Abstract
Background
Autoimmune lymphoproliferative syndrome (ALPS) is a human disorder of T cell homeostasis caused by mutations that impair Fas-mediated apoptosis. A defining characteristic of ALPS is the expansion of double negative T cells (DNTC). Relatively little is known about how defective Fas-driven cell death and the Bcl-2 apoptotic pathway intersect in ALPS patients.
Objective
We studied changes in Bcl-2 family member expression in ALPS to determine whether the Bcl-2 pathway might provide a therapeutic target.
Methods
We used flow cytometry to analyze the expression of pro- and anti-apoptotic Bcl-2 family members in T cells from 12 ALPS patients and determined the in vitro sensitivity of ALPS DNTC to the pro-apoptotic BH3-mimetic, ABT-737.
Results
The pro-apoptotic molecule, Bim was significantly elevated in DNTC. Although no general pattern of individual anti-apoptotic Bcl-2 family members emerged, increased expression of Bim was always accompanied by increased expression of at least one anti-apoptotic Bcl-2 family member. Strikingly, Bim levels in DNTC correlated significantly with serum interleukin-10 (IL-10) in ALPS patients and IL-10 was sufficient to mildly induce Bim in normal and ALPS T cells via a Jak/STAT3-dependent mechanism. Finally, ABT-737 preferentially killed ALPS DNTC in vitro.
Conclusion
Combined, these data show that an IL-10/Jak/STAT3 pathway drives Bim expression in ALPS DNTC, which renders them sensitive to BH3-mimetics, uncovering a potentially novel therapeutic approach to ALPS.
Keywords: ALPS, Bim, Bcl-2 pathway, Interleukin10, BH3- mimetic, ABT-737, T cell homeostasis, double negative T cells, apoptosis
Introduction
Maintenance of T cell homeostasis is critical for normal functioning of the immune system. Regulated induction of apoptosis is required to maintain T cell homeostasis and is controlled largely by two largely independent pathways, the extrinsic, or death receptor-driven pathway and the intrinsic, or Bcl-2-regulated pathway. Defects in either pathway result in disorders that are characterized by lymphoproliferation (including lymphoma), abnormal responses to infection and autoimmunity1, 2. Thus, appropriate regulation of both extrinsic and intrinsic apoptotic pathways is essential for maintaining T cell homeostasis.
Autoimmune Lymphoproliferative Syndrome (ALPS) is an example of a genetic disorder of lymphocyte apoptosis caused by defects in the FAS-mediated cell death pathway3. The majority of ALPS cases are caused by a germline heterozygous mutation in the FAS receptor4, 5. Somatic mutations in the FAS receptor and mutations in FAS ligand or the apoptosis-inducing effector enzyme, caspase-10, are additional causes for ALPS6–9. Mutations in NRAS, KRAS and caspase 8 have also been reported to cause ALPS-like disorders10–13, and about one third of ALPS cases do not have a known genetic cause14. ALPS is manifested by chronic non-malignant lymphoproliferation, autoimmunity and increased risk of lymphoma15, 16. The signature of the disease is an expanded homogenous population of T cells that is T cell receptor (TCR) αβ+ CD4− CD8− and co-expresses B22016–18, so-called double negative T cells (DNTC).
The role of DNTC in ALPS disease is also controversial. In lymphoproliferative (lpr) mice, which have a homozygous Fas receptor mutation, DNTC can make IL-17, which likely contributes to disease progression19. However, in humans DNTC do not appear to be major producers of IL-17 or IFN-γ although they express both granzyme B and perforin18, 20, 21. Further, ALPS patients have dramatic elevations in circulating IL-10 and DNTC are the dominant producers of IL-10 when assessed ex vivo22. Although immunosuppressive therapy of ALPS patients decreases DNTC and serum IL-10 levels23, the roles of DNTC and IL-10 remain unclear.
Although DNTC can arise in animals with defects in either the extrinsic or intrinsic cell death pathway, DNTC origin appears pathway-specific. The presence in most lpr mice of CD8lo cells that express TCRα/β, which are likely DNTC precursors24–26, and the absence of DNTC in β-2microglobulin-deficient lpr mice suggest that DNTC arise from overstimulated CD8+ T cells. Consistent with this, one study found an extensive overlap of particular CDR3 sequences between DNTC and CD8+ T cells in ALPS patients, sequences that were not represented in CD4+ T cells27. Chronic stimulation regimens often induce the death receptor pathway28, suggesting that chronic stimulation of CD8+ T cells may facilitate their elimination via Fas-driven death, while in the absence of Fas signaling, CD8 is lost, and B220+ DNTC accumulate. In contrast, mice that lack expression of Bim or overexpress Bcl-2 also accumulate DNTC, but these cells lack B220 expression29,19. However, why the intrinsic cell death pathway is not sufficient to control DNTC in ALPS patients remains unclear.
Indeed, while the apoptotic defect in ALPS results from defective Fas signaling, the role of the intrinsic apoptotic pathway in ALPS pathophysiology is not known. There appear to be overlapping roles for the extrinsic and intrinsic apoptotic pathways in murine T cell homeostasis30. In ALPS patients, steady-state Bim levels were elevated in some patients and repeated TCR stimulation of CD8+ T cells from these patients slightly increased Bim levels31. However, baseline levels of Bcl-2 family members have not yet been examined extensively in T cell subsets from ALPS patients. Here, we report a dysregulated Bcl-2 pathway in ALPS, most notably that DNTC from ALPS patients have significantly increased levels of Bim. We also show that interleukin-10 signaling through STAT3 contributes to this increased Bim expression. Lastly, we investigated whether this dysregulated expression of Bim could facilitate DNTC targeting by a drug that suppresses the activity of anti-apoptotic Bcl-2 family members32–34. ABT-737 binds to and suppresses the anti-apoptotic effects of Bcl-2, Bcl-xL and Bcl-w (but not Mcl-1 or A134) and prevents their ability to neutralize pro-apoptotic Bcl-2 family members32. Further, we have previously shown that treatment of normal mice with ABT-737 promotes the Bim-dependent loss of T cells35. Our results with this approach suggest that it could be useful for targeting these cells in patients with ALPS or other disorders of immune dysregulation.
Methods
Human Subjects
Patients were evaluated at Cincinnati Children’s Hospital Medical Center. Blood samples were taken after informed consent was obtained according to an Institutional Review Board research protocol. Control samples were obtained from healthy volunteers associated with the Cincinnati Children’s Hospital Diagnostic Immunology Laboratory. Samples were held at room temperature before analysis.
Clinical data, genetic mutations, DNT cell percentage and cytokine level data were obtained from medical records. Peripheral blood mononuclear cells (PBMC) from patients and controls were separated from whole blood by density gradient centrifugation using Ficoll-Paque Plus (GE Healthcare, Pittsburgh, PA). Cells were then washed, pelleted and stained for flow cytometry, cultured or frozen in FBS with 10% DMSO (Sigma-Aldrich, St Louis, MO).
Flow Cytometry
Fresh or thawed human PBMC’s were incubated with human IgG (Sigma-Aldrich, St Louis, MO) to block Fc receptors then stained with combinations of the following cell-surface antibodies: anti-CD3, -CCR7 (BD Biosciences, San Jose, CA), -CD4, -CD45RO (BioLegend, San Diego, CA), -CD8, -CD45RA and -B220 (eBioscience, San Diego, CA). Intracellular staining was performed with one of the following antibodies: anti-Bim, -Bcl-xL (Cell Signaling Technology, Beverly, MA), -Bcl-2 (Caltag Laboratories, Burlingame, CA), -Mcl-1 (Rockland, Gilbertsville, PA), -A-1 (AbCam, Cambridge, MA) or anti IL-10RA (EMD Millipore, Billerica, MA). For detection of Phospho-Stat3, Human PBMC’s were incubated for 2 h at 37°C, stained for cell surface CD3, then stimulated with 50 ng/ml of recombinant human IL-10 (PeproTech, Rocky Hill, NJ) for 20 min. The cells were then washed, fixed, permeabilized, and stained with anti-phospho-Stat3 (Tyr705) (Cell Signaling Technology, Beverly, MA). For evaluation of cell viability, cells were stained with 7AAD stain or the Blue Live/Dead Fixable Dead Cell Stain kit (Life Technologies, Grand Island, NY). A minimum of 2.5 ×105 events was acquired on a BD LSR II or BD FACSCanto flow cytometer and analyzed by FACSDiva (BD Biosciences). In order to convert MFI to MESF values, we used Cyto-Cal™ Multifluor plus Violet Fluorescence Intensity Calibrator (Thermo Scientific, Pittsburgh, PA). The beads were run on the cytometer at the same time as we acquired samples, using the same PMT voltage and compensation settings. The data were analyzed using the Cali curve data analysis program, according to the manufacturer’s instructions.
