Abstract
Mitochondrial DNA (mtDNA) is exposed to reactive oxygen species (ROS) produced during oxidative phosphorylation. Accumulation of several kinds of oxidative lesions, including oxidized pyrimidines, in mtDNA may lead to structural genomic alterations, mitochondrial dysfunction and associated degenerative diseases. In Escherichia coli, oxidative pyrimidines are repaired by endonuclease III (EndoIII) and endonuclease VIII (EndoVIII). To determine whether the overexpression of two bacterial glycosylase/AP lyases which predominantly remove oxidized pyrimidines from DNA, could improve mtDNA repair and cell survival, we constructed vectors containing sequences for the EndoIII and EndoVIII downstream of the mitochondrial targeting sequence (MTS) from manganese superoxide dismutase (MnSOD) and placed them under the control of the tetracycline (Tet)-response element. Successful integrations of MTS–EndoIII or MTS–EndoVIII into the HeLa Tet-On genome were confirmed by Southern blot. Western blots of mitochondrial extracts from MTS–EndoIII and MTS–EndoVIII clones revealed that the recombinant proteins are targeted into mitochondria and their expressions are doxycycline (Dox) dependent. Enzyme activity assays and mtDNA repair studies showed that the Dox-dependent expressions of MTS–EndoIII and MTS–EndoVIII are functional, and both MTS–EndoIII and MTS–EndoVIII (Dox+) clones were significantly more proficient at repair of oxidative damage in their mtDNA. This enhanced repair led to increased cellular resistance to oxidative stress.
INTRODUCTION
MtDNA is a 16.5 kb circular double-stranded DNA molecule which encodes 13 proteins of the respiratory chain complex which are essential for aerobic metabolism. Additionally, it codes for 22 tRNAs and 2 rRNAs (1). Recently, several deletions in mtDNA and point mutations in mitochondrial tRNA and protein-encoding genes have been associated with a variety of diseases including diabetes (2), ischemic heart disease (3), Parkinson's disease (4), Alzheimer's disease (5), and the normal process of aging (6). The damage to mtDNA which leads to these mutations likely results from exposure to reactive oxygen species (ROS). ROS are formed continuously in mitochondria by electron leakage from the respiratory chains. Although oxidative phosphorylation in mitochondria is essential for producing the energy necessary to sustain life, it has been speculated that up to 1–2% of the total oxygen processed by the electron transport chains is reduced via one electron reduction to form ROS. Therefore, mtDNA which is located near the electron transport chains is continuously bombarded by these noxious agents. Although there are antioxidant defenses in mitochondria to protect against the deleterious effects of ROS, damage to mtDNA still results. It has been shown that mtDNA damage is more extensive and persists longer than that to nuclear DNA (7), and the mutation rate is five to ten times greater in mtDNA (8). Therefore, repair mechanisms are imperative to prevent modified lesions from becoming permanent mutations.
Work from our laboratory and that of others has indicated that repair of oxidative DNA damage is via a base excision repair (BER) pathway (9,10). In support of this notion is the finding that mitochondria contain the basic enzymes required for BER (11,12). This repair process for the removal of damaged bases in DNA is highly conserved among species (13). Generally, BER for oxidized bases is initiated by a glycosylase/AP lyase that recognizes and removes damaged bases from DNA. These bifunctional enzymes cleave the glycosidic bond of the adducted base and incise the resulting AP site on the 3′ side of the lesion (14). While oxidative damage-specific DNA glycosylases from bacteria have been extensively studied, more recently interest has begun to focus on mammalian nuclear enzymes and, to a lesser extent, their mitochondrial counterparts (15,16).
The spectrum of ROS-induced damage to DNA is broad and includes a wide variety of modifications to the purine and pyrimidine bases, sites of base loss and single-strand breaks (17). The 5,6 double bond of pyrimidines is particularly vulnerable to oxidative damage and various other pyrimidine modifications generated after ROS attack has been reported (18). Many of the oxidized cytosines are premutagenic lesions; e.g. 5-hydroxycytosine (5-OHC) has been shown to be mutagenic in Escherichia coli (19). Lesions in thymine also have biological consequences. One oxidized thymine, thymine glycol (Tg), is only slightly mutagenic. However, it can block DNA or RNA polymerases and thus be potentially lethal (20). In E.coli, oxidative pyrimidines are repaired by endonuclease III (EndoIII) and endonuclease VIII (EndoVIII) (17,21,22), which share a common range of substrates (23). Additionally, EndoVIII is reported to show some activity toward 8-oxoguanine and formamidopyrymidine (Fapy) lesion. Also, with respect to substrate specificity, it recently has been reported that EndoIII and its mouse homolog can excise the Fapy lesion from DNA (24). These findings raise the possibility that EndoIII and EndoVIII also may serve as back-up enzymes for oxidatively modified purines. Double mutants for these enzymes in E.coli exhibit a mutator phenotype that is 20-fold greater than the wildtype and has an increased sensitivity to hydrogen peroxide and ionizing radiation (22). The mutations observed are mostly C→T transitions probably arising from impaired excision of oxidized cytosine lesions (25).
Previously, we have reported that targeting the DNA repair protein OGG1 to mitochondria augmented DNA repair in this organelle and enhanced cellular survival (26). An obvious question arising about these results is whether this protective effect is unique to the OGG protein or whether other DNA repair proteins also can be protective. In the current studies we investigated whether the targeting of two other glycosylase/AP lyases, which predominantly remove oxidized pyrimidines from DNA, could also improve cell survival. The E.coli BER enzymes EndoIII and EndoVIII were targeted to mitochondria and mtDNA repair and cellular survival evaluated following oxidative stress. Previous studies from our laboratory have revealed that the use of a Tet-regulatable expression system can be a valuable approach for conditional targeting of BER enzymes into mitochondria (26). For the present study, we constructed vectors containing sequences for EndoIII and EndoVIII downstream of the MTS from human MnSOD under the control of the Tet-response element and introduced them into HeLa Tet-On cells. The results showed that conditional targeting of either EndoIII or EndoVIII to mitochondria enhanced repair of oxidative damage to mtDNA and increased cellular resistance to the lethal effects of ROS.
