Abstract
Introduction
HIV testing is the portal to serostatus knowledge that can empower linkage to care for HIV treatment and HIV prevention. However, young people's access to HIV testing is uneven worldwide. The objective of this paper is to review the context and concerns faced by youth around HIV testing in low- as well as high-income country settings.
Discussion
HIV testing is a critical entry point for primary and secondary prevention as well as care and treatment for young people including key populations of vulnerable youth. We provide a framework for thinking about the role of testing in the continuum of prevention and care for young people. Brief case study examples from Kenya and the US illustrate some of the common barriers and issues involved for young people.
Conclusions
Young people worldwide need more routine access to HIV testing services that effectively address the developmental, socio-political and other issues faced by young women and men.
Keywords: HIV testing, HIV continuum of care, youth, adolescents, key populations, development
Introduction
Youth aged 15–24 represent 39% of new HIV infections in people aged 15 years and older (2012) [1]. Among young people with HIV, most (4 million) live in sub-Saharan Africa [2]. Access to HIV testing and to antiretroviral therapy (ART) for youth remains a concern globally. Young people's HIV testing levels in low- and middle-income countries (LMICs), that contain most global HIV disease burden, is uneven [3]. Fewer than one in five boys and one in three girls aged 15–19 years in Africa report ever HIV testing [2]. The United States has poor sexual health statistics including in HIV testing access [4]; the proportion of US youth who HIV test has remained low at 22% and stagnant since 2005 [5]. For key populations (KPs), including males who have sex with males (MSM), people who inject drugs (PWID), transgender people (TG) and sex workers (SW), access to HIV testing is even more challenging due to marginalization and stigma.
The seek, test, treat, retain and suppress continuum has been promulgated as an approach with potential to bend the curve of the HIV epidemic [6]. Knowledge of serostatus is a starting point for lifesaving ART and to reduce sexual, parenteral, or vertical transmission. The particular HIV testing barriers and facilitators for youth in the HIV continuum of care have had less focus, however. Attention to developmental milestones is critical, e.g., yet most of what is known regarding linkage and retention in care has been based on adult, not youth populations. The sense of invulnerability that many adolescents feel – despite epidemiologic risks – also contributes [7,8]. Young people who are part of KP subgroups face overt discrimination and have lower testing rates than general population youth, facing additional barriers including fear, concerns about confidentiality and cost [4], low self-efficacy [9] and lack of KP-youth-friendly services. In this paper, we highlight critical issues involved for youth, including KPs, along the HIV testing–prevention–treatment continuum.
Framework: testing as entry to prevention and treatment
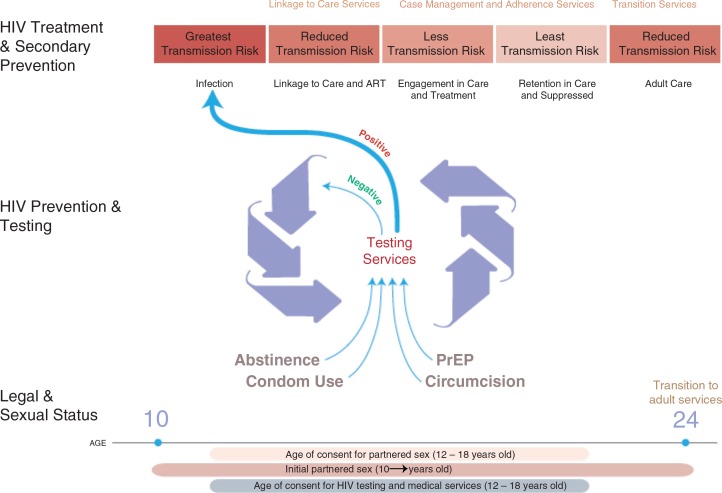
Testing for HIV is offered via provider-initiated testing and counselling (PITC) in health facilities, on a self-initiated basis through voluntary counselling and testing sites (VCT), delivered by staff in homes (home-based or HBCT), through community campaigns and through self-testing. Each modality has benefits and drawbacks specific to youth, yet HIV testing overall serves as a critical core component of the HIV continuum for vulnerable youth. In Figure 1, we graphically summarize a model where testing for HIV functions as a triage portal for needed youth-friendly services around an HIV Continuum of Prevention and Care, including housing, mental health treatment, substance abuse treatment and sexually transmitted infection (STI) services.
Figure 1.
Framework for HIV services for youth in the HIV continuum.
At-risk and KP youth [10] need safe opportunities to test, and re-test, for HIV. Acute HIV infection is important to identify [11] and US guidelines call for 4th generation screening tests [12], though these may not be available in many settings globally. Linkage to care for positive youth is especially challenging. Even with dedicated outreach and youth-friendly clinics, only 70% of HIV-infected youth in the US Adolescent Trials Network were successfully linked [13].
