Abstract
Marginal zone (MZ) B cells, identified as surface (s)IgMhighsIgDlowCD23low/−CD21+CD38− B cells, were purified from human spleens, and the features of their V(D)J gene rearrangements were investigated and compared with those of germinal center (GC), follicular mantle (FM) and switched memory (SM) B cells. Most MZ B cells were CD27+ and exhibited somatic hypermutations (SHM), although to a lower extent than SM B cells. Moreover, among MZ B-cell rearrangements, recurrent sequences were observed, some of which displayed intraclonal diversification. The same diversifying sequences were detected in very low numbers in GC and FM B cells and only when a highly sensitive, gene-specific polymerase chain reaction was used. This result indicates that MZ B cells could expand and diversify in situ and also suggested the presence of a number of activation-induced cytidine deaminase (AID)-expressing B cells in the MZ. The notion of antigen-driven expansion/selection in situ is further supported by the VH CDR3 features of MZ B cells with highly conserved amino acids at specific positions and by the finding of shared (“stereotyped”) sequences in two different spleens. Collectively, the data are consistent with the notion that MZ B cells are a special subset selected by in situ antigenic stimuli.
INTRODUCTION
Human B cells can be subdivided into virgin and memory cells, which generally can be identified by the expression of CD27 (1,2) and FcRL-4 (3,4) antigens by memory B cells. B cells also can be classified based on the anatomic areas where they seed, that is, follicular (FO) and marginal zone (MZ) B cells. FO B cells can be in turn separated into follicular mantle (FM) and germinal center (GC) B cells depending on their location within secondary lymphoid follicles (5–7).
The splenic MZ is defined as the outermost portion of the white pulp, the structure of which permits the transit of B cells from and to the bloodstream and facilitates encounters between blood-borne pathogens with B cells and macrophages (8–10). While FO B cells are believed to be capable of generating plasma cells secreting high-affinity antibodies and switched memory (SM) B cells (7), MZ B cells are thought to produce IgM antibodies in a T cell–independent manner, particularly to polysaccharide antigens of encapsulated bacteria (11–13). This response represents a first defense line to protect the host from bacterial infection spread until an efficient FO response can develop. Special areas of other lymphoid organs, including subepithelial areas of tonsils, the dome region of Peyer patches, the subcapsular areas of lymph nodes and the mucosa-associated lymphoid tissue (MALT) tissue, are believed to be the equivalent of the splenic MZ (14). Studies in mice have determined that MZ B cells have a sIgMhighsIgDlow phenotype with high CD21 and low CD23 expression and respond to T cell–independent antigens in vitro and in vivo(8,15). Studies on genetically manipulated mice also support the notion that FO and MZ B cells represent independent compartments (8,16). Less information is available on human MZ B cells, although their role is supported by the increased incidence of encapsulated bacterial infection in splenectomized or congenitally asplenic patients (17) and in individuals with immature MZ compartments such as neonates (18). In addition, sIgMhighsIgDlow MZ-equivalent B cells respond to polysaccharide antigens in a T cell–independent manner (19). sIgMhighsIgDlow B cells represent a fraction of the B cells located in the MZ or MZ-equivalent areas (20). In addition to these cells, generally called “MZ B cells” (and we maintain this terminology here), the MZ is populated by SM B cells expressing sIgG and sIgA. Therefore, the characterization of MZ B cells requires complex purification procedures (21,22). Thus, studies on the immunoglobulin gene repertoire of MZ B cells are scanty (23–25), and most of the available information comes from studies on MZ-equivalent B cells, particularly from tonsils (26) and circulating sIgMhighsIgDlowCD27+ B cells (2,27,28), for which there is some evidence of an MZ B-cell origin.
Here, we analyzed the V(D)J gene rearrangements used by MZ B cells purified from human spleens. These sequences were compared with those used by FM, GC and SM B cells from the same spleens. Our findings demonstrate that MZ B cells have a particular Ig gene repertoire, possibly shaped by functional selection after stimulation with common antigenic determinants. This stimulation is likely to occur in situ in the MZ, as suggested by both the imprint of somatic hypermutation (SHM) and the existence of clonal families exhibiting an ongoing process of diversification.
MATERIALS AND METHODS
Samples
Five spleens, free of neoplastic cells at histological inspection, were obtained at surgery for cancer (three patients had pancreatic cancer, one metastatic breast cancer and one liposarcoma). Mononuclear cells were isolated by Ficoll-Hypaque (Seromed; Biochrom KG, Berlin, Germany) density gradient centrifugation. Four tonsils were obtained from 5- to 12-year-old children undergoing routine tonsillectomies, and their cells were purified as previously reported (29). The study was approved by the Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Azienda Ospedaliera Universitaria (AOU) San Martino-IST Institutional Review Board and informed consent was obtained from patients.
Flow Cytometry and Cell Sorting
Mononuclear cells were stained and sorted with antibodies by FacsAria 2 (BD, Milan, Italy) to a purity of >98%. A list of all antibodies used is reported in the supplementary materials. Isotypic negative controls were acquired for each fluorochrome. Data were analyzed and normalized by using FlowJo software (TreeStar, Ashland, OR, USA).
Immunohistochemical Staining
Formalin-fixed paraffin-embedded sections (3 μm thick) were subjected to antigen retrieval with citrate buffer at high pH and immunostained with polyclonal anti-δ antibodies (Abs) and anti-CD38 monoclonal antibody (mAb) (Diapath SRL, Martinengo, Italy), with a BenchMark XT automated stainer (Ventana Medical Systems, Strasbourg, France). For activation-induced cytidine deaminase (AID) staining, a mouse anti-AID mAb (Invitrogen/Life Technologies, Carlsbad, CA, USA) was used. Slides were revealed with the ultraView Universal Alkaline Phosphatase Red Detection Kit (Ventana Medical Systems Inc./Roche, Basel, Switzerland) and counterstained with modified Gill’s hematoxylin. The slides were examined with an Olympus microscope (Olympus Italia, Segrate, Italy).
