Abstract
Melatonin and its metabolites including 6-hydroxymelatonin (6(OH)M), N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and 5-methoxytryptamine (5MT) are endogenously produced in human epidermis. This production depends on race, gender and age. The highest melatonin levels are in African-Americans. In each racial group they are highest in young African-Americans [30–50 years old (yo)], old Caucasians (60–90 yo) and Caucasian females. AFMK levels are the highest in African-Americans, while 6(OH)M and 5MT levels are similar in all groups. Testing of their phenotypic effects in normal human melanocytes show that melatonin and its metabolites (10−5 M) inhibit tyrosinase activity and cell growth, and inhibit DNA synthesis in a dose dependent manner with 10−9 M being the lowest effective concentration. In melanoma cells, they inhibited cell growth but had no effect on melanogenesis, except for 5MT which enhanced L-tyrosine induced melanogenesis. In conclusion, melatonin and itsmetabolites [6(OH)M, AFMK and 5MT] are produced endogenously in human epidermis and can affect melanocyte and melanoma behavior.
Keywords: Epidermis, Melatonin, 6-hydroxymelatonin, AFMK, 5-methoxytryptamine, Melanocytes
1. Introduction
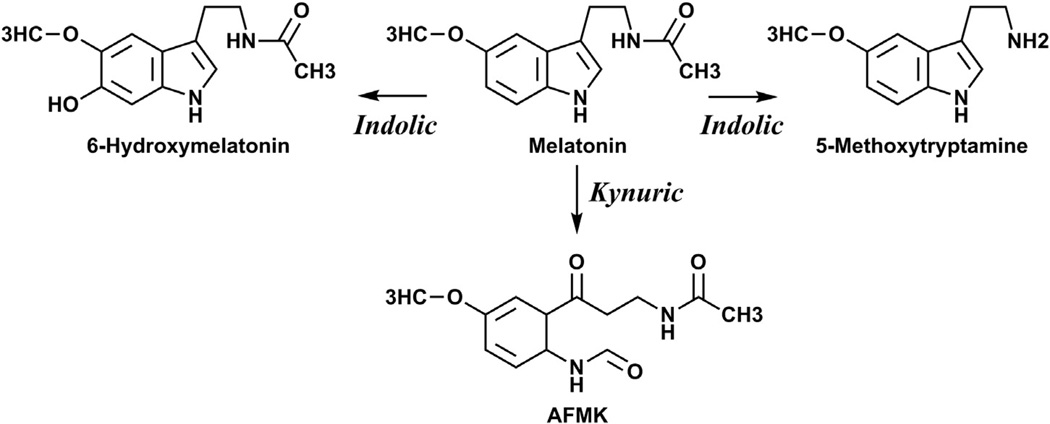
Melatonin is a hormone and a bioregulator with structure of methoxyindole, which is present in almost all biological systems such as animals, plants and microbes (Fischer et al., 2008a; Hardeland et al., 2011; Lanoix et al., 2012a; Lerner et al., 1960; Reiter et al., 2007a, 2007b, 2010; Slominski et al., 2008). It is predominantly synthesized in the pineal gland through a multistep process starting from hydroxylation of tryptophan and culminating with transformation of serotonin to N-acetyl serotonin and further methylation to melatonin (Hardeland et al., 2006; Lerner et al., 1960; Reiter, 1991; Reiter et al., 2007a; Roseboom et al., 1998; Yu and Reiter, 1993). Melatonin is also synthesized in the brain, nerves and peripheral organs (Bubenik, 2008; Hardeland et al., 2011; Konturek et al., 2007; Lanoix et al., 2012a, 2012b; Lerner et al., 1959; Reiter et al., 2010; Tan et al., 2007; Zmijewski et al., 2009) including rodent (Kobayashi et al., 2005; Slominski et al., 1996, 2002a) and human skin (Kobayashi et al., 2005; Slominski et al., 2002b, 2005a). In the periphery and on the central levels, melatonin is metabolized through indolic and kynuric pathways (Fig. 1) with production of 6-hydroxymelatonin, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and 5-methoxytryptamine (5MT) as well as other metabolites (Grace et al., 1991; Hardeland et al., 2006; Hirata et al., 1974; Lerner et al., 1960; Ma et al., 2005; Rogawski et al., 1979; Semak et al., 2005, 2008; Slominski et al., 2008; Young et al., 1985).
Fig. 1.
Main pathways of melatonin metabolism in skin cells.
In humans, melatonin is well known for regulating circadian rhythm. It also has many other effects including regulation of immune and endocrine functions, and it shows anti-oxidative and protective properties against the cellular toxins and internal and environmental insults (Bubenik, 2008; Fischer et al., 2008a; Hardeland et al., 2006, 2011; Lanoix et al., 2012a; Luchetti et al., 2010; Reiter, 1991; Reiter et al., 2010; Slominski et al., 2005a; Tan et al., 2007; Yu and Reiter, 1993). These effects are mediated either through binding to membrane bound melatonin receptors (MT1 and MT2), receptor independent mechanisms or through activation of putative nuclear receptors (Dubocovich and Markowska, 2005; Hardeland et al., 2011; Reiter et al., 2010; Slominski et al., 2012a; Tan et al., 2007).
Extensive studies have been focused on melatonin’s role in general regulation of body homeostasis (Hardeland et al., 2011; Lanoix et al., 2012a; Luchetti et al., 2010; Reiter, 1991; Reiter et al., 2010; Tan et al., 2007; Yu and Reiter, 1993). Skin with subcutaneous adipose tissue is the largest organ in the human body playing an important role in the regulation of local and body homeostasis (Slominski and Wortsman, 2000; Slominski et al., 2012b). Locally produced melatonin plays an important role in the regulation of skin functions (Fischer et al., 2008a, 2008b; Kleszczynski et al., 2011; Kobayashi et al., 2005; Slominski et al., 2005a, 2005b, 2008). Although the role of melatonin and of AFMK in the functions of the epidermis has been extensively investigated (Fischer et al., 2006a, 2008c, 2013; Kim et al., 2013; Kleszczynski et al., 2012, 2013; Slominski et al., 1994, 2003), there is a lack of similar information on functions of other melatonin metabolites. The literature on the role played by melatonin and its metabolites in regulation of behavior of human melanocytes is limited.
Previously we have shown that exogenously applied melatonin in cultured immortalized epidermal (HaCaT) keratinocytes and melanoma cells is metabolized through indolic and kynuric pathways with production of 2-hydroxymelatonin, 4-hydroxymelatonin, 6-hydroxymelatonin, AFMK, and 5MT (Fischer et al., 2006b; Kim et al., 2013; Slominski et al., 2002c). Production of AFMK in HaCaT keratinocytes can be stimulated by ultraviolet radiation (UVB) (Fischer et al., 2006b), and AFMK can also be generated from melatonin through pseudoenzymatic or non-enzymatic processes mediated by free radicals or through photocatalysis induced by UVB (Fischer et al., 2006b; Hardeland et al., 2006; Semak et al., 2005). We have also characterized metabolism of melatonin in normal human primary epidermal keratinocytes, melanocytes, dermal fibroblasts and melanoma cells and show that 6-hydroxymelatonin is the main product of metabolism with lower production of AFMK and 5MT (Kim et al., 2013).