The primary goal of HIV care is viral suppression, with strong evidence for immunological advantage from early suppression [14]. Individuals also gain emotional assurance that viral suppression minimizes risk for transmission. This benefit can be realized on a population level [15]. Lifelong ART is challenging, with high attrition post-ART initiation among youth noted in Africa [16] and elsewhere. Technologies that are highly acceptable to young people, such as text messaging, to support adherence are promising [17]. Technology tools also have been used successfully to support HIV testing uptake among adolescents including in US emergency departments [18]. Partner services are critical for HIV-infected youth. Keeping HIV-negative youth healthy and uninfected remains a key goal.
Since the global burden of HIV disease is in sub-Saharan Africa, with two-thirds of all HIV cases and four-fifths of all young persons living with HIV, making new modalities such as self-tests available may help KP youth gain access to serostatus knowledge. In Malawi, in work done by Choko and colleagues, uptake of HIV self-testing among young people aged 16–24 years was consistently higher than among adults aged ≥25 years: 93.7% among those under age 25 versus 65.5% among adults 25 years and older (p<0.001). However, only 42.4% of the youth in that study had ever HIV tested, compared with 57.6% of those 25 and older (p<0.001) [19], indicating an unmet need as the younger age group is sexually active and exposed to HIV including through sex with older partners more likely to be HIV-infected [20].
In Kenya, where an estimated 100,000 new HIV infections occurred in 2013, girls and KPs are disproportionately affected by HIV [21]. School-based HIV education does not equip youth to seek testing, and there are few youth-friendly facilities available. Policy guidance says minors require parental/guardians’ consent for HIV testing, though Kenya has eliminated age limit as the only criteria. Many healthcare providers are ignorant of this provision, however, and deny unaccompanied adolescents an HIV test. In the country's new HIV roadmap [21], there is commitment to reviewing parental consent for HIV testing for adolescents. Youth who discuss testing with their parents are more likely to HIV test [22]. However, youth often rightfully fear negative reactions from parents and providers, including in schools where they fear isolation and missed opportunities and employment prospects if known to be HIV-positive. In some communities, women cannot give consent without consent by family members (case example, Box 1).
Box 1. Benta [a pseudonym], 17 years old, was admitted with her 2 year-old child into the paediatric ward. She comes from a pastoral community, got married at 15 and never attended antenatal clinic. She does not know her HIV status. She and her child are offered provider-initiated HTC. She has to get permission from her mother-in-law who says Benta and her child can be tested but only if the father of the child consents. He cannot be reached by phone, does not visit the family in the hospital for 10 days, and Benta and her child leave hospital without knowing their HIV status.
KPs including MSM and transgender youth in sub-Saharan Africa are often hidden and it is not safe to self-identify to providers (Box 2). Issues faced by youth for HIV testing cut across country contexts or resource boundaries, especially when in persecuted groups like MSM (Box 3 from high-income US setting).
Box 2. Paul [a pseudonym] is a transgender youth in Kenya. After high school, he was not able to get a job. One day he dressed like a female and got employed as house help looking after two girls. One day the girls and their mother saw Paul in a mall, dressed as a young man. The mother confronted him claiming Paul was masquerading as a woman with intent to abuse her children. A crowd gathered and physically assaulted Paul. Police officers forcibly took him to a nearby clinic where he was tested for STIs including HIV without consent. Results were disclosed without consent. He lost his job and had to re-locate.
Box 3. Michael [a pseudonym] is an 18-year-old who has been homeless for 12 months. He exchanges sex with other men for money in order to survive, and most do not use condoms. He was recently tested for HIV, and he was told it was positive. He is sure it was a mistake, and avoids going to any health facility.
Discussion
Developmental issues relevant to HIV testing
The dividing line of adolescence and adulthood is often seen as a sharp transition (e.g. at 18 or 21 years). These age transitions mark relevant thresholds for age of consent for HIV testing, for HIV medical services, and for partnered sex (Figure 1). Although adolescence is developmentally continuous and subject to substantial individual, cultural and national variation, it is useful to think of the HIV service continuum in the context of early (10–14 years), middle (15–17 years) and late adolescence (18 years and older).
Early adolescence (10–14 years)
Early adolescence is marked by puberty, achievement of adult size and gender-typical body contours with new assumptions about responsibility for sexual behaviour. Family and economic situations may require contributions to household income and sibling care that affect schooling and vocational opportunities [23]. Puberty in many cultures is associated with initiation rites that may not include HIV prevention messages [24–26] and that carry potential risks including non-medical circumcision [27,28]. (Voluntary medical male circumcision plays a critical role in HIV prevention and is well-accepted by many young men and parents [29].)
Development of sexual orientation is a key task. Often heterosexual identity is assumed as the “normal” outcome while other identities may be considered deviant [30]. During early and middle adolescence, sexual orientation has substantial variation and fluidity and often, lack of congruence between identity and behaviour [31].
