Abstract
A series of bromophenol derivatives containing indolin-2-one moiety were designed and evaluated that for their anticancer activities against A549, Bel7402, HepG2, HeLa and HCT116 cancer cell lines using MTT assay in vitro. Among them, seven compounds (4g–4i, 5h, 6d, 7a, 7b) showed potent activity against the tested five human cancer cell lines. Wound-healing assay demonstrated that compound 4g can be used as a potent compound for inactivating invasion and metastasis by inhibiting the migration of cancer cells. The structure–activity relationships (SARs) of bromophenol derivatives had been discussed, which were useful for exploring and developing bromophenol derivatives as novel anticancer drugs.
Keywords: bromophenol, indolin-2-one, anticancer, molecular hybrid, structure–activity relationship
1. Introduction
Cancer is a significant health problem throughout the world, which is a leading cause of death. It is estimated that 14.1 million new cancer cases and 8.2 million cancer-related deaths occurred in 2012 according to the WHO report. The incidence of cancer has been increasing in most regions of the world, which will further increase to 19.3 million new cancer cases per year by 2025 [1]. Although many effective anticancer agents have been developed, therapies for cancer are less than satisfactory due to side effects. Therefore, novel effective agents for treating cancer disease are urgently needed today.
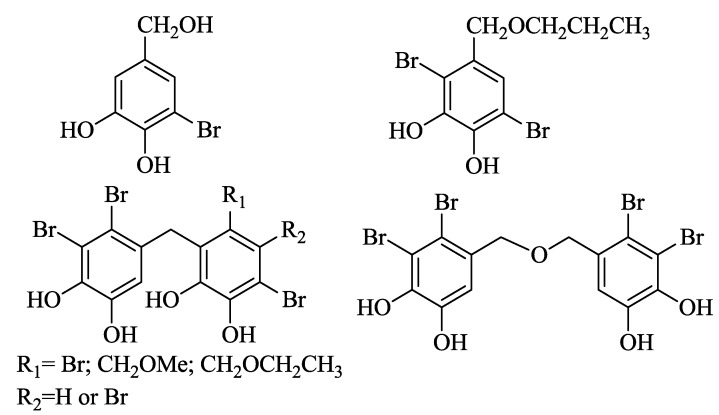
Bromophenols, natural marine products isolated from various marine biology, are known to possess various potent activities including antioxidative, protein tyrosine kinase (PTK) inhibitory, anticancer, protein tyrosine phosphatase 1B inhibitory, antithrombotic, antimicrobial, anti-inflammatory [2], which have attracted much attention, also due to their unique structures. For example, 3-bromo-4,5-dihydroxybenzyl alcohol (Figure 1) showed significant cytotoxicity to KB cells (IC50 = 8.9 μg/mL) [3]. 2,5-dibromo-3,4-dihydroxybenzyl n-propyl ether (Figure 1) synthesized from natural bromophenol demonstrated potent activity against DLD-1 and HCT-116 Cells lines with IC50 values 1.72 and 0.80 µM, respectively [4]. Xu and co-workers reported a series of natural bromophenols (3,4-dibromo-5(methoxymethyl)-1,2-benzenediol, Figure 1) which showed vigorous activities against KB, Bel7402, HELF and A549 cancer cells with IC50 values under 10 μg/mL [5]. In our previous study, a variety of bromophenols (Figure 1) isolated from various marine algae, which exhibited excellent anticancer activity against A549, BGC-823, MCF-7, B16-BL6, HT-1080, A2780, Bel7402 and HCT-8 human cancer cell lines, could be used as potent antitumor agents for PTK over-expression of c-kit and is considered as part of a new therapeutic strategy for treatment of cancer [6].
Figure 1.
Structures of bromophenols possessing potent activity against cancer.
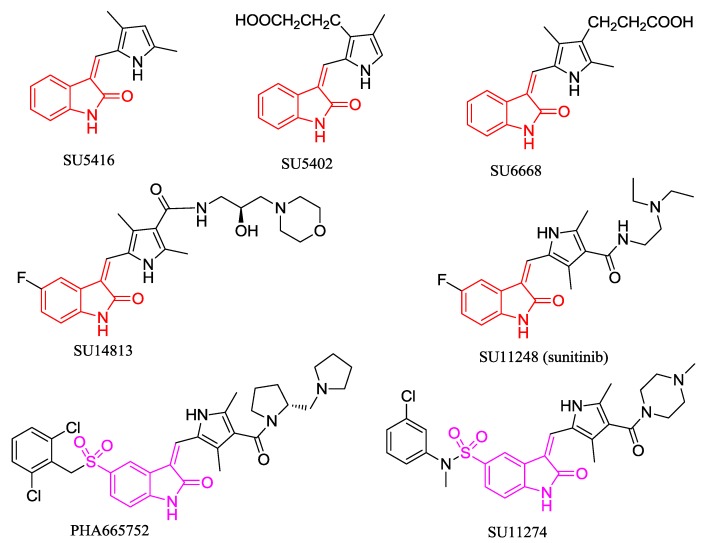
The structural scaffold of oxindole is proven as a core structure in various active compounds that exhibit a wide variety of biological activities including anti-inflammatory [7,8], tuberculosis [9], antiglycation [10], antitumor [11,12,13,14], neurological disorders [15], antioxidant [16], antimicrobial [17], and anticonvulsant activity [18]. Among them, indolin-2-one derivatives have aroused great attention for their anticancer activities and were developed as antitcancer drugs, such as SU5416, SU5402, SU6668and SU14813 (Figure 2), which have been reported as potent and selective inhibitors of different protein kinases and showed a significant cytotoxic activity [19,20]. SU5416 is the first selective kinase insert domain receptor (KDR) inhibitor used in clinical trials. Its derivative SU11248 (sunitinib, Figure 2), an inhibitor of RTK approved by the US Food and Drug Administration (FDA), has been approved and marketed for the treatment of gastrointestinal stromal cancers and renal cell carcinoma [21]. SU11274 and PHA665752 (Figure 2) containing 3-methylene indolin-2-one scaffold with sulfonyl group located on the 5-position were proved as an effective inhibitor of Met tyrosine kinase and have entered preclinical and multi-center clinical studies for anticancer activity [22,23]. Therefore, the indolin-2-one moiety is the pharmacophore in developing anticancer agents.
Figure 2.
Selected indolinone derivatives as anticancer agents in preclinical or clinical trials.
Molecular hybrids with two pharmacophores often lead to synergistic activity. It has been observed that the incorporation of indolin-2-one moiety into the active anticancer moiety (such as pyrrole, oxadiazole, thiazolidinone, etc.) demonstrated profound growth inhibitory activity against different cancer cells, which was an efficient strategy for thedesign of novel antitumor agents [24,25,26].
In view of these observations, a novel series of bromophenols derivatives containing indolin-2-one moiety was designed and evaluated for in vitro anticancer activity against cancer cell lines (Human lung cancer cell line, A549; Human hepatoma cell lines, Bel-7402 and HepG2; human cervical cancer cell line, HeLa; human colon cancer cell line, HCT116) using MTT assay. The most potent activity compound 4g on cancer cell migration was investigated by using the wound-healing assay. Structure–activity relationships (SARs) of these bromophenols analogs are also discussed in this paper.
2. Results and Discussion
2.1. Chemistry
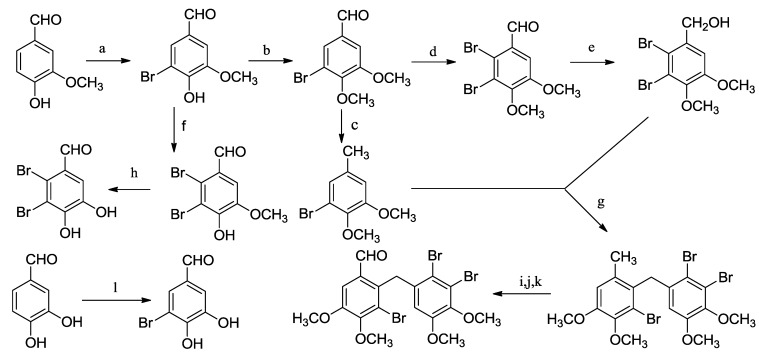
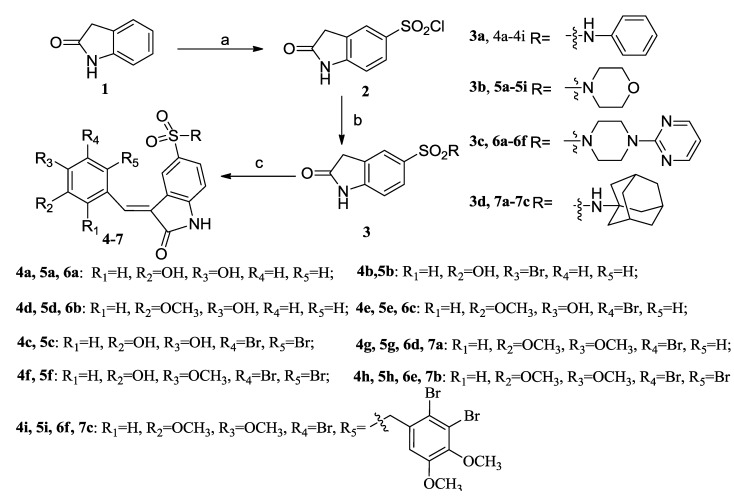
The synthetic procedures for the preparation of aldehydes (Scheme 1) have been publised in our previous reports [27,28,29,30]. As shown in Scheme 2, a series of bromophenol derivatives were synthesized by Knoevenagel condensation between different substituted indolin-2-one and the corresponding aldehydes in order to explore the SARs of these derivatives and obtain the potencial lead compounds. Firstly, oxindole (1) was reacted with ClSO3H to yield compound 2. Then, compound 2 and amines were heated for 3 h in tetrahydrofuran (THF) at 80 °C to afford 5-substituted-indolin-2-one (3). At last, the reaction between 5-substituted-indolin-2-one (3) and the synthesized aldehydes was performed under the condition of Knoevenagel condensation in ethanol with a catalytic amount of piperidine to give the desired bromophenol derivatives 4–7 in good yields. All of the synthesized derivatives were purified and their structures were characterized by spectroscopic means (1H, 13C NMR, MS and HRMS).