Originally melatonin was defined as lightening agent based on its action on amphibian skin (Lerner, 1960; Lerner et al., 1960). In mammalian system, melatonin’s role in fur pigmentation has been well established (Logan and Weatherhead, 1979, 1980) and reviewed (Fischer et al., 2008b; Slominski et al., 2004, 2005c). Also tumorostatic activity of melatonin has been well documented in rodent and human melanomas [reviewed in (Fischer et al., 2006c; Slominski et al., 2005b; Yu and Reiter, 1993)]. However, the role of melatonin in human skin pigmentation is unclear (Slominski et al., 2004) as indicated by lack of effect of orally delivered melatonin on skin melanin pigmentation (McElhinney et al., 1994).
To better understand the role of melatoninergic systemin human epidermis we investigated accumulation of melatonin and its metabolites in the human epidermis from healthy donors of different race, age and sex, and evaluated their effects on proliferation and melanogenesis in human normal epidermal melanocytes in comparison with human melanoma cells.
2. Materials and methods
2.1. Chemicals
Charcoal stripped fetal bovine serum (FBS) was purchased from Atlanta Biologicals, Lawrenceville, GA, USA. Melatonin, 6-hydroxymelatonin and 5-methoxytryptamine (5MT) were purchased from Sigma-Aldrich, St Louis, MO, USA and N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) was purchased from Cayman chemical, Ann Arbor, MI, USA. Acetonitrile, water and acetic acid (Fisher scientific, Pittsburgh, PA) were used for HPLC. For LC-MS system, acetonitrile, water and formic acid (Sigma-Aldrich, St Louis, MO, USA) were used. Trichloroacetic acid (TCA) was purchased from Sigma-Aldrich, St Louis, MO, USA and [3H]-thymidine was purchased from Moravek Biochemicals Inc., Brea, CA, USA.
2.2. Human skins and epidermis preparation
The use of human tissues was approved by UTHSC Institutional Review Boards as an exempt protocol #4. Human skin samples were obtained from the Methodist University Hospital in Memphis, TN. The skin specimens (n = 13) were obtained from both males and females (30–90 years old) of African-American and Caucasian races. The specimens were incubated at 60 °C for 1 h and the epidermis was peeled out and stored at −80 °C for further experiments.
2.3. Extraction of melatonin and its metabolites from human epidermis
The epidermis collected as above was mixed with 3.2 volume (v/w) of PBS and homogenized using Poly Tron PT 2100 (Kinematica, Switzerland). Additional homogenization was performed in 75% acetonitrile. After centrifugation at 4000 rpm, the supernatant was filtered using syringe filter (PES, 0.45 µm, 30 mm; Celltreat, Shirley, MA, USA) and then dried by speedvac drier (Savant Instruments, Inc., Holbrook, NY, USA).
2.4. Detection of melatonin and its metabolites
In order to detect melatonin and its metabolites, the epidermal samples was re-dissolved in methanol. The UPLC (ultra-performance liquid chromatography) separation was performed on a Waters ACQUITY I-Class UPLC system (Waters, Milford, MA, USA) consisting of a binary pump, an autosampler, a column manager, a degasser and a diode-array detector (DAD). An Agilent Zorbax Eclipse Plus C18 column (2.1 × 50 mm, 1.8 µm, Agilent Technologies, Santa Clara, CA, USA) maintained at 35 °C was used with a mobile phase consisting of the following linear gradient of acetonitrile containing 0.1% formic acid: 5–10% for 1 min, 10–15% for 2.5 min, 15–20% for 3.5 min, 20–25% for 0.5 min, 25–70% for 1 min, 70–85% for 1 min, and 85–100% for 0.5 min. The flow rate was 0.3 mL/min and the DAD was operated in the range of 200–400 nm. The UPLC was connected to a Xevo™ G2-S QTof mass spectrometer (Waters), a quadrupole (Q) hybrid with orthogonal acceleration time-of-flight (Tof) tandem mass spectrometer (MS). The scan range was 50–1000 Da in positive mode, and all data were collected in centroid mode. The capillary and cone voltages were 3.0 kV and 30 V, respectively. The desolvation gas was maintained at 1000 L/h at a temperature of 500 °C. The cone gas was 100 L/h with a source temperature of 150 °C. The data acquisition rate was 0.3 s, with a 20 s interval. The lockspray frequency was every 20 s using Leucine Enkephalin solution (100 ng/mL) as the lockspray reference compound (m/z = 556.2771) with a flow rate of 5 µL/min. The MS data were collected with full scan mode with low (6 V) and high (ramp from 20 to 40 V) collision energy (CE) data channels to get both the parent ions (MS) and the daughter ions (MS/MS). All data were acquired and processed by Waters MassLynx v4.1 software. The relative concentrations of products were calculated from MS peak areas in relation to standards curves generated using the corresponding standards at m/z = 233.1 [M+H]+ for melatonin; 287.1 [M+K]+ for 6(OH)M; 287.1 [M+Na]+ for AFMK, and 174.1 [M+H-NH3]+ for 5MT. The values are presented as means ± SE or as individual values.
2.5. DNA synthesis
Primary cultures of normal human epidermal neonatal melanocytes were established as described previously (Slominski et al., 2011). Melanocytes from the passages 2 or 3 were plated (10,000 cells/well) on 24 well plates using MBM-4 with MGM-4 medium (500 µL/well) containing 0.5% charcoal stripped FBS and were grown until reaching 30% of confluence (Kim et al., 2013). To test biological effects media were changed with fresh ones containing melatonin and its metabolites (10−11 to 10−5 M), and the cells were grown for 48 hours. Finally, [3H]-thymidine was added to the media at the concentration of 0.25 µCi/mL and cultures were incubated for additional 4 h. To measure radioactivity incorporated into DNA, media were removed and cells were fixed with 10% TCA in PBS (phosphate-buffered saline) for 30 min followed by two washes with PBS. The fixed cells were lysed by with 1 N NaOH/1% SDS (250 µL/well) and after mixing with Scintiverse cocktail (Fisher scientific, Pittsburgh, PA, USA), radioactivity was counted Packard Matrix 9600 direct beta-counter (Packard, Meridan, CT, USA) (Slominski et al., 2011).
