Abstract
The Ah receptor (AhR) and HLF are transcription factors involved in xenobiotic metabolism and hypoxic response, respectively. AhR and HLF heterodimerize with Arnt as the common partner, and bind to asymmetric E-boxes termed XRE and HRE, respectively. In order to investigate nucleotide preference of the heterodimers, reporter plasmids with oligonucleotides for XREs or HREs with systematic mutations were constructed and their activity was determined. Comparison of the activity revealed that DNA length and nucleotide preference recognized by Arnt subunit in the two heterodimers were largely different between XRE and HRE. We expressed AhR–Arnt and HLF–Arnt in Escherichia coli and used them for DNA binding. The dissociation constant of HLF–Arnt–HRE was 10.4 ± 1.6 nM. Competition activity of mutated XREs or HREs with wild type was consistent with their transcription activity. Bending of XRE and HRE induced by binding of the relevant heterodimers was observed with stronger bending of XRE than of HRE. By deletional and mutational analyses, an alanine and three arginine (Ala 8, Arg 9, Arg 11 and Arg 12) residues in the basic sequence of HLF were found to be indispensable for the transcriptional activity.
INTRODUCTION
The basic helix–loop–helix motif (bHLH) is a frequently used domain structure in transcription factors. This motif mediates dimerization that results in the formation of both homodimers and heterodimers binding to the E-box site CANNTG to regulate transcription (1,2). The bHLH motif consists of the basic sequence followed by two amphipathic α-helices separated by a loop of variable length. The N-terminal basic sequence is rich in basic amino acids and directly contacts bases in the major groove of DNA. It is believed that conformation of the basic region is altered from a random coil to an α-helical structure when it binds to DNA (3,4).
Members of the bHLH–PAS (Per-Arnt-Sim) family constitute a subgroup of the HLH protein family of transcription factors and play key roles in several physiological phenomena including xenobiotic metabolism [Ah receptor (AhR)](5), hypoxic responses such as angiogenesis and erythropoiesis (HIF-1α and HLF) (6,7), maintenance of circadian rhythms (Clock) (8) and neurogenesis (Sim 1 and Sim 2) (9). These transcription factors, except for Clock, form heterodimers with the AhR nuclear translocator (Arnt), which is also a bHLH–PAS domain protein and acts as the common partner protein. Both HLH and PAS domains function to mediate protein–protein interaction for dimerization and the N-terminal basic sequence of each subunit contacts a half-site of the asymmetric E-box (10).
AhR is localized in the cytosol in association with Hsp90 in quiescent cells. When xenobiotics such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and 3-methylcholanthrene (MC) enter the cells, AhR binds to them as ligands and translocates into the nucleus. In the nucleus, AhR dimerizes with Arnt and the AhR–Arnt complex binds to the xenobiotic responsive element (XRE), which is localized in the upstream region of genes for mainly MC-inducible drug metabolizing enzymes such as CYP1A1, glutathione S-transferase Ya subunit and a form of UDP-glucronosyl transferase (11).
HIF-1α and HLF (HIF-2α) are closely related transcription factors that are activated by the reduction of oxygen (12). At normoxia, these two proteins are rapidly degraded through a ubiquitin–proteasome pathway. Under hypoxic conditions, the HIF-1α and HLF stabilize, form heterodimers with Arnt and upregulate genes, such as those for erythropoietin (EPO), vascular endothelial growth factor (VEGF) and glycolytic enzymes, by binding to the hypoxia response element (HRE) in the promoter region. Since HRE has a core sequence different from that of XRE, the two heterodimers, AhR–Arnt and HIF-1α–Arnt or HLF–Arnt, exhibit no cross-binding.
Sim 1 and Sim 2 function negatively on transcription (13), while the Drosophila counterpart dSim is a positive transcription factor. dSim is a master regulatory gene that controls the development of the neurons and glia localized along the midline of the central nervous system (CNS). Drosophila Arnt, a tango gene product (Tgo) that is closely related to mammalian Arnt, dimerizes with dSim to bind the CNS midline element (CME) and activates CNS midline transcription (14). Sim 1 and Sim 2 also function in the development of CNS. Because CME closely resembles HRE in sequence, HIF-1α–Arnt or HLF–Arnt heterodimer could bind to CME. However, the target genes of the dSim–Arnt heterodimer are completely different from those of the HIF-1α–Arnt or HLF–Arnt heterodimer. It is reported that the specificity of target gene selection was performed through the PAS domains of dSim and HIF-1α (15). To date, genes located downstream of mammalian Sims are unknown.
Since the core sequences of XRE (CACGC) and HRE (CACGTA) contain CAC half-sites in common, the CAC half-site was expected to be recognized by Arnt. This expectation was confirmed by DNA–protein cross-linking experiments (16) and the finding that Arnt was able to form a homodimer to bind to a symmetric type 2 E-box, CACGTG (17).
For binding of bHLH–PAS proteins to cognate response elements, flanking sequence is equally important to the core sequence of the response elements. However, the nucleotide preference in the flanking sequence has not been studied so far and is largely unknown. In this work, we have investigated DNA recognition of the AhR–Arnt and HLF–Arnt heterodimers to the flanking sequence of XRE and HRE core sequences, and elucidated the preference for base pairs by monitoring transcription activity of XREs and HREs with mutations in the flanking sequence. Nucleotide preference and length recognized by Arnt in the flanking of XRE core element were strikingly different from those of HRE. DNA binding analyses of bacterially expressed AhR–Arnt and HLF–Arnt to XRE and HRE supported the finding. Furthermore, in accordance with the results, bending of XRE induced by binding of AhR–Arnt was clearly shown in the circular permutation assay.
MATERIALS AND METHODS
Construction of plasmids
Reporter plasmids containing mutated HRE and XRE were constructed by inserting the synthetic oligonucleotides (88mer) which consist of four-tandem repeats of a variety of XRE (18mer; TGAGTTCTCACGCTAGCA) and HRE (18mer; 5′-TGAGACAGCACGTAGGGC-3′) sequences spaced with four nucleotides of GATC and four nucleotides for ligation (CGCG for the coding strand and GATC for the non-coding strand) into the MluI–BglII site of pGL3 Promoter Vector (Promega).
