Ghaedi et al., 2013] derived FOXA2+SOX2+ AFE with high efficiency (more than 90%) followed by cells interpreted as expressing NKX2-1. Exposure to a GF cocktail containing WNT3A, FGF10, KGF and EGF reportedly resulted in SPB and SPC expression in more than 90% of cells. These cells were able to engraft decellularized human lung scaffolds and to differentiate into cells expressing T1a. A major discrepancy between the two papers is the requirement for BMP4 signaling in lung specification media with BMP4 being indispensable in the work of Snoeck and colleagues and dispensable in the work of Ghaedi et al. The necessity of BMP4 in the directed differentiation of PSCs to lung lineages will require further work and the use of careful controls to settle the growing controversy and discrepant results emerging in this nascent field. Fortunately, the very robust efficiencies of differentiation being reported in these latest publications should make it relatively easy to determine the reproducibility and utility of these protocols for generating distal lung lineages.
Another area where significant progress has been made recently is the derivation of thymic epithelium from hES cells [Parent et al., 2013; Sun et al., 2013]. Both groups developed stepwise protocols that recapitulate the developmental trajectory of thymic epithelial progenitor (TEP) derivation through anterior foregut intermediates. In one case, derivation of AFE was accomplished by simultaneous activation of BMP4 and RA signaling. Further differentiation to ventral foregut endoderm and TEPs was achieved by additional signals such as Wnt3a and FGF8 and inhibition of Shh signaling [Parent et al., 2013]. Inhibition of TGF-β signaling was again necessary for the efficient derivation of both AFE and ventral foregut endoderm. In the second study, third pharyngeal pouch endoderm was derived from DE by activation of RA signaling and inhibition of Wnt signaling [Sun et al., 2013]. Subsequent reactivation of Wnt signaling and addition of BMP4 was sufficient to specify TEPs as evidenced by expression of Foxn1, and cytokeratins 5 and 8. Both papers report extensive functional characterization following transplantation of hES cell-derived TEPs to athymic nude mice such as the expression of the TEC markers, MHC II and AIRE, the emergence and maturation of mouse CD4+ and CD8+ T cells, and the ability to support human T cell generation in a humanized mouse model [Sun et al., 2013] or to reject allogeneic skin grafts [Parent et al., 2013].
The most convincing example of a functional anterior foregut derivative originating from ES cells is the recent generation of thyroid follicular cells [Antonica et al., 2012]. By an approach that is more reminiscent of direct reprogramming rather than directed differentiation, the authors used a TetOn system to induce transient expression of Nkx2-1 and Pax8 in undifferentiated mouse ES cells followed by incubation with TSH for two weeks. This treatment resulted in the generation of epithelial thyroid organoids, characterized by robust expression of endogenous Nkx2-1 and Pax8, polarized morphology and secretion of thyroglobulin in the luminal compartment. Most importantly, the ES cell-derived follicular cells could restore plasma T4 levels in a mouse model of hypothyroidism when grafted under the kidney capsule. Whether transient overexpression of tissue-specific combinations of transcription factors in undifferentiated ES cells will lead to the derivation of other mature lineages of anterior foregut tissues will certainly be an area of intense study.
Posterior foregut and hindgut: liver, pancreatic, and intestinal progenitors
Both liver and pancreas originate from the posterior foregut endoderm. The pancreas emerges as separate, ventral and dorsal, anlagen [Zaret and Grompe, 2008]. Fate mapping of the early ventral foregut has demonstrated that liver and ventral pancreas progenitors migrate ventrally from distinct domains, namely the lateral endoderm and the ventral midline [Deutsch et al., 2001; Tremblay and Zaret, 2005]. Although several TFs such as Hhex, Prox1, Onecut1, Gata6 and Tbx3 are expressed in embryonic hepatic progenitors (hepatoblasts) none appears to be necessary for liver specification since expression of the early liver markers, albumin and a-fetoprotein (AFP) appears to be retained in mouse genetic models where these TFs are deleted. Liver organogenesis fails in knock-out mice for these TFs because of abortive primordium formation (Hhex and Gata6) [Martinez-Barbera et al., 2000; Zhao et al., 2005], impaired hepatoblast migration (Prox1 and Onecut1) [Margagliotti et al., 2007; Sosa-Pineda et al., 2000] or preferential cholangiocyte differentiation (Tbx3) [Suzuki et al., 2008]. On the contrary, posterior foregut endoderm-specific double deletion of the pan-endodermal TFs, Foxa1 and Foxa2, results in hepatic bud agenesis [Lee et al., 2005]. Both Foxa and Gata factors act as pioneer factors by binding to the enhancer region of the Alb gene and rendering the pre-specified endoderm competent for liver differentiation [Zaret et al., 2008]. Combined inductive FGF and BMP signals for hepatic specification come from the cardiac mesoderm and the septum transversum, respectively [Gualdi et al., 1996; Jung et al., 1999; Rossi et al., 2001; Serls et al., 2005]. Mouse studies that used explant culture of foregut endoderm and its surrounding mesoderm have demonstrated that FGF signaling (mostly by FGF1 and FGF2) from cardiac mesoderm [Jung et al., 1999; Serls et al., 2005] and BMP signaling (by BMP2 and BMP4) synergize to specify the hepatic primordium and to initiate liver morphogenesis.
Pan-pancreatic progenitors are specified in two different endodermal domains but express the same unique combination of TFs, namely Pdx1, Ptf1a and Sox9 [Burlison et al., 2008; Seymour et al., 2007; Zaret and Grompe, 2008; Zhou et al., 2007]. The combined expression of Pdx1 and Ptf1a is both necessary [Kawaguchi et al., 2002] and sufficient [Afelik et al., 2006] for pancreatic specification within the posterior foregut endoderm. There are distinct differences between dorsal and ventral pancreatic primordia, namely the indispensability of Hlxb9 expression for dorsal pancreas specification and the existence of a Pdx1+/Sox17+ pacreatobiliary progenitor in the posterior ventral foregut.
As pancreas differentiation proceeds, there is progressive fate restriction for the progenitors of the endocrine and exocrine compartments. While both progenitor types arise from the Pdx1+/Ptf1a+ multipotent population, exocrine progenitors maintain Ptf1a expression whereas endocrine progenitors are characterized by the expression of Ngn3, a bHLH TF [Gu et al., 2002; Schwitzgebel et al., 2000]. Nng3 is important both for endocrine cell specification [Gradwohl et al., 2000] and for migration of endocrine cells [Gouzi et al., 2011] during development. Interestingly, duct-residing Ngn3+ progenitors can be activated in a partial duct ligation injury model of the adult mouse pancreas and have been reported to give rise to hormone expressing cells, including β-cells [Xu et al., 2008b]. Another TF, Rfx6, is also expressed in embryonic endocrine progenitors and is necessary for the formation of all endocrine cell types except pancreatic polypeptide cells [Smith et al., 2010].
Signals from adjacent tissues specify and pattern the developing pancreas [Murtaugh, 2007]. Dorsally, notochord-secreted FGF2 and activin B act as permissive signals for pancreatic development of pre-patterned posterior foregut endoderm through suppression of Shh signaling [Hebrok et al., 1998; Kim et al., 1997]. Additionally, aortic endothelium induces and maintains Ptf1a expression within the specified dorsal pancreatic primordium [Yoshitomi and Zaret, 2004]. On the contrary, ventral pancreas appears to be induced by RA, BMP and activin signals derived from the mesenchyme [Kumar et al., 2003b]. Contrary to hepatic progenitors, FGF2 signaling inhibits specification of prospective ventral pancreatic progenitors; the correct positioning of the latter away from the FGF2-producing cardiogenic mesoderm is mediated by Hhex and temporally controlled by TGF-β signaling [Bort et al., 2004; Wandzioch and Zaret, 2009]. Later in development, Notch signaling blocks derivation of committed exocrine and endocrine progenitors from pan-pancreatic Pdx1+ progenitors by trapping the latter in the undifferentiated state [Murtaugh et al., 2003].
Chronic, debilitating diseases of posterior foregut endoderm organs such as end-stage liver disease and diabetes mellitus type I are prime targets for cell replacement therapies, potentially using cells exogenously derived from PSCs in vitro. Early attempts to produce hepatocytes or pancreatic β-cells from ES cells did not include a DE formation step and were characterized by low or artifactual expression of liver/pancreas markers [Abe et al., 1996; Hansson et al., 2004; Rajagopal et al., 2003]. Further potential for misinterpretation of data arose from the fact that several liver markers such as AFP are also expressed in extraembryonic tissues. The realization that induction of DE with activin renders PSC derivatives competent to form downstream endodermal lineages led to the discovery development of more systematic protocols that also recapitulated the specification stages of pancreas and liver development [Agarwal et al., 2008; Cai et al., 2007; D’Amour et al., 2006; Gouon-Evans et al., 2006; Hay et al., 2008; Touboul et al., 2010].
BMP4 was first identified as a potent inducer of hepatic fate in mouse ES cell-derived DE by Gouon-Evans et al. [Gouon-Evans et al., 2006]. Bry+/Foxa2high/c-kithigh cells were found to give rise to hepatocyte-like cells through an AFP+ intermediate post-BMP4 treatment. In this system, FGF2 appeared to be required for expansion of the specified hepatic cell population rather than for specification. More recent papers demonstrated the derivation of AFP+ hepatoblasts from hES cells following treatment of ES-derived DE with FGF4 and BMP2 [Cai et al., 2007], FGF4 and KGF [Agarwal et al., 2008] or Activin and WNT3A [Hay et al., 2008]. In all instances, hepatoblasts were able to advance to more mature hepatocyte-like cells when treated with maturation cocktails containing HGF, Oncostatin M and Dexamethasone. Since the exposure to xenogeneic serum and feeder layers may limit regulatory approval of PSC-derived hepatocytes for clinical use, chemically defined protocols have also been developed and they have been successfully applied for differentiation of patient-derived human iPS cells to hepatocyte-like cells [Rashid et al., 2010; Touboul et al., 2010].