Scheme 1.
Synthesis of aldehydes. Reagents and conditions: (a) Br2, CH3OH, 0 °C; (b) CH3I, K2CO3, DMF, room temperature (rt); (c) 80% N2H4·H2O, KOH, diglycol, 120 °C; (d) Br2, AcOH, 60 °C; (e) NaBH4, CH3OH, 0 °C; (f) Br2, AcOH, Fe, reflux, 12 h; (g) AlCl3, CH2Cl2, rt; (h) BBr3, DCM, −78 °C; (i) NBS, AIBN, CCl4, hv; (j) K2CO3, 1,4-dioxane, H2O, 90 °C; (k) PCC, DCM, rt; (l) Br2, AcOH, rt.
Scheme 2.
Synthesis of bromophenol derivatives 4–7. Reagents and conditions: (a) ClSO3H, 65 °C, 1 h; (b) R1R2NH, THF, reflux; (c) EtOH, aldehyde, piperidine, reflux.
2.2. Anti-Cancer Activity
2.2.1. Antiproliferative Activity
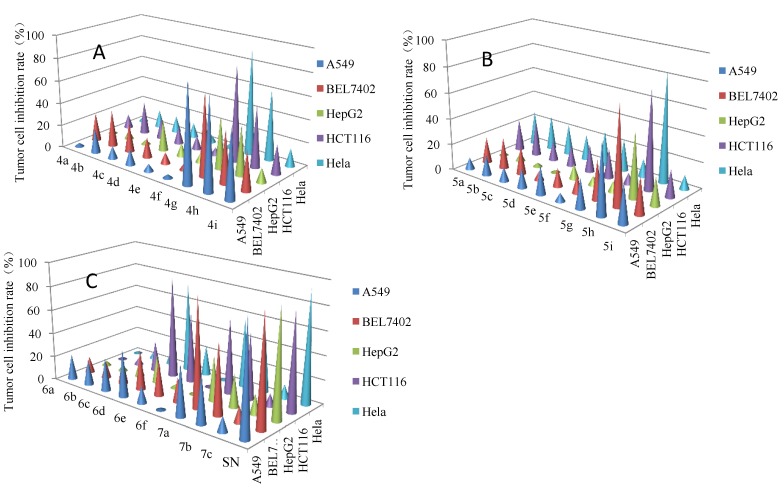
All the synthesized compounds were investigated for their anticancer activity in vitro on five human cancer cell lines, namely against A549, Bel7402, HepG2, HeLa and HCT116 cancer cell lines using MTT assay and sunitinib as a positive control. The results of inhibited ration of comounds 4–7 were listed in Figure 3, and the IC50 values of selective compounds (4g–4i, 5h, 6d, 6e, 7a, 7b) were listed in Table 1.
Figure 3.
Inhibitory activity of bromophenol derivatives (A: compounds 4a–4i; B: compounds 5a–5i; C: compounds 6a–6f, 7a–7c and Sunitinib) against five human cancer cell lines; the percent inhibition rate of tumor cell at 10 µg/mL inhibitor; the cells were seeded in a 96-well plate and incubated overnight. Then, the cells were treated with various amounts of compounds and incubated for 48 h. Cell proliferation was evaluated with the MTT Assay. Sunitinib (SN) as the positive control.
Table 1.
IC50 values of selected bromophenol derivatives against five human cancer cell lines.
| Compd | IC50 (μg/mL) a | ||||
|---|---|---|---|---|---|
| A549 | Bel7402 | HepG2 | Hct116 | Hela | |
| 4g | 6.6 ± 0.82 | 9.2 ± 0.84 | 13.2 ± 2.42 | 9.1 ± 0.13 | 7.4 ± 0.22 |
| 4h | 14.4 ± 1.86 | 12.3 ± 0.23 | 14.3 ± 0.86 | 9.8 ± 0.55 | 8.3 ± 0.67 |
| 4i | 9.9 ± 0.11 | 22.3 ± 1.11 | NA b | NA | NA |
| 5h | 10.1 ± 0.72 | 9.7 ± 2.35 | 11.2 ± 1.26 | 8.6 ± 0.26 | 18.0 ± 0.13 |
| 6d | 25.4 ± 0.82 | 18.6 ± 0.91 | NA | 5.6 ± 0.42 | 17.6 ± 0.89 |
| 6e | NA | NA | NA | 9.8 ± 0.20 | NA |
| 7a | 12.5 ± 0.19 | 7.9 ± 0.26 | 25.0 ± 0.18 | 6.1 ± 0.23 | 8.6 ± 0.14 |
| 7b | 12.5 ± 0.45 | 12.5 ± 0.39 | 14.2 ± 0.77 | 8.2 ± 0.54 | 9.3 ± 0.47 |
| TTEDM c | NA | 44.1 ± 1.19 | NA | 32.3 ± 2.03 | 23.5 ± 1.41 |
| Sunitinib d | 10.4± 0.54 | 3.2 ± 0.15 | 4.5 ± 0.23 | 2.8 ± 0.27 | 1.6 ± 0.08 |
a IC50: Concentration of the compound producing 50% cell growth inhibition after 48 h of drug exposure, as determined by the MTT assay. Each experiment was run at least three times, and the results are presented as average values ± standard deviation; b NA: Compound showing IC50 value > 50 μg/mL; c 2,2',3-tribromo-3',4,4',5-tetrahydroxy-6'-ethyloxymethyldiphenylmethane (TTEDM) is a marine bromophenol compound derived from marine algae. P.; d Sunitinib as the positive control.
As shown in Figure 3, compounds 4g–4i, 5h, 6d, 6e, 7a and 7b exhibited potent anticancer activity against A549, Bel7402, HepG2, HeLa and HCT116 cancer cell lines at 10 μg/mL, respectively. Of them, compound 4g showed significant activity against A549, Bel7402, HepG2, HeLa and HCT116 cancer cell lines with the inhibition ratio of 88.2%, 71.9%, 85.2% and 93.0% at 10 μg/mL, respectively, which were comparable to those of sunitinib. Compounds 4a–4c, 5a–5c, and 6a with two hydroxy groups on phenol ring showed weaker activity against of five cancer cells at 10 μg/mL. After methylation of 3-hydroxyl group on phenol ring of compounds 4d–4f, 5d–5f, 6b, and 6c, the activity have not obviously changed (4d vs. 4a, 4e vs. 4b, 4f vs. 4c, 5d vs. 5a, 5e vs. 5b, 5f vs. 5c, 6b, 6c vs. 6a).
It was inspired that methylation of 4-hydroxyl group on phenol ring (4g, 4h, 5g, 5h, 6d, 6e, 7a, 7b) could significantly enhance their anti-cancer activity, which indicated that the hydrophobic parameter may affect their anticancer activity. However, compounds 4i, 5i, 6f, 7c with four methoxy groups in diphenyl methane moiety showed low anticancer activity, suggesting that many of the hydrophobic groups have a deleterious effect. The steric hindrance may affect their activity also. The derivatives of mono-brominated on phenol ring exhibited better activity than those of di-brominated compounds (4b vs. 4c, 4e vs. 4f, 4g vs. 4h, 5b vs. 5c, 5e vs. 5f, 6d vs. 6e, 7a vs. 7b). On the contrary, compound 5h with di-brominated on phenol moiety showed better inhibitory activity against cancer cells than those of compound 5g with mono-brominated on phenol moiety. These results indicated that the number of bromine atom on phenol moiety could affect the anticancer activities of these hybrid derivatives. Furthermore, it is notable that most compounds introducing secondary amino groups in 5-position of indolin-2-one (4a–4i and 7a–7c) displayed significant activity compared to those of compounds (5a–6f) with tertiary sulfonamide groups, indicating that types of amino groups at 5-position of indolin-2-one could influence the activities of these derivatives. From Table 1, it is also seenthat these synthesized bromophenol derivatives containing indolin-2-one moiety (4g–4i, 5h, 6d, 6e, 7a, 7b) showed better activities than 2,2',3-tribromo-3',4,4',5-tetrahydroxy-6'-ethyloxymethyldiphenylmethane (TTEDM) which was a marine bromophenol compound isolated from marine algae Polysiphonia urcedata.
