Abstract
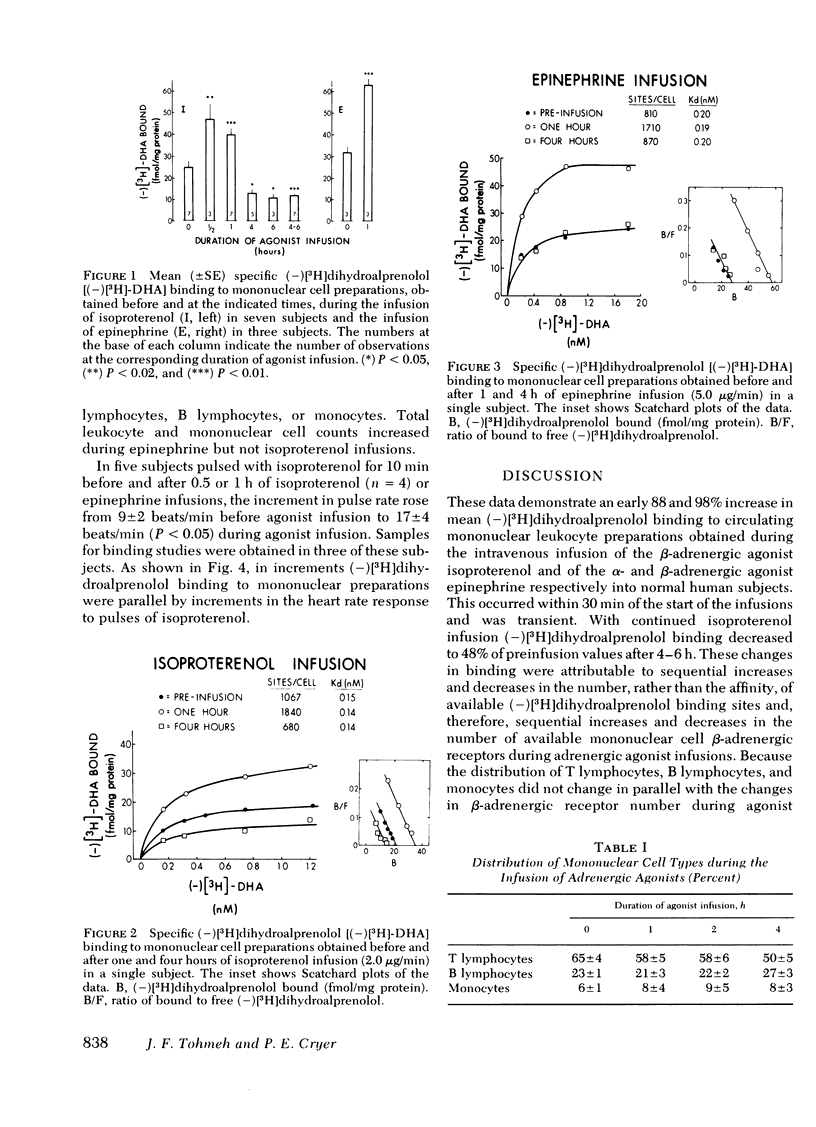
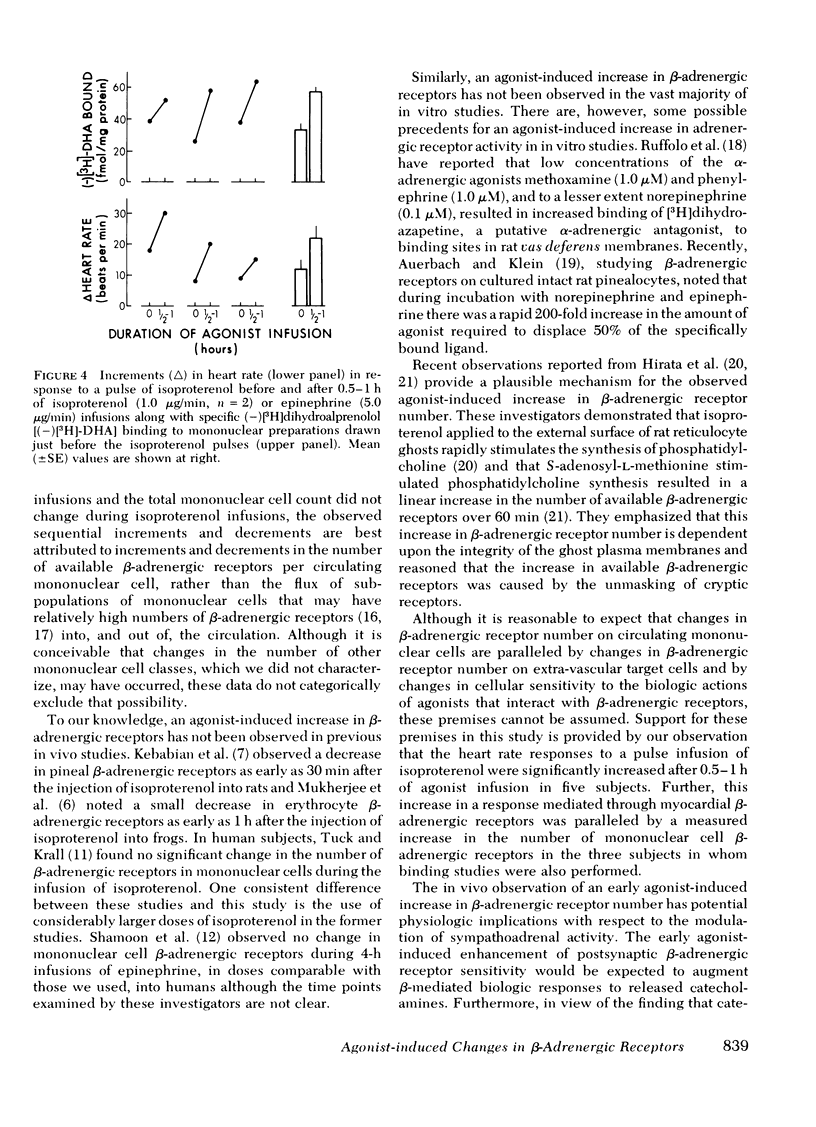
β-Adrenergic receptors in mononuclear leukocyte preparations were assessed with (−)[3H]-dihydroalprenolol binding studies during the infusion of adrenergic agonists into normal human subjects. During the infusion of isoproterenol into seven subjects, mean (±SE) (−)[3H]dihydroalprenolol binding increased from 25±3 fmol/mg protein to 47±8 fmol/mg protein (P < 0.02) at 0.5 h and 40±3 fmol/mg protein (P < 0.01) at 1 h and decreased to 12±1 fmol/mg protein (P < 0.01) at 4-6 h. During the infusion of epinephrine into three subjects, mean (−)[3H]dihydroalprenolol binding increased from 32±3 to 63±3 fmol/mg protein (P < 0.01) at 0.5-1 h. By Scatchard plot analysis, these changes were attributable to changes in the number of available binding sites rather than changes in binding affinity. The observed changes in the number of (−)[3H]dihydroalprenolol binding sites were not paralleled by changes in total mononuclear cell counts or in T lymphocyte, B lymphocyte, and monocyte distributions. Thus, we conclude that adrenergic agonists modulate the number of available β-adrenergic receptors on circulating mononuclear cells in a biphasic manner, with an early increment and a late decrement, in man. Further, the finding that the increase in pulse rate in response to a “pulse” infusion of isoproterenol was significantly greater after 0.5-1 h of agonist infusion suggests that the observed early agonist-induced increment in β-adrenergic receptor number on circulating cells is paralleled by increments in extra-vascular β-adrenergic receptor sensitivity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach M. A. Differences in Cyclic AMP Changes after Stimulation by Prostaglandins and Isoproterenol in Lymphocyte Subpopulations. J Clin Invest. 1975 May;55(5):1074–1081. doi: 10.1172/JCI108008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Galant S. P., Duriseti L., Underwood S., Insel P. A. Decreased beta-adrenergic receptors on polymorphonuclear leukocytes after adrenergic therapy. N Engl J Med. 1978 Oct 26;299(17):933–936. doi: 10.1056/NEJM197810262991707. [DOI] [PubMed] [Google Scholar]
