Abstract
Telomere erosion causes cell mortality, suggesting that longer telomeres enable more cell divisions. In telomerase-positive human cancer cells, however, telomeres are often kept shorter than those of surrounding normal tissues. Recently, we showed that cancer cell telomere elongation represses innate immune genes and promotes their differentiation in vivo. This implies that short telomeres contribute to cancer malignancy, but it is unclear how such genetic repression is caused by elongated telomeres. Here, we report that telomeric repeat-containing RNA (TERRA) induces a genome-wide alteration of gene expression in telomere-elongated cancer cells. Using three different cell lines, we found that telomere elongation up-regulates TERRA signal and down-regulates innate immune genes such as STAT1, ISG15 and OAS3 in vivo. Ectopic TERRA oligonucleotides repressed these genes even in cells with short telomeres under three-dimensional culture conditions. This appeared to occur from the action of G-quadruplexes (G4) in TERRA, because control oligonucleotides had no effect and a nontelomeric G4-forming oligonucleotide phenocopied the TERRA oligonucleotide. Telomere elongation and G4-forming oligonucleotides showed similar gene expression signatures. Most of the commonly suppressed genes were involved in the innate immune system and were up-regulated in various cancers. We propose that TERRA G4 counteracts cancer malignancy by suppressing innate immune genes.
INTRODUCTION
Telomeres are specialized structures at the ends of eukaryotic linear chromosomes that protect these ends from DNA nucleases and DNA double-strand break repair by homologous recombination and nonhomologous end-joining (1). They consist of many tandem repeats of guanine-rich (G-rich) motifs, such as the hexameric repeats of TTAGGG/CCCTAA in vertebrates (1).
Telomeres do not encode a protein and are considered to be transcriptionally silent. However, transcription of these regions, which gives rise to the G-rich long noncoding RNA known as telomeric repeat-containing RNA (TERRA), has been reported (2). TERRA transcripts were shown to interact with telomerase through their complementary sequences to the template of human telomerase RNA (hTR/hTERC), and TERRA-mimicking oligonucleotides inhibit telomerase activity in vitro (3). TERRA has been proposed to repress its own transcription through regulating the structural maintenance of telomeres and heterochromatin formation at telomeres (4). While several functions have been reported for TERRA, its biological significance remains elusive.
The telomere G-rich strand runs 5′ to 3′ toward the distal ends of chromosomes. Such G-rich single-stranded DNA/RNA sequences, including telomeric sequences and TERRA, self-associate in vitro to form four-stranded structures called G-quadruplexes (G4), which are noncanonical nucleic acid secondary structures (1,5). These structures are thought to influence replication and transcription by interrupting the action of DNA/RNA polymerases (5). Accumulating evidence for the in vivo formation of G4 structures also suggests that they have as yet unidentified physiological importance (5,6).
The human telomere length ranges from 5 to 10 kb in somatic cells and 15 to 20 kb in germ cells. Because DNA polymerases cannot begin synthesis de novo, some portions of telomeres are lost during the DNA replication of each cell cycle. This is known as the end replication problem. Eventually, critically shortened telomeres fail to protect chromosomal ends against the DNA damage response, resulting in cellular senescence or apoptosis (7,8). To overcome this, most human cancer cells (80–90%) activate telomerase (9) to elongate their telomeres (10). Thus, longer telomeres confer immortality to cancer cells. However, cancer cells often maintain shortened telomeres even after telomerase reactivation (7,11,12). Recently, we addressed this phenomenon and demonstrated that telomere elongation affects transcription in a genome-wide manner and promotes cancer cell differentiation in vivo (13). Gene ontology analysis revealed that telomere length significantly enriched those transcripts that coded for proteins involved in the immune response. Moreover, according to the Oncomine® database (www.oncomine.org), expression of these genes is up-regulated in several cancers (13), while most were suppressed in telomere-elongated (i.e. well-differentiated) cancer cells compared with parental cells (13). Well-differentiated cancers often have a better prognosis in clinical settings, which at least partially explains why human cancer cells keep telomeres short. Cancer cells with short telomeres might induce immune response genes to remain undifferentiated and therefore enhance tumor malignancy. In this scenario, longer telomeres would suppress those gene expressions and alleviate the malignancy of the cancer. However, it remains unclear how long telomeres that do not encode proteins regulate a genome-wide alteration of gene expression.
In this study, we show that telomere elongation in human cancer cells upregulates TERRA signal detected by northern blot analysis, while telomeric RNA/G4-forming sequences suppress innate immune gene expression in three-dimensional (3D) culture conditions. Based on these observations, we propose that one of the physiological roles of TERRA is to regulate gene expression in a genome-wide manner and epigenetically modulate the cellular phenotype.
MATERIALS AND METHODS
Cell culture, retroviral transduction and viral infection
Cells were maintained in Dulbecco's modified Eagle's medium (DMEM, Nacalai Tesque, Kyoto, Japan) containing 10% heat-inactivated fetal bovine serum (FBS) and 0.1 mg/ml kanamycin. Retroviral infection was performed essentially as previously described (13). Briefly, retroviral supernatants were prepared by the transient transfection of GP2-293 cells with control pLNCX2 (Clontech, Palo Alto, CA, USA) or a series of human telomerase reverse transcriptase (hTERT) vectors with a pVSV-G packaging vector encoding the viral envelope protein (Clontech). Human prostate cancer PC-3, breast cancer HBC4 and gastric cancer MKN74 cells were infected with the retroviral supernatant in the presence of 8 μg/ml polybrene. Infected cells were selected with 400 μg/ml of G418. After positive antibiotic selection, overexpression of exogenous hTERT was verified using western blot analysis and the telomeric repeat amplification protocol (TRAP) assay as detailed below. To excise the exogenous hTERT, PC-3/mock and PC-3/LhTERTL cells (at population doubling [PD] 40) were infected with a recombinant adenovirus, AxCANCre (TaKaRa, Kyoto, Japan) (14), at a multiplicity of infection of 40. After viral adsorption for 60 min, cells were extensively washed with DMEM containing 10% FBS. We verified the identities of cell lines used in this study by DNA fingerprint analysis of short tandem repeat loci. Test of the mycoplasma contamination by the PCR method showed negative in all established cell lines.
Western blot analysis
Cell lysates were prepared and western blot analysis performed as previously described (13) with the following primary antibodies: rabbit anti-hTERT (1531-1, 1:1,000; Epitomics Inc. Cambridge, UK) or mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 10R-G109a, 1.0 μg/ml; Fitzgerald Industries, Sudbury, MA, USA).