2.2.2. Inhibitory Effect of Compound 4g on Cancer Cell Migration
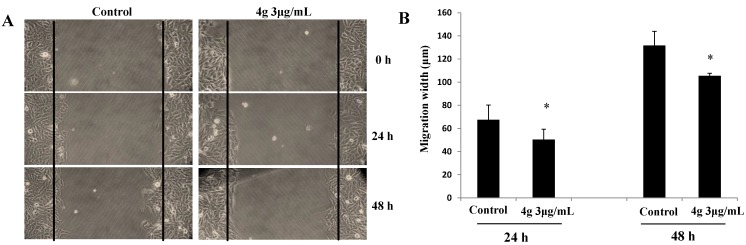
Cancer cell invasion, a hallmark of cancer, plays a critical role during the carcinomas arising from epithelial tissues progressed to higher pathological grades of malignancy [31]. To further investigate the anticancer effect of 4g, we studied the inhibitory effect on cancer cell migration; an invasive process often observed in cancer by using the wound-healing assay [32]. We found that the migration of HepG2 cells was significantly inhibited by 48 h treatment with 4g at 3 μg/mL concentration compared to the untreated control (Figure 4A).
Figure 4.
(A) The HepG2 cells were scraped by a pipette tip to generate wounds. After treatment with or without 4g 3 μg/mL in the presence of 1 μg/mL Mitomycin C, cells migration was recorded by microscopy at 0, 24, 48 h; (B) Results were obtained from three separate experiments. The bar represents the mean ± SE, * (p < 0.05) indicates a significant difference between the control and 4g treated samples.
The migration width (the distances of migrated cells) of cancer cells treated with/without 4g was summarized in Figure 4B. It was demonstrated that ~20% of cell migration was inhibited by treatment with 4g at 3 μg/mL concentration for 48 h. These results suggested that 4g might be used as a potent compound for inactivating invasion and metastasis by inhibiting the migration of cancer cells.
3. Experimental Section
3.1. Chemistry
1H and 13C NMR spectra were recorded on Bruker DRX 500 MHz spectrometers with tetramethylsilane (TMS) as the internal standard (Bruker, Bremerhaven, Germany). MS and HRMS spectra were determined on a LCMS-IT-TOF mass spectrometer (Shimadzu, Kyoto, Japan). Column chromatography (CC): Silica gel (200–300 mesh; Qingdao Makall Group CO., Ltd; Qingdao; China). All reactions were monitored using thin-layer chromatography (TLC) on silica gel plates. Reaction reagents were purchased from J&K Scientific Ltd. (Chaoyang District, Beijing, China) Organic solvents were analytical reagent grade and purchased from Tianjin Chemical Reagent Co., Ltd. (Jinnan District, Tianjin, China). The synthesized compounds were named using ChemBioDraw Ultra software (version 12.0, Waltham, MA, USA).
General Procedure for the Preparation of Derivatives (4–7)
To a 100 mL flask charged with chlorosulfonic acid (25 mL), 2-oxindole (50 mmoL) at 0 °C was added slowly. After the addition, the reaction mixture was stirred at room temperature for 1.5 h. Then, the reaction mixture was heated to 68 °C for 1 h, cooled, and poured into ice water (200 mL). The precipitate was washed with water and dried in a vacuum oven to give 5-chlorosulfonyl-2-oxindole (2) which was used without further purification.
A mixture of 5-chlorosulfonyl-2-oxindole (5 mmoL) and appropriate amine (10 mmoL) in tetrahydrofuran (THF, 50 mL) was heated to refluxing and stirred for 3 h. Then, this mixture was concentrated under reduced pressure, and HCl (pH = 3, 25 mL) was added and stirred for 15 min. The crude product was filtered, washed with ice water (100 mL) and dried in a vacuum oven to give 5-sulfamoyl-2-oxindole (3) which was used without further purification.
Piperidine (50 μL) was added to a mixture of compound 3 (0.5 mmoL) and appropriate aldehyde (0.55 mmoL) in ethanol (5 mL). The reaction mixture was heated to refluxing and stirred for 2 h, and TLC analysis indicated when the reaction was complete. The crude product was filtered, washed with ethanol and dried in a vacuum (if no solid precipitated, the crude product was chromatographed using a silica gel column) to afford the title compound 4–7 as a yellow solid.
(E)-N-(4-Bromophenyl)-3-(3,4-dihydroxybenzylidene)-2-oxoindoline-5-sulfonamide (4a). Yield: 66.1%; 1H NMR (DMSO-d6, 500 MHz, ppm): δ 10.96 (s, 1H), 10.21 (s, 1H), 8.10 (s, 1H), 7.75 (s, 1H), 7.58 (dd, 1H, J = 1.5, 8.5 Hz), 7.56 (s, 1H), 7.38–7.40 (overlap, 3H), 7.06 (d, 1H, J = 9.0 Hz), 7.00 (d, 1H, J = 9.0 Hz), 6.97 (d, 2H, J = 8.5 Hz); 13C NMR (DMSO-d6, 125 MHz, ppm): δ 169.4, 150.3, 148.9, 146.1, 145.2, 137.7, 140.2, 132.4 (2C), 128.8, 125.4, 123.2, 122.5, 122.3 (2C), 121.9, 120.8, 118.0, 116.5, 116.3, 110.5; ESIMS: m/z 484 [M − H]− HRESIMS: Calc for C21H15BrN2O5S [M − H]− 484.9805, found 484.9812.
(E)-3-(3-Bromo-4,5-dihydroxybenzylidene)-N-(4-bromophenyl)-2-oxoindoline-5-sulfonamide (4b). Yield: 67.6%; 1H NMR (DMSO-d6, 500 MHz, ppm): δ 11.00 (s, 1H), 10.24(s, 1H), 8.11 (s, 1H), 7.79(s, 1H), 7.56(dd, 1H, J = 1.5, 8.5 Hz), 7.54 (s, 1H), 7.39 (d, 2H, J = 9.0 Hz), 7.32 (s, 1H), 7.06 (d, 2H, J = 9.0 Hz), 6.99 (d, 1H, J = 8.5 Hz); 13C NMR (DMSO-d6, 125 MHz, ppm): δ 169.1, 147.4, 146.6, 145.8, 139.7, 137.9, 132.4 (2C), 132.1, 131.7,126.6, 126.0, 124.8, 122.7, 122.0 (2C), 121.6, 116.3, 116.1, 110.7, 110.4; ESIMS: m/z 562 [M − H]− HRESIMS: Calc for C21H13BrN2O5S [M − H]− 562.8915, found 562.8917.
(E)-N-(4-Bromophenyl)-3-(2,3-dibromo-4,5-dihydroxybenzylidene)-2-oxoindoline-5-sulfonamide (4c). Yield: 77.4%; 1H NMR (DMSO-d6, 500 MHz, ppm): δ 11.08 (s, 1H), 10.30 (s, 1H), 7.74 (s, 1H), 7.55 (d, 1H, J = 8.5 Hz), 7.54 (s, 1H), 7.33 (d, 2H, J = 8.5 Hz), 7.15(s, 1H), 6.96 (d, 1H, J = 8.5 Hz), 6.93 (d, 2H, J = 8.5 Hz); 13C NMR (DMSO-d6, 125 MHz, ppm): δ 168.7, 147.0, 146.9, 146.0, 138.0, 137.6, 132.4 (2C), 132.1, 129.6, 126.7, 126.0, 121.8 (2C), 121.5, 121.2, 116.4, 115.8, 115.3, 114.4, 110.8; ESIMS: m/z 640 [M − H]− HRESIMS: Calc for C21H12Br3N2O5S [M − H]− 640.8014, found 640.8022.
(E)-N-(4-Bromophenyl)-3-(4-hydroxy-3-methoxybenzylidene)-2-oxoindoline-5-sulfonamide (4d). Yield: 68.0%; 1H NMR (DMSO-d6, 500 MHz, ppm): δ 11.12 (s, 1H), 10.30 (s, 1H), 8.18 (s, 1H), 7.60 (s, 1H), 7.56 (dd, 1H, J = 1.5, 8.5 Hz), 7.32 (s, 1H), 7.31 (d, 1H, J = 8.5 Hz), 7.24 (d, 2H, J = 8.5 Hz), 6.99 (d, 1H, J = 8.5 Hz), 6.92 (d, 1H, J = 8.5 Hz), 6.87 (d, 2H, J = 8.5 Hz), 3.83 (s, 3H); 13C NMR (DMSO-d6, 125 MHz, ppm): δ 169.6, 150.8, 148.2, 147.8, 145.5, 140.8, 139.6, 132.0 (2C), 128.5, 125.4, 124.8, 122.9, 122.5 (2C), 122.2, 120.7, 116.4, 116.1, 113.4, 110.1, 55.9; ESIMS: m/z 498 [M − H]− HRESIMS: Calc for C22H16BrN2O5S [M − H]− 498.9970, found 498.9969.
