Abstract
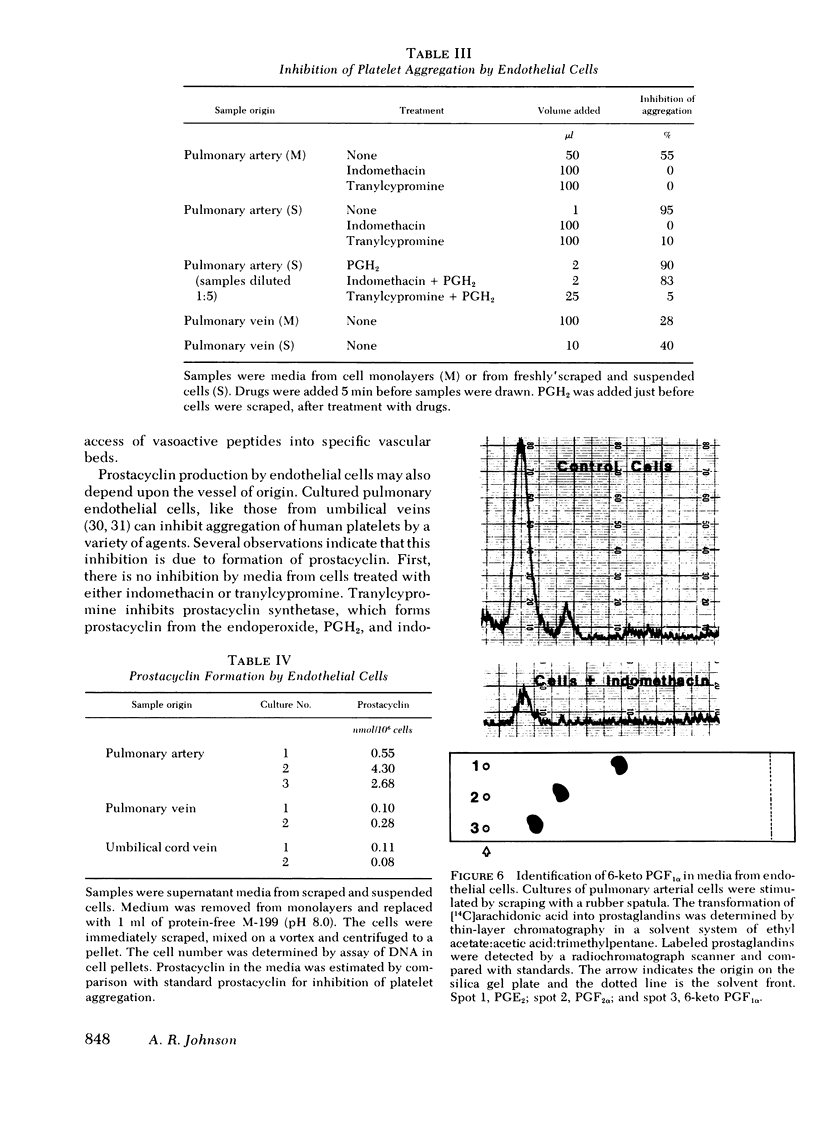
Endothelial cells were cultured from various different human vessels, including aortas, pulmonary, ovarian, and umbilical arteries, and pulmonary, ovarian, and umbilical veins. The cultured cells were identified as endothelial cells by the presence of Factor VIII antigen and antiotensin I converting enzyme (kininase II). They retained these markers for at least five passages in culture, and some cells had them for seven passages or more. Endothelial cells from the various vessels were compared with respect to their ability to metabolize angiotensins I and II and bradykinin. Cells from arteries had three to five times the angiotensin I converting enzyme activity as cells from veins. The activity of angiotensinase A (aspartyl aminopeptidase) had a similar distribution, and cells from arteries were consistently more active than cells from veins. Cultures of endothelial cells from pulmonary and umbilical vessels formed prostacyclin in response to mechanical stimulation. Media from cell monolayers that were subjected to a change of medium and gentle agitation inhibited aggregation of human platelets. This inhibitory activity was generated within 2-5 min, and it was not formed by cells that were treated with indomethacin or tranylcypromine. Addition of prostaglandin (PG)H2 to indomethacin-treated cells restored the ability to form the inhibitor, but cells treated with tranylcypromine were not responsive to PGH2. In experiments where [14C]arachidonic acid was added to the cells before stimulation, the major metabolite identified by thin-layer chromatography was 6-keto PGF1α. Thus, it appears that pulmonary endothelial cells, as well as umbilical cord cells, can form prostacyclin. In experiments comparing the ability of arterial and venous cells to form prostacyclin, arterial cells were more active than venous cells. These studies of cells from various human vessels suggest that the vascular origin of cultured endothelial cells determines how they metabolize vasoactive peptides and form prostacyclin.
