Abstract
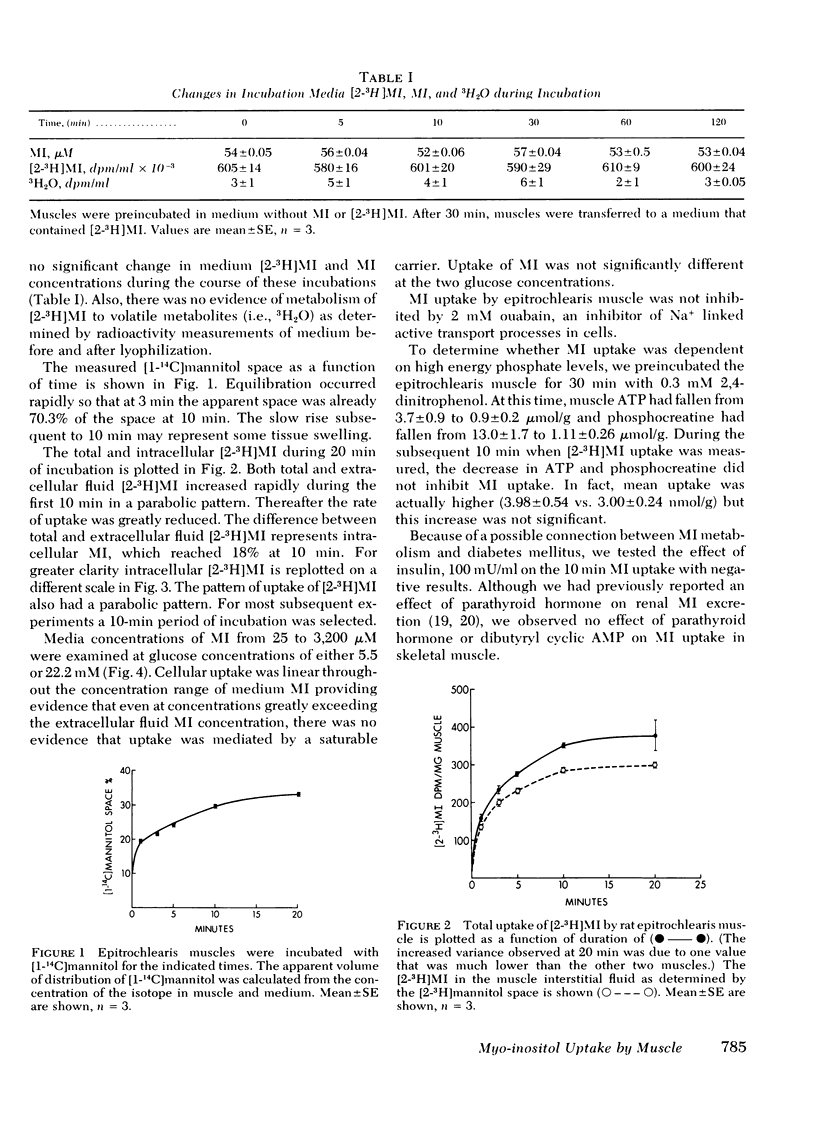
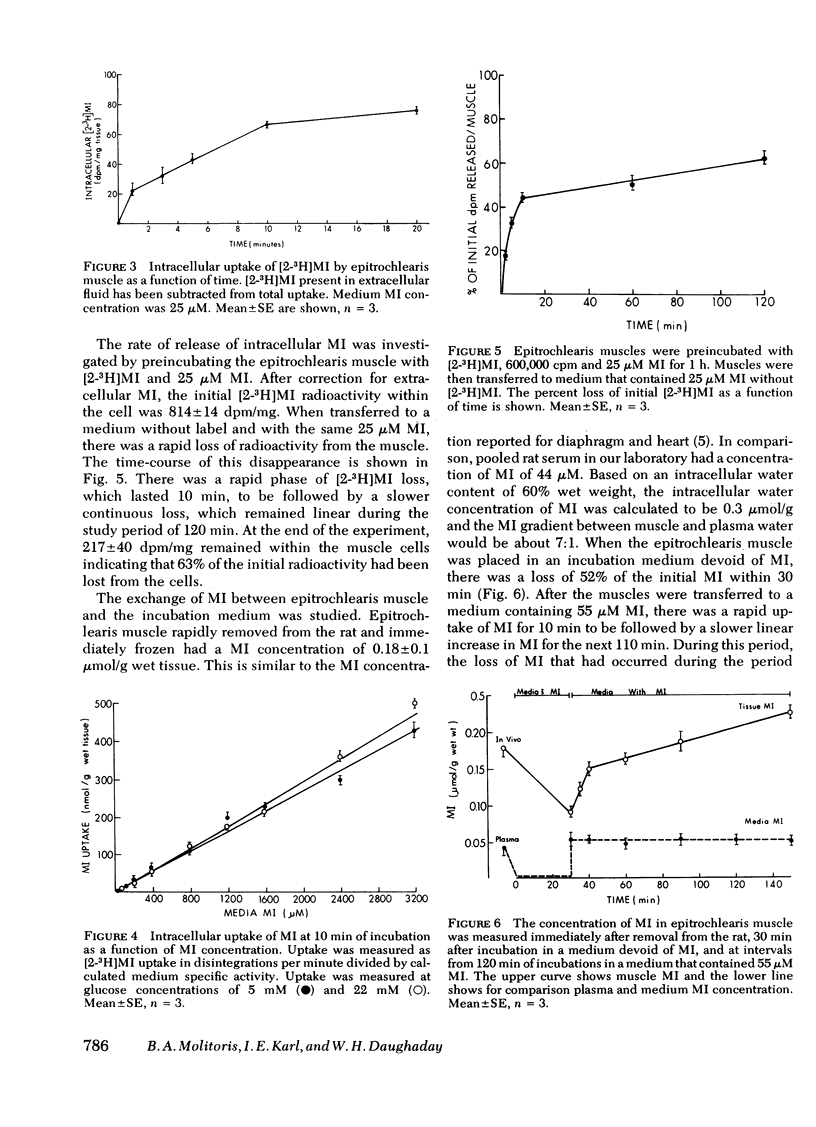
The cellular uptake of nonphosphorylated myo-inositol (MI) and its incorporation into phosphoinositide in the rat epitrochlearis muscle was measured. Cellular uptake of [2-3H]MI was determined by the difference between total uptake and [2-3H]MI present in the extracellular fluid determined with [1-14C]mannitol. Cellular uptake was parabolic and directly proportional to medium MI concentrations between 25 and 3,200 μM. Saturation of a MI carrier was not evident. Moreover, uptake was not inhibited by 2 mM ouabain, 0.3 mM 2,4-dinitrophenol, or 22 mM glucose. Insulin, 100 mU/ml, was without effect on either cellular uptake of [2-3H]MI or its incorporation into phosphoinositides. In muscles that were preloaded with [2-3H]MI and then incubated in media that contained a constant amount of MI but no [2-3H]MI, 44.3% of the [2-3H]MI was released after 10 min increasing to 62.5% by 120 min. Cellular MI concentrations were 0.18 μmol/g wet tissue (four times plasma levels) in rapidly isolated and frozen epitrochlearis muscle. When muscle was incubated without MI, 48% of endogenous MI was lost rapidly. Restoration of cellular MI in 50 μM MI media occurred in two phases, a rapid uptake phase lasting 10 min and a subsequent slow phase of MI uptake.