The bacterial expression plasmid pRSETB-hAhR-hArnt, which encodes amino acids 27–436 of AhR and 82–464 of Arnt, was constructed as described previously (18). pRSETB-mHLF-hArnt was constructed similarly. A DNA fragment of mouse HLF cDNA encoding amino acids 15–360 inclusive of bHLH–PAS regions was amplified by PCR and cloned into the NheI–HindIII of pRSETB vector (Invitrogen). The resultant plasmid, pRSETB-mHLF, was digested with HaeII, and the blunt-ended fragment containing cDNA was cloned into the AflIII site of pRSETB-hArnt (amino acids 82–464) plasmid (18) to generate HLF pRSETB-mHLF-hArnt. pRSETB-mHLF(Δ1–2)-hArnt and pRSETB-mHLF(Κ1Α)-hArnt were constructed by inserting oligonucleotides for mutated basic sequences into the NdeI–BsmBI site of pRSETB-mHLF. Construction of an expression plasmid of Flag-tagged HLF, pBOSFlagmHLF, was performed by replacing DNA (from the EcoRI to the BsmBI site) coding for the N-terminal basic sequence of HLF in pBOSmHLF (12) with a synthetic oligonucleotide for Flag-tag and the basic sequence of HLF. In the oligonucleotide, an NdeI site was introduced just after the C-terminus to the Flag-tag to facilitate construction of HLF mutants. The Flag-tagged N-terminal basic sequence of HLF is as follows: MDYKDDDDKHRKEKSRDAARCRR–. Effector plasmids for Flag-tagged HLF mutant plasmids were constructed by inserting oligonucleotides for mutated basic sequences into the NdeI–BsmBI site of pBOSFlagmHLF.
pBend-HRE and pBend-XRE used in the circular permutation assay were constructed by inserting oligonucleotides of XRE 5′-CTAGATTGATGCTAGCGTGAGAACTCAAATG-3′, 5′-TCGACATTTGAGTTCTCACGCTAGCATCAAT-3′ and of HRE 5′-CTAGATTGAGCCCTACGTGCTGTCT CAAATG-3′, 5′-TCGACATTTGAGACAGCACGTAGGG CTCAAT-3′ between the XbaI and SalI sites of pBend2 (generously provided by Dr S. Adhya). All plasmids constructed were confirmed by sequencing.
Cell culture and reporter assay
Human hepatoma Hep3B cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and 40 µg/ml kanamycin. The cells were transfected using the calcium phosphate method as described previously (19). Cells in a 60 mm dish were transiently transfected with 5 µg of DNA (2 µg of reporter plasmid together with 3 µg pBOS-LacZ, or 2 µg each of effector and reporter plasmids together with 1 µg pBOS-LacZ). Four hours after transfection, the cells received fresh medium. For assays of reporter activity using plasmids containing HRE and XRE, cells were incubated for 18 h in normoxia and for 40 h in the presence or absence of 1 µM MC, respectively, before harvesting. The luciferase activity was determined using a luciferase detection kit (Promega) and normalized to β-galactosidase activity, which was used as an internal control.
Expression of bHLH–PAS proteins in Escherichia coli
pRSETB-hAhR-hArnt, pRSETB-mHLF-hArnt, pRSETB-mHLF(Δ1–2)-hArnt and pRSETB-mHLF(Κ1Α)-hArnt were expressed as described previously (18). In brief, the E.coli strain BL21(DE3), pLysS containing pRSETB-hAhR-hArnt, pRSETB-mHLF-hArnt, pRSETB-mHLF(Δ1–2)-hArnt or pRSETB-mHLF(Κ1Α)-hArnt was grown in 10 ml of L-Broth (50 µg/ml ampicillin, 25 µg/ml chloramphenicol) at 37°C until OD600 > 1.0. Confirming sufficient multiplication, they were grown in 100 ml L-Broth at 20°C, and isopropyl-β-d-thiogalactopyranoside (IPTG) (0.1 mM) was added at OD600 = 0.5 to induce protein expression. After overnight cultivation at 20°C, the bacteria were recovered by centrifugation and lysed by sonication in extract buffer (20 mM HEPES pH 7.9, 500 mM NaCl, 5 mM imidazole, 10% glycerol, 0.1% NP-40, 1mM β-mercaptoethanol). Then the sample was centrifuged at 18 000 r.p.m. for 30 min and the supernatant was added to 0.1 ml Ni-NTA gel (Qiagen). After gentle rotating at 4°C for 1 h, the sample was centrifuged at 1000 r.p.m. for 1 min and the precipitating gel was washed five times with extract buffer. Co-expressed proteins were eluted with elution buffer (20 mM HEPES pH 7.9, 500 mM NaCl, 200 mM imidazole, 10% glycerol, 0.1% NP40, 1 mM β-mercaptoethanol). The purified hAhR-hArnt, mHLF-hArnt, mHLF(Δ1–2)-hArnt and mHLF(Κ1Α)-hArnt proteins were estimated to amount to 30–40% of total protein by SDS–PAGE and subsequent densitometric determination (data not shown).
Gel mobility shift assay (GMSA)
Bacterially expressed HLF–Arnt and AhR–Arnt heterodimers were used for GMSA. The proteins were preincubated at 4°C for 30 min in a total volume of 10 µl of binding buffer (10 mM HEPES pH 7.9, 100 mM NaCl, 0.1 mM EDTA, 3 mM MgCl2 1 mM dithiothreitol, 0.1 mg/ml bovine serum albumin, 0.01 mg/ml salmon sperm DNA, 10% glycerol), followed by incubation with 2 µl of 32P-labeled oligonuculeotide probe (10 000 c.p.m.) at 20°C for 25 min. The reaction mixture was electrophoresed in a non-denaturing 4.5% polyacrylamide gel and the gel was dried and autoradiographed.