Telomere Southern blot analysis and telomerase assay
Terminal restriction fragments were detected using Southern blot analysis with a 32P-labeled (CCCTAA)n probe, as previously described (15). Telomerase activity was detected using the TRAP assay (9). The telomeric products were separated using Tris-borate-EDTA polyacrylamide gel electrophoresis and visualized by staining with SYBR green (TaKaRa).
Generation of subcutaneous xenografts in nude mice
Animal experiments were approved by the Institutional Animal Care and Use Committee of the Japanese Foundation for Cancer Research and conducted in accordance with institutional guidelines. Cells were mechanically dissociated by trypsinization to obtain single-cell suspensions and were diluted in Hanks’ balanced salt solution (Gibco, Life Technologies, Paisley, UK). Then, 1 × 106 cells were subcutaneously injected into 5-week-old female nude mice with a BALB/c genetic background (Charles River Laboratories Japan, Inc., Yokohama, Japan). When the tumor reached at least 5 mm in diameter, mice were euthanized and the tumor tissue was collected.
RNA extraction and northern blot analysis
RNA extraction from cell cultures and tumor xenografts was performed using the RNeasy kit and TissueLyser (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Northern blot analysis was performed essentially as previously described (2). Briefly, RNA (1–4 μg) was denatured with formaldehyde load dye (Ambion, Austin, TX, USA) for 15 min at 96°C, then loaded onto 1.0% formaldehyde agarose gels and separated by electrophoresis. 32P-labeled telomeric and β-actin probes were synthesized from pSP73-Sty11 (15) and the β-actin-human antisense control template (Wako, Osaka, Japan) respectively, using the random primed DNA labeling kit (Roche Applied Science, Basel, Switzerland). For control RNase treatments, samples were incubated for 15 min at 96°C then 18 h at 37°C with 1 mg/ml RNase A (Qiagen).
Quantitative real-time polymerase chain reaction (PCR)
Gene transcripts were detected by quantitative real-time PCR (qPCR) analysis with a LightCycler® 480 real-time PCR system (Roche Applied Science). The amplification conditions used were an initial denaturation at 95°C for 5 min, followed by 45 cycles of denaturation at 95°C for 10 s, annealing at 60°C for 30 s and extension at 72°C for 1 s. Amplification was monitored using Universal ProbeLibrary probes, which were labeled with fluorescein as described in the manufacturer's instructions (Roche Applied Science). All primers and probes for each target gene were taken from the Universal ProbeLibrary Assay Design Center (https://qpcr.probefinder.com/organism.jsp).
Oligonucleotides and circular dichroism spectra
Synthetic telomeric RNA, r(UUAGGGUUAGGGUUAGGGUUAGGG), control RNA, r(UUACCCUUACCCUUACCCUUACCC), telomeric DNA d(TTAGGG), d(TTAGGG)2, d(TTAGGG)4, d(TTAGGG)6, d(TTAGGG)25, control DNA, d(TTACCC)4, d(TTACCC)25, and AS1411, d(GGTGGTGGTGGTTGTGGTGGTGGTGG) (16), were purchased from Greiner Bio-One Co., Ltd. (Tokyo, Japan). Synthetic Cy3-labeled telomeric RNA Cy3-r(UUAGGGUUAGGGUUAGGGUUAGGG), control RNA, Cy3-r(UUACCCUUACCCUUACCCUUACCC), were also purchased from Greiner Bio-One Co., Ltd. Solutions of 150 mM KCl mixed with each oligonucleotide were heated to 95°C for 5 min, cooled slowly at 1°C/min to room temperature and stored at 4°C before use. Circular dichroism (CD) spectra were obtained from three scans on a J-820 spectropolarimeter (Jasco, Tokyo, Japan), with a 2-nm bandwidth, 100-nm/min scan speed and a 0.1-nm step resolution. The CD spectra were measured in a nitrogen environment over 200–320 nm to ascertain the formation of G4 structures in telomeric RNA and AS1411.
Oligonucleotide transfection
Lipofectamine® RNAiMAX (Invitrogen) was used in reverse transfection experiments to transfect 1.0 μg (approximately 100 pmol per well) of the oligonucleotides described above into 0.8 × 105 cells (two-dimensional, 2D culture) or 3.0 × 105 cells (3D culture) as described in the manufacturer's instructions (Invitrogen). Cells were then incubated in a 12-well flat bottom plate (2D culture, Iwaki, AGC Techno Glass, Shizuoka, Japan.) or a 24-well EZ-BindShut II plate (3D culture, Iwaki) in 1 ml DMEM containing 10% heat-inactivated FBS and harvested 72 h after transfection.
Fluorescence in situ hybridization
Cells were fixed with 2% paraformaldehyde phosphate-buffered saline for 10 min and permeabilized with 0.5% Nonidet P-40. Fluorescence in situ hybridization (FISH) analysis with a Cy3-labeled telomere-specific (CCCTAA)3 peptide nucleic acid probe (Greiner Bio-One) was performed essentially as previously described (17).
Microarray and bioinformatic analyses
Total RNA was quantified using the Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA), then labeled and hybridized onto the GeneChip Human Genome U133 Plus 2.0 array (Affymetrix, Santa Clara, CA) according to the manufacturer's instructions. Data normalization, statistical analysis, gene ontology analysis and genome browsing were performed using GeneSpring GX software (Agilent Technologies). All samples were summarized by the Robust Multichip Analysis (RMA) normalization method. The upregulated and downregulated probe set identifiers were used as the input and enrichment was analyzed separately. The results showed significant enrichment using Fisher's exact test, and multiple testing correction was performed using the Benjamini-Hochberg False Discovery Rate method. The P-value for each GO term reflects the enrichment in frequency of that GO term in the input probe list relative to all probes in background list.
Data-mining
Expression of G4-induced suppression signature (G4SS) genes in various human cancer was queried using the Oncomine database (http://www.oncomine.org) in February 2014. This is a publicly available database summarizing microarray experiments across tissue types. The number of significant unique microarray results with changes >2.0-fold (P< 0.0001 by a two-tailed t-test) with increased (red) or decreased (blue) expression in tumors relative to normal tissues were used. The results were extracted directly from the Oncomine analysis and have not been repeated manually.
Data access
The microarray data reported in this study have been deposited in Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO Series accession number GSE56177 (telomere elongation in xenograft) and GSE56239 (G4-forming oligonucleotide in 3D).