Much emphasis is given to timing of coitus [32,33]. Over-emphasis on adolescent coitus complicates appropriate matching of services because many adolescents do not have coitus yet engage in other partnered sexual behaviours associated with HIV risk. Same-sex partnered behaviours often are omitted from sexuality education or relegated to being entirely risky without contribution to sexual or relationship satisfaction.
As pointed out in Figure 1, there may be a mismatch and delay between when young people begin having sex and when they can legally obtain HIV testing independently. HIV tests before first partnered sexual event have unproven benefits (e.g. normalization of testing) and harms (e.g. false security). After first partnered sex, it is unclear when young people begin to seek HIV testing on their own, or when clinicians recommend testing [33], despite guidelines that paediatricians and youth providers offer on HIV testing around age 13 onward [7].
Health providers should ask about sexual activity among younger patients. Many youth are not consensually sexually active and may acquire HIV via sexual abuse, or may have acquired HIV perinatally. In both these cases the young person may not be willing to share their sexual activity history at their first encounter with a new provider. Adolescents’ non-consensual sexual experiences and intimate partner violence (IPV) may increase risky behaviours [34,35]. Few IPV victims report discussions with a provider, demonstrating the importance of routine assessment for partner violence [36].
Sexuality education may occur in secondary schools, although content varies greatly [37–39] and this misses out-of-school youth. Primary emphasis on abstinence-until-marriage is less effective for HIV prevention than age-appropriate, comprehensive programs [40–42]. Informal sources of information including social media are ubiquitous in adolescents’ daily lives worldwide [32,43–45].
Middle adolescence (15–17 years)
By this stage some functional competencies needed to manage one's health may be in place. However, many adolescents lack skills or status to negotiate complex systems [46].
Adolescents’ participation in the HIV continuum of care as consumers of health products (e.g. condoms, pregnancy tests) is infrequently explored. Sale of HIV self-testing kits is not age-restricted although costs, test implementation fidelity and point-of-sale confidentiality have not been fully explored with young, high-risk persons [47–49] . Early data on self-testing acceptability, as seen in the Malawi example, are encouraging.
Many youth in this age group routinely have sex, especially in subgroups where survival depends on sexual exchange, which is often unprotected given power differentials in these encounters. Many KP youth live on their own, though are not yet an age of legal majority.
Late adolescence – youth (18–24 years)
Age 18 often is considered adulthood; however, it is now known if significant brain development including in the prefrontal cortex responsible for decision-making does not actually mature fully until age 25, which may influence vulnerability and resilience of young people [50] in terms of HIV risk and testing decisions.
Legal issues and the HIV continuum of care for adolescents
Three highly variable (from jurisdiction to jurisdiction) milestones dictate legal thresholds for adolescents’ engagement in the HIV continuum of care: age of consent for partnered sex; for HIV testing; and, for HIV medical services (Figure 1). Adolescents’ differential legal access to HIV-related testing and other services is based on traditional assumptions of parental rights as well as restricted autonomy of children [51].
Identification of sexual activity of minors less than the age of consent threshold may mandate reporting to child protection authorities [52]. Given concerns about widespread victimization and HIV (especially of girls and younger adolescents) [53], some countries have enacted “defilement” laws that can be enforced without regard for consensuality of the partnered sex [54].
The ethical concept of “the mature minor,” while infrequently given legal sanction for adolescents’ self-consent for general medical treatment [55], informs legal exceptions to consent requirements for HIV [56,57]. Age thresholds for consent of diagnostic HIV testing are widely variable, often as young as 12 years of age. These laws recognize that parental permission is a critical barrier to HIV testing, and could invoke physical danger if non-marital or same-sex activity is suspected or disclosed. Age thresholds for minor self-consent for HIV is sometimes addressed within the context of laws that allow for STI assessment [58,59]. However, medical HIV treatments are lifelong and expensive, requiring ongoing relationships with providers [60].
Cross-cutting issues for youth that affect HIV testing uptake
Across all age groups, stigma adversely affects each phase of adolescents’ engagement with the HIV continuum [61]. Internalized stigma may particularly effect HIV testing behaviours while anticipated stigma may have especially strong effects on care-seeking and adherence [58,59].
Physical, sexual and emotional aggression is experienced by many youth, especially in KP groups (e.g. sexual minorities), where microaggressions also have a damaging impact [62]. Legal protection, and campaigns to reduce bullying and other forms of aggression, are needed. Finally, many young people are economically disadvantaged relative to adults and cost barriers to HIV testing must be effectively addressed.
