Abstract
A new type of tricyclic azaphenothiazines—1,8-diazaphenothiazines—was obtained in the reaction of 2,3- and 3,4-disubstituted pyridines. The reaction ran as the Smiles rearrangement. The 1,8-diazaphenothiazine system was determined using NOE experiment and 2D NMR spectra (COSY, HSQC, HMBC). 10H-1,8-diazaphenothiazine was transformed into 10-derivatives with alkyl, aminoalkyl, amidoalkyl, sulfonamidoalkyl, and nitrogen half-mustard groups. The compounds were tested for their effects on phytohemagglutinin A-induced proliferative response of human peripheral blood mononuclear cells (PBMC) and lipopolysaccharide-induced tumor necrosis factor alpha production by human whole blood cultures. The compounds exhibited differential, dose-dependent inhibitory activities in these tests. All the compounds were low toxic against PBMC. The compounds showing the highest antiproliferative activity strongly inhibited the growth of leukemia L-1210 and colon cancer SW-948 cell lines, similarly as cisplatin, a reference drug.
Keywords: Phenothiazines, Diazaphenothiazines, Antiproliferative activity, Anticancer activity, Thiazine ring formation
Introduction
Tricyclic phenothiazines attract considerable attention because of their significant biological activities and interesting chemical features. Classical phenothiazines with aminoalkyl substituents at the nitrogen atom are the source of valuable drugs exhibiting neuroleptic, antihistaminic, antitussive, and antiemetic activities (Gupta and Kumar, 1988). The structure modifications of these compounds were carried out by introduction of new substituents, mainly at the thiazine nitrogen atom, and substitution of one or two benzene rings with homoaromatic and heteroaromatic rings. The modifications with azine rings lead to formation of azaphenothiazines. New phenothiazines can contain not only the tricyclic ring system but also tetra and pentacyclic ones with up to four additional nitrogen atoms in the aromatic rings (Silberg et al., 2006; Pluta et al., 2009, 2011). Such modifications can change potency and type of activities of the basic structures. Recent reports describe very promising anticancer, antibacterial, and anti-inflammatory activities, reversal of multidrug resistance and a potential benefit in treatment of Alzheimer’s, Creutzfeldt-Jakob’s and AIDS-associated diseases for the modified phenothiazines (Motohashi et al., 2000, 2006; Dasgupta et al., 2008; Sadandam et al., 2009; Aaron et al., 2009; Tandon et al., 2009; Pluta et al., 2011).
Our strategy for modification of the phenothiazine structure is based on the introduction of two pyridine rings instead of the benzene ones to form dipyrido[1,4]thiazines. Among ten theoretically possible dipyridothiazines types only four have been known before introduction of our research strategy, i.e., 1,6- (Maki, 1957; Takahashi and Maki, 1958a, b; Rodig et al., 1966), 1,9- (Rath, 1957), 2,7- (Kopp et al., 1963; Kopp and Strell, 1962), and 3,6-diazaphenothiazines (Okafor, 1967). Three nomenclature systems of phenothiazines with different atom numbering, valid in the sixties and seventies, were confusing. 2,7-Diazaphenothiazines described by Kopp and co-workers were in fact 3,7-diazaphenothiazines (Pluta et al., 2009). Correct 2,7-diazaphenothiazines were obtained by us and their ring system was confirmed by X-ray analysis (Morak et al., 2002; Morak and Pluta, 2007). The parent compound, 10H-2,7-diazaphenothiazine, was found to be a universal, low-toxic immunosuppressant, inhibiting both humoral and cellular immune responses, and antioxidant property (Zimecki et al., 2009; Morak-Młodawska et al., 2010; Pluta et al., 2010).
In continuation of our studies, we have worked out an efficient synthesis of a new type of dipyridothiazines, 10H-1,8-diazaphenothiazine and its 10-substituted derivatives, possessing alkyl, arylalkyl, aryl, heteroaryl and aminoalkyl, amidoalkyl, sulfonamidoalkyl, and nitrogen half-mustard type substituents. In this work, we discuss their synthesis and structures and test their activities in selected biological assays.
Results and discussion
Chemistry
It is well known that the synthesis of phenothiazines and azaphenothiazines may proceed via cyclization of diphenyl sulfides, phenyl azinyl sulfides, or diazinyl sulfides directly as the Ullmann cyclization or with the Smiles rearrangement of the S → N type depending on the reaction conditions. In the last case, the phenyl or azinyl part migrates from the sulfur atom to the nitrogen atom forming amine and subsequently phenothiazine or azaphenothiazine. The rearrangement proceeds most often under basic but also under acidic and neutral conditions. Sometimes it is impossible to state if a reaction runs with or without the rearrangement because the Ullmann and Smiles products are the same or very similar (Pluta et al., 2009).
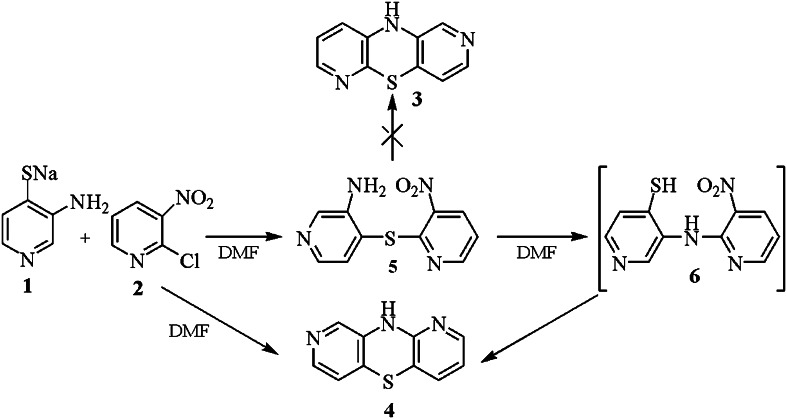
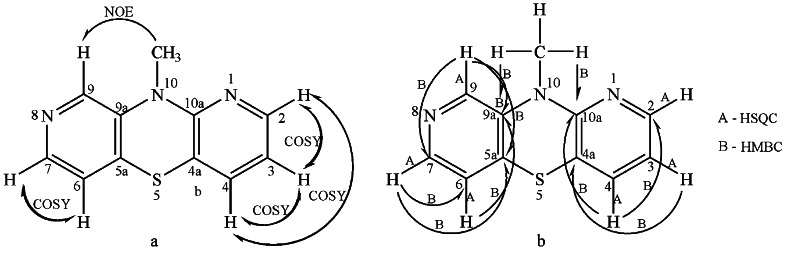
We started the synthesis with a reaction of sodium 3-aminopyridinothiolate (1) with 2-chloro-3-nitropyridine (2) in refluxing DMF. After isolation and purification of the products we found dipyridothiazine (2,6-diazaphenothiazine 3 or 1,8-diazaphenothiazine 4) as the major product in 88 % yield and 3′-amino-3-nitro-2,4′-dipyridyl sulfide (5) in 9 % yield as the minor product (Scheme 1). The mass spectrum confirmed the diazaphenothiazine structure (M = 201) but the 1H NMR spectrum does not point at the structure 3 or 4 as both compounds are built of the 2,3- and 3,4-pyridinediyl units giving a singlet (7.90 ppm), two doublets (7.18, 8.07 ppm), and three doublets of doublet (6.90, 7.26, 8.09 ppm) of the proton signals. To unquestionably determine the diazaphenothiazine structure, we transformed the product into the N-methyl derivative (vide infra). The differentiation between 1,8- and 2,6-diazaphenothiazine system was based on the NOE experiment of this derivative. Irradiation of the methyl protons at 3.44 ppm (Scheme 2) gave an enhancement only of one proton, the singlet signal at 7.90 ppm by 7.06 % what pointed at the 1,8-diazaphenothiazine system and the derivative 7 (Scheme 3).