2.6. Melanogenesis and cell growth
SKMEL-188 human melanoma cells and normal human epidermal melanocytes cells were plated on 25 cm2 flask in corresponding media, see below. Melanoma were grown in Ham’s F-10 media containing 5% charcoal stripped FBS plus 400 µM L-tyrosine and in the presence of 10−5 M melatonin or its metabolites as described previously (Slominski et al., 2009). Normal human melanocytes were cultured in MBM-4with MGM-4 (Lonza, Walkersville, MD, USA) containing 10−5 M melatonin or its metabolites. After 6 days the cells were collected, their number counted in hematocytometer and after centrifugation the pellets were washed with PBS and they were lysed in 0.1M sodium phosphate buffer (pH 6.8, 0.5% Triton X-100 and 0.1 mM PMSF) (Slominski et al., 2009). Cell debris was removed by centrifugation at 16,000 g for 10 min, and the supernatants were used for tyrosinase activity assay as described in Slominski et al. (1988). Briefly, 50 µL of supernatant was added to 950 µL of 1 mM L-DOPA in 0.1M phosphate buffer (pH 6.8), and the absorbance was measured at 475 nm. One unit of enzyme activity was presented as an increase of absorbance/mg protein/hour taken from the linear plot of consecutive measurements.
2.7. Statistics
Student t-test, Mann–Whitney test and one-way ANOVA test were performed as indicated using Prism 4.00 (GraphPad Software, San Diego, CA, USA) presented as means ± SE (n ≥ 3). Statistically significant differences are considered when *, p < 0.05; **, p < 0.01; ***, p < 0.001 (Student t-test) and #, p < 0.05; ##, p < 0.01; ###, p < 0.001 (Mann–Whitney test or one-way ANOVA test).
3. Results and discussion
3.1. Endogenous production of melatonin and its metabolites in the epidermis
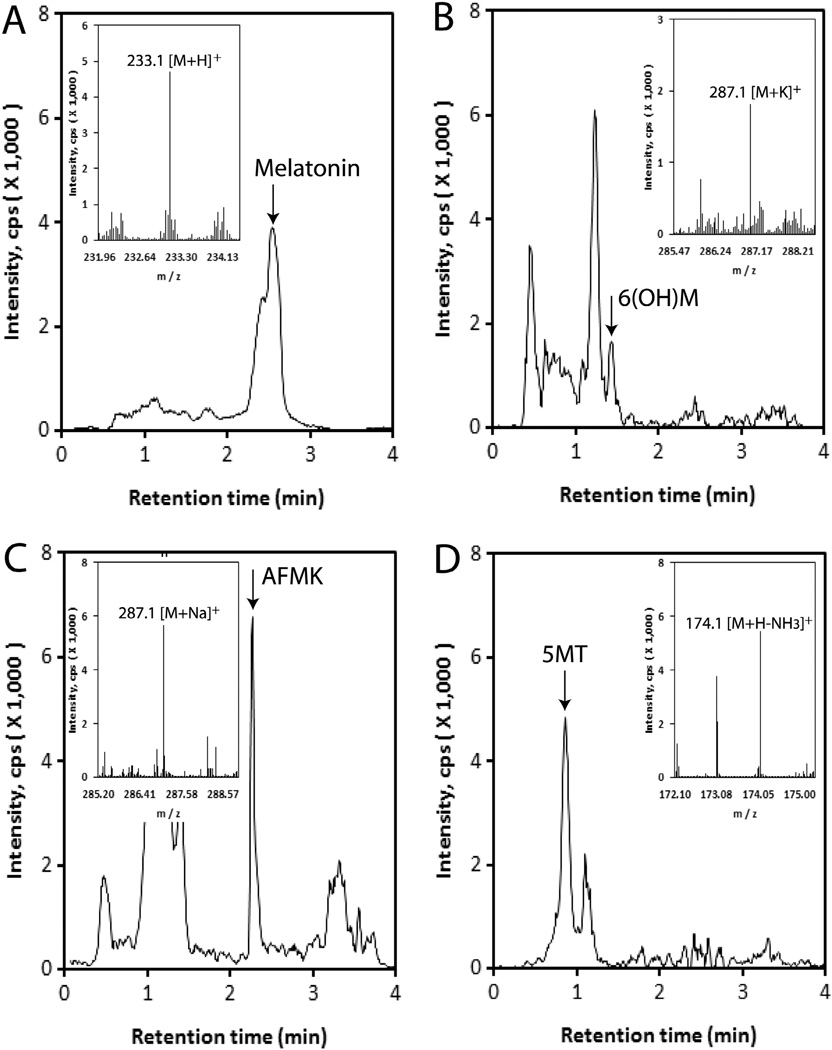
In order to detect melatonin and its metabolites, the extracted samples were re-dissolved in methanol and applied to Waters Acquity™ UPLC system equipped with ESI source using Xevo™ G2 qTOF. As shown in Fig. 2, melatonin, 6(OH)M, AFMK and 5MT were detected, respectively, at m/z = 233.1 [M+H]+, 287.1 [M+K]+, 287.1 [M+Na]+, and 174.1 [M+H-NH3]+ in extracted-ion chromatogram (EIC).
Fig. 2.
Chromatograms of endogenous production of melatonin and its metabolites. Epidermis was extracted with 75% acetonitrile after homogenizing and applied to qTOF LC-MS (EIC) at m/z = 233.1 [M+H]+ for melatonin; 287.1 [M+K]+ for 6(OH)M; 287.1 [M+Na]+ for AFMK; 174.1 [M+H-NH3]+ for 5MT. (A) melatonin; (B) 6-hydroxymelatonin; (C) AFMK; (D) 5-methoxytryptamine.
In previous studies we demonstrated expression of melatoninergic system in the human skin and skin cells with detection of melatonin by mass spectrometry in immortalized epidermal HaCaT keratinocytes and melanoma cells (Slominski et al., 2002b, 2002c, 2005a). We have also demonstrated that skin cells cultured in vitro transform melatonin to 6(OH)M, AFMK and 5MT (Fischer et al., 2006b; Kim et al., 2013). This study represents a milestone in characterization of cutaneous melatoninergic system by actual detection for the first time of melatonin and its metabolites 6(OH)M, AFMK and 5MT in the human epidermis in vivo.
We have then quantified production of these compounds in the epidermis using skin samples obtained from 13 subjects including 6 African-Americans and 7 Caucasians (Table 1). 6(OH)M was the most abundant with concentration significantly higher than melatonin (p < 0.0001) in all subjects (Table 1), and when the population was stratified according to race, e.g., Caucasians (p < 0.0001) and African-Americans (p < 0.01). AFMK was the least abundant species with concentration similar to melatonin but being lower than 6(OH)M either in all subjects (p < 0.001) or in African-Americans (p < 0.01) or Caucasians (p < 0.0001) (Table 1). 5MT concentrations were higher than melatonin and AFMK (p < 0.05) and lower than 6(OH)M levels (p < 0.05) when analysis included all subjects (Table 1). However, after substratification by race only Caucasian samples had 5MT concentration higher than AFMK (p < 0.05) and melatonin levels (p < 0.01). These differences were not detected in African-Americans (p > 0.05).