MATERIALS AND METHODS
Plasmid construction
Genes, nth and nei, encoding EndoIII and EndoVIII from E.coli were amplified by PCR from the chromosome of E.coli LE392 (K-12 strain) using the following primers: EndoIII 5′ primer GGGATCCATGAATAAAGCAAAACGCC, EndoIII 3′ primer GGAATTCAGAGCTCGATGTCAACTTTCTCTTTG for nth gene; EndoVIII 5′ primer GGGATCCATGCCTGAAGGCCCGGAG and EndoVIII 3′primer GGAATTCAGAGCTCGTGCTGGCAGCCAGGGCACC for nei gene. In both cases the 5′ primer incorporated a recognition sequence for BamHI and the 3′ primer incorporated a site for EcoRI (underlined). Amplified PCR-products were cloned into the EcoRV restriction site of pBluescript SK+ (Stratagene). The DNA of the resulting constructs was used as a template for the next PCR. To introduce the human influenza virus hemagglutinin (HA) tag (YPYDVPDYA) (27) to the C- terminus of either EndoIII or EndoVIII the following primers were used: EndoIII 5′ primer (see above), NdeI–EndoVIII 5′ primer CCGCATATGCCTGAAGGCCCGGAGAT, which introduces an NdeI site (underlined) at the start codon for EndoVIII, EndoIII–HA 3′ primer GCCGTCAAGCGTAATCTGGAACATCGTATGGGTAGATGTCAACTTTCTCTTTG, and EndoVIII–HA 3′primer CGCCGCTAAGCGTAATCTGGAACATCGTAGTGCTGGCAGCCAGGGCACC (the HA sequence is underlined). To insert the HA tag after the last codon of the MTS region from human MnSOD, PCR was performed using the MTS 5′ primer GGAATTCATGTTGAGCCGGGCAGTGTGC (EcoRI site underlined) and two different MTS–HA 3′ primers: MTS–HA–BamHI 3′ primer GGCGGGATCCAGCGTAATCTGGAACATCGTATGGGTACTCGGTGGAGCCCAGATAC and MTS–HA–NdeI 3′ primer GGCCCATATGAGCGTAATCTGGAACATCGTATGGGTACTGCCTGGAGCCCAGATAC both of which contain an HA tag and either BamHI or NdeI sites (restriction sites are underlined). As a DNA template for this PCR, a pcDNA3/MTS–OGG1 construct (28) was used. All resulting PCR products were cloned into an EcoRV restriction site in pBluescript SK+ and sequenced to avoid mutations introduced by PCR. To obtain the MTS–HA–EndoIII–HA construct, the BamHI fragment containing the EndoIII–HA sequence was cloned into the BamHI site of the plasmid containing the MTS–HA–BamHI sequence. To generate the MTS–HA– EndoVIII–HA construct, the NdeI/XbaI EndoVIII–HA-containing fragment was cloned into an MTS–HA–NdeI plasmid digested with NdeI and XbaI. EcoRI fragments containing either MTS–HA–EndoIII–HA or MTS–HA–EndoVIII–HA sequences were filled in with Klenow DNA polymerase and ligated into the PvuII restriction site of the pTRE2hyg expression vector (Clontech). The final constructs were sequenced to verify the integrity of the reading frames and fidelity of the sequences.
Cell culture and transfection
HeLa Tet-On cells (Clontech) were transfected with either pTRE2hyg/MTS–EndoIII or pTRE2hyg/MTS–EndoVIII plasmid using Fugene 6 reagent (Roche, Molecular Biochemicals) according to the manufacturer's recommendations. A pTRE2hyg vector without the insert was used to transfect Tet-On HeLa cells as a control. Stable transfectants were selected in DMEM (Invitrogen) supplemented with 10% fetal bovine serum (Clontech), 2 mM l-glutamine (Invitrogen), 1.2 mg/ml G418, (Geneticine), 0.4 mg/ml hygromycin B (Life Technologies, Inc.) and 50 μg/ml penicilline/streptomycine (Sigma), and the surviving cells were tested for the integration of either the MTS–EndoIII or MTS–EndoVIII inserts by Southern hybridization. The selected clones were maintained in the same media with 1 mg/ml G418, and 200–250 μg/ml hygromycin B instead. For conditional expression of the recombinant repair protein, cultures were treated with 1 μg/ml doxycycline hydrochloride (Dox, Sigma).
Preparation of mitochondrial fractions
Mitochondrial fractions were prepared as previously described (26,28). Isolated mitochondria were suspended in a buffer of 10 mM HEPES, pH 7.4, 1 mM EDTA, 5 mM DTT, 100 mM KCl, 5% glycerol, 0.2% TritonX-100 and 5 μl per ml of a mixture of protease inhibitors (Sigma). Mitochondrial pellets were lysed for 30 min at 37°C to disrupt mitochondrial membranes, centrifuged once more at 5000 g to pellet any remaining debris and the supernatant protein was used for western blot assays. The protein concentration was determined using the Bio-Rad protein dye micro-assay (Bio-Rad).
Western blot analysis
SDS–PAGE and transfer of separated proteins to PVDF membranes was performed as previously described (26,28) with some minor modifications. Blocking and antibody immunoblotting were performed in 5% nonfat dry milk. Membranes were incubated with mouse monoclonal anti-HA antibody (Sigma) and anti-cytochrome c monoclonal antibody (PharMingen). The membranes were washed and incubated with anti-mouse IgG secondary antibody (Promega) conjugated with peroxidase, using chemiluminescent reagents (SuperSignal, Pierce).