Solutions to increase HIV testing uptake among youth including KPs
HIV testing services must be available to all young people, particularly those from KPs. Health literacy is an issue for many adolescents [63] which “youth-friendly” programs may address [64]. Demand creation strategies have been used effectively for HIV testing via social marketing campaigns [65] and should be further employed. Testing availability where youth gather, and user-friendly free or subsidized test kits, may increase uptake. Once confirmed positive, linkage to care is critical and youth should access treatment services in whatever clinical venue is preferred, whether paediatric or adult (to reduce loss to follow-up when forced into adult services at arbitrary age cutoffs like 18 years). Testing and care are enhanced by respectful health care teams, and reduction of resource barriers such as transport fees and homelessness, that disrupt treatment continuity. Counselling can address the utility of ART taking into account developmental stages in which many young people may feel invulnerable and find navigating complex health systems overwhelming, especially in the commonly-occurring context of depression, substance use and other co-morbidities. Failure to link/retain adequately has dire consequence; Zanoni and Mayer estimate that only 6% of HIV-positive US youth are virally suppressed. They recommend that HIV testing be integrated wherever youth interact with health systems, as well as in youth venues, to normalize and promote testing and recurrent testing among high-risk and KP youth [66].
Conclusions
Recommendations and research gaps
Adolescents and young adults worldwide deserve better access to HIV testing and re-testing. We recommend that testing venues be made more youth-friendly, and promising new approaches like self-testing be monitored regarding how well they work for youth. Implementation science can identify optimal ways to improve HIV testing access and delivery for youth [67]. HIV testing in prevention of maternal to child transmission (PMTCT), antepartum care and voluntary medical male circumcision (VMMC) campaigns alone is insufficient. For youth, HIV testing is a key portal for linkage to necessary HIV care and prevention services.
Despite international and national guidelines, HIV testing for adolescents is still not consistently done in high- [68] or lower-income countries. Providers worldwide [69] must consistently assess sexual behaviours or partnership risks, so that appropriate counselling based on the young person's actual needs is not pre-empted [10]. HIV testing can be made more youth-friendly [70] even under the constraints of ART scale-up [71], but truly supportive services ultimately must rely on empathetic, self-aware [72] and professional health provider behaviours [73] including assurance of confidentiality around test results [74], reinforcement for those testing HIV-negative, and social and clinical support for those testing HIV-positive. Ensuring youth rights cannot occur only within clinic walls but must extend to the community and to social as well as legal norms [75].
There are social justice and public health imperatives to focus on structural factors that keep young people from freely HIV testing – including laws that harm KPs and program structures and costs that restrict access. The HIV Investment Framework points out that contraceptive services are a cost-effective portal for youth HIV testing [76], of even more importance in LMIC settings where a higher proportion of the population are of reproductive ages [77]. Achieving universal access to youth-friendly services worldwide would cost around US$ 1 per adolescent [78]. Program quality monitoring of HIV testing access [79], and implementation of best HIV testing practices, for young people must a part of the HIV agenda if we are to achieve generations with fewer HIV infections and provide better care of those living with HIV.
Acknowledgements
We thank Nok Chhun for her expert assistance with manuscript construction and submission. We also thank Dr. Elizabeth Ngugi and her counselling staff at the University of Nairobi HIV Prevention Centre for the transgender vignette.
Competing interests
There are no competing interests.
Authors' contributions
AEK organized the paper writing, MAL contributed sections on linkage, ATC provided data from Malawi, II provided examples from Kenya, JDF contributed sections on consent, developmental issues and the initial figure graphic. All authors have read and approved the final manuscript.
References
- 1.UNAIDS. Fact Sheet: UNAIDS's vision: zero new HIV infections. Zero discrimination. 2012. Zero AIDS-related deaths [Internet], [cited 2015 Jan 29]. Available from: http://www.unaids.org/en/media/unaids/contentassets/documents/factsheet/2012/20120417_FS_adolescentsyoungpeoplehiv_en.pdf.
- 2.Idele P, Gillespie A, Porth T, Suzuki C, Mahy M, Kasedde S, et al. Epidemiology of HIV and AIDS among adolescents: current status, inequities, and data gaps. J Acquir Immune Defic Syndr. 2014;66(Suppl 2):S144–53. doi: 10.1097/QAI.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 3.UNICEF. Opportunity in Crisis: preventing HIV from early adolescence to young adulthood [Internet] 2011. [cited 2015 Jan 29]. Available from: http://www.unicef.org/publications/files/Opportunity_in_Crisis-Report_EN_052711.pdf.