(E)-3-(3-Bromo-4-hydroxy-5-methoxybenzylidene)-N-(4-bromophenyl)-2-oxoindoline-5-sulfonamide (4e). Yield: 73.5%; 1H NMR (DMSO-d6, 500 MHz, ppm): δ 11.08 (s, 1H), 10.30 (s, 1H), 7.92 (s, 1H), 7.55(s, 1H), 7.48 (s, 1H), 7.47 (s, 1H), 7.37 (d, 2H, J = 8.5 Hz), 7.32 (d, 1H, J = 8.5 Hz), 7.05 (d, 2H, J = 8.5 Hz), 6.97 (d, 1H, J = 8.5 Hz); 13C NMR (DMSO-d6, 125 MHz, ppm): δ 168.0, 150.3, 144.8, 141.7, 140.8, 138.3, 138.2, 132.3 (2C), 131.2, 128.0, 124.2, 123.1, 121.9 (2C), 119.7, 116.0, 115.9, 113.7, 111.0, 108.7, 55.8; ESIMS: m/z 576 [M − H]− HRESIMS: Calc for C22H15Br2N2O5S [M − H]− 576.9062, found 576.9074.
(E)-N-(4-Bromophenyl)-3-(2,3-dibromo-4-hydroxy-5-methoxybenzylidene)-2-oxoindoline-5-sulfonamide (4f). Yield: 68.9%; 1H NMR (DMSO-d6, 500 MHz, ppm): δ 11.08 (s, 1H), 10.24 (s, 1H), 7.68 (s, 1H), 7.58 (d, 1H, J = 8.5 Hz), 7.55 (s, 1H), 7.53 (s, 1H),7.32 (d, 2H, J = 8.5 Hz), 6.97 (d, 1H, J = 8.5 Hz), 6.92 (d, 2H, J = 8.5 Hz), 3.87 (s, 3H); 13C NMR (DMSO-d6, 125 MHz, ppm): δ 168.5, 152.6, 147.1, 146.0, 137.7, 137.6, 132.4 (2C), 132.2, 131.4, 129.8, 126.8, 122.1 (2C), 121.6, 121.2, 116.9, 116.4, 115.6, 114.2, 110.9, 65.4; ESIMS: m/z 654 [M − H]− HRESIMS: Calc for C22H14Br3N2O5S [M − H]− 654.8168, found 654.8179.
(E)-3-(3-Bromo-4,5-dimethoxybenzylidene-N-(4-bromophenyl)-2-oxoindoline-5-sulfonamide (4g). Yield: 67.2%; 1H NMR (DMSO-d6, 500 MHz, ppm): δ 11.08 (s, 1H), 10.24 (s, 1H), 7.63 (s, 1H), 7.58–7.61 (overlap, 2H), 7.50 (s, 1H), 7.40 (s, 1H), 7.31 (d, 2H, J = 8.5 Hz), 7.05 (d, 1H, J = 9.0 Hz), 6.94 (d, 2H, J = 8.5 Hz), 3.87 (s, 3H), 3.84 (s, 3H); 13C NMR (DMSO-d6, 125 MHz, ppm): δ 168.9, 153.8, 148.3, 147.5, 137.3, 132.3 (2C), 132.1, 131.5, 131.4, 129.6, 126.7, 126.3, 122.4 (2C), 121.4, 121.3, 117.6, 116.5, 113.5, 110.7, 60.8, 56.6; ESIMS: m/z 590 [M − H]− HRESIMS: Calc for C23H17Br2N2O5S [M − H]− 590.9219, found 590.9230.
(E)-N-(4-Bromophenyl)-3-(2,3-dibromo-4,5-dimethoxybenzylidene)-2-oxoindoline-5-sulfonamide (4h). Yield: 82.5%; 1H NMR (DMSO-d6, 500 MHz, ppm): δ 11.16 (s, 1H), 10.24 (s, 1H), 7.66 (s, 1H), 7.60 (d, 1H, J = 8.5 Hz), 7.57 (s, 1H), 7.52 (s, 1H), 7.31 (d, 2H, J = 8.5 Hz), 6.99 (d, 1H, J = 8.5 Hz), 6.90 (d, 2H, J = 8.5 Hz), 3.86 (s, 3H), 3.82 (s, 3H); 13C NMR (DMSO-d6, 125 MHz, ppm): δ 168.5, 152.8, 148.6, 147.2, 137.5, 136.8, 132.4 (2C), 132.2, 131.7, 130.0, 128.0, 122.2 (2C), 122.1, 121.7, 121.2, 117.0, 116.6, 113.9, 110.9, 60.9, 60.7; ESIMS: m/z 668 [M − H]− HRESIMS: Calc for C23H16Br3N2O5S [M − H]− 668.8334, found 668.8335.
(E)-3-(3-Bromo-2-(2,3-dibromo-4,5-dimethoxybenzyl-4,5-dimethoxybenzylidene)-N-(4-bromophenyl)-2-oxoindoline-5-sulfonamide (4i). Yield: 88.2%; 1H NMR (DMSO-d6, 500 MHz, ppm): δ 10.99 (s, 1H), 10.35 (s, 1H), 7.63 (s, 2H), 7.52 (s, 1H), 7.32-7.34 (overlap, 3H), 6.92-6.95 (overlap, 3H), 6.33 (s, 1H), 4.22 (s, 2H), 3.85(s, 3H), 3.80(s, 3H), 3.58(s, 3H), 3.31(s, 3H); 13C NMR (DMSO-d6, 125 MHz, ppm): δ 168.3, 152.4, 152.3, 147.5, 146.9, 145.8, 137.6, 136.9, 136.2, 132.3 (3C), 131.2, 130.5, 129.8, 127.8, 122.5, 121.6 (3C), 121.5, 121.3, 117.0, 116.2, 113.2, 112.9, 110.7, 60.8, 60.2, 56.7, 56.1, 41.3; ESIMS: m/z 896 [M − H]− HRESIMS: Calc for C32H25Br4N2O7S [M − H]− 896.8107, found 896.8121.
(E)-3-(3,4-Dihydroxybenzylidene)-5-(morpholinosulfonyl)indolin-2-one (5a). Yield: 52.3%; 1H NMR (DMSO-d6, 500 MHz, ppm): δ 11.04 (s, 1H), 8.04 (s, 1H), 7.63 (s, 1H), 7.56 (dd, 1H, J = 1.5, 8.5 Hz), 7.14 (s, 1H), 7.06–7.11 (overlap, 2H), 6.87 (d, 1H, J = 8.5 Hz), 3.62 (t, 4H, J = 4.5 Hz), 2.84 (t, 4H, J = 4.5 Hz); 13C NMR (DMSO-d6, 125 MHz, ppm): δ 169.4, 148.9, 145.9, 145.1, 140.2, 129.8, 126.8, 125.4, 123.2, 122.3, 121.5, 121.1, 117.5, 116.2, 110.6, 65.7 (2C), 46.5 (2C); ESIMS: m/z 401 [M − H]− HRESIMS: Calc for C19H17N2O6S [M − H]− 401.0813, found 401.0813.
(E)-3-(3-Bromo-4,5-dihydroxybenzylidene)-5-(morpholinosulfonyl)indolin-2-one (5b). Yield: 66.3%; 1H NMR (DMSO-d6, 500 MHz, ppm): δ 11.10 (s, 1H), 8.08 (s, 1H), 7.60 (s, 1H), 7.54 (dd, 1H, J = 1.5, 8.0 Hz), 7.42 (s, 1H), 7.07 (s, 1H), 7.01 (d, 1H, J = 8.0 Hz), 3.62 (t, 4H, J = 4.5 Hz), 2.84(t, 4H, J = 4.5 Hz); 13C NMR (DMSO-d6, 125 MHz, ppm): δ 169.1, 147.0, 145.8, 144.3, 140.0, 130.0, 129.4, 126.8, 126.3, 124.7, 122.7, 119.4, 116.5, 110.7, 110.3, 65.7 (2C), 46.5 (2C); ESIMS: m/z 478 [M − H]− HRESIMS: Calc for C19H16BrN2O6S [M − H]− 478.9901, found 478.9918.
(E)-3-(2,3-Dibromo-4,5-dihydroxybenzylidene)-5-(morpholinosulfonyl)indolin-2-one (5c). Yield: 68.3%; 1H NMR (DMSO-d6, 500 MHz, ppm): δ 10.95 (s, 1H), 7.79 (s, 1H), 7.67 (s, 1H), 7.55 (dd, 1H, J = 1.5, 8.5 Hz), 7.10 (s, 1H), 6.95 (d, 1H, J = 8.5 Hz), 3.66 (t, 4H, J = 4.5 Hz), 2.85 (t, 4H, J = 4.5 Hz); 13C NMR (DMSO-d6, 125 MHz, ppm): δ 169.7, 146.2, 146.0, 144.4, 139.7, 129.9 128.7, 127.3, 126.6, 125.8, 122.2, 121.9, 117.3, 114.8, 110.2, 65.9 (2C), 46.0 (2C); ESIMS: m/z 556 [M − H]− HRESIMS: Calc for C19H15Br2N2O6S [M − H]− 556.9015, found 556.9023.