Cell culture
PBMC’s from ALPS patients or normal controls were cultured in RPMI 1640 media (supplemented with 2 mM l-glutamine, 10 mM HEPES, 100 IU/ml penicillin, 100 mg/ml streptomycin, 10% FBS) at 37°C in 5% CO2.
ABT-737, a generous gift of Abbott Laboratories, was dissolved in DMSO, and diluted in a solution composed of 35% polyethylene glycol, 5% Tween-80, and 65% dextrose in water. Human cells were cultured in the presence of the indicated ABT-737 concentration or vehicle overnight. Cells were washed, stained and cell viability was determined by flow cytometry as above. In order to account for the rate of spontaneous apoptosis in vitro, the percentage of cell survival was calculated according to the following formula: percentage of cell survival = 100 − {[(% live cells in vehicle−% live cells treated)/ % live cells in vehicle] ×100%}. For exogenous IL-10 culture experiments: human PBMC’s from normal controls were incubated in the presence or absence of 50 ng/ml recombinant human IL-10 for 12 hours before or after T cell activation. Cells were activated for 3 days with 2 µg/ml plate-bound anti-CD3 (OKT3 mAb) and 2 µg/ml anti-CD28 (eBioscience, San Diego, CA), ± 25 IU/ml recombinant human IL-2. ALPS PBMC’s were incubated in the presence or absence of 50 ng/ml recombinant human IL-10 for 12 hours in the presence of IL-2. Cells were then harvested, washed and stained for surface markers and intracellular Bim. In some experiments cells were incubated with 7.5 µM Stattic V, a STAT3 inhibitor (Santa Cruz biotechnology, Santa Cruz, CA) or 250 nM Ruxolitinib, a Jak1 inhibitor (LC Laboratories, Woburn, MA) for 45 minutes prior to adding IL-10.
Statistical analysis
Statistical significance was estimated using a two-sample paired student t-test or Wilcoxon matched-pairs signed rank test. For estimation of significance for Bim elevation, a one-sample t-test was used to compare mean values to a value of 1. Associations between cytokine level and Bim were assessed by separate linear regression of each cytokine to Bim level. All statistical analyses were performed using Prism 6.0 (GraphPad Software Inc.) and differences were considered significant at P ≤ 0.05.
Results
Bim is elevated in ALPS T cells
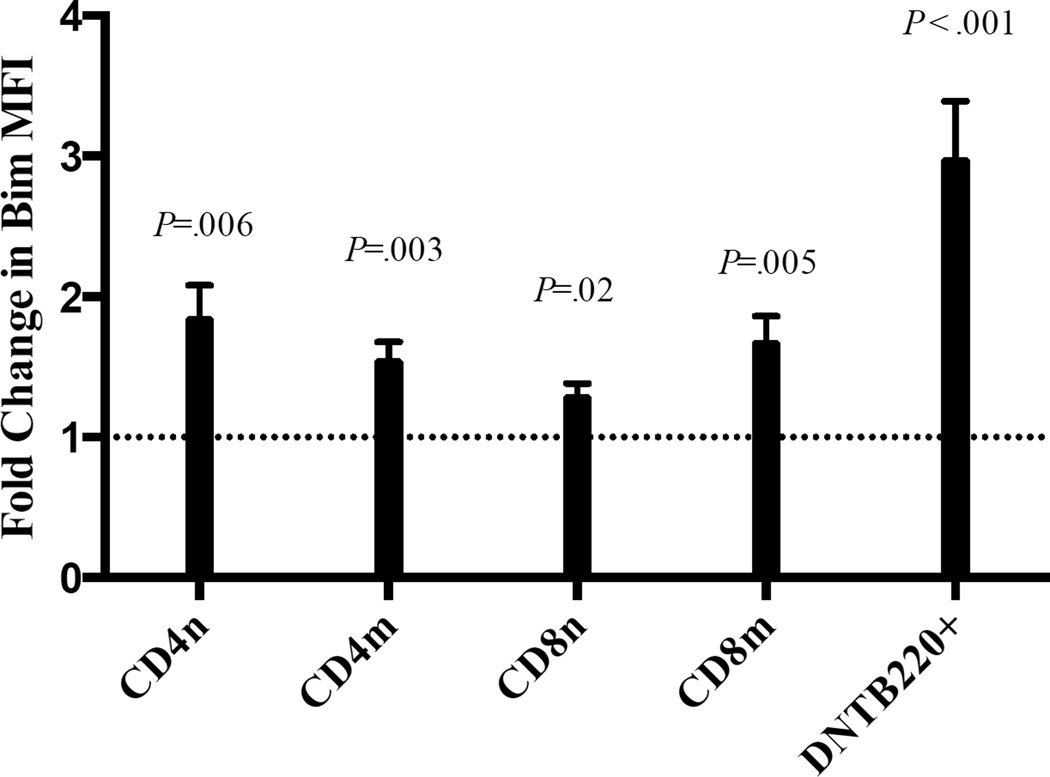
While ALPS is a disorder of the extrinsic apoptotic pathway, little is known about the role of the intrinsic pathway of apoptosis in ALPS. Its importance is suggested by a report that showed that a patient with an ALPS-like syndrome had a mutation in NRAS that resulted in decreased expression of Bim10. Conversely, repeated TCR stimulation of CD8+ T cells from other ALPS patients increased Bim levels31. To determine whether Bim levels were altered in ALPS patients at baseline, we examined the expression of Bim and other Bcl-2 family members in 12 ALPS patients (Table1). The cohort includes patients with germline Fas mutations, one patient with a somatic Fas mutation and one patient with unknown mutations. All patients met the criteria of definite ALPS diagnosis14, and blood draws were performed prior to initiation of significant immunosuppressive therapy. All 12 patients showed lymphoproliferation and 10 out of 12 had autoimmune cytopenia. DNTC percentages ranged from 2.3%–23% of total lymphocytes. Bim levels were elevated in all CD4+ and CD8+ T cell subsets (naïve, memory, DNTC) from ALPS patients relative to healthy controls. As normal controls essentially lack a detectable DNTC B220+ population, but have a detectable population of DNTC, we compared Bim expression in DNTC in normal controls. Interestingly, the largest increase in Bim was observed in DNTC, with a mean 3-fold increase (range = 1.2–5.6 fold) (Figure 1A). Bim expression was significantly elevated in DNTC compared to CD4 and CD8 T cells in ALPS patients (P= .004 and .01 respectively). These data were pooled from several experiments in which one or two ALPS patients were compared with one or two healthy controls; in every instance, Bim levels were consistently higher in the ALPS patients. Similar results were obtained when we used a bead quantification method to convert Bim MFI into MESF (molecules of equivalent soluble fluorochrome) (data not shown). Thus, Bim expression is increased in ALPS.
Table 1.
Clinical characteristics and FAS mutations in ALPS patients.
| Patient | Age (y) | Sex | ALPS type* | FAS mutation | LP | AI cytopenia |
% DNT** |
|---|---|---|---|---|---|---|---|
| 1 | 6 | M | ALPS-sFAS | 863_864insAAT† | + | + | 12.1 |
| 2 | 17 | M | ALPS-FAS | 529(−2)A>G | + | + | 9 |
| 3 | 16 | F | ALPS-U | Unknown | + | − | 3.4 |
| 4 | 0.9 | F | ALPS-FAS | 1009A>G | + | + | 4 |
| 5 | 16 | M | ALPS-FAS | 949A>T† | + | + | 7.8 |
| 6 | 21 | M | ALPS-FAS | 439G>A† | + | + | 6.8 |
| 7 | 5 | M | ALPS-FAS | 851_852delAG | + | + | 15.7 |
| 8 | 11 | M | ALPS-FAS | 669_683delinsA | + | + | 4.3 |
| 9 | 8 | M | ALPS-FAS | 214T>C | + | + | 2.5 |
| 10 | 17 | M | ALPS-FAS | 916C>A | + | + | 2.3 |
| 11 | 6 | M | ALPS-FAS | 762(+4)A>G† | + | − | 3.4 |
| 12 | 3 | M | ALPS-FAS | 652-2A>G | + | + | 23 |
LP indicates lymphoproliferative disease; AI cytopenias, autoimmune cytopenias and %DNT, percentage of double negative T cells.