Commonly used in vitro hepatic progenitor markers are either secreted proteins such as AFP or TFs such as HNF4a. Since cell surface markers can be particularly useful in the purification and study of in vitro and in vivo derived hepatic progenitors, a number of such markers have been identified in the developing liver [Nierhoff et al., 2007; Tanimizu et al., 2003; Terrace et al., 2007]. Dlk/Pref-1 is expressed both in early mouse and human liver [Tanimizu et al., 2003; Terrace et al., 2007] whereas CD24 and Nope are co-expressed with CDH1 (E-cadherin) in E13.5 murine liver [Nierhoff et al., 2007]. On the other hand, the recent discovery of KDR (VEGFR2), a receptor widely believed to be a mesodermal marker, in a subset of endodermal progenitors upstream of the hepatoblast stage exemplifies the pitfalls of “lineage-specific” markers [Goldman et al., 2013]. These progenitors are KDR+CD31−, i.e. they are distinct from both endothelial progenitors and hepatoblasts, and can be found in both mouse and human fetal livers in vivo as well as in PSCs undergoing hepatic directed differentiation in vitro. In the developing mouse liver, they appear to differentiate robustly to DLK+AFP+ hepatoblasts (30%–50% of the total population). Most importantly, their progeny can be found in the adult liver and consists of both hepatocytes and cholangiocytes.
Regarding PSC pancreatic differentiation, the initial protocols for derivation of pancreatic lineages from ES cells were adapted from neuronal differentiation protocols [Hori et al., 2005]. This was due to commonalities of endocrine β-cells and neuronal cells such as expression of the TFs Ngn3 and Neurod1 and brain insulin expression is some species that were attributed to a common embryonic origin [Alpert et al., 1988]. Since the endodermal provenance of the pancreas is currently well established, more convincing demonstrations of the derivation of β-cell-like cells from hES cells, proceeded through important intermediates such as PFE (for example through activation of RA and inhibition of SHH signaling at the DE stage) and pancreatic endocrine progenitors (for example through inhibition of Notch signaling after derivation of PDX1+ cells) [D’Amour et al., 2006]. Subsequent reports used the same stepwise approach with factor variations and also included differentiation of human iPS cells (reviewed in [Van Hoof et al., 2009]). Use of Noggin at the PFE stage increased the derivation efficiency of PDX1+ progenitors [Jiang et al., 2007; Kroon et al., 2008; Zhang et al., 2009], putatively by blocking hepatic specification [Nostro et al., 2011]. Additionally, inhibition of Wnt signaling at the same stage, impaired intestinal differentiation as indicated by reduced levels of CDX2 expression [Nostro et al., 2011]. EGF has also been used to expand Pdx1+ progenitors before downstream differentiation to pancreatic endocrine cells [Zhang et al., 2009]. Inhibition of TGF-β signaling was important post-pancreatic specification, since NGN3+ cell derivation was enhanced by either TGF-β inhibition [Rezania et al., 2011] or simultaneous TGF-β/BMP inhibition [Nostro et al., 2011] resulting in increased hormone production.
While the in vitro production yield of pan-pancreatic/endocrine progenitors from PSCs can be further improved, the most important challenges now lie in subsequent maturation stages, namely in the depletion of polyhormonal cells from the final cell product and in the potentiation of the insulin response of PSC-derived β-cell-like cells.
As with all endodermal tissues, in vitro derivation of intestinal lineages will benefit patients with ailments of the GI tract both in cell therapies and disease modeling. The posterior parts of the adult GI tract arise from the midgut and hindgut which are the most posterior domains of the gut tube during development. Members of the Cdx family of TFs, especially Cdx2, control both intestinal specification and development of mature intestinal lineages [Gao et al., 2009; Zorn and Wells, 2009]. By E8.5 in mouse, non-overlapping Cdx2 and Sox2 expression mark the boundary between mid/hindgut and foregut, respectively [Sherwood et al., 2009]. Inductive signals for midgut/hindgut specification come from lateral plate mesoderm; the latter has posteriorizing effects as indicated by ectopic expression of CdxA, when recombined with more anterior endoderm in chick embryo explants [Kumar et al., 2003b]. Similar effects are observed upon overexpression of activated β-catenin in mouse anterior endoderm, as shown by expression of ectopic Cdx2 and by Sox2 suppression [Sherwood et al., 2011]. On the other hand, conditional ablation of Cdx2 in posterior mouse endoderm expands the anterior foregut caudally as indicated by ectopic Sox2 and Pax9 expression [Gao et al., 2009]. Attempts to identify factors secreted from the adjacent mesoderm that posteriorly patterns the endodermal gut tube have revealed FGF4 as an important inductive factor that posteriorizes endoderm, as evidenced in mouse endoderm-mesoderm recombinant explant cultures (Wells and Melton REF).
Differentiation of both human and mouse PSCs into intestinal lineages has been demonstrated by recapitulation of developmental stages know to occur during in vivo intestinal development. In a landmark study, Wells and colleagues [Spence et al., 2011] were able to adapt their observation that secreted FGF4 could pattern the developing posterior endoderm in mouse embryos, finding media supplemented with FGF4 also could posteriorly pattern DE derived from human PSCs. For example, high levels (500 ng/ml) of Wnt3a and FGF4 specified PSC-derived DE to CDX2+ intestinal progenitors which is turn gave rise to functional differentiated cells expressing a diversity of mature intestinal marker genes and forming self-organized intestinal spheroids. In other work, mouse ES cell differentiation to intestinal lineages has been reported similarly using Cdx2 as a marker of intestinal progenitors [Cao et al., 2011; Sherwood et al., 2011]. Pharmacological activation of Wnt signaling at the DE stage induced Cdx2 with high efficiency by simultaneously suppressing anterior foregut fates [Sherwood et al., 2011]. A similar effect was shown when DE was incubated with fibroblast conditioned media supplemented with Wnt3a [Cao et al., 2011]. The ensuing cells were capable of forming intestinal organoids in culture and of populating mouse colons after in vivo transplantation.
Future research directions
In the years ahead further advances in several emerging key research areas are likely to provide a more detailed basic understanding of endodermal progenitor biology and consequently should result in effective cell therapies and relevant cell culture systems for in vitro disease modeling.
ES versus iPS cells
The advent of iPS cells and the possibility of individualized disease modeling and autologous cell replacement therapy have created excitement among basic researchers and clinicians. Since most directed differentiation protocols have been developed using ES cells, the crucial questions are whether these protocols can successfully be applied for the differentiation of iPS cells and whether the differentiated progeny of ES cells and iPS cells are equivalent in genetic, molecular and functional terms. Extensive characterization of human and mouse iPS cell lines has suggested that genetic and epigenetic differences between ES cells and iPS cells may exist [Bock et al., 2011; Chin et al., 2009] and have produced conflicting interpretations regarding whether iPS cells retain residual epigenetic memory of their cell of origin or whether this memory is only transient [Kim et al., 2010; Polo et al., 2010]. Importantly, several studies demonstrate that any detectable memory of the somatic cell of origin found in reprogrammed progeny appears to be a transient feature of early passage iPS cells since at late passages the genetic differences diminish [Chin et al., 2009], the epigenetic memory is lost and the differentiation potential is indistinguishable from that of ES cells [Polo et al., 2010]. In regard to the ability of iPS cells to differentiate to DE, we have shown that syngeneic mES and iPS cells can form DE with very similar global gene expression programs and can undergo hepatic differentiation with similar efficiencies [Christodoulou et al., 2011]. This was also true for iPS cell clones with aberrant imprinting of the Dlk1-Dio3 gene cluster [Christodoulou et al., 2011; Stadtfeld et al., 2010]. Patient-specific iPS cell lines have been created for a wide variety of diseases, including those affecting endodermally-derived tissues [Inoue and Yamanaka, 2011; Somers et al., 2010]. Furthermore, disease modeling using iPS endoderm-derived cells has already been described [Rashid et al., 2010]. In general, ES cells and iPS cells appear to have similar potential to differentiate to endodermal lineages, such as self-renewing EPs [Cheng et al., 2012], lung progenitors [Mou et al., 2012], hepatocytes [Rashid et al., 2010], β-cells [Nostro et al., 2011], and intestinal lineages [Spence et al., 2011]. Although differences in differentiation capacity to the same lineage for different iPS cell lines have been reported, these differences do not appear to be a generalized feature of iPS cells but rather may reflect the inherent variability ni differentiation potential that exists between PSC lines made from individuals of different genetic backgrounds [Osafune et al., 2008]. For example, it is well known that different ES cell lines, made from human blastocysts of different genetic backgrounds have variable competence to differentiate to particular target lineages [Osafune et al., 2008]. In addition, when comparing the capacity of different iPS cell lines to differentiate to mesodermal lineages, our group has found that different genetic backgrounds rather than clone to clone variability within the same background is more responsible for the variability in differentiation efficiency observed between iPS cell lines [Mills et al., 2013].
Meticulous screening of individual iPS cell lines regarding their differentiation propensity to various endodermal lineages will be necessary in the future. This is particularly important for diseased subject-derived iPS cells to make sure that defects in the differentiated progeny are due to the disease phenotype and not to poor differentiation efficiency. The use of early passage iPS cells from purified mature endodermal cells (e.g. hepatocytes) when the epigenetic memory of the cell of origin is still retained may prove advantageous if iPS cell differentiation to the same cell type is desired.
Bioprocessing of endodermal progenitors
The future use of PSC-derived lineages for cell replacement therapy is an important goal of regenerative medicine and will signify a shift from the realm of basic research to the realm of FDA-regulated therapeutics with the resultant requirements for cGMP (current Good Manufacturing Practice) production, high purity and potency [Halme and Kessler, 2006]. The cell numbers likely to be needed (108–109 cells/patient in the case of diabetes) are several orders of magnitude higher than those currently produced in a typical research laboratory and will require the development of appropriate bioprocesses for the expansion and differentiation of PSCs [Kirouac and Zandstra, 2008]. Hence “bioprocess” research and development, or the development of methods for the production of biological materials for manufacturing, scale-up, or commercialization, is likely to become increasingly required for the successful clinical applications of PSC-derived cells. Current bioprocessing systems involve mostly the use of benchtop stirred bioreactors where cells proliferate or differentiate in suspension as aggregates, on microcarriers (solid or macroporous) or within alginate microcapsules [Serra et al., 2012]. Regarding the bioprocessing of endodermal progenitors, hES cells were expanded on Matrigel-coated microcarriers in spinner flasks and reached confluency before initiation of endodermal differentiation by addition of activin and Wnt3a [Lock and Tzanakakis, 2009]. More than 80% of the cells became FOXA2+/SOX17+ and the numbers of endodermal cells per unit area in microcarrier and static culture were similar, indicating that microcarriers were more efficient in terms of produced cells/unit volume.