Scheme 1.
Synthesis if 10H-diazaphenothiazine 3 from disubstituted pyridines 2 and 3 and dipyridyl sulfide 5
Scheme 2.
The NMR experiments for compound 7: a NOE and COSY, b HSQC and HMBC
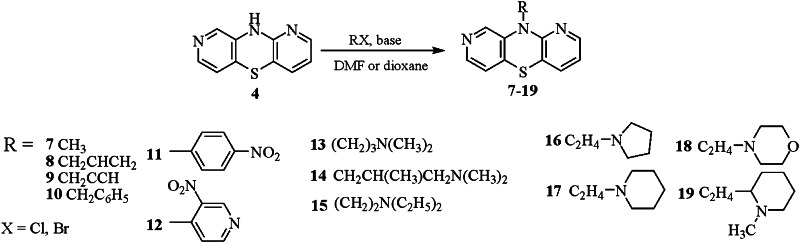
Scheme 3.
Synthesis of 10-dialkylaminoalkyl-1,8-diazaphenothiazines 7–19
The full 1H NMR assignment of the proton signals came from the homonuclear 1H–1H correlation (COSY). Three most deshielded proton signals at 7.90, 8.07, and 8.09 ppm were considered as the α-pyridinyl proton signals. The doublet of doublet signal at 6.90 ppm, considered as the β-pyridinyl proton, was intercorrelated (ortho-coupling) with the signals at 8.09 ppm and at 7.26 ppm (γ-pyridinyl proton) with the coupling constants of 4.9 and 7.2 Hz, respectively. The signal at 7.26 ppm was weak intercorrelated (para-coupling) with the signal at 8.09 ppm with the coupling constant of 1.8 Hz. The protons were assigned as H3, H4, and H2, respectively. The α-pyridinyl proton signal at 8.07 ppm was correlated with the signal at 7.18 ppm (β-pyridinyl proton) with the coupling constant of 5.4 Hz. These protons were assigned as H7 and H6. The proton signal assignment was presented in Scheme 2.
The new diazaphenothiazine system was also determined by the 13C NMR spectrum. The spectrum revealed eleven carbon signals: one primary, six tertiary, and four quaternary. The methyl group was observed at 32.8 ppm. The full assignment of carbon signals came from 2D NMR: HSQC (the tertiary carbon atoms connected with the hydrogen atoms) and HMBC (the tertiary and quaternary carbon atoms correlated with the hydrogen atoms via two and mainly three bonds). The proton-carbon correlation was presented in Scheme 2.
The product structure as 10H-1,8-diazaphenothiazine 4 is the evidence for the Smiles rearrangement of the S–N type of resulted dipyridinyl sulfide 5. Heating sulfide 5 in refluxing DMF gave 10H-1,8-diazaphenothiazine (4) in 88 % yield. The reaction run through the formation of dipyridinyl amine 6 which (not isolated) very easily cyclized to diazaphenothiazine 4 (Scheme 1). The 1,8-diazaphenothiazine ring system was confirmed by X-ray analysis of the nitropyridyl derivative 12 (obtained by independent way from appropriate sulfide containing three nitropyridyl moieties via the double Smiles rearrangement), published separately (Morak-Młodawska et al., 2012).
The parent 10H-1,8-diazaphenothiazine 4 was transformed into 10-derivative in one or three steps. The alkylation with alkyl (methyl, allyl, propargyl, benzyl), aryl (p-nitrophenyl) and heteroaryl (3-nitro-4-pyridinyl) halides and aminoalkyl (3-dimethylaminopropyl, 3-dimethylamino-2-methylpropyl, 2-diethylaminoethyl, 1-pyrrolidinoethyl, 1-piperidinoethyl, 1-methyl-2-piperidinoethyl, 1-morpholinoethyl) in DMF in the presence of sodium hydride or potassium tert-butoxide and in dioxane in the presence of sodium hydroxide gave derivatives 7–19 in good yields Scheme 3).
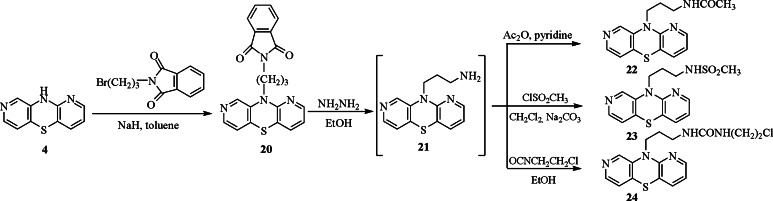
The substrate 4 was also transformed into compounds possessing aminopropyl derivative substituents. Reaction of compound 4 with the phthalimidopropyl bromide in toluene in the presence of sodium hydride gave the phthalimidopropyl derivative 20. The hydrolysis of this compound with hydrazine in ethanol led to aminopropyl derivative 21 which quickly (because of their instability) underwent reactions with acetic anhydride, methanesulfonyl chloride, and 2-chloroethyl isocyanate to give acetamidopropyl, methanesulfonamidopropyl, and chloroethylureidopropyl derivatives 22–24 in 63–80 % yield (Scheme 4).
Scheme 4.
Synthesis of 10-phthalimidopropyl-1,8-diazaphenothiazine 20 and transformations to the acetamidopropyl, methanesulfonamidopropyl, and chloroethylureidopropyl derivatives 22–24
Biological activities
10-substituted 1,8-diazaphenothiazines 4, 7–10, 12–20, and 22–24, possessing various substituents (hydrogen atom, alkyl groups with single, double, and triple bonds, arylalkyl, heteroaryl, alkylaminoalkyl, amidoalkyl, sulfonamidoalkyl and alkyl with a half-mustard-type group) were tested for their biological activities. The tests included the proliferative response of human peripheral blood mononuclear cells (PBMC) induced by phytohemagglutinin A (PHA), the cytotoxic effect on human PBMC and lipopolysaccharide (LPS)-induced production of tumor necrosis factor alpha (TNF-α). The combined results of the tests are presented in Table 1. The most promising compounds, selected on the basis of their strong antiproliferative effects, were tested for growth inhibition of leukemia L-1210 cells and colon carcinoma SW-948 cells in vitro.
Table 1.