RESULTS
Telomere elongation suppresses the up-regulation of innate immune genes in tumors
Previously, we demonstrated that the expression of many innate immune genes, such as STAT1, ISG15 and OAS3, was up-regulated in xenograft tumors formed after the subcutaneous injection of human prostate PC-3 cells into nude mice. Telomere elongation was found to suppress this up-regulation (13). Although long telomeres in yeast and human cells induce the so-called ‘telomere position effect’ (TPE), which results in transcriptional silencing of genes near telomeres (18–20), chromosomal loci of the innate immune genes affected by telomere elongation are randomly distributed along chromosome arms, and there is no preference for the distal ends of the chromosomes (13). Thus, TPE does not seem to account for the transcriptional suppression of innate immune genes in telomere-elongated PC-3 cells. We hypothesized that telomere length modulates gene expression in a TPE-independent manner.
First, we examined whether suppression of the innate immune genes in telomere-elongated PC-3 cells was reproducible in other cell lines. Therefore, we enhanced telomerase activity and elongated telomeres in three human cancer cells, PC-3, HBC4 and MKN74, all of which retained rather short telomeres (see mock in Figure 1A). Human telomerase is a ribonucleoprotein consisting of two essential components, hTR and hTERT (21). Expression of hTR is ubiquitous in human cells, and regulation of telomerase activity is controlled at the level of hTERT transcription (21). Therefore, we established sublines that overexpressed exogenous hTERT (PC-3/LhTERTL, HBC4/hTERT and MKN74/hTERT) (Figure 1A). The up-regulation of telomerase activity and substantial telomere elongation in these cells compared with control cells (PC-3/mock, HBC4/mock and MKN74/mock) was confirmed (Figure 1A). hTERT has been suggested to possess a nontelomeric function through a mechanism independent of telomerase activity (22,23). To distinguish the effect of elongated telomeres from that of hTERT overexpression, we established PC-3/LhTERTL/cre+ cells derived from PC-3/LhTERTL cells by removing the hTERT transgene after telomere elongation using the Cre/loxP system (13). The prominent difference between PC-3/mock/cre+ and PC-3/LhTERTL/cre+ cells is therefore the telomere length (Figure 1A), although we cannot exclude the possibility that hTERT overexpression could induce heritable chromatin changes elsewhere.
Figure 1.
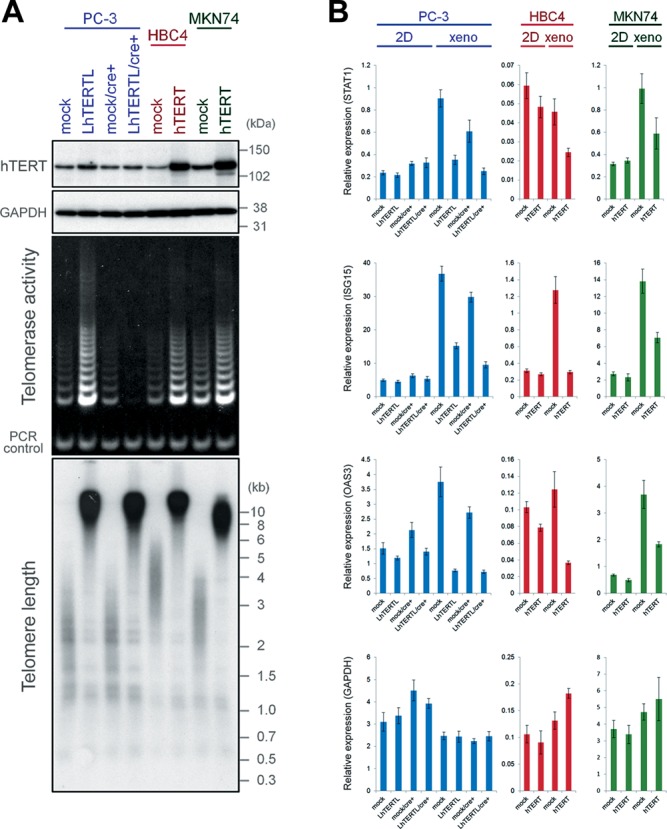
Telomere elongation suppresses the up-regulation of innate immune gene expression in in vivo tumors. (A) Retroviral overexpression of hTERT (upper panels) and up-regulation of telomerase activity (middle panel) at PD 50. Size markers are indicated on the right. After the introduction of Cre at PD 40, only endogenous hTERT and telomerase activities were detected in PC-3/LhTERTL/cre+ cells. Bottom panel: telomere Southern blot analysis at PD 77. PC-3/LhTERTL, HBC4/hTERT and MKN74/hTERT cells show elongated telomeres. PC-3/LhTERTL/cre+ cells also maintained long telomeres even after shutdown of the exogenous hTERT gene at PD 40. (B) Expression of STAT1, ISG15, OAS3 and GAPDH mRNA in telomere-elongated cancer cells in 2D dishes (2D) and xenografts (xeno) were analyzed by qPCR and normalized to GUSB mRNA levels. Error bars indicate standard deviations (n = 3).
To test whether the up-regulation of innate immune genes was suppressed in telomere-elongated cells, we designated STAT1, ISG15 and OAS3 as representative of the innate immune category genes. qPCR analysis confirmed that expression of these genes was up-regulated in vivo in xenograft tumors of PC-3, HBC4 and MKN74 cells, whereas telomere elongation substantially suppressed this up-regulation (Figure 1B), as observed previously in PC-3 cells (13). This effect of long telomeres was not observed in conventional adherent (2D) culture conditions (Figure 1B). As a control, GAPDH expression in both 2D culture and in vivo xenograft tumors was not substantially different in each sample (Figure 1B, bottom). These observations indicate that telomere elongation suppresses the up-regulation of specific innate immune genes in multiple human cancer cells in vivo.
Telomere elongation enhances the levels of TERRA signals in human cancer cells
Yehezkel et al. previously reported that the levels of TERRA are elevated in human induced pluripotent stem cells accompanying telomerase activation and telomere elongation (24). We therefore speculated that long telomeres might influence TERRA expression in cancer cells. To explore this possibility, we monitored TERRA expression in telomere-elongated cells by northern blotting analysis (Figure 2A). RNAs were extracted from cells cultured under conventional 2D conditions and from in vivo xenograft tumors, and telomere elongation was shown to up-regulate the intensities of TERRA signals in both 2D cultures and xenograft tumors (Figure 2A). The observed bands on the northern blots disappeared upon treatment with RNase A (Supplementary Figure S1), indicating that these signals were derived from RNAs rather than from contaminated DNAs. These results suggest that forced elongation of telomeres up-regulates the TERRA signals in multiple human cancer cell lines.
Figure 2.