Static suspension systems have also been used for the scalable expansion and endodermal differentiation of PSCs [Schulz et al., 2012; Ungrin et al., 2012]. Ungrin and co-workers [Ungrin et al., 2012] used microwells to pattern human PSCs to aggregates of defined size and introduced the optimization parameter L (targeted yield loss) to dissect the complex cell dynamics of proliferation, death and differentiation during endodermal differentiation. Combined expansion and DE differentiation under optimal conditions had an output of approximately 65,000 C-KIT+/CXCR4+ cells per input PSC and the resulting cells could undergo pancreatic and hepatic differentiation. In a cGMP environment, Schulz et al. [Schulz et al., 2012] demonstrated that a single starting vial of hES cells could generate more than 108 cells, and the expanded cells could repeatedly differentiate into clusters of functional, glucose-responsive β cell-like cells.
The production-oriented nature of PSC bioprocessing research is likely to be harnessed for both clinical use as well as basic science laboratory explorations. For example, scale-up approaches that rely on optimized PSC bioprocessing can provide the large quantities of cells that are often needed for high throughput screening of small molecule effectors of differentiation, and purification and functional testing of large numbers of specific types of differentiated cells.
Epigenetic networks
Recent studies of endodermal directed differentiation of PSCs indicate dynamic chromatin modifications and activation of enhancers that are likely to regulate the genetic and epigenetic networks that control cell fate decisions [Loh et al., 2014; Longmire et al., 2012; Xie et al., 2013]. Understanding the dynamics of these networks is likely to be increasingly important for precise control of PSC directed differentiation. For example, global mapping of H3K4me3 (activatory) and H3K27me3 (inhibitory) histone modifications from the undifferentiated state to functional endocrine cells during pancreatic differentiation of hES cells revealed interesting correlations between chromatin remodeling and transitions between differentiation stages [Xie et al., 2013]. In addition, derivation of DE required removal of PcG-mediated repression of key DE genes such as EOMES and SOX17. Such genes then reverted to bivalent state as cells moved to the PFE and pancreatic progenitor stages whereas gradual de-repression of key regulators of latter took place. Of note, aberrant retention of the H3K27me3 mark on genes highly expressed in functional endocrine cells appeared to explain the impaired function of polyhormonal cells. In terms of cell fate decisions between closely related endodermal lineages, the decision between a hepatic or pancreatic fate is controlled in part by P300, a histone acetyltransferase, that is responsible for liver-related gene activation and by Ezh2, a methyltransferase, that limits the ventral pancreas specification of DE [Xu et al., 2011].
Detailed mapping of enhancers at the anterior PS, DE, AFE, PFE and midgut/hindgut endoderm stages of human PSC endodermal differentiation revealed that mostly non-overlapping sets of enhancers are activated at each stage [Loh et al., 2014]. At the DE stage, both signaling effectors (SMAD2/3/4) and TFs, such as EOMES and FOXH1 occupied active enhancers. The latter appeared to be associated with various chromatin marks before DE specification, with H2AZ being the predominant one. In an in vitro model of lung progenitor specification [Longmire et al., 2012], pre-specified DE cells demonstrated bivalent histone modifications at the Nkx2-1 promoter area. The bivalent state was resolved post-specification with Nkx2-1+ cells having only the H3K4me3 modification and Nkx2-1-negative cells exhibiting the H3K27me3 modification.
Whether the accruing knowledge of the epigenome during endodermal differentiation can be translated to efficient and robust protocols is a question that will be answered by careful perturbation studies of the epigenomic machinery.
In vitro endodermal organogenesis
The in vitro derivation of endodermal progenitors with broad tissue-specific differentiation repertoire is only the first step in the arduous quest for cell replacement therapies. For the most part, directed differentiation protocols draw upon the concept of cell lineage and do not recapitulate the structural relationships or morphogenetic mechanisms that characterize embryonic development [Salazar-Ciudad et al., 2003]. As the important concepts of emergent properties and self-organization ease their way in the fields of stem cell biology and regenerative medicine [MacArthur et al., 2009], efforts to spatially and temporally organize PSC-derived cells in vitro into functional organoids or complex 3D tissues is likely to become increasingly important [Eiraku et al., 2011; Lancaster et al., 2013; Spence et al., 2011; Suga et al., 2011]. Impressive work using neuroectodermal ES cell derivatives has demonstrated the formation of functional adenohypophysis [Suga et al., 2011], of an optic-cup-like structure composed of correctly positioned retinal pigment epithelium and neural retina layers [Eiraku et al., 2011] and most recently of more complex cerebral organoids with organized forebrain, hindbrain and midbrain domains [Lancaster et al., 2013].
The most striking example of 3D organization in endodermal directed differentiation is the in vitro derivation of intestinal organoids from human PSCs that contained both villus-like structures and crypt-like zones with the right cellular composition and in close contact with a mesenchymal compartment [Spence et al., 2011]. The combined action of soluble factors (WNT3A and FGF4), mesenchymal cells and a 3D matrix (Matrigel) was key to the formation of these organoids where differentiati on and morphogenesis appeared to happen in coordinated fashion.
A recently emerging platform with great potential for the generation of bioartificial organs is the use of 3D ECM scaffolds derived from detergent-mediated decellularization of solid organs[Ott et al., 2010; Ott et al., 2008]. Since the publication of the first proof-of-principle heart decellularization-recellularization paper in 2008 [Ott et al., 2008], the list of successfully decellularized organs has been expanding. Regarding organs with endodermally-derived parenchyma, there have been reports of decellularization-recellularization to engineer lung [Ott et al., 2010; Petersen et al., 2010], esophagus [Totonelli et al., 2013], liver [Uygun et al., 2010], pancreas [Goh et al., 2013; Mirmalek-Sani et al., 2013] and intestine [Totonelli et al., 2012]. As the native organ architecture and most ECM proteins are retained post-decellularization, these scaffolds maintain the various (e.g. parenchymal and vascular) organ compartments. To demonstrate the functional potential of mouse ES cell-derived Nkx2-1+ lung progenitors, we recellularized mouse lung scaffolds with these progenitors [Longmire et al., 2012]. Most of the cells colonized the distal lung and maintained Nkx2-1 expression while we also observed differentiation to Nkx2-1− cells expressing T1a, a marker of Type I alveolar epithelial cells. Detailed studies of the ECM protein composition of and spatial distribution in the various endodermal decellularized scaffolds, and of their mechanical properties have recently appeared [Booth et al., 2012; Gilpin et al., 2014; Petersen et al., 2012] and are needed to understand PSC-derived cell-matrix interactions and deduce principles for PSC-based organ engineering.
Concluding remarks
Clinical applications of hepatocytes and β-cells derived from PSCs appear to be within reach due to the existence of efficient directed differentiation protocols and the relative simplicity of surgical procedures for delivering the cells into any compartment with access to circulating blood, such as subcutaneous implantation. Recent advances in endodermal progenitor biology have also opened up the exciting possibility of iPS cell-based therapies for diseases affecting the GI tract and anterior foregut-derived organs such as the lung, thyroid, and the thymus. To realize this potential, it is necessary to make increasing use of sophisticated tools at all biological scales. These include next generation sequencing to understand cell fate decisions at the single cell level in vitro and in vivo and application of self-organization principles to derive multi-lineage cell assemblies with organ properties in vitro. Although cell/organ replacement therapies for lung, thymus and intestinal regeneration may be many years away, disease modeling and drug screening in vitro using iPS cell-derived progenitors is a realistic, attainable short-term goal that can produce both mechanistic insights and promising drug candidates.
Acknowledgments
We would like to thank Dr. Mohamed Jamal Ahmed for help with Figure 1 design and illustration.
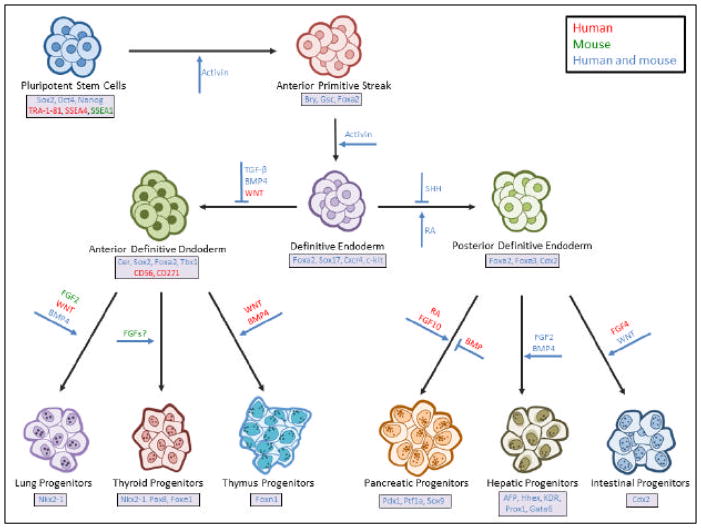
Figure 1.
A model of endodermal progenitor derivation through directed differentiation of pluripotent stem cells.