- 4.Peralta L, Deeds BG, Hipszer S, Ghalib K. Barriers and facilitators to adolescent HIV testing. AIDS Patient Care STDS. 2007;21(6):400–8. doi: 10.1089/apc.2006.0112. [DOI] [PubMed] [Google Scholar]
- 5.Kann L, Kinchen S, Shanklin SL, Flint KH, Kawkins J, Harris WA, et al. Youth risk behavior surveillance – United States, 2013. MMWR Surveill Summ. 2014;63(Suppl 4):1–168. [PubMed] [Google Scholar]
- 6.Hull MW, Wu Z, Montaner JS. Optimizing the engagement of care cascade: a critical step to maximize the impact of HIV treatment as prevention. Curr Opin HIV AIDS. 2012;7(6):579–86. doi: 10.1097/COH.0b013e3283590617. [DOI] [PubMed] [Google Scholar]
- 7.Committee on Pediatric AIDS. Emmanuel PJ, Martinez J. Adolescents and HIV infection: the pediatrician's role in promoting routine testing. Pediatrics. 2011;128(5):1023–9. doi: 10.1542/peds.2011-1761. [DOI] [PubMed] [Google Scholar]
- 8.Mill JE, Jackson RC, Worthington CA, Archibald CP, Wong T, Myers T, et al. HIV testing and care in Canadian Aboriginal youth: a community based mixed methods study. BMC Infect Dis. 2008;8:132. doi: 10.1186/1471-2334-8-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berendes S, Rimal RN. Addressing the slow uptake of HIV testing in Malawi: the role of stigma, self-efficacy, and knowledge in the Malawi BRIDGE Project. J Assoc Nurses AIDS Care. 2011;22(3):215–28. doi: 10.1016/j.jana.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Leonard NR, Rajan S, Gwadz MV, Aregbesola T. HIV testing patterns among urban YMSM of color. Health Educ Behav. 2014;41(6):673–81. doi: 10.1177/1090198114537064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 infection. N Engl J Med. 2011;364(20):1943–54. doi: 10.1056/NEJMra1011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Branson BM, Owen SM, Wesolowski LG, Bennett B, Werner BG, Wroblewski KE, et al. Centers for Disease Control and Prevention and Association of Public Health Laboratories; 2014. Laboratory testing for the diagnosis of HIV infection: updated recommendations. [Internet], [cited 2015 Jan 29]. Available from: http://stacks.cdc.gov/view/cdc/23447. [Google Scholar]
- 13.Philbin MM, Tanner AE, DuVal A, Ellen JM, Xu J, Kapogiannis B, et al. Factors affecting linkage to care and engagement in care for newly diagnosed HIV-positive adolescents within fifteen adolescent medicine clinics in the United States. AIDS Behav. 2014;18(8):1501–10. doi: 10.1007/s10461-013-0650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. Department of Health and Human Services. Panel on Antiretroviral Guidelines for Adults and Adolescents – A Working Group of the Office of AIDS Research Advisory Council. US Department of Health and Human Services; 2014. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. [cited 2015 Jan 30]. Available from: http://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-treatment-guidelines/0. [Google Scholar]
- 15.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamb MR, Fayorsey R, Nuwagaba-Biribonwoha H, Viola V, Mutabazi V, Alwar T, et al. High attrition before and after ART initiation among youth (15–24 years of age) enrolled in HIV care. AIDS. 2014;28(4):559–68. doi: 10.1097/QAD.0000000000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belzer ME, Naar-King S, Olson J, Sarr M, Thornton S, Kahana SY, et al. The use of cell phone support for non-adherent HIV-infected youth and young adults: an initial randomized and controlled intervention trial. AIDS Behav. 2014;18(4):686–96. doi: 10.1007/s10461-013-0661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calderon Y, Cowan E, Nickerson J, Mathew S, Fettig J, Rosenberg M, et al. Educational effectiveness of an HIV pretest video for adolescents: a randomized controlled trial. Pediatrics. 2011;127(5):911–16. doi: 10.1542/peds.2010-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choko AT. PLOS Med. 2011;8(10):e1001102. doi: 10.1371/journal.pmed.1001102. 2014. Age-stratified subanalysis of data from large HIV self-testing. In: Choko AT, Desmond N, Webb EL, et al., editors, The uptake and accuracy of oral kits for HIV self-testing in high HIV prevalence setting: a cross-sectional feasibility study in Blantyre, Malawi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poulin M. Sex, money, and premarital partnerships in southern Malawi. Soc Sci Med. 2007;65(11):2383–93. doi: 10.1016/j.socscimed.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kenya MoH. Kenya HIV prevention revolution road map [Internet] 2014 [cited 2015 Jan 29]. Available from: http://www.nacc.or.ke/attachments/article/418/Kenya_HIV_Prevention_Revolution_Road_Map.pdf. [Google Scholar]