Immunofluorescence Microscopy
Serial splenic OCT cryosections (5–6 μm) were cut, laid on glass cover-slips and stored at −80°C. Sections were thawed briefly and incubated with the primary antibodies for 30 min at room temperature. See the supplementary materials for details of antibodies used. After washing, the sections were incubated with the appropriate secondary antibodies from Invitrogen/Life Technologies. 4′,6-Diamidino-2-phenylindole (DAPI) was used for nuclei staining. Slides were analyzed with a laser scanning confocal microscope (TCS SP2 AOBS [Leica, Heidelberg, Germany], with the following characteristics: HCX PL APO CS 40×/1.25 Oil UV).
Immunoglobulin Heavy Chain Variable (IGHV) Gene Sequencing
RNA isolation from FM, GC, MZ and SM B cells, purified by sorting, and cDNA synthesis were described before (29). First-strand cDNA was amplified in independent polymerase chain reactions (PCRs) by using IGHV1, IGHV3 and IGHV4 subgroup–specific primers in conjunction with the appropriate IGHM or IGHG constant region gene primers (30). Products were cloned into the TOPO-TA vector (Life Technologies) and sequenced (3100 XL genetic analyzer; Applied Biosystems/Life Technologies). Data were analyzed by using ImMunoGeneTics (IMGT) database and tools (http://www.imgt.org/). Only productive rearrangements were evaluated. For the identification of common VH CDR3 sequence patterns, we applied a purpose-built bioinformatics method, which was already used in immunogenetic studies of both normal and malignant B cells (31–34).
Gene-Specific PCR
To search for sequences shared by different B-cell subsets, we looked first for the presence of sequences related to a randomly selected clonal family from the MZ B cells in GC and FM B cells by using clonal family–specific oligonucleotides in quantitative real-time PCR (qRT-PCR) (see Supplementary Data Figure S2). In a second test, we enriched for IGHV1-69 carrying clones in FM and GC B cell subsets by using an IGHV1-69 leader specific primer with IGHM reverse primer followed by a seminested PCR with IGHV1-FR1 forward specific primer. Products were than cloned and sequenced as above.
AID Expression in Different Splenic and Tonsil B-Cell Subsets
qRT-PCR for the human AID and RNA polymerase II (POL2) transcripts was performed in duplicate with specific TaqMan gene expression assays designed by Life Technologies (AID, Hs00757808_m1; POL2, Hs00172187_m1). Samples were obtained from four spleens and three tonsils. Tonsil B-cell subsets were obtained at fluorescence-activated cell sorting by gating CD19+ B cells that were further separated based on the expression of IgD versus CD38 as reported (21). Run was performed on Rotor-Gene Q 5-plex (Corbett Life Science/Qiagen, Milan, Italy), and the cycle threshold (Ct) values were obtained by comparative analysis (Rotor-Gene Q series software). To compare the relative quantity of AID expression between spleens and tonsils, a 2−ΔCt comparative quantification was used (User Bulletin 2; Applied Biosystems/Life Technologies). PCRs with a Ct >35 were considered nonsignificant.
Statistical Analyses
The χ2 test or the Fisher exact test were used to compare differences in frequency between IGHV, IGHD and IGHJ genes. Analysis of variance (ANOVA) and Tamhane post hoc test was used to compare means of VH CDR3 lengths, total number of mutations, transversions and transitions. Statistical significance was considered as a P value ≤0.05. All the statistical tests were two-tailed and performed by using SPSS20 for Windows (SPSS, Chicago, IL, USA).
All supplementary materials are available online at www.molmed.org.
RESULTS
Purification and Phenotypic Characterization of MZ B Cells
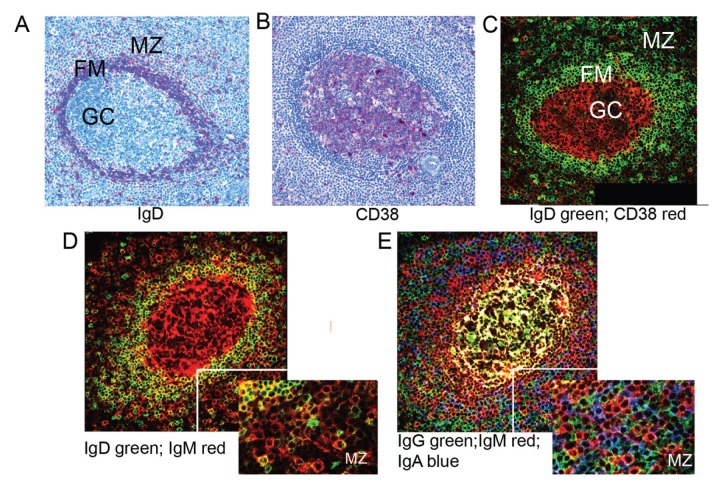
We first investigated the expression of IgD and CD38 in the different splenic areas and subsequently the distribution of the different Ig isotypes. IgD staining was strong in the FM and virtually absent in the GC (Figures 1A, C), whereas CD38 staining was strong in the GC and weak/absent in the FM and MZ (Figures 1B, C). IgD staining was observed in the MZ, albeit at a lower intensity compared with FM (Figure 1D). The distribution of IgM+ cells resembled that of IgD+ cells in the MZ, although with a higher staining intensity. Again, IgM+ cells were predominant in the FM, consistent with FM B cells being strongly positive for both IgM and IgD (40). IgG+ and IgA+ cells also were numerous in the MZ (Figure 1E). IgG and IgM molecules were present in the GC, where they also accumulated in the intracellular spaces (Figure 1E).
Figure 1.
Localization of Ig isotypes in human spleen. (A, B) Paraffin sections of adult spleen stained for IgD and CD38 by immunohistochemistry as indicated (20×). (C–E) Frozen sections from the same spleen were stained for the indicated isotypes and analyzed by confocal microscopy. Insets show higher digital magnification of MZ (30×). Virtual colors are displayed.