Footnotes
This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: [10.1002/jcp.24771]
Bibliography
- Abe K, Niwa H, Iwase K, Takiguchi M, Mori A, Abe S, Yamamura K. Endoderm-specific gene expression in embryonic stem cells differentiated to embryoid bodies. Exp Cell Res. 1996;229:27–34. doi: 10.1006/excr.1996.0340. [DOI] [PubMed] [Google Scholar]
- Afelik S, Chen YL, Pieler T. Combined ectopic expression of Pdx1 and Ptf1a/p48 results in the stable conversion of posterior endoderm into endocrine and exocrine pancreatic tissue. Genes Dev. 2006;20:1441–1446. doi: 10.1101/gad.378706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Holton KL, Lanza R. Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem Cells. 2008;26:1117–1127. doi: 10.1634/stemcells.2007-1102. [DOI] [PubMed] [Google Scholar]
- Alpert S, Hanahan D, Teitelman G. Hybrid insulin genes reveal a developmental lineage for pancreatic endocrine-cells and imply a relationship with neurons. Cell. 1988;53:295–308. doi: 10.1016/0092-8674(88)90391-1. [DOI] [PubMed] [Google Scholar]
- Ang SL, Wierda A, Wong D, Stevens KA, Cascio S, Rossant J, Zaret KS. The formation and maintenance of the definitive endoderm lineage in the mouse - involvement of HNF3/Forkhead proteins. Development. 1993;119:1301–1315. doi: 10.1242/dev.119.4.1301. [DOI] [PubMed] [Google Scholar]
- Antonica F, Kasprzyk DF, Opitz R, Iacovino M, Liao XH, Dumitrescu AM, Refetoff S, Peremans K, Manto M, Kyba M, Costagliola S. Generation of functional thyroid from embryonic stem cells. Nature. 2012;491:66–71. doi: 10.1038/nature11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arufe MC, Lu M, Kubo A, Keller G, Davies TF, Lin RY. Directed differentiation of mouse embryonic stem cells into thyroid follicular cells. Endocrinology. 2006;147:3007–3015. doi: 10.1210/en.2005-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayha E, Jorgensen MC, Serup P, Grapin-Botton A. Retinoic Acid Signaling Organizes Endodermal Organ Specification along the Entire Antero-Posterior Axis. PLoS One. 2009;4 doi: 10.1371/journal.pone.0005845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham EL, Cheng SP, Ignatoski KMW, Doherty GM. Differentiation of Human Embryonic Stem Cells to a Parathyroid-Like Phenotype. Stem Cells Dev. 2009;18:1071–1080. doi: 10.1089/scd.2008.0337. [DOI] [PubMed] [Google Scholar]
- Bock C, Kiskinis E, Verstappen G, Gu HC, Boulting G, Smith ZD, Ziller M, Croft GF, Amoroso MW, Oakley DH, Gnirke A, Eggan K, Meissner A. Reference Maps of Human ES and iPS Cell Variation Enable High-Throughput Characterization of Pluripotent Cell Lines. Cell. 2011;144:439–452. doi: 10.1016/j.cell.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, Weiss K, Horowitz JC, Fiore VF, Barker TH, Moore BB, Martinez FJ, Niklason LE, White ES. Acellular Normal and Fibrotic Human Lung Matrices as a Culture System for In Vitro Investigation. Am J Respir Crit Care Med. 2012;186:866–876. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bort R, Martinez-Barbera JP, Beddington RSP, Zaret KS. Hex homeobox gene-dependent tissue positioning is required for organogenesis of the ventral pancreas. Development. 2004;131:797–806. doi: 10.1242/dev.00965. [DOI] [PubMed] [Google Scholar]
- Bossard P, Zaret KS. GATA transcription factors as potentiators of gut endoderm differentiation. Development. 1998;125:4909–4917. doi: 10.1242/dev.125.24.4909. [DOI] [PubMed] [Google Scholar]
- Brafman DA, Moya N, Allen-Soltero S, Fellner T, Robinson M, McMillen ZL, Gaasterland T, Willert K. Analysis of SOX2-Expressing Cell Populations. Derived from Human Pluripotent Stem Cells. 2013a;1:464–478. doi: 10.1016/j.stemcr.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brafman DA, Phung C, Kumar N, Willert K. Regulation of endodermal differentiation of human embryonic stem cells through integrin-ECM interactions. Cell Death Differ. 2013b;20:369–381. doi: 10.1038/cdd.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Teo A, Pauklin S, Hannan N, Cho CHH, Lim B, Vardy L, Dunn NR, Trotter M, Pedersen R, Vallier L. Activin/Nodal Signaling Controls Divergent Transcriptional Networks in Human Embryonic Stem Cells and in Endoderm Progenitors. Stem Cells. 2011;29:1176–1185. doi: 10.1002/stem.666. [DOI] [PubMed] [Google Scholar]
- Burlison JS, Long QM, Fujitani Y, Wright CVE, Magnuson MA. Pdx-1 and Ptf1a concurrently determine fate specification of pancreatic multipotent progenitor cells. Dev Biol. 2008;316:74–86. doi: 10.1016/j.ydbio.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Yu C, Liu YX, Chen S, Guo YX, Yong J, Lu W, Ding MX, Deng HK. Generation of Homogeneous PDX1(+) Pancreatic Progenitors from Human ES Cell-derived Endoderm Cells. J Mol Cell Biol. 2010;2:50–60. doi: 10.1093/jmcb/mjp037. [DOI] [PubMed] [Google Scholar]
- Cai J, Zhao Y, Liu YX, Ye F, Song ZH, Qin H, Meng S, Chen YZ, Zhou RD, Song XJ, Guo YS, Ding MX, Deng H. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45:1229–1239. doi: 10.1002/hep.21582. [DOI] [PubMed] [Google Scholar]
- Cao L, Gibson JD, Miyamoto S, Sail V, Verma R, Rosenberg DW, Nelson CE, Giardina C. Intestinal lineage commitment of embryonic stem cells. Differentiation. 2011;81:1–10. doi: 10.1016/j.diff.2010.09.182. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Delgado I, Soria B, Martin F, Rojas A. GATA4 and GATA6 control mouse pancreas organogenesis. J Clin Invest. 2012;122:3504–3515. doi: 10.1172/JCI63240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Ying L, Lu L, Galvao AM, Mills JA, Lin HC, Kotton DN, Shen SS, Nostro MC, Choi JK, Weiss MJ, French DL, Gadue P. Self-Renewing Endodermal Progenitor Lines Generated from Human Pluripotent Stem Cells. Cell Stem Cell. 2012;10:371–384. doi: 10.1016/j.stem.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin MH, Mason MJ, Xie W, Volinia S, Singer M, Peterson C, Ambartsumyan G, Aimiuwu O, Richter L, Zhang J, Khvorostov I, Ott V, Grunstein M, Lavon N, Benvenisty N, Croce CM, Clark AT, Baxter T, Pyle AD, Teitell MA, Pelegrini M, Plath K, Lowry WE. Induced Pluripotent Stem Cells and Embryonic Stem Cells Are Distinguished by Gene Expression Signatures. Cell Stem Cell. 2009;5:111–123. doi: 10.1016/j.stem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulou C, Longmire TA, Shen SS, Bourdon A, Sommer CA, Gadue P, Spira A, Gouon-Evans V, Murphy GJ, Mostoslavsky G, Kotton DN. Mouse ES and iPS cells can form similar definitive endoderm despite differences in imprinted genes. J Clin Invest. 2011;121:2313–25. doi: 10.1172/JCI43853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon FL, Lyons KM, Takaesu N, Barth KS, Kispert A, Herrmann B, Robertson EJ. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development. 1994;120:1919–1928. doi: 10.1242/dev.120.7.1919. [DOI] [PubMed] [Google Scholar]
- D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- Desai TJ, Malpel S, Flentke GR, Smith SM, Cardoso WV. Retinoic acid selectively regulates Fgf10 expression and maintains cell identity in the prospective lung field of the developing foregut. Dev Biol. 2004;273:402–415. doi: 10.1016/j.ydbio.2004.04.039. [DOI] [PubMed] [Google Scholar]
- Dessimoz J, Opoka R, Kordich JJ, Grapin-Botton A, Wells JM. FGF signaling is necessary for establishing gut tube domains along the anterior-posterior axis in vivo. Mech Dev. 2006;123:42–55. doi: 10.1016/j.mod.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Deutsch G, Jung JN, Zheng MH, Lora J, Zaret KS. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development. 2001;128:871–881. doi: 10.1242/dev.128.6.871. [DOI] [PubMed] [Google Scholar]
- Domyan ET, Ferretti E, Throckmorton K, Mishina Y, Nicolis SK, Sun X. Signaling through BMP receptors promotes respiratory identity in the foregut via repression of Sox2. Development. 2011;138:971–981. doi: 10.1242/dev.053694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, Sekiguchi K, Adachi T, Sasai Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472:51–U73. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Gadue P, Huber TL, Nostro MC, Kattman S, Keller GM. Germ layer induction from embryonic stem cells. Exp Hematol. 2005;33:955–964. doi: 10.1016/j.exphem.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Gadue P, Huber TL, Paddison PJ, Keller GM. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:16806–16811. doi: 10.1073/pnas.0603916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao N, White P, Kaestner KH. Establishment of Intestinal Identity and Epithelial-Mesenchymal Signaling by Cdx2. Dev Cell. 2009;16:588–599. doi: 10.1016/j.devcel.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaedi M, Calle EA, Mendez JJ, Gard AL, Balestrini J, Booth A, Bove PF, Gui LQ, White ES, Niklason LE. Human iPS cell-derived alveolar epithelium repopulates lung extracellular matrix. J Clin Invest. 2013;123:4950–4962. doi: 10.1172/JCI68793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin SE, Guyette JP, Gonzalez G, Ren X, Asara JM, Mathisen DJ, Vacanti JP, Ott HC. Perfusion decellularization of human and porcine lungs: Bringing the matrix to clinical scale. J Heart Lung Transplant. 2014;33:298–308. doi: 10.1016/j.healun.2013.10.030. [DOI] [PubMed] [Google Scholar]