- 22.Peltzer K, Matseke G. Determinants of HIV testing among young people aged 18–24 years in South Africa. Afr Health Sci. 2013;13(4):1012–20. doi: 10.4314/ahs.v13i4.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sommer M. An overlooked priority: puberty in sub-Saharan Africa. Am J Public Health. 2011;101(6):979–81. doi: 10.2105/AJPH.2010.300092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malisha L, Maharaj P, Rogan M. Rites of passage to adulthood: traditional initiation schools in the context of HIV/AIDS in the Limpopo Province, South Africa. Health Risk Soc. 2008;10(6):585–98. [Google Scholar]
- 25.Munthali AC, Zulu EM. The timing and role of initiation rites in preparing young people for adolescence and responsible sexual and reproductive behaviour in Malawi. Afr J Reprod Health. 2007;11(3):150–67. [PMC free article] [PubMed] [Google Scholar]
- 26.Skinner J, Underwood C, Schwandt H, Magombo A. Transitions to adulthood: examining the influence of initiation rites on the HIV risk of adolescent girls in Mangochi and Thyolo districts of Malawi. AIDS Care. 2013;25(3):296–301. doi: 10.1080/09540121.2012.701721. [DOI] [PubMed] [Google Scholar]
- 27.Mudege NN, Egondi T, Beguy D, Zulu EM. The determinants of female circumcision among adolescents from communities that practice female circumcision in two Nairobi informal settlements. Health Soc Rev. 2012;21(2):240–8. [Google Scholar]
- 28.Martinez Perez G, Namulondo H, Tomas Aznar C. Labia minora elongation as understood by Baganda male and female adolescents in Uganda. Cult Health Sex. 2013;15(10):1191–205. doi: 10.1080/13691058.2013.811613. [DOI] [PubMed] [Google Scholar]
- 29.Corduk N, Unlu G, Sarioglu-Buke A, Buber A, Savran B, Zencir M. Knowledge, attitude and behaviour of boys and parents about circumcision. Acta Paediatr. 2013;102(4):e169–73. doi: 10.1111/apa.12152. [DOI] [PubMed] [Google Scholar]
- 30.Morgan EM. Contemporary issues in sexual orientation and identity development in emerging adulthood. Emerg Adulthood. 2013;1:52–66. [Google Scholar]
- 31.Mustanski B, Birkett M, Greene GJ, Rosario M, Bostwick W, Everett BG. The association between sexual orientation identity and behavior across race/ethnicity, sex, and age in a probability sample of high school students. Am J Public Health. 2014;104(2):237–44. doi: 10.2105/AJPH.2013.301451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Secor-Turner M, Sieving R, Eisenberg ME, Skay C. Associations between sexually experienced adolescents’ sources of information about sex and sexual risk outcomes. Sex Educ. 2011;11(4):489–500. [Google Scholar]
- 33.Tu W, Batteiger BE, Wiehe S, Ofner S, Van Der Pol B, Katz BP, et al. Time from first intercourse to first sexually transmitted infection diagnosis among adolescent women. Arch Pediatr Adolesc Med. 2009;163(12):1106–11. doi: 10.1001/archpediatrics.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young BJ, Furman W, Jones MC. Changes in adolescents’ risk factors following peer sexual coercion: evidence for a feedback loop. Dev Psychopathol. 2012;24(2):559–71. doi: 10.1017/S0954579412000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Decker MR, Miller E, McCauley HL, Tancredi DJ, Anderson H, Levenson RR, et al. Recent partner violence and sexual and drug-related STI/HIV risk among adolescent and young adult women attending family planning clinics. Sex Transm Infect. 2014;90(2):145–9. doi: 10.1136/sextrans-2013-051288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller E, Decker MR, Raj A, Reed E, Marable D, Silverman JG. Intimate partner violence and health care-seeking patterns among female users of urban adolescent clinics. Matern Child Health J. 2010;14(6):910–17. doi: 10.1007/s10995-009-0520-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindberg LD, Santelli JS, Singh S. Changes in formal sex education: 1995–2002. Perspect Sex Reprod Health. 2006;38(4):182–9. doi: 10.1363/psrh.38.182.06. [DOI] [PubMed] [Google Scholar]
- 38.Ott MA, Santelli JS. Abstinence and abstinence-only education. Curr Opin Obstet Gynecol. 2007;19(5):446–52. doi: 10.1097/GCO.0b013e3282efdc0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santelli JS. Medical accuracy in sexuality education: ideology and the scientific process. Am J Public Health. 2008;98(10):1786–92. doi: 10.2105/AJPH.2007.119602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindberg LD, Maddow-Zimet I. Consequences of sex education on teen and young adult sexual behaviors and outcomes. J Adolesc Health. 2012;51(4):332–8. doi: 10.1016/j.jadohealth.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 41.Vivancos R, Abubakar I, Phillips-Howard P, Hunter PR. School-based sex education is associated with reduced risky sexual behaviour and sexually transmitted infections in young adults. Public Health. 2013;127(1):53–7. doi: 10.1016/j.puhe.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 42.Hall KS, Moreau C, Trussell J. Associations between sexual and reproductive health communication and health service use among U.S. adolescent women. Perspect Sex Reprod Health. 2012;44(1):6–12. doi: 10.1363/4400612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bleakley A, Hennessy M, Fishbein M, Jordan A. How sources of sexual information relate to adolescents’ beliefs about sex. Am J Health Behav. 2009;33(1):37–48. doi: 10.5993/ajhb.33.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones RK, Biddlecom AE. Is the internet filling the sexual health information gap for teens? An exploratory study. J Health Commun. 2011;16(2):112–23. doi: 10.1080/10810730.2010.535112. [DOI] [PubMed] [Google Scholar]