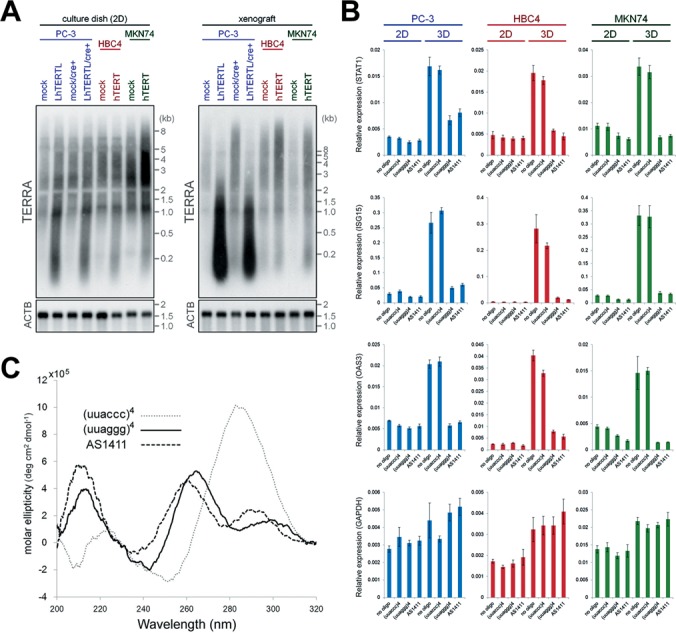
G4-forming nucleic acids suppress the up-regulation of innate immune gene expression in 3D cultures. (A) Northern blot analysis of RNAs from 2D cultures and xenograft tumors. Signals were detected with TERRA and beta-actin (ACTB) mRNA probes. (B) Relative expression levels of STAT1, ISG15, OAS3 and GAPDH mRNA in G4-forming oligonucleotide-transfected cells in conventional 2D dishes and 3D cultures were analyzed by qPCR and normalized to ACTB mRNA levels. Error bars indicate standard deviations (n = 3). (C) CD spectra of telomeric RNA, control RNA and AS1411 in 150 mM KCl solution.
Telomeric RNA suppresses innate immune gene expression in 3D culture conditions
We surmised that TERRA might play a key role in suppression of the innate immune gene expression in telomere-elongated cells. To test this hypothesis, we examined the effect of a synthetic 24mer telomeric RNA oligonucleotide consisting of the same sequence as TERRA (uuaggg repeats), using uuaccc repeats as a control RNA. We introduced these oligonucleotides into parental PC-3, HBC4 and MKN74 cells that retained short telomeres. We then examined the expression of the marker genes STAT1, ISG15, OAS3 and GAPDH as a control, by qPCR analysis after 72 h of oligonucleotide introduction. In these experiments, cells were incubated in either conventional 2D dishes or 3D culture plates, which more closely recapitulate the in vivo environment because cells are permitted to interact with their surroundings in all dimensions. In conventional 2D culture dishes, no difference was seen in the expression levels of the four genes in any cell lines (Figure 2B). However, in 3D culture plates, all of the three marker genes were up-regulated in the no oligonucleotide- or control RNA-transfected cells compared with those in 2D culture dishes (Figure 2B). Importantly, the up-regulation of these marker genes did not occur in telomeric RNA-transfected cells (Figure 2B). The oligonucleotides examined had no effect on GAPDH expression either in 2D or 3D culture conditions (Figure 2B, bottom panel). We evaluated that transfection efficiency of Cy3-labeled RNA oligonucleotides, and obtained similar results between telomeric and control oligonucleotides (Supplementary Figure S2A). These results demonstrate that transfection of telomeric RNA oligonucleotide suppresses the expression of specific genes in 3D culture conditions. Loss of the up-regulation of these genes in telomere-elongated cells under 3D culture conditions is reminiscent of that in xenograft tumors (Figure 1B). Telomere FISH analysis found no difference in the telomeric signal intensity between oligonucleotide-transfected and control cells (Supplementary Figure S2B), suggesting that telomeric RNA suppresses the expression of specific genes independently of the cellular telomere length.
G4-forming oligonucleotides suppress specific gene expression
To examine the length effect of telomeric repeats on the suppression of the marker genes above, we transfected PC-3 cells with various telomeric DNA oligonucleotides that have different numbers of telomere repeats (Supplementary Figure S3). First, similar suppression patterns were observed in four repeats (24mer) telomeric DNA and RNA oligonucleotide-transfected cells (Supplementary Figure S3A). Interestingly, one or two telomeric repeats (hexamer or 12mer) DNA oligonucleotides did not induce such gene suppression, whereas 6 and 25 telomeric repeats (36mer and 150mer) DNA oligonucleotides suppressed the gene expression in 3D culture conditions (Supplementary Figure S3B and SC). Since the suppressive effect of the oligonucleotides corresponded to the ability to form G4(s) within a molecule, these results imply that telomeric oligonucleotide-induced gene suppression is caused by G4.
To investigate the possibility that the G4 structure is involved in telomeric RNA-induced gene suppression, we used a nontelomeric, well-known G4-forming 26mer DNA AS1411 (formerly known as AGRO100) (16). AS1411 is a DNA aptamer that associates with nuclear factor-κB (NF-κB) essential modulator and inhibits NF-κB activation (16). It is currently undergoing phase II clinical trials for cancer treatment (25). Nuclear magnetic resonance studies have revealed that in potassium solution AS1411 forms G4 structures with several different topologies that mimic extracellular fluid (26). We measured the CD spectra of AS1411 and telomeric RNA oligonucleotides in 150 mM KCl solution to determine information about the effects of nucleotide sequences and different cations on G4 structures (Figure 2C). The waveforms of each oligonucleotide and peak were consistent with those in previous reports (26,27), suggesting that the oligonucleotides form G4 structures, at least in vitro. In 2D culture conditions, no difference was seen in the expression of the innate immune genes in oligonucleotide-introduced cells (Figure 2B). However, in 3D culture conditions, transfection of AS1411 into PC-3, HBC4 and MKN74 cells suppressed the upregulation of STAT1, ISG15 and OAS3, as seen with telomeric RNA (Figure 2B). By contrast, no difference was observed in GAPDH expression (Figure 2B, bottom). These results indicate that the observed gene suppression was induced by G4 secondary structures, regardless of their primary sequences or the sugar moieties present in the molecules (i.e. whether DNA or RNA). Moreover, the transfection of G4-forming oligonucleotides appeared to mimic the suppression of marker genes in in vivo xenografts of telomere-elongated cells (Figures 1B and 2B).