- Goh SK, Bertera S, Olsen P, Candiello JE, Halfter W, Uechi G, Balasubramani M, Johnson SA, Sicari BM, Kollar E, Badylak SF, Banerjee I. Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering. Biomaterials. 2013;34:6760–6772. doi: 10.1016/j.biomaterials.2013.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman O, Han S, Sourrisseau M, Dziedzic N, Hamou W, Corneo B, D’Souza S, Sato T, Kotton DN, Bissig KD, Kalir T, Jacobs A, Evans T, Evans MJ, Gouon-Evans V. KDR identifies a conserved human and murine hepatic progenitor and instructs early liver development. Cell stem cell. 2013;12:748–60. doi: 10.1016/j.stem.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J, Manley NR. Mechanisms of thymus organogenesis and morphogenesis. Development. 2011;138:3865–3878. doi: 10.1242/dev.059998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss AM, Tian Y, Tsukiyama T, Cohen ED, Zhou D, Lu MM, Yamaguchi TP, Morrisey EE. Wnt2/2b and beta-Catenin Signaling Are Necessary and Sufficient to Specify Lung Progenitors in the Foregut. Dev Cell. 2009;17:290–298. doi: 10.1016/j.devcel.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouon-Evans V, Boussemart L, Gadue P, Nierhoff D, Koehler CI, Kubo A, Shafritz DA, Keller G. BMP-4 is required for hepatic specification of mouse embryonic stem cell-derived definitive endoderm. Nat Biotechnol. 2006;24:1402–1411. doi: 10.1038/nbt1258. [DOI] [PubMed] [Google Scholar]
- Gouzi M, Kim YH, Katsumoto K, Johansson K, Grapin-Botton A. Neurogenin3 Initiates Stepwise Delamination of Differentiating Endocrine Cells During Pancreas Development. Dev Dyn. 2011;240:589–604. doi: 10.1002/dvdy.22544. [DOI] [PubMed] [Google Scholar]
- Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grapin-Botton A. Community TSCR, editor. StemBook. 2008. Endoderm specification. [PubMed] [Google Scholar]
- Grapin-Botton A, Constam D. Evolution of the mechanisms and molecular control of endoderm formation. Mech Dev. 2007;124:253–278. doi: 10.1016/j.mod.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Green MD, Chen A, Nostro MC, d’Souza SL, Schaniel C, Lemischka IR, Gouon-Evans V, Keller G, Snoeck HW. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat Biotechnol. 2011;29:267–U153. doi: 10.1038/nbt.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu GQ, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- Gualdi R, Bossard P, Zheng MH, Hamada Y, Coleman JR, Zaret KS. Hepatic specification of the gut endoderm in vitro: Cell signaling and transcriptional control. Genes Dev. 1996;10:1670–1682. doi: 10.1101/gad.10.13.1670. [DOI] [PubMed] [Google Scholar]
- Halme DG, Kessler DA. FDA regulation of stem-cell-based therapies. N Engl J Med. 2006;355:1730–1735. doi: 10.1056/NEJMhpr063086. [DOI] [PubMed] [Google Scholar]
- Hannan Nicholas RF, Fordham Robert P, Syed Yasir A, Moignard V, Berry A, Bautista R, Hanley Neil A, Jensen Kim B, Vallier L. Generation of Multipotent Foregut Stem Cells from Human Pluripotent Stem Cells. 2013;1:293–306. doi: 10.1016/j.stemcr.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson M, Tonning A, Frandsen U, Petri A, Rajagopal J, England MCO, Heller RS, Hakansson J, Fleckner J, Skold HN, Melton D, Semb H, Serup P. Artifactual insulin release from differentiated embryonic stem cells. Diabetes. 2004;53:2603–2609. doi: 10.2337/diabetes.53.10.2603. [DOI] [PubMed] [Google Scholar]
- Harris-Johnson KS, Domyan ET, Vezina CM, Sun X. beta-Catenin promotes respiratory progenitor identity in mouse foregut. Proc Natl Acad Sci U S A. 2009;106:16287–16292. doi: 10.1073/pnas.0902274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison KA, Thaler J, Pfaff SL, Gu H, Kehrl JH. Pancreas dorsal lobe agenesis and abnormal islets of Langerhans in Hlxb9-deficient mice. Nature Genet. 1999;23:71–75. doi: 10.1038/12674. [DOI] [PubMed] [Google Scholar]
- Hay DC, Fletcher J, Payne C, Terrace JD, Gallagher RCJ, Snoeys J, Black JR, Wojtacha D, Samuel K, Hannoun Z, Pryde A, Filippi C, Currie IS, Forbes SJ, Ross JA, Newsome PN, Iredale JP. Highly efficient differentiation of hESCs to functional hepatic endoderm requires ActivinA and Wnt3a signaling. Proc Natl Acad Sci U S A. 2008;105:12301–12306. doi: 10.1073/pnas.0806522105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebrok M, Kim SK, Melton DA. Notochord repression of endodermal Sonic hedgehog permits pancreas development. Genes Dev. 1998;12:1705–1713. doi: 10.1101/gad.12.11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka K, Nitta T, Sugawa R, Shirai M, Schwartz RJ, Amagai T, Nitta S, Takahama Y, Morisaki T. Differentiation of Pharyngeal Endoderm from Mouse Embryonic Stem Cell. Stem Cells Dev. 2010;19:1735–1743. doi: 10.1089/scd.2009.0466. [DOI] [PubMed] [Google Scholar]
- Hori Y, Gu XY, Xie XD, Kim SK. Differentiation of insulin-producing cells from human neural progenitor cells. PLos Med. 2005;2:347–356. doi: 10.1371/journal.pmed.0020103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hösgör M, Ijzendoorn Y, Mooi WJ, Tibboel D, de Krijger RR. Thyroid transcription factor-1 expression during normal human lung development and in patients with congenital diaphragmatic hernia. J Pediatr Surg. 2002;37:1258–1262. doi: 10.1053/jpsu.2002.34977. [DOI] [PubMed] [Google Scholar]
- Huang SXL, Islam MN, O’Neill J, Hu Z, Yang YG, Chen YW, Mumau M, Green MD, Vunjak-Novakovic G, Bhattacharya J, Snoeck HW. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol. 2014;32:84-+. doi: 10.1038/nbt.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomou L, Hemnes AR, Bilousova G, Hamid R, Loyd JE, Hatzopoulos AK, Kotton DN, Majka SM, Austin ED. Programmatic change: lung disease research in the era of induced pluripotency. Am J Physiol-Lung Cell Mol Physiol. 2011;301:L830–L835. doi: 10.1152/ajplung.00255.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Yamanaka S. The Use of Induced Pluripotent Stem Cells in Drug Development. Clin Pharmacol Ther. 2011;89:655–661. doi: 10.1038/clpt.2011.38. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Rex M, Scotting PJ, Yasugi S. Region-specific expression of chicken Sox2 in the developing gut and lung epithelium: Regulation by epithelial-mesenchymal interactions. Dev Dyn. 1998;213:464–475. doi: 10.1002/(SICI)1097-0177(199812)213:4<464::AID-AJA11>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Jiang JJ, Au M, Lu KH, Eshpeter A, Korbutt G, Fisk G, Majumdar AS. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells. 2007;25:1940–1953. doi: 10.1634/stemcells.2006-0761. [DOI] [PubMed] [Google Scholar]
- Jung JN, Zheng MH, Goldfarb M, Zaret KS. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science. 1999;284:1998–2003. doi: 10.1126/science.284.5422.1998. [DOI] [PubMed] [Google Scholar]
- Kanai-Azuma M, Kanai Y, Gad JM, Tajima Y, Taya C, Kurohmaru M, Sanai Y, Yonekawa H, Yazaki K, Tam PPL, Hayashi Y. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development. 2002;129:2367–2379. doi: 10.1242/dev.129.10.2367. [DOI] [PubMed] [Google Scholar]
- Kattman SJ, Huber TL, Keller GM. Multipotent Flk-1(+) cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11:723–732. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CVE. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nature Genet. 2002;32:128–134. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- Kearns NA, Genga RMJ, Ziller M, Kapinas K, Peters H, Brehm MA, Meissner A, Maehr R. Generation of organized anterior foregut epithelia from pluripotent stem cells using small molecules. Stem Cell Res. 2013;11:1003–1012. doi: 10.1016/j.scr.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LIR, Yabuuchi A, Takeuchi A, Cunniff KC, Hongguang H, McKinney-Freeman S, Naveiras O, Yoon TJ, Irizarry RA, Jung N, Seita J, Hanna J, Murakami P, Jaenisch R, Weissleder R, Orkin SH, Weissman IL, Feinberg AP, Daley GQ. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–U60. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Hebrok M, Melton DA. Notochord to endoderm signaling is required for pancreas development. Development. 1997;124:4243–4252. doi: 10.1242/dev.124.21.4243. [DOI] [PubMed] [Google Scholar]
- Kimelman D, Griffin KJP. Vertebrate mesendoderm induction and patterning. Curr Opin Genet Dev. 2000;10:350–356. doi: 10.1016/s0959-437x(00)00095-2. [DOI] [PubMed] [Google Scholar]
- Kimura S, Hara Y, Pineau T, FernandezSalguero P, Fox CH, Ward JM, Gonzalez FJ. The T/ebp null mouse thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10:60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- Kimura S, Ward JM, Minoo P. Thyroid-specific enhancer-binding protein thyroid transcription factor 1 is not required for the initial specification of the thyroid and lung primordia. Biochimie. 1999;81:321–327. doi: 10.1016/s0300-9084(99)80077-7. [DOI] [PubMed] [Google Scholar]
- Kimura W, Yasugi S, Fukuda K. Regional specification of the endoderm in the early chick embryo. Dev Growth Diff. 2007;49:365–372. doi: 10.1111/j.1440-169X.2007.00933.x. [DOI] [PubMed] [Google Scholar]
- Kimura W, Yasugi S, Stern CD, Fukuda K. Fate and plasticity of the endoderm in the early chick embryo. Dev Biol. 2006;289:283–295. doi: 10.1016/j.ydbio.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kirouac DC, Zandstra PW. The Systematic Production of Cells for Cell Therapies. Cell Stem Cell. 2008;3:369–381. doi: 10.1016/j.stem.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D’Amour KA, Carpenter MK, Baetge EE. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, Fehling HJ, Keller G. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- Kumar M, Jordan N, Melton D, Grapin-Botton A. Control of A-P identity in endoderm by lateral plate mesoderm signals. Dev Biol. 2003a;259:506. doi: 10.1016/s0012-1606(03)00183-0. [DOI] [PubMed] [Google Scholar]