Preparation of Tg-containing substrate and EndoIII/EndoVIII activity assays
To generate an oligonucleotide duplex containing a single Tg lesion, 1 μg of 5′ 32P end-labeled 24 mer oligonucleotides (Integrated DNA Technologies, Inc.),with a unique thymine at the 11th position (5′-GAAACACAAATGACCACACACAGCGA-3′), were incubated for 30 min at room temperature in a 100 μl reaction volume containing 50 mM OsO4 and 2% pyridine. The oligonucleotide was purified from the reaction mixture using a QIAquick Nucleotide Removal Kit (QIAGEN) and subsequently annealed with the complementary strand. Equal amounts of protein from mitochondrial fractions isolated from transfected cells (Dox±) were used in assays with the labeled duplex oligonucleotide. Activity assays contained 5 nM of labeled duplex oligonucleotide, 2 μl of 10XREC buffer (10 mM HEPES, pH 7.4, 100 mM KCl, 1 mM EDTA) and organelle extract or 5 U of either Endonuclease III or Endonuclease VIII enzyme (Trevigen) in a total volume of 20 μl. Reaction mixes were incubated for 6 and 20 h at 37°C for extracts isolated from EndoIII and EndoVIII transfected clones, respectively. Reactions were stopped by addition of formamide/bromphenol (80%/0.2%) dye, samples were heated at 80°C for 2 min, and reaction products were separated by 20% polyacrylamide/8 M urea gel electrophoresis (PAGE). Following PAGE, the resultant images were scanned on a Bio-Rad GS-250 molecular imaging system.
Drug preparation and exposure
For oxidative damage and repair experiments cells were grown in 100 mm dishes for 4 days in media ± Dox until 70–80% confluent. Cells were exposed to 800 μM of menadione (2-methyl-1, 4-naphthoquinone sodium bisulfite, Sigma) in HBSS for 20 min in a 37°C, 5% CO2 incubator. Control cultures were exposed to HBSS under the same conditions. After incubation, cells were either rinsed or lysed immediately or allowed to repair for 2 or 6 h in normal growth media ± Dox.
Quantitative and neutral Southern blots
MTS–EndoIII or MTS–EndoVIII or vector-only transfected cells were exposed to menadione as described above. DNA isolation, quantitative and neutral Southern blots were performed as previously described (26,28) with some modifications. For the quantitative Southern blots, DNA samples were digested with the restriction endonuclease BamHI (10 U per microgram of DNA). DNA damage and repair were determined as described previously (29). To confirm the integration of the pTRE2hyg/MTS–EndoIII plasmid, DNA samples were digested with EcoRI and for the MTS–EndoVIII transfectants, DNA was digested with HindIII. Hybridizations were performed with 32P-labeled probe for either MTS–EndoIII or MTS–EndoVIII fragments generated using the RadPrime Labelling System (Invitrogen).
Clonogenic assays
Two hundred and fifty cells were plated in a 60 mm dish to achieve sparse distribution and incubated for 24 h in normal culture medium to allow them to adhere to the culture vessel. Cells were exposed to menadione as described above with the exception that lower concentrations of the drug were used due to the greater sensitivity of cells to oxidative stress when plated at the low density required for the clonogenic assay. For each cell type, controls (no menadione) and cultures receiving various concentrations of menadione were studied in triplicate. After 20 min of treatment, the menadione solution was replaced with normal culture medium (Dox±) and cells were grown for eight days. The colonies formed were stained with hematoxylin and counted.
Statistical analysis
Data are presented as the means ± standard errors of three independent experiments. Data were compared using two-way ANOVA followed by Bonferroni analysis. Statistical significance was determined at 0.01 level.
RESULTS
Generation of double transfected cell lines
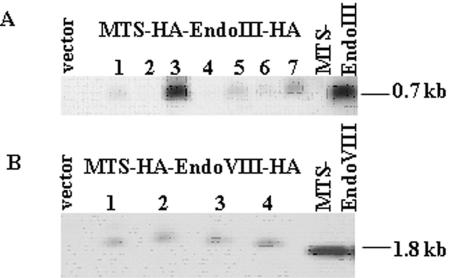
To fully evaluate the potential of using recombinant EndoIII or EndoVIII proteins to alter mtDNA repair, we used HeLa Tet-On cells transfected with either MTS–EndoIII or MTS–EndoVIII under the control of a Tet-mediated promoter. HeLa Tet-On cells were stably cotransfected with either pTRE2hyg/MTS–EndoIII or pTRE2hyg/MTS–EndoVIII plasmid, or a control vector (pTRE2hyg); double-transfected clones were selected in hygromycin. Total DNA was isolated from HeLa Tet-On cells transfected with vector only and seven clones of MTS–EndoIII, and the presence of the insert was confirmed in the MTS–EndoIII transfected clones by hybridizing with a 0.7 kb fragment corresponding to a partial MTS–EndoIII sequence (Figure 1A). The presence of the MTS–EndoVIII insert was confirmed in four MTS–EndoVIII transfected clones by hybridizing with a 1.8 kb fragment containing an MTS–EndoVIII sequence (Figure 1B). Clone 3 of MTS–EndoIII (Figure 1A) and clone 4 of MTS–EndoVIII (Figure 1B) were chosen for subsequent study because they had the highest levels of MTS–EndoIII or MTS–EndoVIII incorporated into their genomes (Figure 1), and produced the greatest amount of recombinant EndoIII or EndoVIII proteins in mitochondria after induction (Figure 2).
Figure 1.
Incorporation of MTS–EndoIII and MTS–EndoVIII inserts into HeLa Tet-On Cells. Southern blots of vector and (A) seven HeLa Tet-On clones transfected with MTS–EndoIII, hybridized with an MTS–EndoIII probe; (B) vector and four HeLa Tet-On/MTS–EndoVIII clones, hybridized with a MTS–EndoVIII-specific probe.
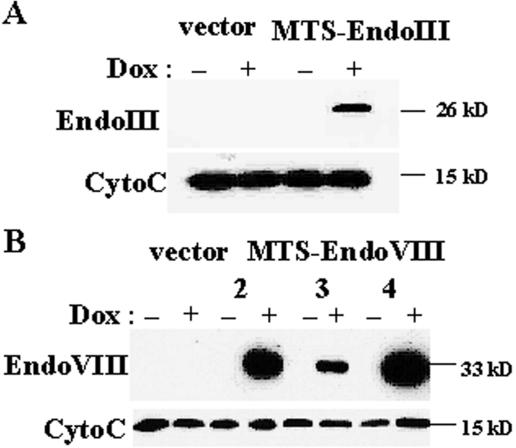
Figure 2.