ALPS type according to revised ALPS classification: ALPS-FAS: germline mutation in FAS, ALPS-sFAS: somatic mutation in ALPS and ALPS-U: patient fulfilling ALPS criteria but no mutation is identified.
% DNT normal values <1.5% of total lymphocytes.
Mutation unreported previously.
Figure 1. Bim is increased in all T cell subsets of ALPS patients.
The average fold increase in Bim mean fluorescence intensity (MFI, ± standard error of mean) in naïve CD4+ (CD4n), memory CD4+ (CD4m), naïve CD8+ (CD8n), memory CD8+ (CD8m) and double negative T cells: CD3+CD4−CD8−B220+(DNTB220+) from PBMC’s. CD3+CD4−CD8−B220+ cells in ALPS patients were compared to CD3+CD4−CD8− cells in healthy controls, because the former are immeasurable in healthy controls.
Different anti-apoptotic Bcl-2 members are elevated in ALPS
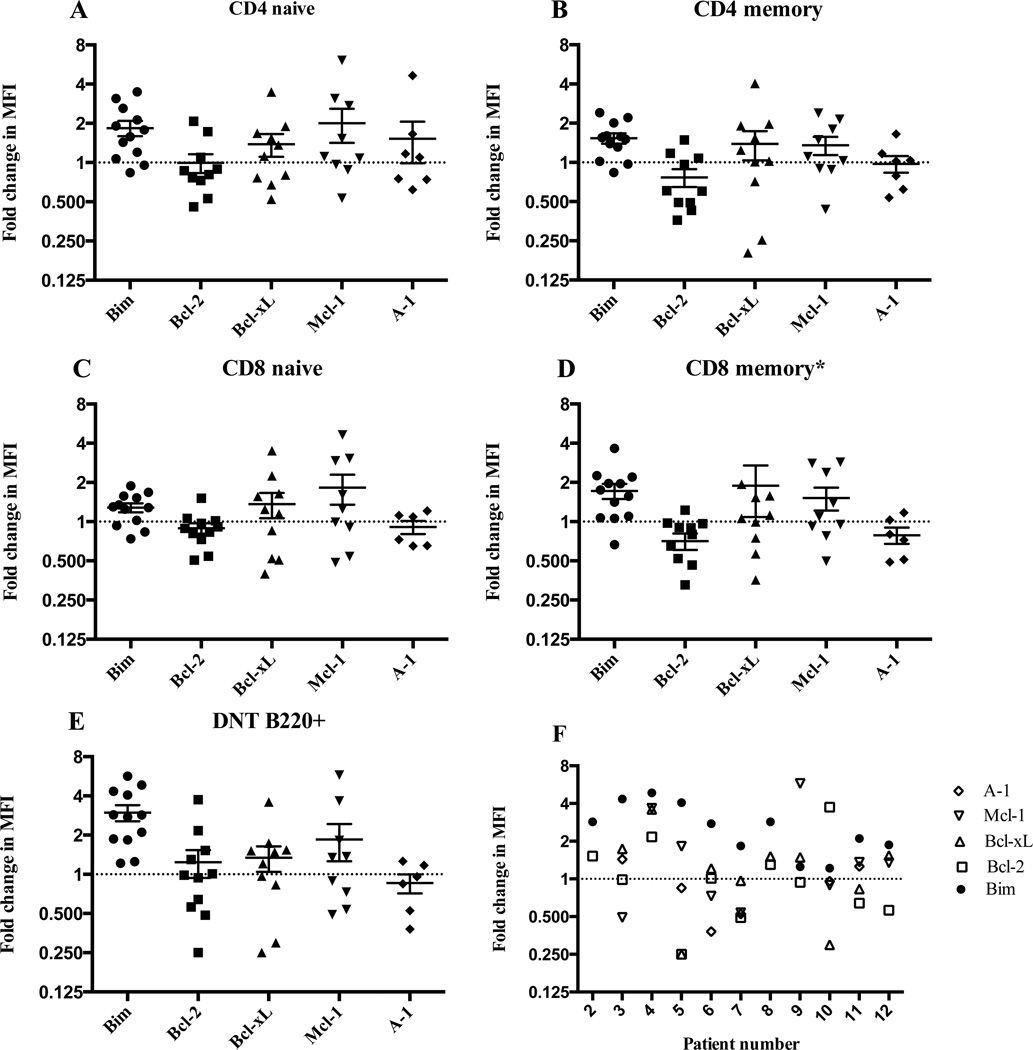
It seemed paradoxical that Bim was elevated in DNTC despite their accumulation in ALPS. However, our previous work showed that Bim is also increased in long-lived memory T cells, but is balanced by increased levels of the anti-apoptotic molecule, Bcl-2, which allows cells to survive high levels of Bim by blocking Bim induction of apoptosis36. To determine whether increased expression of an anti-apoptotic Bcl-2 family member similarly accompanies the increased expression of Bim within DNTC, we measured the levels of four anti-apoptotic Bcl-2 family members (Bcl-2, Bcl-xL, Mcl-1 and A-1) in DNTC. In contrast to our previous observation with CD8+ T cells in normal mice, in which increased Bim levels were generally antagonized by increased levels of Bcl-2 is the major antagonist of Bim35, 36, no single anti-apoptotic molecule was consistently elevated in DNTC from all ALPS patients. Instead, different anti-apoptotic molecules were elevated in different ALPS patients (Figure 2). Usually, the same anti-apoptotic molecule(s) was increased in the different T cell subsets in the same patient. In every ALPS patient, at least one anti-apoptotic molecule was elevated in DNTC (Figure 2F), suggesting that, depending upon the ALPS patient, different anti-apoptotic molecules antagonize Bim to allow T cell survival.
Figure 2. The expression of anti-apoptotic Bcl-2 molecules is heterogeneous in ALPS.
The fold change in the MFI (± SEM) of different Bcl-2 family members in PBMC’s of 12 ALPS patients versus healthy controls in (A) naïve or (B) memory CD4 cells, (C) naïve or (D) memory CD8 cells, and (E) CD3+CD4−CD8−B220+ cells. (F) The relative expression of Bim and different anti-apoptotic molecules in DNTB220+ cells per patient (X-axis). Dashed horizontal line represents the expression in healthy controls (ratio of 1). * Two data points are outside the axis limit.