- Kumar M, Jordan N, Melton D, Grapin-Botton A. Signals from lateral plate mesoderm instruct endoderm toward a pancreatic fate. Dev Biol. 2003b;259:109–122. doi: 10.1016/s0012-1606(03)00183-0. [DOI] [PubMed] [Google Scholar]
- Kuo CT, Morrisey EE, Anandappa R, Sigrist K, Lu MM, Parmacek MS, Soudais C, Leiden JM. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1997;11:1048–1060. doi: 10.1101/gad.11.8.1048. [DOI] [PubMed] [Google Scholar]
- Lai LJ, Jin JJ. Generation of Thymic Epithelial Cell Progenitors by Mouse Embryonic Stem Cells. Stem Cells. 2009;27:3012–3020. doi: 10.1002/stem.238. [DOI] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin C-A, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA. Cerebral organoids model human brain development and microcephaly. 2013 doi: 10.1038/nature12517. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson KA, Pedersen RA. Cell fate, morphogenetic movement and population-kinetics of embryonic endoderm at the time of germ layer formation in the mouse. Development. 1987;101:627–652. doi: 10.1242/dev.101.3.627. [DOI] [PubMed] [Google Scholar]
- Lazzaro D, Price M, Defelice M, Dilauro R. The Transcription Factor-Ttf-1 Is Expressed at the Onset of Thyroid and Lung Morphogenesis and in Restricted Regions of the Fetal Brain. Development. 1991;113:1093–&. doi: 10.1242/dev.113.4.1093. [DOI] [PubMed] [Google Scholar]
- Lee CS, Friedman JR, Fulmer JT, Kaestner KH. The initiation of liver development is dependent on Foxa transcription factors. Nature. 2005;435:944–947. doi: 10.1038/nature03649. [DOI] [PubMed] [Google Scholar]
- Lenas P, Moos M, Luyten FP. Developmental Engineering: A New Paradigm for the Design and Manufacturing of Cell-Based Products. Part I: From Three-Dimensional Cell Growth to Biomimetics of In Vivo Development. Tissue Eng Part B-Rev. 2009;15:381–394. doi: 10.1089/ten.TEB.2008.0575. [DOI] [PubMed] [Google Scholar]
- Li H, Arber S, Jessell TM, Edlund H. Selective agenesis of the dorsal pancreas in mice lacking homeobox gene Hlxb9. Nature Genet. 1999;23:67–70. doi: 10.1038/12669. [DOI] [PubMed] [Google Scholar]
- Litingtung Y, Lei L, Westphal H, Chiang C. Sonic hedgehog is essential to foregut development. Nature Genet. 1998;20:58–61. doi: 10.1038/1717. [DOI] [PubMed] [Google Scholar]
- Liu PT, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nature Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- Lock LT, Tzanakakis ES. Expansion and Differentiation of Human Embryonic Stem Cells to Endoderm Progeny in a Microcarrier Stirred-Suspension Culture. Tissue Eng Part A. 2009;15:2051–2063. doi: 10.1089/ten.tea.2008.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh KM, Ang LT, Zhang J, Kumar V, Ang J, Auyeong JQ, Lee KL, Choo SH, Lim CYY, Nichane M, Tan J, Noghabi MS, Azzola L, Ng ES, Durruthy-Durruthy J, Sebastiano V, Poellinger L, Elefanty AG, Stanley EG, Chen Q, Prabhakar S, Weissman IL, Lim B. Efficient Endoderm Induction from Human Pluripotent Stem Cells by Logically Directing Signals Controlling Lineage Bifurcations. 2014;14:237–252. doi: 10.1016/j.stem.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longmire TA, Ikonomou L, Hawkins F, Christodoulou C, Cao YX, Jean JC, Kwok LW, Mou HM, Rajagopal J, Shen SS, Dowton AA, Serra M, Weiss DJ, Green MD, Snoeck HW, Ramirez MI, Kotton DN. Efficient Derivation of Purified Lung and Thyroid Progenitors from Embryonic Stem Cells. Cell Stem Cell. 2012;10:398–411. doi: 10.1016/j.stem.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma RS, Latif R, Davies TF. Thyrotropin-Independent Induction of Thyroid Endoderm from Embryonic Stem Cells by Activin A. Endocrinology. 2009;150:1970–1975. doi: 10.1210/en.2008-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur BD, Ma’ayan A, Lemischka IR. Systems biology of stem cell fate and cellular reprogramming. Nat Rev Mol Cell Biol. 2009;10:672–681. doi: 10.1038/nrm2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manis JP. Knock out, knock in, knock down - Genetically manipulated mice and the Nobel Prize. N Engl J Med. 2007;357:2426–2429. doi: 10.1056/NEJMp0707712. [DOI] [PubMed] [Google Scholar]
- Mansouri A, Chowdhury K, Gruss P. Follicular cells of the thyroid gland require Pax8 gene function. Nature Genet. 1998;19:87–90. doi: 10.1038/ng0598-87. [DOI] [PubMed] [Google Scholar]
- Margagliotti S, Clotman F, Pierreux CE, Beaudry JB, Jacquemin P, Rousseau GG, Lemaigre FP. The Onecut transcription factors HNF-6/OC-1 and OC-2 regulate early liver expansion by controlling hepatoblast migration. Dev Biol. 2007;311:579–589. doi: 10.1016/j.ydbio.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Martin GR. Isolation of a pluripotent cell-line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem-cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Barbera JP, Clements M, Thomas P, Rodriguez T, Meloy D, Kioussis D, Beddington RSP. The homeobox gene Hex is required in definitive endodermal tissues for normal forebrain, liver and thyroid formation. Development. 2000;127:2433–2445. doi: 10.1242/dev.127.11.2433. [DOI] [PubMed] [Google Scholar]
- Matsushita S, Urase K, Komatsu A, Scotting PJ, Kuroiwa A, Yasugi S. Foregut endoderm is specified early in avian development through signal(s) emanating from Hensen’s node or its derivatives. Mech Dev. 2008;125:377–395. doi: 10.1016/j.mod.2008.02.003. [DOI] [PubMed] [Google Scholar]
- McLin VA, Rankin SA, Zorn AM. Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development. 2007;134:2207–2217. doi: 10.1242/dev.001230. [DOI] [PubMed] [Google Scholar]
- Micallef SJ, Janes ME, Knezevic K, Davis RP, Elefanty AG, Stanley EG. Retinoic acid induces Pdx1-positive endoderm in differentiating mouse embryonic stem cells. Diabetes. 2005;54:301–305. doi: 10.2337/diabetes.54.2.301. [DOI] [PubMed] [Google Scholar]
- Mills JA, Wang K, Paluru P, Ying L, Lu L, Galvao AM, Xu DB, Yao Y, Sullivan SK, Sullivan LM, Mac H, Omari A, Jean JC, Shen S, Gower A, Spira A, Mostoslavsky G, Kotton DN, French DL, Weiss MJ, Gadue P. Clonal genetic and hematopoietic heterogeneity among human-induced pluripotent stem cell lines. Blood. 2013;122:2047–2051. doi: 10.1182/blood-2013-02-484444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirmalek-Sani SH, Orlando G, McQuilling JP, Pareta R, Mack DL, Salvatori M, Famey AC, Stratta RJ, Atala A, Opara EC, Soker S. Porcine pancreas extracellular matrix as a platform for endocrine pancreas bioengineering. Biomaterials. 2013;34:5488–5495. doi: 10.1016/j.biomaterials.2013.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan AP, Kaestner KH, Grau E, Schutz G. Postimplantation expression patterns indicate a role for the mouse forkhead/HNF-3 a, β and ? genes in determination of the definitive endoderm, chordamesoderm and neuroectoderm. Development. 1993;119:567–578. doi: 10.1242/dev.119.3.567. [DOI] [PubMed] [Google Scholar]
- Morrisey EE, Tang ZH, Sigrist K, Lu MM, Jiang F, Ip HS, Parmacek MS. GATA6 regulates HNF4 and is required for differentiation of visceral endoderm in the mouse embryo. Genes Dev. 1998;12:3579–3590. doi: 10.1101/gad.12.22.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison GM, Oikonomopoulou I, Migueles RP, Soneji S, Livigni A, Enver T, Brickman JM. Anterior Definitive Endoderm from ESCs Reveals a Role for FGF Signaling. Cell Stem Cell. 2008;3:402–415. doi: 10.1016/j.stem.2008.07.021. [DOI] [PubMed] [Google Scholar]
- Motoyama J, Liu J, Mo R, Ding Q, Post M, Hui CC. Essential function of Gli2 and Gli3 in the formation of lung, trachea and oesophagus. Nature Genet. 1998;20:54–57. doi: 10.1038/1711. [DOI] [PubMed] [Google Scholar]
- Mou HM, Zhao R, Sherwood R, Ahfeldt T, Lapey A, Wain J, Sicilian L, Izvolsky K, Musunuru K, Cowan C, Rajagopal J. Generation of Multipotent Lung and Airway Progenitors from Mouse ESCs and Patient-Specific Cystic Fibrosis iPSCs. Cell Stem Cell. 2012;10:385–397. doi: 10.1016/j.stem.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: Lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Murtaugh LC. Pancreas and beta-cell development: from the actual to the possible. Development. 2007;134:427–438. doi: 10.1242/dev.02770. [DOI] [PubMed] [Google Scholar]
- Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A. 2003;100:14920–14925. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierhoff D, Levoci L, Schulte S, Goeser T, Rogler LE, Shafritz DA. New cell surface markers for murine fetal hepatic stem cells identified through high density complementary DNA microarrays. Hepatology. 2007;46:535–547. doi: 10.1002/hep.21721. [DOI] [PubMed] [Google Scholar]
- Nostro MC, Sarangi F, Ogawa S, Holtzinger A, Corneo B, Li XL, Micallef SJ, Park IH, Basford C, Wheeler MB, Daley GQ, Elefanty AG, Stanley EG, Keller G. Stage-specific signaling through TGF beta family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development. 2011;138:861–871. doi: 10.1242/dev.055236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osafune K, Caron L, Borowiak M, Martinez RJ, Fitz-Gerald CS, Sato Y, Cowan CA, Chien KR, Melton DA. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat Biotechnol. 2008;26:313–315. doi: 10.1038/nbt1383. [DOI] [PubMed] [Google Scholar]
- Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–U131. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- Parent AV, Russ HA, Khan IS, Laflam TN, Metzger TC, Anderson MS, Hebrok M. Generation of Functional Thymic Epithelium from Human Embryonic Stem Cells that Supports Host T Cell Development. Cell Stem Cell. 2013;13:219–29. doi: 10.1016/j.stem.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patient RK, McGhee JD. The GATA family (vertebrates and invertebrates) Curr Opin Genet Dev. 2002;12:416–422. doi: 10.1016/s0959-437x(02)00319-2. [DOI] [PubMed] [Google Scholar]
- Perea-Gomez A, Vella FDJ, Shawlot W, Oulad-Abdelghani M, Chazaud C, Meno C, Pfister V, Chen L, Robertson E, Hamada H, Behringer RR, Ang SL. Nodal antagonists in the anterior visceral endoderm prevent the formation of multiple primitive streaks. Dev Cell. 2002;3:745–756. doi: 10.1016/s1534-5807(02)00321-0. [DOI] [PubMed] [Google Scholar]
- Petersen TH, Calle EA, Colehour MB, Niklason LE. Matrix Composition and Mechanics of Decellularized Lung Scaffolds. Cells Tissues Organs. 2012;195:222–231. doi: 10.1159/000324896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen TH, Calle EA, Zhao LP, Lee EJ, Gui LQ, Raredon MB, Gavrilov K, Yi T, Zhuang ZW, Breuer C, Herzog E, Niklason LE. Tissue-Engineered Lungs for in Vivo Implantation. Science. 2010;329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, Tan KY, Apostolou E, Stadtfeld M, Li YS, Shioda T, Natesan S, Wagers AJ, Melnick A, Evans T, Hochedlinger K. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol. 2010;28:848–U130. doi: 10.1038/nbt.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que J, Okubo T, Goldenring JR, Nam KT, Kurotani R, Morrisey EE, Taranova O, Pevny LH, Hogan BLM. Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development. 2007;134:2521–2531. doi: 10.1242/dev.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que JW, Choi MR, Ziel JW, Klingensmith J, Hogan BLM. Morphogenesis of the trachea and esophagus: current players and new roles for noggin and Bmps. Development. 2006;74:422–437. doi: 10.1111/j.1432-0436.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- Que JW, Luo XY, Schwartz RJ, Hogan BLM. Multiple roles for Sox2 in the developing and adult mouse trachea. Development. 2009;136:1899–1907. doi: 10.1242/dev.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal J, Anderson WJ, Kume S, Martinez OI, Melton DA. Insulin staining of ES cell progeny from insulin uptake. Science. 2003;299:363–363. doi: 10.1126/science.1077838. [DOI] [PubMed] [Google Scholar]
- Rashid ST, Corbineau S, Hannan N, Marciniak SJ, Miranda E, Alexander G, Huang-Doran I, Griffin J, Ahrlund-Richter L, Skepper J, Semple R, Weber A, Lomas DA, Vallier L. Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. J Clin Invest. 2010;120:3127–3136. doi: 10.1172/JCI43122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezania A, Riedel MJ, Wideman RD, Karanu F, Ao ZL, Warnock GL, Kieffer TJ. Production of Functional Glucagon-Secreting alpha-Cells From Human Embryonic Stem Cells. Diabetes. 2011;60:239–247. doi: 10.2337/db10-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodaway A, Patient R. Mesendoderm: An ancient germ layer? Cell. 2001;105:169–172. doi: 10.1016/s0092-8674(01)00307-5. [DOI] [PubMed] [Google Scholar]
- Rodewald HR. Annual Review of Immunology. Palo Alto: Annual Reviews; 2008. Thymus organogenesis; pp. 355–388. [DOI] [PubMed] [Google Scholar]
- Rossi JM, Dunn NR, Hogan BLM, Zaret KS. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001;15:1998–2009. doi: 10.1101/gad.904601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi SW, Jenkinson WE, Anderson G, Jenkinson EJ. Clonal analysis reveals a common progenitor for thymic cortical and medullary epithelium. Nature. 2006;441:988–991. doi: 10.1038/nature04813. [DOI] [PubMed] [Google Scholar]
- Salazar-Ciudad I, Jernvall J, Newman SA. Mechanisms of pattern formation in development and evolution. Development. 2003;130:2027–2037. doi: 10.1242/dev.00425. [DOI] [PubMed] [Google Scholar]
- Schier AF. Nodal signaling in vertebrate development. Annu Rev Cell Dev Biol. 2003;19:589–621. doi: 10.1146/annurev.cellbio.19.041603.094522. [DOI] [PubMed] [Google Scholar]
- Schulz TC, Young HY, Agulnick AD, Babin MJ, Baetge EE, Bang AG, Bhoumik A, Cepa I, Cesario RM, Haakmeester C, Kadoya K, Kelly JR, Kerr J, Martinson LA, McLean AB, Moorman MA, Payne JK, Richardson M, Ross KG, Sherrer ES, Song XH, Wilson AZ, Brandon EP, Green CE, Kroon EJ, Kelly OG, D’Amour KA, Robins AJ. A Scalable System for Production of Functional Pancreatic Progenitors from Human Embryonic Stem Cells. PLoS One. 2012;7 doi: 10.1371/journal.pone.0037004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwitzgebel VM, Scheel DW, Conners JR, Kalamaras J, Lee JE, Anderson DJ, Sussel L, Johnson JD, German MS. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127:3533–3542. doi: 10.1242/dev.127.16.3533. [DOI] [PubMed] [Google Scholar]
- Seguin CA, Draper JS, Nagy A, Rossant J. Establishment of endoderm progenitors by SOX transcription factor expression in human embryonic stem cells. Cell Stem Cell. 2008;3:182–195. doi: 10.1016/j.stem.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Semb H. Expandable Endodermal Progenitors: New Tools to Explore Endoderm and Its Derivatives. Cell Stem Cell. 2008;3:355–356. doi: 10.1016/j.stem.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Serls AE, Doherty S, Parvatiyar P, Wells JM, Deutsch GH. Different thresholds of fibroblast growth factors pattern the ventral foregut into liver and lung. Development. 2005;132:35–47. doi: 10.1242/dev.01570. [DOI] [PubMed] [Google Scholar]
- Serra M, Brito C, Correia C, Alves PM. Process engineering of human pluripotent stem cells for clinical application. Trends Biotechnol. 2012;30:350–359. doi: 10.1016/j.tibtech.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R, Scherer G, Sander M. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci U S A. 2007;104:1865–1870. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood RI, Chen TYA, Melton DA. Transcriptional Dynamics of Endodermal Organ Formation. Dev Dyn. 2009;238:29–42. doi: 10.1002/dvdy.21810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood RI, Maehr R, Mazzoni EO, Melton DA. Wnt signaling specifies and patterns intestinal endoderm. Mech Dev. 2011;128:387–400. doi: 10.1016/j.mod.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AG. Embryo-derived stem cells: Of mice and men. Annu Rev Cell Dev Biol. 2001;17:435–462. doi: 10.1146/annurev.cellbio.17.1.435. [DOI] [PubMed] [Google Scholar]
- Smith SB, Qu HQ, Taleb N, Kishimoto NY, Scheel DW, Lu Y, Patch AM, Grabs R, Wang JH, Lynn FC, Miyatsuka T, Mitchell J, Seerke R, Desir J, Eijnden SV, Abramowicz M, Kacet N, Weill J, Renard ME, Gentile M, Hansen I, Dewar K, Hattersley AT, Wang R, Wilson ME, Johnson JD, Polychronakos C, German MS. Rfx6 directs islet formation and insulin production in mice and humans. Nature. 2010;463:775–780. doi: 10.1038/nature08748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon JB, Borowiak M, Melton DA. Self-renewal of embryonic-stem-cell-derived progenitors by organ-matched mesenchyme. Nature. 2012;491:765–768. doi: 10.1038/nature11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers A, Jean JC, Sommer CA, Omari A, Ford CC, Mills JA, Ying L, Sommer AG, Jean JM, Smith BW, Lafyatis R, Demierre MF, Weiss DJ, French DL, Gadue P, Murphy GJ, Mostoslavsky G, Kotton DN. Generation of Transgene-Free Lung Disease-Specific Human Induced Pluripotent Stem Cells Using a Single Excisable Lentiviral Stem Cell Cassette. Stem Cells. 2010;28:1728–1740. doi: 10.1002/stem.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa-Pineda B, Wigle JT, Oliver G. Hepatocyte migration during liver development requires Prox1. Nature Genet. 2000;25:254–255. doi: 10.1038/76996. [DOI] [PubMed] [Google Scholar]
- Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, Wells JM. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–U120. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M, Apostolou E, Akutsu H, Fukuda A, Follett P, Natesan S, Kono T, Shioda T, Hochedlinger K. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature. 2010;465:175–U55. doi: 10.1038/nature09017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford D, Prince VE. Retinoic acid signaling is required for a critical early step in zebrafish pancreatic development. Curr Biol. 2002;12:1215–1220. doi: 10.1016/s0960-9822(02)00929-6. [DOI] [PubMed] [Google Scholar]
- Suga H, Kadoshima T, Minaguchi M, Ohgushi M, Soen M, Nakano T, Takata N, Wataya T, Muguruma K, Miyoshi H, Yonemura S, Oiso Y, Sasai Y. Self-formation of functional adenohypophysis in three-dimensional culture. Nature. 2011;480:57–U215. doi: 10.1038/nature10637. [DOI] [PubMed] [Google Scholar]