DNase I footprinting
A DNA fragment containing a single copy of the XRE or HRE sequence was prepared by cleaving pBend-XRE or pBend-HRE with XhoI and Asp718. The fragment was labeled at the 5′ end of the coding or non-coding strand by treating it with [32P]ATP and T4 polynucleotide kinase and then received a second digestion by BglII and BamHI, respectively. The labeled fragment was purified on agarose gels and incubated with purified AhR–Arnt or HLF–Arnt in a total volume of 40 µl of binding buffer (10 mM HEPES pH 7.9, 100 mM NaCl, 0.1 mM EDTA, 3 mM MgCl2, 1 mM dithiothreitol, 0.1 mg/ml bovine serum albumin, 10% glycerol, 4 µg/ml poly[dI-dC]) at 4°C for 20 min. Next, 2 µl of a DNase I solution (5 mM CaCl2, 10 mM MgCl2, 17.5 U/ml DNase I) were added to the mixture and incubated for 5 s. Reactions were terminated by the addition of 40 µl of phenol saturated with 3% NaCl. Digested DNA was fractionated through an 8% polyacrylamide–8 M urea sequencing gel (acrylamide: bisacrylamide = 19:1). Chemical cleavage of another portion of the labeled DNA at purine residues (A + G) was performed according to the procedure described by maxam and Gilbert (20), and the resultant DNA was run in parallel with the samples of digested DNA. After electrophoresis, gels were dried and autoradiographed.
Circular permutation assay
A circular permutation assay was performed as described previously (21). The plasmids pBend-HRE and pBend-XRE were each separately digested with five restriction enzymes listed in the legend to Figure 5, and DNA fragments were purified by agarose gel electrophoresis and labeled with 32P-ATP. Using these DNA fragments and purifed proteins expressed in E.coli, gel mobility shift assay was conducted as described above.
Figure 5.
Bending of DNA bound by the AhR–Arnt or HLF–Arnt heterodimer. (A) Structure of probes used for permutation assays. Oligonucleotides of XRE or HRE were cloned between the XbaI and SalI sites of pBEND2, and the resultant plasmids were digested with restriction enzymes (probe 1, MluI; probe 2, XhoI; probe 3, NruI; probe 4, Asp718; probe 5, BamHI) and purified on agarose gel. (B) Gel shift analysis of AhR–Arnt–XRE and HLF–Arnt–HRE complexes. Probes 1–5 were end-labeled with [32P]ATP and T4 polynucleotide kinase and were incubated with corresponding bacterially expressed and purified heterodimers at 4°C for 30 min. The reaction mixture was separated in non-denaturating acrylamide gel and autoradiographed. Filled and open arrowheads show retarded bands and free probes, respectively. (C) Electrophoresis of probe DNA. Probes 1–5 end-labeled as shown in (B) were electrophoresed without incubation with heterodimers in non-denaturing acrylamide gel and autoradiographed. The filled arrowhead shows probes.
RESULTS
Mutational analysis of HRE and XRE
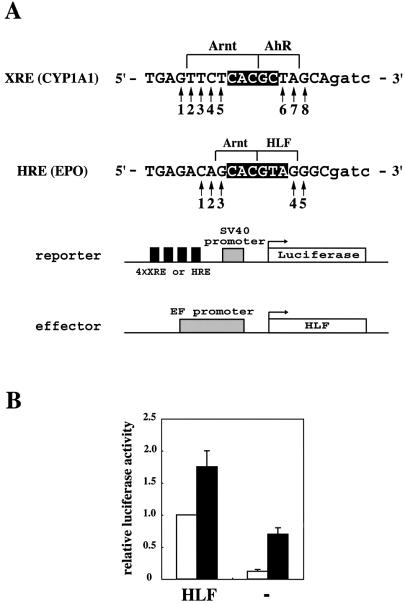
In order to estimate the nucleotide preference that AhR–Arnt and HLF–Arnt heterodimers exhibit in the flanking region of XRE and HRE, respectively, systematic mutations were introduced to the response elements in the promoter of reporter plasmids as shown in Figure 1A. As parental response elements, XRE1 in the rat CYP1A1 gene and the HRE sequence in the human erythropoietin gene that are known to act as potent enhancers were selected and used as wild types. Each nucleotide in the flanking sequence of wild-type XRE and HRE core sequence changed to three other nucleotides (positions 1–8 for XRE and 1–5 for HRE). These reporter plasmids were introduced into Hep3B cells which express endogenous AhR, HLF and HIF-1α and show a strong response to xenobiotic and hypoxic stimuli. In the case of reporter assays of plasmids containing HRE sequences, wild-type HLF was coexpressed to minimize the effect of endogenous HIF-1α. As shown in Figure 1B, the expressed luciferase activity in normoxic cells was increased about eight times by the coexpression of HLF. The activity was further increased 2.5-fold in hypoxic conditions. As the effect of exogeneous HLF emerged more strongly in the normoxic condition than the hypoxic condition, we chose the normoxic condition to determine reporter activity of mutated HREs driven by HLF. We first introduced mutations at position 3 of wild-type HRE (Fig. 1C). When compared with wild type, the three mutants exhibited decreased luciferase activity. In particular, mutation to C largely decreased the activity of wild type to 13.1%, suggesting the presence of an interaction between the base pair at this position and the HLF–Arnt heterodimer. Mutations at position 2 were then introduced and luciferase activity was assayed. Mutations of A in wild type to C, G and T showed activity similar to wild type, suggesting that the heterodimer did not recognize the base pair at position 2. Since a weak augmentation in activity was found when mutated to T at position 2, we further introduced similar mutations at position 1. Nearly the same amounts of activity of mutants as for the wild type was found, suggesting no interaction between DNA and the heterodimer at this position. The mutation at position 4 from G of wild type to A and T decreased its activity to 35% and 37% of wild type, respectively, suggesting interaction of the heterodimer with HRE at this position. Mutations at position 5 to other nucleotides did not affect the activity, suggesting no interaction between HRE and the dimer at position 5. Taken together, these results suggest that the HLF–Arnt heterodimer contacted the eight base pairs 5′-GCACGTAG-3′ in HRE. For recognition of HRE by HLF–Arnt, four base pairs were recognized by each subunit as shown in Figure 1A.