Activities of 10-substituted 1,8-diazaphenothiazines in selected immunological assays
| No. | Cytotoxicity against PBMC | Inhibition of PHA-induced PBMC proliferation | TNF-α inhibition | |||
|---|---|---|---|---|---|---|
| 10 µg/ml | 50 µg/ml | 1 µg/ml | 10 µg/ml | 50 µg/ml | 5 µg/ml | |
| 4 | 6.7 | 21.4 | 5.0 | 74.4 | 78.6 | 50.4 |
| 7 | 0.8 | 1.7 | 9.6 | 22.9 | 45.6 | 76.4 |
| 8 | −0.3 | −6.0 | 19.0 | 26.0 | 55.6 | 89.3 |
| 9 | −1.1 | 8.8 | 9.3 | 24.4 | 41.2 | 87.4 |
| 10 | 2.0 | 2.6 | 13.6 | 26.8 | 45.5 | 85.9 |
| 12 | 6.6 | 8.1 | 4.1 | 5.2 | 26.2 | 54.8 |
| 13 | −3.6 | 15.0 | 5.7 | 20.9 | 81.1 | 86.7 |
| 14 | −0.7 | 11.9 | 1.4 | 19.2 | 59.4 | 89.1 |
| 15 | 1.3 | 12.1 | −6.8 | −5.4 | 59.6 | 75.0 |
| 16 | 0.9 | 10.0 | −0.9 | −2.9 | 47.0 | 85.6 |
| 17 | 1.5 | 7.3 | −0.9 | −0.5 | 18.0 | 47.6 |
| 18 | −1.4 | 18.7 | −3.4 | 5.1 | 67.4 | 73.1 |
| 19 | −4.5 | 4.8 | −0.9 | 7.0 | 18.2 | 46.1 |
| 20 | −2.0 | −0.1 | 3.6 | 12.5 | 42.2 | 76.0 |
| 22 | −5.0 | 6.7 | 8.9 | 16.2 | 62.5 | 5.8 |
| 23 | −0.9 | 12.5 | 9.4 | 19.3 | 50.2 | 48.6 |
| 24 | −1.6 | 4.5 | 8.4 | 12.4 | 46.8 | 7.3 |
The table shows the degree of cytotoxicity against PBMC, effects on PHA-induced proliferative response of human PBMC and LPS-induced TNF-α production by these cells. The results are given in percentage inhibition as compared with appropriate DMSO controls. Positive values denote inhibition, negative stimulation
The proliferation test was performed at the concentrations of 1, 10, and 50 µg/ml. A strong activity (inhibition over 70 %) was exhibited by compound: 4 at 10 µg/ml and compound 13 at 50 µg/ml in comparison with the control cultures (culture medium containing appropriate dilution of DMSO). These compounds possess the hydrogen atom and dimethylaminopropyl groups at position 10. A moderate activity (inhibition about 60 % at 50 µg/ml) was exhibited by compounds: 14, 15, 18, and 22 (the dimethylamino-2-methylpropyl, diethylaminoethyl, 1-methyl-2-piperidinoethyl, and acetamidopropyl groups). Other compounds were weakly active or inactive.
In order to check whether the inhibitory effects of the compounds were not caused by cytotoxicity, the compounds were tested for their effects on viability of PBMC. All the compounds exhibited very weak cytotoxic properties with the inhibition of cell viability not exceeding 22 % even at 50 μg/ml. Because lack of toxicity at 1 μg/ml that concentration of the compounds was deleted in Table 1.
The compounds were also tested for their inhibitory effects on LPS-induced TNF-α production at the concentrations of 5 and 25 μg/ml. No further inhibition of TNF-α production was registered for 25 μg/ml and, therefore, not shown in Table 1. Compounds 8–10, 13, 14, and 16 showed inhibitions of over 85 % at 5 μg/ml.
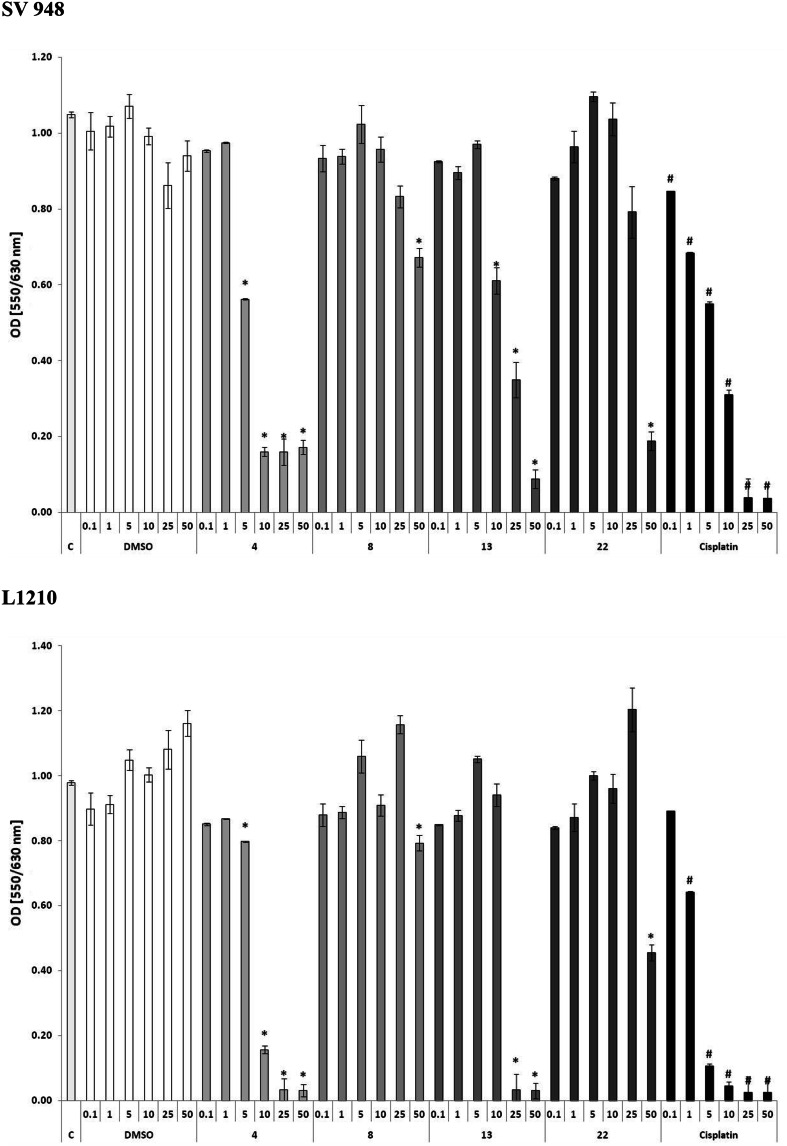
The most promising compounds 4, 8, 13, and 22 (being strongly antiproliferative and low cytotoxic) were selected for evaluation of anticancer activities against the cancer cell lines at the concentrations of 0.1–50 µg/ml using cisplatin as the reference drug (Fig. 1). The most active was compound 4, exhibiting similar anticancer activity to cisplatin against colon carcinoma SV-948 cells at the concentration of 5 µg/ml and against leukemia L-1210 cells at 10 µg/ml (Table 2). Compounds 13 and 22 showed strong inhibition at 10 µg/ml. It is worth noting that cisplatin showed high toxicity killing of 50 % of granulocyte/macrophage progenitor cells already at 0.9 μg/ml after 1 h of culture (Umbach et al., 1984). The drug is also nephrotoxic (Yao et al., 2007). The ability of the compounds (in particular 4 and 13) to strongly inhibit TNF-α may be of additional advantage in anti-tumor therapy. Although TNF-α may have a dual role in tumor progression (Wajant, 2009) some anti-tumor strategies aim at inhibition of its activity (Guadagni et al., 2007).
Fig. 1.
The anticancer activities of selected compounds at concentrations of 0.1–50 µg/ml. L-1210 and SW-948 cell lines were used in the study. The results are presented as the mean optical density ± SE (*versus DMSO; #versus Control, p < 0.001)
Table 2.
Anticancer activity (IC50) of selected compounds 4 and 13 and cisplatin as a reference drug against cancer lines SW-948 and L-1210
| Compound | IC50 (μg/ml) | |
|---|---|---|
| SW-948 | L-1210 | |
| 4 | 5.47 | 7.41 |
| 13 | 14.95 | 6.03 |
| Cisplatin | 5.52 | 2.13 |
It is interesting that the most active was compound 4, possessing the hydrogen atom instead of the pharmacophoric aminoalkyl substituents at the thiazine nitrogen atom. It seems that compound 4 displays a different mechanism of action than that found for substituted phenothiazines and azaphenothiazines with the acylaminoalkyl and chloroethylureidoalkyl groups (Motohashi et al., 2000, 2006; Pluta et al., 2011).
Conclusion
We report here a few step synthesis and biological activity of novel tricyclic 10H- and 10-substituted 1,8-diazaphenothiazines. The synthesis was run through the Smiles rearrangement of S–N type. The structure diazaphenothiazine system was elucidated using the NOE experiment and 2D (1H–1H and 1H–13C) spectra. Some 1,8-diazaphenothiazines exhibited antiproliferative, anticancer, TNF-α inhibitory activities with low cytotoxicity. The new diazaphenothiazine system was found to be pharmacophoric as 10H-1,8-diazaphenothiazine was the most active, with anticancer activities comparable to that of cisplatin. This compound seems to be a useful starting point for further study to found more potent anticancer agents by introduction of new substituents at the thiazine nitrogen atom.