It is concluded that MI enters and leaves skeletal muscle cells freely by a process that does not involve active transport. Neither insulin nor hyperglycemia affected MI transport nor its incorporation into phosphoinositides. The intracellular to medium concentration gradient may be dependent on reversible binding to tubulin and possibly to other intracellular components.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burton L. E., Ray R. E., Bradford J. R., Orr J. P., Nickerson J. A., Wells W. W. myo-Inositol metabolism in the neonatal and developing rat fed a myo-inositol-free diet. J Nutr. 1976 Nov;106(11):1610–1616. doi: 10.1093/jn/106.11.1610. [DOI] [PubMed] [Google Scholar]
- Caspary W. F., Crane R. K. Active transport of myo-inositol and its relation to the sugar transport system in hamster small intestine. Biochim Biophys Acta. 1970 Apr 21;203(2):308–316. doi: 10.1016/0005-2736(70)90145-8. [DOI] [PubMed] [Google Scholar]
- Clements R. S., Jr Diabetic neuropathy--new concepts of its etiology. Diabetes. 1979 Jun;28(6):604–611. doi: 10.2337/diab.28.6.604. [DOI] [PubMed] [Google Scholar]
- Clements R. S., Jr, Reynertson R. Myoinositol metabolism in diabetes mellitus. Effect of insulin treatment. Diabetes. 1977 Mar;26(3):215–221. doi: 10.2337/diab.26.3.215. [DOI] [PubMed] [Google Scholar]
- DAWSON R. M., FREINKEL N. The distribution of free mesoinositol in mammalian tissues, including some observations on the lactating rat. Biochem J. 1961 Mar;78:606–610. doi: 10.1042/bj0780606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber A. J., Karl I. E., Kipnis D. M. Alanine and glutamine synthesis and release from skeletal muscle. I. Glycolysis and amino acid release. J Biol Chem. 1976 Feb 10;251(3):826–835. [PubMed] [Google Scholar]
- Hauser G. Energy- and sodium-dependent uptake of inositol by kidney cortex slices. Biochem Biophys Res Commun. 1965 Jun 9;19(6):696–701. doi: 10.1016/0006-291x(65)90313-x. [DOI] [PubMed] [Google Scholar]
- Hauser G. Myo-inositol transport in slices of rat kidney cortex. I. Effect of incubation conditions and inhibitors. Biochim Biophys Acta. 1969 Mar 11;173(2):257–266. doi: 10.1016/0005-2736(69)90109-6. [DOI] [PubMed] [Google Scholar]
- Howard C. F., Jr, Anderson L. Metabolism of myo-inositol in animals. II. Complete catabolism of myo-inositol-14C by rat kidney slices. Arch Biochem Biophys. 1967 Feb;118(2):332–339. doi: 10.1016/0003-9861(67)90357-8. [DOI] [PubMed] [Google Scholar]
- Kirazov E. P., Lagnado J. R. Interactions of myo-inositol with brain microtubules. FEBS Lett. 1977 Sep 1;81(1):173–178. doi: 10.1016/0014-5793(77)80953-8. [DOI] [PubMed] [Google Scholar]
- Molitoris B. A., Hruska K. A., Fishman N., Daughaday W. H. Effects of glucose and parathyroid hormone on the renal handling of myoinositol by isolated perfused dog kidneys. J Clin Invest. 1979 Jun;63(6):1110–1118. doi: 10.1172/JCI109403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmano K. P., Whiting P. H., Hawthorne J. N. Free and lipid myo-inositol in tissues from rats with acute and less severe streptozotocin-induced diabetes. Biochem J. 1977 Oct 1;167(1):229–235. doi: 10.1042/bj1670229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard M. R., Hawthorne J. N. Does myo-inositol specifically interact with brain microtubules? FEBS Lett. 1978 Sep 1;93(1):78–80. doi: 10.1016/0014-5793(78)80809-6. [DOI] [PubMed] [Google Scholar]
- Sherman W. R., Stewart M. A., Kurien M. M., Goodwin S. L. The measurement of myo-inositol, myo-inosose-2 and scyllo-inositol in mammalian tissues. Biochim Biophys Acta. 1968 May;158(2):197–205. doi: 10.1016/0304-4165(68)90131-1. [DOI] [PubMed] [Google Scholar]
- Spector R. Inositol accumulation by brain slices in vitro. J Neurochem. 1976 Nov;27(5):1273–1276. doi: 10.1111/j.1471-4159.1976.tb00343.x. [DOI] [PubMed] [Google Scholar]
- Spector R., Lorenzo A. V. Myo-inositol transport in the central nervous system. Am J Physiol. 1975 May;228(5):1510–1518. doi: 10.1152/ajplegacy.1975.228.5.1510. [DOI] [PubMed] [Google Scholar]
- Takenawa T., Wada E., Tsumita T. myo-Inositol binding and transport in brush border membranes of rat kidney. Biochim Biophys Acta. 1977 Jan 4;464(1):108–117. doi: 10.1016/0005-2736(77)90374-1. [DOI] [PubMed] [Google Scholar]
- Warfield A., Hwang S. M., Segal S. On the uptake of inositol by rat brain synaptosomes. J Neurochem. 1978 Oct;31(4):957–960. doi: 10.1111/j.1471-4159.1978.tb00133.x. [DOI] [PubMed] [Google Scholar]