Table 1.
Production of melatonin and its metabolites in the human epidermis.
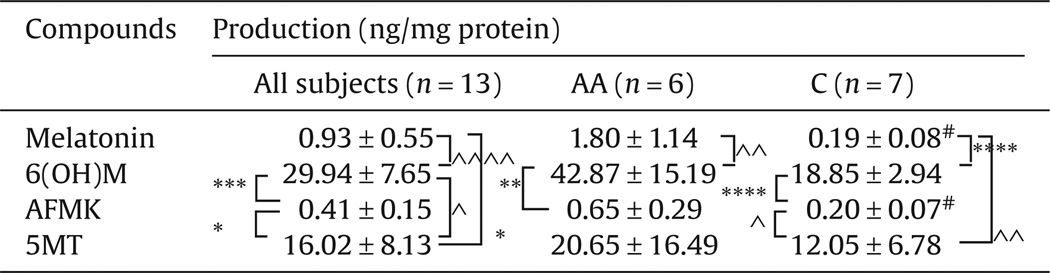 |
, p < 0.05 African-Americans (AA) vs Caucasians (C) using Mann–Whitney test.
, p < 0.05;
, p < 0.01;
, p < 0.001;
, p < 0.0001 metabolite vs melatonin or another metabolite using Student t-test.
, p < 0.05;
, p < 0.01;
, p < 0.0001 metabolite vs melatonin or another metabolite using Mann–Whitney test.
Our recent in vitro testing have demonstrated that the major product of melatonin metabolism in immortalized epidermal keratinocytes is 6(OH)M with AFMK and 5MT representing minor product (Kim et al., 2013). The in vivo data are in general agreement with cell culture studies showing the highest production of 6(OH)M in the epidermis and lowest production of AFMK and they demonstrate that epidermal melatonin is metabolized predominantly through the indolic pathway with kynuric pathway playing a minor role. Relatively, high level of 5MT production may represent evolutionary conservation of melatonin metabolism through deacetylation by tissues producing it as described for brain, retina and skin of amphibians (Grace et al., 1991).
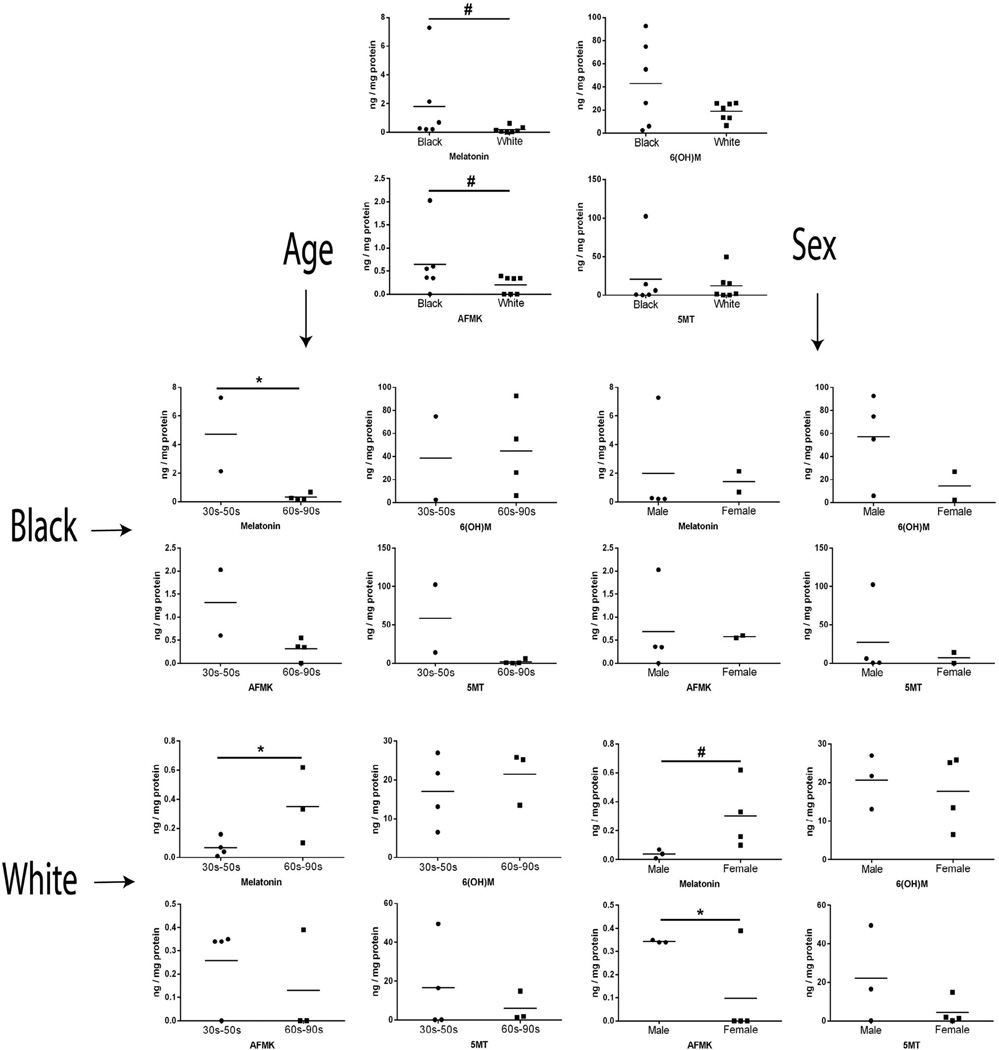
The production of melatonin and AFMK, but not of 6(OH)M and 5MT, was significantly higher (p < 0.05) in African-Americans in comparison with Caucasians (Table 1 and Fig. 3). The samples were further substratified according to gender and age (Fig. 3). Melatonin content was the highest in groups of young African-Americans (30–50 years old) and older Caucasians (60–90 years old) and in Caucasians females in comparison with Caucasians males (Fig. 3). In case of AFMK, the production was statistically higher in Caucasians males compared with Caucasians females. However, the production of 6(OH)M and 5MT was similar (p > 0.05) between young and old, African-Americans and Caucasians, and male and female people (Fig. 3).
Fig. 3.
Levels of endogenous production of melatonin and its metabolites depend on race, age and gender. Epidermis was extracted with 75% acetonitrile after homogenizing and applied to qTOF LC-MS (EIC) for quantification using corresponding standards (m/z = 233.1 [M+H]+ for melatonin; 287.1 [M+K]+ for 6(OH)M; 287.1 [M+Na]+ for AFMK, and 174.1 [M+H-NH3]+ for 5MT). *, p < 0.05; ***, p < 0.001 at Student t-test and #, p < 0.05 at Mann–Whitney test. Black (African-Americans) and white (Caucasians): race comparison; Age: age comparison; Sex: male and female comparison.