Figure 1.
Mutational analysis of HRE and XRE. (A) Sequence of wild-type XRE and HRE and structure of reporter and effector plasmids. A 22mer of oligonucleotides for XRE from XRE 1 of the CYP1A1 gene and HRE originating from the EPO gene is shown. Mutated nucleotides are shown by 1–8 for XRE and 1–5 for HRE. Regions contacted with Arnt and AhR or Arnt and HLF are shown by bars above the sequences. Reporter plasmids contain four tandemly repeated XREs or HREs upstream of the SV40 promoter and luciferase structural gene. The effector plasmid contains elongation factor enhancer and promoter to induce expression of HLF in Hep3B cells. (B) Effect of coexpression of HLF on reporter activity. The effector plasmid (2 µg) shown in (A) was cotransfected with 2 µg of HRE reporter plasmid in (A) and 1 µg of pBOSlacZ, and expressed luciferase activity was determined. Open and filled boxes show luciferase activity from cells incubated in normoxic and hypoxic conditions, respectively. The activity was shown relative to the activity from cells transfected with HLF effector plasmid and incubated in normoxia. The luciferase activity was normalized with β-galactosidase activity used as an internal control. The activity is the average of at least three independent experiments with SD. (C) Transcriptional activity of plasmids with mutated HRE. The position of the mutated nucleotide was shown above each diagram. A, C, G and T below diagrams denote nucleotides changed in HRE. The original nucleotide used in wild-type HRE is shown as (WT). Luciferase activity was determined using cells incubated in normoxic conditions, and is shown relative to the activity of wild type. The luciferase activity was normalized with β-galactosidase activity used as an internal control. The activity is the average of at least three independent experiments with SD. (D) Transcriptional activity of plasmids with mutated XRE. The position of the mutated nucleotide is shown above each diagram. A, C, G and T below diagrams denote nucleotides changed in XRE. The original nucleotide used in wild-type HRE is shown as (WT). Luciferase activity was determined from cells incubated in the presence or absence of MC for 40 h after transfection and is shown relative to the activity of the wild type induced by MC. The luciferase activity was normalized with β-galactosidase activity used as an internal control. The activity is the average of at least three independent experiments with SD.
We performed similar experiments to investigate recognition of the AhR–Arnt heterodimer to XRE, using the luciferase reporter plasmids containing mutated XRE sequences (Fig. 1D). Strict preference for base-pair recognition by AhR–Arnt was observed in the flanking region. Selection of T and C at positions 4 and 5 in XRE greatly decreased the activity. At position 3, changes to A or G led to a large decrease in activity. Furthermore, mutation to A or C at position 2 also led to a slight decrease in activity. These results strongly suggest that recognition of Arnt to XRE extends to position 2. Mutations at position 6 did not affect activity, and mutations to C, G or T at position 7 led to a large reduction in activity. In particular, mutation to G diminished activity nearly to the background level. Taken together, the AhR–Arnt heterodimer recognized 10 base pairs of XRE (positions 2–7, excluding position 6), and at positions 1 and 8 the heterodimer did not show any base-pair preference.
In vitro DNA binding of HLF–Arnt and AhR–Arnt
Before doing experiments on DNA binding of heterodimers, the dissociation constant of the bacterially expressed HLF–Arnt heterodimer–HRE complex was determined. As shown in Figure 2A, the Kd value was 10.4 ± 1.6 nM. This value was slightly lower than that of the AhR–Arnt to XRE (18). This result strongly suggests that the recombinant HLF–Arnt heterodimer has a DNA binding property quite similar to that of cellular origin, although to date no Kd values using a cellular HLF–Arnt heterodimer have been reported. Methylation of the CpG sequence in the oligonucleotides of HRE greatly reduced the ability to compete with unmethylated HRE (Fig. 2B). A similar decrease in the binding activity of the AhR–Arnt heterodimer to XRE has already been reported (18).
Figure 2.
Binding of the bacterially expressed HLF–Arnt heterodimer to HRE. (A) The dissociation constant was determined using the HLF–Arnt expressed in E.coli and synthetic oligonucleotides of HRE in GMSA. A representative diagram of a Scatchard plot and a typical autoradiographic result of GMSA are shown on the left and right, respectively. The HRE concentrations in lanes 1–6 were 25, 78.8, 14, 10.5, 7.9 and 5.9 nM, respectively. Protein concentration in the reaction mixture was 35 ng/µl. The filled and open arrowheads show the bound and free probes, respectively. The bands indicated by an arrow were not reproducible. (B) Effect of CpG methylation in the HRE sequence on binding. The position of 5-methyl cytosine in HRE is marked with arrows. All combinations of oligonucleotides with methylated and unmethylated strands of HRE were used as competitors in GMSA where unmethylated HRE was used as the probe. Numbers with an asterisk above autoradiography show methylated strands of HRE. Increasing amounts of competitors of 12.5-fold (lanes 2, 5, 8 and 11), 25-fold (lanes 3, 6, 9 and 12) and 50-fold (lanes 4, 7, 10 and 13) over probe were added to the pre-incubation mixture.
Recombinant AhR–Arnt heterodimer was incubated with 32P-labeled probe for wild-type XRE, and increasing amounts of competitors were added (Fig. 3). Similar experiments were performed using a probe for HRE and bacterially expressed HLF and Arnt. We chose three different types of competitors in affinity with mutations in the region mainly contacted by Arnt: in the first type the transcriptional activity of the sequence was similar to or higher than wild type (m1, m6), in the second it was lower (m2, m4, m5, m7 and m8) and in the third it was much lower (m3). We could not find mutant competitors with extreme low activity for HRE. We used m18 (22) as a negative control in competition with XRE. Competition with m1 and m6 showed more intense competition than that with wild type (Fig. 3A and B, lanes 5–7). The competitive activity of m2, m4, m5, m7 and m8 was lower than wild type and correlated roughly with their transcriptional activity. No or very weak competitive activity, similar to that of m18, was observed with m3 which showed essentially no transcriptional activity. These results demonstrate that the binding activity of AhR–Arnt and HLF–Arnt heterodimer to the mutated XRE and HRE, respectively, is highly correlated with the transcriptional activity shown in Figure 1B and C.