Conditional and targeted expression of EndoIII and EndoVIII into mitochondria. Western blots containing 20 μg of mitochondrial extracts isolated from (A) Dox ± vector and clone 3 of HeLa Tet-On/MTS–EndoIII cells; and (B) Dox ± vector and three separate clones (2, 3 and 4) of HeLa Tet-On/MTS–EndoVIII transfected cells using anti-HA antibodies. Immunodetection of cytochrome c was performed to show that the recombinant proteins are in mitochondria.
Dox-dependent overexpression of EndoIII and EndoVIII in mitochondria
To show that the recombinant EndoIII and EndoVIII enzymes were targeted to mitochondria and their expression was regulated by Dox, mitochondrial extracts were isolated from vector and either MTS–EndoIII- or MTS–EndoVIII-transfected clones which were grown in media with or without Dox for four days. Western blot analysis, with mouse monoclonal anti-HA antibody, detected bands at the predicted molecular mass of ∼26 kDa in MTS–EndoIII (Dox+) transfectants (Figure 2A) and ∼33 kDa in MTS–EndoVIII (Dox+)-transfected clones (Figure 2B). No band was present in the mitochondrial extracts isolated from the same transfected clones cultured without Dox or vector transfected cells. Even loading was confirmed by Coomassie Blue staining of the gel and Ponceau staining of the membrane following transfer. These data establish that the recombinant EndoIII and EndoVIII enzymes were targeted to mitochondria and their expression was regulated by Dox.
Dox-dependent EndoIII and EndoVIII activities in mitochondria
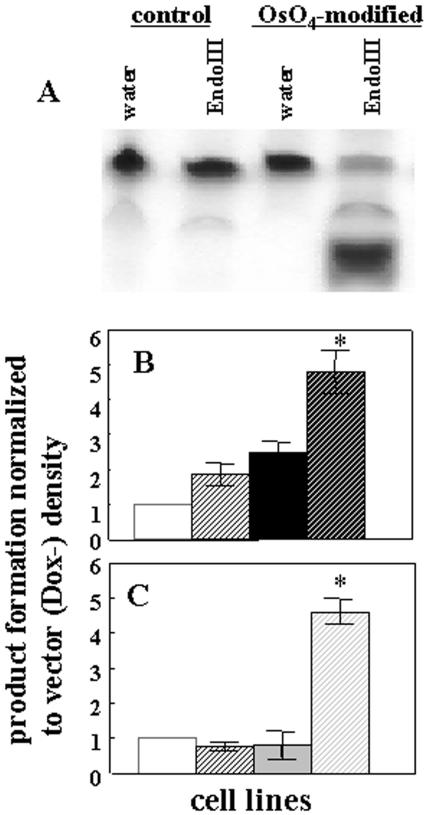
To determine whether the recombinant EndoIII and EndoVIII were functional in mitochondria, activity assays for these enzymes were performed. Because OsO4 reacts almost exclusively with thymine and ∼85% of the modified thymines are thymine glycol (30), enzymatic activity was followed using a 32P end-labeled oligonucleotide containing a single thymine which had been pretreated with OsO4. To confirm the conversion of thymine to Tg upon OsO4 treatment the following studies were performed. After modification by OsO4, 5′ 32P end-labeling and annealing to the complementary oligonucleotide, the Tg-containing 24 mer was digested with E.coli EndoIII (Trevigen). Only Tg-containing substrate was incised by EndoIII. No cleavage was observed when the control thymine-containing substrate was digested with EndoIII or when duplexes with control and modified oligos were incubated with reaction buffer (Figure 3A). Mitochondrial fractions were isolated from vector, MTS–EndoIII- and MTS–EndoVIII-transfected clones that had been maintained with or without Dox for four days. Of each mitochondrial extract 20 μg was then incubated with labeled Tg substrate. As a positive control E.coli EndoIII and EndoVIII (Trevigen) were used. Reaction mixes were incubated for 6 and 20 h at 37°C for extracts isolated from MTS–EndoIII and MTS–EndoVIII transfected clones, respectively. A longer incubation time (20 h) has been used in the reactions where mitochondrial extracts were isolated from MTS–EndoVIII transfected clones due to the better cleavage of Tg substrate by EndoVIII (Trevigen) at 20 versus 6 h (data not shown). As shown in Figure 3, mitochondrial extracts isolated from either MTS–EndoIII (B) or MTS–EndoVIII (C) (Dox+) clones were better able to cleave the DNA substrate than were the same cells grown in the absence of Dox or vector only transfected cells. Thus, these data reveal that the recombinant EndoIII and EndoVIII proteins are functional enzymes.
Figure 3.
Dox-regulated EndoIII and EndoVIII activities in mitochondria. Enzyme activity was measured using a labeled 24 mer containing Tg. (A) After OsO4 treatment, 5′ 32P end-labeling and annealing to the complementary oligonucleotide, the Tg-containing duplex was digested with EndoIII. Mitochondrial fractions were isolated from vector (Dox−) (white solid bar), (Dox+) (white dashed bar) and (B) either MTS–EndoIII (Dox−) (black solid bar), (Dox+) (black dashed bar) or (C) MTS–EndoVIII (Dox−) (gray solid bar), Dox+ (gray dashed bar) clones and the extracts incubated with labeled substrate. The data represent three independent experiments. An asterisk indicates a significant difference (p < 0.01).