(E)-3-(4-Hydroxy-3-methoxybenzylidene)-5-(morpholinosulfonyl)indolin-2-one (5d). Yield: 77.5%; 1H NMR (DMSO-d6, 500 MHz, ppm): δ 11.03 (s, 1H), 8.00(s, 1H), 7.69 (s, 1H), 7.52(dd, 1H, J = 1.5, 8.5 Hz), 7.38 (s, 1H), 7.26 (dd, 1H, J = 1.5, 8.0 Hz), 7.09 (d, 1H, J = 8.0 Hz), 6.90 (d, 1H, J = 8.0 Hz), 3.84 (s, 3H), 3.62 (t, 4H, J = 4.5 Hz), 2.86(t, 4H, J = 4.5 Hz); 13C NMR (DMSO-d6, 125 MHz, ppm): δ 169.8, 150.4, 147.8, 144.3, 140.4, 129.9, 127.2, 125.7, 125.6, 123.6, 122.5, 121.6, 116.7, 113.9, 110.0, 66.0 (2C), 56.4, 46.7 (2C); ESIMS: m/z 415 [M − H]− HRESIMS: Calc for C20H19N2O6S [M − H]− 415.0955, found 415.0969.
(E)-3-(3-Bromo-4-hydroxy-5-methoxybenzylidene)-5-(morpholinosulfonyl)indolin-2-one (5e). Yield: 67.2%; 1H NMR (DMSO-d6, 500 MHz, ppm): δ 11.10 (s, 1H), 8.07 (s, 1H), 7.67 (s, 1H), 7.59 (s, 1H), 7.55 (d, 1H, J = 8.0 Hz), 7.40 (s, 1H), 7.04 (d, 1H, J = 8.0 Hz), 3.90 (s, 3H), 3.62 (t, 4H, J = 4.5 Hz), 2.86 (t, 4H, J = 4.5 Hz); 13C NMR (DMSO-d6, 125 MHz, ppm): δ 169.1, 148.7, 148.0, 144.2, 139.8, 131.0, 128.8, 127.0, 126.4, 124.9, 123.0, 119.3, 115.6, 109.8, 109.3, 65.7 (2C), 56.6, 46.4 (2C); ESIMS: m/z 493 [M − H]− HRESIMS: Calc for C20H18BrN2O6S [M − H]− 493.0073, found 493.0074.
(E)-3-(2,3-Dibromo-4-hydroxy-5-methoxybenzylidene)-5-(morpholinosulfonyl)indolin-2-one (5f). Yield: 78.3%; 1H NMR (DMSO-d6, 500 MHz, ppm): δ 10.95 (s, 1H), 8.02 (s, 1H), 7.67 (s, 1H), 7.56 (dd, 1H, J = 1.5, 8.0 Hz), 7.39 (s, 1H), 7.04 (d, 1H, J = 8.0 Hz), 3.90 (s, 3H), 3.62 (t, 4H, J = 4.5 Hz), 2.86 (t, 4H, J = 4.5 Hz); 13C NMR (DMSO-d6, 125 MHz, ppm): δ 169.2, 148.8, 147.5, 144.3, 139.8, 130.9 127.1, 126.4, 124.9, 123.0, 121.9, 121.7, 119.3, 113.1, 109.9, 65.7 (2C), 56.6, 46.4 (2C); ESIMS: m/z 570 [M − H]− HRESIMS: Calc for C20H17Br2N2O6S [M − H]− 570.9172, found 570.9180.
(E)-3-(3-Bromo-4,5-dimethoxybenzylidene)-5-(morpholinosulfonyl)indolin-2-one (5g). Yield: 82.6%; 1H NMR (DMSO-d6, 500 MHz, ppm): δ 11.00 (s, 1H), 8.07 (s, 1H), 7.70 (s, 1H), 7.62 (dd, 1H, J = 1.5, 8.5 Hz), 7.60 (s, 1H), 7.46 (s, 1H), 7.05 (d, 1H, J = 8.5 Hz), 3.89 (s, 3H), 3.82 (s, 3H), 3.62 (t, 4H, J = 4.5 Hz), 2.87 (t, 4H, J = 4.5 Hz); 13C NMR (DMSO-d6, 125 MHz, ppm): δ 168.9, 153.9, 148.3, 144.8, 138.7, 131.7, 130.7, 126.9, 126.0, 125.5, 122.1, 121.7, 116.6, 114.4, 110.1, 65.7 (2C), 60.8, 56.8, 46.4 (2C); ESIMS: m/z 507 [M − H]− HRESIMS: Calc for C21H20BrN2O6S [M − H]− 507.0227, found 507.0231.
(E)-3-(2,3-Dibromo-4,5-dimethoxybenzylidene)-5-(morpholinosulfonyl)indolin-2-one (5h). Yield: 88.2%; 1H NMR (CDCl3, 500 MHz, ppm): δ 10.96 (s, 1H), 7.89 (s, 1H), 7.67 (dd, 1H, J = 1.5, 8.5 Hz), 7.63 (s, 1H), 7.29 (s, 1H), 7.11 (d, 1H, J = 8.5 Hz) , 3.97 (s, 3H) , 3.93 (s, 3H), 3.75 (t, 4H, J = 4.5 Hz), 2.93 (t, 4H, J = 4.5 Hz); 13C NMR (CDCl3, 125 MHz, ppm): δ 169.7, 153.0, 149.4, 147.1, 138.6, 131.9, 130.8, 128.3, 128.0, 123.0, 122.9, 122.0, 117.9, 112.9, 111.0, 66.3 (2C), 61.1, 56.8, 46.3 (2C); ESIMS: m/z 584 [M − H]− HRESIMS: Calc for C21H19Br2N2O6S [M − H]− 584.9325, found 584.9336.
(E)-3-(3-Bromo-2-(2,3-dibromo-4,5-dimethoxybenzyl)-4,5-dimethoxybenzylidene)-5-(morpholinosulfonyl) indolin-2-one (5i). Yield: 84.3%; 1H NMR (DMSO-d6, 500 MHz, ppm): δ 10.96 (s, 1H), 7.62 (s, 1H), 7.53 (dd, 1H, J = 1.5, 8.5 Hz), 7.31 (s, 1H), 7.21 (s, 1H), 6.99 (d, 1H, J = 8.5 Hz), 6.56 (s, 1H), 4.26 (s, 2H), 3.80 (s, 3H), 3.79 (s, 3H), 3.60 (t, 4H, J = 4.5 Hz), 3.54 (s, 3H), 3.52 (s, 3H), 2.75 (t, 4H, J = 4.5 Hz); 13C NMR (DMSO-d6, 125 MHz, ppm): δ 168.2, 152.3 (2C), 147.4, 147.1, 145.8, 137.5, 136.8, 131.5, 130.7, 130.6, 127.9, 126.9, 122.6, 122.3, 121.6, 121.3, 116.7, 113.7, 113.4, 110.6, 65.7 (2C), 60.7, 60.1, 56.9, 56.2, 41.4, 46.2 (2C); ESIMS: m/z 812 [M − H]− HRESIMS: Calc for C30H28Br3N2O8S [M − H]− 812.9113, found 812.9122.
(E)-3-(3,4-Dihydroxybenzylidene)-5-((4-(pyrimidin-2-yl)piperazin-1-yl) sulfonyl) indolin-2-one (6a). Yield: 55.1%; 1H NMR (DMSO-d6, 500 MHz, ppm): δ 11.11 (s, 1H), 8.30 (d, 2H, J = 5.0 Hz), 7.77 (s, 1H), 7.57 (s, 1H), 7.52 (d, 1H, J = 8.0 Hz), 7.14 (s, 1H), 7.02 (d, 1H, J = 8.0 Hz), 6.94 (d, 1H, J = 8.0 Hz), 6.73 (d, 1H, J = 8.0 Hz),6.60 (t, 1H, J = 5.0 Hz), 2.95 (t, 4H, J = 4.5 Hz), 2.92 (t, 4H, J = 4.5 Hz); 13C NMR (DMSO-d6, 125 MHz, ppm): δ 167.9, 161.2, 158.4 (2C), 147.1, 145.9, 143.0, 141.1, 130.5, 127.7, 126.5, 125.4, 122.9, 117.6, 116.4, 115.8, 111.1, 110.2, 109.0, 46.2 (2C), 43.0 (2C); ESIMS: m/z 478 [M − H]− HRESIMS: Calc for C23H20N5O5S [M − H]− 478.1184, found 478.1191.