Figure 3.
Competition of mutated XRE or HRE with wild type. (A) Competitive GMSA was performed using representative mutated XRE as competitors and bacterially expressed AhR–Arnt heterodimer. Purified protein (40 ng) was mixed with buffer A containing mutated oligonucleotides on ice for 30 min. About 133 pg of 32P-labeled (10 000 c.p.m.) XRE (wild type) was added to the mixture which was incubated at 20°C for 25 min. The mutated XRE sequence was shown, and only mutated nucleotides in mutants were represented. The transcriptional activity obtained in Figure 1 is also shown. A representative autoradiograph of GMSA is shown. Increasing amounts of competitors [30-fold (lanes 2, 5, 8 and 11), 100-fold (lanes 3, 6, 9 and 12) and 250-fold (lanes 4, 7, 10 and 13)] were used for competition. The arrow shows the retarded band. (B) Competitive GMSA was performed using representative mutated HRE as competitors. The experiment was performed using the same procedure as in (A) using bacterial expressed HLF–Arnt heterodimer and mutated HRE. Increasing amounts of competitors [100-fold (lanes 2, 5, 8 and 11), 250-fold (lanes 3, 6, 9 and 12) and 500-fold (lanes 4, 7, 10 and 13)] were used for competition assay. The arrow shows the retarded band.
DNase I footprinting analysis of XRE and HRE
To further investigate the region recognized by HLF–Arnt or AhR–Arnt, DNase I footprinting was performed using the purified proteins expressed in E.coli and DNA fragments, including a single copy of HRE or XRE. When the protected region of HRE and XRE was compared, we found that the coding strand of XRE, especially the 3′-flanking region of XRE, was more protected than that of HRE from DNase I digestion as shown in Figure 4. Hypersensitive sites were found 5′ upstream of XRE and HRE in the coding strand and 3′ downstream of XRE and HRE in the non-coding strand. Weak signals for hypersensitive sites were also observed 3′ downstream of XRE and HRE. Weak protection of bases was found in the 5′ region in the coding sequence of XRE and HRE. This protection was probably due to non-specific binding of protein.
Figure 4.
DNase I foot printing analysis. A DNA fragment (117 bp for the coding strand and 112 bp for the non-coding strand) containing a single copy of HRE or XRE in the central part was 5′ end-labeled with [32P]ATP and T4 polynucleotide kinase and incubated with DNase I in the presence or absence of the corresponding heterodimer that was bacterially expressed and purified. After incubation for 5 s at 20°C, the reaction was terminated by the addition of 3% NaCl-saturated phenol. The digested DNA was precipitated with ethanol and separated in the denaturating acrylamide gel containing 8 M urea. A portion of labeled DNA was modified with formic acid which resulted in cleavage of DNA at positions G and A. The protected regions are shown by parentheses on the right side of DNA ladders and the regions are also shown above and below the XRE and HRE sequences. The nucleotides contacted by heterodimers found in Figure 1 are boxed, with shading for the core sequence of XRE and HRE.
Bending of HRE and XRE
As the recognized sequence of XRE by the AhR–Arnt heterodimer was unexpectedly long, as shown in Figure 1C, the DNA fragments containing XRE were expected to bend when interacting with the heterodimer. To investigate the DNA bending of XRE and HRE, we conducted a circular permutation assay. The structures of the probes, including XRE and HRE, are shown in Figure 5A. Five types of probes in which the binding site of the protein was moved from one end to the other were prepared for each response element. As shown in Figure 5B, the two heterodimers bent the corresponding DNA and the migration pattern of the AhR–Arnt–XRE complex was more progressive than that of HLF–Arnt–HRE. This result shows that XRE is more strongly bent than HRE when interacting with protein. The DNA fragments used as probes showed almost the same mobility when they were electrophoresed without heterodimers, as shown in Figure 5C, indicating that the bending is protein dependent.
Amino acids responsible for DNA recognition in the basic sequence of HLF
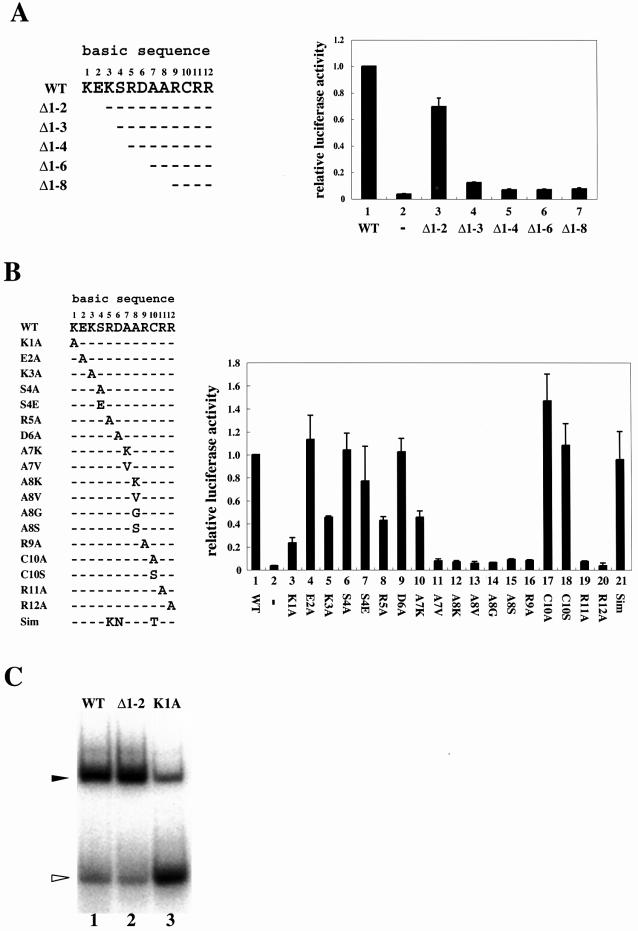
To determine which amino acids in the basic sequence of HLF are important for contacting HRE, we performed transient transfection assays in Hep3B cells using plasmids with mutation or deletion in the basic sequence of HLF as shown in Figure 6. Mutants of HLF were nearly equally expressed as determined by western blotting using anti-Flag-tag antibody (data not shown). Figure 6A shows that deletion of the three N-terminal amino acids (Δ1–3) results in almost complete loss of the reporter activity so that it was reduced to the basal level, although deletion of the first two amino acids did not affect the activity. As shown in Figure 6B, mutations of Ala 8, Arg 9, Arg 11 and Arg 12 abolished the luciferase activity (Fig. 6B, lanes 12–17 and 20), suggesting that these amino acids are responsible for DNA binding. Although mutation of Ala 7 to Val led to a large decrease in activity, mutation to Lys showed significant luciferase activity (Figure 6B, lanes 10 and 11). When basic amino acids Lys 3 and