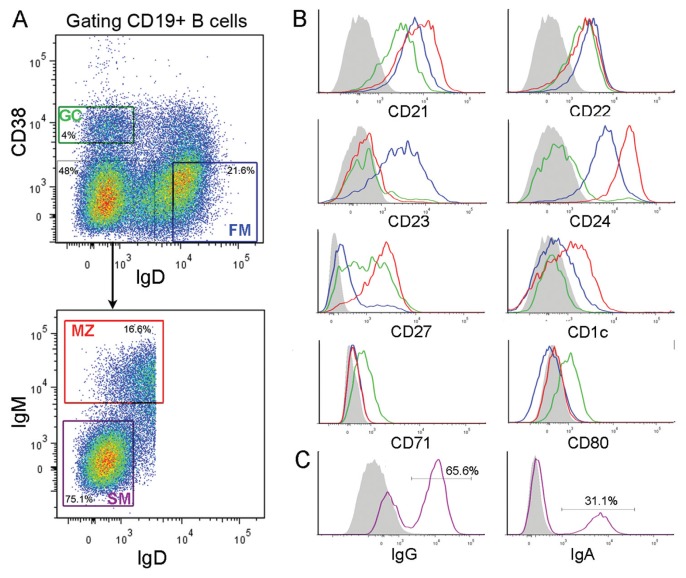
On the basis of the above information, splenic B-cell suspensions were stained with anti-δ plus anti-CD38 mAbs and sorted. Three major cell subsets were isolated: GC B cells (sIgD−CD38+), FM B cells (CD38−sIgD+) and sIgDlow/−CD38− B cells (Figure 2A). GC B cells were purified further by removing CD24+ cells (35) (not shown). sIgDlow/−CD38− B cells were fractionated further according to the expression of sIgM molecules to yield two cell subsets, that is, sIgMhighsIgDlow and SM B cells expressing IgG or IgA (Figures 2A, C). sIgMhighsIgDlow B cells, which comprised a small minority of sIgD− cells (Figure 2A), will be called MZ B cells. Surface marker analysis of the sorted B cells (Figure 2B) revealed that MZ B cells exhibited high levels of CD1c, CD21 and low/negative levels of CD23. Moreover, they had high surface CD27 and low CD71 and CD80, which were detected solely on GC B cells. CD22 was high in all the B-cell subsets investigated, whereas CD23 was high in FM B cells only, and CD24 was elevated in FM and particularly in MZ B cells. SM B cells, as isolated in Figure 2A, shared the same phenotype as MZ B cells (not shown), but had elevated levels of sIgG and sIgA (Figure 2C). These results were similar for all of the five spleens examined, except for variability in CD21 expression (range 20–80%) by GC B cells.
Figure 2.
Fluorescence-activated cell sorting and phenotypic characterization of MZ, FM and GC B cells. (A) Plots shown are gated on CD19+ B cells. Staining for sIgD/CD38 expression enables the separation of FM, GC and IgD−CD38− B cells. The latter cells are further separated into MZ and SM B cells as indicated. Numbers indicate the percentage of cells in the boxes. (B) Cell surface marker analysis of the purified B-cell subsets. (C) Staining for sIgG or sIgA of SM B cells. The gray area represents the negative control staining with an unrelated mAb. Red, green, blue and purple line colors correspond to MZ, GC, FM and SM, respectively.
Identification of Unique or Recurrent V(D)J Rearrangements in MZ B Cells
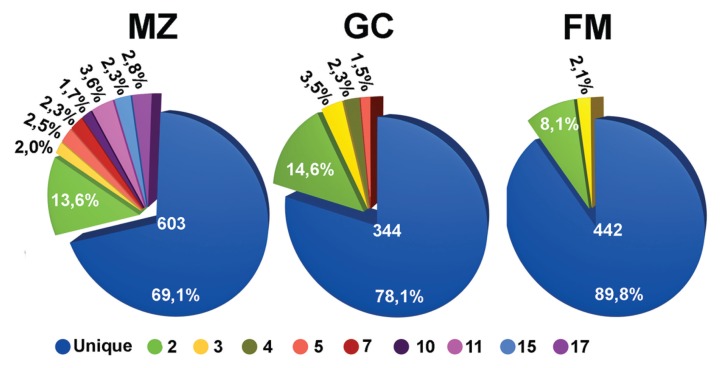
A total of 1,389 productive IGHV1, IGHV3 or IGHV4 rearrangements from μ cDNA of five spleens were obtained and analyzed (603 from MZ, 334 from GC and 443 from FM B cells). Sequences were classified by a VH CDR3 amino acid sequence and by IGHV gene utilization. Two types of Ig rearrangements were identified, that is, unique Ig rearrangements, found only once in the set analyzed, and recurrent Ig rearrangements that were considered to belong to a clonal family. These included identical and related sequences. The latter were characterized by VH CDR3 of identical length and ≥60% amino acids (aa) identity and by the same IGV gene combinations, which may differ for somatic mutations consistent with an intraclonal diversification process. Recurrent sequences were more abundant and were comprised of more numerous clones in the MZ B cells compared with the other B-cell subsets (Figure 3).
Figure 3.
Frequency of recurrent clones in different B-cell subpopulations. Pie charts summarizing the proportion of unique (blue) and recurrent (other colors) sequences in the FM, GC and MZ B cell subsets. The total number of sequences analyzed is indicated in the center. Different colors indicate the clonal families characterized by a different number of recurrent sequences, as specified at the bottom of the figure.
Recurrent sequences also were observed in gDNA from MZ B cells. With this approach, which used IGHV1 and IGHV4 gene–specific primers in conjunction with IGHJ specific primers, 111 gDNA clones were analyzed. A total of 47 of 72 (65%) of the IGHV1 and 32 of 39 (82%) of IGHV4 rearrangements, respectively, were productive, and there were 38% (18/47) and 12.5% (4/32) recurrent sequences in the IGHV1 and IGHV4 gene subgroups, respectively. These data further excluded that the presence of recurrent sequences could be attributed merely to the presence of cells with abundant Ig mRNA, such as plasma cells, although plasma cells expressing very high CD38 levels were already removed from MZ B cells by the purification procedure (Figure 2A).
IGHV Gene of MZ B Cells
An arbitrary cutoff of three mutations was used to operationally distinguish unmutated (zero to three mutations; 1.05% difference from the germ line) from mutated sequences (three or more mutations) (29). To determine the relative frequencies of mutated or unmutated sequences, for each group of the recurrent sequences, only one was randomly considered. This was feasible because all of the recurrent sequences from a given group were invariably mutated or unmutated according to the set criteria.