Dox-mediated overexpression of EndoIII and EndoVIII in mitochondria enhance mtDNA repair
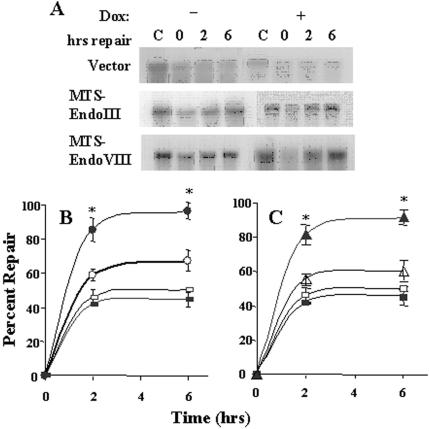
Because oxidatively modified pyrimidines are removed from DNA by EndoIII and EndoVIII in E.coli, we tested whether the conditional overexpression of foreign repair enzymes in human mitochondria would have an effect upon mtDNA repair capacity. To detect mtDNA damage and repair a quantitative Southern blot technique was used. First, dose response experiments were performed using different concentrations of menadione, which redox cycles with complex I of the electron transport chain and forms superoxide radical (31). A concentration of 800 μM menadione was chosen for the repair experiments because it produced an appropriate amount of lesions (∼1 lesion per 104 normal nucleotides) for evaluation of repair. There was no significant difference in the initial break frequencies between clones which were grown in the absence or presence of Dox. Repair experiments were performed in which the MTS–EndoIII or MTS–EndoVIII or vector transfectants were exposed to 800 μM menadione for 20 min and either lysed immediately or allowed to repair for 2 or 6 h in normal culture media with or without Dox. Control cells were incubated with drug diluent only. Total DNA was isolated from the lysed cells and quantitative Southern blot hybridizations performed. As shown in Figure 4A, both MTS–EndoIII and MTS–EndoVIII clones treated with Dox repaired most of the damage within the initial 6 h following drug removal, whereas the same clones not exposed to Dox or the vector transfectants did not repair an appreciable amount of the damage to their mtDNA. The average amount of repair from three separate experiments for each cell type is shown in Figure 4B and C. These results document that the recombinant E.coli enzymes EndoIII and EndoVIII, when conditionally overexpressed and targeted to mitochondria, enhance mtDNA repair of oxidative damage.
Figure 4.
Conditional Expression of EndoIII and EndoVIII in mitochondria and mtDNA repair. MTS–EndoIII, MTS–EndoVIII and vector transfectants were treated with 800 μM menadione for 20 min and lysed immediately or allowed to repair for 2 or 6 h in Dox ± media. Control cells were exposed to the drug diluent only. Total DNA was isolated from the lysed cells and quantitative Southern blot analysis with mtDNA specific probe performed. (A) Representative autoradiographs for each cell type are displayed. (B) The percentage of repair for MTS–EndoIII clones (Dox−) (open circles), (Dox+) (solid circles). (C) The percentage of repair for MTS–EndoVIII transfectants (Dox−) (open triangles), Dox+ (filled triangles). The percentages of repair for vector transfectants (Dox-) (open squares), Dox+ (filled squares) are shown in both (B) and (C).The data are the means ± SE of three separate experiments. An asterisk indicates a significant difference (p < 0.01).
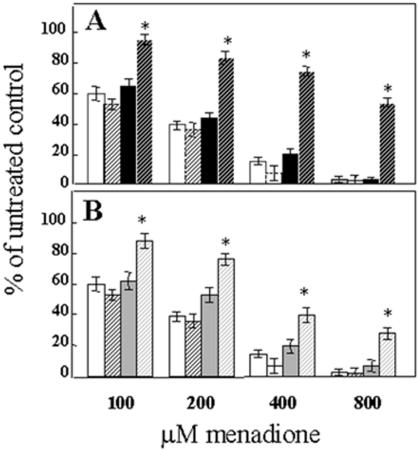
The effect of conditional overexpression of EndoIII and EndoVIII in mitochondria on cellular viability
To determine whether the increase in mtDNA repair enhanced cellular survival following oxidative stress, we used a clonogenic assay which assesses both long-term survival and proliferation. Because sparsely plated cells are more sensitive to menadione than confluent cells, we used lower doses of menadione than were required for the DNA repair experiments. The colonies formed from cells that survived and proliferated following oxidative stress induced by menadione were counted after eight days of culture. Figure 5A and B show that there is a significant enhancement in survival in the MTS–EndoIII- and MTS–EndoVIII-Dox-treated transfectants. These viability data establish that conditional transduction of either EndoIII or EndoVIII into mitochondria leads to increased cellular survival after an oxidative challenge.
Figure 5.
EndoIII and EndoVIII Dox-regulated expression and cell survival. (A) MTS–EndoIII clones (Dox−) (black solid bar), Dox+ (black dashed bar), and (B) represents data for MTS–EndoVIII clones (Dox−) (gray solid bar), Dox+ (gray dashed bar). Results of the clonogenic assay for vector clones (Dox−) (white solid bar), Dox+ (white dashed bar) are shown in both (A) and (B). Mean ± SE from three separate clonogenic assays is shown. An asterisk indicates a significant difference (p < 0.01).