- 45.Lagus KA, Bernat DH, Bearinger LH, Resnick MD, Eisenberg ME. Parental perspectives on sources of sex information for young people. J Adolesc Health. 2011;49(1):87–9. doi: 10.1016/j.jadohealth.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 46.Parker R, Ratzan SC. Health literacy: a second decade of distinction for Americans. J Health Commun. 2010;15(Suppl 2):20–33. doi: 10.1080/10810730.2010.501094. [DOI] [PubMed] [Google Scholar]
- 47.Mavedzenge SN, Luecke E, Ross DA. Effective approaches for programming to reduce adolescent vulnerability to HIV infection, HIV risk, and HIV-related morbidity and mortality: a systematic review of systematic reviews. J Acquir Immune Defic Syndr. 2014;66(Suppl 2):S154–69. doi: 10.1097/QAI.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 48.Meyerson B, Barnes P, Emetu R, Bailey M, Ohmit A, Gillespie A. Institutional and structural barriers to HIV testing: elements for a theoretical framework. AIDS Patient Care STDS. 2014;28(1):22–7. doi: 10.1089/apc.2013.0238. [DOI] [PubMed] [Google Scholar]
- 49.Pant Pai N, Sharma J, Shivkumar S, Pillay S, Vadnais C, Joseph L, et al. Supervised and unsupervised self-testing for HIV in high- and low-risk populations: a systematic review. PLoS Med. 2013;10(4):e1001414. doi: 10.1371/journal.pmed.1001414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson SB, Blum RW, Giedd JN. Adolescent maturity and the brain: the promise and pitfalls of neuroscience research in adolescent health policy. J Adolesc Health. 2009;45(3):216–21. doi: 10.1016/j.jadohealth.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iltis AS. Introduction: vulnerability in biomedical research. J Law Med Ethics. 2009;37(1):6–11. doi: 10.1111/j.1748-720X.2009.00345.x. [DOI] [PubMed] [Google Scholar]
- 52.Miller BB, Cox DN, Saewyc EM. Age of sexual consent law in Canada: population-based evidence for law and policy. Can J Human Sexuality. 2010;19(3):105–19. [PMC free article] [PubMed] [Google Scholar]
- 53.Oudekerk BA, Guarnera LA, Reppucci ND. Older opposite-sex romantic partners, sexual risk, and victimization in adolescence. Child Abuse Negl. 2014;38(7):1238–48. doi: 10.1016/j.chiabu.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 54.Parikh SA. “They arrested me for loving a schoolgirl”: ethnography, HIV, and a feminist assessment of the age of consent law as a gender-based structural intervention in Uganda. Soc Sci Med. 2012;74(11):1774–82. doi: 10.1016/j.socscimed.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coleman DL, Rosoff PM. The legal authority of mature minors to consent to general medical treatment. Pediatrics. 2013;131(4):786–93. doi: 10.1542/peds.2012-2470. [DOI] [PubMed] [Google Scholar]
- 56.English A. State minor consent laws: a summary. 3rd ed. Chapel Hill, NC: Center for Health and the Law; 2010. [Google Scholar]
- 57.WHO. HIV and adolescents: guidance for HIV testing and counselling and care for adolescents living with HIV; Geneva: WHO; 2013. [Google Scholar]
- 58.Earnshaw VA, Smith LR, Chaudoir SR, Amico KR, Copenhaver MM. HIV stigma mechanisms and well-being among PLWH: a test of the HIV stigma framework. AIDS Behav. 2013;17(5):1785–95. doi: 10.1007/s10461-013-0437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nelson RM, Lewis LL, Struble K, Wood SF. Ethical and regulatory considerations for the inclusion of adolescents in HIV biomedical prevention research. J Acquir Immune Defic Syndr. 2010;54(Suppl 1):S18–24. doi: 10.1097/QAI.0b013e3181e2012e. [DOI] [PubMed] [Google Scholar]
- 60.Fortenberry JD, Martinez J, Rudy BJ, Monte D. Adolescent Trials Network for HIVAI. Linkage to care for HIV-positive adolescents: a multisite study of the adolescent medicine trials units of the adolescent trials network. J Adolesc Health. 2012;51(6):551–6. doi: 10.1016/j.jadohealth.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fortenberry JD. Health care seeking behaviors related to sexually transmitted diseases among adolescents. Am J Public Health. 1997;87(3):417–20. doi: 10.2105/ajph.87.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nadal KL. That's so Gay! Microaggressions and the Lesbian, Gay, Bisexual, and Transgender Community. Washington, DC: American Psychological Association; 2013. [Google Scholar]