A total of 1,179 (460 from MZ, 300 from GC and 419 from FM B cells) sequences were analyzed. The majority of IGHV gene sequences from MZ B cells were mutated (361 of 460, or 78.5%). Similar values were found in GC B cells (225 of 300 mutated sequences, or 75%), whereas lower values (123 of 419, or 29.4%) were detected in FM B cells. Moreover, the mean mutation number of MZ and GC B cells was higher than that of FM B cells (FM versus GC, p < 0.001; FM versus MZ, p < 0.001), and MZ B cells exhibited more mutations than GC B cells (MZ versus GC, p < 0.05). Finally, the mean mutation number in the MZ B cell–mutated sequences was similar to that of GC B cells but higher than that of FM B cells (p < 0.001). Data are summarized in the supporting information (Supplementary Table S1).
Transitions predominated over transversions in all B-cell subsets. The majority of mutations occurred in FRs rather than CDRs; however, most mutations in CDRs were R rather than S, leading to higher R/S ratios (>3.0). In contrast, this R/S ratio was lower in FRs (<3.0) (not shown). The only exception to this rule concerned the R/S ratios (<3.0) in the VH CDR1 of mutated FM and MZ B cells (not shown).
Correlation Between CD27 Expression and Utilization of Mutated IGHV Genes in MZ B Cells
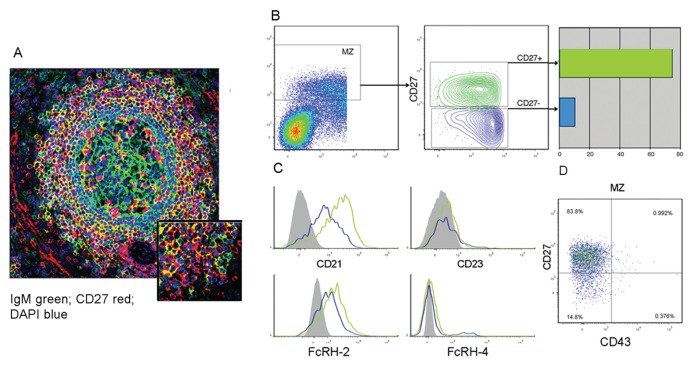
Most, although not all, of the B cells in the MZ were CD27+, as assessed by flow cytometry and in situ staining (Figures 2B, 4A). To find a possible correlation between IGHV gene mutational status and CD27 expression, sIgDlowCD38− cells were fractionated into sIgMhighCD27+ and sIgMhighCD27−B cells (Figure 4B). cDNA was amplified using IGHV1 gene subgroup–specific primers in conjunction with IGHM-specific primers and sequenced. Mutated IGHV sequences were found mainly in the sIgMhigh+CD27+ B cells (30 of 40, or 75%), whereas the proportion of mutated sequences was much lower in the sIgMhighCD27− B cells (2 of 28, or 7%). Notably, sIgMhighCD27+ and sIgMhighCD27− MZ B cells were both characterized by low CD23 and high CD21 expression (with marginal differences for the latter marker between the two subsets). FcRL-4 was virtually absent in the two groups of cells that stained for FcRL-2 (Figure 4C) (3,36). Recently, it has been proposed that CD27+ B cells expressing the CD43 marker could represent the human counterpart of mouse B1 B cells (37). These may possibly have a close relationship with MZ B cells (12). However, in the spleen, both sIgMhighCD27+ and sIgMhighCD27− B cells were negative for this marker (Figure 4D).
Figure 4.
Correlation between CD27 expression and SHM in MZ B cells. (A) Splenic frozen sections are stained for the indicated markers and analyzed by confocal microscopy (20×). A higher magnification (30×) of the MZ is shown in the inset. (B) sIgDlowCD38− B cells were gated to separate sIgMhighCD27+ and sIgMhighCD27− MZ B cells. The IGHV genes amplified from the two subsets were investigated for the presence of SHM. (C) Sorted sIgMhighCD27+ and sIgMhgihCD27− B cells were studied for the expression of the indicated markers (D). Staining of MZ B cells for anti-CD27 and -CD43.
V(D)J Gene Usage and VH CDR3 Features of MZ B Cells
The frequency of individual IGHV, IGHD and IGHJ genes in MZ B cells was determined and compared with that found in FM and GC B cells. Unique Ig rearrangements and a single representative sequence from groups of recurrent sequences were considered in this analysis. The IGHV gene frequencies were for the most part similar in the three cell subsets and spleens. The only exception concerned the IGHV1-3 gene, which was significantly more frequent in MZ (and FM) than in GC B cells (p < 0.05), but this difference was not confirmed when the analysis was repeated for each spleen taken separately. No significant differences were found regarding the usage of IGHJ and IGHD genes.
The average VH CDR3 length of MZ B cells was similar to that of GC B cells (15.01 versus 14.75 aa); both of these values were significantly lower than those observed in FM B cells (15.83 aa; MZ versus FM, p < 0.05; GC versus FM, p = 0.001). Notably, there was a significant difference in VH CDR3 length between the mutated and unmutated sequences of MZ B cells (14.79 versus 15.83; p < 0.05).
No difference in the number of N insertions were observed between subsets, although MZ and FM B cells exhibited more GC (guanine-cytosine)-N insertions than GC B cells (p < 0.05).
Diversification of Clonal Families in MZ B Cells
A total of 115 groups of recurrent clones were identified; 97 of 115 were identical (45, 31 and 21 from MZ, GC and FM B cells, respectively). The remaining 18 groups fulfilled the definition of related sequences.
Groups of identical sequences were usually small (range 2–5), with the exception of a group of 15 rearrangements in the MZ B cells that used the IGHV3-7 gene. Identical sequences were either mutated or unmutated. Mutated sequences were observed in 9 of 21 (42%), 26 of 31 (83.8%) and 36 of 45 (80%) groups from FM, GC and MZ B cells, respectively.
Of 18 groups of related sequences, 16 were from the MZ, 1 from GC and 1 from FM B cells. Related sequences exhibited SHM with both shared and unique mutations, indicative of intraclonal diversification (a representative MZ clonal family is reported in Supplementary Figure S1).