Experimental
Chemistry
Melting points were determined in open capillary tubes on a Boetius melting point apparatus and are uncorrected. The 1H NMR, COSY, NOE HSQC, HMBC spectra were recorded on a Bruker Fourier 300 and Bruker DRX spectrometers at 300 and 600 MHz in deuteriochloroform with tetramethylsilane as the internal standard. The 13C NMR spectrum was recorded at 75 MHz. Electron Impact mass spectra (EI MS) and Fast Atom Bombardment mass spectra (FAB MS, in glycerol) were run on a Finnigan MAT 95 spectrometer at 70 eV. The thin layer chromatography were performed on silica gel 60 F254 (Merck 1.05735) with CHCl3-EtOH (5:1 and 10:1 v/v) and on aluminum oxide 60 F254 neutral (type E) (Merck 1.05581) with CHCl3-EtOH (10:1 v/v) as eluents.
Synthesis of 10H-1,8-diazaphenothiazine (4)
From sodium 3-amino-4-pyridinethiolate (1) and 2-chloro-3-nitropyridine (2)
To a solution of 148 mg (1 mmol) sodium 3-amino-4-pyridinethiolate (1) in 10 ml dry DMF was added 158 mg (1 mmol) 2-chloro-3-nitropyridine (2). The mixture was stirred at rt 3 h and next was refluxed 3 h. After cooling, the reaction mixture was evaporated in vacuo. The dry residue was dissolved in CHCl3 and purified by column chromatography (aluminum oxide, CHCl3) to give
10H-1,8-diazaphenothiazine (4) (0.125 g, 62 %) mp 135–136 °C.
1H NMR (CDCl3) δ 6.73 (dd, J = 7.5 Hz, J = 5.1 Hz, 1H, H3), 6.84 (d, J = 5.0 Hz, 1H, H6), 7.11 (dd, J = 7.5 Hz, J = 1.5 Hz, 1H, H4), 7.69 (board s, 1H, N–H), 7.84 (dd, J = 5.1 Hz, J = 1.5, 1H, H2), 7.89 (s, 1H, H9), 7.95 (d, J = 5,0 Hz, 1H, H7). 13C NMR (CDCl3) δ 112.2 (C4a), 118.9 (C3), 120.5 (C6), 128.9 (C5a), 134.3 (C4), 134.4 (C9), 136.9 (C9a), 143.1 (C7), 145.9 (C2), 152.1 (C10a). EI MS m/z: 201 (M, 100), 174 (M-HCN, 30). Anal. Calcd for: C10H7N3S, C 59.68, H 3.51, N 20.88; S 15.93. Found: C 59.49, H 3.53, N 20.80; S 15.79.
-
(b)
3-amino-3′-nitro-2,4′-dipyridinyl sulfide (5) (0.025 g, 9 %) mp 147–148 °C.
In cyclization of 3-amino-3′-nitro-2,4′-dipyridinyl sulfide (5)
The brown solution of 124 mg (0.5 mmol) 3-amino-3′-nitro-2,4′-dipyridinyl sulfide 5 in 5 ml dry DMF was refluxed for 4 h. After cooling, the reaction mixture was evaporated in vacuo. The dry residue was dissolved in CHCl3 and purified by column chromatography (aluminum oxide, CHCl3) to give 10H-1,8-diazaphenothiazine (4) (0.088 g, 88 %)
Synthesis of 10-substituted 1,8-diazaphenothiazines 7, 8, and 10–12
To a solution of 10H-1,8-diazaphenothiazine (4) (0.100 g, 0.5 mmol) in dry DMF (5 ml) NaH (0.024 g, 1 mmol, 60 % NaH in mineral oil was washed out with hexane) was added. The reaction mixture was stirred at room temperature for 1 h and then alkyl, aryl, and heteroaryl halides (methyl iodide, allyl bromide, benzyl chloride, 1-fluoro-4-nitrobenzene, 4-chloro-3-pyridine, 1.5 mmol) were added and the stirring was continued for 24 h. The mixture was poured into water (15 ml), extracted with CHCl3 (3 × 10 ml), and dried using Na2SO4. The obtained product was purified by column chromatography (aluminum oxide, CHCl3) to give
10-Methyl-1,8-diazaphenothiazine (7) (0.085 g, 79 %); mp 82–83 °C
1H NMR (CDCl3) δ 3.44 (s, 3H, CH3), 6.90 (dd, J = 7.2 Hz, J = 4.9 Hz, 1H, H3), 7.18 (d, J = 5.4 Hz, 1H, H6), 7.26 (dd, J = 7.8 Hz, J = 1.8 Hz, 1H, H4), 7.90 (s, 1H, H9), 8.07 (d, J = 5.4 Hz, 1H, H7), 8.09 (dd, J = 4.9 Hz, J = 1.8 Hz, 1H, H2). 13C NMR (CDCl3) δ 32.8 (NCH3), 115.0 (C4a), 118.2 (C3), 120.8 (C6), 131.9 (C5a), 134.4 (C4), 135.2 (C9), 139.9 (C9a), 143.9 (C7), 145.8 (C2), 154.3 (C10a). EI MS m/z: 215 (M, 100), 200 (M-CH3, 80). Anal. Calcd for: C11H9N3S C 61.37, H 4.21, N 19.52. Found: C 61.22; H 4.23; N 19.41.
10-Allyl-1,8-diazaphenothiazine (8) (0.085 g, 70 %); an oil
1H NMR (CDCl3) δ 4.66 (m, 2H, N-CH2), 5.32 (m, 2H, =CH2), 5.96 (m, 1H, CH), 6.82 (dd, J = 7.5 Hz, J = 5.1 Hz, 1H, H3), 7.04 (d, J = 5.0 Hz, 1H, H6), 7.18 (dd, J = 7.5 Hz, J = 1.5 Hz, 1H, H4), 7.89 (s, 1H, H9), 8.02 (m, 2H, H2, H7). 13C NMR (CDCl3) δ 47.6 (NCH2), 113.0 (C4a), 118.1 (C3), 119.2 (C6), 121.1 (CH2=), 130.2 (C5a), 131.2 (C4), 134.5 (C9), 137.9(–CH=), 138.8 (C9a), 140.2 (C7), 146.4 (C2), 151.9 (C10a). EI MS m/z: 241 (M, 50), 200 (M-CH2CHCH2, 100). Anal. Calcd for: C13H11N3S C 64.70, H 4.59, N 17.41. Found: 64.58; H 4.58; N 17.31.
10-Benzyl-1,8-diazaphenothiazine (10) (0.095 g, 65 %); an oil
1H NMR (CDCl3) δ 5.34 (s, 2H, CH2), 6.76 (dd, J = 7.2 Hz, J = 4.8 Hz, 1H, H3), 6.87 (d, J = 5.0 Hz, 1H, H6), 7.22 (dd, J = 7.2 Hz, J = 1.4 Hz, 1H, H4), 7.29 (m, 5H, C6H5), 7.81 (s, 1H, H9), 7.96 (m, 2H, H2, H7). EI MS m/z: 291 (M, 80), 200 (M-CH2C6H5, 100). Anal. Calcd for: C17H13N3S C 70.08, H 4.50, N 14.42. Found: C 70.00; H 4.52; N 14.29.