- Galant S. P., Underwood S., Duriseti L., Insel P. A. Characterization of high-affinity beta2-adrenergic receptor binding of (-)-[3H]-dihydroalprenolol to human polymorphonuclear cell particulates. J Lab Clin Med. 1978 Oct;92(4):613–618. [PubMed] [Google Scholar]
- Hirata F., Strittmatter W. J., Axelrod J. beta-Adrenergic receptor agonists increase phospholipid methylation, membrane fluidity, and beta-adrenergic receptor-adenylate cyclase coupling. Proc Natl Acad Sci U S A. 1979 Jan;76(1):368–372. doi: 10.1073/pnas.76.1.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn C. R. Membrane receptors for hormones and neurotransmitters. J Cell Biol. 1976 Aug;70(2 Pt 1):261–286. doi: 10.1083/jcb.70.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebabian J. W., Zatz M., Romero J. A., Axelrod J. Rapid changes in rat pineal beta-adrenergic receptor: alterations in l-(3H)alprenolol binding and adenylate cyclase. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3735–3739. doi: 10.1073/pnas.72.9.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MacDermott R. P., Nash G. S., Bertovich M. J., Merkel N. S., Weinrieb I. J. Human B-cell mitogenic responsiveness to lectins: the requirement for T cells. Cell Immunol. 1978 Jun;38(1):198–202. doi: 10.1016/0008-8749(78)90047-3. [DOI] [PubMed] [Google Scholar]
- Mendelsohn J., Nordberg J. Adenylate cyclase in thymus-derived and bone marrow-derived lymphocytes from normal donors and patients with chronic lymphocytic leukemia. J Clin Invest. 1979 Jun;63(6):1124–1132. doi: 10.1172/JCI109405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickey J., Tate R., Lefkowitz R. J. Subsensitivity of adenylate cyclase and decreased beta-adrenergic receptor binding after chronic exposure to (minus)-isoproterenol in vitro. J Biol Chem. 1975 Jul 25;250(14):5727–5729. [PubMed] [Google Scholar]