DISCUSSION
To our knowledge, this is the first report to show conditional expression in mitochondria of two recombinant DNA repair enzymes from E.coli, EndoIII and EndoVIII, which have substrate specificity for oxidatively modified pyrimidines. For the present study, we constructed vectors containing sequences for EndoIII and EndoVIII downstream of the human MnSOD mitochondrial targeting sequence (MTS) under the control of the Tet-response element and introduced them into HeLa Tet-On cells. A general concern regarding cells that have been stably transfected to constitutively express a recombinant protein is that the effects observed are due to insertional mutagenesis rather than the effects of the recombinant protein. To overcome this problem, we employed a strategy of exogenous regulation of EndoIII and EndoVIII expression in mitochondria, using the Tet-controlled system for transcriptional activation. This permits the control of expression of individual gene products quantitatively and reversibly in a temporal manner (32). HeLa cells were chosen for this study because we previously discovered that these cells do not proficiently repair oxidative damage in their mtDNA (26,28), and it has been reported that in these cells Tet-systems exhibit no measurable ‘intrinsic leakiness’ (33). Additionally, previously we have used these cells for the stable (28) and conditional expression of hOGG1 in mitochondria. Moreover, repair of oxidative damage in the nucleus of these cells appears to be normal (26). This makes these cells preferable to cells in which mammalian homologs of EndoIII (Nth1) or EndoVIII (Nei1) have been knocked out because those cells would be defective in both nuclear and mitochondrial repair. Although the reason that HeLa cells are defective in the repair of oxidative damage in mtDNA has yet to be fully elucidated, we believe a plausible explanation is that there is a defect in the regulatory process controlling the formation of the alternatively spliced transcripts for mitochondrial localization. In support of this notion is our finding that HeLa cells have low levels of OGG1 protein in their mitochondria (28). Additionally, studies reported by others have shown that hNth1 can be targeted to both nucleus and mitochondria in some cells (34). However, in HeLa cells, enzyme localization appears to be only in the nucleus (35). Previously, we showed that a Tet-controlled expression system can be a useful approach for conditional targeting of BER enzymes to mitochondria. Conditional targeting of hOGG1 to mitochondria was found to increase mtDNA repair and cellular survival (26). In the present study, we evaluated mtDNA repair and cellular viability following conditional expression in mitochondria of two DNA glycosylase/AP lyases enzymes from E.coli, EndoIII and EndoVIII, which predominantly repair oxidized pyrimidines. The results from both enzyme activity assays, as well as DNA repair studies, revealed that Dox-dependent expression of either the MTS–EndoIII or MTS–EndoVIII transgenes produced functional enzyme that was targeted to mitochondria. Mitochondrial extracts isolated from MTS–EndoIII and MTS–EndoVIII (Dox+) cells were markedly better able to cleave a Tg-containing oligonucleotide than the same cells grown without Dox or vector-only transfected cells. Although we have added an additional nine amino acids from the HA tag to both the N- and C-termini of either construct, the fact that there was a significant increase of cleaved products following reactions with mitochondrial extracts from Dox-treated cells transfected with either the MTS–EndoIII or MTS–EndoVIII transgene reveals that both enzymes were not impaired. We believe that these data are not in conflict with previously reported studies concerning the role of the N-terminal Pro1 which works as a nucleophile in the catalytic activity of EndoVIII (36). We believe that the N-terminal HA tag sequence as well as the MTS are cleaved from EndoVIII following localization in the mitochondria. Studies focused upon mtDNA repair show that both MTS–EndoIII and MTS–EndoVIII (Dox+) cells were significantly more proficient at repairing oxidative damage in their mitochondrial genome. When these results are combined with those from the viability studies, it can be concluded that the MTS–EndoIII and MTS–EndoVIII recombinant enzymes were conditionally expressed under the control of the Dox-inducible promoter and targeted to mitochondria in an active form. Furthermore, they enhanced the repair of oxidative damage to the sugar–phosphate backbone of mtDNA, and this augmented repair rendered the cells more resistant to oxidative stress.
Recently, much attention has been directed toward the identification and characterization of enzymes involved in BER of mtDNA. Although many of the enzymes that are involved have been identified (11,37) and characterized in vitro, a thorough understanding of how BER works in mitochondria and the biological consequences of perturbations in this system remains incomplete. To better understand the role that different mtDNA repair enzymes play in preserving cellular functions and enhancing cellular survival, we and others have conducted studies targeting recombinant DNA repair proteins to mitochondria (26,38–41). Different enzymes have been found to have different effects. For instance, the glycosylase hMPG and the bacterial AP endonuclease ExoIII were both found to sensitize cells to specific genotoxic agents. hMPG appears to work through the generation of an intolerable number of abasic sites in mtDNA (41) following treatment with a simple alkylating toxin. In the case of ExoIII, we found that this enzyme, when directed to mitochondria, sensitizes breast cancer cells to reactive oxygen species. It has been speculated that the exonuclease activity in this enzyme generates long gaps in mtDNA following oxidative damage which are difficult to repair. This renders the cell more sensitive to oxidative stress (40). In contrast to these findings, we have discovered that overexpression of hOGG1 targeted to mitochondria in a variety of cells protects these cells from oxidative stress (26,28,38,39). The present work shows that two other glycosylase/AP lyases with different base specificities also exert a protective effect. These findings suggest that glycosylase/AP lyases may work through at least two different mechanisms which are not necessarily mutually exclusive. First, it is possible that oxidative damage leads to the formation of a critical amount of damage to bases in mtDNA. Although the three different enzymes recognize different base lesions, each enzyme, acting separately, is able to remove a sufficient number of the overall base lesions to lower the total burden below a toxic threshold. A second mechanism is related to the shared activity of the three enzymes. All three have AP lyase activity. This activity may be crucial for the repair of damage to the sugar–phosphate backbone which comprises a significant proportion of the total lesions in DNA following oxidative stress (30). In support of this notion are the results of the repair studies performed here with EndoIII- or EndoVIII-expressing cells or previously with cells that overexpress OGG1 (26,28,39). Under the alkali conditions used for the quantitative Southern blot repair assay, only single-strand breaks and abasic sites were revealed. Removal of these lesions was accelerated by conditional expression of any of the three recombinant enzymes. The glycosylase activity of these enzymes would not be expected to have any role in the removal of these lesions. Only the lyase activity should be involved in this repair. Further support for a protective role of the AP lyase activity comes from a study in which it was shown that overexpression of EndoIII in yeast defective in the AP endonuclease APN1 reduces sensitivity to methyl methanesulfonate (MMS) (42). The protective effect of EndoIII expression was ascribed to the AP lyase activity of EndoIII. It is possible that the 3′-incised AP site is processed more efficiently by other BER enzymes and therefore is less toxic to the cells than 5′-incised AP sites. Future studies using a recombinant enzyme with only lyase activity should help resolve this issue.
The use of bacterial enzymes has afforded us a unique opportunity to look at mechanisms of base excision repair in mitochondria. In BER, in the nucleus, it has been speculated that for repair to take place one enzyme in the process must ‘hand off’ to the next one (43). Because we were able to augment mtDNA repair with a bacterial enzyme, it less likely that ‘hand off’ is required for efficient repair in mitochondria. Thus, it appears that there may be functional differences between how BER proceeds in the nucleus and mitochondria.
The importance of mtDNA integrity for normal cellular homeostasis is only beginning to be appreciated. Because an increased number of oxidative lesions are found in mtDNA in individuals with a variety of chronic diseases, regulated expression of mitochondrial repair enzymes may provide a beneficial gene therapeutic strategy for preventing or delaying the symptoms of these diseases. Additionally, conditional expression of repair enzymes may be a viable approach for protecting normal cells during cancer therapy, or sensitizing cancer cells to this treatment.