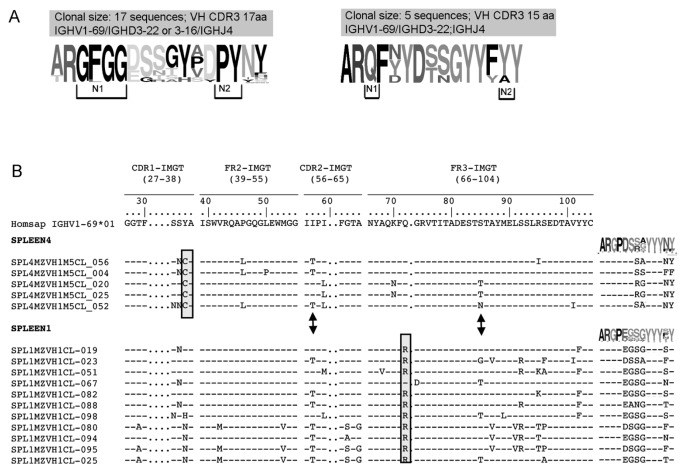
In certain clonal families, the VH CDR3 sequences from MZ B cells exhibited variability, possibly related to an active SHM process. Because of this variability, in certain clone members, different IGHD segments were assigned based on IMGT algorithm. Figure 5A shows an example of the VH CDR3s of a clonal family with highly conserved N1 and N2 regions. In contrast, the IGHD gene sequence was highly mutated, although a conservative aa change (that is, D→E, both negatively charged) was observed in certain clones. The pattern of mutations on the V region, however, with shared and unique mutations, clearly indicates they belong to a clonal family (not shown). In another MZ clonal family, three VH CDR3 aa positions, likely originating from the IGHD gene, exhibited great variability, whereas others were conserved (Figure 5A).
Figure 5.
Clonal diversification in MZ. (A) VH CDR3 logos of two different clonal families isolated from MZ B cells of spleen 1 are reported. The IGHD gene has been modified by SHM leading to a number of amino acid changes while conserved amino acids are found at N1 and N2 positions. The height of symbols within the stack indicates the relative frequency of each amino acid at that position. Amino acid position is according to IMGT numbering for the V domain. Logos were created by using the WebLogo tool (http://weblogo.berkeley.edu). (B) Stereotyped sequences in spleen 1 and 4. They are identified as stereotyped based on the VH CDR3 similarities and utilization of the same V(D)J gene rearrangement. Amino acids in the boxes indicate spleen-specific SHM; arrows highlight the presence of common SHM on the IGHV gene when aligned with the closest germ line gene (IGHV1-69). VH CDR3 logos for each spleen are shown.
A preferential usage of IGHV1-69 (9/16) was observed in the group of related sequences from MZ B cells. Sequence groups using the IGHV1-69 gene and displaying very similar VH CDR3 were observed in different spleen samples. Indeed, two groups of 11 and 4 such sequences were observed in spleen 1 and 4, respectively (Figure 5B). Each clonal family was characterized by spleen-specific somatic mutations, as evidenced by aa replacements Q→R at codon 65 in spleen 1 and Y→C at codon 32 in spleen 4. However, common aa replacements between the two spleens also were observed (that is, S→N at codon 31; I→T at codon 52; S→T at codon 77; Figure 5B). Notably, the VH CDR3s not only presented >60% homology, but they had very similar motifs. For example, the first amino acids (Gly G107, Pro P108) of the N1 region at the IGHV-IGHD junction were highly conserved in the two spleens (Figure 5B).
Finally, in view of the predominant IGHV1-69 gene utilization by clonally related families from MZ B cells, we used a clone-specific qRT-PCR methodology and IGHV1-69–specific PCR to search for these sequences in GC and FM B cells in two different spleens (see the supplementary materials). No clone-specific amplification was observed in GC and in FM B-cell subsets (Supplementary Figure S2) by qRT-PCR. However, by using a nested IGHV1-69–specific PCR, few clones related to MZ were found in GC and FM; even no progenitor/effector relationship could be established (supplementary materials and Supplementary Table S2 and Figure S3).
AID Expression by MZ B Cells
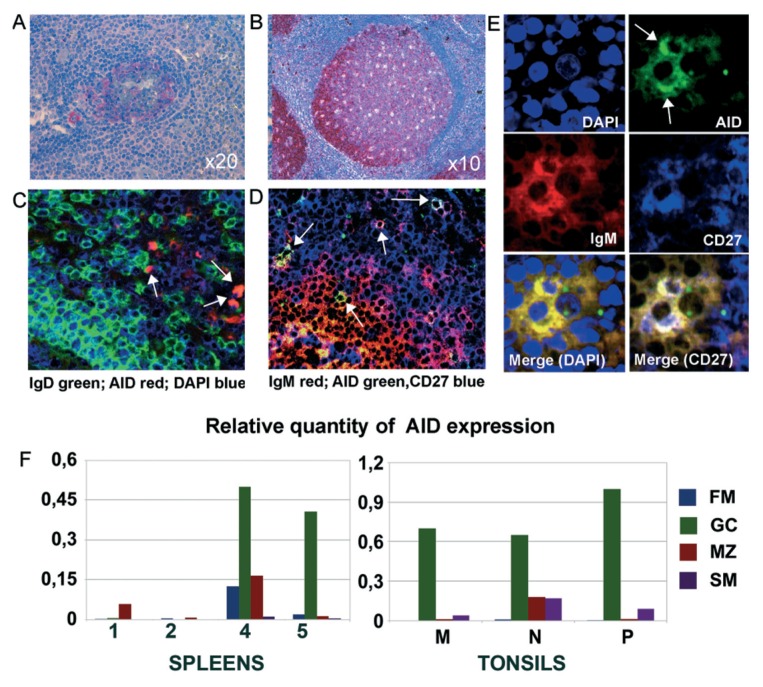
SHM depends on the expression and activity of AID. On the basis of the observation of the ongoing SHM in certain MZ B cell clones in situ, a positivity for AID had to be expected in a number of MZ B cells. As shown in Figure 6A, AID positivity was observed by in situ staining in many GC B cells, albeit the staining intensity was much lower than that observed in tonsil sections (Figure 6B). Some AID+ cells were observed in the splenic MZ (Figure 6A). Sparse AID+ cells also were seen in the interfollicular and subepithelial areas of tonsils (Figure 6B). AID+ B cells in the splenic MZ were IgM+CD27+ (Figures 6D, E), whereas their IgD expression was low to negative (Figure 6C).