- Mukherjee C., Caron M. G., Lefkowitz R. J. Regulation of adenylate cyclase coupled beta-adrenergic receptors by beta-adrenergic catecholamines. Endocrinology. 1976 Aug;99(2):347–357. doi: 10.1210/endo-99-2-347. [DOI] [PubMed] [Google Scholar]
- Posner B. I., Kelly P. A., Friesen H. G. Induction of a lactogenic receptor in rat liver: influence of estrogen and the pituitary. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2407–2410. doi: 10.1073/pnas.71.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A. K., Milin B. S., McMinn D. M. Androgen receptor in rat liver: hormonal and developmental regulation of the cytoplasmic receptor and its correlation with the androgen-dependent synthesis of alpha2u-globulin. Biochim Biophys Acta. 1974 Jul 4;354(2):213–232. doi: 10.1016/0304-4165(74)90008-7. [DOI] [PubMed] [Google Scholar]
- Ruffolo R. R., Jr, Fowble J. W., Miller D. D., Patil P. N. Binding of [3H]dihydroazapetine to alpha-adrenoreceptor-related proteins from rat vas deferens. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2730–2734. doi: 10.1073/pnas.73.8.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter W. J., Hirata F., Axelrod J. Phospholipid methylation unmasks cryptic beta-adrenergic receptors in rat reticulocytes. Science. 1979 Jun 15;204(4398):1205–1207. doi: 10.1126/science.221977. [DOI] [PubMed] [Google Scholar]
- Williams L. T., Snyderman R., Lefkowitz R. J. Identification of beta-adrenergic receptors in human lymphocytes by (-) (3H) alprenolol binding. J Clin Invest. 1976 Jan;57(1):149–155. doi: 10.1172/JCI108254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe B. B., Harden T. K., Molinoff P. B. In vitro study of beta-adrenergic receptors. Annu Rev Pharmacol Toxicol. 1977;17:575–604. doi: 10.1146/annurev.pa.17.040177.003043. [DOI] [PubMed] [Google Scholar]