10-(4′-Nitrophenyl)-1,8-diazaphenothiazine (11) (0.120 g, 74 %); mp 171–172 °C
1H NMR (CDCl3) δ 6.88 (dd, J = 7.2 Hz, J = 5.0 Hz, 1H, H3), 6.95 (d, J = 5.0 Hz, 1H, H6), 7.21 (dd, J = 7.2 Hz, J = 1.6 Hz, 1H, H4), 7.55 (m, 2H, 2H C6H4), 7.81 (dd, J = 5.0 Hz, J = 1.6 Hz, 1H, H2), 7.96 (d, J = 5.0 Hz, 1H, H7), 8.15 (s, 1H, H9), 8.50 (m, 2H, 2H C6H4). EI MS m/z: 322 (M, 100), 276 (M-NO2, 30), 200 (M-NO2C6H4, 18). Anal. Calcd for: C16H10N4O2S C 59.62, H 3.13, N 17.38. Found: C 59.44; H 3.12; N 17.29.
10-(3′-Nitro-4′-pyridinyl)-1,8-diazaphenothiazine (12) (0.130 g, 80 %); mp 189–190 °C
lit. (Morak-Młodawska et al., 2012) mp 189–190 °C.
Synthesis of 10-propargyl-1,8-diazaphenothiazines (9)
To a suspension of 100 mg (0.5 mmol) 10H-1,8-diazaphenothiazine (4) in 10 ml DMF was added 80 mg (0.72 mmol) potassium tert-butoxide. The mixture was stirred at room temperature for 1 h. Then to the solution was added dropwise a solution of propargyl bromide 80 mg (0.64 mmol) in toluene. The solution stirred at room temperature for 24 h and poured onto water (20 ml), extracted with methylene chloride (20 ml), dried with Na2SO4, and evaporated to the brown oil. The residue was purified by column chromatography (silica gel, CHCl3) to yield 85 mg (71 %) of 10-propargyl-1,8-diazaphenothiazine (9), mp 96–97 °C.
1H NMR: δ 2.39 (t, J = 2.5 Hz, 1H), 4.61 (t, J = 2.5 Hz, 2H), 6.92 (dd, J = 7.5 Hz, J = 5.1 Hz 1H, H3), 7.23 (m, 2H, H4, H6), 8.10 (d, J = 5.5 Hz, 1H, H7), 8.13 (s,1H, H9), 8.15 (dd, J = 5.1 Hz, J = 1.3 Hz, 1H, H2). EI MS: 239 (M, 100), 200 (M-CH2CCH, 85). Anal. Calcd for: C13H9N3S C 65.25, H 3.79, N 17.56. Found: C 65.20; H 3.77; N 17.39.
Synthesis of 10-substituted 1,8-diazaphenothiazines 13–19
To a solution of 10H-1,8-diazaphenothiazine (4) (0.100 g, 0.5 mmol) in dry dioxane (10 ml) NaOH (0.20 g, 5 mmol) was added. The mixture was refluxed 1 and 5 h then the hydrochlorides of dialkylaminoalkyl chloride (3-dimethylaminopropyl, 2-diethylaminoethyl, 3-dimethylamino-2-methylpropyl) and hydrochlorides of cycloaminoethyl chloride (N-(2-chloroethyl)-pyrrolidine, 2-(1-methyl-2′-piperydinyl)ethylchloride, N-(2-chloroethyl)piperidine, N-(2-chloroethyl)morpholine, 1.5 mmol) were added. The reaction mixture was refluxed for 24 h. After cooling, dioxane was evaporated in vacuo and residue was dissolved in CHCl3 (10 ml). The extracts were washed with water, dried with anhydrous sodium sulfate, and evaporated in vacuo. The obtained product was purified by column chromatography (aluminum oxide, CHCl3-EtOH 10:1) to give
10-(3′-Dimethylaminopropyl)-1,8-diazaphenothiazine (13) (0.100 g, 70 %); an oil
1H NMR: δ 2.00 (m, 2H, CH2), 2.26 (s, 6H, 2CH3), 2.44 (t, J = 7.5 Hz, 2H, NCH2), 4.10 (t, J = 7.5 Hz, 2H, NCH2), 6.73 (m, 1H, H3), 6.89 (d, J = 4.8 Hz, 1H, H6), 7.16 (d, J = 7.2 Hz, 1H, H4), 7.99 (m, 2H, H2, H7), 8.08 (s, 1H, H9). 13C NMR (CDCl3) δ 24.2 (CH2), 42.9 (CH2), 45.5 (N(CH3)2), 57.13 (CH2), 114.6 (C4a), 118.1 (C3), 120.8 (C6), 131.8 (C5a), 134.7 (C4), 135.5 (C9), 138.7 (C9a), 143.6 (C7), 145.6 (C2), 153.6 (C10a). FAB MS m/z: 287 (M+1, 100), 202 (M+1-C3H6NC2H6, 19). Anal. Calcd for C15H18N4S C 62.91; H 6.33; N 19.56. Found: C 62.78; H 6.30; N 19.39.
10-(3′-Dimethylamino-2′-methylpropyl)-1,8-diazaphenothiazine (14) (0.125 g, 83 %); an oil
1H NMR: δ 1.02 (d, J = 6.5 Hz, 3H, CH3), 2.39 (m, 9H, 2CH3, CH2, CH), 4.15 (m, 2H, CH2), 6.80 (dd, J = 7.4 Hz, J = 5.2 Hz, 1H, H3), 6.85 (d, J = 5.0 Hz, 1H, H6), 7.20 (dd, J = 7.4 Hz, J = 1.4 Hz, 1H, H4), 8.02 (dd, J = 5.2 Hz, J = 1.4 Hz, 1H, H2), 8.09 (d, J = 5.0 Hz, 1H, H7), 8.15 (s, 1H, H9). FAB MS m/z: 301 (M+1, 100), 202 (M+1-C2H4NC4H10, 18). Anal. Calcd for: C16H20N4S C 63.97; H 6.71; N 18.65. Found: C 63.80; H 6.73; N 18.42.
10-(2′-Diethylaminoethyl)-1,8-diazaphenothiazine (15) (0.113 g, 75 %); an oil
1H NMR: δ 1.04 (t, J = 7.3 Hz, 6H, 2CH3), 2.62 (q, J = 7.3 Hz, 4H, 2CH2), 3.62 (t, J = 7.4 Hz, 2H, CH2), 4.15 (t, J = 7.4 Hz, 2H, CH2), 6.76 (dd, J = 7.2 Hz, J = 5.1 Hz, 1H, H3), 6.83 (d, J = 5.0 Hz, 1H, H6), 7.16 (dd, J = 7.2 Hz, J = 1.2 Hz, 1H, H4), 7.96 (dd, J = 5.1 Hz, J = 1.6 Hz, 1H, H2), 8.03 (d, J = 5.0 Hz, 1H, H7), 8.09 (s, 1H, H9). FAB MS m/z: 301 (M+1, 100), 202 (M+1-C2H4NC4H10, 25). Anal. Calcd for: C16H20N4S C 63.97; H 6.71; N 18.65. Found: C 63.81; H 6.73; N 18.41.
10-(2′-Pyrrolidinylethyl)-1,8-diazaphenothiazine (16) (0.110 g, 75 %); an oil
1H NMR (CDCl3) δ 1.90 (m, 4H, 2CH2), 2.72 (m, 4H, 2CH2), 3.09 (t, J = 7.2 Hz, 2H, CH2), 4.35 (t, J = 7.2 Hz, 2H, NCH2), 6.70 (dd, J = 7.6 Hz, J = 5.0 Hz, 1H, H3), 6.83 (d, J = 5.0 Hz, 1H, H6), 7.17 (dd, J = 7.2 Hz, J = 1.5 Hz 1H, H4), 7.97 (dd, J = 5.0 Hz, J = 1.5 Hz, 1H, H2), 8.04 (d, J = 5.0 Hz, 1H, H7), 8.19 (s, 1H, H9). FAB MS m/z: 299 (M+1, 100), 202 (M+1-C2H4NC4H8, 29). Anal. Calcd for: C16H18N4S C 64.40; H 6.08; N 18.78. Found: C 64.25; H 6.05; N 18.55.