Figure 6.
AID expression in MZ B cells by in situ staining and qRT- PCR. (A) Paraffin sections of spleen and (B) tonsil stained for AID by immunohistochemistry (20×). Staining is very weak in spleen and much stronger in tonsil GC. Sparse AID+ cells are localized in the tonsil intrafollicular areas and in the splenic MZ. (C, D) Confocal microscopy view of frozen spleen sections from a different spleen stained for AID and the indicated Ig isotypes. (E) Higher magnification view of two AID+ MZ B cells stained as reported. (F) AID relative expression measured by qRT-PCR analysis in different B-cell subsets isolated from spleens and tonsils. Bars represent the relative quantity of AID normalized to POL2 calculated as 2−ΔCt.
We also determined the relative expression of AID by qRT-PCR in different splenic B-cell subsets. The same tonsil B-cell subsets (GC-, FM- and MZ-like B cells) were investigated for comparison. The relative expression level of AID was calculated, by normalizing AID versus POL2. The AID expression level in spleens was lower than in tonsils, with variability between samples. GC B cells from spleen 4 and 5 displayed the highest AID levels (Figure 6F). Moreover, a weakly expression of AID also was observed in the MZ from the spleens, in particular, from spleen 4 and 1. The latter results are perhaps consistent with the sequence data indicating a more robust ongoing intraclonal diversification in these spleens. Tonsil B-cell subsets displayed a more uniform AID distribution, with GC B cells exhibiting consistently the highest AID levels (Figure 6F).
Molecular Features of V(D)J Rearrangements from SM B Cells
cDNA from SM B cells and GC B cells (Figure 2) was amplified with IGHV1 or IGHV4 subgroup primers together with IGHG-specific primers and sequenced. Overall, 130 and 119 productive rearrangements from SM B cells and IgG-GC B cells, respectively, were investigated, and all of them exhibited SHM. The average mutation number was higher than in MZ B cells (24.92 and 20.67 for SM and IgG-GC B cells respectively, versus 11.76 for MZ B cells). Strikingly, the mean VH CDR3 length for SM and IgG-GC B cells was of 16.25 and 17.04 aa, respectively, unexpectedly higher than that of IgM-GC (14.74) and MZ (15.01) and similar to that of FM B cells (15.83). Recurrent sequences also were found, although most of the γ sequences were unique (84.9% and 76.27% in SM and IgG-GC, respectively), with evidence for antigen selection indicated by a CDR R/S ratio >3. Groups of related sequences also were found, that is, one group in SM B cells and three groups in the IgG-GC. They exhibited intraclonal diversification, but were not related to those detected in the other B-cell subsets.
DISCUSSION
In this study, we describe features of the IGHV gene repertoire of sIgM+ MZ B cells, which include evidence of in situ diversification and of Ag-driven stimulation/selection. The fluorescence-activated cell sorting procedure yielded an MZ B-cell subset that was homogeneous, with a CD1c+, CD21+, CD23− surface phenotype, typical of MZ B cells (8,10,14). Notably, unlike that observed for tonsil MZ-like B cells, IgM-only MZ B cells were very few in the spleen and could not be separated from the bulk of sIgMhighsIgDlow MZ B cells (26,27,38).
CD27 was not used for the purification of splenic MZ B cells. Although circulating sIgMhighsIgDlow B cells, which may be splenic MZ B-cell derivatives, express CD27 and exhibit SHM (2,27), it was still to be ascertained whether all splenic MZ B cells display SHM. Indeed, SHM was observed in most, but not all, of the MZ B cells, and a correlation could be established between CD27 expression and SHM. Because these CD27− cells did not express FcRL-4, which is a marker of CD27− memory B cells (3), it is likely that unmutated, CD27− MZ B cells were virgin B cells. However, a progenitor/effector relationship between unmutated and mutated MZ B cells based on similarities of IGHV gene structure could not be established.
SM B cells exhibited limited expansion/diversification compared with MZ B cells and a higher level of SHM, stronger evidence for antigen selection and substantially longer VH CDR3s. This last finding, which is somewhat striking, was confirmed by preliminary next-generation sequencing (NGS) tests (GS Junior; 454 sequencing; Roche), which will be the subject of a separate study, in two different spleens. The mean of VH CDR3 length of MZ B cells (14.87) was significantly lower than SM (15.35, p = 0.001) and IgG-GC (16.01, p < 0.0001) in 881, 1,150 and 3,553 sequences from MZ, SM and IgG-GC, respectively. This result seems to exclude a progenitor/effector relationship between the MZ and SM B-cell subsets. Collectively, the data suggest that the majority of SM B cells in spleen are post-GC B cells (21,39), although MZ B cells can undergo isotype switch in certain circumstances (31,40).
MZ B cells displayed similar IGHV gene usage compared with GC B cells. Notably, however, recurrent clonal sequences were found in the MZ B cells at a frequency of 31%, which is higher than that of GC B cells (22%) (Figure 3). Such recurrent sequences also were described in MZ-equivalent B cells from tonsils, indicating that MZ B cells are stimulated in situ(26). This concept also is reinforced by the studies on gDNA from MZ B cells (24). After expansion in lymphoid organs, MZ B cells may reach the circulation, because accumulation of related clones has been reported for the IgM+IgDlowCD27+ circulating B cells (41). However, the available data on these circulating clones are not sufficient to assess their relationships with resident B cells, although the impact of SHM is similar for both populations (2,27).
Some of the expanding clones in the splenic MZ exhibited diversification in the context of ongoing SHM, likely driven by antigen. The same expanding and diversifying clones were not observed or were detected in very low proportions in GC and FM B cells from the same spleens by using very sensitive PCR methodologies.
CONCLUSION
The data provide evidence for an ongoing SHM in the MZ B cells. This finding may be explained by the considerable expansion of the MZ in the spleens we studied that contrasted with the paucity and small size of GC at microscopical inspection (data not shown). In addition, maybe GC B cells are not rescued from apoptosis as effectively (42). Notably, through in situ microdissection tests, which will be the subject of a separate study, we confirmed the presence of an ongoing clonal diversification in the MZ. Further evidence in favor of an in situ diversification/selection of the MZ B cells is the observation of sequence restriction at certain positions (often junctional) of the VH CDR3 within the diversifying clonal families.