G4-forming oligonucleotides mimic the genome-wide alteration of gene expression profiles of TERRA-overexpressing cells in vivo
We next compared the gene expression profiles of TERRA-overexpressing cells in in vivo xenografts and G4-forming oligonucleotide-transfected cells in 3D culture conditions. First, we analyzed the similarity of altered gene expression patterns between two different G4-forming oligonucleotides. The comparison of telomeric RNA (versus control RNA) and AS1411 (versus no oligonucleotide) gene expression profiles identified several hundreds of probes with >2.0-fold expression changes in each of the three cancer cell lines (Figure 3A). Approximately half of those probes identified overlapped between telomeric RNA- and AS1411-transfected cells (199, 184 and 115 probes in PC-3, HBC4 and MKN74 cells, respectively). These findings strongly suggest that the genome-wide regulation of gene expression by G-rich oligonucleotides is mediated by G4 structures in the oligonucleotides.
Figure 3.
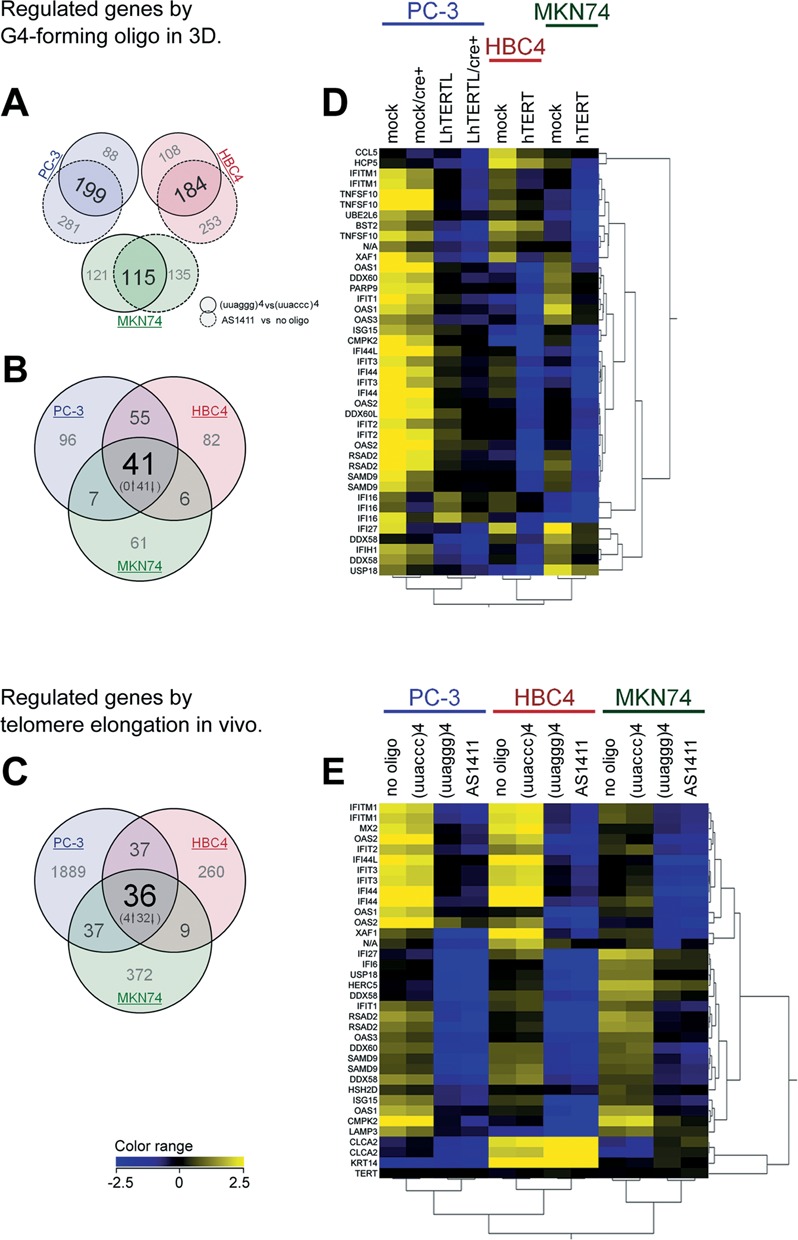
Comparison of gene expression profiles between G4-forming oligonucleotide-transfected cells in 3D cultures and telomere-elongated cells in in vivo xenografts. (A) Gene expression profiles were compared between telomeric RNA versus control RNA, as well as between AS1411 versus no oligonucleotide-transfected cells. With changes of >2.0-fold, the expression of 199 (PC-3), 184 (HBC4) and 115 (MKN74) probes was affected by both telomeric RNA and AS1411. (B) Identification of regulated gene sets in G4-forming oligonucleotide-transfected cells. With changes of >2.0-fold, the expression of 41 probes was affected in three cancer cell lines. (C) Identification of regulated gene sets in telomere-elongated cells. With changes of >2.0-fold, the expression of 36 probes was affected in three cancer cell lines. (D) Hierarchical cluster analysis (Pearson centered, average linkage) using 41 identified genes in G4-forming oligonucleotide-transfected cells (left) as shown in Figure 3B. Heat map colors indicate gene expression patterns in telomere-elongated (i.e. TERRA signal-enhanced) cells. (E) Hierarchical cluster analysis (Pearson centered, average linkage) using 41 identified genes in G4-forming oligonucleotide-transfected cells (left) as shown in Figure 3B. Heat map colors indicate gene expression patterns in G4-forming oligonucleotide-transfected cells.
We then compared the gene expression profiles of PC-3, HBC4 and MKN74 cells in both G4-forming oligonucleotide-transfected cells in 3D cultures (Figure 3B) and telomere-elongated cells overexpressing TERRA in in vivo xenografts (Figure 3C). We identified 41 probes (all down-regulated; Figure 3D) in G4-forming oligonucleotide-transfected cells, and 36 (two up-regulated probes and 34 down-regulated probes; Figure 3E) in telomere-elongated cells with >2.0-fold expression changes (Figure 3B and C). Most of the 41 probes identified in G4-forming oligonucleotide-transfected cells were also suppressed in telomere-elongated cells (Figure 3D, top of heat map). Similarly, most of the 36 probes identified in telomere-elongated cells were also suppressed by G4-forming oligonucleotides under 3D culture conditions (Figure 3E, top of heat map). These results indicate that the ectopic introduction of G4-forming oligonucleotides can mimic the genome-wide gene expression profiles of telomere-elongated cancer cells, which overexpress endogenous TERRA.