(E)-3-(4-Hydroxy-3-methoxybenzylidene)-5-((4-(pyrimidin-2-yl) piperazin-1-yl) sulfonyl) indolin-2-one (6b). Yield: 61.5%; 1H NMR (DMSO-d6, 500 MHz, ppm): δ 11.00 (s, 1H), 8.30 (d, 2H, J = 5.0 Hz), 8.00 (s, 1H), 7.68 (s, 1H), 7.58 (d, 1H, J = 8.5 Hz), 7.39 (s, 1H), 7.05 (d, 1H, J = 8.5 Hz), 6.99 (d, 1H, J = 8.0 Hz), 6.87 (d, 1H, J = 8.0 Hz), 6.60 (t, 1H, J = 5.0 Hz), 3.84 (s, 3H), 2.94 (t, 4H, J = 4.5 Hz), 2.89 (t, 4H, J = 4.5 Hz); 13C NMR (DMSO-d6, 125 MHz, ppm): δ 167.9, 161.2, 158.4 (2C), 150.1, 147.5, 144.0, 141.4, 129.8, 127.2, 126.3, 125.3, 123.4, 121.5, 121.3, 116.7, 115.7, 111.1, 110.7, 56.1, 46.2 (2C), 43.0 (2C); ESIMS: m/z 492 [M − H]− HRESIMS: Calc for C24H22N5O5S [M − H]− 492.1341, found 492.1347.
(E)-3-(3-Bromo-4-hydroxy-5-methoxybenzylidene)-5-((4-(pyrimidin-2-yl)piperazin-1-yl) sulfonyl) indolin-2-one (6c). Yield: 70.2%; 1H NMR (DMSO-d6, 500 MHz, ppm): δ 10.70 (s, 1H), 8.29 (d, 2H, J = 5.0 Hz), 7.88 (s, 1H), 7.70 (s, 1H), 7.50 (s, 1H),7.36 (d, 1H, J = 8.0 Hz), 7.14 (s, 1H), 6.91 (d, 1H, J = 8.0 Hz), 6.58 (t, 1H, J = 5.0 Hz), 3.81 (s, 3H), 2.97 (t, 4H, J = 4.5 Hz), 2.91 (t, 4H, J = 4.5 Hz); 13C NMR (DMSO-d6, 125 MHz, ppm): δ 168.0, 161.2, 158.4 (2C), 150.4, 145.1, 142.0, 141.4, 128.4, 126.2, 125.1, 123.5, 120.0, 117.8, 116.7, 113.7, 111.0, 1109.8, 108.7, 55.7, 46.2 (2C), 43.0 (2C); ESIMS: m/z 570 [M − H]− HRESIMS: Calc for C24H21BrN5O5S [M − H]− 570.0433, found 570.0452.
(E)-3-(3-Bromo-4,5-dimethoxybenzylidene)-5-((4-(pyrimidin-2-yl)piperazin-1-yl) sulfonyl) indolin-2-one (6d). Yield: 76.2%; 1H NMR (DMSO-d6, 500 MHz, ppm): δ 11.11 (s, 1H), 8.31 (d, 2H, J = 5.0 Hz), 8.05 (s, 1H), 7.68(s, 1H), 7.58-7.60 (overlap, 2H), 7.45 (s, 1H), 7.01 (d, 1H, J = 8.5 Hz), 6.60 (t, 1H, J = 5.0 Hz), 3.89(s, 3H), 3.82(s, 3H), 2.95 (t, 4H, J = 4.5 Hz), 2.91 (t, 4H, J = 4.5 Hz); 13C NMR (DMSO-d6, 125 MHz, ppm): δ 168.9, 161.2, 158.4 (2C), 154.0, 148.3, 144.8, 137.3, 131.7, 130.7, 127.6, 127.3, 125.5, 121.7, 122.0, 116.6, 114.4, 111.1, 110.1, 60.8, 56.8, 46.1 (2C), 42.9 (2C); ESIMS: m/z 584 [M − H]− HRESIMS: Calc for C25H23BrN5O5S [M − H]− 584.0606, found 584.0609.
(E)-3-(2,3-Dibromo-4,5-dimethoxybenzylidene)-5-((4-(pyrimidin-2-yl)piperazin-1-yl) sulfonyl) indolin-2-one (6e). Yield: 78.3%; 1H NMR (Pyridine-d5, 500 MHz, ppm): δ 10.16 (s, 1H), 8.14 (d, 2H, J = 5.0 Hz), 7.99 (s, 1H), 7.67 (d, 1H, J = 8.0 Hz), 7.42 (s, 1H), 7.03 (s, 1H), 6.97 (d, 1H, J = 8.0 Hz), 6.25 (t, 1H, J = 5.0 Hz), 3.84 (s, 3H), 3.78 (s, 3H), 3.04 (t, 4H, J = 4.5 Hz), 2.90 (t, 4H, J = 4.5 Hz); 13C NMR (Pyridine-d5, 125 MHz, ppm): δ 169.6, 161.6, 158.2 (2C), 153.4, 150.3, 148.3, 137.3, 132.4, 131.0, 128.9, 128.4, 123.2, 123.1, 122.3, 118.2, 113.8, 110.93, 110.86, 60.9, 57.0, 46.5 (2C), 43.2 (2C); ESIMS: m/z 661 [M − H]− HRESIMS: Calc for C25H22Br2N5O5S [M − H]− 661.9690, found 661.9714.
(E)-3-(3-Bromo-2-(2,3-dibromo-4,5-dimethoxybenzyl)-4,5-dimethoxybenzylidene)-5-((4-(pyrimidin-2-yl) piperazin-1-yl) sulfonyl) indolin-2-one (6f). Yield: 85.2%; 1H NMR (DMSO-d6, 500 MHz, ppm): δ 10.90 (s, 1H), 8.31 (d, 2H, J = 5.0 Hz), 7.59 (s, 1H), 7.53 (dd, 1H, J = 1.5, 8.5 Hz), 7.28 (s, 1H), 7.21 (s, 1H), 6.96 (d, 1H, J = 8.5 Hz), 6.60–6.62 (overlap, 2H), 4.26 (s, 2H), 3.83 (s, 3H), 3.80 (s, 3H), 3.54 (s, 3H), 3.51 (s, 3H), 2.92 (t, 4H, J = 4.5 Hz), 2.81 (t, 4H, J = 4.5 Hz); 13C NMR (DMSO-d6, 125 MHz, ppm): δ 168.2, 161.1, 158.4 (2C), 152.3, 152.2, 147.3, 147.0, 145.7, 137.6, 137.0, 131.5, 130.6, 127.7, 127.2, 122.7, 122.2, 121.6, 121.2, 116.6, 114.0, 113.4, 111.1, 110.5, 60.7, 60.0, 56.9, 56.2, 46.1 (2C), 42.8 (2C), 41.5; ESIMS: m/z 889 [M − H]− HRESIMS: Calc for C34H31Br3N5O7S [M − H]− 889.9522, found 889.9500.
(E)-N-(Adamantan-1-yl)-3-(3-bromo-4,5-dimethoxybenzylidene)-2-oxoindoline-5-sulfonamide (7a). Yield: 42.6%; 1H NMR (DMSO-d6, 500 MHz, ppm): δ 11.09 (s, 1H), 8.07 (s, 1H), 7.78–7.71 (overlap, 2H), 7.66 (s, 1H), 7.46 (s, 1H), 7.02 (d, 1H, J = 8.0 Hz), 3.94 (s, 3H), 3.93 (s, 3H), 1.99 (m, 6H), 1.57 (m, 3H), 1.25 (m, 6H); 13C NMR (DMSO-d6, 125 MHz, ppm): δ 168.1, 153.8, 148.3, 144.7, 138.2, 137.6, 130.9, 130.1, 126.7, 126.0, 121.7, 121.6, 118.0, 115.3, 110.3, 60.8, 56.3, 55.3, 43.1 (3C), 35.8 (3C), 29.5 (3C); ESIMS: m/z 571 [M − H]− HRESIMS: Calc for C27H28BrN2O5S [M − H]− 571.0907, found 571.0908.
(E)-N-(Adamantan-1-yl)-3-(2,3-dibromo-4,5-dimethoxybenzylidene)-2-oxoindoline-5-sulfonamide (7b). Yield: 52.5%; 1H NMR (DMSO-d6, 500 MHz, ppm): δ 11.08 (s, 1H), 7.85 (s, 1H), 7.70 (d, 1H, 8.0 Hz), 7.64 (s, 1H), 7.28 (s, 1H), 7.01(d, 1H, J = 8.0 Hz), 3.80 (s, 3H) , 3.83 (s, 3H), 1.85 (m, 6H), 1.70 (m, 3H), 1.40 (m, 6H); 13C NMR (DMSO-d6, 125 MHz, ppm): δ 168.6, 152.9, 148.4, 146.1, 136.3, 132.1, 129.6, 128.8, 128.6, 121.9, 121.4, 120.8, 116.6, 113.9, 111.7, 60.8, 56.9, 54.1, 42.9 (3C), 35.9 (3C), 29.3 (3C); ESIMS: m/z 649 [M − H]− HRESIMS: Calc for C27H27Br2N2O5S [M − H]− 648.9996, found 649.0013.