These results indicate that production of melatonin and AFMK is dependent on race, age and sex of the donors. The higher production of melatonin and AFMK in African-Americans vs Caucasians is intriguing. Since both compounds play important role in antioxidative responses in various organs (Hardeland et al., 2011; Lanoix et al., 2012a; Luchetti et al., 2010; Tan et al., 2007) including protection against UVR (Kleszczynski et al., 2011), we suggest that both compounds could also contribute to higher resistance of skin of African-Americans to UVR induced carcinogenesis. The age related differences in melatonin within both racial groups, and gender related differences in melatonin and AFMK levels for Caucasians only, may be explained by corresponding changes in local neurohormonal regulation of melatoninergic system, taking into consideration neuroendocrine capabilities of the skin (Slominski et al., 2012b).
3.2. Effects of melatonin and its metabolites on proliferation and melanogenesis in human melanoma cells and epidermal melanocytes
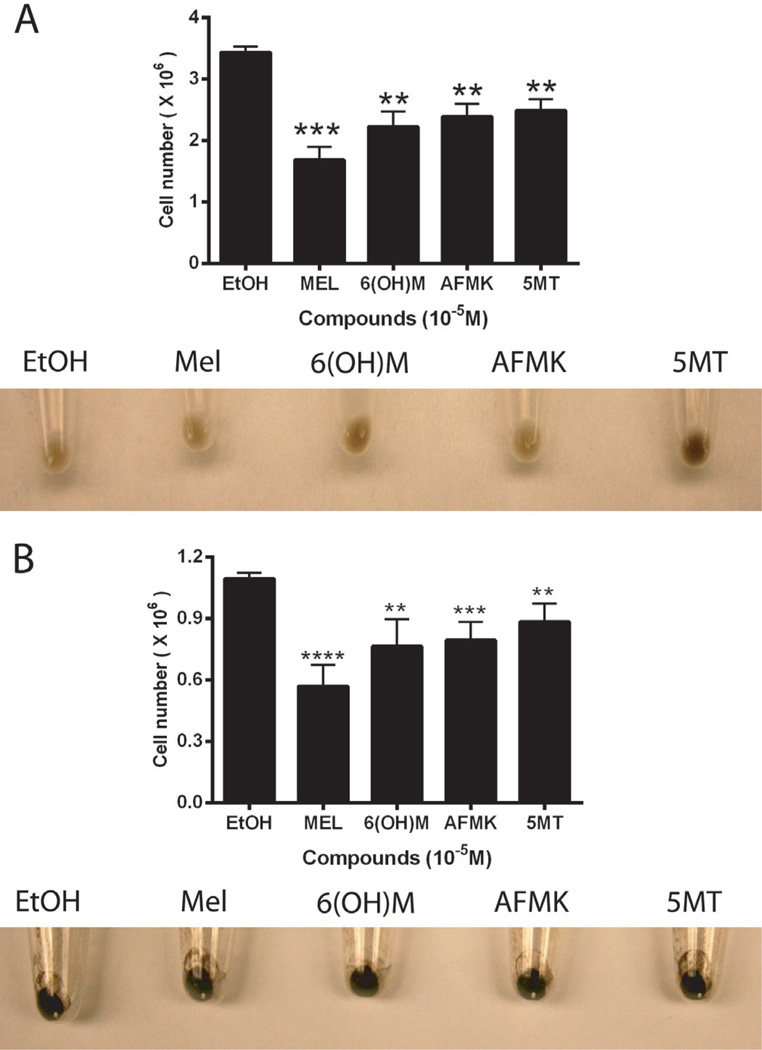
Combined results of the effects of melatonin and its metabolites in normal epidermal melanocytes and melanoma cells are shown in Fig. 4 and Table 2.
Fig. 4.
Effects of melatonin and its metabolites on melanogenesis and cell growth of human SKMEL-188 melanoma (A) and normal human epidermal melanocytes (B). Melanoma cells were grown in Ham’s F10 media containing 400 µM L-tyrosine with 10−5M of test compounds. Normal human melanocytes cells were grown in MBM-4 media supplemented with MGM-4 containing10−5 M of test compounds.
Table 2.
Effects of melatonin and its metabolites on tyrosinase activity in melanoma cells and normal human epidermal melanocytes.
| Compounds (10−5 M) | Tyrosinase activity (units/mg protein) | |
|---|---|---|
| SKMEL-188 | Melanocytes | |
| Ethanol | 0.39 ± 0.05 | 1.54 ± 0.11 |
| Melatonin | 0.36 ± 0.09 | 1.18 ± 0.05** |
| 6(OH)M | 0.41 ± 0.08 | 0.83 ± 0.02*** |
| AFMK | 0.34 ± 0.08 | 1.26 ± 0.05** |
| 5MT | 0.58 ± 0.05** | 1.29 ± 0.03* |
, <0.05;
, <0.01;
, <0.001 at Student t-test vs ethanol control.
3.2.1. Studies on human melanoma cells
Production of melanin pigment was induced by addition of 400 µM L-tyrosine to Ham’s F-10 medium (Slominski et al., 1988, 2009). Although all tested compounds inhibited growth of human melanoma cells (Fig. 4A), melatonin, 6(OH)M and AFMK had no significant effect on melanin pigmentation and tyrosinase activity (Fig. 4A, Table 2). However, 5MT enhanced both melanin pigmentation (Fig 4A, lower panel) and tyrosinase activity (Table 2, p < 0.05).
These results are not only consistent with our previous antiproliferative activity of melatonin against human and rodent melanomas (Fischer et al., 2006c; Slominski and Pruski, 1993) but also extend this anti-melanoma activity to its metabolites including 6(OH)M, AFMK and 5MT. However, in contrast to previous studies on rodent melanomas (Slominski and Pruski, 1993; Valverde et al., 1995) melatonin had no effect on melanin pigmentation in human melanoma cells. However, this is consistent with other reports on human melanomas [reviewed in (Slominski et al., 2004)]. Although the stimulatory effect of 5MT on melanogenesis was unexpected, it may explain the single report of stimulation of melanogenesis by melatonin in human melanoma in vitro (Cabrera et al., 2010), apparently through its metabolism to 5MT.