- Sun X, Xu J, Lu H, Liu W, Miao Z, Sui X, Liu H, Su L, Du W, He Q, Chen F, Shi Y, Deng H. Directed Differentiation of Human Embryonic Stem Cells into Thymic Epithelial Progenitor-like Cells Reconstitutes the Thymic Microenvironment In Vivo. Cell stem cell. 2013;13:230–6. doi: 10.1016/j.stem.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Sekiya S, Buscher D, Belmonte JCI, Taniguchi H. Tbx3 controls the fate of hepatic progenitor cells in liver development by suppressing p19(ARF) expression. Development. 2008;135:1589–1595. doi: 10.1242/dev.016634. [DOI] [PubMed] [Google Scholar]
- Tada S, Era T, Furusawa C, Sakurai H, Nishikawa S, Kinoshita M, Nakao K, Chiba T, Nishikawa SI. Characterization of mesendoderm: a diverging point of the definitive endoderm and mesoderm in embryonic stem cell differentiation culture. Development. 2005;132:4363–4374. doi: 10.1242/dev.02005. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tanimizu N, Nishikawa M, Saito H, Tsujimura T, Miyajima A. Isolation of hepatoblasts based on the expression of Dlk/Pref-1. J Cell Sci. 2003;116:1775–1786. doi: 10.1242/jcs.00388. [DOI] [PubMed] [Google Scholar]
- Terrace JD, Currie IS, Hay DC, Masson NM, Anderson RA, Forbes SJ, Parks RW, Ross JA. Progenitor cell characterization and location in the developing human liver. Stem Cells Dev. 2007;16:771–778. doi: 10.1089/scd.2007.0016. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Totonelli G, Maghsoudlou P, Garriboli M, Riegler J, Orlando G, Burns AJ, Sebire NJ, Smith VV, Fishman JM, Ghionzoli M, Turmaine M, Birchall MA, Atala A, Soker S, Lythgoe MF, Seifalian A, Pierro A, Eaton S, De Coppi P. A rat decellularized small bowel scaffold that preserves villus-crypt architecture for intestinal regeneration. Biomaterials. 2012;33:3401–3410. doi: 10.1016/j.biomaterials.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totonelli G, Maghsoudlou P, Georgiades F, Garriboli M, Koshy K, Turmaine M, Ashworth M, Sebire NJ, Pierro A, Eaton S, De Coppi P. Detergent enzymatic treatment for the development of a natural acellular matrix for oesophageal regeneration. Pediatr Surg Int. 2013;29:87–95. doi: 10.1007/s00383-012-3194-3. [DOI] [PubMed] [Google Scholar]
- Touboul T, Hannan NRF, Corbineau S, Martinez A, Martinet C, Branchereau S, Mainot S, Strick-Marchand H, Pedersen R, Di Santo J, Weber A, Vallier L. Generation of Functional Hepatocytes from Human Embryonic Stem Cells Under Chemically Defined Conditions that Recapitulate Liver Development. Hepatology. 2010;51:1754–1765. doi: 10.1002/hep.23506. [DOI] [PubMed] [Google Scholar]
- Tremblay KD, Zaret KS. Distinct populations of endoderm cells converge to generate the embryonic liver bud and ventral foregut tissues. Dev Biol. 2005;280:87–99. doi: 10.1016/j.ydbio.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Ungrin MD, Clarke G, Yin T, Niebrugge S, Nostro MC, Sarangi F, Wood G, Keller G, Zandstra PW. Rational bioprocess design for human pluripotent stem cell expansion and endoderm differentiation based on cellular dynamics. Biotechnol Bioeng. 2012;109:853–866. doi: 10.1002/bit.24375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, Milwid J, Kobayashi N, Tilles A, Berthiaume F, Hertl M, Nahmias Y, Yarmush ML, Uygun K. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814–U120. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci. 2005;118:4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- Van Hoof D, D’Amour KA, German MS. Derivation of insulin-producing cells from human embryonic stem cells. Stem Cell Res. 2009;3:73–87. doi: 10.1016/j.scr.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Wan HJ, Dingle S, Xu Y, Besnard V, Kaestner KH, Ang SL, Wert S, Stahlman MT, Whitsett JA. Compensatory roles of Foxa1 and Foxa2 during lung morphogenesis. J Biol Chem. 2005;280:13809–13816. doi: 10.1074/jbc.M414122200. [DOI] [PubMed] [Google Scholar]
- Wandzioch E, Zaret KS. Dynamic Signaling Network for the Specification of Embryonic Pancreas and Liver Progenitors. Science. 2009;324:1707–1710. doi: 10.1126/science.1174497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Rodriguez RT, Wang J, Ghodasara A, Kim SK. Targeting SOX17 in Human Embryonic Stem Cells Creates Unique Strategies for Isolating and Analyzing Developing Endoderm. Cell Stem Cell. 2011;8:335–346. doi: 10.1016/j.stem.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Tian Y, Morley MP, Lu MM, DeMayo FJ, Olson EN, Morrisey EE. Development and Regeneration of Sox2+ Endoderm Progenitors Are Regulated by a HDAC1/2-Bmp4/Rb1 Regulatory Pathway. Dev Cell. 2013;24:345–358. doi: 10.1016/j.devcel.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt AJ, Zhao R, Li JX, Duncan SA. Development of the mammalian liver and ventral pancreas is dependent on GATA4. BMC Dev Biol. 2007;7 doi: 10.1186/1471-213X-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein DC, Altaba ARI, Chen WS, Hoodless P, Prezioso VR, Jessell TM, Darnell JE. The winged-helix transcription factor HNF-3-beta is required for notochord development in the mouse embryo. Cell. 1994;78:575–588. doi: 10.1016/0092-8674(94)90523-1. [DOI] [PubMed] [Google Scholar]
- Wells JM, Melton DA. Early mouse endoderm is patterned by soluble factors from adjacent germ layers. Development. 2000;127:1563–1572. doi: 10.1242/dev.127.8.1563. [DOI] [PubMed] [Google Scholar]
- Wetsel RA, Wang DC, Calame DG. Therapeutic Potential of Lung Epithelial Proge nitor Cells Derived from Embryonic and Induced Pluripotent Stem Cells. In: Caskey CT, editor. Annual Review of Medicine. Vol. 62. Palo Alto: Annual Reviews; 2011. pp. 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Wong AP, Bear CE, Chin S, Pasceri P, Thompson TO, Huan LJ, Ratjen F, Ellis J, Rossant J. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat Biotechnol. 2012;30:876–U108. doi: 10.1038/nbt.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SM, Hochedlinger K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nat Cell Biol. 2011;13:497–505. doi: 10.1038/ncb0511-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie R, Everett LJ, Lim HW, Patel NA, Schug J, Kroon E, Kelly OG, Wang A, D’Amour KA, Robins AJ, Won KJ, Kaestner KH, Sander M. Dynamic chromatin remodeling mediated by polycomb proteins orchestrates pancreatic differentiation of human embryonic stem cells. Cell Stem Cell. 2013;12:224–37. doi: 10.1016/j.stem.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu CR, Cole PA, Meyers DJ, Kormish J, Dent S, Zaret KS. Chromatin “Prepattern” and Histone Modifiers in a Fate Choice for Liver and Pancreas. Science. 2011;332:963–966. doi: 10.1126/science.1202845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu RH, Sampsell-Barron TL, Gu F, Root S, Peck RM, Pan GJ, Yu JY, Antosiewicz-Bourget J, Tian SL, Stewart R, Thomson JA. NANOG is a direct target of TGF beta/Activin-mediated SMAD signaling in human ESCs. Cell Stem Cell. 2008a;3:196–206. doi: 10.1016/j.stem.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XB, D’Hoker J, Stange G, Bonne S, De Leu N, Xiao XW, De Casteele MV, Mellitzer G, Ling ZD, Pipeleers D, Bouwens L, Scharfmann R, Gradwohl G, Heimberg H. beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008b;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Xuan SH, Borok MJ, Decker KJ, Battle MA, Duncan SA, Hale MA, Macdonald RJ, Sussel L. Pancreas-specific deletion of mouse Gata4 and Gata6 causes pancreatic agenesis. J Clin Invest. 2012;122:3516–3528. doi: 10.1172/JCI63352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasunaga M, Tada S, Nishikawa ST, Nakano Y, Okada M, Jakt LM, Nishikawa S, Chiba T, Era T, Nishikawa SI. Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat Biotechnol. 2005;23:1542–1550. doi: 10.1038/nbt1167. [DOI] [PubMed] [Google Scholar]
- Yoshitomi H, Zaret KS. Endothelial cell interactions initiate dorsal pancreas development by selectively inducing the transcription factor Ptf1a. Development. 2004;131:807–817. doi: 10.1242/dev.00960. [DOI] [PubMed] [Google Scholar]
- Yu JY, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zaret KS, Grompe M. Generation and Regeneration of Cells of the Liver and Pancreas. Science. 2008;322:1490–1494. doi: 10.1126/science.1161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret KS, Watts J, Xu J, Wandzioch E, Smale ST, Sekiya T. Pioneer Factors, Genetic Competence, and Inductive Signaling: Programming Liver and Pancreas Progenitors from the Endoderm. In: Stillman B, Stewart S, Grodzicker T, editors. Control and Regulation of Stem Cells. Plainview: Cold Spring Harbor Laboratory Press; 2008. pp. 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DH, Jiang W, Liu M, Sui X, Yin XL, Chen S, Shi Y, Deng HK. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 2009;19:429–438. doi: 10.1038/cr.2009.28. [DOI] [PubMed] [Google Scholar]
- Zhao RO, Watt AJ, Li JX, Luebke-Wheeler J, Morrisey EE, Duncan SA. GATA6 is essential for embryonic development of the liver but dispensable for early heart formation. Mol Cell Biol. 2005;25:2622–2631. doi: 10.1128/MCB.25.7.2622-2631.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Law AC, Rajagopal J, Anderson WJ, Gray PA, Melton DA. A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell. 2007;13:103–114. doi: 10.1016/j.devcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Zorn AM, Wells JM. Vertebrate Endoderm Development and Organ Formation. Annu Rev Cell Dev Biol. 2009;25:221–251. doi: 10.1146/annurev.cellbio.042308.113344. [DOI] [PMC free article] [PubMed] [Google Scholar]