- 63.Massey P, Prelip M, Calimlim B, Afifi A, Quiter E, Nessim S, et al. Findings toward a multidimensional measure of adolescent health literacy. Am J Health Behav. 2013;37(3):342–50. doi: 10.5993/AJHB.37.3.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tylee A, Haller DM, Graham T, Churchill R, Sanci LA. Youth-friendly primary-care services: how are we doing and what more needs to be done? Lancet. 2007;369(9572):1565–73. doi: 10.1016/S0140-6736(07)60371-7. [DOI] [PubMed] [Google Scholar]
- 65.Futterman DC, Peralta L, Rudy BJ, Wolfson S, Guttmacher S, Rogers AS. The ACCESS (Adolescents Connected to Care, Evaluation, and Special Services) project: social marketing to promote HIV testing to adolescents, methods and first year results from a six city campaign. J Adolesc Health. 2001;29(Suppl 3):19–29. doi: 10.1016/s1054-139x(01)00290-7. [DOI] [PubMed] [Google Scholar]
- 66.Zanoni BC, Mayer KH. The adolescent and young adult HIV cascade of care in the United States: exaggerated health disparities. AIDS Patient Care STDS. 2014;28(3):128–35. doi: 10.1089/apc.2013.0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kapogiannis BG, Legins KE, Chandan U, Lee S. Evidence-based programming for adolescent HIV prevention and care: operational research to inform best practices. J Acquir Immune Defic Syndr. 2014;66(Suppl 2):S228–35. doi: 10.1097/QAI.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 68.Coeytaux K, Kramer MR, Sullivan PS. HIV testing among United States high school students at the state and national level, Youth Risk Behavior Survey 2005–2011. Springerplus. 2014;3:202. doi: 10.1186/2193-1801-3-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Godia PM, Olenja JM, Lavussa JA, Quinney D, Hofman JJ, van den Broek N. Sexual reproductive health service provision to young people in Kenya; health service providers’ experiences. BMC Health Serv Res. 2013;13:476. doi: 10.1186/1472-6963-13-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.MacPhail CL, Pettifor A, Coates T, Rees H. “You must do the test to know your status”: attitudes to HIV voluntary counseling and testing for adolescents among South African youth and parents. Health Educ Behav. 2008;35(1):87–104. doi: 10.1177/1090198106286442. [DOI] [PubMed] [Google Scholar]
- 71.Renju J, Andrew B, Nyalali K, Kishamawe C, Kato C, Changalucha J, et al. A process evaluation of the scale up of a youth-friendly health services initiative in northern Tanzania. J Int AIDS Soc. 2010;13:32. doi: 10.1186/1758-2652-13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parker L, Maman S, Pettifor A, Chalachala JL, Edmonds A, Golin CE, et al. Barriers to provider-delivered sexual behavior counseling for youth living with HIV/AIDS in the Democratic Republic of the Congo. J HIV AIDS Soc Serv. 2013;12(3–4):1–15. doi: 10.1080/15381501.2012.748585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mathews C, Guttmacher SJ, Flisher AJ, Mtshizana YY, Nelson T, McCarthy J, et al. The quality of HIV testing services for adolescents in Cape Town, South Africa: do adolescent-friendly services make a difference? J Adolesc Health. 2009;44(2):188–90. doi: 10.1016/j.jadohealth.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 74.Ntsepe Y, Simbayi LC, Shisana O, Rehle T, Mabaso M, Ncitakalo N, et al. Perceptions about the acceptability and prevalence of HIV testing and factors influencing them in different communities in South Africa. SAHARA J. 2014;11:138–47. doi: 10.1080/17290376.2014.937355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shaw D. Access to sexual and reproductive health for young people: bridging the disconnect between rights and reality. Int J Gynaecol Obstet. 2009;106(2):132–6. doi: 10.1016/j.ijgo.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 76.Hainsworth G, Engel DM, Simon C, Rahimtoola M, Ghiron LJ. Scale-up of adolescent contraceptive services: lessons from a 5-country comparative analysis. J Acquir Immune Defic Syndr. 2014;66(Suppl 2):S200–8. doi: 10.1097/QAI.0000000000000180. [DOI] [PubMed] [Google Scholar]
- 77.Laski L, Wong S. Addressing diversity in adolescent sexual and reproductive health services. Int J Gynaecol Obstet. 2010;110(Suppl):10–12. doi: 10.1016/j.ijgo.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 78.Deogan C, Ferguson J, Stenberg K. Resource needs for adolescent friendly health services: estimates for 74 low- and middle-income countries. PLoS One. 2012;7(12):e51420. doi: 10.1371/journal.pone.0051420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Naidoo P, Chirinda W, McHunu G, Swartz S, Anderson J. Social and structural factors associated with vulnerability to HIV infection among young adults in South Africa. Psychol Health Med. 2014;19:1–11. doi: 10.1080/13548506.2014.936883. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]