3.2.2. Studies on human epidermal melanocytes
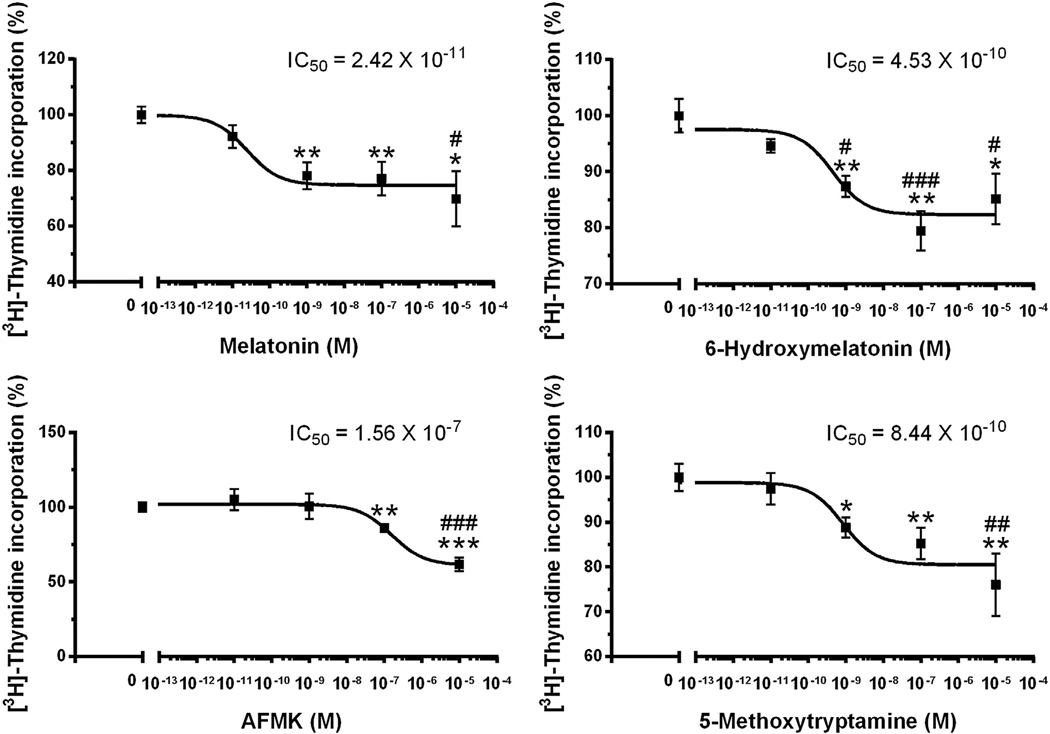
In normal human epidermal melatonin and its metabolites diminished significantly tyrosinase activity (Table 2), without morphologically detectable effect on melanin content (Fig. 4B, see equally black color of cell pellets). 6(OH)M was the most effective compound causing 50% reduction in tyrosinase activity (Table 2). The inhibitory effect on tyrosinase was accompanied by growth inhibition as estimated by number of the cells in comparison with control. Anti-proliferative effect was further confirmed using incorporation of [3H]-thymidine into DNA (Fig. 5). All compounds inhibited proliferation in a dose dependent manner, with 1 nM being the lowest effective concentration of melatonin, 6(OH)M or 5MT, and 100 nM for AFMK treatment in normal epidermal melanocytes. The corresponding IC50 values were 2.42 × 10−11 (melatonin), 4.53 × 10−10 [6(OH)M], 1.56 × 10−7(AFMK) and 8.44 × 10−10 (5MT) M. Antiproliferative effects shown in Figs. 4 and 5 indicate that melatonin is more effective in inhibition of cell proliferation in comparison to its metabolites.
Fig. 5.
Dose dependent effects of melatonin and its metabolites on DNA synthesis in normal human epidermal melanocytes. The cells were grown in MBM-4 media supplemented with MGM-4 containing melatonin and its metabolites at the concentrations listed. *, p < 0.05; **, p < 0.01; ***, p < 0.001 at Student t-test. #, p < 0.05; ##, p < 0.01; ###, p < 0.001 at one-way ANOVA test.
The inhibitory effect on tyrosinase activity in normal is in agreement with described inhibitory role of melatonin on melanin pigmentation in vertebrates [reviewed in Slominski et al. (2004, 2005a)]. The combined inhibitory effect of melatonin and metabolites on normal epidermal melanocytes proliferation and tyrosinase activity suggests that these compounds can be used as adjuvants in treatment of skin hyperpigmentation or they could attenuate malignant transformation of epidermal melanocytes.
4. Concluding remarks
In this study, using skin samples from 13 donors we show for the first time endogenous production of melatonin and its metabolites including 6(OH)M, AFMK and 5MT in the human epidermis in vivo. The level of production was dependent on race, gender and age as well as on chemical structure of the compound. These results substantiate previous molecular, histochemical and biochemical studies on human cutaneous melatoninergic system (Fischer et al., 2006b; Kim et al., 2013; Kobayashi et al., 2005; Slominski et al., 2002b, 2005a, 2014). They also provide an initial proof-of-the concept that this system operates in vivo in the human epidermis in a context dependent manner, being affected by race, age and gender. Even though the endogenous production of melatonin and its metabolites are dependent on the demographics of the humans, all metabolites inhibited proliferation of human normal epidermal melanocytes and melanoma cells. Furthermore, a moderate inhibitory effect on tyrosinase activity was observed in normal epidermal melanocytes.
Thus the above findings open new exciting possibilities on the in vivo role of endogenous melatonin synthesis and metabolism systems in the regulation of epidermal functions. These would include regulation of its barrier function, anti-carcinogenic activity and tuning up epidermal pigmentary system. Furthermore, regulation of their endogenous production/metabolism can serve as a rationale strategy for cosmetic or therapeutic purposes.
Acknowledgementss
This paper is dedicated to Dr. Aaron B. Lerner, a discoverer of melatonin. The work was supported by NIH grant 1R01AR056666-01A2 to AS and NIH instrument grant 1S10OD010678-01 to WL. We thank Dr. Dianne Kovacic, Dermatopathology fellow, for collecting human tissue for the studies.