(E)-N-(Adamantan-1-yl)-3-(3-bromo-2-(2,3-dibromo-4,5-dimethoxybenzyl)-4,5-dimethoxybenzylidene)-2-oxoindoline-5-sulfonamide (7c). Yield: 57.6%; 1H NMR (CDCl3, 500 MHz, ppm): δ 10.00 (s, 1H), 9.43 (s, 1H), 7.87 (s, 1H), 7.69 (s, 1H), 7.61 (d, 1H, J = 8.0 Hz), 7.26 (s, 1H), 7.21 (s, 1H), 7.16 (d, 1H, J = 8.0 Hz), 4.29 (s, 2H), 3.90 (s, 3H), 3.75 (s, 3H), 3.54 (s, 3H), 3.52 (s, 3H), 2.03 (m, 6H), 1.75 (m, 3H), 1.25 (m, 6H); 13C NMR (CDCl3, 125 MHz, ppm): δ 169.4, 152.5, 152.3, 148.4, 146.1, 144.7, 137.1, 135.4, 131.4, 129.2 130.6, 127.7, 127.6, 123.0, 122.3, 121.6, 121.3, 117.6, 111.8, 111.6, 110.1, 60.9, 60.4, 56.5, 56.1, 55.3, 46.9, 43.1 (3C), 35.8 (3C), 29.5 (3C); ESIMS: m/z 876 [M − H]− HRESIMS: Calc for C36H36Br3N2O7S [M − H]− 876.9793, found 876.9793.
3.2. Antiproliferative Activities Assays
3.2.1. Cell Culture and Proliferation Assay
The cells including Human lung cancer cell line, A549; Human hepatoma cell lines, Bel-7402 and HepG2; human cervical cancer cell line, HeLa; human colon cancer cell line, HCT116 were purchased from BOSTER, Ltd (Wuhan, China). The cells were maintained in DMEM supplemented with 10% FBS and 1% antibiotics at 37 °C and in an atmosphere containing 5% CO2. The cells were split 1:3 when they reached 80%–90% confluence. Cell proliferation was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay. The IC50 was calculated by using the SPSS 17.0 (SPSS, Inc., Chicago, IL, USA).
The cells (4 × 103 cells/well) with 10% FBS culture medium were seeded in a 96-well plate and incubated overnight. Next, the cells were treated with various amounts of compounds and incubated for 48 h. Subsequently, 20 μL of 5 mg/mL MTT was transferred into each well, and the cells were incubated for 4 h. The medium in each well was carefully removed, and 150 μL DMSO was then added to each well. The samples were thoroughly agitated for 10 min on a shaker. Finally, the absorbance of the samples at 490 and 690 nm was measured against a background control (blank) using a microplate reader.
3.2.2. Wound-Healing Assay
The HepG2 cells (1 × 105 cells/well) were cultured in 6-well plates rinsed with PBS and then starved overnight in 2% FBS medium until they reached 95% confluence. A single wound was then scratched in the center of the cell monolayers with a 200 μL sterile plastic pipette tip. The wounded monolayers were washed twice to remove the non-adherent cells and were incubated with various concentrations of compound 4g in the presence of 1 μg/mL of mitomycin C. To measure the length of the endothelial cells that had migrated from the edge of the injured monolayer, images were obtained immediately after wounding and after a 24, 48 h incubation period, using a phase-contrast microscope (Olympus, Tokyo, Japan). The length was measured by the Image-Pro Plus v 6.0 (Media Cybernetics, Inc., Bethesda, MD, USA). Each experiment was repeated at least three times.
3.2.3. Statistical Analysis
All the experiments were performed at least three times, and the data are presented as mean ± SD values. Differences between the mean values were assessed using one-way analysis of variance. For all the analyses, p < 0.05 was considered significant. Statistical analyses were performed using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA).
4. Conclusions
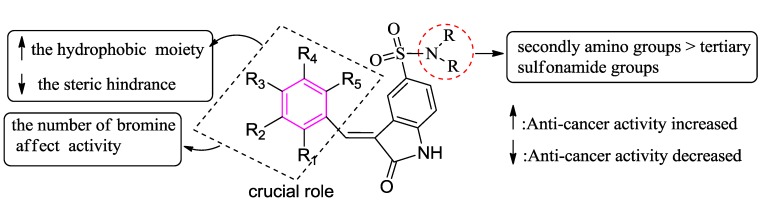
In summary, a series of bromophenols derivatives containing indolin-2-one moiety were designed and evaluated for their anticancer activities against A549, Bel7402, HepG2, HeLa and HCT116 cancer cell lines using MTT assay in vitro. The preliminary SAR analysis (summarized in Figure 5) reveals that: (i) the hydrophobic parameter may affect their anticancer activity; (ii) the steric hindrance may also affect their activity; (iii) the number of bromine atoms on phenol moiety could affect the anticancer activities of these hybrid derivatives; (iv) the types of amino groups at 5-position of indolin-2-one could influence the activities; (v) the bromophenol moiety played a crucial role in maintaining anticancer activities of the conjugated derivatives. Among them, seven compounds (4g–4i, 5h, 6d, 7a, 7b) showed potent activity. Wound-healing assay demonstrated that compound 4g can be used as a potent compound for inactivating invasion and metastasis by inhibiting the migration of cancer cells. These active derivatives targeting properties needs to be further investigated. Potentially, this finding may aid in the design of novel agents for the intervention of cancer.
Figure 5.
Structure–Activity relationships of bromophenol derivatives anticancer activity.
Acknowledgments
The work was supported by the National Natural Science Foundation of China (No. 41276167, 41206066 and 41306157), China Postdoctoral Science Foundation (No. 2014M551971 and 2014M551972), Innovation Project Special Fund for Post Doctors of Shandong Province (No. 201302017 and 201401019), the National science and technology support project (2013BAB01B02-2) and the Natural Science Foundation of Jiangsu Province (Grant No. BK 2012223).
Author Contributions
Li-Jun Wang, Shuai-Yu Wang and Da-Yong Shi contributed to the study concept and design, and the manuscript preparation. Li-Jun Wang and Shuai-Yu Wang performed the experimental studies and analyzed the data. Bo Jiang, Ning Wu, Xiang-Qian Li, Bao-Cheng Wang, Jiao Luo, Meng Yang and Shui-Hua Jin contributed in critical reading and discussion on the manuscript. All the authors approved the final version.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ferlay J.S.I., Ervik M., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]; GLOBOCAN 2012 v1.0 2013. International Agency for Research on Cancer; Lyon, France: 2013. [(accessed on 25 December 2014)]. Available online: http://globocan.iarc.fr. [Google Scholar]
- 2.Liu M., Hansen P.E., Lin X. Bromophenols in marine algae and their Bioactivities. Mar. Drugs. 2011;9:1273–1292. doi: 10.3390/md9071273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colon M., Guevara P., Gerwick W.H., Ballantine D. 5'-Hydroxyisoavrainvilleol, a new diphenylmethane derivative from the tropical green-alga avrainvillea-nigricans. J. Nat. Prod. 1987;50:368–374. doi: 10.1021/np50051a005. [DOI] [PubMed] [Google Scholar]
- 4.Shoeib N.A., Bibby M.C., Blunden G., Linley P.A., Swaine D.J., Wheelhouse R.T., Wright C.W. In-vitro cytotoxic activities of the major bromophenols of the red alga Polysiphonia lanosa and some novel synthetic isomers. J. Nat. Prod. 2004;67:1445–1449. doi: 10.1021/np0305268. [DOI] [PubMed] [Google Scholar]
- 5.Xu N.J., Fan X., Yan X.J., Tseng C.K. Screening marine algae from China for their antitumor activities. J. Appl. Phycol. 2004;16:451–456. doi: 10.1007/s10811-004-5508-x. [DOI] [Google Scholar]
- 6.Shi D.Y., Li J., Guo S.J., Su H., Fan X. The antitumor effect of bromophenol derivatives in vitro and Leathesia nana extract in vivo. Chin. J. Oceanol. Limnol. 2009;27:277–282. doi: 10.1007/s00343-009-9119-x. [DOI] [Google Scholar]
- 7.Eastwood P., Gonzalez J., Gomez E., Vidal B., Caturla F., Roca R., Balague C., Orellana A. Indolin-2-one p38α inhibitors I: Design, profiling and crystallographic binding mode. Bioorg. Med. Chem. Lett. 2011;21:4130–4133. doi: 10.1016/j.bmcl.2011.05.114. [DOI] [PubMed] [Google Scholar]