10-(2′-Piperydinylethyl)-1,8-diazaphenothiazine (17) (0.110 g, 70 %); an oil
1H NMR (CDCl3) δ 1.47 (m, 2H, CH2),1.63 (m, 4H, 2CH2) 2.54 (m, 4H, 2CH2), 2.75 (t, J = 6.8 Hz, 2H, CH2), 4.22 (t, J = 6.8 Hz, 2H, NCH2), 6.73 (dd, J = 7.6 Hz, J = 5.0 Hz, 1H, H3), 6.85 (d, J = 5.0 Hz, 1H, H6), 7.14 (dd, J = 7.6 Hz, J = 1.6 Hz 1H, H4), 7.97 (dd, J = 5.0 Hz, J = 1.6 Hz, 1H, H2), 8.03 (d, J = 5.0 Hz, 1H, H7), 8.18 (s, 1H, H9). FAB MS m/z: 313 (M+1, 100), 202 (M+1-C2H4NC5H10, 20). Anal. Calcd for: C17H20N4S: C 65.35; H 6.45; N 17.93. Found: C 65.22; H 6.47; N 17.80.
10-(1′-Methyl-2′-piperydinylethyl)-1,8-diazaphenothiazine (18) (0.116 g, 72 %); an oil
1H NMR (CDCl3) δ 1.30–2.15 (m, 7H), 2.36 (s, 3H, NCH3), 2.85 (m, 1H, CH), 4.0 (m, 2H, NCH2), 6.73 (dd, J = 7.6 Hz, J = 5.1 Hz, 1H, H3), 6.87 (d, J = 5.0 Hz, 1H, H6), 7.14 (dd, J = 7.6 Hz, J = 1.6 Hz, 1H, H4), 7.97 (dd, J = 5.1 Hz, J = 1.6 Hz, 1H, H2), 8.03 (d, J = 5.0 Hz, 1H, H7), 8.06 (s, 1H, H9). FAB MS. 327 (M+H, 80), 313 (M+1-CH3 100). Anal. Calcd for: C18H22N4S C 66.22; H 6.79; N 17.16. Found: C 66.17; H 6.75; N 17.03.
10-(2′-Morpholinylethyl)-1,8-diazaphenothiazine (19) (0.106 g, 68 %); an oil
1H NMR (CDCl3) δ 1.67 (m, 4H, 2CH2), 2.59 (m, 4H, 2CH2), 2.82 (t, J = 6.6 Hz, 2H, CH2), 4.22 (t, J = 6.6 Hz, 2H, NCH2) 6.75 (dd, J = 7.2 Hz, J = 5.1 Hz, 1H, H3), 6.88 (d, J = 5.0 Hz, 1H, H6), 7.15 (dd, J = 7.6 Hz, J = 1.6 Hz 1H, H4), 7.97 (dd, J = 5.1 Hz, J = 1.6 Hz, 1H, H2), 8.03 (d, J = 5.0 Hz, 1H, H7), 8.16 (s, 1H, H9). FAB MS m/z: 315 (M+1, 40), 202 (M+1-C2H4NOC4H8, 15), 114 (C2H4NC5H10, 100). Anal. Calcd for: C16H18N4OS: C 61.12; H 5.77; N 17.82. Found: C 61.03; H 5.73; N 17.68.
Synthesis of 10-phthalimidopropyl-1,8-diazaphenothiazines (20)
To a stirred solution of 10H-1,8-diazaphenothiazine (4) (0.100 g, 0.5 mmol) in dry toluene (20 ml) NaH (0.12 g, 5 mmol, washed out with hexane) was added. The mixture was stirred for 20 min at rt, then refluxed for 1 h and a solution of 1.5 mmol, N-(3-bromopropyl) phthalimide 0.405 g, in toluene (10 ml) was added. The mixture was refluxed for 24 h. After cooling, the resulted solid was filtered off, toluene was evaporated in vacuo and the residue was purified by column chromatography (aluminum oxide, CHCl3) to give 10-(3′-phthalimidopropyl)-1,8-diazaphenothiazine (20) (0.110 g, 70 %), mp 40–41 °C.
1H NMR (CDCl3) δ 2.39 (m, 2H, CH2), 3.86 (t, J = 6.1 Hz, 2H, NCH2), 4.13 (t, J = 6.0 Hz, 2H, NCH2), 6.77 (dd, J = 7.1 Hz, J = 4.9 Hz Hz, 1H, H3), 6.88 (d, J = 5.0 Hz, 1H, H6), 7.14 (dd, J = 7.1 Hz, J = 1.4 Hz, 1H, H4), 7.71 (m, 2Hphthalimide), 7.79 (dd, dd, J = 4.9 Hz, J = 1.4 Hz, 1H, H2), 7.82 (m, 2Hphthalimide), 7.98 (s, 1H, H9), 8.07 (d, J = 5.0 Hz, 1H, H7). FAB MS m/z: 389 (M+H, 100), 201 (M+1-(CH2)3N(CO)2C6H4, 30). Anal. calcd. For C21H16N4O2S: C 64.93, H 4.15, N 14.42. Found: C 64.82; H 4.14; N 14.29.
Hydrolysis of 10-phthalimidopropyl-1,8-diazaphenothiazine (20)
To a solution of 10-phthalimidopropyl-1,8-diazaphenothiazine (20) (0.388 g, 1 mmol) in EtOH (20 ml) 80 % aqueous solution of hydrazine (0.2 ml, 5 mmol) was added. The mixture was refluxed for 2 h. After cooling, the reaction mixture was acidified with conc. hydrochloric acid to pH 2. The solution was concentrated and the resulted solid was filtered off. The filtrate was alkalized with 10 % aqueous solution of sodium hydroxide and extracted with CHCl3 (3 × 10 ml). The extracts were washed with water, dried with anhydrous sodium sulfate, and evaporated in vacuo. The obtained residue with 10-aminopropyl-1,8-diazaphenothiazine (21) was fast used in the synthesis of the amide derivatives of 1,8-diazaphenothiazines (22–24).
Synthesis of 10-(3′-acetamidopropyl)-1,8-diazaphenothiazine (22)
To a suspension with the oil of 10-aminopropyl-1,8-diazaphenothiazine (21)(0.129 g, 0.5 mmol) in pyridine (5 ml) acetic anhydride (1.48 ml, 1.5 mmol) was added and the mixture was stirred at rt for 2 h. After evaporation of pyridine in vacuo the residue was dissolved in CHCl3 (10 ml). The solution was washed with water, dried with anhydrous sodium sulfate, and evaporated in vacuo. The residue was purified by column chromatography (aluminum oxide, CHCl3) to give 0.120 g (80 %) 10-(3′-acetamidopropyl)-2,7-diazaphenothiazine (22), mp 120 °C.
1H NMR (CDCl3) δ 2.05 (s, 3H, CH3), 2.07 (m, 2H, CH2), 3.44 (m, 2H, NCH2), 3.96 (t, J = 6.6 Hz, 2H, NCH2), 5.99 (broad s, 1H, NH), 6.73 (dd, J = 7.2 Hz, J = 5.0 Hz, 1H, H3), 6.85 (d, J = 5.0 Hz, 1H, H6), 7.14 (dd, J = 7.2 Hz, J = 1.4 Hz, 1H, H4), 7.97 (dd, J = 5.0 Hz, J = 1.4 Hz 1H, H2), 8.03 (d, J = 5.0 Hz, 1H, H7), 8.18 (s, 1H, H9). FAB MS m/z: 301 (M+H, 100), 202 (M+1–C3H5NHCOCH3, 30). Anal. calcd. For C15H16N4OS: C 59.98; H 5.37; N 18.65. Found: C 59.83; H 5.35; N 18.55.