Abbreviations
- 6(OH)M
6-hydroxymelatonin
- AFMK
N1-acetyl-N2-formyl-5-methoxykynuramine
- 5MT
5-methoxytryptamine
- yo
years old
- UVB
ultraviolet B
- FBS
fetal bovine serum
- TCA
trichloroacetic acid
- SDS
sodium dodecyl sulfate
- ESI
electrospray ionization
- MS
mass spectrometry
- LC-MS
liquid chromatography mass spectrometry
- HPLC
high-performance liquid chromatography
- UPLC
ultra-performance liquid chromatography
- qTOF
quadrupole time-of-flight
References
- Bubenik GA. Thirty four years since the discovery of gastrointestinal melatonin. J. Physiol. Pharmacol. 2008;59(Suppl. 2):33–51. [PubMed] [Google Scholar]
- Cabrera J, Negrin G, Estevez F, et al. Melatonin decreases cell proliferation and induces melanogenesis in human melanoma SK-MEL-1 cells. J. Pineal Res. 2010;49:45–54. doi: 10.1111/j.1600-079X.2010.00765.x. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27:101–110. doi: 10.1385/ENDO:27:2:101. [DOI] [PubMed] [Google Scholar]
- Fischer TW, Zbytek B, Sayre RM, et al. Melatonin increases survival of HaCaT keratinocytes by suppressing UV-induced apoptosis. J. Pineal Res. 2006a;40:18–26. doi: 10.1111/j.1600-079X.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- Fischer TW, Sweatman TW, Semak I, et al. Constitutive and UV-induced metabolism of melatonin in keratinocytes and cell-free systems. FASEB J. 2006b;20:1564–1566. doi: 10.1096/fj.05-5227fje. [DOI] [PubMed] [Google Scholar]
- Fischer TW, Zmijewski MA, Zbytek B, et al. Oncostatic effects of the indole melatonin and expression of its cytosolic and nuclear receptors in cultured human melanoma cell lines. Int. J. Oncol. 2006c;29:665–672. doi: 10.3892/ijo.29.3.665. [DOI] [PubMed] [Google Scholar]
- Fischer TW, Slominski A, Zmijewski MA, et al. Melatonin as a major skin protectant: from free radical scavenging to DNA damage repair. Exp. Dermatol. 2008a;17:713–730. doi: 10.1111/j.1600-0625.2008.00767.x. [DOI] [PubMed] [Google Scholar]
- Fischer TW, Slominski A, Tobin DJ, et al. Melatonin and the hair follicle. J. Pineal Res. 2008b;44:1–15. doi: 10.1111/j.1600-079X.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- Fischer TW, Zmijewski MA, Wortsman J, et al. Melatonin maintains mitochondrial membrane potential and attenuates activation of initiator (casp-9) and effector caspases (casp-3/casp-7) and PARP in UVR-exposed HaCaT keratinocytes. J. Pineal Res. 2008c;44:397–407. doi: 10.1111/j.1600-079X.2007.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer TW, Kleszczynski K, Hardkop LH, et al. Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2’-deoxyguanosine) in ex vivo human skin. J. Pineal Res. 2013;54:303–312. doi: 10.1111/jpi.12018. [DOI] [PubMed] [Google Scholar]
- Grace MS, Cahill GM, Besharse JC. Melatonin deacetylation: retinal vertebrate class distribution and Xenopus laevis tissue distribution. Brain Res. 1991;559:56–63. doi: 10.1016/0006-8993(91)90286-5. [DOI] [PubMed] [Google Scholar]
- Hardeland R, Pandi-Perumal SR, Cardinali DP. Melatonin. Int. J. Biochem. Cell Biol. 2006;38:313–316. doi: 10.1016/j.biocel.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Hardeland R, Cardinali DP, Srinivasan V, et al. Melatonin – a pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011;93:350–384. doi: 10.1016/j.pneurobio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Hirata F, Hayaishi O, Tokuyama T, et al. In vitro and in vivo formation of two new metabolites of melatonin. J. Biol. Chem. 1974;249:1311–1313. [PubMed] [Google Scholar]
- Kim TK, Kleszczynski K, Janjetovic Z, et al. Metabolism of melatonin and biological activity of intermediates of melatoninergic pathway in human skin cells. FASEB J. 2013;27:2742–2755. doi: 10.1096/fj.12-224691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleszczynski K, Hardkop LH, Fischer TW. Differential effects of melatonin as a broad range UV-damage preventive dermato-endocrine regulator. Dermatoendocrinol. 2011;3:27–31. doi: 10.4161/derm.3.1.14842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleszczynski K, Tukaj S, Kruse N, et al. Melatonin prevents ultraviolet radiation-induced alterations in plasma membrane potential and intracellular pH in human keratinocytes. J. Pineal Res. 2012 doi: 10.1111/j.1600-079X.2012.01028.x. doi:10.1111/j.1600-079X.2012.01028.x. [DOI] [PubMed] [Google Scholar]
- Kleszczynski K, Tukaj S, Kruse N, et al. Melatonin prevents ultraviolet radiation-induced alterations in plasma membrane potential and intracellular pH in human keratinocytes. J. Pineal Res. 2013;54:89–99. doi: 10.1111/j.1600-079X.2012.01028.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Kromminga A, Dunlop TW, et al. A role of melatonin in neuroectodermal-mesodermal interactions: the hair follicle synthesizes melatonin and expresses functional melatonin receptors. FASEB J. 2005;19:1710–1712. doi: 10.1096/fj.04-2293fje. [DOI] [PubMed] [Google Scholar]
- Konturek SJ, Konturek PC, Brzozowska I, et al. Localization and biological activities of melatonin in intact and diseased gastrointestinal tract (GIT) J. Physiol. Pharmacol. 2007;58:381–405. [PubMed] [Google Scholar]
- Lanoix D, Lacasse AA, Reiter RJ, et al. Melatonin: the smart killer: the human trophoblast as a model. Mol. Cell. Endocrinol. 2012a;348:1–11. doi: 10.1016/j.mce.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Lanoix D, Guerin P, Vaillancourt C. Placental melatonin production and melatonin receptor expression are altered in preeclampsia: new insights into the role of this hormone in pregnancy. J. Pineal Res. 2012b;53:417–425. doi: 10.1111/j.1600-079X.2012.01012.x. [DOI] [PubMed] [Google Scholar]
- Lerner AB. Hormonal control of pigmentation. Annu. Rev. Med. 1960;11:187–194. doi: 10.1146/annurev.me.11.020160.001155. [DOI] [PubMed] [Google Scholar]
- Lerner AB, Case JD, Mori W, et al. Melatonin in peripheral nerve. Nature. 1959;183:1821. doi: 10.1038/1831821a0. [DOI] [PubMed] [Google Scholar]
- Lerner AB, Case JD, Takahashi Y. Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands. J. Biol. Chem. 1960;235:1992–1997. [PubMed] [Google Scholar]
- Logan A, Weatherhead B. Melatonin-induced inhibition of melanogenesis in hair follicles in vitro [proceedings] J. Endocrinol. 1979;81:168P. [PubMed] [Google Scholar]
- Logan A, Weatherhead B. Post-tyrosinase inhibition of melanogenesis by melatonin in hair follicles in vitro. J. Invest. Dermatol. 1980;74:47–50. doi: 10.1111/1523-1747.ep12514608. [DOI] [PubMed] [Google Scholar]
- Luchetti F, Canonico B, Betti M, et al. Melatonin signaling and cell protection function. FASEB J. 2010;24:3603–3624. doi: 10.1096/fj.10-154450. [DOI] [PubMed] [Google Scholar]
- Ma X, Idle JR, Krausz KW, et al. Metabolism of melatonin by human cytochromes p450. Drug Metab. Dispos. 2005;33:489–494. doi: 10.1124/dmd.104.002410. [DOI] [PubMed] [Google Scholar]