Full text
PDF



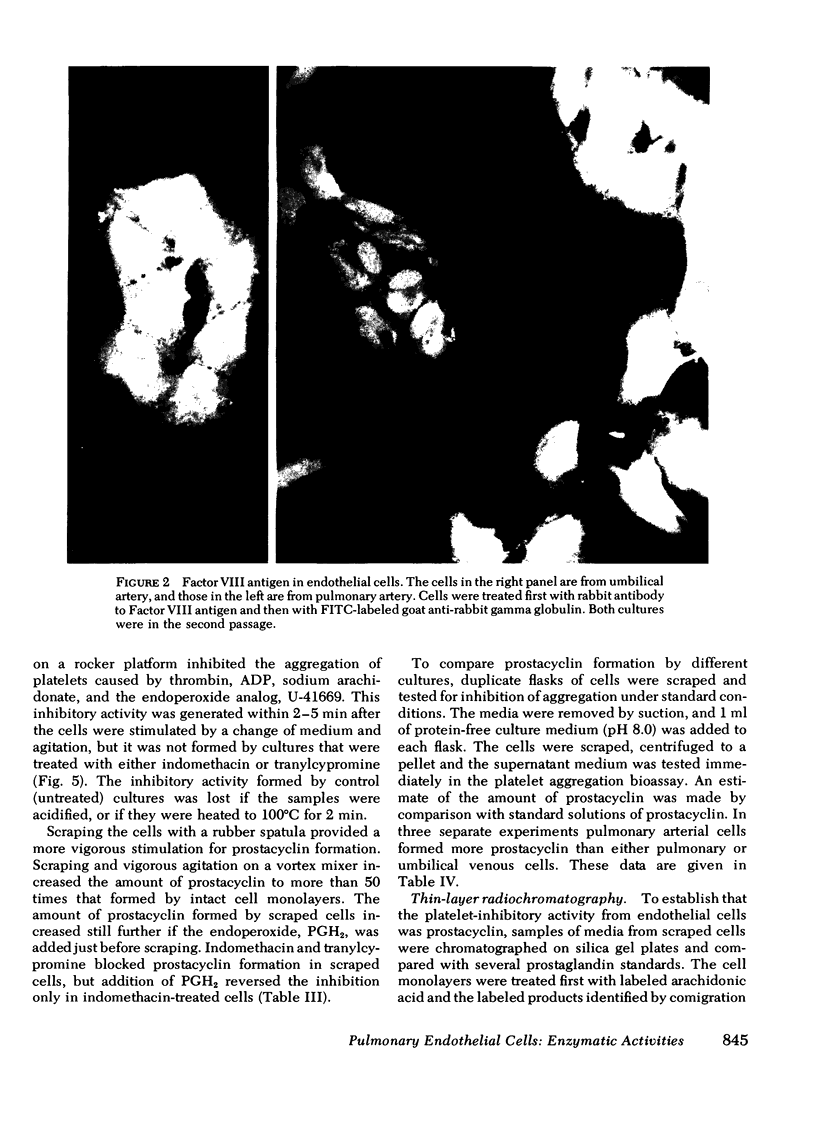
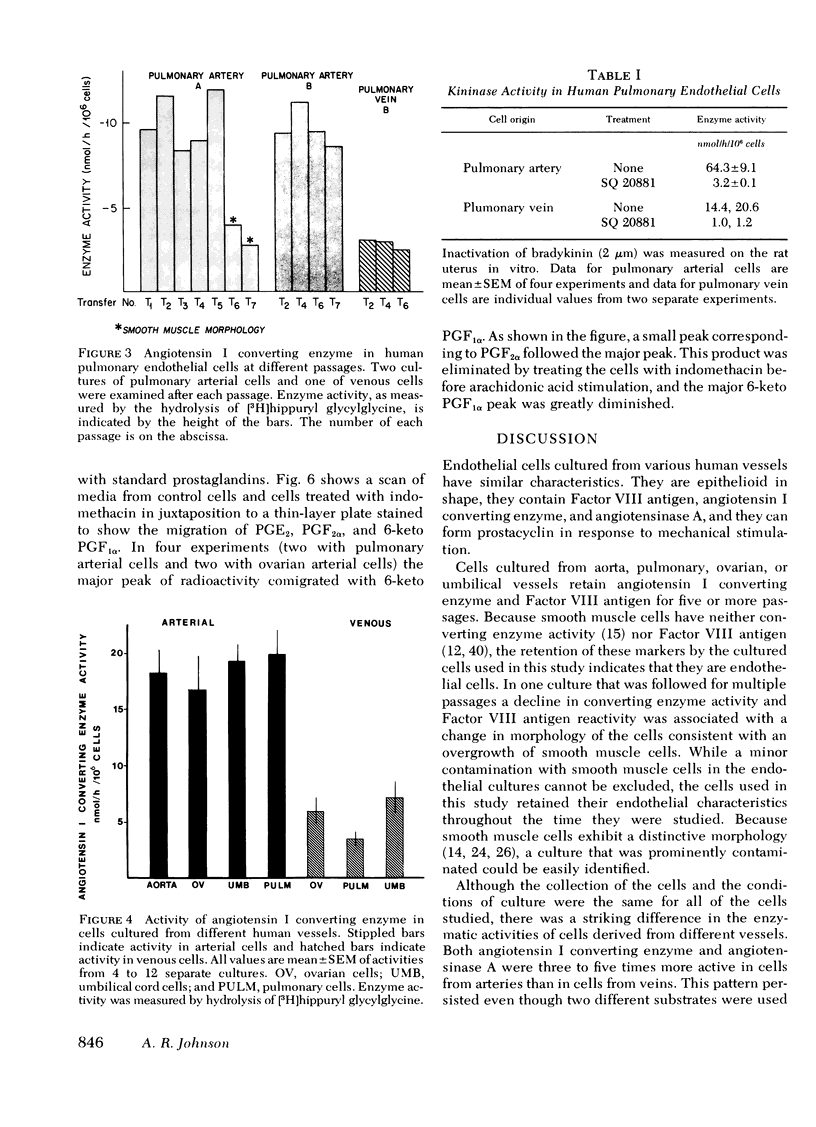
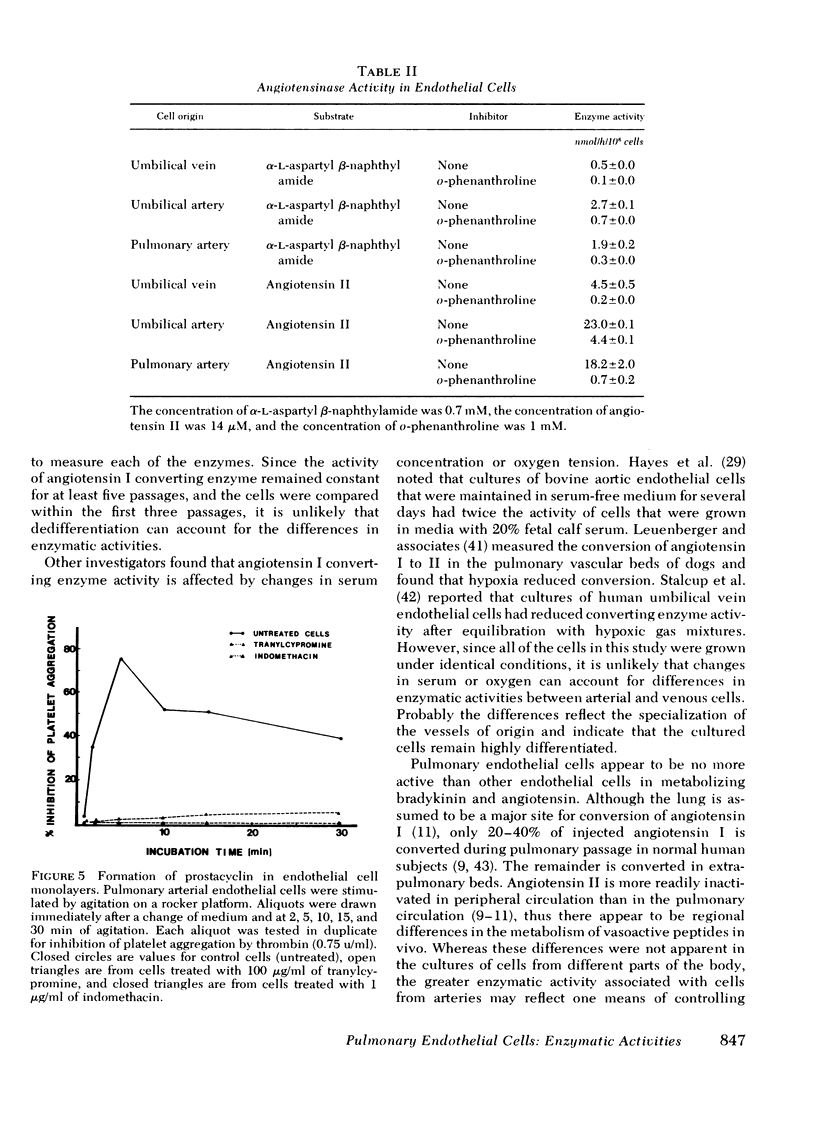


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiken J. W., Vane J. R. Inhibition of converting enzyme of the renin-angiotensin system in kidneys and hindlegs of dogs. Circ Res. 1972 Mar;30(3):263–273. doi: 10.1161/01.res.30.3.263. [DOI] [PubMed] [Google Scholar]
- Baenziger N. L., Becherer P. R., Majerus P. W. Characterization of prostacyclin synthesis in cultured human arterial smooth muscle cells, venous endothelial cells and skin fibroblasts. Cell. 1979 Apr;16(4):967–974. doi: 10.1016/0092-8674(79)90111-9. [DOI] [PubMed] [Google Scholar]
- Baenziger N. L., Dillender M. J., Majerus P. W. Cultured human skin fibroblasts and arterial cells produce a labile platelet-inhibitory prostaglandin. Biochem Biophys Res Commun. 1977 Sep 9;78(1):294–301. doi: 10.1016/0006-291x(77)91253-0. [DOI] [PubMed] [Google Scholar]
- Bakhle Y. S., Reynard A. M., Vane J. R. Metabolism of the angiotensins in isolated perfused tissues. Nature. 1969 Jun 7;222(5197):956–959. doi: 10.1038/222956a0. [DOI] [PubMed] [Google Scholar]
- Biron P., Campeau L., David P. Fate of angiotensin I and II in the human pulmonary circulation. Am J Cardiol. 1969 Oct;24(4):544–547. doi: 10.1016/0002-9149(69)90498-6. [DOI] [PubMed] [Google Scholar]
- Biron P., Campeau L. Pulmonary and extrapulmonary fate of angiotensin I. Rev Can Biol. 1971 Mar;30(1):27–34. [PubMed] [Google Scholar]
- Biron P., Huggins C. G. Pulmonary activation of synthetic angiotensin I. Life Sci. 1968 Sep 1;7(17):965–970. doi: 10.1016/0024-3205(68)90103-3. [DOI] [PubMed] [Google Scholar]
- Booyse F. M., Quarfoot A. J., Bell S., Fass D. N., Lewis J. C., Mann K. G., Bowie E. J. Cultured aortic endothelial cells from pigs with von Willebrand disease: in vitro model for studying the molecular defect(s) of the disease. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5702–5706. doi: 10.1073/pnas.74.12.5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booyse F. M., Sedlak B. J., Rafelson M. E., Jr Culture of arterial endothelial cells: characterization and growth of bovine aortic cells. Thromb Diath Haemorrh. 1975 Dec 15;34(3):825–839. [PubMed] [Google Scholar]