IL-10 levels are correlated with Bim levels in DNTC and both are reduced with immunosuppressive therapy
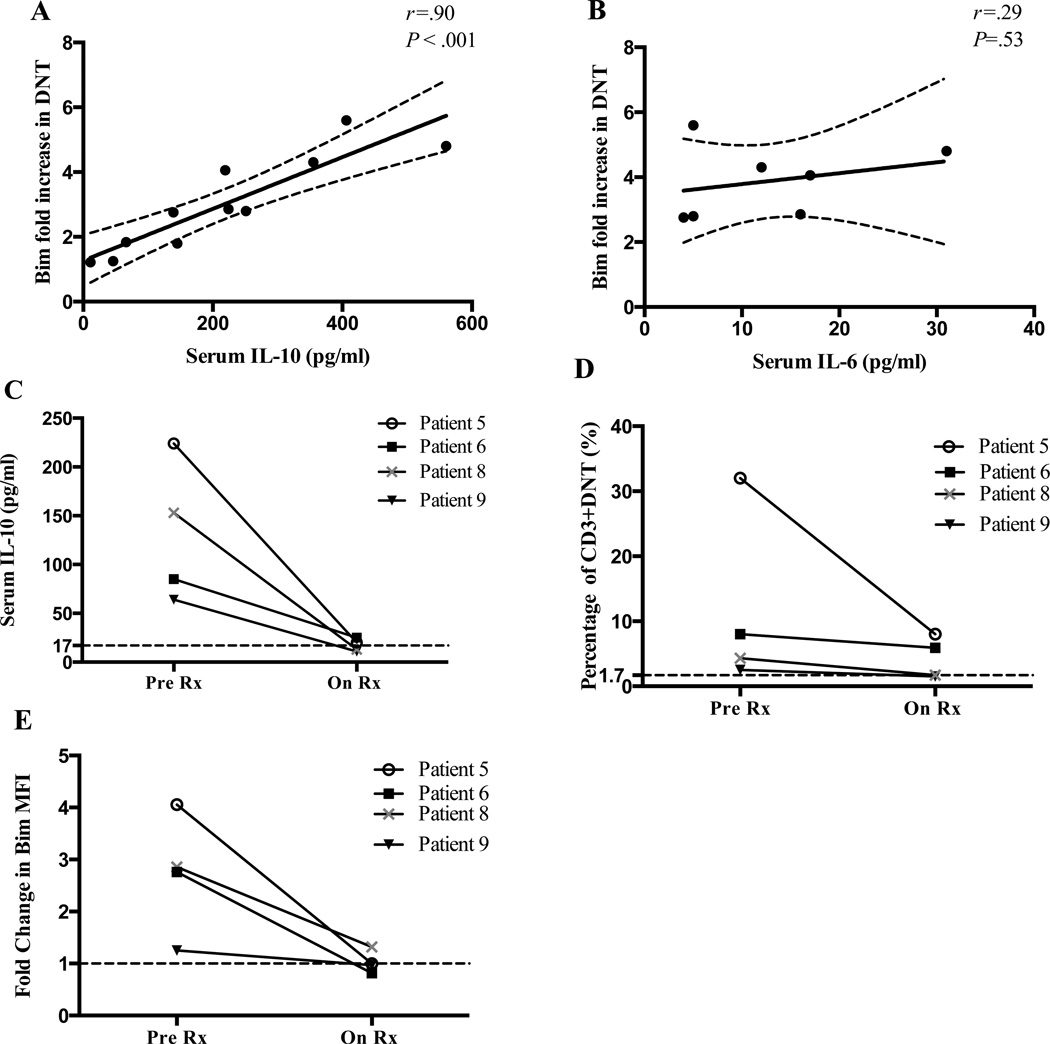
Because serum IL-10 is elevated in ALPS patients22, 23, 37 and DNTC are thought to be the major source of IL-10,20, 22 we evaluated whether serum IL-10 levels correlated with DNTC Bim levels. Strikingly, there was a strong correlation between serum IL-10 levels and DNTC Bim levels in DNTC in our ALPS patients (Figure 3A). Patients with the highest IL-10 level had the largest increase in Bim (P=0.0002 and r=0.90). This strong correlation was not seen with IL-6, another serum cytokine that was elevated in some of our patients (n=7) (Figure 3B). As previously reported,23 we noted a decrease in serum IL-10 level and DNTC in four ALPS patients after immunosuppressive therapy was started (Figure 3C and D). Interestingly, Bim levels in DNTC also decreased to almost normal levels in these patients (Figure 3E). Together, these data show that immunosuppressive therapy decreases IL-10 levels, reduces DNTC levels, and decreases DNTC Bim expression in vivo.
Figure 3. Serum IL-10 level correlates with Bim before and after therapy.
Results show the correlation of the increase in Bim expression in DNTC and serum (A) IL-10 or (B) IL-6 levels. Dashed lines represent the 95% confidence interval. The change in (C) serum IL-10, (D) the percentage of CD3+CD4−CD8− T cells and (E) Bim fold increase in DNTC of four ALPS patients after therapy. Dashed horizontal line represents the expression in healthy controls.
IL-10 mildly increases Bim level in normal and ALPS T cells
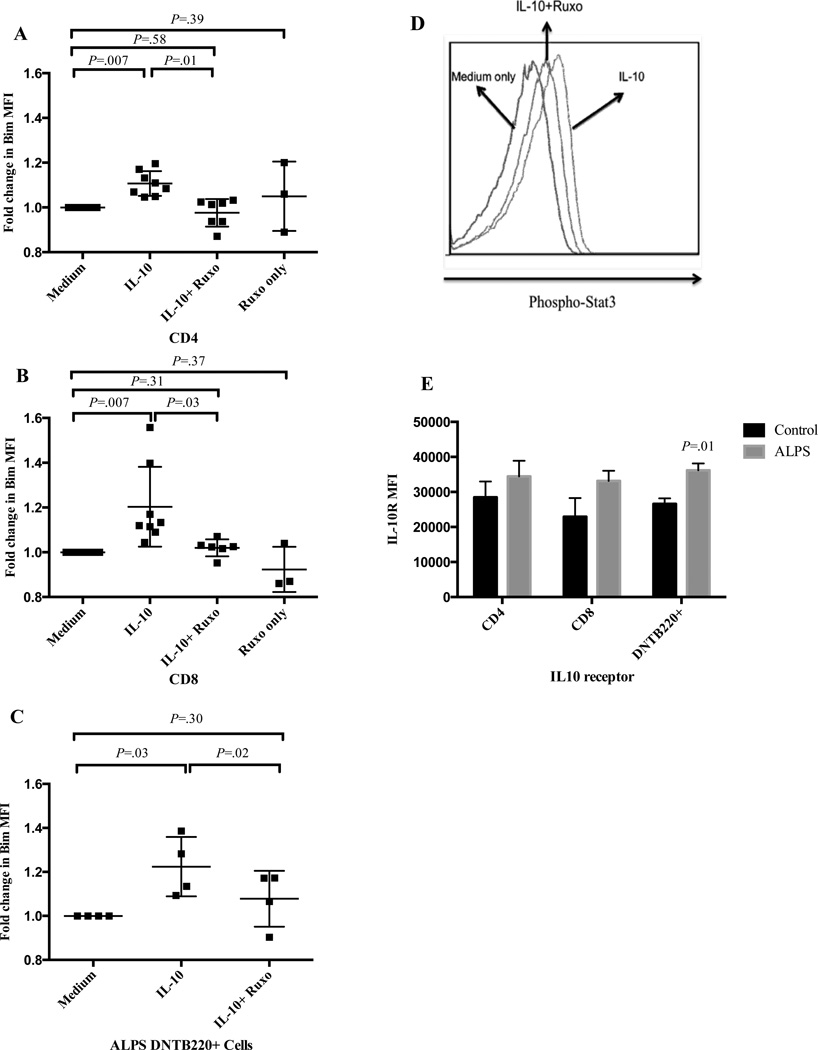
These observations raised the possibility that IL-10 might promote T cell Bim expression. To evaluate this possibility, we separately cultured PBMCs from normal controls and ALPS patients with or without IL-10 and measured Bim levels 24 hours later. IL-10 increased Bim levels in CD4+ and CD8+ T cells from normal controls (Figure 4A, B), as well as in DNTC from ALPS patients (Figure 4C). Because IL-10 signals via a Jak1-Stat3 pathway38, we next used pharmacologic inhibition to block signaling through Jak1 (Ruxolitinib)39. As expected, IL-10 drove phosphorylation of STAT3, which was largely inhibited by ruxolitinib (Figure 4D). Further, ruxolitinib significantly reduced IL-10-driven Bim expression in both normal and ALPS patients’ T cells, but ruxolitinib did not significantly affect Bim levels in the absence of IL10 (Figure 4A–C). Similar effects were seen with other Jak and Stat3 inhibitors (data not shown). It has also been shown that up-regulation of the IL-10 receptor (IL-10R) is essential for its function in neutrophils40 and that IL-10R down regulation can render T cells insensitive to IL-1041, 42.
Figure 4. Exogenous IL-10 partially up-regulates Bim in normal T cells and ALPS DNTC in a Jak-dependent manner.
Fold change in Bim expression in (A) CD4+, (B) CD8+, and (C) DNTC after culture with IL-10 +/− ruxolitinib. (D) pSTAT3 staining in T cells cultured in media or IL-10 +/− ruxolitinib. (E) IL-10 receptor MFI (± SEM) in CD3+CD4+, CD3+CD8+ and CD3+CD4−CD8−B220+ in ALPS patients and normal controls. Representative of three independent experiments with similar results.
This suggested that enhanced Bim upregulation in vivo might be partly related to upregulation of IL-10R in ALPS DNTC. To examine this, we assessed the expression of IL-10R in different T cell subsets in 4 ALPS patients. DNTC from ALPS patients expressed slightly, albeit significantly, more IL-10R than normal controls (Figure 4E) Together, these data show that IL-10 can promote Bim expression in a Jak1/STAT3-dependent manner and may have this effect in ALPS.