- 8.Lai Y., Ma L., Huang W., Yu X., Zhang Y., Ji H., Tian J. Synthesis and biological evaluation of 3-4-(amino/methylsulfonyl) phenyl methylene-indolin-2-one derivatives as novel COX-1/2 and 5-LOX inhibitors. Bioorg. Med. Chem. Lett. 2010;20:7349–7353. doi: 10.1016/j.bmcl.2010.10.056. [DOI] [PubMed] [Google Scholar]
- 9.Jeankumar V.U., Alokam R., Sridevi J.P., Suryadevara P., Matikonda S.S., Peddi S., Sahithi S., Alvala M., Yogeeswari P., Sriram D. Discovery and structure optimization of a series of isatin derivatives as mycobacterium tuberculosis chorismate mutase inhibitors. Chem. Biol. Drug Des. 2014;83:498–506. doi: 10.1111/cbdd.12265. [DOI] [PubMed] [Google Scholar]
- 10.Kaur A., Singh B., Vyas B., Silakari O. Synthesis and biological activity of 4-aryl-3-benzoyl-5-phenylspiro pyrrolidine-2.3 “-indolin-2”-one derivatives as novel potent inhibitors of advanced glycation end product. Eur. J. Med. Chem. 2014;79:282–289. doi: 10.1016/j.ejmech.2014.04.022. [DOI] [PubMed] [Google Scholar]
- 11.Kim M.H., Tsuhako A.L., Co E.W., Aftab D.T., Bentzien F., Chen J., Cheng W., Engst S., Goon L., Klein R.R., et al. The design, synthesis, and biological evaluation of potent receptor tyrosine kinase inhibitors. Bioorg. Med. Chem. Lett. 2012;22:4979–4985. doi: 10.1016/j.bmcl.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 12.Lin H.-H., Wu W.-Y., Cao S.-L., Liao J., Ma L., Gao M., Li Z.-F., Xu X. Synthesis and antiproliferative evaluation of piperazine-1-carbothiohydrazide derivatives of indolin-2-one. Bioorg. Med. Chem. Lett. 2013;23:3304–3307. doi: 10.1016/j.bmcl.2013.03.099. [DOI] [PubMed] [Google Scholar]
- 13.Lv K., Wang L.-L., Liu M.-L., Zhou X.-B., Fan S.-Y., Liu H.-Y., Zheng Z.-B., Li S. Synthesis and antitumor activity of 5-1-(3-(dimethylamino)propyl)-5-halogenated-2-oxoindolin-(3Z)-ylideneme thyl-2,4-dimethyl-1H-pyrrole-3-carboxamides. Bioorg. Med. Chem. Lett. 2011;21:3062–3065. doi: 10.1016/j.bmcl.2011.03.031. [DOI] [PubMed] [Google Scholar]
- 14.Penthala N.R., Yerramreddy T.R., Madadi N.R., Crooks P.A. Synthesis and in vitro evaluation of N-alkyl-3-hydroxy-3-(2-imino-3-methyl-5-oxoimidazolidin-4-yl)indolin-2-one analogs as potential anticancer agents. Bioorg. Med. Chem. Lett. 2010;20:4468–4471. doi: 10.1016/j.bmcl.2010.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McComas C.C., Vu A.T., Mahaney P.E., Cohn S.T., Fensome A., Marella M.A., Nogle L., Trybulski E.J., Ye F., Zhang P., et al. Synthesis and activity of 1-(3-amino-1-phenylpropyl)indolin-2-ones: A new class of selective norepinephrine reuptake inhibitors. Bioorg. Med. Chem. Lett. 2008;18:4929–4931. doi: 10.1016/j.bmcl.2008.08.060. [DOI] [PubMed] [Google Scholar]
- 16.Najafi M. On the antioxidant activity of ortho- and meta-substituted indolin-2-one derivatives. Monatsh. Chem. 2014;145:291–299. doi: 10.1007/s00706-013-1099-z. [DOI] [Google Scholar]
- 17.Prakash C.R., Raja S. Synthesis, characterization and in vitro antimicrobial activity of some novel 5-substituted Schiff and Mannich base of isatin derivatives. J. Saudi Chem. Soc. 2013;17:337–344. doi: 10.1016/j.jscs.2011.10.022. [DOI] [Google Scholar]
- 18.Praveen C., Ayyanar A., Perumal P.T. Practical synthesis, anticonvulsant, and antimicrobial activity of N-allyl and N-propargyl di(indolyl)indolin-2-ones. Bioorg. Med. Chem. Lett. 2011;21:4072–4077. doi: 10.1016/j.bmcl.2011.04.117. [DOI] [PubMed] [Google Scholar]
- 19.Li P.K., Xiao Z.L., Hu Z.G., Pandit B., Sun Y.J., Sackett D.L., Werbovetz K., Lewis A., Johnsamuel J. Conformationally restricted analogs of Combretastatin A-4 derived from SU5416. Bioorg. Med. Chem. Lett. 2005;15:5382–5385. doi: 10.1016/j.bmcl.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Dweedar H.E., Mahrous H., Ibrahim H.S., Abdel-Aziz H.A. Analogue-based design, synthesis and biological evaluation of 3-substituted-(methylenehydrazono)indolin-2-ones as anticanceragents. Eur. J. Med. Chem. 2014;78:275–280. doi: 10.1016/j.ejmech.2014.03.058. [DOI] [PubMed] [Google Scholar]
- 21.Chow L.Q., Jonker D.J., Laurie S.A., Call J.A., Diab S.G., Goss G., McWilliam M., Wang E., Chao R., Eckhardt S.G., et al. Sunitinib (SU) in combination with pemetrexed (P) in patients (pts) with advanced solid malignancies: A phase I dose escalation study. J. Clin. Oncol. 2008;26:3566. doi: 10.1200/JCO.2008.17.1363. [DOI] [Google Scholar]
- 22.Sattler M., Pride Y.B., Ma P., Gramlich J.L., Chu S.C., Quinnan L.A., Shirazian S., Liang C.X., Podar K., Christensen J.G., et al. A novel small molecule met inhibitor induces apoptosis in cells transformed by the oncogenic TPR-MET tyrosine kinase. Cancer Res. 2003;63:5462–5469. [PubMed] [Google Scholar]
- 23.Christensen J.G., Schreck R., Burrows J., Kuruganti P., Chan E., Le P., Chen J., Wang X.Y., Ruslim L., Blake R., et al. A selective small molecule inhibitor of c-Met kinase inhibits c-Met-dependent phenotypes in vitro and exhibits cytoreductive antitumor activity in vivo. Cancer Res. 2003;63:7345–7355. [PubMed] [Google Scholar]
- 24.Wang S., Zhao Y., Zhang G., Lv Y., Zhang N., Gong P. Design, synthesis and biological evaluation of novel 4-thiazolidinones containing indolin-2-one moiety as potential antitumor agent. Eur. J. Med. Chem. 2011;46:3509–3518. doi: 10.1016/j.ejmech.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 25.Wang S., Zhao Y., Zhu W., Liu Y., Guo K., Gong P. Synthesis and anticancer activity of indolin-2-one derivatives bearing the 4-thiazolidinone moiety. Arch. Pharm. 2012;345:73–80. doi: 10.1002/ardp.201100082. [DOI] [PubMed] [Google Scholar]
- 26.Singh P., Kaur M., Holzer W. Synthesis and evaluation of indole, pyrazole, chromone and pyrimidine based conjugates for tumor growth inhibitory activities—Development of highly efficacious cytotoxic agents. Eur. J. Med. Chem. 2010;45:4968–4982. doi: 10.1016/j.ejmech.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Jiang B., Guo S.J., Shi D.Y., Guo C., Wang T. Discovery of novel bromophenol 3,4-dibromo-5-(2-bromo-3,4-dihydroxy-6-(isobutoxymethyl)benzyl)benzene-1,2-diol as protein tyrosine phosphatase 1B inhibitor and its anti-diabetic properties in C57BL/KsJ-db/db mice. Eur. J. Med. Chem. 2013;64:129–136. doi: 10.1016/j.ejmech.2013.03.037. [DOI] [PubMed] [Google Scholar]
- 28.Cui Y.C., Shi D.Y., Hu Z.Q. Synthesis and protein tyrosine phosphatase 1B inhibition activities of two new synthetic bromophenols and their methoxy derivatives. Chin. J. Oceanol. Limn. 2011;29:1237–1242. doi: 10.1007/s00343-011-0271-8. [DOI] [Google Scholar]
- 29.Guo S.J., Li J., Li T., Shi D.Y., Han L.J. Synthesis of three bromophenols from red algae as PTP1B inhibitors. Chin. J. Oceanol. Limn. 2011;29:68–74. doi: 10.1007/s00343-011-9996-7. [DOI] [Google Scholar]
- 30.Shi D.Y., Guo S.J., Jiang B., Guo C., Wang T., Zhang L.Y., Li J.Y. HPN, a synthetic analogue of bromophenol from red alga rhodomela confervoides: Synthesis and anti-diabetic effects in C57BL/KsJ-db/db mice. Mar. Drugs. 2013;11:350–362. doi: 10.3390/md11020350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Astin J.W., Batson J., Kadir S., Charlet J., Persad R.A., Gillatt D., Oxley J.D., Nobes C.D. Competition amongst Eph receptors regulates contact inhibition of locomotion and invasiveness in prostate cancer cells. Nat. Cell. Biol. 2010;12:1194–1204. doi: 10.1038/ncb2122. [DOI] [PubMed] [Google Scholar]