Finally, unlike that observed in tonsils (Figure 6) (43), GC B cells in the spleen exhibited relatively little AID expression consistent with the notion of a putatively weak antigenic stimulation. Relatively low and heterogeneous expression of AID by splenic GC B cells was also confirmed by qRT-PCR. It is of note that in situ staining demonstrated AID positivity in certain IgMhighIgDlowCD27+ cells in the MZ (Figure 6). The stained cells in the MZ were very few, similar to previous findings (44). Although these data have been used in the past to argue against the capacity of MZ B cells of undergoing SHM in situ and have raised considerable controversy (27,38,45), they could now be reevaluated in light of the sequence data. Considering only about 10% of recurrent MZ B cells exhibited intraclonal diversification, the expected rate of diversification could be likely ascribed to 1–2% of the total B cells residing in the MZ area (that is, MZ plus SM B cells) in accordance with the low percentage of AID+ cells in the MZ. Whether the induction of SHM in MZ B cells requires special antigenic stimuli and the assistance of particular accessory cells and/or of cytokines is open to speculation. In addition, it remains to be ascertained whether all the mutated IgMhigh-IgDlow MZ B cells are generated by B cells diversifying in situ or whether a proportion of them derive from B cells that exited from GC.
Shared sequences were observed in MZ B cells from two different spleens. These sequences, which could be defined as stereotyped according to a terminology used for lymphoproliferative disorders, were consistently found in samples analyzed in different tests at different times, thus ruling out PCR-related artifacts. Selective antigenic pressures underlying the emergence of stereotyped gene rearrangements have been proposed both in zebra fish and humans (46,47). Furthermore, stereotyped B cell receptors (BCRs) are frequent in clones from different patients with chronic lymphocytic leukemia (CLL) (30,48,49) and also mantle cell lymphoma (MCL) (34) and splenic marginal zone lymphoma (SMZL) (32,50), suggesting stimulation/selection by common antigens during lymphomagenesis. Notably, our preliminary observations indicate similarities between the IGHV region gene repertoire of normal splenic MZ cells and of SMZL, although the stereotyped IGHV sequences described in this study were not found in these lymphomas nor in the CLL (33) and MCL (34) clones.
Supplementary Material
ACKNOWLEDGMENTS
Grant support was provided by the Italian Association for Cancer Research (AIRC) “Special Program Molecular Clinical Oncology–5 per mille,” number 9980, 2010/15 (to M Colombo); by Compagnia di San Paolo 4824 SD/CV, 2007.2880 (to F Fais and G Cutrona); and by Cariplo Foundation, Milan, Italy (to K Stamatopoulos) and the ENosAI project (code 09SYN-13-880), cofunded by the European Union and the Hellenic General Secretariat for Research and Technology (to K Stamatopoulos).
The authors would like to thank Nicholas Chiorazzi for revising the manuscript. Special thanks go to Laura Veroni for excellent secretarial support.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Tangye SG, Liu Y-J, Aversa G, Phillips JH, de Vries JE. Identification of functional human splenic memory B cells by expression of CD148 and CD27. J Exp Med. 1998;188:1691–703. doi: 10.1084/jem.188.9.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–89. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehrhardt GR, et al. Expression of the immunoregulatory molecule FcRH4 defines a distinctive tissue-based population of memory B cells. J Exp Med. 2005;202:783–91. doi: 10.1084/jem.20050879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moir S, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205:1797–805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8:22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- 7.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–57. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 8.Martin F, Kearney JF. Marginal-zone B cells. Nat Rev Immunol. 2002;2:323–35. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 9.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–16. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 10.Pillai S, Cariappa A, Moran ST. Marginal zone B cells. Annu Rev Immunol. 2005;23:161. doi: 10.1146/annurev.immunol.23.021704.115728. [DOI] [PubMed] [Google Scholar]
- 11.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–29. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 12.Hardy RR. B-1 B cell development. J Immunol. 2006;177:2749–54. doi: 10.4049/jimmunol.177.5.2749. [DOI] [PubMed] [Google Scholar]
- 13.Cerutti A, Puga I, Cols M. New helping friends for B cells. Eur J Immunol. 2012;42:1956–68. doi: 10.1002/eji.201242594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Annu Rev Immunol. 2009;27:267–85. doi: 10.1146/annurev.immunol.021908.132607. [DOI] [PubMed] [Google Scholar]
- 15.Oliver AM, Martin F, Kearney JF. IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J Immunol. 1999;162:7198–207. [PubMed] [Google Scholar]
- 16.Pillai S, Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat Rev Immunol. 2009;9:767–77. doi: 10.1038/nri2656. [DOI] [PubMed] [Google Scholar]
- 17.Kruetzmann S, et al. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med. 2003;197:939–45. doi: 10.1084/jem.20022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Timens W, Boes A, Rozeboom-Uiterwijk T, Poppema S. Immaturity of the human splenic marginal zone in infancy: possible contribution to the deficient infant immune response. J Immunol. 1989;143:3200–6. [PubMed] [Google Scholar]
- 19.Dono M, et al. Subepithelial B cells in the human palatine tonsil. II. Functional characterization. Eur J Immunol. 1996;26:2043–9. doi: 10.1002/eji.1830260912. [DOI] [PubMed] [Google Scholar]