DNTC in ALPS are preferentially sensitive to the BH3 mimetic agent ABT-737
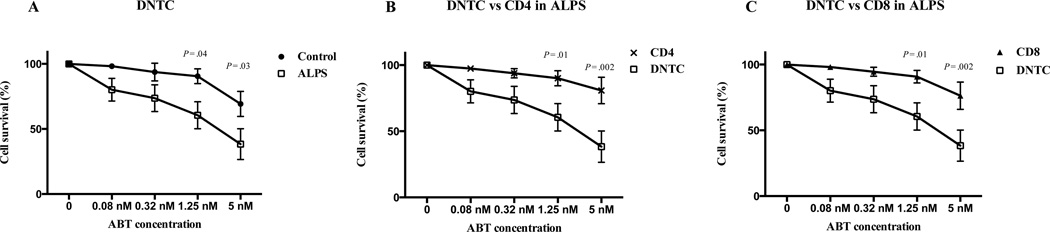
The increased expression of Bim within DNTC should increase their sensitivity to drugs such as ABT-737, which specifically targets the endogenous Bim antagonists Bcl-2 and Bcl-xL, and, by doing so, should promote DNTC death. To test this, we cultured PBMC’s from six different ALPS patients with increasing concentrations of ABT-737. DNTC from ALPS patients showed increased sensitivity to killing by ABT-737 compared to cells from normal controls (Figure 5A). Interestingly, CD4 and CD8 T cells from ALPS patients were relatively spared at concentrations that significantly killed DNTC (Figure 5B and C). This effect of ABT-737 on DNTC is consistent with the higher expression of Bim in DNTC compared to CD4 and CD8 T cells (as noted in Figure 1, Bim expression was significantly higher in DNTC than either CD4+ or CD8+ T cells from ALPS patients). These results suggest that the Bcl-2 pathway may be a potential therapeutic target in ALPS that can facilitate the selective targeting of DNTC population in ALPS.
Figure 5. DNTC in ALPS are more sensitive to killing by ABT-737.
(A) The percentage of DNTC survival after culture with ABT-737 in ALPs patients and controls. (B) The percentage of DNTC and CD4 T cell survival after culture with ABT-737 in ALPS patients. (C) The percentage of DNTC and CD8 T cell survival after culture with ABT-737 in ALPS patients. Data are representative of 3 independent experiments in 6 ALPS patients. Points represent mean ± SEM
Discussion
T cell homeostasis is ultimately controlled by two distinct apoptotic pathways, the death-receptor or extrinsic pathway, and the Bcl-2-regulated or intrinsic pathway. While it is well-known that defects in either pathway are sufficient to disrupt T cell homeostasis, recent work has suggested that there are partially redundant roles of both pathways. Indeed, repeated stimulation of ALPS patients’ T cells increased expression of Bim, suggesting potential involvement of Bim in Fas-driven death signaling31. Further, although ALPS is generally due to defects in Fas signaling, one report showed that a patient with an ALPS-like disorder had a mutation in N-RAS which resulted in decreased expression of Bim10. Moreover, combined loss of both apoptotic pathways in mice leads to more severe accumulation of T cells43–45; however, the degree to which the Bcl-2 pathway is disturbed in humans with death receptor defects has not previously been investigated. Here, we found that Bim is significantly increased in DNTC and may make anti-apoptotic molecules in these cells a therapeutic target.
Given previous data showing the importance of the anti-apoptotic molecule Bcl-2 in antagonizing Bim35, 36, it is intriguing that we failed to observe consistently increased expression of Bcl-2 in DNTC in all of our ALPS patients. Instead, in this cohort, we did not find a unique anti-apoptotic molecule that antagonizes Bim, but rather that individuals had distinct elevations in one or more anti-apoptotic molecules. Because Bcl-xL, Mcl-1, and A1 can all antagonize Bim46–48, it is possible that increases in any of these molecules may allow DNTC in ALPS patients to tolerate increased Bim expression. Interestingly, the two ALPS patients without germline FAS mutations had elevations in Bcl-xL and A1 – two molecules that are controlled by co-stimulatory molecule-driven NF-kB signaling49–51– while the majority of patients with germline mutations in Fas had more impressive increases in Bcl-2 or Mcl-1 – molecules that are largely controlled by cytokine signaling52, 53. However, more somatic ALPS patients will need to be studied to determine whether this is the case.
Our data also show a significant correlation between Bim level in DNTC and serum IL-10, but not IL-6. Further, immunosuppressive treatment normalized DNTC, IL-10 levels and Bim expression. While the treatment data are correlative, our culture data show that IL-10 is sufficient to increase Bim expression in DNTC and that blockade of Jak/STAT3 signaling prevents IL-10-driven increased Bim expression. In the course of this work, another study was published showing an IL-21/STAT3 pathway leading to increased expression of Bim in mouse dendritic cells54. Our data are consistent with this and suggest that IL-10/Jak1/STAT3 signaling also contributes to increased Bim expression in DNTC in ALPS patients. In addition, dysregulation of Jak/STAT signaling is reported in lpr mice, and is thought to play role in mediating kidney damage55, 56. Jak inhibitors and the tyrosine kinase inhibitor imatinib were successful in ameliorating renal disease in lpr mice56, 57. This suggests that dysregulated Jak/STAT signaling can serve as a potential therapeutic target in ALPS in future studies.
The ability of IL-10 to increase Bim expression is also consistent with previous data describing pro-apoptotic effects of IL-10. Overexpression of IL-10 in cardiac allograft mouse models showed significantly increased apoptosis of alloreactive CD4+ and CD8+ T cells58–60, with an accompanying increased expression of another pro-apoptotic Bcl-2 family member, Bax59. In addition, IL-10 enhances lymphocyte apoptosis from SLE patients and other patients with activated lymphocytes but has no effect on apoptosis of normal lymphocytes in vitro61. Moreover, IL-10 enhances the sensitivity of immunoregulatory double negative T cells to activation-induced cell death62, 63. However, consistent with previous studies, we found that while DNTC have an increased rate of spontaneous apoptosis in vitro, IL-10 does not appear to alter this, despite mildly increasing Bim expression22, 64.
While Bim levels are clearly increased in DNTC in ALPS patients and IL-10 significantly increases Bim levels in DNTC in vitro, the magnitude of increased Bim expression was much greater in the former. We envision several potential explanations for this discrepancy. First, our in vitro model does not mimic prolonged exposure to the high circulating levels of IL-10 that are observed in vivo. Second, the effect of IL-10 in vivo may involve the effects of IL-10 on other cells that synergizes with the effects of IL-10 on DNTC. Third, IL-10 may be partially responsible for Bim induction and additional, IL-10-independent factors are needed to fully induce Bim. For example, cytokine withdrawal can lead to increased expression of Bim via AKT/FOXO signaling65, and while IL-10 is in abundance, other homeostatic cytokines such as IL-7 and/or IL-15 may be limiting due to lymphadenopathy and expansion of DNTC. Such limited cytokine availability may enhance expression of Bim in DNTC. Alternatively, because TCR stimulation has also been shown to increase expression of Bim66, 67, chronic TCR stimulation of DNTC may underlie their increased Bim expression. Thus, while IL-10 likely contributes to increased expression of Bim in T cells, further work is required to determine whether additional mechanism(s) contribute.
Lastly, we explored the dysregulated Bcl-2 pathway as a potential therapeutic target in ALPS. Because we had previously shown that the effect of ABT-737 required Bim35, we reasoned that the increased expression of Bim in ALPS DNTC might render them hypersensitive to ABT-737. Because ABT-737 inhibits Bcl-2, Bcl-xL and Bcl-w, but not Mcl-1 or A-168, we also tracked expression of these molecules in ALPS patients’ DNTC, with the idea that ABT-737 should be more effective in patients whose T cells has elevated levels of Bcl-2, Bcl-xL, and/or Bcl-w. As expected, at higher doses, ABT-737 killed all T cells and acted as a lympholytic agent in all patient samples and in normal controls. Notably, ABT-737 demonstrated selective killing properties in ALPS. Indeed, in ALPS patients the DNTC were more sensitive to killing by ABT-737 compared to normal controls. In addition, we were able to target DNTC at doses that relatively spared their counterparts in normal controls and the other T cell subsets in ALPS patients. Interestingly, we did see a difference in the sensitivity of DNTC in ALPS patients with high Bcl2 and/or Bcl-xL compared to one patient with low expression of both Bcl-2 and Bcl-xL, suggesting that anti-apoptotic expression, in addition to Bim, determines the sensitivity of DNTC to this agent. Thus, while DNTC with high Bcl-2 and/or Bcl-xL can be targeted by ABT-737, cells in patients expressing higher levels of Mcl-1 may be targeted by the Mcl-1 inhibitor, obatoclax.69 Moreover, a new chemical probe has been tested in vitro that has successfully targeted the anti-apoptotic molecule A-1 with high specificity70. The difference in DNTC sensitivity observed in patients with different Bcl-2 and Bcl-xL expression was promising. However this needs to be studied in a larger cohort of patients, to prove the effect of anti-apoptotic molecules expression on killing properties of BH3 mimetics in ALPS.