- Caldwell P. R., Seegal B. C., Hsu K. C., Das M., Soffer R. L. Angiotensin-converting enzyme: vascular endothelial localization. Science. 1976 Mar 12;191(4231):1050–1051. doi: 10.1126/science.175444. [DOI] [PubMed] [Google Scholar]
- Collier J. G., Robinson B. F. Comparison of effects of locally infused angiotensin I and II on hand veins and forearm arteries in man: evidence for converting enzyme activity in limb vessels. Clin Sci Mol Med. 1974 Aug;47(2):189–192. doi: 10.1042/cs0470189. [DOI] [PubMed] [Google Scholar]
- Czervionke R. L., Smith J. B., Hoak J. C., Fry G. L., Haycraft D. L. Use of a radioimmunoassay to study thrombin-induced release of PGI2 from cultured endothelium. Thromb Res. 1979;14(4-5):781–786. doi: 10.1016/0049-3848(79)90132-4. [DOI] [PubMed] [Google Scholar]
- Dieterle Y., Ody C., Ehrensberger A., Stalder H., Junod A. F. Metabolism and uptake of adenosine triphosphate and adenosine by porcine aortic and pulmonary endothelial cells and fibroblasts in culture. Circ Res. 1978 Jun;42(6):869–876. doi: 10.1161/01.res.42.6.869. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Cotran R. S., Folkman J. Human vascular endothelial cells in culture. Growth and DNA synthesis. J Cell Biol. 1974 Mar;60(3):673–684. doi: 10.1083/jcb.60.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Bialecki H., Zetter B. R. Factors involved in the modulation of cell proliferation in vivo and in vitro: the role of fibroblast and epidermal growth factors in the proliferative response of mammalian cells. In Vitro. 1978 Jan;14(1):85–118. doi: 10.1007/BF02618177. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Moran J., Braun D., Birdwell C. Clonal growth of bovine vascular endothelial cells: fibroblast growth factor as a survival agent. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4120–4124. doi: 10.1073/pnas.73.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haudenschild C. C., Cotran R. S., Gimbrone M. A., Jr, Folkman J. Fine structure of vascular endothelium in culture. J Ultrastruct Res. 1975 Jan;50(1):22–32. doi: 10.1016/s0022-5320(75)90004-0. [DOI] [PubMed] [Google Scholar]
- Hayes L. W., Goguen C. A., Ching S. F., Slakey L. L. Angiotensin-converting enzyme: accumulation in medium from cultured endothelial cells. Biochem Biophys Res Commun. 1978 Jun 29;82(4):1147–1153. doi: 10.1016/0006-291x(78)90306-6. [DOI] [PubMed] [Google Scholar]
- Hoyer L. W., De los Santos R. P., Hoyer J. R. Antihemophilic factor antigen. Localization in endothelial cells by immunofluorescent microscopy. J Clin Invest. 1973 Nov;52(11):2737–2744. doi: 10.1172/JCI107469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igic R., Erdös E. G., Yeh H. S., Sorrells K., Nakajima T. Angiotensin I converting enzyme of the lung. Circ Res. 1972 Sep;31(9 Suppl):51–61. [PubMed] [Google Scholar]
- Jaffe E. A., Hoyer L. W., Nachman R. L. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest. 1973 Nov;52(11):2757–2764. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. R., Erdös E. G. Metabolism of vasoactive peptides by human endothelial cells in culture. Angiotensin I converting enzyme (kininase II) and angiotensinase. J Clin Invest. 1977 Apr;59(4):684–695. doi: 10.1172/JCI108687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuenberger P. J., Stalcup S. A., Mellins R. B., Greenbaum L. M., Turino G. M. Decrease in angiotensin I conversion by acute hypoxia in dogs. Proc Soc Exp Biol Med. 1978 Sep;158(4):586–589. doi: 10.3181/00379727-158-40252. [DOI] [PubMed] [Google Scholar]
- Lewis L. J., Hoak J. C., Maca R. D., Fry G. L. Replication of human endothelial cells in culture. Science. 1973 Aug 3;181(4098):453–454. doi: 10.1126/science.181.4098.453. [DOI] [PubMed] [Google Scholar]