- McElhinney DB, Hoffman SJ, Robinson WA, et al. Effect of melatonin on human skin color. J. Invest. Dermatol. 1994;102:258–259. doi: 10.1111/1523-1747.ep12371773. [DOI] [PubMed] [Google Scholar]
- Reiter RJ. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 1991;12:151–180. doi: 10.1210/edrv-12-2-151. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Manchester LC, et al. Melatonin in edible plants (phytomelatonin): identification, concentrations, bioavailability and proposed functions. World Rev. Nutr. Diet. 2007a;97:211–230. doi: 10.1159/000097917. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Manchester LC, et al. Medical implications of melatonin: receptor-mediated and receptor-independent actions. Adv. Med. Sci. 2007b;52:11–28. [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Fuentes-Broto L. Melatonin: a multitasking molecule. Prog. Brain Res. 2010;181:127–151. doi: 10.1016/S0079-6123(08)81008-4. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Roth RH, Aghajanian GK. Melatonin: deacetylation to 5-methoxytryptamine by liver but not brain aryl acylamidase. J. Neurochem. 1979;32:1219–1226. doi: 10.1111/j.1471-4159.1979.tb11049.x. [DOI] [PubMed] [Google Scholar]
- Roseboom PH, Namboodiri MA, Zimonjic DB, et al. Natural melatonin ‘knockdown’ in C57BL/6J mice: rare mechanism truncates serotonin N-acetyltransferase. Brain Res. Mol. Brain Res. 1998;63:189–197. doi: 10.1016/s0169-328x(98)00273-3. [DOI] [PubMed] [Google Scholar]
- Semak I, Naumova M, Korik E, et al. A novel metabolic pathway of melatonin: oxidation by cytochrome C. Biochemistry. 2005;44:9300–9307. doi: 10.1021/bi050202d. [DOI] [PubMed] [Google Scholar]
- Semak I, Korik E, Antonova M, et al. Metabolism of melatonin by cytochrome P450s in rat liver mitochondria and microsomes. J. Pineal Res. 2008;45:515–523. doi: 10.1111/j.1600-079X.2008.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Pruski D. Melatonin inhibits proliferation and melanogenesis in rodent melanoma cells. Exp. Cell Res. 1993;206:189–194. doi: 10.1006/excr.1993.1137. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr. Rev. 2000;21:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- Slominski A, Moellman G, Kuklinska E, et al. Positive regulation of melanin pigmentation by two key substrates of the melanogenic pathway: L-tyrosine and L-dopa. J. Cell Sci. 1988;89:287–296. doi: 10.1242/jcs.89.3.287. [DOI] [PubMed] [Google Scholar]
- Slominski A, Chassalevris N, Mazurkiewicz J, et al. Murine skin as a target for melatonin bioregulation. Exp. Dermatol. 1994;3:45–50. doi: 10.1111/j.1600-0625.1994.tb00265.x. [DOI] [PubMed] [Google Scholar]
- Slominski A, Baker J, Rosano TG, et al. Metabolism of serotonin to N-acetylserotonin, melatonin, and 5-methoxytryptamine in hamster skin culture. J. Biol. Chem. 1996;271:12281–12286. doi: 10.1074/jbc.271.21.12281. [DOI] [PubMed] [Google Scholar]
- Slominski A, Pisarchik A, Semak I, et al. Serotoninergic system in hamster skin. J. Invest. Dermatol. 2002a;119:934–942. doi: 10.1046/j.1523-1747.2002.00156.x. [DOI] [PubMed] [Google Scholar]
- Slominski A, Pisarchik A, Semak I, et al. Serotoninergic and melatoninergic systems are fully expressed in human skin. FASEB J. 2002b;16:896–898. doi: 10.1096/fj.01-0952fje. [DOI] [PubMed] [Google Scholar]
- Slominski A, Semak I, Pisarchik A, et al. Conversion of L-tryptophan to serotonin and melatonin in human melanoma cells. FEBS Lett. 2002c;511:102–106. doi: 10.1016/s0014-5793(01)03319-1. [DOI] [PubMed] [Google Scholar]
- Slominski A, Pisarchik A, Zbytek B, et al. Functional activity of serotoninergic and melatoninergic systems expressed in the skin. J. Cell. Physiol. 2003;196:144–153. doi: 10.1002/jcp.10287. [DOI] [PubMed] [Google Scholar]
- Slominski A, Tobin DJ, Shibahara S, et al. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Tobin DJ. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J. 2005a;19:176–194. doi: 10.1096/fj.04-2079rev. [DOI] [PubMed] [Google Scholar]
- Slominski A, Fischer TW, Zmijewski MA, et al. On the role of melatonin in skin physiology and pathology. Endocrine. 2005b;27:137–148. doi: 10.1385/ENDO:27:2:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Plonka PM, et al. Hair follicle pigmentation. J. Invest. Dermatol. 2005c;124:13–21. doi: 10.1111/j.0022-202X.2004.23528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Tobin DJ, Zmijewski MA, et al. Melatonin in the skin: synthesis, metabolism and functions. Trends Endocrinol. Metab. 2008;19:17–24. doi: 10.1016/j.tem.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Slominski R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int. J. Cancer. 2009;124:1470–1477. doi: 10.1002/ijc.24005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Kim TK, Janjetovic Z, et al. 20-Hydroxyvitamin D2 is a noncalcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells. Am. J. Physiol. Cell Physiol. 2011;300:C526–C541. doi: 10.1152/ajpcell.00203.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Zmijewski MA, Skobowiat C, et al. Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv. Anat. Embryol. Cell Biol. 2012b;212:1–115. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Zmijewski MA, Semak I, et al. Cytochromes p450 and skin cancer: role of local endocrine pathways. Anticancer Agents Med. Chem. 2014;14:77–96. doi: 10.2174/18715206113139990308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, et al. Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol. Cell. Endocrinol. 2012a;351:152–166. doi: 10.1016/j.mce.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan DX, Manchester LC, Terron MP, et al. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007;42:28–42. doi: 10.1111/j.1600-079X.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- Valverde P, Benedito E, Solano F, et al. Melatonin antagonizes alpha-melanocyte-stimulating hormone enhancement of melanogenesis in mouse melanoma cells by blocking the hormone-induced accumulation of the c locus tyrosinase. Eur. J. Biochem. 1995;232:257–263. doi: 10.1111/j.1432-1033.1995.tb20807.x. [DOI] [PubMed] [Google Scholar]
- Young IM, Leone RM, Francis P, et al. Melatonin is metabolized to N-acetyl serotonin and 6-hydroxymelatonin in man. J. Clin. Endocrinol. Metab. 1985;60:114–119. doi: 10.1210/jcem-60-1-114. [DOI] [PubMed] [Google Scholar]
- Yu HS, Reiter RJ. Melatonin Biosynthesis, Physiological Effects, and Clinical Implications. Boca Raton, FL: CRC Press; 1993. [Google Scholar]
- Zmijewski MA, Sweatman TW, Slominski AT. The melatonin-producing system is fully functional in retinal pigment epithelium (ARPE-19) Mol. Cell. Endocrinol. 2009;307:211–216. doi: 10.1016/j.mce.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]