- 20.Reynaud CA, et al. IgM memory B cells: a mouse/human paradox. Cell Mol Life Sci. 2012;69:1625–34. doi: 10.1007/s00018-012-0971-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pascual V, et al. Analysis of somatic mutation in five B cell subsets of human tonsil. J Exp Med. 1994;180:329–39. doi: 10.1084/jem.180.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arpin C, Banchereau J, Liu YJ. Memory B cells are biased towards terminal differentiation: a strategy that may prevent repertoire freezing. J Exp Med. 1997;186:931–40. doi: 10.1084/jem.186.6.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunn-Walters DK, Isaacson PG, Spencer J. Analysis of mutations in immunoglobulin heavy chain variable region genes of microdissected marginal zone (MGZ) B cells suggests that the MGZ of human spleen is a reservoir of memory B cells. J Exp Med. 1995;182:559–66. doi: 10.1084/jem.182.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tierens A, Delabie J, Michiels L, Vandenberghe P, De Wolf-Peeters C. Marginal-zone B cells in the human lymph node and spleen show somatic hypermutations and display clonal expansion. Blood. 1999;93:226–34. [PubMed] [Google Scholar]
- 25.Weller S, et al. Somatic diversification in the absence of antigen-driven responses is the hallmark of the IgM+ IgD+ CD27+ B cell repertoire in infants. J Exp Med. 2008;205:1331–42. doi: 10.1084/jem.20071555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dono M, et al. Heterogeneity of tonsillar subepithelial B lymphocytes, the splenic marginal zone equivalents. J Immunol. 2000;164:5596–604. doi: 10.4049/jimmunol.164.11.5596. [DOI] [PubMed] [Google Scholar]
- 27.Weller S, et al. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104:3647–54. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man. Immunol Rev. 2004;197:179–91. doi: 10.1111/j.0105-2896.2004.0109.x. [DOI] [PubMed] [Google Scholar]
- 29.Dono M, et al. CD5+ B cells with the features of subepithelial B cells found in human tonsils. Eur J Immunol. 2007;37:2138–47. doi: 10.1002/eji.200636887. [DOI] [PubMed] [Google Scholar]
- 30.Fais F, et al. Chronic lymphocytic leukemia B cells express restricted sets of mutated and unmutated antigen receptors. J Clin Invest. 1998;102:1515–25. doi: 10.1172/JCI3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berkowska MA, et al. Human memory B cells originate from three distinct germinal center-dependent and -independent maturation pathways. Blood. 2011;118:2150–8. doi: 10.1182/blood-2011-04-345579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bikos V, et al. Over 30% of patients with splenic marginal zone lymphoma express the same immunoglobulin heavy variable gene: ontogenetic implications. Leukemia. 2012;26:1638–46. doi: 10.1038/leu.2012.3. [DOI] [PubMed] [Google Scholar]
- 33.Darzentas N, et al. A different ontogenesis for chronic lymphocytic leukemia cases carrying stereotyped antigen receptors: molecular and computational evidence. Leukemia. 2010;24:125–32. doi: 10.1038/leu.2009.186. [DOI] [PubMed] [Google Scholar]
- 34.Hadzidimitriou A, et al. Is there a role for antigen selection in mantle cell lymphoma? Immunogenetic support from a series of 807 cases. Blood. 2011;118:3088–95. doi: 10.1182/blood-2011-03-343434. [DOI] [PubMed] [Google Scholar]
- 35.Galibert L, et al. Negative selection of human germinal center B cells by prolonged BCR cross-linking. J Exp Med. 1996;183:2075–85. doi: 10.1084/jem.183.5.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Falini B, et al. Expression of the IRTA1 receptor identifies intraepithelial and subepithelial marginal zone B cells of the mucosa-associated lymphoid tissue (MALT) Blood. 2003;102:3684–92. doi: 10.1182/blood-2003-03-0750. [DOI] [PubMed] [Google Scholar]
- 37.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seifert M, Kuppers R. Molecular footprints of a germinal center derivation of human IgM+(IgD+)CD27+ B cells and the dynamics of memory B cell generation. J Exp Med. 2009;206:2659–69. doi: 10.1084/jem.20091087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu YJ, et al. Within germinal centers, isotype switching of immunoglobulin genes occurs after the onset of somatic mutation. Immunity. 1996;4:241–50. doi: 10.1016/s1074-7613(00)80432-x. [DOI] [PubMed] [Google Scholar]
- 40.Obukhanych TV, Nussenzweig MC. T-independent type II immune responses generate memory B cells. J Exp Med. 2006;203:305–10. doi: 10.1084/jem.20052036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu YC, et al. High-throughput immunoglobulin repertoire analysis distinguishes between human IgM memory and switched memory B-cell populations. Blood. 2010;116:1070–8. doi: 10.1182/blood-2010-03-275859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cutrona G, et al. The propensity to apoptosis of centrocytes and centroblasts correlates with elevated levels of intracellular myc protein. Eur J Immunol. 1997;27:234–8. doi: 10.1002/eji.1830270135. [DOI] [PubMed] [Google Scholar]
- 43.Cattoretti G, et al. Nuclear and cytoplasmic AID in extrafollicular and germinal center B cells. Blood. 2006;107:3967–75. doi: 10.1182/blood-2005-10-4170. [DOI] [PubMed] [Google Scholar]
- 44.Willenbrock K, Jungnickel B, Hansmann ML, Kuppers R. Human splenic marginal zone B cells lack expression of activation-induced cytidine deaminase. Eur J Immunol. 2005;35:3002–7. doi: 10.1002/eji.200535134. [DOI] [PubMed] [Google Scholar]
- 45.Puga I, et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol. 2012;13:170–80. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weinstein JA, Jiang N, White RA, 3rd, Fisher DS, Quake SR. High-throughput sequencing of the zebrafish antibody repertoire. Science. 2009;324:807–10. doi: 10.1126/science.1170020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Briney BS, Willis JR, McKinney BA, Crowe JE., Jr High-throughput antibody sequencing reveals genetic evidence of global regulation of the naive and memory repertoires that extends across individuals. Genes and immunity. 2012;13:469–73. doi: 10.1038/gene.2012.20. [DOI] [PubMed] [Google Scholar]
- 48.Messmer BT, et al. Multiple distinct sets of stereotyped antigen receptors indicate a role for antigen in promoting chronic lymphocytic leukemia. J Exp Med. 2004;200:519–25. doi: 10.1084/jem.20040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stamatopoulos K, et al. Over 20% of patients with chronic lymphocytic leukemia carry stereotyped receptors: pathogenetic implications and clinical correlations. Blood. 2007;109:259–70. doi: 10.1182/blood-2006-03-012948. [DOI] [PubMed] [Google Scholar]
- 50.Zibellini S, et al. Stereotyped patterns of B-cell receptor in splenic marginal zone lymphoma. Haematologica. 2010;95:1792–6. doi: 10.3324/haematol.2010.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.