G4-induced suppression signature genes reside in innate immune system categories and are overexpressed in malignant tumors
We identified 26 probes that overlapped between the 41 probes identified in G4-forming oligonucleotide-transfected cells and 36 probes identified in telomere-elongated cells (Figure 4A). We hereafter refer to these probes (representing 18 genes) as ‘G4-induced suppression signature (G4SS) genes’. Gene ontology analysis revealed that the innate immune response-related categories were significantly enriched in the G4SS genes out a total of 54 675 probe sets (P < 1E-7 by Fisher's exact test; Figure 4B). These categories were also significantly enriched by any of the probe sets in each of the three G4-forming oligonucleotide-transfected cell lines (P< 1E-7 by Fisher's exact test, data not shown). Similarly, the top three categories, all related to type I interferon (GO:0034340, response to type I interferon; GO:0071357, cellular response to type I interferon and GO:0060337, type I interferon signaling pathway), and several other categories (GO:0009615 in PC-3, GO:0009615, GO:0045087, GO:0006955, GO:0006952 and GO:0002376 in HBC4, GO:0009615, GO:0045087, GO:0051707, GO:0009607, GO:0006955, GO:0019221, GO:0006952, and GO:0002376 and GO:0034097 in MKN74) were significantly enriched by any of the probe sets in each of the telomere-elongated cell lines line (P< 1E-7 by Fisher's exact test).
Figure 4.
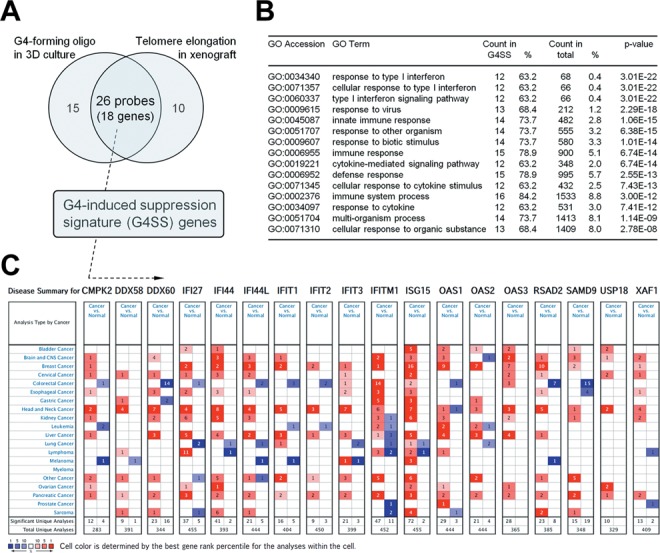
G4-induced suppression signature (G4SS) and its global association with human cancer. (A) Identification of G4SS genes affected by both G4-forming oligonucleotide transfection in 3D culture and telomere elongation in in vivo xenografts with expression changes >2.0-fold. (B) Gene ontology (GO) analysis of G4SS genes. Enriched GO categories were sorted by increasing P value (a total of 54 675 probe sets, P< 0.0001, Fisher's exact test). All enriched categories were related to the innate immune system. (C) Disease summary of 18 G4SS genes using the Oncomine database, which indicates the number of significant unique microarray results with changes of >2.0-fold (P< 1E-7 by the two-tailed t-test) with increased (red) or decreased (blue) expression in tumors relative to normal tissues.
According to the Oncomine database, most of the G4SS genes exhibited increased expression in various tumors compared with respective normal tissues (Figure 4C). This suggests that the G4SS genes may contribute to cancer malignancy in vivo, and that G4-forming sequences, including TERRA, have a negative effect on human cancer cells through the suppression of innate immune genes.
DISCUSSION
In the present study, we demonstrated that telomere elongation suppressed the induction of specific innate immune gene expression in multiple human cancer cells in vivo xenografts (Figure 1). We focused on TERRA and found that this telomeric noncoding RNA plays a key role in the phenomenon. First, telomere elongation was shown to enhance TERRA signals detected by northern blot analysis in three different cancer cell lines (Figures 1A and 2A). After Cre recombinase-mediated shutdown of the forced expression of hTERT, PC-3/LhTERTL/cre+ cells still exhibited the enhanced TERRA levels and suppressed innate immune gene expression (Figures 1 and 2A). Moreover, no alterations in hTERT expression were observed in G4-forming oligonucleotide-transfected cells (Figure 3E, bottom probe). These results suggest that specific gene suppression in vivo and increased TERRA levels are accompanied by telomere elongation in multiple cancer cells.
Telomeric RNA oligonucleotides suppressed the induction of STAT1, ISG15 and OAS3 expression in 3D culture conditions, mimicking the cellular environment of in vivo tumors (Figure 2B). Because this suppression was not observed in conventional 2D culture dishes, we speculated that the tumor microenvironment is an important factor for telomeric RNA-mediated gene suppression. It is possible that control RNA (uuaccc)4 oligonucleotide hybridizes to the endogenous TERRA. However, the telomeric cytosine-rich strand sequence (i.e. complementary sequence of TERRA) is (uaaccc)n, 17% mismatch to the control RNA. Furthermore, TERRA can self-assemble to form four-stranded G-quadruplexes, which would interrupt intermolecular hybridization with this control oligonucleotide. Thus we consider that the control RNA (uuaccc)4 oligonucleotide has only a negligible effect, if any, on endogenous TERRA. Indeed, no differences were observed in the innate immune gene expression between control RNA and no oligonucleotide-transfected cells in 3D culture conditions (Figure 2B).
Strikingly, this suppression was similarly induced by the nontelomeric G-rich DNA aptamer AS1411. CD spectra of telomeric RNA and AS1411 indicated that both oligonucleotides form G4 structures at least in vitro, which appear to suppress the induction of specific innate immune genes in cancer cells. Again, we speculate that the enhanced levels of TERRA cause this suppression in telomere-elongated cells in vivo. Although the longest telomeric oligonucleotide examined in this study is 150mer, still shorter than the endogenous TERRA, the former mimics the latter in terms of its ability to form multiple (up to six) G4s within a molecule.
Genome-wide microarray analyses showed that G4-forming oligonucleotides induced specific and very similar expression patterns to those in telomere-elongated and TERRA-overexpressing cancer cells (Figure 3). A common property of these nucleotides is their single-stranded state and the presence of tandem arrays of G4-forming sequences. Because the transcriptional alteration in G4-forming oligonucleotide-transfected cells was induced without telomere elongation, we reasoned that TERRA could play a key role in the alteration of genome-wide gene expression in telomere-elongated cells through its G4 secondary structure.