- Loskutoff D. J., Edgington T. E. Synthesis of a fibrinolytic activator and inhibitor by endothelial cells. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3903–3907. doi: 10.1073/pnas.74.9.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macarak E. J., Howard B. V., Kefalides N. A. Properties of calf endothelial cells in culture. Lab Invest. 1977 Jan;36(1):62–67. [PubMed] [Google Scholar]
- Marcus A. J. The role of lipids in platelet function: with particular reference to the arachidonic acid pathway. J Lipid Res. 1978 Sep;19(7):793–826. [PubMed] [Google Scholar]
- Marcus A. J., Weksler B. B., Jaffe E. A. Enzymatic conversion of prostaglandin endoperoxide H2 and arachidonic acid to prostacyclin by cultured human endothelial cells. J Biol Chem. 1978 Oct 25;253(20):7138–7141. [PubMed] [Google Scholar]
- Nagatsu I., Gillespie L., George J. M., Folk J. E., Glenner G. G. Serum aminopeptidases, "angiotensinase," and hypertension. II. Amino acid beta-napthylamide hydrolysis by normal and hypertensive serum. Biochem Pharmacol. 1965 May;14(5):853–861. doi: 10.1016/0006-2952(65)90105-x. [DOI] [PubMed] [Google Scholar]
- Ondetti M. A., Williams N. J., Sabo E. F., Pluscec J., Weaver E. R., Kocy O. Angiotensin-converting enzyme inhibitors from the venom of Bothrops jararaca. Isolation, elucidation of structure, and synthesis. Biochemistry. 1971 Oct 26;10(22):4033–4039. doi: 10.1021/bi00798a004. [DOI] [PubMed] [Google Scholar]
- Pearson J. D., Olverman H. J., Gordon J. L. Transport of 5-hydroxytryptamine by endothelial cells [proceedings]. Biochem Soc Trans. 1977;5(4):1181–1183. doi: 10.1042/bst0051181. [DOI] [PubMed] [Google Scholar]
- Ryan J. W., Chung A., Ammons C., Carlton M. L. A simple radioassay for angiotensin-converting enzyme. Biochem J. 1977 Nov 1;167(2):501–504. doi: 10.1042/bj1670501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J. W., Ryan U. S. Pulmonary endothelial cells. Fed Proc. 1977 Dec;36(13):2683–2691. [PubMed] [Google Scholar]