Thrombocytopenia has been proven to be a dose-limiting toxicity with ABT-737 and its related oral agent, navitoclax, in cancer clinical trials due to their effect on Bcl-xL71. However, ABT-199 has recently been developed that retains much of the anti-tumor efficacy of ABT-737, but lacks the substantial thrombocytopenic effect72, likely due to its Bcl-2 specificity. Further, the continued discovery of new compounds that target various anti-apoptotic Bcl-2 members with high specificity70, 73 and potentially novel delivery methods to enhance target cell specificity, such off target effects of anti-Bcl-2 family member drugs may be substantially reduced. In support of our findings, another group presented data at a recent meeting of the American Society for Hematology meeting that ABT-737 caused significant reduction in lymphoproliferation in lpr mice and that DNTC were more sensitive to killing by ABT-737 (Dowdell et al. abstract 695. ASH 2011). Further, ABT-737 was also effective in treating animal models of arthritis and lupus.74, suggesting potential roles in restraining autoreactive T cells. Together, these data suggest a promising role for Bcl-2 family inhibitors in treating non-malignant disorders of immune dysregulation, although more pre-clinical studies are necessary.
In summary, we show that the Bcl-2 pathway is dysregulated in ALPS, a human disorder of the extrinsic apoptotic pathway. Bim is significantly elevated in T cells of ALPS patients, at least partially through an IL-10/Jak/Stat3 mechanism. We also show that ABT-737, a pro-apoptotic BH3-mimetic that targets the Bcl-2 pathway, can have a promising role in treating ALPS by targeting the pathologic lymphoproliferative DNTC, rather than global lympholysis. Finally, our work provides new insight into the overlap between Fas and Bcl-2 pathways in a human disorder of T cell homeostasis.
Key messages.
We identify an unappreciated dysregulation of the Bcl-2 pathway in ALPS.
IL-10/Jak /SAT3 signaling contributes to increased levels of Bim in DNTC.
Targeted BH3 agonists may provide patient-specific therapeutic benefit.
Acknowledgments
We thank Lin Fei, PhD for reviewing statistical methods, the diagnostic immunology lab at Cincinnati Children’s Hospital for providing control samples, Abbott Laboratories for their gift of ABT-737, and the Hildeman lab members for the helpful suggestions and comments. We also thank Drs. Michael Jordan, Fred Finkelman and Claire Chougnet for helpful comments. All flow cytometric data were acquired using equipment maintained by the Research Flow Cytometry Core in the Division of Rheumatology at Cincinnati Children’s Hospital Medical Center, supported in part by NIH grants AR47363, DK78392 and DK90971.
Funding
This project was supported by a grant from primary immune deficiency transplant consortium (PIDTC) and by United States Public Health Service Grants AI057753 and DK081175 (to D.A.H.).
Abbreviations
- 7-AAD
7- Aminoactinomycin D
- ALPS
Autoimmune lymphoproliferative syndrome
- CDR
Complementarity determining region
- DMSO
Dimethyl sulfoxide
- DNTC
Double negative T cells
- IL10R
IL-10 receptor
- Jak
Janus kinase
- MESF
Molecules of equivalent soluble fluorochrome
- MFI
Mean fluorescence intensity
- NF-kB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- PBMC
Peripheral blood mononuclear cell
- SLE
Systemic lupus erythematosus
- STAT
Signal transducer and activator of transcription
- TCR
T cell receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no competing financial interest.
References
- 1.Fleisher TA, Oliveira JB. Monogenic defects in lymphocyte apoptosis. Curr Opin Allergy Clin Immunol. 2012;12:609–615. doi: 10.1097/ACI.0b013e3283588da0. [DOI] [PubMed] [Google Scholar]
- 2.Kurtulus S, Tripathi P, Hildeman DA. Protecting and rescuing the effectors: roles of differentiation and survival in the control of memory T cell development. Front Immunol. 2012;3:404. doi: 10.3389/fimmu.2012.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher GH, Rosenberg FJ, Straus SE, Dale JK, Middleton LA, Lin AY, et al. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995;81:935–946. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 4.Price S, Shaw PA, Seitz A, Joshi G, Davis J, Niemela JE, et al. Natural history of autoimmune lymphoproliferative syndrome associated with FAS gene mutations. Blood. 2014;123:1989–1999. doi: 10.1182/blood-2013-10-535393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson CE, Fischer RE, Hsu AP, Anderson SM, Choi Y, Wang J, et al. Autoimmune lymphoproliferative syndrome with defective Fas: genotype influences penetrance. Am J Hum Genet. 1999;64:1002–1014. doi: 10.1086/302333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Del-Rey M, Ruiz-Contreras J, Bosque A, Calleja S, Gomez-Rial J, Roldan E, et al. A homozygous Fas ligand gene mutation in a patient causes a new type of autoimmune lymphoproliferative syndrome. Blood. 2006;108:1306–1312. doi: 10.1182/blood-2006-04-015776. [DOI] [PubMed] [Google Scholar]
- 7.Dowdell KC, Niemela JE, Price S, Davis J, Hornung RL, Oliveira JB, et al. Somatic FAS mutations are common in patients with genetically undefined autoimmune lymphoproliferative syndrome. Blood. 2010;115:5164–5169. doi: 10.1182/blood-2010-01-263145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holzelova E, Vonarbourg C, Stolzenberg MC, Arkwright PD, Selz F, Prieur AM, et al. Autoimmune lymphoproliferative syndrome with somatic Fas mutations. N Engl J Med. 2004;351:1409–1418. doi: 10.1056/NEJMoa040036. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Zheng L, Lobito A, Chan FK, Dale J, Sneller M, et al. Inherited human Caspase 10 mutations underlie defective lymphocyte and dendritic cell apoptosis in autoimmune lymphoproliferative syndrome type II. Cell. 1999;98:47–58. doi: 10.1016/S0092-8674(00)80605-4. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira JB, Bidere N, Niemela JE, Zheng L, Sakai K, Nix CP, et al. NRAS mutation causes a human autoimmune lymphoproliferative syndrome. Proc Natl Acad Sci U S A. 2007;104:8953–8958. doi: 10.1073/pnas.0702975104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takagi M, Shinoda K, Piao J, Mitsuiki N, Takagi M, Matsuda K, et al. Autoimmune lymphoproliferative syndrome-like disease with somatic KRAS mutation. Blood. 2011;117:2887–2890. doi: 10.1182/blood-2010-08-301515. [DOI] [PubMed] [Google Scholar]
- 12.Bidere N, Su HC, Lenardo MJ. Genetic disorders of programmed cell death in the immune system. Annu Rev Immunol. 2006;24:321–352. doi: 10.1146/annurev.immunol.24.021605.090513. [DOI] [PubMed] [Google Scholar]
- 13.Salmena L, Hakem R. Caspase-8 deficiency in T cells leads to a lethal lymphoinfiltrative immune disorder. J Exp Med. 2005;202:727–732. doi: 10.1084/jem.20050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oliveira JB, Bleesing JJ, Dianzani U, Fleisher TA, Jaffe ES, Lenardo MJ, et al. Revised diagnostic criteria and classification for the autoimmune lymphoproliferative syndrome (ALPS): report from the 2009 NIH International Workshop. Blood. 2010;116:e35–e40. doi: 10.1182/blood-2010-04-280347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sneller MC, Straus SE, Jaffe ES, Jaffe JS, Fleisher TA, Stetler-Stevenson M, et al. A novel lymphoproliferative/autoimmune syndrome resembling murine lpr/gld disease. J Clin Invest. 1992;90:334–341. doi: 10.1172/JCI115867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sneller MC, Wang J, Dale JK, Strober W, Middelton LA, Choi Y, et al. Clincal, immunologic, and genetic features of an autoimmune lymphoproliferative syndrome associated with abnormal lymphocyte apoptosis. Blood. 1997;89:1341–1348. [PubMed] [Google Scholar]