Synthesis of 10-(3′-methanesulfonamidopropyl)-1,8-diazaphenothiazine (23)
To a stirred solution of oil with 10-aminopropyl-1,8-diazaphenothiazine (21) (0.129 g, 0.5 mmol) in a mixture of CH2Cl2 (5 ml) and 10 % aqueous Na2CO3 solution (5 ml) a solution of methanesulfonyl chloride (0.12 ml, 1.5 mmol) in CH2Cl2 (3 ml) was added. The solutions were stirred at rt for 24 h. The organic phase was separated and aqueous phase was extracted with CH2Cl2 (2 × 5 ml). The combined extracts were washed with water (10 ml) and dried with anhydrous sodium sulfate and evaporated in vacuo. The residue was purified by column chromatography (aluminum oxide, CH2Cl2) to give 0.125 g (74 %) 10-(3′-methanesulfonamidopropyl-1,8-diazaphenothiazine (23) as an oil.
1H NMR (CDCl3) δ 2.08 (m, 2H, CH2), 2.94 (s, 3H, CH3), 3.42 (m, 2H, NCH2), 4.02 (t, J = 6.9 Hz, 2H, NCH2), 5.57 (broad s, 1H, NH), 6.74 (dd, J = 7.2 Hz, J = 5.0 Hz, 1H, H3), 6.84 (d, J = 5.0 Hz, 1H, H6), 7.14 (dd, J = 7.2 Hz, J = 1.4 Hz 1H, H4), 7.97 (dd, J = 5.0 Hz, J = 1.4 Hz 1H, H2), 8.03 (d, J = 5.0 Hz, 1H, H7), 8.18 (s, 1H, H9). FAB MS m/z: 337 (M+1, 100), 202 (M+1-C3H5NHSO2CH3,30). Anal. calcd. For C14H16N4O2S2: C 49.98; H 4.79; N 16.65. Found: C 49.88; H 4.74; N 16.39.
Synthesis of 10-(3′-chloroethylureidopropyl)-1,8-diazaphenothiazine (24)
To a stirred solution of 10-aminopropyl-1,8-diazaphenothiazine (21) (0.129 g, 0.5 mmol) in dry EtOH (10 ml) at 0 °C 2-chloroethyl isocyanate (0.87 ml, 1 mmol) was added. The mixture was stirred at 0 °C for 0.5 h and at rt for 24 h. After evaporation of EtOH in vacuo the residue was purified by column chromatography (aluminum oxide, CH2Cl2) to give 0.120 g (63 %) 10-chloroethylureidopropyl-1,8-diazaphenothiazine (24), mp 103 °C.
1H NMR (CDCl3) δ 1.75 (m, 2H, CH2), 2.10 (m, 2H, CH2), 3.49 (m, 4H, 2CH2), 4.46 (m, 2H, CH2), 6.76 (dd, J = 7.2 Hz, J = 5.1 Hz, 1H, H3), 6.84 (d, J = 5.0 Hz, 1H, H6), 7.14 (dd, J = 7.2 Hz, J = 1.4 Hz 1H, H4), 7.96 (dd, J = 5.1 Hz, J = 1.4 Hz 1H, H2), 8.01 (d, J = 5.0 Hz, 1H, H7), 8.17 (s, 1H, H9). FAB MS m/z: 364 (M+1, 30), 202 (M+H-C3H6NHCONHCH2CH2Cl, 10), 185 (2gly + H, 100). Anal. calcd. for C16H18ClN5OS: C 52.82, H 4.99, N 19.25. Found: C 52.77; H 4.97; N 19.11.
Biological assays
Preparation of the compounds for biological assays
The compounds were dissolved in DMSO (10 mg/ml) and subsequently diluted in RPMI-1640 cell culture medium (see below).
Isolation of the peripheral blood mononuclear cells
Venous blood from a single donor was withdrawn into heparinized syringes and diluted twice with phosphate-buffered saline. PBMC were isolated by centrifugation on Ficoll-uropoline gradient (density 1.077 g/ml) and centrifuged at 800×g for 20 min at 4 °C. The interphase cells, consisting of lymphocytes (20 %) and monocytes (80 %) were then washed three times with Hanks’ medium and re-suspended in a culture medium, referred to below as the culture medium, consisting of RPMI-1640, supplemented with 10 % fetal calf serum, l-glutamine, sodium pyruvate, 2-mercaptoethanol, and antibiotics, at density of 2 × 106 cells/ml.
PHA-induced proliferation of human blood mononuclear cells
The isolated PBMC were distributed into 96-well flat-bottom plates in 100 µL aliquots (2 × 105 cells/well). PHA was added at a concentration of 5 µg/ml. The compounds were tested at doses of 1, 10, and 50 µg/ml. DMSO at appropriate dilutions served as control. After a four-day incubation in a cell culture incubator, the proliferative response of the cells was determined by the colorimetric MTT method (Hansen et al., 1989). The results are given in percentage inhibition as compared with appropriate DMSO controls.
Cytotoxicity of the compounds against human blood mononuclear cells
PBMC at density of 2 × 105/well, re-suspended in the culture medium, were cultured for 24 h in a cell culture incubator with the preparations at indicated concentrations. Cell survival was determined by MTT colorimetric method (Hansen et al., 1989). The results are given in percentage inhibition as compared with appropriate DMSO controls.
Lipopolysaccharide-induced TNF-a production in whole blood cell culture
Venous blood from a single donor was diluted 10× with RPMI-1640 medium and distributed in 1 ml aliquots in 24-well culture plates. The cultures were stimulated by addition of 1 µg/ml of LPS from E. coli, O111:B4. The compounds were added to the cultures at concentrations of 5 and 25 µg/ml. Higher concentrations of the compounds could not be used because of inhibitory effects on TNF-α production by corresponding DMSO (the solvent) dilutions. Appropriate dilutions of DMSO served as controls. After overnight incubation in a cell culture incubator, the supernatants were harvested and frozen at −20 °C until cytokine determination by a biological assay (Espevik and Nissen-Meyer, 1986). The results are given in percentage inhibition as compared with appropriate DMSO controls.
Growth inhibition of tumor cell lines
L-1210 lymphoma and SW-948 colon tumor cell lines derived from the Collection of Cell Lines of The Institute of Immunology and Experimental Therapy, Wrocław, Poland. The lines were re-suspended in the culture medium and distributed into 96-well flat-bottom plates. L-1210 was present at 1.5 × 104 cells/well while SW-948 and at 2.5 × 104 cells/well. The preparations were added to the wells at the concentration range of 0.1–50 µg/ml. Cisplatin was used as a reference drug in the same concentrations. After 3-day incubation in a cell culture incubator, the proliferation was determined using MTT colorimetric method. The data are presented as a mean OD value from quadruplicate wells ± SE.
Statistics
The results are presented as mean values ± standard error (SE) or percentage inhibition = [(control value − tested value)/control value] × 100. Brown-Forsyth’s test was used to determine the homogeneity of variance between groups. When the variance was homogenous, analysis of variance (One-way ANOVA) was applied, followed by post-hoc comparisons with the Tukey’s test to estimate the significance of the difference between groups. Nonparametric data were evaluated with the Kruskal–Wallis’ analysis of variance. Significance was determined at p < 0.05. Statistical analysis was performed using STATISTICA 6.1 for Windows.
Acknowledgments
The work was supported by the Medical University of Silesia (Grant KNW-1-006/P/2/0).