Arg 5 were mutated to Ala, the luciferase activity decreased by about 55%, suggesting a considerable role in the interaction of these amino acids with DNA. Mutation of Lys 1 to Ala caused a considerable reduction in luciferase activity, although Lys 1 was shown to be dispensable for the activity by the analysis of a deletion mutant (Δ1–2). To solve the discrepancy, we expressed two mutants of HLF, Δ1–2 and K1A, with Arnt in E.coli and used the expressed heterodimers to examine the DNA binding activity to HRE. As shown in Figure 6C, the DNA binding activity of the mutants was roughly associated with the transcription activity. From the result, we concluded that the first two amino acids in the basic sequence of HLF were not involved in direct DNA binding, and that mutation of Lys 1 to Ala indirectly damaged the DNA binding.
Figure 6.
Deletional and mutational analyses of the basic sequence of HLF. (A) Deletional analysis of the HLF basic sequence. The amino acid sequence (1–12) of the basic region of HLF and the deleted amino acids are shown on the left. Effector plasmids (2 µg) with corresponding deletions were cotransfected into Hep3B cells together with 2 µg of the HRE reporter plasmid shown in Figure 1A and 1 µg of pBOSlacZ using the same procedure as shown in Figure 1. The cells were incubated in normoxic conditions for 18 h after transfection. The luciferase activity was normalized with β-galactosidase activity used as an internal control. The activity is the average of at least three independent experiments with SD. Effector plasmids with or without full-length HLF cDNA were used as positive and negative controls, respectively, and the activity is shown in lanes 1 and 2. (B) Mutational analysis of the HLF basic sequence. Mutated amino acids are shown on the left. The amino acids of basic sequence were replaced with Ala. The alanine residues in Ala 7 and Ala 8 in the wild type were changed to K or V for Ala 7 and K, V, G or S for Ala 8. The structure of the basic sequence of Sim 1 is also shown. The transfection procedures and culture conditions of cells were the same as that in (A). Effector plasmids with or without full-length HLF cDNA were used as positive and negative controls, respectively, and the activity is shown in lanes 1 and 2. The luciferase activity was normalized with β-galactosidase activity used as an internal control. The activity is the average of at least three independent experiments with SD. (C) Binding activity of HLF mutants to HRE. Mutants of HLF, Δ1–2 and K1A were generated with Arnt in E.coli and purified. The DNA binding activity of the mutants was examined in GMSA according to the same procedure as shown in Figure 3B. Filled and open arrowheads show retarded bands and free probes, respectively.
The sequence of CME is known to be closely related to, or nearly the same as, that of HRE (15), and three amino acids in the basic sequence are different between HLF and Sim 1 (Fig. 6B). We mutated the three amino acids in HLF to that of Sim 1, and the expressed luciferase activity was determined. As shown in Figure 6B, the mutated HLF with basic sequence of Sim 1 showed activity similar to wild-type HLF (Fig. 6B, lane 21). This result suggests that the amino acid substitution between HLF and Sim 1 did not affect DNA binding activity, and that the two factors have quite similar DNA binding specificity.
DISCUSSION
bHLH–PAS proteins constitute a subgroup in the bHLH superfamily and bind to the asymmetric E-box. Of the bHLH–PAS proteins, AhR and HLF have key roles in xenobiotic metabolism and hypoxic response, respectively, by activating transcription. AhR and HLF form heterodimers with Arnt and bind to DNA elements termed XRE and HRE, respectively. These elements have the core sequence CACGC (XRE) or CACGTA (HRE). Although the occurrence of the core sequence in the DNA is necessary for specific binding, it is not enough to assure efficient binding of the factors, but its flanking sequence must be considered. However, a detailed analysis for flanking sequences has not been performed. We systematically mutated XRE and HRE in their flanking region (Fig. 1), and found that the AhR–Arnt and HLF–Arnt heterodimers recognize 10 bp and 8 bp DNA, respectively. Sequence specificity of Arnt subunits was clearly different between AhR–Arnt and HLF–Arnt.
Flanking sequences of the XRE core have been analyzed according to the method of DNA selection and amplification (23). The DNA sequences selected by several rounds of selection were to some extent compatible with those shown in Figure 1D. However, a closer comparison showed clear differences probably due to overly repeated selection and amplification and/or a bias rising from the strategy used. In the literature, certain base pairs were not used for XRE even in the sites distant from the core sequence. Extended contact of Arnt in the AhR–Arnt to the DNA has been reported (24) with a limited number of mutated XRE sequences, consistent with the results of this study. A survey of known enhancer sequences containing XRE or HRE found in various genes for drug metabolism and hypoxia satisfied the preferable sequences elucidated in this study (25–27).