Acknowledgments
ACKNOWLEDGEMENTS
We appreciate the assistance of Renee Ho and Viktoriya Solodushko in the preparation of this manuscript. This work was supported in part by Public Health Service Grants ES-03456 and ES-05865 from the National Institute of Environmental Health Sciences and AG-19602 from the National Institute on Aging.
REFERENCES
- 1.Anderson S., Bankier,A.T., Barrell,B.G., de Bruijn,M.H., Coulson,A.R., Drouin,J., Eperon,I.C., Nierlich,D.P., Roe,B.A. et al. (1981) Sequence and organization of the human mitochondrial genome. Nature, 290, 457–465. [DOI] [PubMed] [Google Scholar]
- 2.Wallace D.C. (1999) Mitochondrial diseases in man and mouse. Science, 283, 1482–1488. [DOI] [PubMed] [Google Scholar]
- 3.Wallace D.C. (2000) Mitochondrial defects in cardiomyopathy and neuromuscular disease. Am. Heart J., 139, S70–S85. [DOI] [PubMed] [Google Scholar]
- 4.Schapira A.H. (1999) Mitochondrial involvement in Parkinson's disease, Huntington's disease, hereditary spastic paraplegia and Friedreich's ataxia. Biochim. Biophys. Acta, 1410, 159–170. [DOI] [PubMed] [Google Scholar]
- 5.Hutchin T. and Cortopassi,G.A. (1995) mitochondrial DNA clone is associated with increased risk for Alzheimer disease. Proc. Natl Acad. Sci. USA, 92, 6892–6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hudson E.K., Hogue,B.A., Souza-Pinto,N.C., Croteau,D.L., Anson,R.M., Bohr,V.A. and Hansford,R.G. (1998) Age-associated change in mitochondrial DNA damage. Free Radic. Res., 29, 573–579. [DOI] [PubMed] [Google Scholar]
- 7.Yakes F.M. and Van Houten,B. (1997) Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl Acad. Sci. USA, 94, 514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallace D.C. (1989) Mitochondrial DNA mutations and neuromuscular disease. Trends Genet., 5, 9–13. [DOI] [PubMed] [Google Scholar]
- 9.Bogenhagen D.F. (1999) Repair of mtDNA in vertebrates. Am. J. Hum. Genet., 64, 1276–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pettepher C.C., LeDoux,S.P., Bohr,V.A. and Wilson,G.L. (1991) Repair of alkali-labile sites within the mitochondrial DNA of RINr 38 cells after exposure to the nitrosourea streptozotocin. J. Biol. Chem., 266, 3113–3117. [PubMed] [Google Scholar]
- 11.Pinz K.G. and Bogenhagen,D.F. (1998) Efficient repair of abasic sites in DNA by mitochondrial enzymes. Mol. Cell Biol., 18, 1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stierum R.H., Dianov,G.L. and Bohr,V.A. (1999) Single-nucleotide patch base excision repair of uracil in DNA by mitochondrial protein extracts. Nucleic Acids Res., 27, 3712–3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Memisoglu A. and Samson,L. (2000) Base excision repair in yeast and mammals. Mutat. Res., 451, 39–51. [DOI] [PubMed] [Google Scholar]
- 14.McCullough A.K., Dodson,M.L. and Lloyd,R.S. (1999) Initiation of base excision repair: glycosylase mechanisms and structures. Annu. Rev. Biochem., 68, 255–285. [DOI] [PubMed] [Google Scholar]
- 15.Croteau D.L., ap Rhys,C.M., Hudson,E.K., Dianov,G.L., Hansford,R.G. and Bohr,V.A. (1997) An oxidative damage-specific endonuclease from rat liver mitochondria. J. Biol. Chem., 272, 27338–27344. [DOI] [PubMed] [Google Scholar]
- 16.de Souza-Pinto N.C., Eide,L., Hogue,B.A., Thybo,T., Stevnsner,T., Seeberg,E., Klungland,A. and Bohr,V.A. (2001) Repair of 8-oxodeoxyguanosine lesions in mitochondrial DNA depends on the oxoguanine dna glycosylase (OGG1) gene and 8-oxoguanine accumulates in the mitochondrial dna of OGG1-defective mice. Cancer Res., 61, 5378–5381. [PubMed] [Google Scholar]
- 17.Demple B. and Harrison,L. (1994) Repair of oxidative damage to DNA: enzymology and biology. Annu. Rev. Biochem., 63, 915–948. [DOI] [PubMed] [Google Scholar]
- 18.Boiteux S., Gajewski,E., Laval,J. and Dizdaroglu,M. (1992) Substrate specificity of the Escherichia coli Fpg protein (formamidopyrimidine-DNA glycosylase): excision of purine lesions in DNA produced by ionizing radiation or photosensitization. Biochemistry, 31, 106–110. [DOI] [PubMed] [Google Scholar]
- 19.Feig D.I., Sowers,L.C. and Loeb,L.A. (1994) Reverse chemical mutagenesis: identification of the mutagenic lesions resulting from reactive oxygen species-mediated damage to DNA. Proc. Natl Acad. Sci. USA, 91, 6609–6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ide H., Kow,Y.W. and Wallace,S.S. (1985) Thymine glycols and urea residues in M13 DNA constitute replicative blocks in vitro. Nucleic Acids Res., 13, 8035–8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang D., Hatahet,Z., Melamede,R.J., Kow,Y.W. and Wallace,S.S. (1997) Characterization of Escherichia coli endonuclease VIII. J. Biol. Chem., 272, 32230–32239. [DOI] [PubMed] [Google Scholar]