- 17.Bleesing JJ, Brown MR, Dale JK, Straus SE, Lenardo MJ, Puck JM, et al. TcR-alpha/beta(+) CD4(−)CD8(−) T cells in humans with the autoimmune lymphoproliferative syndrome express a novel CD45 isoform that is analogous to murine B220 and represents a marker of altered O-glycan biosynthesis. Clin Immunol. 2001;100:314–324. doi: 10.1006/clim.2001.5069. [DOI] [PubMed] [Google Scholar]
- 18.Bleesing JJ, Brown MR, Novicio C, Guarraia D, Dale JK, Straus SE, et al. A composite picture of TcR alpha/beta(+) CD4(−)CD8(−) T Cells (alpha/beta-DNTCs) in humans with autoimmune lymphoproliferative syndrome. Clin Immunol. 2002;104:21–30. doi: 10.1006/clim.2002.5225. [DOI] [PubMed] [Google Scholar]
- 19.Kyttaris VC, Kampagianni O, Tsokos GC. Treatment with anti-interleukin 23 antibody ameliorates disease in lupus-prone mice. Biomed Res Int. 2013;2013:861028. doi: 10.1155/2013/861028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohga S, Nomura A, Takahata Y, Ihara K, Takada H, Wakiguchi H, et al. Dominant expression of interleukin 10 but not interferon gamma in CD4(−)CD8(−)alphabetaT cells of autoimmune lymphoproliferative syndrome. Br J Haematol. 2002;119:535–538. doi: 10.1046/j.1365-2141.2002.03848.x. [DOI] [PubMed] [Google Scholar]
- 21.Andre N, Roquelaure B, Thuret I, Ziol M, Rieux-Laucat F, Le Deist F. Expression of Granzyme B in viral hepatitis in patients with ALPS. Hepatology. 2004;39:864–865. doi: 10.1002/hep.20100. [DOI] [PubMed] [Google Scholar]
- 22.Lopatin U, Yao X, Williams RK, Bleesing JJ, Dale JK, Wong D, et al. Increases in circulating and lymphoid tissue interleukin-10 in autoimmune lymphoproliferative syndrome are associated with disease expression. Blood. 2001;97:3161–3170. doi: 10.1182/blood.v97.10.3161. [DOI] [PubMed] [Google Scholar]
- 23.Magerus-Chatinet A, Stolzenberg MC, Loffredo MS, Neven B, Schaffner C, Ducrot N, et al. FAS-L, IL-10, and double-negative CD4− CD8− TCR alpha/beta+ T cells are reliable markers of autoimmune lymphoproliferative syndrome (ALPS) associated with FAS loss of function. Blood. 2009;113:3027–3030. doi: 10.1182/blood-2008-09-179630. [DOI] [PubMed] [Google Scholar]
- 24.Mixter PF, Russell JQ, Durie FH, Budd RC. Decreased CD4−CD8− TCR-alpha beta + cells in lpr/lpr mice lacking beta 2-microglobulin. J Immunol. 1995;154:2063–2074. [PubMed] [Google Scholar]
- 25.Maldonado MA, Eisenberg RA, Roper E, Cohen PL, Kotzin BL. Greatly reduced lymphoproliferation in lpr mice lacking major histocompatibility complex class I. J Exp Med. 1995;181:641–648. doi: 10.1084/jem.181.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohteki T, Iwamoto M, Izui S, MacDonald HR. Reduced development of CD4−8−B220+ T cells but normal autoantibody production in lpr/lpr mice lacking major histocompatibility complex class I molecules. Eur J Immunol. 1995;25:37–41. doi: 10.1002/eji.1830250108. [DOI] [PubMed] [Google Scholar]
- 27.Bristeau-Leprince A, Mateo V, Lim A, Magerus-Chatinet A, Solary E, Fischer A, et al. Human TCR alpha/beta+ CD4−CD8− double-negative T cells in patients with autoimmune lymphoproliferative syndrome express restricted Vbeta TCR diversity and are clonally related to CD8+ T cells. J Immunol. 2008;181:440–448. doi: 10.4049/jimmunol.181.1.440. [DOI] [PubMed] [Google Scholar]
- 28.Alderson MR, Tough TW, Davis-Smith T, Braddy S, Falk B, Schooley KA, et al. Fas ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med. 1995;181:71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 30.Fortner KA, Bouillet P, Strasser A, Budd RC. Apoptosis regulators Fas and Bim synergistically control T-lymphocyte homeostatic proliferation. Eur J Immunol. 2010 doi: 10.1002/eji.201040577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snow AL, Oliveira JB, Zheng L, Dale JK, Fleisher TA, Lenardo MJ. Critical role for BIM in T cell receptor restimulation-induced death. Biol Direct. 2008;3:34. doi: 10.1186/1745-6150-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carrington EM, Vikstrom IB, Light A, Sutherland RM, Londrigan SL, Mason KD, et al. BH3 mimetics antagonizing restricted prosurvival Bcl-2 proteins represent another class of selective immune modulatory drugs. Proc Natl Acad Sci U S A. 2010;107:10967–10971. doi: 10.1073/pnas.1005256107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res. 2009;15:1126–1132. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 35.Wojciechowski S, Tripathi P, Bourdeau T, Acero L, Grimes HL, Katz JD, et al. Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis. J Exp Med. 2007;204:1665–1675. doi: 10.1084/jem.20070618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurtulus S, Tripathi P, Moreno-Fernandez ME, Sholl A, Katz JD, Grimes HL, et al. Bcl-2 allows effector and memory CD8+ T cells to tolerate higher expression of Bim. J Immunol. 2011;186:5729–5737. doi: 10.4049/jimmunol.1100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caminha I, Fleisher TA, Hornung RL, Dale JK, Niemela JE, Price S, et al. Using biomarkers to predict the presence of FAS mutations in patients with features of the autoimmune lymphoproliferative syndrome. J Allergy Clin Immunol. 2010;125:946–949. e6. doi: 10.1016/j.jaci.2009.12.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weber-Nordt RM, Riley JK, Greenlund AC, Moore KW, Darnell JE, Schreiber RD. Stat3 recruitment by two distinct ligand-induced, tyrosine-phosphorylated docking sites in the interleukin-10 receptor intracellular domain. J Biol Chem. 1996;271:27954–27961. doi: 10.1074/jbc.271.44.27954. [DOI] [PubMed] [Google Scholar]
- 39.Deisseroth A, Kaminskas E, Grillo J, Chen W, Saber H, Lu HL, et al. U.S. Food and Drug Administration approval: ruxolitinib for the treatment of patients with intermediate and high-risk myelofibrosis. Clin Cancer Res. 2012;18:3212–3217. doi: 10.1158/1078-0432.CCR-12-0653. [DOI] [PubMed] [Google Scholar]
- 40.Crepaldi L, Gasperini S, Lapinet JA, Calzetti F, Pinardi C, Liu Y, et al. Up-regulation of IL-10R1 expression is required to render human neutrophils fully responsive to IL-10. J Immunol. 2001;167:2312–2322. doi: 10.4049/jimmunol.167.4.2312. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Wei SH, Ho AS, de Waal Malefyt R, Moore KW. Expression cloning and characterization of a human IL-10 receptor. J Immunol. 1994;152:1821–1829. [PubMed] [Google Scholar]