DNA recognition of the AhR–Arnt heterodimer was unexpectedly long, and it exceeded one helical turn of DNA. This long DNA recognition by AhR–Arnt was mainly performed by the Arnt subunit. Presumably, Arnt in the heterodimer with AhR contacts the DNA beyond the major groove by bending DNA. Rather strong DNA bending by the AhR–Arnt was observed (Fig. 5). It is not known why the difference in DNA recognition of Arnt between XRE and HRE arose. A subtle conformational change or positioning of the basic region caused by partner proteins could generate the difference. In the bHLH-zip protein family, MAX has a similar function to Arnt. MAX can heterodimerize to c-Myc and MAD, and also form a homodimer. However, unlike Arnt, the recognition sequence of MAX seems identical in heterodimers or homodimers (4), and no DNA bending was reported.
The three-dimensional structures of the DNA–protein complexes of several bHLH-domain-containing transcription factors, including MAX, USF, MyoD and PHO4 homodimers, have been elucidated (28–31). The DNA recognition helix of the basic region was inserted into the corresponding major groove of DNA. The liganded DNA was essentially B form and was not bent by the binding of proteins. The discovery of the bending of XRE and HRE, as shown in this study, was an extraordinary finding and may be characteristic of an asymmetric E-box. The X-ray crystallographic studies demonstrated that conserved amino acids His, Glu and Arg at positions 4, 8 and 12, respectively, directly interacted in basic sequences with bases in the major groove (31). These amino acids were not conserved in the basic sequence of HLF except for Arg 12. Mutational analysis of the amino acids in positions 4, 8 and 12 of HLF clarified that mutation of Ser 4 to Ala was not effective in the transcriptional activity. Instead, mutation or deletion of the surrounding basic amino acids at positions 3 and 5 reduced the transcriptional activity. Mutation of Ala 8 to Lys, Val, Gly and Ser showed extensive reduction in transcription activity, suggesting the necessary interaction of Ala 8 with DNA albeit with the charge changed from negative to neutral when compared with Glu 8 in bHLH-zip proteins. It has been shown that mutations of Ser 4 in the HIF-1α basic region showed no effect on acticity (32). Although the report did not show the importance of basic amino acids surrounding Ser 4, the contact between the amino acids and the DNA might be conserved between HLF and HIF-1α.
To date, no data have been available on dissociation constant of HLF–Arnt–HRE. Thus HLF and Arnt were co-expressed in E.coli, purified and used for determination of the dissociation constant of the HLF–Arnt–HRE complex. The dissociation constant of HLF–Arnt–HRE was similar to that of AhR–Arnt–XRE (18). Essentially no different dissociation constants were exhibited between cellular and recombinant AhR–Arnt heterodimers [see (18) and references cited therein]. CpG methylation of each strand of HRE largely reduced the affinity, suggesting a regulation mechanism of transcription via methylation of DNA. Reduction of expression of CYP1A1 presumably through CpG methylation of XRE was reported in senescent model animals (33).
In expectation of the occurrence of XRE or HRE in the promoter of newly identified target genes, sequence information carried by the core sequences was, to some extent, useful for a rough estimation of presence of the response elements, but the information was not enough for accurate identification and estimation of strength of the response elements. The base-pair preference in the flanking sequence of XRE and HRE elucidated in this study, would help to identify the response elements in the genes, although estimated XRE and HRE consensus sequences are not complete because interactions between neighboring amino acids in the basic sequence were not considered in the experiments.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Dr O. Gotoh and Dr K. Yasumoto for helpful discussions and Dr S. Adhya for the kind gift of pBEND2 vector. This work was supported in part by Grant-in-Aid for research on priority area (A) from the Ministry of Education, Science, Sports and Culture of Japan, and research for the Future Program of the Japan Society for the Promotion of Science.
REFERENCES
- 1.Baxevanis A.D. and Vinson,C.R. (1993) Interactions of coiled coils in transcription factors: where is the specificity? Curr. Opin. Genet. Dev., 3, 278–285. [DOI] [PubMed] [Google Scholar]
- 2.Blackwell T.K. and Weintraub,H. (1990) Differences and similarities in DNA-binding preferences of MyoD and E2A protein complexes revealed by binding site selection. Science, 250, 1104–1110. [DOI] [PubMed] [Google Scholar]
- 3.Murre C., McCaw,P.S. and Baltimore,D. (1989) A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD and myc proteins. Cell, 56, 777–783. [DOI] [PubMed] [Google Scholar]
- 4.Ferre-D’Amare A.R., Prendergast,G.C., Ziff,E.B. and Burley,S.K. (1993) Recognition by Max of its cognate DNA through a dimeric b/HLH/Z domain. Nature, 363, 38–45. [DOI] [PubMed] [Google Scholar]
- 5.Whitlock J.P. Jr (1999) Induction of cytochrome P4501A1. Annu. Rev. Pharmacol., 39, 103–125. [DOI] [PubMed] [Google Scholar]
- 6.Hochachka P.W., Buck,L.T., Doll,C.J. and Land,S.C. (1996) Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc. Natl Acad. Sci. USA, 93, 9493–9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guillemin K. and Krasnow,M.A. (1997) The hypoxic response: huffing and HIFing. Cell, 89, 9–12. [DOI] [PubMed] [Google Scholar]