Nucleic acids often influence cellular gene expression and innate immune systems. One example is the RNA interference or small interfering RNA machinery. Unmethylated CpG DNA in the bacterial genome can be recognized by Toll-like receptor (TLR) 9, which in turn activates genes of the innate immune system (28,29). Similarly, foreign viral RNA can stimulate innate immune responses through TLR3, TLR7, or RIG-I-like receptors (28). In this study, three cancer cell lines upregulated the innate immune genes without any nucleic acids in 3D culture conditions (Figure 2B). Thus, this does not seem to be the classical antiviral-type response to nucleic acids. Furthermore, our GeneChip microarray (Figure 3 and (13)) and RT-qPCR (Figure 2B) analyses clearly indicate that the oligonucleotides per se do not upregulate the innate immune genes in 2D culture conditions. Meanwhile, the telomere RNA and AS1411 DNA oligonucleotides inhibited (not enhanced) the upregulation of those innate immune genes (Figures 2B, 3 and 4): this is reminiscent to that telomeric repeats have ability to downregulate the bacterial CpG-induced immune activation, where the suppression correlates with G4 formation (29). Therefore, although the stimuli in 3D culture conditions are different from bacteria or viruses, they may share the same activating pathway, which is downregulated by the G4-forming oligonucleotides. Although the underlying mechanism has not yet been addressed, Tseng et al. demonstrated by in-cell optical imaging that exogenous G4-forming oligonucleotides, including AS1411, localize mainly at the lysosomes and mitochondria (6). Based on these observations, we propose that the G4-forming sequence-mediated regulation of transcription does not result from a direct effect on nuclear genomes but occurs through G4 recognizing proteins such as nucleolin (AS1411 putative target) (16,25) or TLS/FUS (TERRA putative target) (30).
ISG15, one of the three representative marker genes used in this study, is located near the p-arm telomere of chromosome 1 (1p36.33). The ISG15 gene is the first human endogenous gene that has been identified whose expression is inversely correlated with telomere length (31). Since the expression of genes located between ISG15 locus and its distal telomere was not altered by telomere length, it seems that the classical TPE is not involved in the regulation of ISG15 expression (13,31). Recently, Robin et al. reported a novel TPE mechanism, called ‘TPE over long distances’, under which telomere length influences the gene expression through the formation of chromatin loops (32). 3D-FISH demonstrated that ISG15 gene and the telomeric end of the same chromosome arm are adjacent in cells with long telomeres and separated in cells with short telomeres (32). The G4-forming sequences, including TERRA, may regulate the expression of ISG15 (and many other genes, such as G4SS genes) through the formation of chromatin loop structure.
Our meta-analysis using the Oncomine database revealed that most of the G4SS genes were overexpressed in various malignant tumors (Figure 4C). These findings suggest that G4-forming sequences may possess some tumor suppressive potential. Indeed, our previous studies showed that telomere elongation induces cancer cell differentiation in vivo (13). In clinical settings, well-differentiated tumors often have a good prognosis. Moreover, AS1411 is the first oligodeoxynucleotide aptamer to reach phase II clinical trials for treatment of relapsed or refractory acute myeloid leukemia, and for renal cell carcinoma (25).
Because of its ability to form noncanonical four-stranded structures, G4-forming motifs on nuclear double-stranded genomic sequences are thought to inhibit DNA replication by interrupting DNA polymerases, and to regulate transcription by switching RNA polymerases (5). Meanwhile, our findings demonstrate that TERRA, single-stranded endogenous transcripts and exogenous G4-forming oligonucleotides alter specific gene expression at distinct intracellular sites from the nuclear genome in human cancer cells in vivo and in 3D culture conditions. These results indicate that single-stranded RNAs containing the G4-forming sequence may have physiological significance. In particular, the G4-forming sequence is highly conserved among most eukaryotic organisms, while TERRA (and its template telomeres) repeat sequences can form many G4 structures. In conclusion, we propose a new model for the role of G4-forming RNAs, including TERRA, that influence eukaryotic cellular behaviors through the regulation of gene expression.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
Acknowledgments
We thank Takashi Tsuruo, Mitsuaki Yoshida and Haruo Sugano for critical comments and discussions, Sachiko Okabe and Yukiko Muramatsu for maintenance of cell lines and authentication, and Keisuke Iida and Takahiro Nakamura for technical advice on CD spectra.
FUNDING
Grant-in-Aid for Challenging Exploratory Research, Japan Society for the Promotion of Science (JSPS) [25640071 to K.H., 24650639 to H.S.]; Grant-in-Aid for Scientific Research (B), JSPS [25290060 to H.S.]. Funding for open access charge: Grant-in-Aid for Challenging Exploratory Research, Japan Society for the Promotion of Science (JSPS) [25640071 to K.H., 24650639 to H.S.]; Grant-in-Aid for Scientific Research (B), JSPS [25290060 to H.S.].
Conflict of interest statement. None declared.
REFERENCES
- 1.Blackburn E.H. Telomeres: structure and synthesis. J. Biol. Chem. 1990;265:5919–5921. [PubMed] [Google Scholar]
- 2.Azzalin C.M., Reichenbach P., Khoriauli L., Giulotto E., Lingner J. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science. 2007;318:798–801. doi: 10.1126/science.1147182. [DOI] [PubMed] [Google Scholar]
- 3.Redon S., Reichenbach P., Lingner J. The non-coding RNA TERRA is a natural ligand and direct inhibitor of human telomerase. Nucleic Acids Res. 2010;38:5797–5806. doi: 10.1093/nar/gkq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnoult N., Van Beneden A., Decottignies A. Telomere length regulates TERRA levels through increased trimethylation of telomeric H3K9 and HP1α. Nat. Struct. Mol. Biol. 2012;19:948–956. doi: 10.1038/nsmb.2364. [DOI] [PubMed] [Google Scholar]
- 5.Lipps H.J., Rhodes D. G-quadruplex structures: in vivo evidence and function. Trends Cell Biol. 2009;19:414–422. doi: 10.1016/j.tcb.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Tseng T.Y., Wang Z.F., Chien C.H., Chang T.C. In-cell optical imaging of exogenous G-quadruplex DNA by fluorogenic ligands. Nucleic Acids Res. 2013;41:10605–10618. doi: 10.1093/nar/gkt814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maser R.S., DePinho R.A. Connecting chromosomes, crisis, and cancer. Science. 2002;297:565–569. doi: 10.1126/science.297.5581.565. [DOI] [PubMed] [Google Scholar]