- 42.Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy--review of a new approach. Pharmacol Rev. 2003;55:241–269. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- 43.Hughes PD, Belz GT, Fortner KA, Budd RC, Strasser A, Bouillet P. Apoptosis regulators Fas and Bim cooperate in shutdown of chronic immune responses and prevention of autoimmunity. Immunity. 2008;28:197–205. doi: 10.1016/j.immuni.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hutcheson J, Scatizzi JC, Siddiqui AM, Haines GK, 3rd, Wu T, Li QZ, et al. Combined deficiency of proapoptotic regulators Bim and Fas results in the early onset of systemic autoimmunity. Immunity. 2008;28:206–217. doi: 10.1016/j.immuni.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 45.Weant AE, Michalek RD, Khan IU, Holbrook BC, Willingham MC, Grayson JM. Apoptosis regulators Bim and Fas function concurrently to control autoimmunity and CD8+ T cell contraction. Immunity. 2008;28:218–230. doi: 10.1016/j.immuni.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 46.Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 47.Zhu Y, Swanson BJ, Wang M, Hildeman DA, Schaefer BC, Liu X, et al. Constitutive association of the proapoptotic protein Bim with Bcl-2-related proteins on mitochondria in T cells. Proc Natl Acad Sci U S A. 2004;101:7681–7686. doi: 10.1073/pnas.0402293101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herman MD, Nyman T, Welin M, Lehtio L, Flodin S, Tresaugues L, et al. Completing the family portrait of the anti-apoptotic Bcl-2 proteins: crystal structure of human Bfl-1 in complex with Bim. FEBS Lett. 2008;582:3590–3594. doi: 10.1016/j.febslet.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 49.Khoshnan A, Tindell C, Laux I, Bae D, Bennett B, Nel AE. The NF-kappa B cascade is important in Bcl-xL expression and for the anti-apoptotic effects of the CD28 receptor in primary human CD4+ lymphocytes. J Immunol. 2000;165:1743–1754. doi: 10.4049/jimmunol.165.4.1743. [DOI] [PubMed] [Google Scholar]
- 50.Chen C, Edelstein LC, Gelinas C. The Rel/NF-kappaB family directly activates expression of the apoptosis inhibitor Bcl-x(L) Mol Cell Biol. 2000;20:2687–2695. doi: 10.1128/mcb.20.8.2687-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zong WX, Edelstein LC, Chen C, Bash J, Gelinas C. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-kappaB that blocks TNFalpha-induced apoptosis. Genes Dev. 1999;13:382–387. doi: 10.1101/gad.13.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitchell T, Kappler J, Marrack P. Bystander virus infection prolongs activated T cell survival. J Immunol. 1999;162:4527–4535. [PubMed] [Google Scholar]
- 53.Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 54.Wan CK, Oh J, Li P, West EE, Wong EA, Andraski AB, et al. The cytokines IL-21 and GM-CSF have opposing regulatory roles in the apoptosis of conventional dendritic cells. Immunity. 2013;38:514–527. doi: 10.1016/j.immuni.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dong J, Wang QX, Zhou CY, Ma XF, Zhang YC. Activation of the STAT1 signalling pathway in lupus nephritis in MRL/lpr mice. Lupus. 2007;16:101–109. doi: 10.1177/0961203306075383. [DOI] [PubMed] [Google Scholar]
- 56.Wang S, Yang N, Zhang L, Huang B, Tan H, Liang Y, et al. Jak/STAT signaling is involved in the inflammatory infiltration of the kidneys in MRL/lpr mice. Lupus. 2010;19:1171–1180. doi: 10.1177/0961203310367660. [DOI] [PubMed] [Google Scholar]
- 57.Sadanaga A, Nakashima H, Masutani K, Miyake K, Shimizu S, Igawa T, et al. Amelioration of autoimmune nephritis by imatinib in MRL/lpr mice. Arthritis Rheum. 2005;52:3987–3996. doi: 10.1002/art.21424. [DOI] [PubMed] [Google Scholar]
- 58.Furukawa H, Oshima K, Tung T, Cui G, Laks H, Sen L. Overexpressed exogenous IL-4 And IL-10 paradoxically regulate allogenic T-cell and cardiac myocytes apoptosis through FAS/FASL pathway. Transplantation. 2008;85:437–446. doi: 10.1097/TP.0b013e31816026e7. [DOI] [PubMed] [Google Scholar]
- 59.Tung TC, Oshima K, Cui G, Laks H, Sen L. Dual upregulation of Fas and Bax promotes alloreactive T cell apoptosis in IL-10 gene targeting of cardiac allografts. Am J Physiol Heart Circ Physiol. 2003;285:H964–H973. doi: 10.1152/ajpheart.00976.2002. [DOI] [PubMed] [Google Scholar]
- 60.Oshima K, Sen L, Cui G, Tung T, Sacks BM, Arellano-Kruse A, et al. Localized interleukin-10 gene transfer induces apoptosis of alloreactive T cells via FAS/FASL pathway, improves function, and prolongs survival of cardiac allograft. Transplantation. 2002;73:1019–1026. doi: 10.1097/00007890-200204150-00002. [DOI] [PubMed] [Google Scholar]
- 61.Georgescu L, Vakkalanka RK, Elkon KB, Crow MK. Interleukin-10 promotes activation-induced cell death of SLE lymphocytes mediated by Fas ligand. J Clin Invest. 1997;100:2622–2633. doi: 10.1172/JCI119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marra LE, Zhang ZX, Joe B, Campbell J, Levy GA, Penninger J, et al. IL-10 induces regulatory T cell apoptosis by up-regulation of the membrane form of TNF-alpha. J Immunol. 2004;172:1028–1035. doi: 10.4049/jimmunol.172.2.1028. [DOI] [PubMed] [Google Scholar]
- 63.Hillhouse EE, Beauchamp C, Chabot-Roy G, Dugas V, Lesage S. Interleukin-10 limits the expansion of immunoregulatory CD4−CD8− T cells in autoimmune-prone non-obese diabetic mice. Immunol Cell Biol. 2010;88:771–780. doi: 10.1038/icb.2010.84. [DOI] [PubMed] [Google Scholar]
- 64.Haas JP, Grunke M, Frank C, Kolowos W, Dirnecker D, Leipold G, et al. Increased spontaneous in vitro apoptosis in double negative T cells of humans with a fas/apo-1 mutation. Cell Death Differ. 1998;5:751–757. doi: 10.1038/sj.cdd.4400426. [DOI] [PubMed] [Google Scholar]
- 65.Stahl M, Dijkers PF, Kops GJ, Lens SM, Coffer PJ, Burgering BM, et al. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J Immunol. 2002;168:5024–5031. doi: 10.4049/jimmunol.168.10.5024. [DOI] [PubMed] [Google Scholar]
- 66.Sandalova E, Wei CH, Masucci MG, Levitsky V. Regulation of expression of Bcl-2 protein family member Bim by T cell receptor triggering. Proc Natl Acad Sci U S A. 2004;101:3011–3016. doi: 10.1073/pnas.0400005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O'Reilly LA, Kruse EA, Puthalakath H, Kelly PN, Kaufmann T, Huang DC, et al. MEK/ERK-mediated phosphorylation of Bim is required to ensure survival of T and B lymphocytes during mitogenic stimulation. J Immunol. 2009;183:261–269. doi: 10.4049/jimmunol.0803853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Billard C. BH3 Mimetics: Status of the Field and New Developments. Mol Cancer Ther. 2013 doi: 10.1158/1535-7163.MCT-13-0058. [DOI] [PubMed] [Google Scholar]
- 69.Nguyen M, Marcellus RC, Roulston A, Watson M, Serfass L, Murthy Madiraju SR, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A. 2007;104:19512–19517. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bittker JA, Weiwer M, Wei G, Germain A, Brown E, Dandapani S, et al. Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD): 2010. Discovery of Inhibitors of Anti-Apoptotic Protein A1. [Google Scholar]
- 71.Schoenwaelder SM, Jarman KE, Gardiner EE, Hua M, Qiao J, White MJ, et al. Bcl-xL-inhibitory BH3 mimetics can induce a transient thrombocytopathy that undermines the hemostatic function of platelets. Blood. 2011;118:1663–1674. doi: 10.1182/blood-2011-04-347849. [DOI] [PubMed] [Google Scholar]
- 72.Vandenberg CJ, Cory S. ABT-199, a new Bcl-2-specific BH3 mimetic, has in vivo efficacy against aggressive Myc-driven mouse lymphomas without provoking thrombocytopenia. Blood. 2013;121:2285–2288. doi: 10.1182/blood-2013-01-475855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bajwa N, Liao C, Nikolovska-Coleska Z. Inhibitors of the anti-apoptotic Bcl-2 proteins: a patent review. Expert Opin Ther Pat. 2012;22:37–55. doi: 10.1517/13543776.2012.644274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bardwell PD, Gu J, McCarthy D, Wallace C, Bryant S, Goess C, et al. The Bcl-2 family antagonist ABT-737 significantly inhibits multiple animal models of autoimmunity. J Immunol. 2009;182:7482–7489. doi: 10.4049/jimmunol.0802813. [DOI] [PubMed] [Google Scholar]