- 8.King D.P., Zhao,Y., Sangoram,A.M., Wilsbacher,L.D., Tanaka,M., Antoch,M.P., Steeves,T.D., Vitaterna,M.H., Kornhauser,J.M., Lowrey,P.L., et al. (1997) Positional cloning of the mouse circadian clock gene. Cell, 16, 641–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stark K.A., Yee,G.H., Roote,C.E., Williams,E.L., Zusman,S. and Hynes,R.O. (1997) A novel alpha integrin subunit associates with betaPS and functions in tissue morphogenesis and movement during Drosophila development. Development, 124, 4583–4594. [DOI] [PubMed] [Google Scholar]
- 10.Swanson H.I. (2002) DNA binding and protein interactions of the AHR/ARNT heterodimer that facilitate gene activation. Chem. Biol. Interact., 141, 63–76. [DOI] [PubMed] [Google Scholar]
- 11.Mimura J. and Fujii-Kuriyama,Y. (2003) Functional role of AhR in the expression of toxic effects by TCDD. Biochim. Biophys. Acta, 1619, 263–268. [DOI] [PubMed] [Google Scholar]
- 12.Ema M., Taya,S., Yokotani,N., Sogawa,K., Matsuda,Y. and Fujii-Kuriyama,Y. (1997) Proc. Natl Acad. Sci. USA, 94, 4273–4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ema M., Morita,M., Ikawa,S., Tanaka,M., Matsuda,Y., Gotoh,O., Saijoh,Y., Fujii,H., Hamada,H., Kikuchi,Y. et al. (1996) Two new members of the murine Sim gene family are transcriptional repressors and show different expression patterns during mouse embryogenesis. Mol. Cell. Biol., 16, 5865–5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wharton K.A. Jr, Franks,R.G., Kasai,Y. and Crews,S.T. (1994) Control of CNS midline transcription by asymmetric E-box-like elements: similarity to xenobiotic responsive regulation. Development, 120, 3563–3569. [DOI] [PubMed] [Google Scholar]
- 15.Zelzer E., Wappner,P. and Shilo BZ. (1997) The PAS domain confers target gene specificity of Drosophila bHLH/PAS proteins. Genes Dev., 11, 2079–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bacsi S.G., Reisz-Porszasz,S. and Hankinson,O. (1995) Orientation of the heterodimeric aryl hydrocarbon (dioxin) receptor complex on its asymmetric DNA recognition sequence. Mol. Pharmacol., 47, 432–438. [PubMed] [Google Scholar]
- 17.Sogawa K., Nakano,R., Kobayashi,A., Kikuchi,Y., Ohe,N., Matsushita,N. and Fujii-Kuriyama,Y. (1995) Possible function of Ah receptor nuclear translocator (Arnt) homodimer in transcriptional regulation. Proc. Natl Acad. Sci. USA, 92, 1936–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kikuchi Y., Ohsawa,S., Mimura,J., Ema,M., Takasaki,C., Sogawa,K. and Fujii-Kuriyama,Y. (2003) Heterodimers of bHLH–PAS protein fragments derived from AhR, AhRR and Arnt prepared by co-expression in Escherichia coli: characterization of their DNA binding activity and preparation of a DNA complex. J. Biochem. (Tokyo), 134, 83–90. [DOI] [PubMed] [Google Scholar]
- 19.Fujisawa-Sehara A., Sogawa,K., Nishi,C. and Fujii-Kuriyama,Y. (1986) Regulatory DNA elements localized remotely upstream from the drug-metabolizing cytochrome P-450c gene. Nucleic Acids Res., 14, 1465–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maxam A.M. and Gilbert,W. (1980) Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol., 65, 499–560. [DOI] [PubMed] [Google Scholar]
- 21.Kim J., Zwieb,C., Wu,C. and Adhya,S. (1989) Bending of DNA by gene-regulatory proteins: construction and use of a DNA bending vector. Gene, 85,15–23. [DOI] [PubMed] [Google Scholar]
- 22.Semenza G.L. and Wang,G.L. (1992) A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell. Biol., 12, 5447–5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swanson H.I., Chan,W.K. and Bradfield,C.A. (1995) DNA binding specificities and pairing rules of the Ah receptor, ARNT and SIM proteins. J. Biol. Chem., 270, 26292–26302. [DOI] [PubMed] [Google Scholar]
- 24.Shen E.S. and Whitlock,J.P.,Jr (1992) Protein–DNA interactions at a dioxin-responsive enhancer. Mutational analysis of the DNA-binding site for the liganded Ah receptor. J. Biol. Chem., 267, 6815–6819. [PubMed] [Google Scholar]
- 25.Kress S., Reichert,J. and Schwarz,M. (1998) Functional analysis of the human cytochrome P4501A1 (CYP1A1) gene enhancer. Eur. J. Biochem., 258, 803–812. [DOI] [PubMed] [Google Scholar]
- 26.Camenisch G., Stroka,D.M., Gassmann,M. and Wenger,R.H. (2001) Attenuation of HIF-1 DNA-binding activity limits hypoxia-inducible endothelin-1 expression. Pflugers Arch., 443, 240–249. [DOI] [PubMed] [Google Scholar]
- 27.Baba T., Mimura,J., Gradin,K., Kuroiwa,A., Watanabe,T., Matsuda,Y., Inazawa,J., Sogawa,K. and Fujii-Kuriyama,Y. (2001) Structure and expression of the Ah receptor repressor gene. J. Biol. Chem., 276, 33101–33110. [DOI] [PubMed] [Google Scholar]
- 28.Nair S.K. and Burley,S.K. (2003) X-ray structures of Myc-Max and Mad-Max recognizing DNA: molecular bases of regulation by proto-oncogenic transcription factors. Cell, 112, 193–205. [DOI] [PubMed] [Google Scholar]
- 29.Ferre-D’Amare A.R., Pognonec,P., Roeder,R.G. and Burley,S.K. (1994) Structure and function of the b/HLH/Z domain of USF. EMBO J., 13, 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma P.C., Rould,M.A., Weintraub,H. and Pabo,C.O. (1994) Crystal structure of MyoD bHLH domain-DNA complex: perspectives on DNA recognition and implications for transcriptional activation. Cell, 77, 451–459. [DOI] [PubMed] [Google Scholar]
- 31.Shimizu T., Toumoto,A., Ihara,K., Shimizu,M., Kyogoku,Y., Ogawa,N., Oshima,Y. and Hakoshima,T. (1997) Crystal structure of PHO4 bHLH domain–DNA complex: flanking base recognition. EMBO J., 16, 4689–4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michel G., Minet,E., Ernest,I., Roland,I., Durant,F., Remacle,J. and Michiels,C. (2000) A model for the complex between the hypoxia-inducible factor-1 (HIF-1) and its consensus DNA sequence. J. Biomol. Struct. Dyn., 18, 169–179. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi Y., Suzuki,C. and Kamataki,T. (1998) Silencing of CYP1A1 expression in rabbits by DNA methylation. Biochem. Biophys. Res. Commun., 247, 383–386. [DOI] [PubMed] [Google Scholar]