- 22.Saito Y., Uraki,F., Nakajima,S., Asaeda,A., Ono,K., Kubo,K. and Yamamoto,K. (1997) Characterization of endonuclease III (nth) and endonuclease VIII (nei) mutants of Escherichia coli K-12. J. Bacteriology, 179, 3783–3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisen J.A. and Hanawalt,P.C. (1999) A phylogenomic study of DNA repair genes, proteins, and processes. Mutat. Res., 435, 171–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asagoshi K., Yamada,T., Okada,Y., Terato,H., Ohyama,Y., Seki,S. and Ide,H. (2000) Recognition of formamidopyrimidine by Escherichia coli and mammalian thymine glycol glycosylases. Distinctive paired base effects and biological and mechanistic implications. J. Biol. Chem., 275, 24781–24786. [DOI] [PubMed] [Google Scholar]
- 25.Blaisdell J.O., Hatahet,Z. and Wallace,S.S. (1999) A novel role for Escherichia coli endonuclease VIII in prevention of spontaneous G–>T transversions. J. Bacteriol., 181, 6396–6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rachek L.I., Grishko,V.I., Musiyenko,S.I., Kelley,M.R., LeDoux,S.P. and Wilson,G.L. (2002) Conditional targeting of the DNA repair enzyme hOGG1 into mitochondria. J. Biol. Chem., 277, 44932–44937. [DOI] [PubMed] [Google Scholar]
- 27.Wilson I.A., Niman,H.L., Houghten,R.A., Cherenson,A.R., Connolly,M.L. and Lerner,R.A. (1984) The structure of an antigenic determinant in a protein. Cell, 37, 767–778. [DOI] [PubMed] [Google Scholar]
- 28.Dobson A.W., Xu,Y., Kelley,M.R., LeDoux,S.P. and Wilson,G.L. (2000) Enhanced mitochondrial DNA repair and cellular survival after oxidative stress by targeting the human 8-oxoguanine glycosylase repair enzyme to mitochondria. J. Biol. Chem., 275, 37518–37523. [DOI] [PubMed] [Google Scholar]
- 29.Driggers W.J., LeDoux,S.P. and Wilson,G.L. (1993) Repair of oxidative damage within the mitochondrial DNA of RINr 38 cells. J. Biol. Chem., 268, 22042–22045. [PubMed] [Google Scholar]
- 30.Dizdaroglu M. (1991) Chemical determination of free radical-induced damage to DNA. Free Radic. Biol. Med., 10, 225–242. [DOI] [PubMed] [Google Scholar]
- 31.Frei B., Winterhalter,K.H. and Richter,C. (1986) Menadione (2-methyl-1, 4-naphthoquinone)-dependent enzymatic redox cycling and calcium release by mitochondria. Biochemistry, 25, 4438–4443. [DOI] [PubMed] [Google Scholar]
- 32.Resnitzky D., Gossen,M., Bujard,H. and Reed,S.I. (1994) Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol. Cell Biol., 14, 1669–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gossen M. and Bujard,H. (1992) Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA, 89, 5547–5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ikeda S., Kohmoto,T., Tabata,R. and Seki,Y. (2002) Differential intracellular localization of the human and mouse endonuclease III homologs and analysis of the sorting signals. DNA Repair (Amst), 1, 847–854. [DOI] [PubMed] [Google Scholar]
- 35.Luna L., Bjoras,M., Hoff,E., Rognes,T. and Seeberg,E. (2000) Cell-cycle regulation, intracellular sorting and induced overexpression of the human NTH1 DNA glycosylase involved in removal of formamidopyrimidine residues from DNA. Mutat. Res., 460, 95–104. [DOI] [PubMed] [Google Scholar]
- 36.Rieger R.A., McTigue,M.M., Kycia,J.H., Gerchman,S.E., Grollman,A.P. and Iden,C.R. (2000) Characterization of a cross-linked DNA-endonuclease VIII repair complex by electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom., 11, 505–515. [DOI] [PubMed] [Google Scholar]
- 37.Longley M.J., Prasad,R., Srivastava,D.K., Wilson,S.H. and Copeland,W.C. (1998) Identification of 5′-deoxyribose phosphate lyase activity in human DNA polymerase gamma and its role in mitochondrial base excision repair in vitro. Proc. Natl Acad. Sci. USA, 95, 12244–12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dobson A.W., Grishko,V., LeDoux,S.P., Kelley,M.R., Wilson,G.L. and Gillespie,M.N. (2002) Enhanced mtDNA repair capacity protects pulmonary artery endothelial cells from oxidant-mediated death. Am. J. Physiol. Lung Cell Mol. Physiol., 283, L205–L210. [DOI] [PubMed] [Google Scholar]
- 39.Druzhyna N.M., Hollensworth,S.B., Kelley,M.R., Wilson,G.L. and Ledoux,S.P. (2003) Targeting human 8-oxoguanine glycosylase to mitochondria of oligodendrocytes protects against menadione-induced oxidative stress. Glia, 42, 370–378. [DOI] [PubMed] [Google Scholar]
- 40.Shokolenko I.N., Alexeyev,M.F., Robertson,F.M., LeDoux,S.P. and Wilson,G.L. (2003) The expression of Exonuclease III from E.coli in mitochondria of breast cancer cells diminishes mitochondrial DNA repair capacity and cell survival after oxidative stress. DNA Repair (Amst), 2, 471–482. [DOI] [PubMed] [Google Scholar]
- 41.Fishel M.L., Seo,Y.R., Smith,M.L. and Kelley,M.R. (2003) Imbalancing the DNA base excision repair pathway in the mitochondria; targeting and overexpressing N-methylpurine DNA glycosylase in mitochondria leads to enhanced cell killing. Cancer Res., 63, 608–615. [PubMed] [Google Scholar]
- 42.Masson J.Y. and Ramotar,D. (1997) Normal processing of AP sites in Apn1-deficient Saccharomyces cerevisiae is restored by Escherichia coli genes expressing either exonuclease III or endonuclease III. Mol. Microbiol., 24, 711–721. [DOI] [PubMed] [Google Scholar]
- 43.Hazra T.K., Hill,J.W., Izumi,T. and Mitra,S. (2001) Multiple DNA glycosylases for repair of 8-oxoguanine and their potential in vivo functions. Prog. Nucleic Acid Res. Mol. Biol., 68, 193–205. [DOI] [PubMed] [Google Scholar]