- 8.d'Adda di Fagagna F., Reaper P.M., Clay-Farrace L., Fiegler H., Carr P., Von Zglinicki T., Saretzki G., Carter N.P., Jackson S.P. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 9.Kim N.W., Piatyszek M.A., Prowse K.R., Harley C.B., West M.D., Ho P.L., Coviello G.M., Wright W.E., Weinrich S.L., Shay J.W. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 10.Bodnar A.G., Ouellette M., Frolkis M., Holt S.E., Chiu C.P., Morin G.B., Harley C.B., Shay J.W., Lichtsteiner S., Wright W.E. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 11.Meeker A.K., Hicks J.L., Platz E.A., March G.E., Bennett C.J., Delannoy M.J., De Marzo A.M. Telomere shortening is an early somatic DNA alteration in human prostate tumorigenesis. Cancer Res. 2002;62:6405–6409. [PubMed] [Google Scholar]
- 12.Sommerfeld H.J., Meeker A.K., Piatyszek M.A., Bova G.S., Shay J.W., Coffey D.S. Telomerase activity: a prevalent marker of malignant human prostate tissue. Cancer Res. 1996;56:218–222. [PubMed] [Google Scholar]
- 13.Hirashima K., Migita T., Sato S., Muramatsu Y., Ishikawa Y., Seimiya H. Telomere length influences cancer cell differentiation in vivo. Mol. Cell. Biol. 2013;33:2988–2995. doi: 10.1128/MCB.00136-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka M., Kagawa H., Yamanashi Y., Sata T., Kawaguchi Y. Construction of an excisable bacterial artificial chromosome containing a full-length infectious clone of herpes simplex virus type 1: viruses reconstituted from the clone exhibit wild-type properties in vitro and in vivo. J. Virol. 2003;77:1382–1391. doi: 10.1128/JVI.77.2.1382-1391.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Lange T. Human telomeres are attached to the nuclear matrix. EMBO J. 1992;11:717–724. doi: 10.1002/j.1460-2075.1992.tb05104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girvan A.C., Teng Y., Casson L.K., Thomas S.D., Ju"liger S., Ball M.W., Klein J.B., Pierce W.M., Barve S.S., Bates P.J. AGRO100 inhibits activation of nuclear factor-kappaB (NF-kappaB) by forming a complex with NF-kappaB essential modulator (NEMO) and nucleolin. Mol. Cancer. Ther. 2006;5:1790–1799. doi: 10.1158/1535-7163.MCT-05-0361. [DOI] [PubMed] [Google Scholar]
- 17.Meeker A.K., Gage W.R., Hicks J.L., Simon I., Coffman J.R., Platz E.A., March G.E., De Marzo A.M. Telomere length assessment in human archival tissues: combined telomere fluorescence in situ hybridization and immunostaining. Am. J. Pathol. 2002;160:1259–1268. doi: 10.1016/S0002-9440(10)62553-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baur J.A., Zou Y., Shay J.W., Wright W.E. Telomere position effect in human cells. Science. 2001;292:2075–2077. doi: 10.1126/science.1062329. [DOI] [PubMed] [Google Scholar]
- 19.Tennen R.I., Bua D.J., Wright W.E., Chua K.F. SIRT6 is required for maintenance of telomere position effect in human cells. Nat. Commun. 2011;2:433. doi: 10.1038/ncomms1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stadler G., Rahimov F., King O.D., Chen J.C., Robin J.D., Wagner K.R., Shay J.W., Emerson C.P., Wright W.E. Telomere position effect regulates DUX4 in human facioscapulohumeral muscular dystrophy. Nat. Struct. Mol. Biol. 2013;20:671–678. doi: 10.1038/nsmb.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nugent C.I., Lundblad V. The telomerase reverse transcriptase: components and regulation. Genes Dev. 1998;12:1073–1085. doi: 10.1101/gad.12.8.1073. [DOI] [PubMed] [Google Scholar]
- 22.Choi J., Southworth L.K., Sarin K.Y., Venteicher A.S., Ma W., Chang W., Cheung P., Jun S., Artandi M.K., Shah N., et al. TERT promotes epithelial proliferation through transcriptional control of a Myc- and Wnt-related developmental program. PLoS Genet. 2008;4:e10. doi: 10.1371/journal.pgen.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maida Y., Yasukawa M., Furuuchi M., Lassmann T., Possemato R., Okamoto N., Kasim V., Hayashizaki Y., Hahn W.C., Masutomi K. An RNA-dependent RNA polymerase formed by TERT and the RMRP RNA. Nature. 2009;461:230–235. doi: 10.1038/nature08283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yehezkel S., Rebibo-Sabbah A., Segev Y., Tzukerman M., Shaked R., Huber I., Gepstein L., Skorecki K., Selig S. Reprogramming of telomeric regions during the generation of human induced pluripotent stem cells and subsequent differentiation into fibroblast-like derivatives. Epigenetics. 2011;6:63–75. doi: 10.4161/epi.6.1.13390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reyes-Reyes E.M., Teng Y., Bates P.J. A new paradigm for aptamer therapeutic AS1411 action: uptake by macropinocytosis and its stimulation by a nucleolin-dependent mechanism. Cancer Res. 2010;70:8617–8629. doi: 10.1158/0008-5472.CAN-10-0920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dailey M.M., Miller M.C., Bates P.J., Lane A.N., Trent J.O. Resolution and characterization of the structural polymorphism of a single quadruplex-forming sequence. Nucleic Acids Res. 2010;38:4877–4888. doi: 10.1093/nar/gkq166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biffi G., Tannahill D., Balasubramanian S. An intramolecular G-quadruplex structure is required for binding of telomeric repeat-containing RNA to the telomeric protein TRF2. J. Am. Chem. Soc. 2012;134:11974–11976. doi: 10.1021/ja305734x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meylan E., Tschopp J. Toll-like receptors and RNA helicases: two parallel ways to trigger antiviral responses. Mol. Cell. 2006;22:561–569. doi: 10.1016/j.molcel.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Gursel I., Gursel M., Yamada H., Ishii K.J., Takeshita F., Klinman D.M. Repetitive elements in mammalian telomeres suppress bacterial DNA-induced immune activation. J. Immunol. 2003;171:1393–1400. doi: 10.4049/jimmunol.171.3.1393. [DOI] [PubMed] [Google Scholar]
- 30.Takahama K., Takada A., Tada S., Shimizu M., Sayama K., Kurokawa R., Oyoshi T. Regulation of telomere length by G-quadruplex telomere DNA- and TERRA-binding protein TLS/FUS. Chem. Biol. 2013;20:341–350. doi: 10.1016/j.chembiol.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Lou Z., Wei J., Riethman H., Baur J.A., Voglauer R., Shay J.W., Wright W.E. Telomere length regulates ISG15 expression in human cells. Aging. 2009;1:608–621. doi: 10.18632/aging.100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robin J.D., Ludlow A.T., Batten K., Magdinier F., Stadler G., Wagner K.R., Shay J.W., Wright W.E. Telomere position effect: regulation of gene expression with progressive telomere shortening over long distances. Genes Dev. 2014;28:2464–2476. doi: 10.1101/gad.251041.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