References
Part CXXXVIII in the series of Azinyl Sulfides
- Aaron JJ, Gaye Seye MD, Trajkovska S, Motohashi N (2009) Bioactive Phenothiazines and Benzo[a]phenothiazines: spectroscopic studies and biological and biomedical properties and applications. Topics in Heterocyclic Chemistry, vol 16. Springer-Verlag, Berlin, pp 153–231
- Dasgupta A, Dastridara SG, Shirataki Y, Motohashi N (2008) Antibacterial activity of artificial phenothiazines and isoflavones from plants. Topics in Heterocyclic Chemistry, vol 15. Springer-Verlag, Berlin, pp 67–132
- Espevik T, Nissen-Meyer J. A highly sensitive cell line WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986;95:99–103. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Guadagni F, Ferroni P, Palmirotta R, Portarena I, Formica V, Roselli M. Review. TNF/VEGF cross-talk in chronic inflammation-related cancer initiation and progression: an early target in anticancer therapeutic strategy. In Vivo. 2007;21:147–161. [PubMed] [Google Scholar]
- Gupta RR, Kumar M. Synthesis, properties and reactions of phenothiazines. In: Gupta RR, editor. Phenothiazines and 1,4-benzothiazines: chemical and biological aspects. Amsterdam: Elsevier; 1988. pp. 1–161. [Google Scholar]
- Hansen MB, Nielsen SE, Berg K. Reexamination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989;119:203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- Kopp E, Strell M. Über 2,7-Diazaphenothiazin. Reaktionen in der pyridinreihe. Arch Pharm. 1962;295:99–107. doi: 10.1002/ardp.19622950204. [DOI] [PubMed] [Google Scholar]
- Kopp E, Strell M, Janson R. Verfahren zur Herstellung von 2,7-Diazaphenothiazinen. German Patent DE. 1963;1(147):235. [Google Scholar]
- Maki Y. Sulfur-containing pyridine derivatives. Smiles rearrangement in pyridine derivatives and synthesis of azaphenothiazine derivatives. Yakugaku Zasshi. 1957;77:485–490. [Google Scholar]
- Morak B, Pluta K. Synthesis of novel dipyrido-1,4-thiazines. Heterocycles. 2007;71:1347–1361. doi: 10.3987/COM-07-11035. [DOI] [Google Scholar]
- Morak B, Pluta K, Suwinska K. Unexpected simple route to novel dipyrido-1,4-thiazines. Heterocycl Commun. 2002;8:331–334. doi: 10.1515/HC.2002.8.4.331. [DOI] [Google Scholar]
- Morak-Młodawska B, Pluta K, Matralis AN, Kourounakis AP. Antioxidant activity of newly synthesized 2,7-diazaphenothiazines. Archiv Pharm Chem Life Sci. 2010;343:268–273. doi: 10.1002/ardp.200900253. [DOI] [PubMed] [Google Scholar]
- Morak-Młodawska B, Suwińska K, Pluta K, Jeleń M. 10-(3′-Nitro-4′-pyridyl)-1,8-diazaphenothiazine as the double Smiles rearrangement. J Mol Struct. 2012;1015:94–98. doi: 10.1016/j.molstruc.2012.02.022. [DOI] [Google Scholar]
- Motohashi N, Kawase M, Saito S, Sakagami H. Antitumor potential and possible targets of phenothiazine-related compounds. Curr Drug Targets. 2000;1:237–245. doi: 10.2174/1389450003349191. [DOI] [PubMed] [Google Scholar]
- Motohashi N, Kawase M, Satoh K, Sakagami H. Cytotoxic potential of phenothiazines. Curr Drug Targets. 2006;7:1055–1066. doi: 10.2174/138945006778226624. [DOI] [PubMed] [Google Scholar]
- Okafor C. Studies in the heterocyclic series. A novel diazaphenothiazine system. J Org Chem. 1967;32:2006–2007. doi: 10.1021/jo01281a074. [DOI] [Google Scholar]
- Pluta K, Jeleń M, Morak-Młodawska B, Zimecki M, Artym J, Kocięba M. Anticancer activity of newly synthesized azaphenothiazines in NCI’s anticancer screening. Pharmacol Rep. 2010;62:319–332. doi: 10.1016/S1734-1140(10)70272-3. [DOI] [PubMed] [Google Scholar]
- Pluta K, Morak-Młodawska B, Jeleń M. Synthesis and properties of diaza-, triaza- and tetraazaphenothiazines. J Heterocycl Chem. 2009;46:355–391. doi: 10.1002/jhet.42. [DOI] [Google Scholar]
- Pluta K, Morak-Młodawska B, Jeleń M. Recent progress in biological activities of synthesized phenothiazines. Eur J Med Chem. 2011;46:3179–3189. doi: 10.1016/j.ejmech.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Rath S (1957) Dimethylaminopropyl-dipyridothiazane. US Patent 2,789,978
- Rodig OR, Collier RE, Schlatzer RK. Pyridine chemistry. Further studies on the Smiles rearrangement of the 3-amino-2,2′-dipyridyl sulfide system. The synthesis of some 1,6-diazaphenothiazines. J Med Chem. 1966;9:116–120. doi: 10.1021/jm00319a028. [DOI] [PubMed] [Google Scholar]
- Sadandam YS, Shetty MM, Bhaskar Rao A. 10H-Phenothiazines: a new class of enzyme inhibitors for inflammatory diseases. Eur J Med Chem. 2009;44:197–202. doi: 10.1016/j.ejmech.2008.02.028. [DOI] [PubMed] [Google Scholar]
- Silberg IA, Cormos G, Oniciu DC (2006) Retrosynthetic approach to the synthesis of phenothiazines. In Advances in heterocyclic chemistry; Katritzky AR (ed.), Elsevier, New York, vol 90, pp 205–237, and Biological evaluation as potential antiproliferative and antifungal agents. Eur J Med Chem 44:1086–1092
- Takahashi T, Maki Y. Smiles rearrangement in pyridine derivatives and synthesis of benzopyrido- and dipyridothiazine derivatives. Yakugaku Zasshi. 1958;78:417–421. [Google Scholar]
- Takahashi T, Maki Y. Sulfur-containing pyridine derivatives. Smiles rearrangement of pyridine derivatives and synthesis of benzopyrido- and dipyrido-1,4-thiazine derivatives. Chem Pharm Bull. 1958;6:369–373. doi: 10.1248/cpb.6.369. [DOI] [PubMed] [Google Scholar]
- Tandon VK, Maurya HK, Tripathi A, Shiva Keshava GB, Shukla PK, Srivastava P, Panda D (2009) 2,3-Disubstituted-1,4-naphthoquinones, 12H-benzo[b]phenothiazine-6,11-diones and related compounds: synthesis. Eur J Med Chem 44(3):1086–1092 [DOI] [PubMed]
- Umbach GE, Singletary SE, Tomasovic B, Spitzer G, Hug V, Drevinko B. Dose-survival curves of cis-platinum, melphalan, and velban in human granulocyte/macrophage progenitor cells. Int J Cell Cloning. 1984;2:335–340. doi: 10.1002/stem.5530020601. [DOI] [PubMed] [Google Scholar]
- Wajant H. The role of TNF in cancer. Results Probl Cell Differ. 2009;49:1–15. doi: 10.1007/400_2008_26. [DOI] [PubMed] [Google Scholar]
- Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am J Med Sci. 2007;334:12–115. doi: 10.1097/MAJ.0b013e318093e609. [DOI] [PubMed] [Google Scholar]
- Zimecki M, Artym J, Kocięba M, Pluta K, Morak-Młodawska B, Jeleń M. Immunosupressive activities of newly synthesized azaphenothiazines in human and mouse models. Cell Mol Biol Lett. 2009;14:622–635. doi: 10.2478/s11658-009-0025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]