- Ryan J. W., Ryan U. S., Schultz D. R., Whitaker C., Chung A. Subcellular localization of pulmonary antiotensin-converting enzyme (kininase II). Biochem J. 1975 Feb;146(2):497–499. doi: 10.1042/bj1460497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan U. S., Ryan J. W., Whitaker C., Chiu A. Localization of angiotensin converting enzyme (kininase II). II. Immunocytochemistry and immunofluorescence. Tissue Cell. 1976;8(1):125–145. doi: 10.1016/0040-8166(76)90025-2. [DOI] [PubMed] [Google Scholar]
- Shepro D., Batbouta J. C., Robblee L. S., Carson M. P., Belamarich F. A. Serotonin transport by cultured bovine aortic endothelium. Circ Res. 1975 Jun;36(6):799–806. doi: 10.1161/01.res.36.6.799. [DOI] [PubMed] [Google Scholar]
- Skidgel R. A., Printz M. P. PGI2 production by rat blood vessels: diminished prostacyclin formation in veins compared to arteries. Prostaglandins. 1978 Jul;16(1):1–16. doi: 10.1016/0090-6980(78)90196-x. [DOI] [PubMed] [Google Scholar]
- Stalcup S. A., Lipset J. S., Woan J. M., Leuenberger P., Mellins R. B. Inhibition of angiotensin converting enzyme activity in cultured endothelial cells by hypoxia. J Clin Invest. 1979 May;63(5):966–976. doi: 10.1172/JCI109397. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Vane J. R. The release and fate of vaso-active hormones in the circulation. Br J Pharmacol. 1969 Feb;35(2):209–242. doi: 10.1111/j.1476-5381.1969.tb07982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler B. B., Ley C. W., Jaffe E. A. Stimulation of endothelial cell prostacyclin production by thrombin, trypsin, and the ionophore A 23187. J Clin Invest. 1978 Nov;62(5):923–930. doi: 10.1172/JCI109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler B. B., Marcus A. J., Jaffe E. A. Synthesis of prostaglandin I2 (prostacyclin) by cultured human and bovine endothelial cells. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3922–3926. doi: 10.1073/pnas.74.9.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. Y., Erdös E. G., Levin Y. Characterization of a dipeptide hydrolase (kininase II: angiotensin I converting enzyme). J Pharmacol Exp Ther. 1971 Apr;177(1):291–300. [PubMed] [Google Scholar]